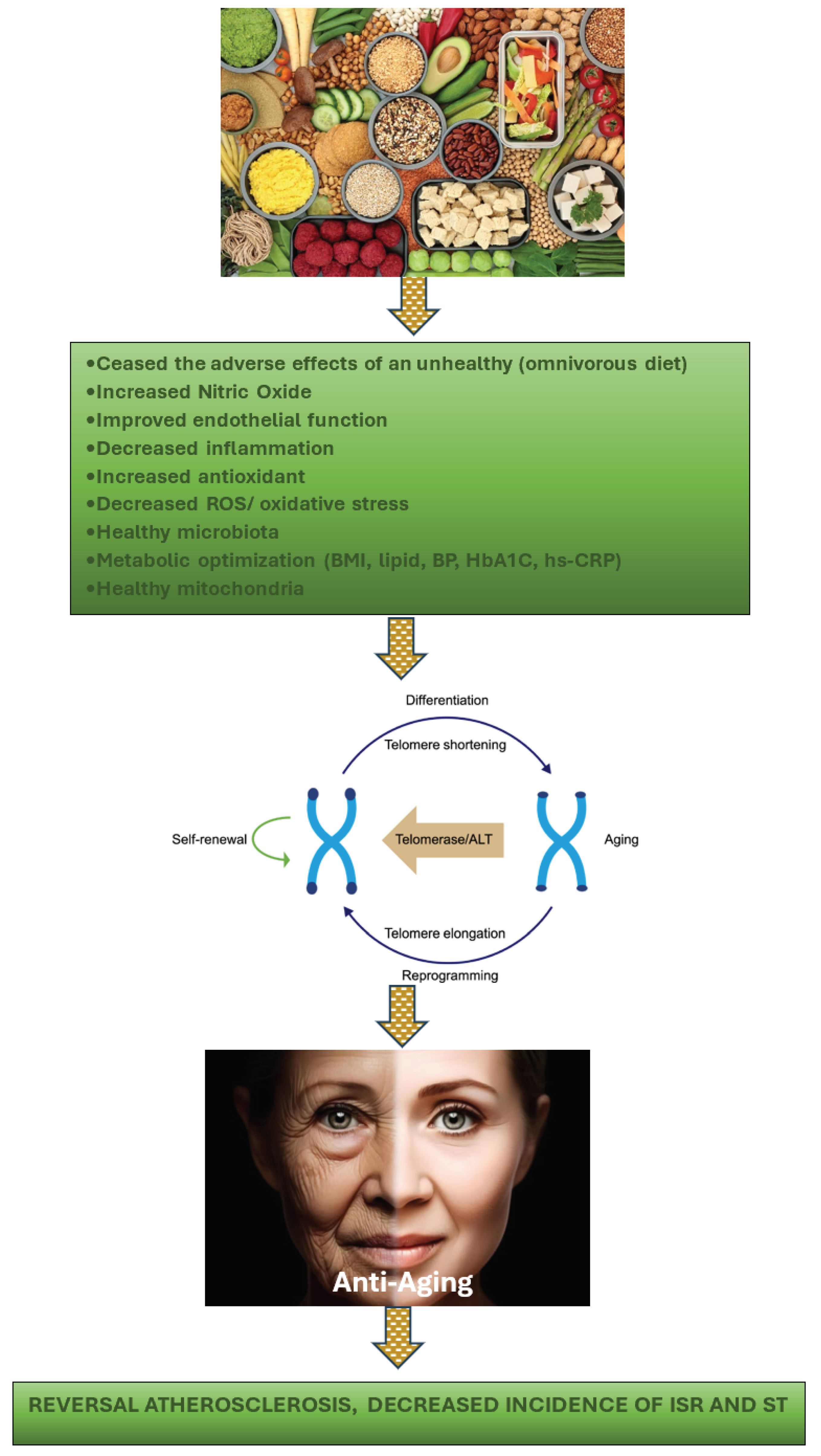
1. Introduction
The American Heart Association reports that roughly 600,000 coronary stent procedures are conducted annually in the United States [
1], with more than three million stents utilized globally. Despite progress in cardiovascular health and interventional cardiology, coronary in-stent restenosis (ISR) and stent thrombosis (ST) continue to present a challenge to interventional cardiologists. Despite the significant advancements in interventional techniques and intracoronary imaging, there is no universally accepted approach to managing either condition. The lack of a definitive strategy is a testament to the underlying mechanisms' complexity [
2].
Balloon dilation and stent implantation may result in vascular injury, which can lead to restenosis. Mechanical stretch, endothelial denudation, and subintimal hemorrhage may trigger an inflammatory response, which subsequently induces a proliferative process. The activation of vascular smooth muscle cells (VSMCs), including proliferation, migration, and differentiation, as well as matrix metalloproteinase activation, DNA, and extracellular matrix synthesis, can all contribute to neointimal hyperplasia formation [
3,
4]. Drug-eluting stents (DES) have anti-inflammatory, immunomodulatory, and antiproliferative properties, which help the coronary vessel heal properly. Nonetheless, if the suitable dose of the medication does not elute at the appropriate moment, in cases of drug resistance or if persistent systemic inflammation exists, or if the patient exhibits an allergy to the stent platform, stent polymer, or in the formation of neoatherosclerosis, all of these factors can contribute to the development of ISR and ST.
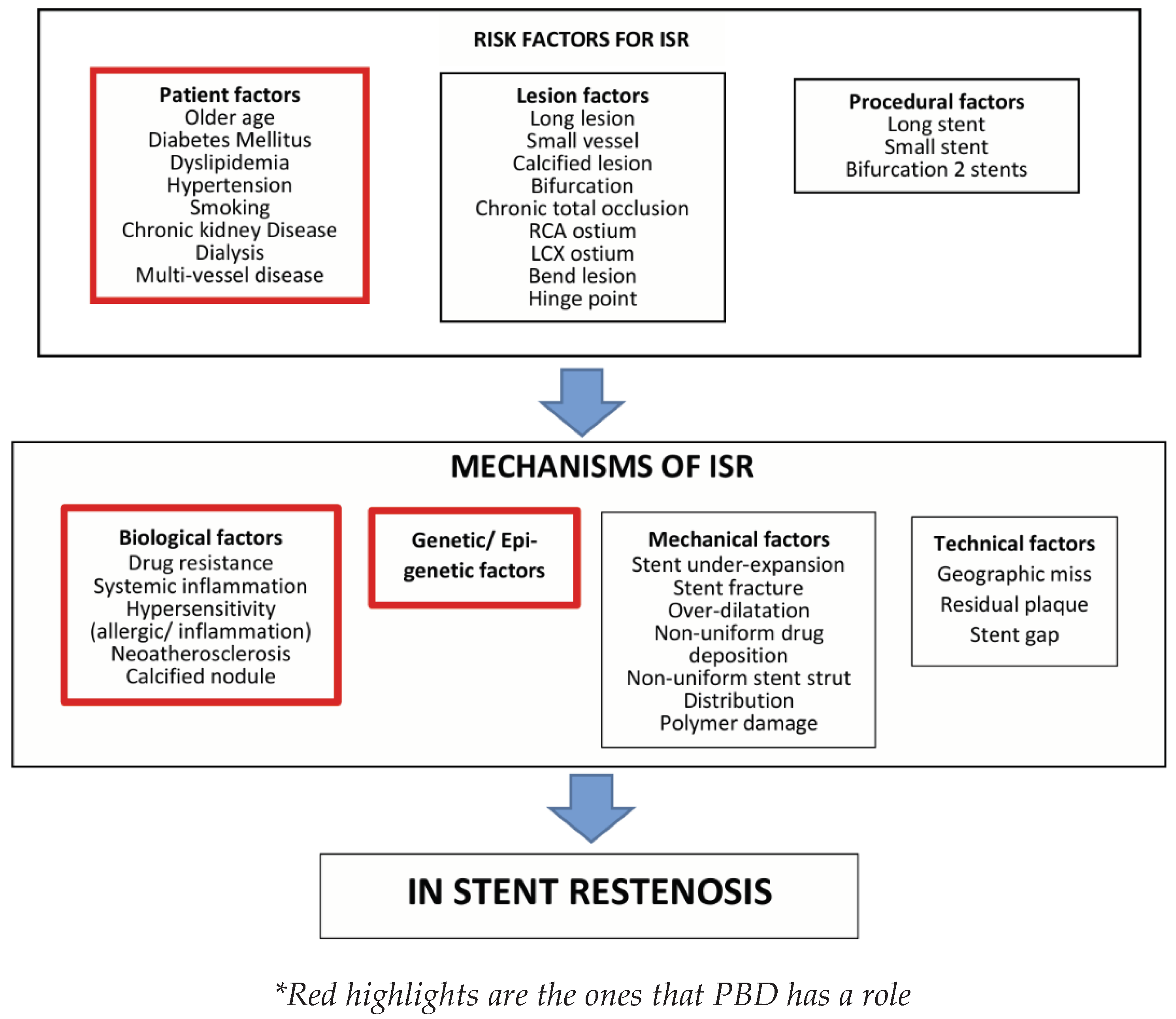
Various factors may be implicated in the development of DES restenosis, including patient factors, lesion factors, procedural factors, biological, genetic (epi-genetic), mechanical, and technical elements. In the context of this paper, we will focus on the patient, biological, and epigenetic factors that can be effectively managed with the help of a PBD. The biological mechanisms that could play a role in the emergence of ISR and ST involve inflammatory/ immunity processes, heightened sensitivity, drug resistance, the development of neoatherosclerosis, and defective re-endothelialization within the coronary artery where the stent was placed [
5] (Please refer to
Figure 1). Consequently, either the vessel wall heals or neointimal hyperplasia and/or neo-atherosclerosis develops. Identifying the core molecular and signaling pathways responsible for inflammatory, immune, hypersensitivity, vascular healing, and thrombotic processes is crucial for developing effective therapeutic approaches aimed at overcoming the primary challenge to the success of percutaneous coronary intervention (PCI). This is necessary to prevent the occurrence of ISR and ST as effectively as possible [
6,
7,
8].
