Introduction
Melanoma belongs to the most aggressive human cancers. In a metastatic phase it is resistant to the conventional treatment. Clinically it is a highly heterogeneous disease, which may translate into the systemic therapy failures. Despite a recent progress in the identification of a number of therapeutic targets or inhibitors of immune check-points, metastatic melanoma is still incurable. So far little is known about its pathogenesis, also the specific biomarkers which would allow for a better diagnosis and prediction of disease course are missing. Such a high invasiveness and metastatic potential of melanoma result from mutations and activation of different signal transduction pathways. The Hippo signaling is responsible for a growth control and differentiation of tissues and organs. It is also largely involved in tumor formation and metastasis. However, the specific mechanisms how the Hippo pathway controls melanoma are not known. Characterization of the key proteins involved in major signaling pathways and the analysis of their interactions will allow for a better understanding of the mechanisms of melanoma pathogenesis and identification of its specific clinical subtypes. This in turn will allow in the future for identification of the specific molecular targets for a more effective treatment.
The Hippo pathway
The Hippo signaling was initially discovered in
Drosophila melanogaster. It is an evolutionary conserved pathway which controls organ growth during the development of all metazoans [
1,
2]. The main role of the Hippo pathway is a thorough supervision and regulation of organ size, cell proliferation, apoptosis, and stemness in response to intrinsic and extrinsic signals that act through G-protein-coupled receptors [3-5]. Dysregulation of the pathway results in organ and tissue hypertrophy and is associated broadly with neoplastic phenotype and cancer metastasis [
6].
Main Components
Human Hippo pathway is composed of three interlinked parts, including upstream regulatory components, the core kinase cascade, and the downstream transcriptional machinery. The core of the Hippo pathway acts rather as a signalling network than a definite transduction cascade [
7]. It contains a conserved kinase cassette consisting of Mst1/2, Lats1/2, and MOBKL1A/B and MOBKL1B (Mob1) kinases [
8,
9]. MST1/2 (Mammalian Ste20-like kinases 1/2) forms the complex with SAV1 (
Salvador Family WW Domain Containing Protein 1) which phosphorylates and activates the kinase complex composed of LATS1/2 (Large tumour suppressor 1/2) and MOB1 (
MOB Kinase Activator) [10-14]. While actived LATS1/2 phosphorylates and inactivates YAP1 (Yes association protein 1) and its paralog TAZ (also known as WWTR1) [
11,
15]. In their unphosphorylated states, YAP1 and TAZ easily enter the nucleus and function as transcriptional activators. However, while phosphorylated, YAP1 and TAZ are bound by 14-3-3 proteins, and consequently sequestered in the cytosol and degraded by proteasome complex [
14,
16].
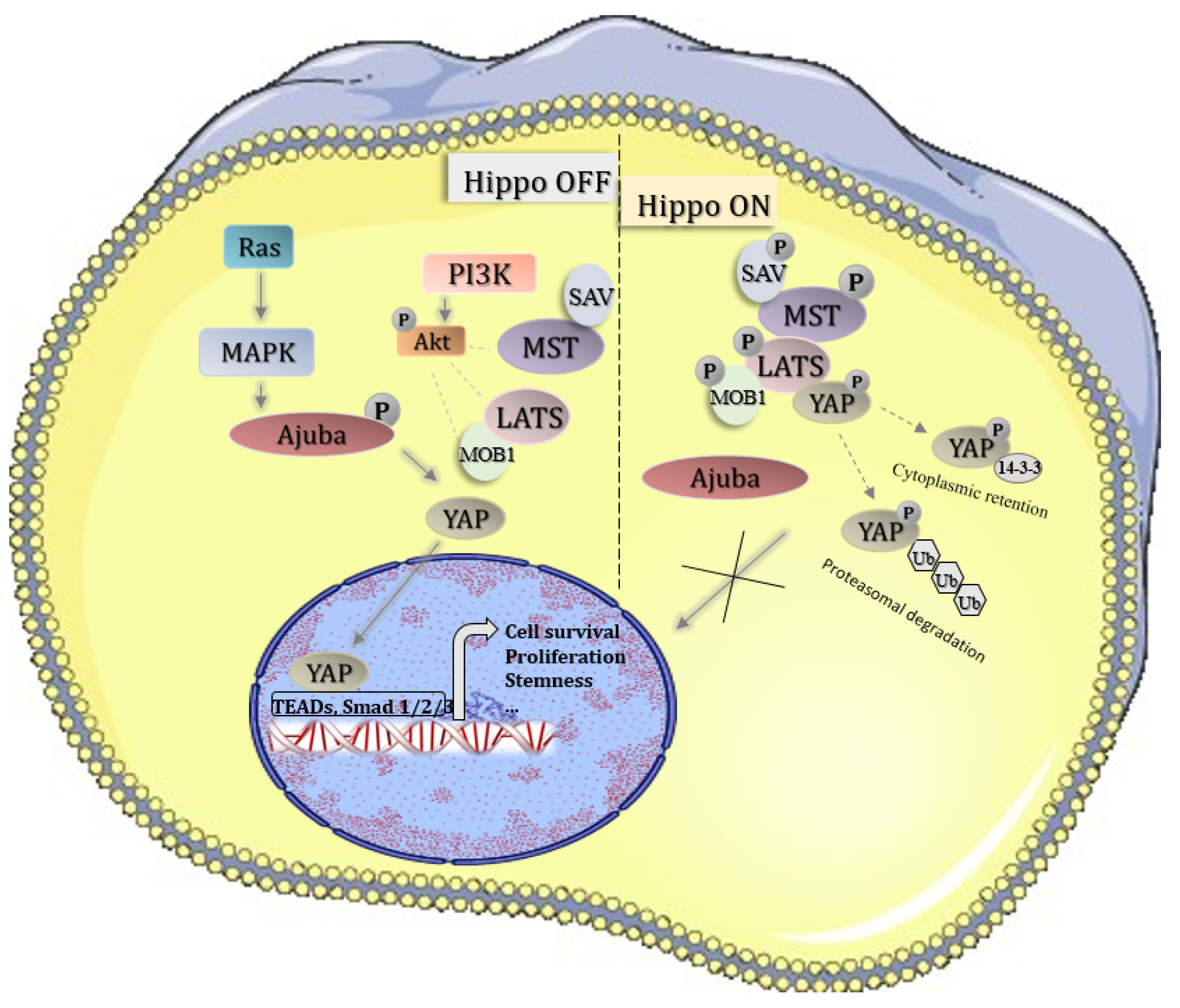
Figure 1.
Hippo pathway signaling; inactive Hippo leads to YAP oncogene transport to the nucleus and activation of pro-survival genes; active Hippo leads to YAP phosphorylation and its degradation in cytoplasm .
Figure 1.
Hippo pathway signaling; inactive Hippo leads to YAP oncogene transport to the nucleus and activation of pro-survival genes; active Hippo leads to YAP phosphorylation and its degradation in cytoplasm .
Upstream Regulators of the Core Kinase Cascade
The first identified upstream components of
the Hippo pathway in Drosophila were two FERM-domain containing proteins: Merlin (NF2 in mammals) and Expanded (FRMD6 in mammals) [
17]. NF2 (Merlin) is the product of the Neurofibromatosis type 2 (NF2) tumour suppressor gene and a member of ezrin-radixin-moesin (ERM) protein family [
18,
19]. In its closed inactive conformation, the CTD is joined with the N-terminal FERM domain. Phosphorylation of C-terminal residues disrupts the connection and liberates FERM to bind cell adhesion molecules. Mer/NF2 functions in complex with WWC1 (Kibra) and FRMD6 as an upstream effector of Hippo pathway in order to regulate cell and growth and suppress tumour development. WWC1-NF2-FMRD6 protein complex activates MST1 protein through autophosphorylation [
18,
20]. WWC1 is an apical protein containing two amino-terminal WW domains, an internal C2-like domain and carboxy-terminal glutamic acid-rich region. To promote Hippo signalling, NF2 binds to Kibra (WWC1 in mammals) [
17]. The last upstream regulator of the Hippo pathway is a transmembrane protein Crumbs (Crb) which functions in a complex with FRMD6 [
17].
The Core of the Hippo Signaling
The Mammalian Ste20-Like Kinases (MST) 1/2
Mammalian Ste20-like kinases (MST1/2) are classified among STE20 kinases. Primarily MST1/2 were described as elemental proapoptotic kinases, which are activated through signals linked to stress-induced apoptosis [
21,
22]. Mst1/2 activity is modulated both by autophosphorylation and caspase-cleavage [
23]. Following autophosphorylation of their threonine residues (Tre183 for MST1 and Tre180 for MST2), MST1/2 phosphorylates protein SAV1 (also referred to as WW45), thus initiating the Hippo pathway [
24]. Conversely, following caspase-cleavage, modified MST protein with liberated N-terminal kinase domain enters the nucleus and induces chromatin condensation by phosphorylating histone H2B, therefore causing DNA fragmentation and apoptosis [
11,
25]. Mst1/2 activity is also regulated by heterodimerization with RASSF family proteins, which are tumour suppressors. Frequently, in human cancers expression of RASSF proteins is restricted through epigenetic silencing [
24]. The experiments performed on mice proved that MST1/2 proteins are essential for proper development. Single knock-out of one MST1 or MST2 allele does not lead to any disorders. However, mice having both alleles knocked-out die in embryonal stadium due to developmental defects [
26].
