Submitted:
28 March 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
1. The Mask Issue
1.1. RCTs on Masks
1.2. The Cochrane Review on Mask RCTs
1.3. Meta-Analyses that Support Masking
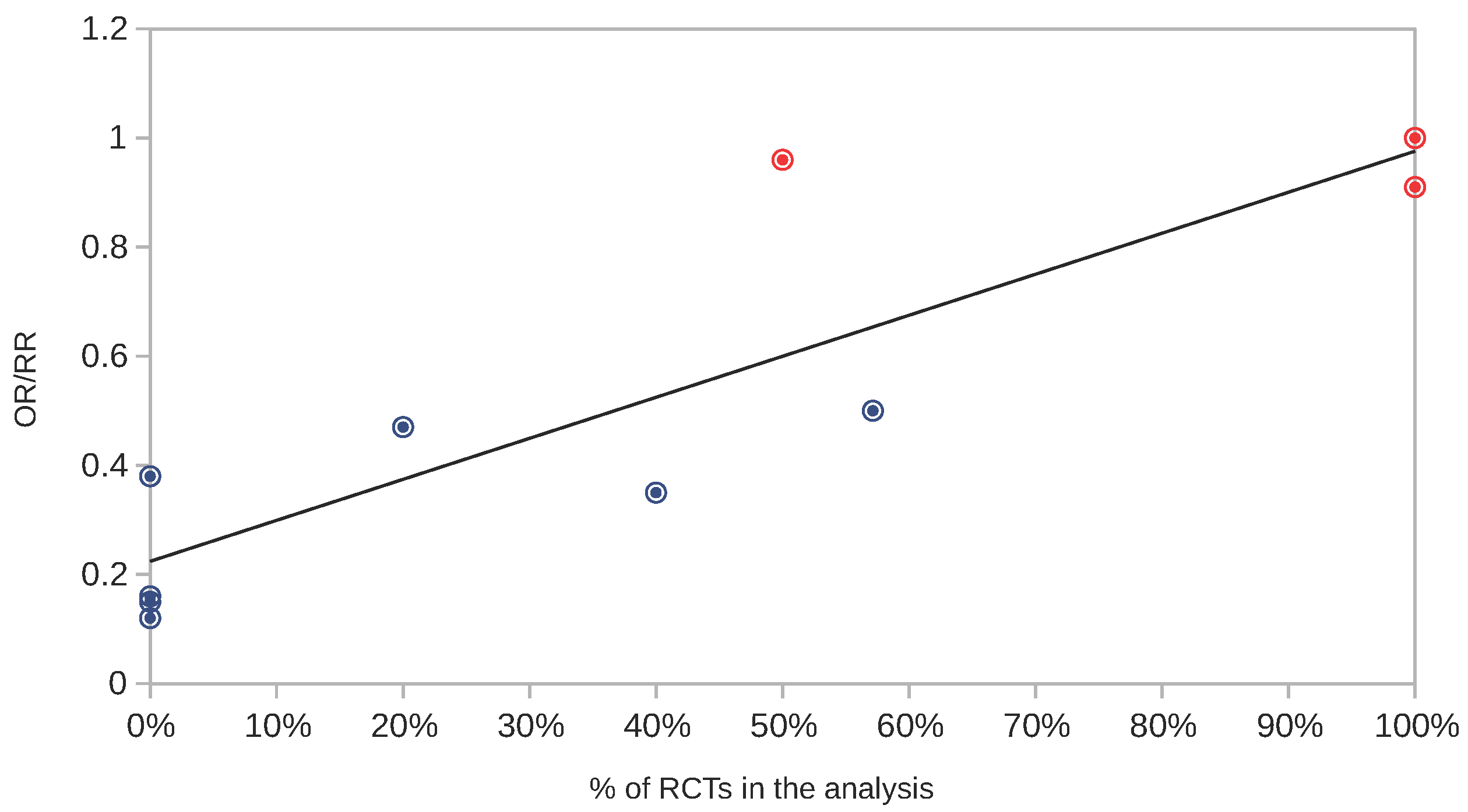
1.4. Randomised Trials with Positive Results of Masks and Their Statistical Significance
1.5. On Masks and Condoms
1.6. Masking as an “Alternative Therapy”
1.7. Masking Priors
1.8. Mask in the Lab – Filtering Efficacy as a Secondary Outcome
References
- Ernst, E.; Smith, K. Research Fundamentals. In More Harm than Good? Springer International Publishing: Cham, 2018; pp. 25–60. [Google Scholar] [CrossRef]
- Fanaroff, A.C.; Califf, R.M.; Harrington, R.A.; Granger, C.B.; McMurray, J.J.; Patel, M.R.; Bhatt, D.L.; Windecker, S.; Hernandez, A.F.; Gibson, C.M.; Alexander, J.H.; Lopes, R.D. Randomized Trials Versus Common Sense and Clinical Observation. Journal of the American College of Cardiology 2020, 76, 580–589. [Google Scholar] [CrossRef] [PubMed]
- Guyatt, G.H.; Oxman, A.D.; Montori, V.; Vist, G.; Kunz, R.; Brozek, J.; Alonso-Coello, P.; Djulbegovic, B.; Atkins, D.; Falck-Ytter, Y.; Williams, J.W.; Meerpohl, J.; Norris, S.L.; Akl, E.A.; Schünemann, H.J. GRADE guidelines: 5. Rating the quality of evidence—publication bias. Journal of Clinical Epidemiology 2011, 64, 1277–1282. [Google Scholar] [CrossRef] [PubMed]
- Razavi, M.; Glasziou, P.; Klocksieben, F.A.; Ioannidis, J.P.A.; Chalmers, I.; Djulbegovic, B. US Food and Drug Administration Approvals of Drugs and Devices Based on Nonrandomized Clinical Trials: A Systematic Review and Meta-analysis. JAMA Netw Open 2019, 2, e1911111. [Google Scholar] [CrossRef] [PubMed]
