Sleep, often overlooked in its significance, stands as a cornerstone of human biology, playing a pivotal role in our physical, mental, and emotional well-being. Beyond its apparent state of restfulness, sleep is a complex and dynamic process, characterized by distinct stages and intricate physiological mechanisms. At its core, sleep is not merely a passive state of unconsciousness but rather an active and essential process vital for sustaining life.
Human sleep comprises two primary states namely, rapid eye movement (REM) and non-rapid eye movement (NREM) sleep. Non-Rapid Eye Movement (Non-REM or NREM) sleep progresses through three stages (Stage S1, Stage S2, and Stage S3), and culminates in slow wave sleep (SWS or deep sleep) characterized by delta waves inducing relaxation. This phase occurs when one feels tired, signaling the body's need for restoration, and exhibits decreased activity in the reticular formation of the brain, leading to a dreamless and restful state. Accompanied by reduced blood pressure and respiratory rate, NREM sleep advances through light to heavy stages, crucial for physical recovery. Alternating with Rapid Eye Movement (REM) sleep, Non-REM plays a vital role in the sleep cycle's rhythm. In contrast, REM sleep (also known as paradoxical sleep), shows heightened brain activity alongside muscle atonia, resulting in irregular heart and respiratory rates. During REM sleep, vivid dreaming occurs, serving as a mechanism for processing emotions, memories, and cognitive functions. Recent research has underscored the critical role of sleep in various physiological processes, including hormone regulation, immune function, and cellular repair and reorganization.
Genetic factors intricately influence our sleep patterns, as evidenced by studies linking specific genes to circadian rhythms—the internal sleep clock regulating our sleep-wake cycles [
1]. These genetic predispositions, observed across species from fruit flies to humans, shed light on the biological underpinnings of sleep and its variations among individuals. Moreover, sleep is governed by built-in regulatory mechanisms, including circadian rhythms and sleep drive [
2,
3,
4]. The circadian rhythm, orchestrated by the suprachiasmatic nucleus (“master clock” or “brain's biological clock”), responds to external light cues, while the sleep drive accumulates throughout the day, compelling us to rest [
5]. These processes work in tandem to synchronize our sleep patterns with the natural day-night cycle, ensuring optimal physiological functioning.
The purposes of sleep have long intrigued scientists and philosophers alike, leading to several prominent theories attempting to elucidate its underlying mechanisms [
6]. The inactivity theory posits that sleep evolved as a mechanism to protect organisms from predation during periods of vulnerability and inactivity. Conversely, the energy conservation theory suggests that sleep serves to reduce energy expenditure during times of low metabolic efficiency, contributing to overall energy conservation. Furthermore, the restoration theory highlights sleep's role in cellular repair and replenishment, essential for maintaining optimal biological function. This theory aligns with empirical evidence demonstrating the body's heightened physiological processes during sleep, including muscle repair, tissue growth, and hormone release. Additionally, the brain plasticity theory proposes that sleep is crucial for neural reorganization and the growth of brain structure and function, particularly during critical periods of development in infants and children.
Despite the advancements in sleep research, the exact purpose of sleep remains elusive, underscoring its multifaceted nature and complexity. It is increasingly evident that no single theory can fully encapsulate the myriad functions and implications of sleep. Instead, a holistic understanding incorporating elements of various theories is essential to unraveling the mysteries of sleep.