Large Tumor Suppressors (LATS) 1/2
LATS1 and LATS2 belong to the serine-threonine kinases family and are involved in cell proliferation, apoptosis and regulation of the cell cycle. They are mainly active in the early prophase as LATS1 cooperates with CDC2/cyclin A and LATS2 binds to the proteins in centrosome region enabling the formation of kinetic spindle. Additionally, LATS1 negatively regulates G2/M transition by down-regulating CDK1 kinase activity [
27]. Correspondingly, LATS2 inhibits cell growth at the G1/S control point via downregulating cyclin E/CDK2 kinase activity [
28]. In regard of the Hippo pathway, LATS1 and LATS2 are tumour suppressors that inhibit the oncogenic nuclear function of YAP/TAZ and TEAD. Following phosphorylation by MST1/2, LATS1/2 connect with their coactivator MOB1 and undergo autophosphorylation. Simultaneously, SAV plays an important role of a scaffold protein for assembling MST1/2 and LATS1/2 [
29,
30]. Consequently, this active LATS1/2-MOB1 complex phosphorylates transcriptional coactivators YAP and TAZ on their multiple HxRxxS motifs, thereby leading to their inactivation [
31].
Mps One Binder Kinase Activator (MOB) 1
MOB 1 is an essential coactivator of LATS kinases. Overexpression of MOB1 triggers LATS1/2 activation and consequently leads to proliferation inhibition or even to apoptosis. Contrarily, decrease of MOB1 expression increases the rate of cell division. MOB1 deficiency or reduction is frequently associated with tumorigenesis, especially in human skin melanoma, colorectal cancer and non-small cell lung cancer [
32].
Hippo Pathway Effectors
Yes-Associated Protein (YAP)
YAP is an effector protein in the Hippo pathway. As it does not have a DNA-binding domain, it is classified as a transcriptional coactivator, which interacts with transcriptional factors in order to regulate gene expression [
33]. There were two isoforms of YAP identified: YAP1 with single WW domain (domain consisting of 40 amino acids, rich in proline and with two conservative tryptophan residues – WW) and YAP2 with double WW domain [
34]. WW domain proteins have an ability to bind other proteins that exhibit PY motifs, being proline-rich modules [
35]. Besides the WW domain, YAP1 and YAP2, contain an SH3 domain-binding motif and PDZ-binding motif in their C-terminus [
36]. YAP activity depends on its cellular localisation, since only while being in cell nucleus it may exert its functions. Contrarily, following the phosphorylation of serine residue in position 127, YAP binds with 14-3-3 protein and therefore it is inactivated and restrained outside nucleus. Apart from serine 127, there are 4 other consensus HXRXXS motifs, that may be phosphorylated (S61, S109, S164, and S381). Nonetheless, S127 and S381 are regarded as the critical sites related to oncogenic phenotype. Phosphorylation of solely S127 suffices to impede YAP activity [
37]. Phosphorylated YAP is either sequestrated in the cytoplasm via interaction with 14-3-3 protein or subjected to proteasomal or autophagy-induced degradation [
38]. As YAP chromosomal locus 11q22 is commonly amplified in cancer cells, its expression and nuclear localization is frequently elevated in human cancers [
39]. YAP performs a role of an oncogene in medulloblastoma, glioblastoma multiforme, squamous cell carcinoma, small cell lung cancer, liver cancer, colorectal cancer, pancreatic cancer and ovarian cancer [
40]. Noteworthy YAP overexpression leads to neoplastic transformation as cells gain ability to proliferate infinitely and uncontrollably, to undergo epithelial–mesenchymal transition (EMT) and to evade apoptosis [
41]. Additionally, YAP and its paralog TAZ make cancer cells evade immune surveillance [42-44]. Adversely, while in most solid tumours, YAP is an oncogene, in haematological cancers it plays a tumour-suppressor role [
45].
TEA Domain (TEAD) Factors
TEAD belongs to a family of transcriptional factors, which mediate the main transcriptional output of the Hippo pathway in response to YAP and TAZ activity [
46,
47]. All members of TEAD family contain identical DNA-binding domain, which connects with M-CAT motif (5’-CATTCCT-3’). TEAD transcription factors are expressed in every kind of tissue. However, depending on tissue type, the levels of particular TEAD factors vary and their physiological response may differ. For example YAP protein is indispensable for the full transcriptional activity of TEAD2. Interaction between TEAD1–4 and their major binding partners, YAP and TAZ, causes VGLL4 dissociation from TEAD1–4 and anchorage of YAP/TAZ to chromatin. Consequently TEAD-mediated gene transcription induces cell proliferation and inhibits apoptosis [
4,
48].
Response to Cell Density
As it is known that organ growth is controlled by the intrinsic network of pathways of which the Hippo pathway plays the pivotal role. The organ development depends on cell number and density, cell size as well as extracellular factors [
49]. The increased adherens and tight junctions in confluent cells contribute to activation of LATS and inactivation of YAP/TAZ [
50]. Detachment of the cells inactivates YAP/TAZ and triggers anoikis [
51]. At low cell density LATS kinase remains inactive, which leads to YAP/TAZ activation and evasion from cell-contact inhibition [
16].
Hippo Pathway in Cancer
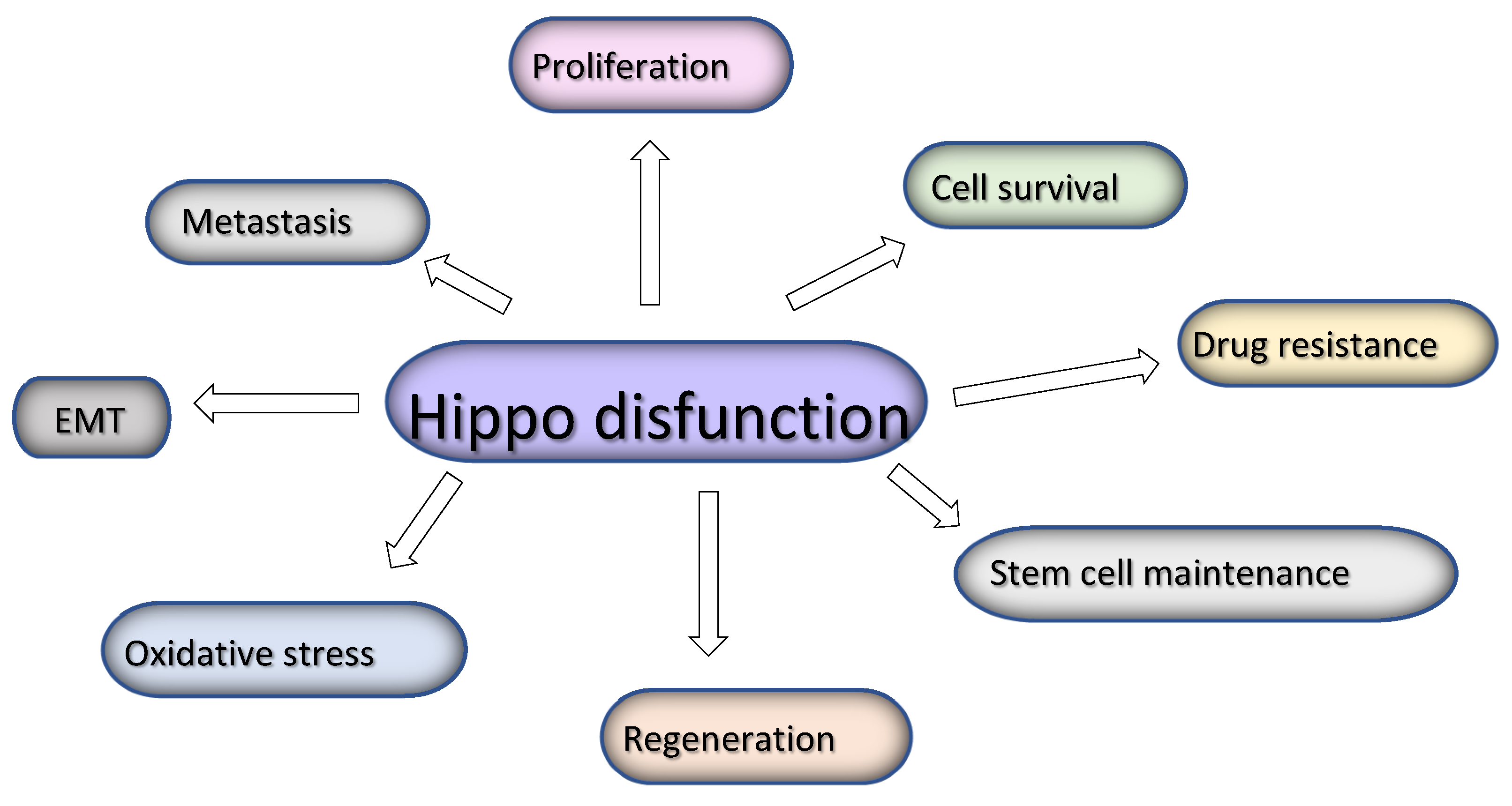
The Hippo pathway signaling is dysregulated in a variety of cancers leading to YAP/TAZ activation which in turn leads to hyperproliferation of cancer cells and lack of apoptosis, stem cell maintainance and epithelial-to–mesenchymal transition, increased invasion and metastatic potential, oxidative stress and finally resistance to therapies [
52].