- Djulbegovic, B.; Glasziou, P.; Klocksieben, F.A.; Reljic, T.; VanDenBergh, M.; Mhaskar, R.; Ioannidis, J.P.; Chalmers, I. Larger effect sizes in nonrandomized studies are associated with higher rates of EMA licensing approval. Journal of Clinical Epidemiology 2018, 98, 24–32. [Google Scholar] [CrossRef] [PubMed]
- Bundgaard, H.; Bundgaard, J.S.; Raaschou-Pedersen, D.E.T.; von Buchwald, C.; Todsen, T.; Norsk, J.B.; Pries-Heje, M.M.; Vissing, C.R.; Nielsen, P.B.; Winsløw, U.C. Effectiveness of adding a mask recommendation to other public health measures to prevent SARS-CoV-2 infection in Danish mask wearers: a randomized controlled trial. Annals of internal medicine 2021, 174, 335–343, Publisher: American College of Physicians. [Google Scholar] [CrossRef] [PubMed]
- Abaluck, J.; Kwong, L.H.; Styczynski, A.; Haque, A.; Kabir, M.A.; Bates-Jefferys, E.; Crawford, E.; Benjamin-Chung, J.; Raihan, S.; Rahman, S. Impact of community masking on COVID-19: A cluster-randomized trial in Bangladesh. Science 2021. p. eabi9069. Publisher: American Association for the Advancement of Science. [Google Scholar]
- Unherd. danish-mask-study-professor-protective-effect-may-be-small-but-masks-are-worthwhile, 2020.
- Thornley, S.; Jackson, M.D.; Sundborn, G. Danish mask study: masks, media, fact checkers, and the interpretation of scientific evidence. BMJ 2020. p. m4919. Publisher: BMJ. [Google Scholar] [CrossRef]
- O’Grady, C. ‘It’s misinformation at worst.’ Weak health studies can do more harm than good, scientists say, 2021.