Numerous studies have underscored the critical significance of PBDs in managing chronic inflammatory diseases, including atherosclerosis, hyperlipidemia, obesity, non-insulin-dependent diabetes mellitus (NIDDM), and hypertension. It has been widely acknowledged that PBD may not only contribute to the prevention of atherosclerosis but may also cause regression of coronary plaques that have occurred [
9,
10,
11,
12,
13,
14,
15]. To our knowledge, no published work has explored the role of PBDs in mitigating ISR and ST. The role of PBDs as an anti-inflammatory, immunomodulatory, and assisting vascular healing has been recognized and published as beneficial in chronic inflammatory diseases, especially atherosclerosis. Consuming PBD in conjunction with anti-platelet drugs may aid in the mitigation of thrombosis processes, ultimately reducing the risk of developing acute coronary syndrome (ACS) and subacute thrombosis [
16,
17,
18,
19,
20,
21,
22].
Atherosclerosis is a systemic disease resulting from metabolic dysfunction and persistent inflammation rather than a localized process. Consequently, it is classified as a metabolic chronic inflammatory condition. Patients who have experienced coronary obstruction due to the atherosclerosis process have typically exhibited a state of metabolic disorder and systemic inflammation for a prolonged period. Cardiologists who exclusively employ interventions such as stent implantation to manage systemic diseases are likely to be disappointed if they do not address these systemic issues. Following stent implantation, the artery is disturbed locally by the balloon, and foreign body implantation (stent) will undergo a healing process for the local injury. Nevertheless, the systemic environment will significantly influence the local healing process. At the very least, the atherosclerosis process, which is a systemic process, will still be developing. Even if patients do not experience ISR or ST, there may still be an atherosclerosis process occurring that could cause coronary stenosis in new vessels different from the vessels that have been treated with coronary interventions. Therefore, it is not Judicious to manage ISR and ST solely by addressing the local vessel issue.
Our cardiac center, Bethsaida Hospital in Indonesia, conducted interventional studies on approximately 500 cardiology patients. These patients implemented PBDs months before they underwent their PCI procedures using various second and third-generation DES. Our research has been ongoing for nearly five years, making our center the only one in Indonesia to pioneer PBDs for patients who receive PCI procedures. Our data suggests that only a small percentage of individuals who adhere to PBD experience ISR in their subsequent annual coronary angiography follow-up. Our research findings suggest that the occurrence of ISR is significantly lower in patients who adhere to our PBD interventions, at approximately 2-3%, as compared to those who do not follow our interventions, the non-PBD (NPBD) or omnivorous patients, who have an ISR rate of 10-20%; similar with the results of other studies [
23,
24]. We did not observe any instances of early ST among the participants in our PBD intervention within the first year following their stent implantations. In contrast, the rate of ST in the NPBD group was 1% [
25].
It would undoubtedly be more prudent to initiate anti-atherosclerosis, anti-ISR, and anti-ST management as early as feasible in relation to the development of ISR and ST. It is the responsibility of the interventionalist to manage the patient's metabolic and inflammatory condition well in advance of their scheduled intervention. In the acute setting, for instance, when performing an intervention on patients with acute coronary syndrome, similar issues arise. Sadly, interventionists often underestimate the importance of managing the patient's metabolic and chronic inflammatory condition post-intervention to minimize the occurrence of ISR and ST in the long run. This paper emphasizes the significance of launching metabolic interventions as soon as feasible, with the aim of rectifying metabolic abnormalities, restoring endothelial function, reducing systemic inflammation, preventing allergic reactions, enhancing the availability of NO, fostering a healthy microbiota, correcting mitochondrial dysfunction, and improving telomere as part of epigenetic adjustments. By doing so, a solid foundation can be established prior to the occurrence of injuries resulting from coronary interventions.
Given the promising outcomes that our cardiac center has achieved, additional research is warranted to assess the efficacy of PBDs in reducing the incidence of ISR and ST before broader application of this intervention in the cardiology community, particularly among interventional cardiologists. This would ultimately benefit numerous patients in the future and elevate the reputation of the interventional cardiology society as a pioneer in promoting PBDs, thereby surpassing other methods (optimal medical therapy (OMT) and bypass surgery) in achieving optimal coronary intervention results.
2. Mechanism PBD in Reducing ISR and ST
2.1. The Most Important Mechanism of How PBD Decreases the Risk of Atherosclerosis, ISR, and ST
The key mechanism by which a healthy PBD can reduce the probability of atherosclerosis, ISR, and ST is by changing one's diet to PBD, thereby eliminating the consumption of foods that contribute to coronary heart disease in the first place. Adhering to an omnivorous diet and consuming foods such as sugary products, processed meat, processed snacks, red meats, poultry, dairy products, and eggs significantly increases the risk of developing atherosclerosis, as well as the likelihood of ISR and ST. These unhealthy eating behaviors have been linked to dyslipidemia, insulin resistance, hypertension, glucose intolerance, endothelial dysfunction, chronic systemic inflammation, increased oxidative stress, elevated trimethylamine N-oxide (TMAO), low nitric oxide (NO), gut dysbiosis, mitochondrial damage, and shortened telomeres (accelerating the aging process) [
26,
27,
28,
29,
30]. All of these are widely accepted as risk factors for the development of atherosclerosis.
2.2. Mechanism of Nitric Oxide (NO) in Reducing Atherosclerosis, ISR, and ST
The endothelium exhibits anti-atherosclerotic properties, which can be ascribed to the presence of nitric oxide (NO). NO prevents monocytes and leukocytes from adhering to the endothelium, inhibits platelet-vessel wall interactions, and suppresses the proliferation of VSMCs, important processes in developing ISR and ST. Additionally, NO promotes coronary dilation and increases coronary blood flow [
31]. Furthermore, NO plays a vital role in endothelial regeneration, which is crucial for the healing of the coronary artery after stent placement [
5].
Conditions such as obesity, hypertension, hyperlipidemia, insulin resistance, and glucose intolerance, which are associated with atherosclerosis risk factors, can decrease NO release into the coronary wall due to impaired synthesis or excessive degradation [
32]. PBD has been found to improve chronic inflammatory diseases, including obesity, hypertension, hyperlipidemia, insulin resistance, and elevated glucose levels, and it may also reverse atherosclerosis [
8,
9,
10,
11,
12,
13,
14,
15]. Notably, NO production decreases with age, which is significant since atherosclerosis primarily affects older populations [
33]. To measure NO, we may use a salivary strip, which has a 96% accuracy rate [
34].
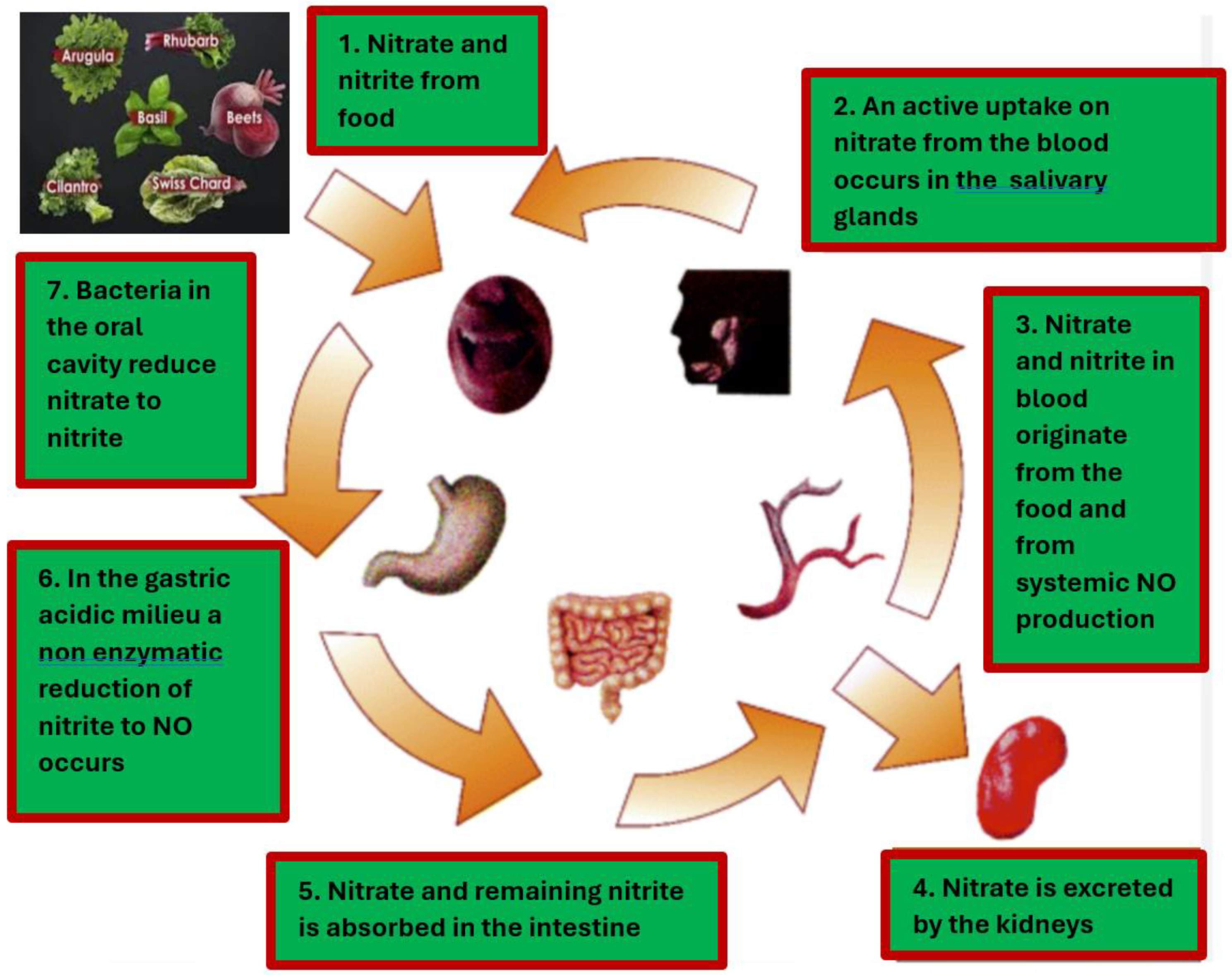
We can enhance our patients' NO availability by altering their diet and lifestyle. The process of converting nitrate-rich foods, such as green leafy vegetables, into nitric oxide (NO) is illustrated in
Figure 2. Please note that it is crucial to consider that certain vegetables that are high in nitrates can have their nitrate levels reduced through cooking.
Food preparation is also a significant factor in determining the effectiveness or beneficial effects of the food [
35]. Many studies of PBDs have not given much consideration to the quality of the food consumed by their participants, including
the selection, quantity, and processing of the food. This lack of attention may fail to maximize the benefits of PBDs. Another important factor is the potential
deficiency of certain vitamins, minerals, and micronutrients often present in PBD. Therefore, it is crucial to ensure that these important nutrients are adequately supplemented, as a deficiency in these nutrients may not only decrease the efficacy of a PBD but can also be potentially harmful [
36,
37].