Figure 2.
Hippo pathway dysfunction leads to several events linked with cancer progressio.
Figure 2.
Hippo pathway dysfunction leads to several events linked with cancer progressio.
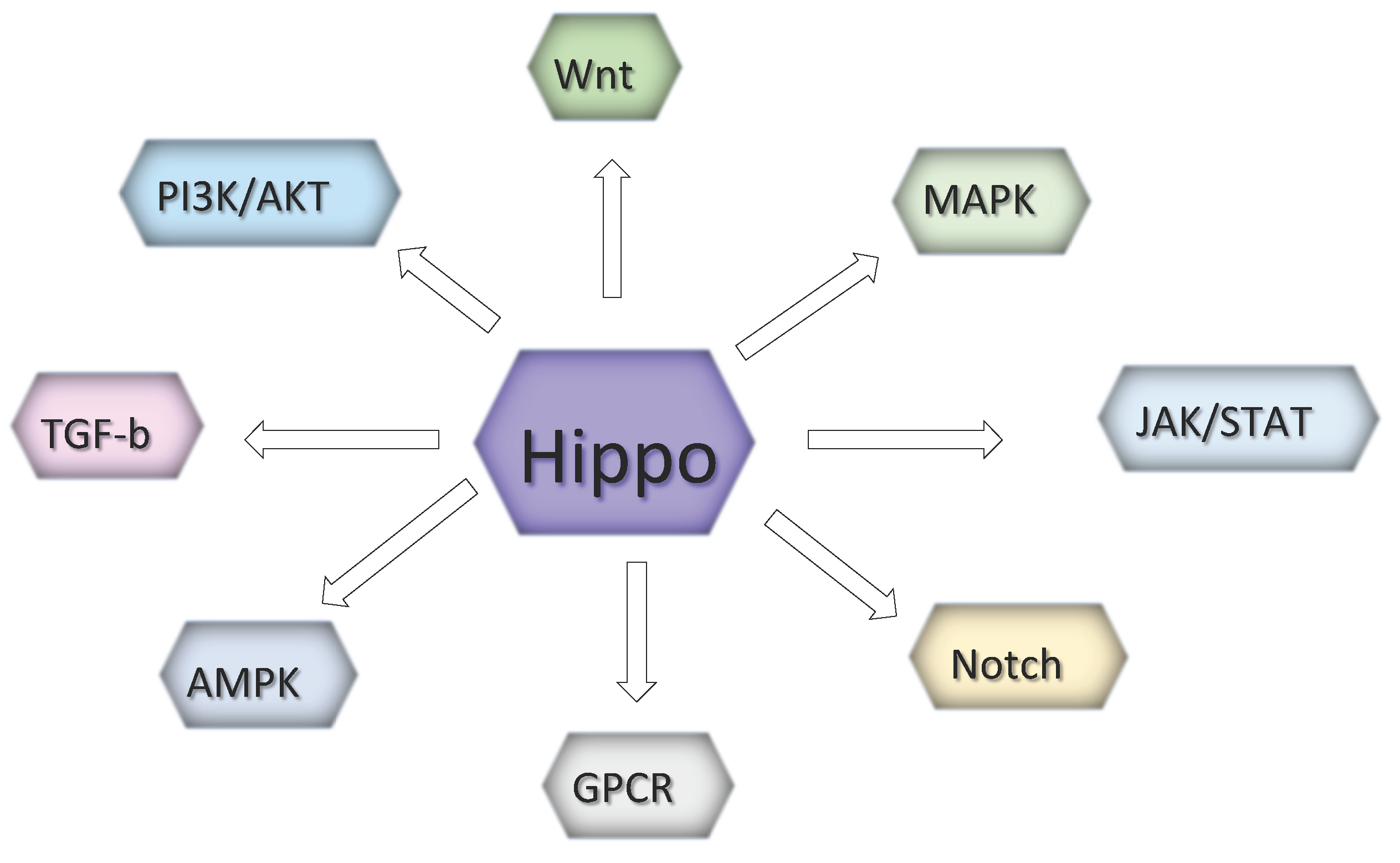
However, those event result from the mutual cooperation with other prominent signaling pathways such as the Wnt, G protein-coupled receptor (GPCR), epidermal growth factor (EGF), BMP/transforming growth factor beta (TGFβ), JAK/STAT and Notch pathways [
52,
53]. In melanoma, the interaction between the Hippo with PI3K/Akt (Akt) and ERK/Raf/Ras (MAPK) pathways is essential for tumor growth control [
54].
Figure 3.
Hippo signaling highly impacts several pathways involved in melanoma development.
Figure 3.
Hippo signaling highly impacts several pathways involved in melanoma development.
Hippo Pathway in Melanoma Biology and Treatment
Hippo pathway components are involved in determination of the overall number of cutaneous melanocytes. Mechanical signals mediated by contacts between melanocytes and the basement membrane, other melanocytes and keratinocytes are integrated in space and time to activate the Hippo signaling as it takes place in other tissues. These signals are complemented by biochemical markers send from keratinocytes, and transferred from surface receptors to cellular components through protein phosphorylation [
55]. The clinical data confirmed that the deregulation of the Hippo pathway often correlated with poor prognosis for melanoma patients [
56].
Hippo Signaling and BRAF/MEK Inhibitor Resistance
Targeted therapy with BRAF and MEK inhibitors significantly improved the prognosis of melanoma patients. However, it still demonstrates some limitations [
57,
58]. The vast majority of patients treated with BRAF inhibitors acquires the resistance within 1 or 2 years. Defining the mechanisms of that phenomenon is an emerging issue to improve the outcomes. It has been shown previously shown that YAP promote resistance to anti-cancer therapies. In the past decade, the YAP mechanotransduction pathway has been implicated in the resistance to BRAF and MEK inhibitors. Understanding the mechanisms leading to YAP-related resistance to RAF and MEK inhibitors is essential to develop more effective treatment. Lin et al. confirmed the unanticipated functional cross-talk between YAP and RAF-MEK signaling. The authors investigated whether YAP regulates the response to targeted inhibition of BRAF signaling in several tumor cell lines harboring BRAF, KRAS or NRAS activating mutations [
59]. They found that YAP1 suppression enhanced the response to both vemurafenib and trametinib in A2058 and WM793 melanoma cell lines, and A2058 melanoma xenografts. Most likely YAP enhances the expression of antiapoptotic factors such as BCL2 family member protein BCL-xL in resistant cells and YAP knockdown prevent that process. Moreover, YAP upregulation in BRAF- or RAS-mutant tumor cells might promote resistance to RAF and MEK inhibitors in patients. Accordingly, the they observed high levels of YAP in the majority of melanoma samples with mutant BRAF V600E. Interestingly, patients harboring BRAF V600E mutation who responded to RAF and MEK inhibitors (CR) exhibited lower YAP expression in the pretreatment tumor samples, and those with incomplete response had higher YAP expression before treatment [
59]. The above study clearly shows that YAP acts a survival factor in melanoma cells and its downregulation may be an effective way to enhance the therapeutic effects of RAF and MEK inhibitor based treatment.
Searching deeper into the mechanisms of BRAFi resistance, Kim et al. looked at the connection between Hippo pathway and anti-melanoma immune response. They genetically modified three melanoma cell lines (SKMEL28, WM3248, and A375) to activate YAP, and performed a set of in vitro experiments to test whether YAP activation changes the cytokine release by those cells, and how it influences the cytotoxic abilities of lymphocytes. They developed that YAP activation in BRAFi-resistant melanoma cells directly inhibited cytotoxic T-cell responses, and the mechanism they proposed was the induction of PD-L1 expression on tumor cells [
60,
61].
Kim et. al. studied the mechanism of BRAF inhibitor PLX4032 resistance in melanoma cell lines and linked it with actin cytoskeleton remodeling. They demonstrated that melanoma cells resistant to PLX4032 exhibit an increased content of stress fibers in actin cytoskeleton, which appears to promote the nuclear accumulation of YAP/TAZ [62-64]. After knocking down of YAP in PLX4032-resistant SK-MEL28 and WM3248 cell lines, they observed the reduced viability of the cells, whereas overexpression of constitutively active YAP induced resistance of the parental melanoma cell lines. YAP/TAZ knockdown influenced also EGFR and phosphorylated AKT levels, and resulted in significant enrichment of downregulated genes related with cell cycle and mitosis in PLX4032-resistant cells. Activated YAP overexpression suppressed the expression of SOX10 and MITF, and upregulating EGFR. The authors also showed that both c-MYC protein and mRNA levels are dependent on YAP/TAZ. Conversely, inhibition of actin polymerization and actomyosin tension suppresses both YAP/TAZ activation and PLX4032 resistance. The results of the above study suggest that inhibition of actin remodeling may be a potential strategy to suppress the resistance to BRAF-inhibitor based therapies [
62]. Also Monaghan-Benson found the connection between Hippo pathway and BRAF-related modification of cell cytoskeleton. They found that mutated BRAF was associated with a Rac-dependent cadherin switch in melanoma cells. Rac1 acts to modify the cytoskeleton. This gene is highly mutated in melanoma cells and loss of its function can change Hippo pathway regulation [
55,
65].