- Head, M.; Gill, J. expert reaction to paper using an RCT to assess mask use as a public health measure to help control SARS-CoV-2 spread (DANMASK-19), 2020.
- Ontario, P.H. Review of “Effectiveness of Adding a MaskRecommendation to Other Public HealthMeasures to Prevent SARS-CoV-2 Infection inDanish Mask Wearers”, 2020.
- Leech, G.; Rogers-Smith, C.; Monrad, J.T.; Sandbrink, J.B.; Snodin, B.; Zinkov, R.; Rader, B.; Brownstein, J.S.; Gal, Y.; Bhatt, S.; Sharma, M.; Mindermann, S.; Brauner, J.M.; Aitchison, L. Mask wearing in community settings reduces SARS-CoV-2 transmission. Proceedings of the National Academy of Sciences 2022, 119. Publisher: Proceedings of the National Academy of Sciences. [Google Scholar] [CrossRef] [PubMed]
- Peeples, L. Face masks for COVID pass their largest test yet, 2021.
- Conger, K. Surgical masks reduce COVID-19 spread, large-scale study shows, 2021.
- Chikina, M.; Pegden, W.; Recht, B. Re-analysis on the statistical sampling biases of a mask promotion trial in Bangladesh: a statistical replication. Trials 2022, 23. Publisher: Springer Science and Business Media LLC. [Google Scholar] [CrossRef] [PubMed]
- Loeb, M.; Bartholomew, A.; Hashmi, M.; Tarhuni, W.; Hassany, M.; Youngster, I.; Somayaji, R.; Larios, O.; Kim, J.; Missaghi, B.; others. Medical masks versus N95 respirators for preventing COVID-19 among health care workers: a randomized trial. Annals of Internal Medicine 2022, 175, 1629–1638, Publisher: American College of Physicians. [CrossRef] [PubMed]
- Jefferson, T.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; van Driel, M.L.; Bawazeer, G.A.; Jones, M.A.; Hoffmann, T.C.; Clark, J.; Beller, E.M.; Glasziou, P.P.; Conly, J.M. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2023, 2023. [Google Scholar] [CrossRef]
- Jefferson, T.; Mar, C.B.D.; Dooley, L.; Ferroni, E.; Al-Ansary, L.A.; Bawazeer, G.A.; Driel, M.L.v.; Jones, M.A.; Thorning, S.; Beller, E.M.; Clark, J.; Hoffmann, T.C.; Glasziou, P.P.; Conly, J.M. Physical interventions to interrupt or reduce the spread of respiratory viruses. Cochrane Database of Systematic Reviews 2020, 2020. Publisher: Wiley. [Google Scholar] [CrossRef]
- Alfelali, M.; Haworth, E.A.; Barasheed, O.; Badahdah, A.M.; Bokhary, H.; Tashani, M.; Azeem, M.I.; Kok, J.; Taylor, J.; Barnes, E.H.; El Bashir, H.; Khandaker, G.; Holmes, E.C.; Dwyer, D.E.; Heron, L.G.; Wilson, G.J.; Booy, R.; Rashid, H.; on behalf of the Hajj Research Team. Facemask against viral respiratory infections among Hajj pilgrims: A challenging cluster-randomized trial. PLoS ONE 2020, 15, e0240287. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.; Akl, E.A.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J.; Chu, D.K.; Akl, E.A.; El-harakeh, A.; Bognanni, A.; Lotfi, T.; Loeb, M.; Hajizadeh, A.; Bak, A.; Izcovich, A.; Cuello-Garcia, C.A.; Chen, C.; Harris, D.J.; Borowiack, E.; Chamseddine, F.; Schünemann, F.; Morgano, G.P.; Schünemann, G.E.U.M.; Chen, G.; Zhao, H.; Neumann, I.; Chan, J.; Khabsa, J.; Hneiny, L.; Harrison, L.; Smith, M.; Rizk, N.; Rossi, P.G.; AbiHanna, P.; El-khoury, R.; Stalteri, R.; Baldeh, T.; Piggott, T.; Zhang, Y.; Saad, Z.; Khamis, A.; Reinap, M.; Duda, S.; Solo, K.; Yaacoub, S.; Schünemann, H.J. Physical distancing, face masks, and eye protection to prevent person-to-person transmission of SARS-CoV-2 and COVID-19: a systematic review and meta-analysis. The Lancet 2020, 395, 1973–1987, Publisher: Elsevier BV. [Google Scholar] [CrossRef]