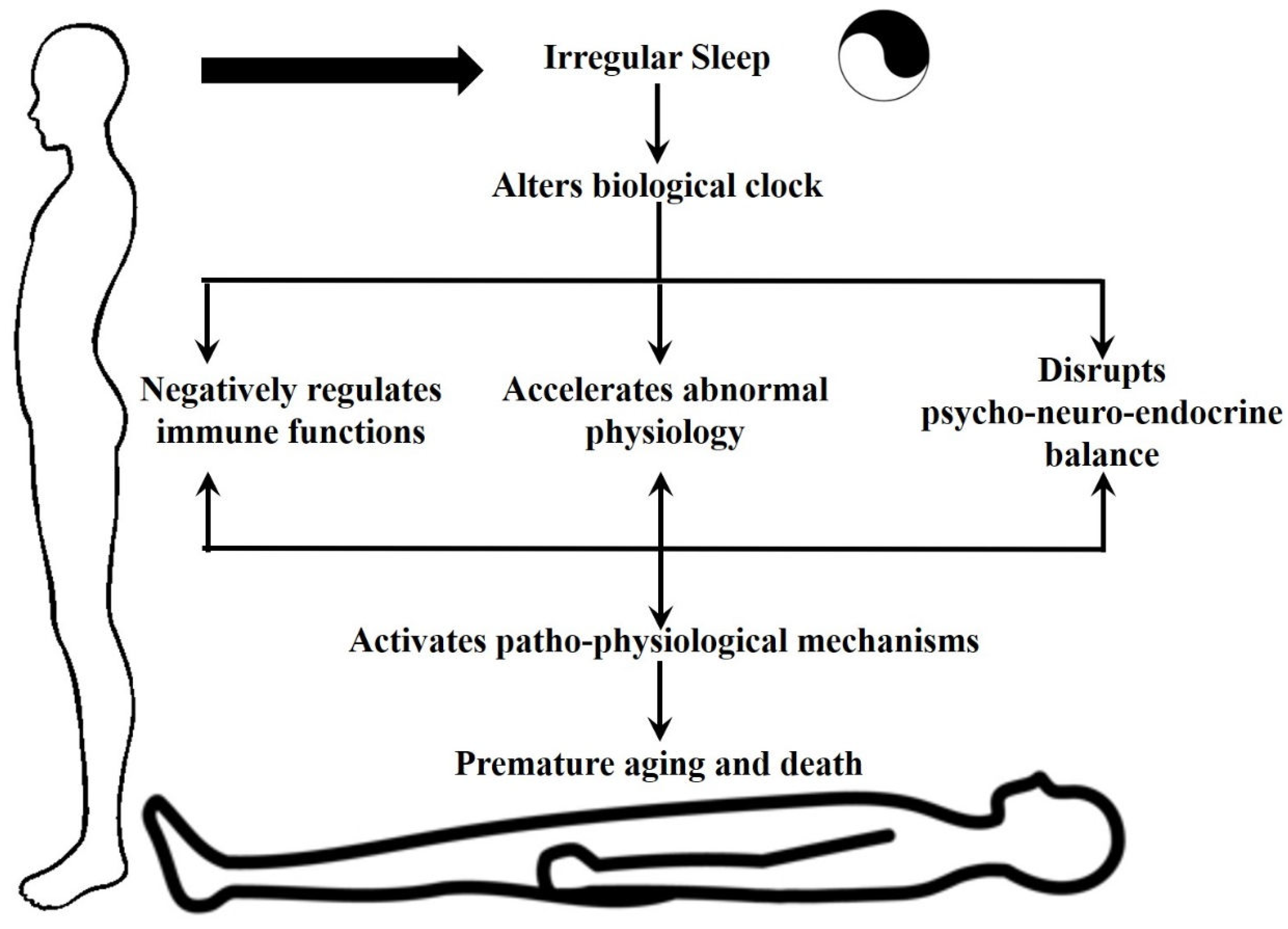
The consequences of sleep deprivation on human health are profound and far-reaching. Chronic sleep disturbances have been linked to a myriad of adverse health outcomes, including cognitive impairment, mood disorders, cardiovascular disease, and compromised immune function (
Figure 1). In today's fast-paced society, characterized by round-the-clock connectivity and demanding schedules, sleep deprivation has emerged as a pervasive public health concern warranting urgent attention.
With current understanding, sleep stands as a cornerstone of human physiology, essential for maintaining optimal health and well-being across the lifespan. Its intricate mechanisms and profound implications underscore the need for continued research and public awareness. By unraveling the mysteries of sleep and fostering healthy sleep habits, we can unlock its full potential to enhance human health and quality of life. In this article, we delve into the fascinating realm of sleep, exploring its fundamental importance, and the profound consequences of its deprivation on the overall health of humans.