Recently Dieter et al. have described in Nature a novel approach for breaking YAP/TAZ-driven resistance to MAPK pathway inhibitor treatment. First, they transiently activated YAP1 in BRAFV600E mutant melanoma cell lines and established that it is YAP1 who induces the resistance to BRAF and MEK inhibitors. Moreover, YAP1 showed to drive the MITFlow/AXLhigh phenotype which seems to be a potential biomarker predicting response to MAPKi therapeutic approaches. Next, using a genome-wide CRISPR/Cas9 functional screen the authors identified the essential genes in MAPKi-resistant cells with activated YAP1. They distinguished
SLC35B2 gene which appeared to play a crucial role in that process. SLC35B2 is responsible for heparan sulfation expression and positively correlates with tumor progression. After suppressing the gene they noticed that such modification sensitizes the cells to BRAF inhibition [
66].
BRAF inhibitor resistance can be also triggered by the ubiquitin ligase- dependent degradation of LATS1 and MST2 in BRAFi resistant cell lines. Romano et. al. demonstrated that mutant BRAFV600E binds to and inhibits MST2 preventing the MST2-dependent apoptosis. Treatment of BRAFi resistant cells with proteasome inhibitors resulted in the rescue of proapoptotic MST2 and LATS1 signalling. The results from a small cohort of patients with resistance to BRAFi confirm that MST2 downregulation might be associated with the acquisition of resistance in human melanoma [
67].
YAP/TAZ in Melanoma Invasion and Metastasis
A connection between the Hippo signaling targets and melanoma pathogenesis has been subject of several studies. Nallet-Staub et al. provided an evidence that endogenous YAP/TAZ contribute to the invasiveness and metastatic behavior of melanoma cells. They performed profiling of human melanocytic lesions and melanoma cell lines for endogenous YAP and TAZ expression, followed by specific YAP/TAZ knockdown and overexpression experiments in melanoma cell lines to analyze the anchorage-independent growth, invasion into Matrigel, human skin reconstructs, and in vivo tumor growth and metastasis formation. They found that knockdown of either of these Hippo effectors lead to reduced clonogenic and invasive capacity in vitro, and decreased their ability to form lung metastases after intravenous injection in mice, while YAP/TAZ overexpression had the opposite effects. [
68,
69]. Interestingly, TAZ levels were higher than YAP in melanoma cells, and its knockdown gave better anti-melanoma response than YAP knockdown. The authors of the above study noticed that overexpression of both YAP and TAZ leads to enhancement of their nuclear activity despite the presence of their phosphorylated forms in the cytoplasm [
68,
70] YAP/TAZ overexpression increased anchorage-independent growth and invasion into Matrigel, hereby confirming a direct role for endogenous YAP and TAZ in controlling melanoma cell metastatic potential. This study corroborates the findings of Lamar
et al. from 2012 who showed that overexpression of YAP enhanced the frequency and size of melanoma tumors in lungs after intra-vein injection, in a TEAD binding dependent manner [
71]. They used A375 melanoma cell line for a Luminex-based in vivo assays and found that the domain of YAP that interacts with the TEAD/TEF family of transcription factors is essential for tumor cell proliferation, transformation, migration, and invasion. Thay also observed that the ability of melanoma cells to form metastases is significantly correlated with increased TEAD transcriptional activity [
71]. They generated an activated form of YAP by introducing the S127A mutation into YAP and found that it dramatically enhanced tumor growth and metastasis in mice. Mutation of the TEAD-interaction domain prevented tumor formation. Concluding, it seems that YAP-mediated melanoma growth and metastasis requires a functional TEAD-interaction domain. In addition, YAP exerts its prometastatic effects at both the primary and metastatic site, and enhances several processes which contribute to metastasis.
YAP/TAZ and CSC and EMT Phenotype
Kim et al. studied whether upstream elements of the Hippo pathway and the markers of epithelial-to-mesenchymal transition E-cadherin and N-cadherin (Kim et al., submitted) are correlated with YAP1 and TAZ expression. They found that the majority of melanoma cell lines tested had lost E-cadherin expression which was replaced with the expression of N-cadherin, which in turn was associated with a more invasive phenotype. Regarding YAP/TAZ, all cell lines strongly expressed TAZ and many additionally expressed YAP [
72].
A group of scientists from the University of Maryland School of Medicine searched for the role of YAP/TAZ in cancer stem cells (CSC). They studied BRAFi resistant melanoma stem cell spheroids (MCS) and noticed their enhanced growth and matrigel invasion, together with elevated YAP1, TAZ and TEAD levels. That effect was reversed after knockdown of TEAD proteins.They also performed knockdown and overexpression experiments to confirm a BRAFi resistance role of YAP1 and TAZ, since the inhibition of YAP1 function with verteporfin reduced YAP1/TAZ level and restored sensitivity to BRAFi [
73]. YAP1 expression can be downregulated after treatment with the combination of a histone deacetylase inhibitor LBH589 and a bromodomain inhibitor I-BET151 as it was shown in melanoma xenografts. Both drugs induced a caspase-dependent apoptosis which except of YAP1 decrease involved the downregulation of AKT pathway [
74].
YAP/TAZ as Prognostic Factors
Menzel et. al. used the comparative genomic hybridization (CGH) data of 48 human primary melanomas and 10 melanoma metastases to analyze the involvment of YAP1 gene in malanoma development. They found that in twenty tumors YAP1 gene or YAP1-activating PAK1 gene were amplificated or there was a copy number loss of LATS2 or NF2 suppressor genes. Also, patients with high YAP1 expression showed decreased survival [
75].
YAP1 activity has been shown to be elevated especially in constitutively invasive melanoma cells. Zhang et. al. demonstrated that YAP1 hyperactivity can switch melanoma cells from proliferative to invasive phenotypes and promote spontaneous melanoma metastasis
in vivo while compromising the growth of primary tumors. Moreover, that switch cannot be reversed and in consequence such invasive cells either die or downregulate YAP1 overexpression to increase the chance of survival [
76].
Zhang et. al. depleted YAP and/or TAZ in several melanoma cell lines, and used patient-derived xenografts (PDX), to test the therapeutic effects of YAP targeting. They discovered that the sensitivity of YAP1 or TAZ targeting was independent of classical BRAF/NRAS drivermutations; however, it was variable between different cell lines or patients. They also showed that YAP1 is elevated in most melanomas, and may be considered as a survival factor for melanoma cells. From the other side, YAP1 level was also increased in benign nevi, the vast majority of which do not transform to invasive state. From that view, YAP1 does not seem to be a good prognostic biomarker for YAP1-targeted therapy and other marker proteins need to be elucidated [
77].
Yuan et al. analyzed different genetic variants (single nucleotide polymorphisms) of the Hippo pahway genes in melanoma patients to check whether they were associated with survival rates. The authors demonstrated that
YAP1 rs11225163 C>T,
TEAD1 rs7944031 A>G and
TEAD4 rs1990330 C>A significantly modulated survival rates of melanoma patients. Moreover, based on genotyping data they could distinguish different prognostic groups. This study confirmed the importance of combining genetic features with clinicopathological characteristics while assessing the patient prognosis [
30].
YAP/TAZ Signaling Regulation
The evidence that YAP overexpression is an essential factor for tumor progression and targeted drug resistance to targeted drugs is obvious. However, little is known about the regulatory mechanisms of YAP/TAZ signaling. Studies has shown that it can be directly or indirectly regulated by non-coding RNAa (ncRNAs). Choe et al. demonstrated that miR-550a-3-5p directly suppressed oncogenic YAP and exerted tumor-suppressive activity. Treatment of vemurafenib-resistant melanoma cells with miR-550a-3-5p reduced phosphorylation of AKT, and marginally modulated phosphorylated ERK, and in consequence improved the sensitivity of the drug. The authors also observed that the resistant WM3248 melanoma cell line showed a higher level of EGFR and maintained ERK/AKT activity in response to vemurafenib comparing with control cells. miR-550a-3-5p overexpression inhibited various oncogenic properties, including cell proliferation, anti-apoptosis, migration, invasion, and cancer stemness, which was consistent with the effects of YAP inhibition [
78].
Hippo signaling regulation is clearly essential for melanoma development. Kim et. al. indicated that in melanoma cells the transcription of TAZ/TEAD target genes may be regulated by PIN1-STK3 axis. PIN1 is one of the factors which inhibit the Hippo pathway. It associates with STK3 (MST2) and promotes its ubiquitin-mediated degradation, to induce the nuclear translocation of TAZ oncogene and subsequent transcriptional activation of the TAZ/TEAD complex. TAZ/TEAD enhances the transcription of specific genes such as
CTGF which promotes cell proliferation, epithelial–mesenchymal transition, invasion, and cellular transformation [
79,
80]. Conversely, depletion of PIN1 in melanoma cells leads to retention of TAZ in the cytoplasm followed by CTGF decrease and final inhibition of melanoma development. The authors demonstrated that PIN1 increases the mRNA and protein expression of TAZ/TEAD target genes in A375 cells promoting tumorigenicity, and STK3 overexpression suppressed the tumorigenicity of those cells. These results were further confirmed by
in vivo experiments with syngeneic mouse model. Moreover, TCGA analysis of SKCM patients revealed a significant negative correlation between PIN1 and STK3 mRNA levels [
81].