Recent progress has been made in the application of NO coatings on stents, which has demonstrated the potential for preventing ISR and ST [
38,
39]. NO strategies promote normal endothelial cell growth, prevent neointimal hyperplasia formation, inhibit the proliferation of VSMCs, increase vasodilation, and decrease platelet activation and aggregation. This research should enlighten those who advocate for the administration of PBD to their patients, as PBD has the potential to increase circulating levels of NO, which may be more effective than local NO administration. This is because the pathogenesis of atherosclerosis and the occurrence of ISR and ST are driven by systemic processes rather than by a local reaction post-coronary intervention.
2.3. PBD as an Anti-Inflammation, Antioxidant, and a Crucial Factor in Repairing Endothelial Dysfunction
In 1998, a small randomized controlled trial known as the Lifestyle Heart Trial was conducted by Ornish et al. The study's results demonstrated that intensive lifestyle changes in conjunction with PBD could reverse coronary atherosclerosis. The most important mechanism of this process is by repairing endothelial dysfunction [
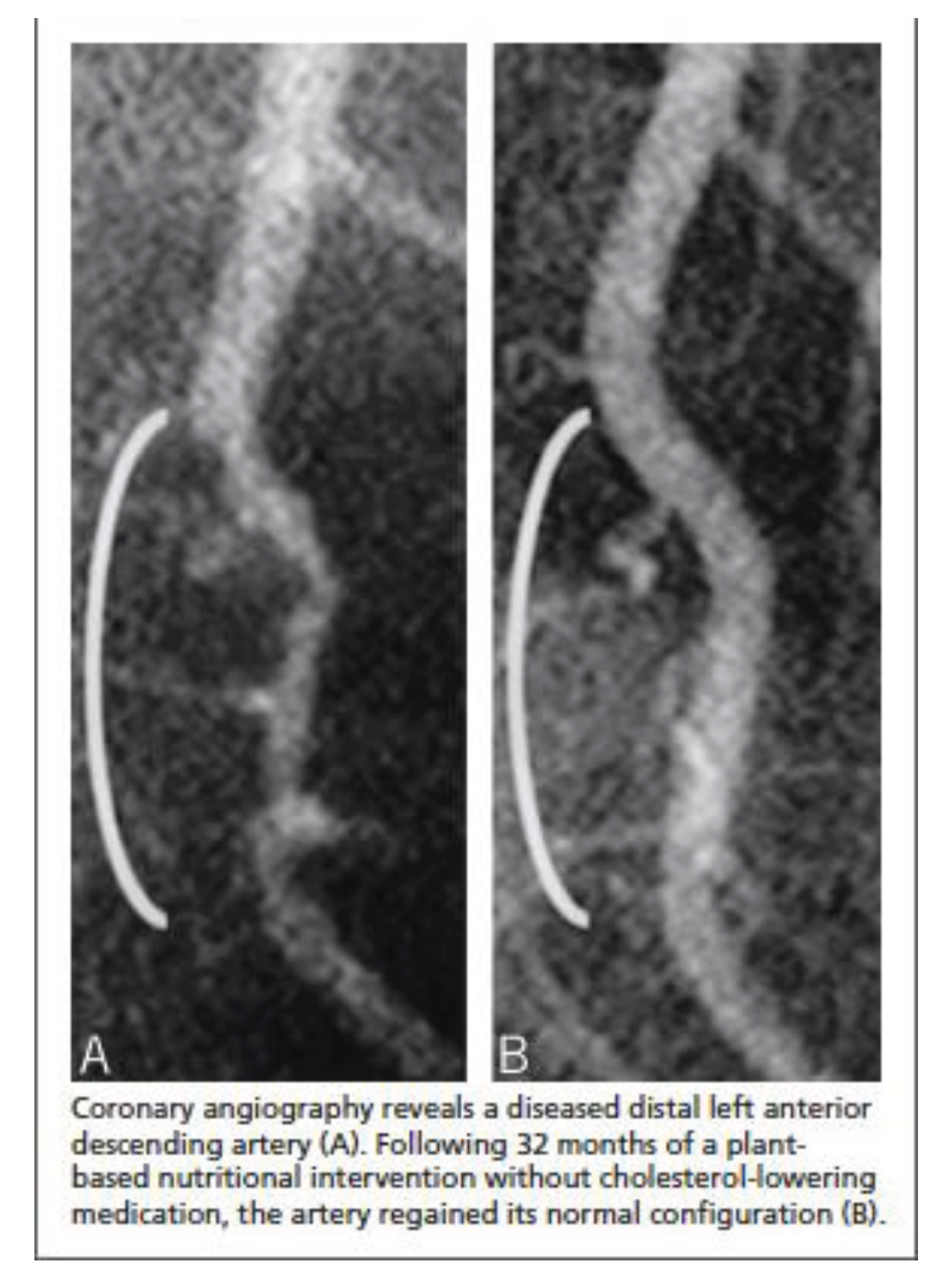
40]. In 2014, Esselstyn et al. presented a famous image, as depicted in
Figure 3, which illustrated strict PBD (without oil) can regress coronary stenosis [
41].
It has been a decade since the remarkable research studies on atherosclerosis have garnered significant praise and acceptance within the PBD community and among practitioners who recommend PBD. Despite this, the widespread adoption of recommending PBD to patients has not been extensively embraced within the interventional cardiology community. As an interventional cardiologist myself, I initially had reservations about these findings. However, after being diagnosed with coronary stenosis on my coronary multi-slice CT angiography, I decided to follow the advice of these PBD experts. To my surprise, after three years, my LAD stenosis of 50% had regressed to 20%, and my CT calcium reading had decreased by 20%. Moreover, I found that this method effectively regressed atherosclerosis and reduced the incidence of my patient’s ISR and ST.
Eating poor-quality food that is high in sugar (refined carbohydrate), devoid of fiber-phytonutrients, highly processed, and contains saturated and trans fats, cholesterol, and chemicals that promote chronic inflammation is a major contributor to the development of atherosclerosis. The consumption of these unhealthy foods has been demonstrated to increase levels of LDL cholesterol, triglycerides, apolipoprotein (a), apolipoprotein (b), C-reactive protein (CRP), pro-inflammatory mediators, pro-inflammatory chemokines/ cytokines, Trimethylamine N-oxide (TMAO), persistent organic pollutants (POPs), oxidative stress, tumor promotion, and cell proliferation, among other factors. All of these factors play significant role in the development of atherosclerosis, ISR, and ST [
42,
43]. On the contrary, eating healthy PBD with adequate supplementations such as vitamins B12 and D and minerals will enhance our body to fight against inflammation. Healthy foods such as vegetables, fruits, and legumes contain carotenoids, isoflavones, phytoestrogens, and phytosterols, which have been shown to prevent atherosclerosis. These polyphenols and phytochemicals' role in molecular signaling are anti-inflammatory, antiplatelet aggregation, inhibitor to VSMCs proliferation and migration, and safeguard for lipid oxidation (ox-LDL) [
44,
45]. Oxidized LDL, in addition to its infamous role in causing atherosclerosis, also plays a significant part in the progression of ISR [
46]. Healthy PBD will also help to restore endothelial function and enhance the process of endothelialization post-traumatic balloon inflation and stent insertion during coronary intervention.
Paclitaxel and sirolimus, which are used in the coating of DES, possess therapeutic properties that can reduce intimal hyperplasia and promote re-endothelialization, thereby decreasing the incidence of ISR in bare-metal stents from 30-50% to 10-20% in DES.
The formation of an excessive amount of reactive oxygen species (ROS) results in oxidative stress, a significant contributing factor to the emergence and progression of atherosclerosis [
47]. Robust evidence indicates that ROS, through their pro-atherogenic effects, play a crucial role in several pathological processes, including inflammation, endothelial dysfunction, and dysregulated lipid metabolism. Moreover, ROS has been demonstrated to impair mitochondrial function, which is critical to ensure effective healing following coronary interventions. Tissue damage resulting from coronary intervention and stent implantation triggers increased production of ROS, which plays a role in the initial proliferation, migration, and apoptosis of VSMCs, contributing to the development of ISR [
48]. Vascular damage during coronary intervention often results in the overproduction of ROS, leading to platelet dysfunction and subsequent abnormal activation and aggregation, which can contribute to the formation of blood clots and ST [
49].