1.Adequate Sleep Is Necessary for Maintaining Psychological Balance
Sleep is a fundamental aspect of human life, essential for physical and mental well-being. In recent years, sleep disorders and disturbances have become increasingly prevalent in modern society, affecting individuals of all ages from different walks of life. A systematic review by Biabani et al. [
7] delved into the neurophysiological landscape of sleep onset, highlighting the intricate processes involved in transitioning from wakefulness to sleep. They found that the activation of the prefrontal cortex, thalamus, and hypothalamus plays a crucial role in the transition from wakefulness to sleep. They also reported that changes in brain waves, specifically an increase in theta and delta waves, are associated with sleep onset. This provides valuable insights into the neural basis of sleep onset and highlights the importance of understanding these mechanisms in the diagnosis and treatment of sleep disorders and related mental health issues. Thangaleela et al. [
8] focused on sleep disorders in individuals with Parkinson's disease. They identified the potential mechanisms underlying sleep disturbances specifically in Parkinson’s patients, including changes in neurotransmitter levels and disruptions in the sleep-wake cycle. It was also identified that sleep disorders had an impact on the overall quality of life and treatment outcomes in Parkinson's disease patients. This emphasizes the need for targeted interventions to improve sleep quality and overall disease management.
The prevalence of delayed sleep-wake phase disorder (DSWPD) and its related sleep behaviors in the younger generation was explored by Futenma et al. [
9]. DSWPD is a circadian rhythm sleep disorder characterized by a delay in the timing of sleep onset and offset. This disorder is becoming increasingly prevalent among the younger generation due to lifestyle factors, such as the use of electronic devices before bedtime. This observation highlights the need for early public health interventions to promote healthy sleep habits and prevent the onset of DSWPD in young individuals. Sleep disturbances in individuals at risk of mental illness and those experiencing their first episode of psychosis has been reported by several researchers [
10]. Several interventions have been tried with this population to improve sleep, including cognitive-behavioral therapy for insomnia and mindfulness-based interventions. Improved sleep was also found to have a positive impact on mental health outcomes. Moreover, sleep deprivation and insomnia in adolescence have significant implications for mental health [
11]. This finding stresses the importance of addressing sleep issues during this critical developmental period to mitigate the risk of mental health disorders. Huang et al. [
12] investigated the effects of physical activity on sleep disturbance in various populations. A review of randomized clinical trials found that physical activity can improve sleep quality and reduce sleep disturbances in individuals with different health conditions, including insomnia, depression, and chronic pain. These studies suggest that addressing sleep disturbances, as well as sleep deprivation, may be a crucial aspect of early interventions for individuals at risk of mental illness.
Non-pharmacological perinatal interventions also showed promise in impacting maternal sleep and mental health [
13]. Addressing sleep issues during the perinatal period is crucial for promoting maternal well-being and preventing postpartum mental health disorders. It was also identified that addressing sleep disturbances in current and former athletes, optimized their performance and overall well-being [
14]. The relationship between sleep disturbances and suicidal ideation was explored by Ballard et al. [
15] and Killgore et al. [
16]. Both studies demonstrated the association between nocturnal wakefulness, trait extraversion, and suicidal ideation in individuals with mood disorders.
These studies provide valuable insights into the complex interplay between sleep and mental health across various populations and conditions. Understanding the mechanisms underlying sleep disturbances and implementing targeted interventions is crucial for promoting mental health and overall well-being. It is clear that the sleep disturbances increase at-risk mental states and first episodes of psychosis. Thus, highlighting the importance of incorporating sleep-focused strategies into early intervention programs for psychosis prevention.
2. Sleep Pattern and Metabolic Syndrome
The link between sleep and metabolic syndrome has been a topic of interest for researchers for many years. Metabolic syndrome (MetS) is a cluster of conditions that include high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels. It increases the risk of developing chronic diseases such as diabetes, heart disease, and stroke. According to the World Health Organization (WHO), about 1.9 billion adults worldwide have excess body weight, and 463 million are obese. This has led to an increased prevalence of MetS, making it a major public health concern.
Recent studies have shown that there is a strong association between sleep duration and MetS [
17]. It was found that individuals who slept less than 7 hours per night had a higher risk of developing MetS compared to those who slept for 7-9 hours per night. This was further supported by a meta-analysis conducted by Che et al. [
18], which also found a positive correlation between sleep duration and MetS. Not only does sleep duration play a role in MetS, but also the quality of sleep. Poor sleep quality has been found to have a positive association with MetS [
19]. Hua et al. [
20] found that individuals with shorter sleep duration and poor sleep quality had a higher risk of developing metabolic syndrome. In addition to sleep duration and quality, irregular sleep patterns have also been linked to MetS. Ogura et al. [
21] found that subjective irregular sleep was associated with a higher risk of MetS. This highlights the importance of maintaining a regular sleep schedule for overall health and well-being.