While studying the mechanisms of melanoma pathogenesis the researchers from China focused on the role of the latent transforming growth factor binding protein (LTBP). They have previously demonstrated that LTBP4 may serve as a diagnostic marker for melanoma in the GSE46517 dataset [
82]. Patients with low expression of that gene had poorer prognosis. It has been shown that LTBP4 stabilizes the TGF-β receptor complex and regulates TGF-β1 activity, while TGF-β1 regulates the nuclear translocation of YAP1 protein [
83]. Thus, LTBP4 regulates the Hippo pathway by affecting TGF-β1 activity. Wang with his group silenced LTBP4 in melanoma cells and observed the enrichment of YAP1 in the nucleus and inhibited phosphorylation of YAP1 protein in the cytoplasm. Thus, they indicated that LTBP4 promotes the phosphorylation of YAP1 and reduces its nuclear translocation, leading to the activation of the Hippo signaling [
84].
The Core of the Hippo Pathway in Melanoma Suppression
LATS1/2 in Tumor Growth Regulation
Although most studies on the Hippo singnaling engagement in melanoma is focused on YAP1 oncotarget, several evidences point to the high importance of its core kinases in the regulation of that cancer. Hippo pathway consists of four tumor suppressors: SAV1, MST1/2, LATS1/2, and MOB kinase activators. The role of the upstream kinases is to degradate their oncotargets (YAP/TAZ), and inhibit melanoma growth and metastasis. SAV1 is involved early in the Hippo pathway and is required for activation of LATS1/2. Moreover, SAV1 directly interacts with AKT kinase and suppresses AKT-mediated tumorigenesis; also, it promotes apoptosis. It is clear that SAV1 and LATS1/2 play essential roles in tumor suppression.Romano et al. demonstrated that SAV1 and LATS1/2 expression can be induced by triptonide. Triptonide is a minor component in the Chinese herb Tripterygium wilfordii and exerts a strong anti-cancer effect with low toxicity. Enhanced expression of SAV1 and LATS1/2 leads to degradation of oncogenic YAP, and suppression of MITF and AKT. These events strongly inhibit melanoma cell tumorigenicity, migration, invasion, and lung metastasis [
85].
Our previous study showed that LATS1 silencing reduces the expression of the melanogenesis markers and melanin synthesis in primary melanocytes and melanoma cells. That phenomenon was associated with increased growth of LATS1 knocked down human xenografts in nude mice. Moreover, silencing LATS1 resulted in enhanced oxidative stress [
86].
Vittoria et. al. studied the controlled BRAFV600E expression in a melanocyte cell line and discovered that the expression of oncogenic BRAFV600E leads to the significant increase in LATS phosphorylation and in a consequence the nuclear exclusion of YAP and decrease in the expression of the YAP target genes CYR61 and AMOTL2. Also, it restrains oncogenic melanocyte proliferation in vitro due to the Hippo pathway activation. Next, the authors searched for the mechanism of that activation and found that since inhibition of MEK1/2 or ERK1/2 kinases resulted in YAP/TAZ phosphorylation, Hippo pathway activation is entirely mediated by the general hyperactivation of MAPK signaling. In vivo experiments showed that the functional impairment of the Hippo pathway in melanocytes promoted cutaneous melanomagenesis. They showed that Lats1/2−/− mice formed tumors with strong p-ERK staining suggesting that LATS1/2 depleted melanocytes evolved to hyperactivate the MAPK pathway. In addition to highlighting the role of LATS1/2 kinases in melanoma development, that study also pointed to YAP/TAZ as promising therapeutic targets as constitutivly active YAP caused extended tumor formation in zebrafish.
In 2016 Moroishi et. al. published a prominent paper which describes a suppressing role of LATS1/2 in melanoma immunity. They discovered that inactivation of LATS1/2 in tumor cells strongly suppresses tumor growth in immune-competent, but not immune-compromised mice, which is caused by the induction of anti-tumor immune responses. LATS1/2-deficient melanomas secreted nucleic-acid-rich extracellular vesicles (EVs) that stimulated the host TLRs-MYD88/TRIF-IFN nucleic-acid-sensing pathways, inducing anti-tumor inflammatory responses. Using three different melanoma cell lines in different syngeneic mouse models they showed that LATS1/2 deletion abolished the growth of SCC7 tumors and highly reduced tumor growth and the metastasis of B16 and 4T1 cells [
87].
It has been recently demonstrated that LATS1 kinase directly links Hippo pathway with apoptosis. Garcia-Gutierrez et al. performed the set of experiments experiments to identify new interactions of LATS1 with apoptotic markers. They found that LATS1 forms complexes with SMAC which is dependent on RASSF1A expression. SMAC is a mitochondrial protein that promotes cytochrome c-dependent caspase activation. The LATS1-SMAC complex requiresthe presence of the IAP family member XIAP. Although XIAP inhibits SMAC alone, it seems that LATS1 bound to SMAC counteracts that effect resulting in an increase of SMAC levels due to protein stabilization. SMAC then promotes the ubiquitination and subsequent degradation of XIAP leading to apoptosis. In a parallel study, the authors observed the loss ofMST2 and LATS1 expression in BRAFi resistant melanoma cell lines. They showed that oncogenic BRAF can inhibit MST1/2 in these cell lines, prevents MST2-LATS1 interactions and LATS1-dependent apoptosis [
88].
LATS and microRNA in Melanoma Tumorigenesis
Hu et al. using an online tool for target prediction analysis of microRNA (miRDB) linked LATS2 kinase with miR-135b expression and showed its role in melanoma tumorigenesis. The authors compared miR-135b levels in 20 melanoma tissues and analyzed the impact of miR-135b on cell proliferation, migration, or apoptosis in either primary melanocytes or the A-375 melanoma cell line. MiR-135b expression was significantly upregulated in melanoma tissue. Overexpression of miR-135b in primary melanocytes promoted cell proliferation and migration, and miR-135b inhibition reversed that effect. The proposed mechanism of such process was that miR135b through targeting LATS2 prevents inhibitory effects of that kinase [
89].
Surprisingly, LATS1 phosphorylation of YAP1 may also indirectly promote the growth of a small population of cancer cells named Tumor Repopulating Cells (TRCs). TRCs were discovered by Jiu et. al. who mechanically selected a small sub-population of cancer cells with high tumorigenic and proliferative capacity and named it Tumor Repopulating Cells (TRCs). Those cells showed to efficiently repopulate tumors at distant organs in mice, and were independent of surface CSC markers. Using microarrays the authors screened for potential factors contributing to enhanced proliferation of TRCs and identified Nuclear protein 1 (Nupr1) which was markedly reduced in that population. YAP1 phosphorylation by LATS1 leads to the downregulation of Nupr1, leading to the downregulation of p53 and upregulation of Nestin and Tert. All those events help to promote the proliferation and self-renewal of TRCs [
90].
MST1/2 Activation and Suppression of Tumor Growth
The efficacy of the Hippo pathway is significantly dependent on Merlin which is an upstream regulator of MST1/2 Ser/Thr kinases and responds for inhibition of cell proliferation, and invasiveness [
91]. Murray et al. assessed the correlation between Merlin expression and response to oxidative stress. They found that higher merlin levels in human melanoma cells promote the H2O2-induced activation of MST1/2 and suppression of tumor growth. Merlin knockdown promoted post-confluence cell proliferation and subcutaneous growth of WM1552C human melanoma cells.
Feng et. al. analyzed the interactions between the MAPK and Hippo pathways in four melanoma cell lines. They found that RAF-1 forms a complex with MST-2. The suppression of RAF-1 prevents RAF-1/MST-2 complex formation leading to MST-2 activation, and subsequent inhibition of cell proliferation, migration and invasion, and apoptosis promotion. [
92] These results stay in agreement with another study on interactions between different signaling pathways. Notably, Romano et al. reported that RAF-1 mutation stimulates both the Hippo and MAPK pathways, simultaneously driving apoptosis and proliferation, whereas concomitant MST-2 downregulation switches signaling to cell proliferation, transformation and survival [
93].
Yu et al. linked Hippo pathway with curcumin-induced melanoma cell apoptosis. They found that curcumin activated MST1-dependent cell death in cultured melanoma cells. Curcumin enhances ROS production which activates MST1. Activated MST1 then promotes cell apoptosis probably through inducing JNK activation, Foxo3a nuclear accumulation, and Bim-1 expression. MST1 knockdown prevented curcumin-induced activation of caspase-3 and caspase-9 and Bim expression, which contributed to cancer cell apoptosis [
94].
Kang et. al. studied a link between the Hippo pathway and fascin in melanoma. Fascin is upregulated in various cancers including melanoma. It increases melanoma tumorigenesis and stemness via interaction with MST2 and inhibition of MST2 homodimer formation and kinase activity, and thus reduction of LATS activity and enhancement of TAZ (but not YAP1) stability. The results point to fascin/Hippo axis as a potential therapeutic target for melanoma. Knockout of fascin significantly reduces tumorigenecity and stemness of melanoma whereas ectopic expression of fascin exerts opposite effects. Thus, targeting fascin could also become an effective strategy for advanced melanoma [
95].
Summary
Hippo pathway has been investigated in tumorigenesis due to its particular role in cell growth and metastasis. It affects several biological processes such as cell proliferation, migration and invasion, epithelial-to-mesenchymal transition, cancer stem cell formation, apoptosis, cell cycle and polarity. Hippo pathway effector components are often found mutated or downregulated in a variety of cancers including melanoma, and the target genes are upregulated. Therapies targeted to the Hippo pathway components may largely improve the survival rates of melanoma patients. The thorough analysis of the Hippo pathway in melanoma and investigation of its relationship with other key signaling pathways involved in the tumor formation will provide a better understanding of the mechanisms of melanoma pathogenesis and will help to develop more effective treatments.