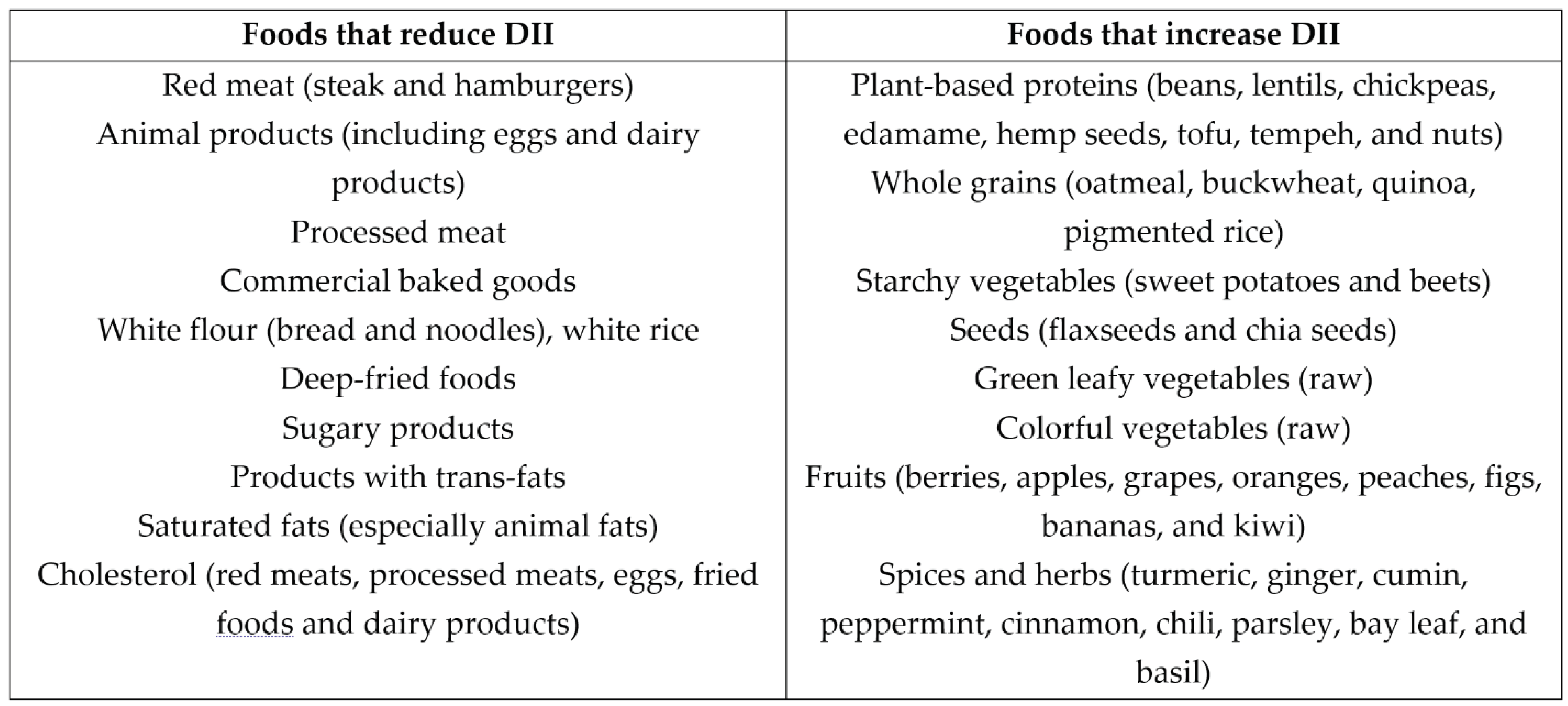
Carotenoids, prominently present in foods like pumpkin, carrots, tomatoes, green leafy vegetables, broccoli, and bell peppers, are lipophilic antioxidants that can mitigate the detrimental effects of ROS. Citrus fruits, bell peppers, strawberries, kiwis, broccoli, green leafy vegetables, grains, and legumes are rich in vitamin C and the B-complex group. Both vitamins function as potent antioxidants, helping combat ROS's negative effects. Adopting healthy dietary habits in conjunction with PBD can not only guard against the onset of atherosclerosis but can also protect against the potential complications of coronary interventions such as ISR or ST. It is important to note that individuals who have adopted healthy and correct PBDs may never need coronary intervention in their lifetimes. Consuming foods that elevate the dietary inflammatory index (DII) is advisable to counteract the inflammation and ROS implications in the progression of atherosclerosis and the development of ISR and ST, as depicted in
Table 1.
2.4. PBD Decreases Systemic Allergic Reactions and May Prevent Stent Hypersensitivity/Allergic Reaction
In contrast to the omnivorous diet, which contains a high amount of pro-inflammatory nutrients. PBDs are enriched with micronutrients and dietary flavonoids that are associated with not only anti-inflammatory but also anti-allergy effects.
There have been suggestions that ISR and ST may be linked to allergies or hypersensitivity to stent materials, including metal, polymer, and eluting drugs. Identifying whether individuals who experience ISR or ST are allergic to these materials is a complex challenge, and it has only recently been raised as a potential cause. In certain circumstances, an allergic patch test may be administered; however, even if the test returns a negative result, the likelihood of the patient being allergic to the stent materials cannot be entirely eliminated. The most common allergic reaction to stent materials is type 4 hypersensitivity, which is T-helper cell-mediated [
50]. This type of allergic reaction can be inhibited by flavonoids, which are found in abundance in PBDs. Flavonoids can inhibit the activation of T cells, reduce the production of inflammatory biomarkers, decrease IgE production and binding, and prevent degranulation of mast cells that release histamine, IL-4, IL-5, IL-6, IL-13, and MCP-1 [
51].
Given that it is challenging to predict which patients will develop hypersensitivity to the stent we employ, it is prudent to adopt a diet that generally possesses anti-allergic properties. This approach helps minimize the likelihood of patients developing ISR and ST due to allergic reactions [
51,
52]. Moreover, using drugs to eliminate allergies, as experienced in the past, normally carries side effects and will reduce patients' compliance to adhere.
2.5. The Connection between Microbiota and ISR- ST
The gut microbiota is a complex community of microorganisms that inhabit the gastrointestinal tract and play a crucial role in regulating various physiological processes, including metabolism, inflammation, and immunity. Recent research has revealed that the bacteria in atherosclerotic plaques share DNA similarities with gut bacteria. One of the metabolites produced by the gut microbiota, TMAO, is primarily derived from dietary sources such as choline, betaine, and L-carnitine found in an omnivorous diet. TMAO has been linked to the development and progression of atherosclerosis due to its pro-inflammatory properties and inhibition of reverse cholesterol transport. Additionally, elevated levels of plasma TMAO have been associated with neoatherosclerosis, ruptured plaque, and thrombosis, which may contribute to ISR and ST [
53,
54]. With a high intake of red meat, Omnivore's risk for developing atherosclerosis is much higher compared to those who consume PBDs [
55]. Switching to a PBD may protect against atherosclerosis by promoting endothelial protective mechanisms and lowering TMAO, a pro-atherosclerotic metabolite.
Atherosclerosis patients often exhibit an elevated production of TMAO-producing microbiomes and a reduced of short-chain fatty acid (SCFA)- producing species. SCFAs, a class of anti-inflammatory metabolites, are generated through a fiber-rich diet. By increasing the expression of anti-inflammatory factors, improving the integrity of the intestinal barrier, and inhibiting pro-inflammatory cytokines, SCFAs help to reduce inflammation, a key risk factor for coronary heart disease (CHD). An imbalance between SCFAs and TMAOs can contribute to inflammation and exacerbate the risk of CHD.
Plant-based dieters have been shown to possess a microbiome capable of producing a significant amount of SCFAs, including butyrate, and increased Acetyl-CoA and X4-aminobutyrate-succinate pathways. Additionally, PBDs have been linked to a lowered post-prandial glycemic response (PPGR), lowered inflammatory markers, improved cardio-metabolic health, low T-cell repertoire diversity, and low IgE expression levels. These beneficial microbiome characteristics may help protect against the development of CHD and other chronic inflammatory conditions like obesity, hypertension, hyperlipidemia, glucose intolerance, insulin resistance, allergic reaction, and the likelihood of ISR and ST.
Changes in gut microbiota have the potential to impact gut permeability, which can lead to the translocation of bacterial DNA, lipopolysaccharides (LPS), and proinflammatory cytokines into circulation. This phenomenon, known as
leaky gut syndrome, can result in the absorption of metabolites and endotoxins into the bloodstream.
Leaky gut syndrome has been linked to the development of atherosclerosis and acute coronary syndrome [
56]. SCFAs can reduce gut permeability by decreasing nuclear factor-kappa B (NF-kB) activation and reducing proinflammatory cytokines such as IL-1b, IL-6, IL-8, and TNF-α [
57]. Low consumption of plant-based foods may lead to increased penetration of the intestinal barrier, as a low-fiber diet triggers a shift from fiber-degrading to mucus-degrading bacteria. This could promote a hyperactive immune response, conceivably with the production of pro-inflammatory metabolites that fuel the disease process.
A healthy diet includes non-digestible carbohydrates, such as resistant starch, soluble and insoluble dietary fiber, and plant wall polysaccharides and oligosaccharides, fermented by beneficial gut bacteria to produce butyrate and other SCFAs. These SCFAs possess anti-inflammatory properties and have the ability to strengthen the intestinal barrier, thereby promoting optimal gut health. Moreover, SCFAs can inhibit the formation of foam cells by stimulating the expression of interleukin-10 (IL-10) and decreasing the production of pro-inflammatory cytokines by the endothelium. This contributes to the recovery of endothelial damage post-coronary intervention and reduces the occurrence of atherosclerosis, ISR, and ST.
Foods high in fiber, including barley, wheat bran, brown rice, and other whole grains, legumes, fruits, and vegetables, as well as prebiotics like fructo-oligosaccharides, are commonly consumed by individuals following a PBD diet. Plant-based foods also contain polyphenols such as lignans, isoflavones, anthocyanins, and flavonols, as well as other phytochemicals like carotenoids and phytosterols, which are metabolized into bioactive compounds by beneficial microbes, conferring health benefits and exhibiting anti-inflammatory and antioxidant activity. Phytochemicals have been shown to increase the populations of beneficial bacteria such as
Lactobacillus and
Bifidobacterium, which are the primary species found in probiotic supplements that are taken to improve gut health. In addition, fiber-rich plant foods like nuts (including walnuts, almonds, and pistachios) have been found to have prebiotic effects, leading to increases in butyrate-producing microbes and other beneficial microbes. Overall, the composition of the gut microbiome is greatly influenced by dietary fiber, polyphenols, and other phytochemicals and their metabolites, consumed in greater quantities by those following PBDs [
58]. Certainly, an omnivorous diet that is low in fiber, devoid of polyphenols and phytochemicals, and deficient in SCFAs while being high in TMAO can contribute to the development of new atherosclerotic plaques and increase the incidence of ISR and ST in patients who have undergone coronary interventions. Thus, the gut and the heart interaction is known as the gut-heart axis [
59,
60].
Figure 4.
The gut-heart axis, in which diet affects the development of atherosclerosis, ISR and ST through gut microbiota.
Figure 4.
The gut-heart axis, in which diet affects the development of atherosclerosis, ISR and ST through gut microbiota.
2.6. Caloric Restriction Is an Essential Factor in Combating Inflammation
In 2022, 43% of adults aged 18 years or older were considered overweight, and 16% were diagnosed with obesity [
61]. Overeating is a pervasive issue in contemporary society, influenced by various factors, including the availability of copious amounts of food, stress, emotional eating, and social tradition. While partaking in occasional feasts is an ingrained aspect of human nature, persistent overeating can severely affect an individual's health and society. Based on our analysis of over 10,000 patients who have visited our cardiology clinic, we have discovered that a significant majority, or 90%, would be classified as overweight using the BMI cutoff point of 21 kg/m2. This finding is not surprising, given the prevalence of CHD among this group. Our clinic offers a lifestyle program to help patients maintain their ideal weight by restricting their calorie intake.