Interestingly, the association between sleep and MetS seems to differ depending on age. Liang et al. [
22] conducted a Mendelian randomization analysis and found a linear association between genetics, sleep duration and metabolic syndrome in individuals under the age of 60, but a U-shaped association in those over 60. This suggests that the relationship between sleep and MetS may vary among different age groups. Kim et al. [
23] conducted a cross-sectional study that found a significant association between sleep duration and metabolic syndrome, even after adjusting for lifestyle factors. This suggests that sleep itself may have a direct impact on metabolic health.
Though the exact mechanisms behind irregular sleep and MetS are still not clear, one possible explanation is the disruption of the body's circadian rhythm. Depner et al. [
24] suggest that sleep deprivation and circadian misalignment can lead to metabolic disturbances, such as insulin resistance and dysregulation of hunger hormones, which can contribute to the development of MetS. Moreover, inadequate sleep may also lead to unhealthy lifestyle choices, such as poor dietary habits and sedentary behavior, which are known risk factors for MetS. Chaput et al. [
25] found that insufficient sleep and circadian misalignment were associated with increased caloric intake and decreased physical activity, which can ultimately lead to weight gain and metabolic disturbances.
In large cohort studies carried out in different parts of the world [
26,
27,
28], it was observed that there are sex specific variations in the severity score of MetS induced by short or long duration sleep in different geographical regions. These findings underscore the relevance of considering sociodemographic factors when examining the relationship between sleep and metabolic health. Various studies support the association between sleep and metabolic syndrome. Adequate sleep duration and quality, as well as maintaining a regular sleep schedule, play an important role in preventing the development of metabolic syndrome. Therefore, the importance of sleep and promoting healthy sleep habits to reduce the risk of MetS and its associated chronic diseases. Further research is needed to better understand the underlying mechanisms and to develop effective interventions for improving sleep and metabolic health.
Cancer is a disease that occurs when cells in the body grow uncontrollably, forming tumors or invading other tissues. It is the second leading cause of death globally, with an estimated to be more than 10 million deaths per year globally. While many factors can contribute to the development of cancer, irregular sleep patterns have been identified as a potential risk factor. Sleep patterns can lead to a decrease in the production of the hormone melatonin, which regulates the body's internal clock and has anti-cancer properties. Irregular sleep patterns can also down-regulate the body's immune system, making it more vulnerable to cancer and facilitating the progression of the disease [
29]. Reports also indicate that sleep patterns play an important role in determining the treatment outcomes of cancer treatment [
30].
3. The Role of Sleep in Immune Homeostasis
Sleep is an essential biological process that allows the body to rest and repair itself. It is crucial for maintaining health, as well as for proper immune function. In recent years, research has shown that sleep deprivation can have a significant impact on the immune system, increasing the risk of immune-related diseases and negatively affecting outcomes. Multiple studies have shown that sleep has a significant impact on immune function, with both short-term and long-term effects. Sleep deprivation was found to increase the chances of infection and aggravate autoimmune diseases [
31]. Chronic sleep deprivation can lead to long-term changes in immune function, potentially leading to a higher susceptibility to infections and chronic inflammation. Besedovsky et al. [
33] discussed the intricate crosstalk between sleep and the immune system, emphasizing the impact of sleep disturbances on immune homeostasis.
Different stages of sleep have varying effects on the immune system. Rapid eye movement (REM) sleep, for example, has been linked to increased production of cytokines, which are proteins that help to regulate the immune response [
33]. Sleep loss can cause a decrease in the production of immune cells and cytokines, making the body less able to fight off infections. A longitudinal study conducted with age 5 preschool children indicated that sleep duration had an impact on cytokine levels [
34]. As cytokines play a major role in shaping the immune system at an early age, sleep habits may have long-term effects on immune function. Schmitz et al., [
35] identified that disruptions in the sleep-wake cycle can impair the effectiveness of vaccinations against viral infections, leading to a decreased immune response. Proper sleep hygiene and alignment with circadian rhythms were shown to enhance vaccine efficacy and improve immune responses. In addition to the physical effects of sleep on the immune system, there is also evidence that sleep can impact mental health and emotional well-being. Poor sleep can lead to emotional disturbances and mental health issues, which can, in turn, affect immune function [
36]. Besedovsky et al., [
32] described the complex interactions between sleep, the immune system, and the central nervous system, and how they impact each other. The mechanisms underlying the interaction between sleep and immunity are multifaceted. Irwin and Opp [
37] proposed reciprocal regulation between sleep and innate immunity, wherein sleep disturbances can lead to dysregulation of immune responses, and vice versa. Additionally, Dempsey [
38] suggested that sleep plays a crucial role in regulating innate immunity, which is the first line of immune protection in the body.