Author Contributions
UK created the study idea, and prepared the manuscript in cooperation with AP and MK. TK supported the project with his critical expertise. AM supervised the whole project.
Funding
This work was supported by the National Science Centre, Poland under Grant: OPUS 8, 2014/15/B/NZ5/03563.
Acknowledgments
The authors thank prof. Antonis Koromilas, McGill University, Montreal, Canada, for providing his invaluable scientific expertise and supervision of UK’s post-doctoral training.
Conflicts of Interest
The authors declare that there is no conflict of interest.
References
- Staley, B.K.; Irvine, K.D. Hippo signaling in Drosophila: recent advances and insights. Developmental dynamics : an official publication of the American Association of Anatomists 2012, 241, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Hilman, D.; Gat, U. The evolutionary history of YAP and the hippo/YAP pathway. Molecular biology and evolution 2011, 28, 2403–2417. [Google Scholar] [CrossRef]
- Kim, H.Y.; Sah, S.K.; Choi, S.S.; Kim, T.Y. Inhibitory effects of extracellular superoxide dismutase on ultraviolet B-induced melanogenesis in murine skin and melanocytes. Life sciences 2018, 210, 201–208. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Moroishi, T.; Guan, K.L. Mechanisms of Hippo pathway regulation. Genes & development 2016, 30, 1–17. [Google Scholar] [CrossRef]
- Yu, F.X.; Zhao, B.; Guan, K.L. Hippo Pathway in Organ Size Control, Tissue Homeostasis, and Cancer. Cell 2015, 163, 811–828. [Google Scholar] [CrossRef]
- Han, Y. Analysis of the role of the Hippo pathway in cancer. Journal of translational medicine 2019, 17, 116. [Google Scholar] [CrossRef]
- Gomez, M.; Gomez, V.; Hergovich, A. The Hippo pathway in disease and therapy: cancer and beyond. Clinical and translational medicine 2014, 3, 22. [Google Scholar] [CrossRef]
- Callus, B.A.; Verhagen, A.M.; Vaux, D.L. Association of mammalian sterile twenty kinases, Mst1 and Mst2, with hSalvador via C-terminal coiled-coil domains, leads to its stabilization and phosphorylation. The FEBS journal 2006, 273, 4264–4276. [Google Scholar] [CrossRef] [PubMed]
- Praskova, M.; Xia, F.; Avruch, J. MOBKL1A/MOBKL1B phosphorylation by MST1 and MST2 inhibits cell proliferation. Current biology : CB 2008, 18, 311–321. [Google Scholar] [CrossRef]
- Hao, Y.; Chun, A.; Cheung, K.; Rashidi, B.; Yang, X. Tumor suppressor LATS1 is a negative regulator of oncogene YAP. The Journal of biological chemistry 2008, 283, 5496–5509. [Google Scholar] [CrossRef]
- Pan, D. The hippo signaling pathway in development and cancer. Developmental cell 2010, 19, 491–505. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Shimizu, T.; Lai, Z.C. Mob as tumor suppressor is activated by Hippo kinase for growth inhibition in Drosophila. The EMBO journal 2007, 26, 1772–1781. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Liu, Y.; Zheng, Y.; Dong, J.; Pan, D. The TEAD/TEF family protein Scalloped mediates transcriptional output of the Hippo growth-regulatory pathway. Developmental cell 2008, 14, 388–398. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.; Li, L.; Zhao, B. The regulation and function of YAP transcription co-activator. Acta biochimica et biophysica Sinica 2015, 47, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Lei, Q.Y.; Zhang, H.; Zhao, B.; Zha, Z.Y.; Bai, F.; Pei, X.H.; Zhao, S.; Xiong, Y.; Guan, K.L. TAZ promotes cell proliferation and epithelial-mesenchymal transition and is inhibited by the hippo pathway. Molecular and cellular biology 2008, 28, 2426–2436. [Google Scholar] [CrossRef] [PubMed]
- Zhao, B.; Wei, X.; Li, W.; Udan, R.S.; Yang, Q.; Kim, J.; Xie, J.; Ikenoue, T.; Yu, J.; Li, L.; et al. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & development 2007, 21, 2747–2761. [Google Scholar] [CrossRef]
- Elbediwy, A.; Thompson, B.J. Evolution of mechanotransduction via YAP/TAZ in animal epithelia. Current opinion in cell biology 2018, 51, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Golovnina, K.; Blinov, A.; Akhmametyeva, E.M.; Omelyanchuk, L.V.; Chang, L.S. Evolution and origin of merlin, the product of the Neurofibromatosis type 2 (NF2) tumor-suppressor gene. BMC evolutionary biology 2005, 5, 69. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Sekido, Y. NF2/Merlin Inactivation and Potential Therapeutic Targets in Mesothelioma. International journal of molecular sciences 2018, 19. [Google Scholar] [CrossRef]
- Hamaratoglu, F.; Willecke, M.; Kango-Singh, M.; Nolo, R.; Hyun, E.; Tao, C.; Jafar-Nejad, H.; Halder, G. The tumour-suppressor genes NF2/Merlin and Expanded act through Hippo signalling to regulate cell proliferation and apoptosis. Nature cell biology 2006, 8, 27–36. [Google Scholar] [CrossRef]
- Radu, M.; Chernoff, J. The DeMSTification of mammalian Ste20 kinases. Current biology : CB 2009, 19, R421–425. [Google Scholar] [CrossRef] [PubMed]
- Rybarczyk, A.; Wierzbicki, P.; Kowalczyk, A.; Kmieć, Z. [Role of the Hippo pathway in cell proliferation and organ size control. Disorders of the pathway in cancer diseases]. Postepy higieny i medycyny doswiadczalnej (Online) 2014, 68, 503–515. [Google Scholar] [CrossRef]
- Ura, S.; Masuyama, N.; Graves, J.D.; Gotoh, Y. Caspase cleavage of MST1 promotes nuclear translocation and chromatin condensation. Proceedings of the National Academy of Sciences of the United States of America 2001, 98, 10148–10153. [Google Scholar] [CrossRef] [PubMed]
- Praskova, M.; Khoklatchev, A.; Ortiz-Vega, S.; Avruch, J. Regulation of the MST1 kinase by autophosphorylation, by the growth inhibitory proteins, RASSF1 and NORE1, and by Ras. The Biochemical journal 2004, 381, 453–462. [Google Scholar] [CrossRef]
- Cinar, B.; Fang, P.K.; Lutchman, M.; Di Vizio, D.; Adam, R.M.; Pavlova, N.; Rubin, M.A.; Yelick, P.C.; Freeman, M.R. The pro-apoptotic kinase Mst1 and its caspase cleavage products are direct inhibitors of Akt1. The EMBO journal 2007, 26, 4523–4534. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.; Lee, D.; Kim, T.; Kim, T.S.; Oh, H.J.; Hwang, C.Y.; Kong, Y.Y.; Kwon, K.S.; Lim, D.S. Crucial role for Mst1 and Mst2 kinases in early embryonic development of the mouse. Molecular and cellular biology 2009, 29, 6309–6320. [Google Scholar] [CrossRef] [PubMed]
- Furth, N.; Aylon, Y. The LATS1 and LATS2 tumor suppressors: beyond the Hippo pathway. Cell death and differentiation 2017, 24, 1488–1501. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Pei, J.; Xia, H.; Ke, H.; Wang, H.; Tao, W. Lats2, a putative tumor suppressor, inhibits G1/S transition. Oncogene 2003, 22, 4398–4405. [Google Scholar] [CrossRef] [PubMed]
- Park, B.H.; Lee, Y.H. Phosphorylation of SAV1 by mammalian ste20-like kinase promotes cell death. BMB reports 2011, 44, 584–589. [Google Scholar] [CrossRef]
- Yuan, H.; Liu, H.; Liu, Z.; Zhu, D.; Amos, C.I.; Fang, S.; Lee, J.E.; Wei, Q. Genetic variants in Hippo pathway genes YAP1, TEAD1 and TEAD4 are associated with melanoma-specific survival. International journal of cancer 2015, 137, 638–645. [Google Scholar] [CrossRef]
- Chan, S.W.; Lim, C.J.; Chong, Y.F.; Pobbati, A.V.; Huang, C.; Hong, W. Hippo pathway-independent restriction of TAZ and YAP by angiomotin. The Journal of biological chemistry 2011, 286, 7018–7026. [Google Scholar] [CrossRef] [PubMed]
- Couzens, A.L.; Xiong, S.; Knight, J.D.R.; Mao, D.Y.; Guettler, S.; Picaud, S.; Kurinov, I.; Filippakopoulos, P.; Sicheri, F.; Gingras, A.C. MOB1 Mediated Phospho-recognition in the Core Mammalian Hippo Pathway. Molecular & cellular proteomics : MCP 2017, 16, 1098–1110. [Google Scholar] [CrossRef]