Caloric Restriction (CR), which involves reducing the intake of calories without depriving essential nutrients, has consistently demonstrated anti-aging effects across a broad range of organisms. Furthermore, it has been shown to protect against age-related diseases such as cardiovascular disease, diabetes, hypertension, hypercholesterolemia, and cancer. CR achieves this by reducing oxidative stress and inflammation while enhancing the production and activity of antioxidant and anti-inflammatory substances, thereby improving the body's overall balance. Research has also shown that CR can improve overall health and well-being, optimize energy metabolism, enhance cellular protection, improve insulin sensitivity and glucose regulation, induce functional changes in the neuroendocrine systems, reduce oxidative damage and inflammation, and even shape the gut microbiota [
62].
Implementing CR may improve cardiovascular health. By promoting the activity of endothelial nitric oxide synthase (eNOS) and sirtuin 1 (SIRT1), CR can enhance endothelial function, leading to vasodilation, regulation of blood pressure, and improved blood flow. Additionally, CR may also reduce the development of atherosclerosis [
63]. Eating an unhealthy diet with calorie excess will certainly promote the development of atherosclerosis, ISR, and ST [
64]. CR protects DNA methylation, histone modification, and non-coding RNA (nc-RNA), which prevent VSMCs proliferation, migration, and inflammation, which play an important role in the development of atherosclerosis and ISR [
65,
66]. The vast majority of individuals who follow plant-based diets (PBDs) generally do not necessitate caloric restriction (CR) since these diets inherently promote a sense of satiety. Additionally, the majority of items on PBD menus are low in calories. In stark contrast, most omnivorous individuals frequently experience feelings of hunger due to the malfunctioning of their hormonal regulators, which are typically caused by their consumption of unhealthy foods with minimal nutrients and empty calories.
2.7. The Role of PBD as Mitochondrial Protectors
As we age, the powerhouses of our cells, called mitochondria, undergo changes that lead to a decline in their function. This decline is caused by the accumulation of oxidative damage and mutations that induce ROS. As a result, the volume, integrity, and functionality of mitochondrial DNA (mtDNA) decrease. In older adults, mitochondria are characterized by significant increases in ROS and decreased antioxidant defense, which lead to impaired functions. These include decreased ATP production, lowered oxidative capacity, and reduced oxidative phosphorylation. Additionally, with aging, mitochondrial biogenesis will decline due to inhibition of mitophagy and alterations in mitochondrial dynamics (fission and fusion). Mitophagy, an autophagy process that eliminates defective mitochondria, also deteriorates with aging. An excessive generation of ROS characterizes acute and chronic inflammatory diseases, which can cause damage to mtDNA, mitochondrial proteins, and lipids. This negatively affects normal mitochondrial function and dynamics. Inflammation is generated by various mitochondrial products called damage-associated molecular patterns (DAMPs) and is released into the cytosol or extracellular environment. Protective measures are in place to prevent mitochondria from triggering harmful inflammatory responses, such as disposing of damaged mitochondria through mitophagy. However, if these mechanisms are overwhelmed or not functioning correctly, inflammatory reactions instigated by mitochondria can become problematic, contribute to developing disorders, and impede healing [
67].
Atherosclerosis arises due to the malfunction of endothelial cells and the accumulation of lipids. Mitochondrial malfunction can have deleterious effects on various cells in the arterial wall, such as endothelial cells, smooth muscle cells, macrophages, monocytes, and lymphocytes. This can result in increased levels of ROS, chronic inflammation, oxidative stress, and intracellular lipid deposition. Also, mitochondrial dysfunction is crucial in chronic inflammatory diseases, including hypertension, obesity, hyperlipidemia, insulin resistance, and atherosclerosis. Mitochondria are essential for cellular metabolism, energy production, and cell survival. When these mechanisms are impaired, it can lead to cellular dysfunction, excessive ROS production, cellular damage, and the initiation of the inflammatory response. Mitochondria have been implicated in the pathogenesis of cardiovascular diseases [
68].
Normal mitochondrial functioning is vital for cell survival, and it is determined by the equilibrium between mitophagy (the autophagic degradation of mitochondria) and mitochondria biogenesis (fusion and fission). Mitophagy plays a crucial role in eliminating damaged mitochondria, preventing their accumulation and associated malfunction of the cell and subsequent apoptosis. Mitophagy acts as a barrier against ROS accumulation in damaged mitochondria. Inadequate mitophagy has been linked to the development of atherosclerosis, which contributes to progressive cell death, cell stress, and ROS accumulation. This results in the formation of a necrotic core and the destabilization of the atherosclerosis plaque. The mitochondrial dysfunction resulting in increased ROS generation causes damage to mtDNA, which is more susceptible to mutagenesis than nuclear DNA due to differences in DNA packaging and repair mechanisms. The accumulation of specific mtDNA mutations further contributes to mitochondrial dysfunction and the progression of atherosclerotic lesions and is involved in plaque destabilization processes. Mutations in mtDNA can decrease the synthesis of respiratory complexes and weaken mitochondrial respiration in VSMCs and macrophages. ROS plays a crucial role in damaging mtDNA, decreasing the amount of coding mtDNA, impairing mitochondrial protein synthesis, altering mitochondrial membrane potential, and decreasing total ATP production in smooth muscle and endothelial cells, all of which are important factors in the development of atherosclerosis. Mitochondrial genome changes, such as an increase in mtDNA copy number, mtDNA methylation, and the appearance of mutations (insertions, deletions, and insertions), are associated with the development of atherosclerosis. Damaged mtDNA was detected before the appearance of other atherosclerotic signs, suggesting that mtDNA damage may be the primary event that induces excessive ROS generation, violates mitochondrial membrane potential, and causes mitochondrial dysfunction, followed by the release of cytochrome C and the activation of apoptotic pathways. Furthermore, damaged mtDNA can be recognized by the body as an endogenous DAMP, which triggers the inflammatory response [
69].
Vascular endothelial cells play a crucial role in regulating apoptosis and NO production. They are also important in modulating cell signaling and the cellular response to stress, which this type of cell is particularly sensitive to. In addition to their barrier function, endothelial cells are involved in regulating vascular tone, the transport of blood plasma molecules, hemostasis, inflammation, and lipid metabolism. A healthy endothelial barrier prevents the infiltration of circulating cells, such as monocytes/macrophages, into the vascular wall. Endothelial dysfunction is one of the earliest signs of mitochondrial disorder [
70].
Mitochondrial dysfunction was observed in VSMCs isolated from human atherosclerotic plaques. These cells exhibited the presence of mtDNA mutations, reduced mitochondrial mass, and defects in ATP synthase. Impaired mitochondrial dynamics in SMCs from atherosclerotic plaques can contribute to their proliferation. The morphology of the mitochondria is determined by fusion and fission processes, which also control the effectiveness of ATP synthesis, oxygen consumption, and the potential of the mitochondrial membrane. These processes are regulated by mitochondria-associated small GTPases, including the mitochondrial fusion protein mitofusin 2 (Mfn2), which reduces proliferation and promotes apoptosis of SMCs. Mitochondrial malfunctioning could potentially exacerbate the activation of Poly (ADP-ribose) polymerase-1 (PARP-1), a critical component in the pathogenesis of atherosclerosis.
Mitochondrial oxidative stress associated with atherosclerosis contributes to inflammation activation through the NF-kB-mediated pathway in macrophages. This process is characterized by a system of pro-inflammatory cytokines, adhesion molecules, and growth factors that, in turn, can trigger inflammatory signaling [
71].
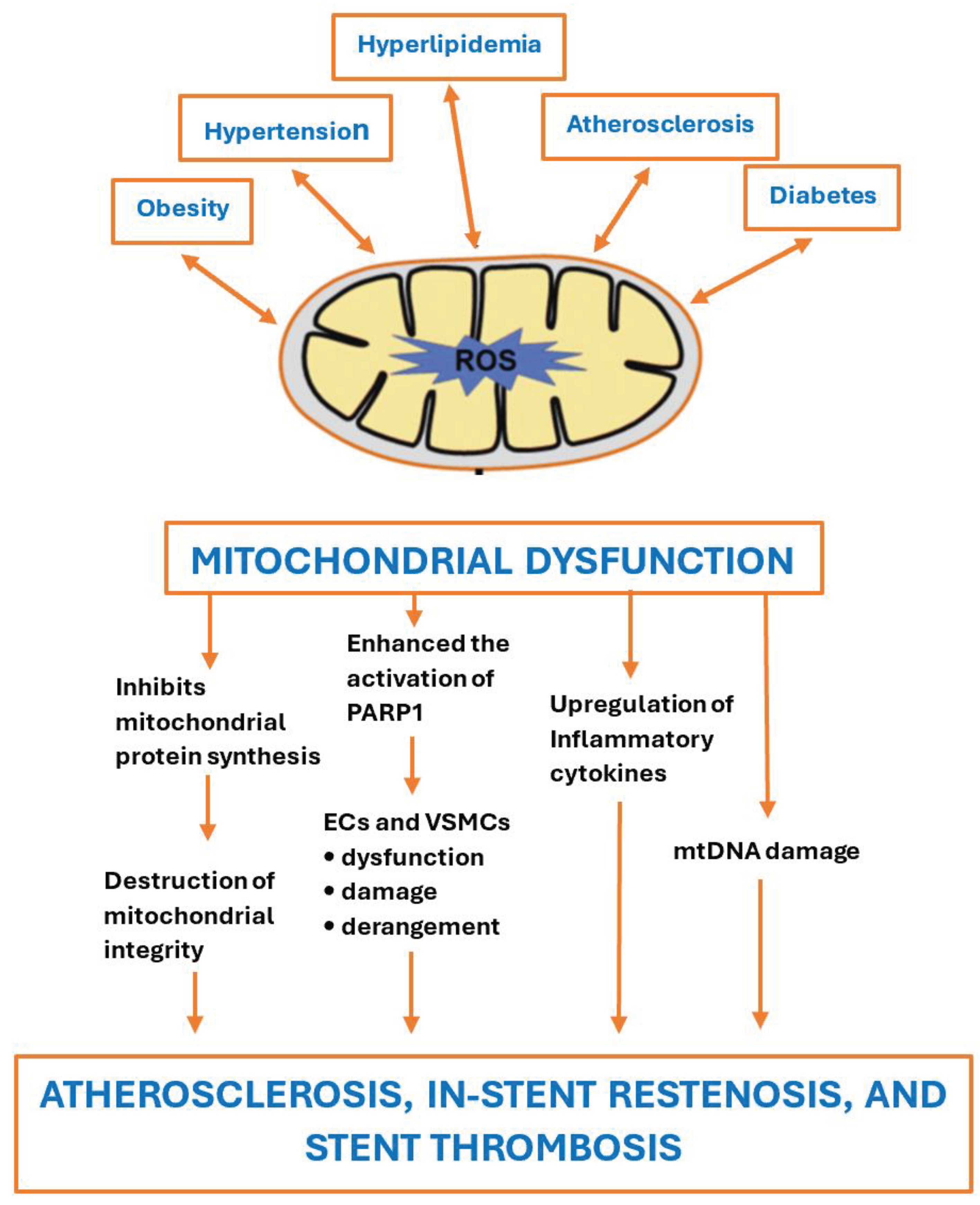
Chronic metabolic-inflammatory conditions can impact mitochondrial functionality and energy production capacity. Addressing these conditions, including obesity, hypertension, glucose intolerance, hyperlipidemia, and atherosclerosis, can aid in mitochondrial regeneration. The relationship between mitochondria and metabolic-inflammatory disorders is reciprocal in that both conditions can exacerbate one another. Metabolic-inflammatory disorders can lead to mitochondrial dysfunction, and conversely, mitochondrial dysfunction can contribute to the development of these disorders, as illustrated in
Figure 5. Thus, the term secondary mitochondrial dysfunction is used since, typically, it is acquired during a person's lifetime and is often associated with chronic diseases and age-related changes.