- Liang, M.; Gao, L.; Cheng, C.; Zhou, Q.; Uy, J.P.; Heiner, K.; Sun, C. Efficacy of face mask in preventing respiratory virus transmission: A systematic review and meta-analysis. Travel Medicine and Infectious Disease 2020, 36, 101751, Publisher: Elsevier BV. [Google Scholar] [CrossRef]
- Nanda, A.; Hung, I.; Kwong, A.; Man, V.C.; Roy, P.; Davies, L.; Douek, M. Efficacy of surgical masks or cloth masks in the prevention of viral transmission: Systematic review, meta-analysis, and proposal for future trial. J Evid Based Med. 2021, 14, 97–111. [Google Scholar] [CrossRef]
- Li, Y.; Liang, M.; Gao, L.; Ahmed, M.A.; Uy, J.P.; Cheng, C.; Zhou, Q.; Sun, C. Face masks to prevent transmission of COVID-19: A systematic review and meta-analysis. American Journal of Infection Control 2021, 49, 900–906, Publisher: Elsevier BV. [Google Scholar] [CrossRef] [PubMed]
- Talic, S.; Shah, S.; Wild, H.; Gasevic, D.; Maharaj, A.; Ademi, Z.; Li, X.; Xu, W.; Mesa-Eguiagaray, I.; Rostron, J.; Theodoratou, E.; Zhang, X.; Motee, A.; Liew, D.; Ilic, D. Effectiveness of public health measures in reducing the incidence of covid-19, SARS-CoV-2 transmission, and covid-19 mortality: systematic review and meta-analysis. BMJ, 2021; p. e068302. [Google Scholar] [CrossRef]
- Tabatabaeizadeh, S.A. Airborne transmission of COVID-19 and the role of face mask to prevent it: a systematic review and meta-analysis. Eur J Med Res 2021, 26, 1. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.S.; Seong, D.; Li, H.; Chung, S.K.; Park, Y.; Lee, M.; Lee, S.W.; Yon, D.K.; Kim, J.H.; Lee, K.H.; Solmi, M.; Dragioti, E.; Koyanagi, A.; Jacob, L.; Kronbichler, A.; Tizaoui, K.; Cargnin, S.; Terrazzino, S.; Hong, S.H.; Abou Ghayda, R.; Radua, J.; Oh, H.; Kostev, K.; Ogino, S.; Lee, I.; Giovannucci, E.; Barnett, Y.; Butler, L.; McDermott, D.; Ilie, P.; Shin, J.I.; Smith, L. Comparative effectiveness of N95, surgical or medical, and non-medical facemasks in protection against respiratory virus infection: A systematic review and network meta-analysis. Reviews in Medical Virology 2022, 32. [Google Scholar] [CrossRef]
- Schoberer, D.; Osmancevic, S.; Reiter, L.; Thonhofer, N.; Hoedl, M. Rapid review and meta-analysis of the effectiveness of personal protective equipment for healthcare workers during the COVID-19 pandemic. Public Health in Practice 2022, 4, 100280. [Google Scholar] [CrossRef]
- Brainard, J.; Jones, N.R.; Lake, I.R.; Hooper, L.; Hunter, P.R. Community use of face masks and similar barriers to prevent respiratory illness such as COVID-19: a rapid scoping review. Eurosurveillance 2020, 25. Publisher: European Centre for Disease Control and Prevention (ECDC). [Google Scholar] [CrossRef]