- Plouffe, S.W.; Lin, K.C.; Moore, J.L., 3rd; Tan, F.E.; Ma, S.; Ye, Z.; Qiu, Y.; Ren, B.; Guan, K.L. The Hippo pathway effector proteins YAP and TAZ have both distinct and overlapping functions in the cell. The Journal of biological chemistry 2018, 293, 11230–11240. [Google Scholar] [CrossRef]
- Iglesias-Bexiga, M.; Castillo, F.; Cobos, E.S.; Oka, T.; Sudol, M.; Luque, I. WW domains of the yes-kinase-associated-protein (YAP) transcriptional regulator behave as independent units with different binding preferences for PPxY motif-containing ligands. PloS one 2015, 10, e0113828. [Google Scholar] [CrossRef] [PubMed]
- Ingham, R.J.; Colwill, K.; Howard, C.; Dettwiler, S.; Lim, C.S.; Yu, J.; Hersi, K.; Raaijmakers, J.; Gish, G.; Mbamalu, G.; et al. WW domains provide a platform for the assembly of multiprotein networks. Molecular and cellular biology 2005, 25, 7092–7106. [Google Scholar] [CrossRef] [PubMed]
- Gaffney, C.J.; Oka, T.; Mazack, V.; Hilman, D.; Gat, U.; Muramatsu, T.; Inazawa, J.; Golden, A.; Carey, D.J.; Farooq, A.; et al. Identification, basic characterization and evolutionary analysis of differentially spliced mRNA isoforms of human YAP1 gene. Gene 2012, 509, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Bae, J.S.; Kim, S.M.; Lee, H. The Hippo signaling pathway provides novel anti-cancer drug targets. Oncotarget 2017, 8, 16084–16098. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Shin, J.E.; Park, H.W. The Role of Hippo Pathway in Cancer Stem Cell Biology. Molecules and cells 2018, 41, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Overholtzer, M.; Zhang, J.; Smolen, G.A.; Muir, B.; Li, W.; Sgroi, D.C.; Deng, C.X.; Brugge, J.S.; Haber, D.A. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proceedings of the National Academy of Sciences of the United States of America 2006, 103, 12405–12410. [Google Scholar] [CrossRef]
- Piccolo, S.; Panciera, T.; Contessotto, P.; Cordenonsi, M. YAP/TAZ as master regulators in cancer: modulation, function and therapeutic approaches. Nature cancer 2023, 4, 9–26. [Google Scholar] [CrossRef]
- Akrida, I.; Bravou, V.; Papadaki, H. The deadly cross-talk between Hippo pathway and epithelial-mesenchymal transition (EMT) in cancer. Molecular biology reports 2022, 49, 10065–10076. [Google Scholar] [CrossRef] [PubMed]
- Hata, Y. Hippo Pathway in Cancer, towards the Realization of Hippo-Targeted Therapy. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Pan, Z.; Tian, Y.; Cao, C.; Niu, G. The Emerging Role of YAP/TAZ in Tumor Immunity. Molecular cancer research : MCR 2019, 17, 1777–1786. [Google Scholar] [CrossRef] [PubMed]
- Szulzewsky, F.; Holland, E.C.; Vasioukhin, V. YAP1 and its fusion proteins in cancer initiation, progression and therapeutic resistance. Developmental biology 2021, 475, 205–221. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Deng, L.; Zou, H.; Guo, Y.; Tong, T.; Huang, M.; Ling, G.; Li, P. New insights into the ambivalent role of YAP/TAZ in human cancers. Journal of experimental & clinical cancer research : CR 2023, 42, 130. [Google Scholar] [CrossRef]
- Cunningham, R.; Hansen, C.G. The Hippo pathway in cancer: YAP/TAZ and TEAD as therapeutic targets in cancer. Clinical science (London, England : 1979) 2022, 136, 197–222. [Google Scholar] [CrossRef]
- Zhao, B.; Ye, X.; Yu, J.; Li, L.; Li, W.; Li, S.; Yu, J.; Lin, J.D.; Wang, C.Y.; Chinnaiyan, A.M.; et al. TEAD mediates YAP-dependent gene induction and growth control. Genes & development 2008, 22, 1962–1971. [Google Scholar] [CrossRef]
- Holden, J.K.; Cunningham, C.N. Targeting the Hippo Pathway and Cancer through the TEAD Family of Transcription Factors. Cancers 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Alberts B, J.A., Lewis J. Extracellular Control of Cell Division, Cell Growth, and Apoptosis.; New York: Garland Science: Molecular Biology of the Cell. 4th edition. 2002.
- Karaman, R.; Halder, G. Cell Junctions in Hippo Signaling. Cold Spring Harbor perspectives in biology 2018, 10. [Google Scholar] [CrossRef]
- Frisch, S.M.; Screaton, R.A. Anoikis mechanisms. Current opinion in cell biology 2001, 13, 555–562. [Google Scholar] [CrossRef]
- Noorbakhsh, N.; Hayatmoghadam, B.; Jamali, M.; Golmohammadi, M.; Kavianpour, M. The Hippo signaling pathway in leukemia: function, interaction, and carcinogenesis. Cancer cell international 2021, 21, 705. [Google Scholar] [CrossRef] [PubMed]
- Hansen, C.G.; Moroishi, T.; Guan, K.L. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends in cell biology 2015, 25, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Wang, Y.; Su, R.; Jia, Y.; Lai, X.; Su, H.; Fan, Y.; Wang, Y.; Xing, W.; Qin, J. Dimethyl Fumarate Combined With Vemurafenib Enhances Anti-Melanoma Efficacy via Inhibiting the Hippo/YAP, NRF2-ARE, and AKT/mTOR/ERK Pathways in A375 Melanoma Cells. Frontiers in oncology 2022, 12, 794216. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.E.; Finlay, G.J.; Baguley, B.C. The role of the hippo pathway in melanocytes and melanoma. Frontiers in oncology 2013, 3, 123. [Google Scholar] [CrossRef] [PubMed]
- Vittoria, M.A.; Kingston, N.; Kotynkova, K.; Xia, E.; Hong, R.; Huang, L.; McDonald, S.; Tilston-Lunel, A.; Darp, R.; Campbell, J.D.; et al. Inactivation of the Hippo tumor suppressor pathway promotes melanoma. Nature communications 2022, 13, 3732. [Google Scholar] [CrossRef] [PubMed]
- Rozeman, E.A.; Dekker, T.J.A.; Haanen, J.; Blank, C.U. Advanced Melanoma: Current Treatment Options, Biomarkers, and Future Perspectives. American journal of clinical dermatology 2018, 19, 303–317. [Google Scholar] [CrossRef] [PubMed]
- Mackiewicz, J.; Mackiewicz, A. BRAF and MEK inhibitors in the era of immunotherapy in melanoma patients. Contemporary oncology (Poznan, Poland) 2018, 22, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Sabnis, A.J.; Chan, E.; Olivas, V.; Cade, L.; Pazarentzos, E.; Asthana, S.; Neel, D.; Yan, J.J.; Lu, X.; et al. The Hippo effector YAP promotes resistance to RAF- and MEK-targeted cancer therapies. Nature genetics 2015, 47, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Kim, C.G.; Kim, S.K.; Shin, S.J.; Choe, E.A.; Park, S.H.; Shin, E.C.; Kim, J. YAP-Induced PD-L1 Expression Drives Immune Evasion in BRAFi-Resistant Melanoma. Cancer immunology research 2018, 6, 255–266. [Google Scholar] [CrossRef]
- Taha, Z.; Janse van Rensburg, H.J.; Yang, X. The Hippo Pathway: Immunity and Cancer. Cancers 2018, 10. [Google Scholar] [CrossRef]
- Kim, M.H.; Kim, J.; Hong, H.; Lee, S.H.; Lee, J.K.; Jung, E.; Kim, J. Actin remodeling confers BRAF inhibitor resistance to melanoma cells through YAP/TAZ activation. The EMBO journal 2016, 35, 462–478. [Google Scholar] [CrossRef]
- Zanconato, F.; Battilana, G.; Cordenonsi, M.; Piccolo, S. YAP/TAZ as therapeutic targets in cancer. Current opinion in pharmacology 2016, 29, 26–33. [Google Scholar] [CrossRef]
- Zanconato, F.; Cordenonsi, M.; Piccolo, S. YAP/TAZ at the Roots of Cancer. Cancer cell 2016, 29, 783–803. [Google Scholar] [CrossRef] [PubMed]
- Monaghan-Benson, E.; Burridge, K. Mutant B-RAF regulates a Rac-dependent cadherin switch in melanoma. Oncogene 2013, 32, 4836–4844. [Google Scholar] [CrossRef]
- Dieter, S.M.; Lovecchio, D.; Pataskar, A.; Zowada, M.K.; Körner, P.R.; Khalizieva, A.; van Tellingen, O.; Jäger, D.; Glimm, H.; Agami, R. Suppression of heparan sulfation re-sensitizes YAP1-driven melanoma to MAPK pathway inhibitors. Oncogene 2022, 41, 3953–3968. [Google Scholar] [CrossRef]
- Romano, D.; García-Gutiérrez, L.; Aboud, N.; Duffy, D.J.; Flaherty, K.T.; Frederick, D.T.; Kolch, W.; Matallanas, D. Proteasomal down-regulation of the proapoptotic MST2 pathway contributes to BRAF inhibitor resistance in melanoma. Life science alliance 2022, 5. [Google Scholar] [CrossRef]