Promoting healthy mitochondrial function can be achieved through various lifestyle adjustments, including following a balanced diet, engaging in regular physical activity, and taking supplements containing vitamins (such as vitamin C, E, and biotin), minerals (zinc), or nutraceuticals (ubiquinone or CoQ10, NMN-precursor of NAD+, Quercetin, Astaxanthin, Resveratrol) [
72]. The concept of Mitochondrial nutrients has gained momentum in recent years and refers to the essential nutrients necessary to maintain optimal mitochondrial function. Recent investigations have demonstrated that a Mediterranean diet, characterized by the consumption of plant-derived compounds such as vegetables, fruits, and nuts, which are rich in polyphenols (isoflavones, phytoestrogens) and other phytochemicals (phytosterols, carotenoids, luteolin, organosulfur, terpenes, saponins) as well as polyunsaturated fatty acids from flaxseeds and sunflower seeds and can enhance mitochondrial function [
73]. Polyphenols have been demonstrated to hinder the activity of PARP-1, a crucial component in the pathogenesis of atherosclerosis [
74].
Enhancing mitochondrial function can potentially expedite the healing process after coronary interventions, such as ballooning and stent implantation. As illustrated in
Figure 5, this could substantially decrease the incidence of new atherosclerosis, in-stent restenosis, and stent thrombosis.
2.8. Potential Benefits of Telomere Manipulation in Atherosclerosis, ISR, and ST Utilizing PBD
Aging is a progressive deterioration of biological functions that results in cellular malfunction and can lead to diseases such as hypertension, CHD, and stroke. Numerous physiological changes occur as time passes, including genomic instability, epigenetic irregularities, loss of proteostasis, altered intercellular communication, abnormal nutrient absorption, altered mitochondrial function, depletion of stem cells, cellular senescence, and shortened telomeres. Telomeres are the protective caps at the ends of chromosomes, consisting of non-coding DNA sequences that prevent chromosomal breaks and clustering. With each cell division, telomeres become shorter, resulting in faster aging. Telomerase, a ribonucleoprotein enzyme, can synthesize telomeric repeats at the ends of chromosomes, slowing down the shortening of telomeres. By increasing telomerase enzyme activity, the rate of
telomere shortening (TeS) can be slowed [
75].
Various external factors, including lifestyle, nutrition, genetics, and heredity, can influence aging. Research on twin subjects has revealed that only 20-30% of an individual's lifespan is determined by genetic factors, while a significant portion depends on behavior and environmental factors. Some age-related diseases, such as hypertension, obesity, hyperlipidemia, insulin resistance, and atherosclerosis, are associated with increased inflammation, oxidative stress, decreased telomerase activity, and
TeS. Certain lifestyle habits, such as leading a sedentary lifestyle, smoking, experiencing emotional stress, being exposed to pollution, and engaging in unhealthy eating behaviors, can significantly increase oxidative stress and
TeS, accelerating the aging and disease process [
76].
Several longitudinal studies have demonstrated that
TeS is strongly associated with mortality and age-related diseases such as acute myocardial infarction (AMI), atherosclerosis, stroke, hypertension, and type 2 diabetes mellitus. In one population-based study, individuals with the longest telomeres had the lowest risk of dying during a 10-year follow-up period. In another study, participants with
TeS had the lowest survival rates. Large-scale studies have also found that subjects with the shortest telomeres had a 17-66% increase in mortality risk compared to those with the longest telomeres. Thus,
TeS are associated with early death, most likely due to cardiovascular risk factors, including obesity, coronary heart disease (CHD), AMI, hyperlipidemia, hypertension, and diabetes mellitus. Consequently, individuals with the longest telomeres had the lowest risk of developing CHD, AMI, stroke, and vascular death [
77].
Oxidative stress and persistent inflammation have the potential to lead to TeS, dysregulation of genes associated with telomeres, and a decrease in telomerase activity. The relationship between inflammation and TeS is reciprocal. Inflammatory stimuli can accelerate telomere attrition and result in telomere dysfunction and shortening. Conversely,
TeS can contribute to low-grade inflammation [
78].
Eating edible plants can be advantageous because they are abundant in compounds with antioxidant and anti-inflammatory properties. These compounds can counteract the negative impact of oxidative stress and persistent inflammation, both of which contribute to the TeS. According to observational studies, consuming polyphenol/ phytochemicals-rich diets, such as vegetables, fruits, nuts, seeds, and their derivatives, can prevent the TeS. This, in turn, can result in improved overall health and longevity. [
79].
Elizabeth Blackburn, a Nobel laureate, discovered that adopting a vegan diet can activate over 500 genes within a three-month period. This diet has the ability to activate genes that help prevent diseases and deactivate genes that cause chronic inflammatory diseases. There are various methods for strengthening telomeres, such as regular exercise, avoiding smoking, and consuming a diet rich in plant-based foods that protect telomeres. Dean Ornish and Elizabeth Blackburn conducted a study demonstrating how a vegan diet can increase telomerase activity, the enzyme responsible for maintaining the length of telomeres. The ability to lengthen telomeres is critical for longevity. Although we cannot reverse our chronological age, we can reverse our biological age, which can help us reverse chronic illnesses in our patients. Furthermore, reducing our patients' biological age by one or two decades will automatically decrease their risk of developing atherosclerosis, ISR, and ST [
80].
Figure 6 demonstrates the advantages of a healthy lifestyle, specifically adopting a well-balanced diet, in preventing TeS. By doing so, the aging process is slowed down or halted, thereby inhibiting the progression of atherosclerosis. Moreover, this approach can prevent the onset of ISR and the incidence of ST.
Although our current capabilities do not permit altering our patients' genetics through the manipulation of mitochondria and telomeres, there is potential for modifying epigenetic markers to activate healthy genes and deactivate unhealthy ones. Epigenetics has the power to influence the body's interpretation of specific DNA sequences. As research progresses, the possibility of manipulating mitochondria and telomeres and even altering genes may become a reality.
3. Conclusions
The significance of the relationship between nutrition and health should be acknowledged. Research has demonstrated that an unhealthy diet can result in a heightened risk of developing metabolic chronic inflammatory diseases, such as hypertension, hyperlipidemia, obesity, insulin resistance, and atherosclerosis. To promote optimal health and prevent these conditions, it is essential to adopt a healthy lifestyle that includes balanced-proper nutrition, regular exercise, abstinence from harmful substances (such as smoking, alcohol, and unhealthy foods), stress management, adequate sleep, and social support.
Managing atherosclerosis and preventing ISR and ST solely by concentrating on the local coronary vessel is no longer justifiable in modern medical practice. Trials from Courage (2007) to Ischemia (2019) have demonstrated that optimal medical therapies are as effective as performing coronary intervention in stable coronary disease patients with chronic coronary syndrome [
81,
82,
83]. Thus, the prerequisites for conducting coronary interventions have become progressively more stringent [
84]. Providing optimal medical treatment (OMT) prior to performing an intervention in stable patients is a reasonable choice. Given that most interventional cardiologists have traditionally concentrated on the local concerns of their patients rather than addressing the metabolic-chronic inflammation issues. The ACC/AHA guidelines recommend that patients with coronary artery disease should primarily consume PBDs (COR 1 and LOE B-R), but it is essential to note that the majority of patients who have received coronary interventions have not adhered to this recommendation. Furthermore, many interventional cardiologists do not follow this nutrient recommendation themselves. Although medication is crucial for addressing systemic issues, neglecting a patient's dietary habits may not be considered ethical or noble. Unhealthy diets significantly contribute to the development of atherosclerosis and the processes of ISR and ST.
Last year, Rathod et al. from St. Bartholomew's Hospital, London, presented the NITRATE-OCT findings, an ISR study at TCT 2023, demonstrating that those who consumed a daily serving of beet juice experienced significantly less late lumen loss compared to those who received a nitrate-depleted placebo. Additionally, there was a trend towards fewer major adverse cardiovascular events at two years. After six months, the median stent late lumen loss in the nitrate-depleted beet juice group was 0.244 mm, while it was 0.117 mm in the natural beet juice group (P = .0165). Similarly, the mean segment late lumen loss favored the natural beet juice group (0.269 vs. 0.050 mm; P = .0011). Over the 24-month follow-up period, there were 18 major adverse cardiovascular events in the control group and 9 in the group randomized to dietary nitrate (P = .0718). Although no in-stent thromboses were observed in either group, death, myocardial infarction, and target-vessel revascularization were all numerically lower in the group receiving dietary nitrate. The results of the study have not yet been disclosed in a final report. However, these findings are of great significance, as they indicate that a daily serving of beet juice may significantly decrease the likelihood of experiencing ISR. Compared to a limited serving of beet juice, the whole food form of PBD contains more antioxidants, anti-inflammatory compounds, and other beneficial components, providing even greater protection against ISR.
Elucidating the pathways by which beneficial nutrients exert a substantial impact on the progression of coronary artery disease and its role in mitigating the occurrence of ISR and ST is of paramount importance. Acquiring this knowledge will facilitate larger-scale studies and encourage the adoption of healthy lifestyle choices within the interventional cardiac community.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data sharing is not applicable to this article. No new data were created in this review study.
Conflict of Interests
The author declares no conflict of interest.