- Wang, M.X.; Gwee, S.X.W.; Chua, P.E.Y.; Pang, J. Effectiveness of Surgical Face Masks in Reducing Acute Respiratory Infections in Non-Healthcare Settings: A Systematic Review and Meta-Analysis. Frontiers in Medicine 2020, 7. Publisher: Frontiers Media SA. [Google Scholar] [CrossRef]
- Spira, B. Correlation Between Mask Compliance and COVID-19 Outcomes in Europe. Cureus 2022. [Google Scholar] [CrossRef]
- Iwasaki, A.; Grubaugh, N.D. Why does Japan have so few cases of COVID-19? EMBO Mol Med 2020, 12. [Google Scholar] [CrossRef] [PubMed]
- Konishi, Y.; Saito, T.; Ishikawa, T.; Kanai, H.; Igei, N. How Did Japan Cope with COVID-19? Big Data and Purchasing Behavior*. Asian Economic Papers 2021, 20, 146–167. [Google Scholar] [CrossRef]
- IHME. Institute for Health Metrics and Evaluation. University of Washington, 2022.
- MacIntyre, C.R.; Wang, Q.; Seale, H.; Yang, P.; Shi, W.; Gao, Z.; Rahman, B.; Zhang, Y.; Wang, X.; Newall, A.T.; Heywood, A.; Dwyer, D.E. A Randomized Clinical Trial of Three Options for N95 Respirators and Medical Masks in Health Workers. Am J Respir Crit Care Med 2013, 187, 960–966. [Google Scholar] [CrossRef] [PubMed]
- Colquhoun, D. The reproducibility of research and the misinterpretation of p -values. R. Soc. open sci. 2017, 4, 171085. [Google Scholar] [CrossRef]
- Syed, Q. Behind the mask. Journey through an epidemic: some observations of contrasting public health responses to SARS. Journal of Epidemiology & Community Health 2003, 57, 855–856. [Google Scholar] [CrossRef]
- Valdiserri, R.O.; Holtgrave, D.R.; Kalichman, S.C. Barrier Methods for the Prevention of Infectious Diseases: Decades of Condom Research can Inform the Promotion of Face Mask Use. AIDS Behav 2020, 24, 3283–3287. [Google Scholar] [CrossRef]
- Yan, Y.; Bayham, J.; Richter, A.; Fenichel, E.P. Risk compensation and face mask mandates during the COVID-19 pandemic. Sci Rep 2021, 11, 3174. [Google Scholar] [CrossRef]
- Weller, S.C.; Davis-Beaty, K. Condom effectiveness in reducing heterosexual HIV transmission. Cochrane Database of Systematic Reviews 2002, 2012. [Google Scholar] [CrossRef]
- Gallo, M.F.; Grimes, D.A.; Lopez, L.M.; Schulz, K.F. Nonlatex versus latex male condoms for contraception. Cochrane Database of Systematic Reviews 2006. [Google Scholar] [CrossRef]
- Steiner, M.; Trussell, J.; Glover, L.; Joanis, C.; Spruyt, A.; Dorflinger, L. Standardized protocols for condom breakage and slippage trials: a proposal. Am J Public Health 1994, 84, 1897–1900. [Google Scholar] [CrossRef] [PubMed]
- Shang, A.; Huwiler-Müntener, K.; Nartey, L.; Jüni, P.; Dörig, S.; Sterne, J.A.; Pewsner, D.; Egger, M. Are the clinical effects of homoeopathy placebo effects? Comparative study of placebo-controlled trials of homoeopathy and allopathy. The Lancet 2005, 366, 726–732. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Reyes, M.X.; Granados Rugeles, C. Oral antibiotics versus parenteral antibiotics for severe pneumonia in children. Cochrane Database of Systematic Reviews 2006. [Google Scholar] [CrossRef] [PubMed]