- Nallet-Staub, F.; Marsaud, V.; Li, L.; Gilbert, C.; Dodier, S.; Bataille, V.; Sudol, M.; Herlyn, M.; Mauviel, A. Pro-invasive activity of the Hippo pathway effectors YAP and TAZ in cutaneous melanoma. The Journal of investigative dermatology 2014, 134, 123–132. [Google Scholar] [CrossRef] [PubMed]
- Thompson, B.J. YAP/TAZ: Drivers of Tumor Growth, Metastasis, and Resistance to Therapy. BioEssays : news and reviews in molecular, cellular and developmental biology 2020, 42, e1900162. [Google Scholar] [CrossRef] [PubMed]
- Sanchez, I.M.; Aplin, A.E. Hippo: hungry, hungry for melanoma invasion. The Journal of investigative dermatology 2014, 134, 14–16. [Google Scholar] [CrossRef]
- Lamar, J.M.; Stern, P.; Liu, H.; Schindler, J.W.; Jiang, Z.G.; Hynes, R.O. The Hippo pathway target, YAP, promotes metastasis through its TEAD-interaction domain. Proceedings of the National Academy of Sciences of the United States of America 2012, 109, E2441–E2450. [Google Scholar] [CrossRef]
- Kim, J.E.; Stones, C.; Joseph, W.R.; Leung, E.; Finlay, G.J.; Shelling, A.N.; Phillips, W.A.; Shepherd, P.R.; Baguley, B.C. Comparison of growth factor signalling pathway utilisation in cultured normal melanocytes and melanoma cell lines. BMC cancer 2012, 12, 141. [Google Scholar] [CrossRef]
- Fisher, M.L.; Grun, D.; Adhikary, G.; Xu, W.; Eckert, R.L. Inhibition of YAP function overcomes BRAF inhibitor resistance in melanoma cancer stem cells. Oncotarget 2017, 8, 110257–110272. [Google Scholar] [CrossRef]
- Heinemann, A.; Cullinane, C.; De Paoli-Iseppi, R.; Wilmott, J.S.; Gunatilake, D.; Madore, J.; Strbenac, D.; Yang, J.Y.; Gowrishankar, K.; Tiffen, J.C.; et al. Combining BET and HDAC inhibitors synergistically induces apoptosis of melanoma and suppresses AKT and YAP signaling. Oncotarget 2015, 6, 21507–21521. [Google Scholar] [CrossRef]
- Menzel, M.; Meckbach, D.; Weide, B.; Toussaint, N.C.; Schilbach, K.; Noor, S.; Eigentler, T.; Ikenberg, K.; Busch, C.; Quintanilla-Martinez, L.; et al. In melanoma, Hippo signaling is affected by copy number alterations and YAP1 overexpression impairs patient survival. Pigment cell & melanoma research 2014, 27, 671–673. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, L.; Szeto, P.; Abali, G.K.; Zhang, Y.; Kulkarni, A.; Amarasinghe, K.; Li, J.; Vergara, I.A.; Molania, R.; et al. The Hippo pathway oncoprotein YAP promotes melanoma cell invasion and spontaneous metastasis. Oncogene 2020, 39, 5267–5281. [Google Scholar] [CrossRef]
- Zhang, X.; Tang, J.Z.; Vergara, I.A.; Zhang, Y.; Szeto, P.; Yang, L.; Mintoff, C.; Colebatch, A.; McIntosh, L.; Mitchell, K.A.; et al. Somatic Hypermutation of the YAP Oncogene in a Human Cutaneous Melanoma. Molecular cancer research : MCR 2019, 17, 1435–1449. [Google Scholar] [CrossRef] [PubMed]
- Choe, M.H.; Yoon, Y.; Kim, J.; Hwang, S.G.; Han, Y.H.; Kim, J.S. miR-550a-3-5p acts as a tumor suppressor and reverses BRAF inhibitor resistance through the direct targeting of YAP. Cell death & disease 2018, 9, 640. [Google Scholar] [CrossRef]
- Blume-Jensen, P.; Hunter, T. Oncogenic kinase signalling. Nature 2001, 411, 355–365. [Google Scholar] [CrossRef]
- Zanconato, F.; Forcato, M.; Battilana, G.; Azzolin, L.; Quaranta, E.; Bodega, B.; Rosato, A.; Bicciato, S.; Cordenonsi, M.; Piccolo, S. Genome-wide association between YAP/TAZ/TEAD and AP-1 at enhancers drives oncogenic growth. Nature cell biology 2015, 17, 1218–1227. [Google Scholar] [CrossRef]
- Kim, G.; Bhattarai, P.Y.; Lim, S.C.; Kim, J.Y.; Choi, H.S. PIN1 facilitates ubiquitin-mediated degradation of serine/threonine kinase 3 and promotes melanoma development via TAZ activation. Cancer letters 2021, 499, 164–174. [Google Scholar] [CrossRef]
- Gutzmer, R.; Stroyakovskiy, D.; Gogas, H.; Robert, C.; Lewis, K.; Protsenko, S.; Pereira, R.P.; Eigentler, T.; Rutkowski, P.; Demidov, L.; et al. Atezolizumab, vemurafenib, and cobimetinib as first-line treatment for unresectable advanced BRAF(V600) mutation-positive melanoma (IMspire150): primary analysis of the randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (London, England) 2020, 395, 1835–1844. [Google Scholar] [CrossRef]
- Patel, S.; Tang, J.; Overstreet, J.M.; Anorga, S.; Lian, F.; Arnouk, A.; Goldschmeding, R.; Higgins, P.J.; Samarakoon, R. Rac-GTPase promotes fibrotic TGF-β1 signaling and chronic kidney disease via EGFR, p53, and Hippo/YAP/TAZ pathways. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2019, 33, 9797–9810. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Tang, D.; Wu, T.; Sun, F. Disruption of LTBP4 Inhibition-Induced TGFβ1 Activation Promoted Cell Proliferation and Metastasis in Skin Melanoma by Inhibiting the Activation of the Hippo-YAP1 Signaling Pathway. Frontiers in cell and developmental biology 2021, 9, 673904. [Google Scholar] [CrossRef] [PubMed]
- Tan, S.; Zhao, Z.; Qiao, Y.; Zhang, B.; Zhang, T.; Zhang, M.; Qi, J.; Wang, X.; Meng, M.; Zhou, Q. Activation of the tumor suppressive Hippo pathway by triptonide as a new strategy to potently inhibit aggressive melanoma cell metastasis. Biochemical pharmacology 2021, 185, 114423. [Google Scholar] [CrossRef] [PubMed]
- Kazimierczak, U.; Dondajewska, E.; Zajaczkowska, M.; Karwacka, M.; Kolenda, T.; Mackiewicz, A. LATS1 Is a Mediator of Melanogenesis in Response to Oxidative Stress and Regulator of Melanoma Growth. International journal of molecular sciences 2021, 22. [Google Scholar] [CrossRef] [PubMed]
- Moroishi, T.; Hayashi, T.; Pan, W.W.; Fujita, Y.; Holt, M.V.; Qin, J.; Carson, D.A.; Guan, K.L. The Hippo Pathway Kinases LATS1/2 Suppress Cancer Immunity. Cell 2016, 167, 1525–1539. [Google Scholar] [CrossRef]
- García-Gutiérrez, L.; Fallahi, E.; Aboud, N.; Quinn, N.; Matallanas, D. Interaction of LATS1 with SMAC links the MST2/Hippo pathway with apoptosis in an IAP-dependent manner. Cell death & disease 2022, 13, 692. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Q.; Zhu, X.H. MiR-135b is a novel oncogenic factor in cutaneous melanoma by targeting LATS2. Melanoma research 2019, 29, 119–125. [Google Scholar] [CrossRef] [PubMed]
- Jia, Q.; Zhou, W.; Yao, W.; Yang, F.; Zhang, S.; Singh, R.; Chen, J.; Chen, J.J.; Zhang, Y.; Wei, F.; et al. Downregulation of YAP-dependent Nupr1 promotes tumor-repopulating cell growth in soft matrices. Oncogenesis 2016, 5, e220. [Google Scholar] [CrossRef]
- Murray, L.B.; Lau, Y.K.; Yu, Q. Merlin is a negative regulator of human melanoma growth. PloS one 2012, 7, e43295. [Google Scholar] [CrossRef]
- Feng, R.; Gong, J.; Wu, L.; Wang, L.; Zhang, B.; Liang, G.; Zheng, H.; Xiao, H. MAPK and Hippo signaling pathways crosstalk via the RAF-1/MST-2 interaction in malignant melanoma. Oncology reports 2017, 38, 1199–1205. [Google Scholar] [CrossRef] [PubMed]
- Romano, D.; Nguyen, L.K.; Matallanas, D.; Halasz, M.; Doherty, C.; Kholodenko, B.N.; Kolch, W. Protein interaction switches coordinate Raf-1 and MST2/Hippo signalling. Nature cell biology 2014, 16, 673–684. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Ji, J.; Guo, Y.L. MST1 activation by curcumin mediates JNK activation, Foxo3a nuclear translocation and apoptosis in melanoma cells. Biochemical and biophysical research communications 2013, 441, 53–58. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Wang, J.; Yao, Z.; Hu, Y.; Ma, S.; Fan, Q.; Gao, F.; Sun, Y.; Sun, J. Fascin induces melanoma tumorigenesis and stemness through regulating the Hippo pathway. Cell communication and signaling : CCS 2018, 16, 37. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).