References
- Mohamad, T.; Jyotsna, F.; Farooq, U.; et al. Individualizing Medicinal Therapy Post Heart Stent Implantation: Tailoring for Patient Factors. Cureus. 2023, 15, e43977. [Google Scholar] [CrossRef] [PubMed]
- Klein, L.W.; Nathan, S.; Maehera, A.; et al. SCAI Expert Consensus Statement on Management of In-Stent Restenosis and Stent Thrombosis. JSCAI 2023, 2, 100971. [Google Scholar] [CrossRef]
- Pelliccia, F.; Zimarino, M.; Niccoli, G.; et al. In-stent restenosis after percutaneous coronary intervention: emerging knowledge on biological pathways. Eur Heart J Open. 2023, 3, oead083. [Google Scholar] [CrossRef] [PubMed]
- Wolf, D.; Ley, K. Immunity and Inflammation in Atherosclerosis. Circ Res. 2019, 124, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Aoki, J.; Tanabe, K. Mechanisms of drug-eluting stent restenosis. Cardiovasc Interv Ther. 2021, 36, 23–29. [Google Scholar] [CrossRef] [PubMed]
- Zheng, P.G.; Chen, P.; Wang, L.J.; et al. The association of the systemic immune-inflammation index and stent thrombosis in myocardial infarction patients after coronary stent implantation-a retrospectively study. J Thorac Dis. 2023, 15, 1726–1733. [Google Scholar] [CrossRef]
- Condello, F.; Spaccarotella, C.; Sorrentino, S.; et al. Stent Thrombosis and Restenosis with Contemporary Drug-Eluting Stents: Predictors and Current Evidence. J Clin Med. 2023, 12, 1238. [Google Scholar] [CrossRef]
- Khalid, W.; Arshad, M.S.; Ranjha, M.M.A.N.; et al. Functional constituents of plant-based foods boost immunity against acute and chronic disorders. Open Life Sci. 2022, 17, 1075–1093. [Google Scholar] [CrossRef]
- Peña-Jorquera, H.; Cid-Jofré, V.; Landaeta-Díaz, L.; et al. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome—A Comprehensive Review. Nutrients 2023, 15, 3244. [Google Scholar] [CrossRef]
- Salehin, S.; Rasmussen, P.; Mai, S.; et al. Plant Based Diet and Its Effect on Cardiovascular Disease. Int J Environ Res Public Health. 2023, 20, 3337. [Google Scholar] [CrossRef]
- Koutentakis, M.; Surma, S.; Rogula, S.; et al. The Effect of a Vegan Diet on the Cardiovascular System. J Cardiovasc Dev Dis. 2023, 10, 94. [Google Scholar] [CrossRef] [PubMed]
- Tucci, M.; Marino, M.; Martini, D.; et al. Plant-Based Foods and Vascular Function: A Systematic Review of Dietary Intervention Trials in Older Subjects and Hypothesized Mechanisms of Action. Nutrients. 2022, 14, 2615. [Google Scholar] [CrossRef] [PubMed]
- Islam, S.U.; Ahmed, M.B.; Ahsan, H.; et al. Recent Molecular Mechanisms and Beneficial Effects of Phytochemicals and Plant-Based Whole Foods in Reducing LDL-C and Preventing Cardiovascular Disease. Antioxidants (Basel). 2021, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Tawfeeq, S.; Padte, S.; et al. Plant-based diet and its effect on coronary artery disease: A narrative review. World J Clin Cases. 2023, 11, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Bruns, A.; Greupner, T.; Nebl, J.; et al. Plant-based diets and cardiovascular risk factors: a comparison of flexitarians, vegans and omnivores in a cross-sectional study. BMC Nutr 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Di Sotto, A.; Vitalone, A.; Di Giacomo, S. Plant-Derived Nutraceuticals and Immune System Modulation: An Evidence-Based Overview. Vaccines (Basel). 2020, 8, 468. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Kh Al-ani, M.; Pan, X.; et al. Plant-Derived Products for Treatment of Vascular Intima Hyperplasia Selectively Inhibit Vascular Smooth Muscle Cell Functions. Evidence-Based Complementary and Alternative Medicine 2018, 2018, 3549312. [Google Scholar] [CrossRef]
- Monsalve, B.; Concha-Meyer, A.; Palomo, I.; et al. Mechanisms of Endothelial Protection by Natural Bioactive Compounds from Fruit and Vegetables. An Acad Bras Cienc. 2017, 89 (1 Suppl 0), 615–633. [Google Scholar] [CrossRef] [PubMed]
- Aquila, G.; Marracino, L.; Martino, V.; et al. The Use of Nutraceuticals to Counteract Atherosclerosis: The Role of the Notch Pathway. Oxid Med Cell Longev. 2019, 2019, 5470470. [Google Scholar] [CrossRef]
- Moss, J.; Dipak, R. Nutraceutical therapies for atherosclerosis. Nature Reviews Cardiology 2016, 13, 513–532. [Google Scholar] [CrossRef]
- Wei, T.; Liu, J.; Zhang, D.; et al. The Relationship Between Nutrition and Atherosclerosis. Front Bioeng Biotechnol. 2021, 9, 635504. [Google Scholar] [CrossRef] [PubMed]
- Mitu, O.; Cirneala, I.A.; Lupsan, A.I.; et al. The Effect of Vitamin Supplementation on Subclinical Atherosclerosis in Patients without Manifest Cardiovascular Diseases: Never-ending Hope or Underestimated Effect? Molecules 2020, 25, 1717. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hou, J.; Gu, X.; et al. Incidence and risk factors of in-stent restenosis after percutaneous coronary intervention in patients from southern China. Eur J Med Res. 2022, 27, 12. [Google Scholar] [CrossRef] [PubMed]
- Rohman, M.S.; Waranugraha, Y.; Masbuchin, A.N.; et al. Coronary In-Stent Restenosis Predictors following Drug-Eluting Stent Implantation: A Meta-Analysis Study. J. Vasc. Dis. 2023, 2, 266–281. [Google Scholar] [CrossRef]
- Saleh, A.; Hammoudeh, A.; Tabbalat, R.; et al. Incidence and prognosis of stent thrombosis following percutaneous coronary intervention in Middle Eastern patients: The First Jordanian Percutaneous Coronary Intervention Registry (JoPCR1). Ann Saudi Med. 2016, 36, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Marchio, P.; Guerra-Ojeda, S.; Vila, J.M.; et al. Targeting Early Atherosclerosis: A Focus on Oxidative Stress and Inflammation. Oxid Med Cell Longev. 2019, 2019, 8563845. [Google Scholar] [CrossRef]
- Adarsh Ray, Krushna Ch. Maharana.; et al. Endothelial dysfunction and its relation in different disorders: Recent update. Health Sciences Review 2023, 7, 100084. [CrossRef]
- Almeida, C.; Barata, P.; Fernandes, R. The influence of gut microbiota in cardiovascular diseases-a brief review. Porto Biomed J. 2021, 6, e106. [Google Scholar] [CrossRef] [PubMed]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; et al. Mediterranean diet and mitochondria: New findings. Exp Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef] [PubMed]
- Cinegaglia, N.; Antoniazzi, L.; Rosa, D.; et al. Shortening telomere is associated with subclinical atherosclerosis biomarker in omnivorous but not in vegetarian healthy men. Aging (Albany NY). 2019, 11, 5070–5080. [Google Scholar] [CrossRef]
- Napoli, C.; de Nigris, F.; Williams-Ignarro, S.; et al. Nitric oxide and atherosclerosis: an update. Nitric Oxide. 2006, 15, 265–79. [Google Scholar] [CrossRef] [PubMed]
- Naseem, K.M. The role of nitric oxide in cardiovascular diseases. Mol Aspects Med. 2005, 2, 33–65. [Google Scholar] [CrossRef] [PubMed]
- Torregrossa, A.C.; Aranke, M.; Bryan, N.S. Nitric Oxide and geriatric: Implications in diagnostics and treatment of the elderly. J Geriatr Cardiol. 2011, 8, 230–242. [Google Scholar] [PubMed]
- Babateen, A.M.; Shannon, O.M.; Mathers, J.C.; et al. Validity and reliability of test strips for the measurement of salivary nitrite concentration with and without the use of mouthwash in healthy adults. Nitric Oxide. 2019, 91, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Salehzadeh, H.; Maleki, A.; Rezaee, R.; et al. The nitrate content of fresh and cooked vegetables and their health-related risks. PLoS One. 2020, 15, e0227551. [Google Scholar] [CrossRef] [PubMed]
- Neufingerl, N.; Eilander, A. Nutrient Intake and Status in Adults Consuming Plant-Based Diets Compared to Meat-Eaters: A Systematic Review. Nutrients. 2021, 14, 29. [Google Scholar] [CrossRef] [PubMed]
- Rajendran, S.; Shen, X.; Glawe, J.; et al. Nitric Oxide and Hydrogen Sulfide Regulation of Ischemic Vascular Growth and Remodeling. Compr Physiol. 2019, 9, 1213–1247. [Google Scholar] [CrossRef] [PubMed]
- Rao, J.; Pan Bei, H.; Yang, Y.; et al. Nitric Oxide-Producing Cardiovascular Stent Coatings for Prevention of Thrombosis and Restenosis. Front Bioeng Biotechnol. 2020, 8, 578. [Google Scholar] [CrossRef] [PubMed]
- Kabirian, F.; Brouki Milan, P.; Zamanian, A.; et al. Nitric oxide-releasing vascular grafts: A therapeutic strategy to promote angiogenic activity and endothelium regeneration. Acta Biomater. 2019, 92, 82–91. [Google Scholar] [CrossRef] [PubMed]
- Ornish, D.; Scherwitz, L.W.; Billings, J.H.; et al. Intensive lifestyle changes for reversal of coronary heart disease. JAMA. 1998, 280, 2001–7. [Google Scholar] [CrossRef] [PubMed]
- Esselstyn, C.B., Jr.; Gendy, G; Doyle, J; et al. A way to reverse CAD? J Fam Pract. 2014, 63, 356–364b. [Google Scholar] [PubMed]
- Rose, Stewart D. A Comprehensive Review of the Prevention and Treatment of Heart Disease with a Plant-Based Diet. Journal of Cardiology & Cardiovascular Therapy 2018. n. pag. [Google Scholar]