- El Sayed, I.; Liu, Q.; Wee, I.; Hine, P. Antibiotics for treating scrub typhus. Cochrane Database of Systematic Reviews 2018, 2018. [Google Scholar] [CrossRef] [PubMed]
- Shah, Y.; Kurelek, J.W.; Peterson, S.D.; Yarusevych, S. Experimental investigation of indoor aerosol dispersion and accumulation in the context of COVID-19: Effects of masks and ventilation. Physics of Fluids 2021, 33, 073315. [Google Scholar] [CrossRef]
- Drewnick, F.; Pikmann, J.; Fachinger, F.; Moormann, L.; Sprang, F.; Borrmann, S. Aerosol filtration efficiency of household materials for homemade face masks: Influence of material properties, particle size, particle electrical charge, face velocity, and leaks. Aerosol Science and Technology 2021, 55, 63–79. [Google Scholar] [CrossRef]
- Sankhyan, S.; Heinselman, K.N.; Ciesielski, P.N.; Barnes, T.; Himmel, M.E.; Teed, H.; Patel, S.; Vance, M.E. Filtration Performance of Layering Masks and Face Coverings and the Reusability of Cotton Masks after Repeated Washing and Drying. Aerosol Air Qual. Res. 2021, 21, 210117. [Google Scholar] [CrossRef]
- Kwong, L.H.; Wilson, R.; Kumar, S.; Crider, Y.S.; Reyes Sanchez, Y.; Rempel, D.; Pillarisetti, A. Review of the Breathability and Filtration Efficiency of Common Household Materials for Face Masks. ACS Nano 2021, 15, 5904–5924. [Google Scholar] [CrossRef] [PubMed]
- Joo, T.; Takeuchi, M.; Liu, F.; Rivera, M.P.; Barr, J.; Blum, E.S.; Parker, E.; Tipton, J.H.; Varnedoe, J.; Dutta, B.; Lively, R.P.; Ng, N.L. Evaluation of particle filtration efficiency of commercially available materials for homemade face mask usage. Aerosol Science and Technology 2021, 55, 930–942. [Google Scholar] [CrossRef]
- Greenhalgh, T.; Fisman, D.; Cane, D.J.; Oliver, M.; Macintyre, C.R. Adapt or die: how the pandemic made the shift from EBM to EBM+ more urgent. BMJ EBM 2022, 27, 253–260. [Google Scholar] [CrossRef]
- Ioannidis, J.P.A. Why Science Is Not Necessarily Self-Correcting. Perspect Psychol Sci 2012, 7, 645–654. [Google Scholar] [CrossRef]
- Gall, T.; Ioannidis, J.P.A.; Maniadis, Z. The credibility crisis in research: Can economics tools help? PLoS Biol 2017, 15, e2001846. [Google Scholar] [CrossRef]
- Wong, C.H.; Siah, K.W.; Lo, A.W. Estimation of clinical trial success rates and related parameters. Biostatistics 2019, 20, 273–286. [Google Scholar] [CrossRef]
- Becker, R.E.; Greig, N.H. Fire in the ashes: Can failed Alzheimer’s disease drugs succeed with second chances? Alzheimer’s & Dementia 2013, 9, 50–57. [Google Scholar] [CrossRef]
- Müller, T.D.; Blüher, M.; Tschöp, M.H.; DiMarchi, R.D. Anti-obesity drug discovery: advances and challenges. Nat Rev Drug Discov 2022, 21, 201–223. [Google Scholar] [CrossRef]
- Beauchamp, J.D.; Mayhew, C.A. Revisiting the rationale of mandatory masking. Journal of Breath Research 2023, 17, 042001. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