- Bilal, M.; Ashraf, S.; Zhao, X. Dietary Component-Induced Inflammation and Its Amelioration by Prebiotics, Probiotics, and Synbiotics. Front Nutr. 2022, 9, 931458. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, S.; Dixit, M. Role of Polyphenols and Other Phytochemicals on Molecular Signaling. Oxid Med Cell Longev. 2015, 2015, 504253. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.T.; Chen, L.; Tan, Z.B.; et al. Luteolin Inhibits Vascular Smooth Muscle Cell Proliferation and Migration by Inhibiting TGFBR1 Signaling. Front Pharmacol. 2018, 9, 1059. [Google Scholar] [CrossRef]
- Naruko, T.; Ueda, M.; Ehara, S.; et al. Persistent high levels of plasma oxidized low-density lipoprotein after acute myocardial infarction predict stent restenosis. Arterioscler Thromb Vasc Biol. 2006, 26, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells. 2022, 11, 3843. [Google Scholar] [CrossRef] [PubMed]
- Ganjali, S.; Mansouri, A.; Abbasifard, M.; et al. Association between Oxidative Burden and Restenosis: A Case-Control Study. Oxid Med Cell Longev. 2022, 2022, 3577761. [Google Scholar] [CrossRef] [PubMed]
- Li, P.; Ma, X.; Huang, G. Understanding thrombosis: the critical role of oxidative stress. Hematology 2024, 29, 2301633. [Google Scholar] [CrossRef] [PubMed]
- Chioncel, V.; Andrei, C.L.; Brezeanu, R.; et al. Some Perspectives on Hypersensitivity to Coronary Stents. Int J Gen Med. 2021, 14, 4327–4336. [Google Scholar] [CrossRef]
- Rakha, A.; Umar, N.; Rabail, R.; et al. Anti-inflammatory and anti-allergic potential of dietary flavonoids: A review. Biomed Pharmacother. 2022; 156, 113945. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P. The Role of Diet and Nutrition in Allergic Diseases. Nutrients. 2023, 15, 3683. [Google Scholar] [CrossRef] [PubMed]
- Mehta, P.; Tawfeeq, S.; Padte, S.; et al. Plant-based diet and its effect on coronary artery disease: A narrative review. World J Clin Cases. 2023, 11, 4752–4762. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.; Zhou, J.; Liu, C.; et al. Association Between Plasma Trimethylamine N-oxide and Neoatherosclerosis in Patients With Very Late Stent Thrombosis. Can J Cardiol. 2020, 36, 1252–1260. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Chidambaram, V.; Mehta, J.L. Vegetarianism, microbiota, and cardiovascular health: looking back, and forward. Eur J Prev Cardiol. 2022, 29, 1895–1910. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Li, L.; Sun, Z.; Zang, G.; et al. Gut Microbiota and Atherosclerosis-Focusing on the Plaque Stability. Front Cardiovasc Med. 2021, 8, 668532. [Google Scholar] [CrossRef] [PubMed]
- Peña-Jorquera, H.; Cid-Jofré, V.; Landaeta-Díaz, L.; et al. Plant-Based Nutrition: Exploring Health Benefits for Atherosclerosis, Chronic Diseases, and Metabolic Syndrome-A Comprehensive Review. Nutrients. 2023, 15, 3244. [Google Scholar] [CrossRef]
- Craig, W.J.; Mangels, A.R.; Fresán, U.; et al. The Safe and Effective Use of Plant-Based Diets with Guidelines for Health Professionals. Nutrients. 2021, 13, 4144. [Google Scholar] [CrossRef] [PubMed]
- Desai, D.; Desai, A.; Jamil, A.; et al. Re-defining the Gut Heart Axis: A Systematic Review of the Literature on the Role of Gut Microbial Dysbiosis in Patients With Heart Failure. Cureus. 2023, 15, e34902. [Google Scholar] [CrossRef] [PubMed]
- Bui, T.V.A.; Hwangbo, H.; Lai, Y.; et al. The Gut-Heart Axis: Updated Review for The Roles of Microbiome in Cardiovascular Health. Korean Circ J. 2023, 53, 499–518. [Google Scholar] [CrossRef]
- Sørensen, T.I.A.; Martinez, A.R.; Jørgensen, T.S.H. Epidemiology of Obesity. Handb Exp Pharmacol. 2022, 74, 3–27. [Google Scholar] [CrossRef] [PubMed]
- Kökten, T.; Hansmannel, F.; Ndiaye, N.C.; et al. Calorie Restriction as a New Treatment of Inflammatory Diseases. Adv Nutr. 2021, 12, 1558–1570. [Google Scholar] [CrossRef]
- Perry, C.A.; Gadde, K.M. The Role of Calorie Restriction in the Prevention of Cardiovascular Disease. Curr Atheroscler Rep. 2022, 24, 235–242. [Google Scholar] [CrossRef] [PubMed]
- Samad, F.; Ruf, W. Inflammation, obesity, and thrombosis. Blood. 2013, 122, 3415–22. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yang, Y.; Guo, J.; et al. Targeting the epigenome in in-stent restenosis: from mechanisms to therapy. Mol Ther Nucleic Acids. 2021, 23, 1136–1160. [Google Scholar] [CrossRef] [PubMed]
- Zhai, J.; Kongsberg, W.H.; Pan, Y.; et al. Caloric restriction induced epigenetic effects on aging. Front Cell Dev Biol. 2023, 10, 1079920. [Google Scholar] [CrossRef]
- Marchi, S.; Guilbaud, E.; Tait, S.W.G.; et al. Mitochondrial control of inflammation. Nat Rev Immunol. 2023, 23, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Ciccarelli, G.; Conte, S.; Cimmino, G.; et al. Mitochondrial Dysfunction: The Hidden Player in the Pathogenesis of Atherosclerosis? Int J Mol Sci. 2023, 24, 1086. [Google Scholar] [CrossRef]
- Shemiakova, T.; Ivanova, E.; Wu, W.K.; et al. Atherosclerosis as Mitochondriopathy: Repositioning the Disease to Help Finding New Therapies. Front Cardiovasc Med. 2021, 8, 660473. [Google Scholar] [CrossRef]
- Qu, K.; Yan, F.; Qin, X.; et al. Mitochondrial dysfunction in vascular endothelial cells and its role in atherosclerosis. Front Physiol. 2022, 13, 1084604. [Google Scholar] [CrossRef]
- Suárez-Rivero, J.M.; Pastor-Maldonado, C.J.; Povea-Cabello, S.; et al. From Mitochondria to Atherosclerosis: The Inflammation Path. Biomedicines. 2021, 9, 258. [Google Scholar] [CrossRef]
- Niyazov, D.M.; Kahler, S.G.; Frye, R.E. Primary Mitochondrial Disease and Secondary Mitochondrial Dysfunction: Importance of Distinction for Diagnosis and Treatment. Mol Syndromol. 2016, 7, 122–137. [Google Scholar] [CrossRef]
- Pollicino, F.; Veronese, N.; Dominguez, L.J.; et al. Mediterranean diet and mitochondria: New findings. Exp Gerontol. 2023, 176, 112165. [Google Scholar] [CrossRef] [PubMed]
- Khalil, M.; Shanmugam, H.; Abdallah, H.; et al. The Potential of the Mediterranean Diet to Improve Mitochondrial Function in Experimental Models of Obesity and Metabolic Syndrome. Nutrients. 2022, 14, 3112. [Google Scholar] [CrossRef] [PubMed]
- Maluchenko, N.V.; Feofanov, A.V.; Studitsky, V.M. PARP-1-Associated Pathological Processes: Inhibition by Natural Polyphenols. Int J Mol Sci. 2021, 22, 11441. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- D'Angelo, S. Diet and Aging: The Role of Polyphenol-Rich Diets in Slow Down the Shortening of Telomeres: A Review. Antioxidants (Basel). 2023, 12, 2086. [Google Scholar] [CrossRef]
- Herrmann, W.; Herrmann, M. The Importance of Telomere Shortening for Atherosclerosis and Mortality. J Cardiovasc Dev Dis. 2020, 7, 29. [Google Scholar] [CrossRef]
- Liu, S.; Nong, W.; Ji, L.; et al. The regulatory feedback of inflammatory signaling and telomere/telomerase complex dysfunction in chronic inflammatory diseases. Exp Gerontol. 2023, 174, 112132. [Google Scholar] [CrossRef] [PubMed]
- Crous-Bou, M.; Molinuevo, J.L.; Sala-Vila, A. Plant-Rich Dietary Patterns, Plant Foods and Nutrients, and Telomere Length. Adv Nutr. 2019, 10 (Suppl_4), S296–S303. [Google Scholar] [CrossRef]
- Varun, B. Dwaraka, Lucia Aronica, Natalia Carreras-Gallo, Jennifer L Robinson, Tayler Hennings, Aaron Lin, Logan Turner, Ryan Smith, Tavis L. Mendez, Hannah Went, Emily R. Ebel, Matthew M. Carter, Erica D. Sonnenburg, Justin L. Sonnenburg, Christopher D. Gardner. Unveiling the Epigenetic Impact of Vegan vs. Omnivorous Diets on Aging: Insights from the Twins Nutrition Study (TwiNS). medRxiv 2023.12.26.23300543. [CrossRef]
- Boden, W.E. Interpreting the results of the COURAGE trial: a non-interventionalist perspective. Rev Cardiovasc Med. 2009, 10 (Suppl 2), S34–44. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Sendon, J.; Moreno, R.; Tamargo, J. ISCHEMIA Trial: Key Questions and Answers. Eur Cardiol. 2021, 16, e34. [CrossRef] [PubMed] [PubMed Central]
- Pham, V.; Moroni, A.; Gall, E.; Benedetti, A.; Zivelonghi, C.; Picard, F. Revascularization and Medical Therapy for Chronic Coronary Syndromes: Lessons Learnt from Recent Trials, a Literature Review. J Clin Med. 2023, 12, 2833. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.S.; Newby, L.K.; Arnold, S.V.; et al. 2023 AHA/ACC/ACCP/ASPC/NLA/PCNA Guideline for the Management of Patients With Chronic Coronary Disease: A Report of the American Heart Association/American College of Cardiology Joint Committee on Clinical Practice Guidelines. Circulation 2023, 148, e9–e119. [Google Scholar] [CrossRef] [PubMed]
- Rathod, K.S.; Jones, D.A.; Van-Eijl, T.J.; et al. Randomised, double-blind, placebo-controlled study investigating the effects of inorganic nitrate on vascular function, platelet reactivity and restenosis in stable angina: protocol of the NITRATE-OCT study. BMJ Open. 2016, 6, e012728. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).