The impact of sleep on immunity has also been studied in athletes, who often have disrupted sleep due to training and competition schedules. Adequate sleep duration and quality were associated with enhanced immune function, emphasizing the importance of sleep in optimizing athletic performance and recovery in adolescent track and field athletes [
39]. This study suggests that prioritizing sleep hygiene may help to maintain immune health. The relationship between sleep and immunity is vast and consistently shows that sleep plays a crucial role in immune function. Garbarino et al. [
31] further support this evidence and highlight the importance of addressing sleep deprivation in the prevention and management of immune-related diseases. Adequate sleep is not only essential for physical and mental health but also for maintaining a strong and effective immune system and for overall well-being.
4. Irregular Sleep Accelerates Aging Phenotype
Aging is a natural and inevitable process that affects all living organisms. With the advancement of medical science and better living conditions, the human lifespan has significantly increased over the years. However, with aging comes a decline in physical and cognitive abilities, which can greatly impact the quality of life in older adults. The process of aging is complex and involves various physiological changes in different organ systems, including the brain.
Sleep is a vital physiological process that plays a crucial role in maintaining overall health and well-being. As we age, there is a natural decline in sleep quality, with older adults experiencing shorter sleep duration, frequent awakenings, and less deep sleep. Casagrande et al. [
40] conducted a systematic review of the relationship between sleep quality and aging. They found that poor sleep quality was associated with an increased risk of cognitive decline and Alzheimer's disease in older adults. Candidate mechanisms underlying the association between sleep-wake disruptions and Alzheimer's disease have been explored, providing valuable insights into the pathophysiology of neurodegenerative diseases [
41]. Sleep disturbances can also contribute to the development and progression of Alzheimer's disease through various mechanisms, including amyloid-beta accumulation, inflammation, and oxidative stress [
42]. Understanding the bidirectional relationship between sleep disturbances and Alzheimer's disease pathology is important for identifying newer and more effective targets for therapeutic interventions. It was also found that sleep disturbances can accelerate the aging process by promoting inflammation and oxidative stress in the brain. Recent research based on animal studies also indicates that the brain purges waste during sleep. This is an essential mechanism for brain regeneration and to maintain central nervous system health.
As we age, there is a natural decline in brain volume, which is associated with cognitive decline and neurodegenerative diseases. Kokošová et al. [
43] conducted a study to investigate the bidirectional association between sleep and brain atrophy in aging. They found that poor sleep quality was associated with accelerated brain atrophy, particularly in the prefrontal cortex and hippocampus, which are crucial for cognitive functions. On the other hand, brain atrophy can also lead to sleep disturbances, creating a vicious cycle that can further accelerate the aging process.
Poor sleep quality can accelerate the aging process and contribute to the development of age-related diseases such as Alzheimer's disease. Early diagnosis and management of sleep disorders, along with lifestyle factors such as exercise and stress management, can help in promoting healthy aging and preventing age-related cognitive decline. Major Depressive Disorder (MDD) is a common mental health disorder in older adults and its prevalence increases with age. Irregular sleep alters the melatonergic system leading to depression [
44,
45]. Practicing yoga was found to improve brain function, reduce oxidative stress, and promote neuroplasticity, thereby helping in the management of MDD symptoms and possibly slowing down the aging process [
46].
The role of oxidative stress, inflammation, and neuro-progression in chronic post-traumatic stress disorder (PTSD) further underscores the intricate relationship between sleep disturbances, aging, and cognitive health [
47]. Their research highlights the potential long-term consequences of sleep disruptions on cognitive function and mental health outcomes in aging populations. Integrative neurobiology approaches offer a comprehensive framework for understanding the complex interplay between metabolic diseases, neuroinflammation, and neurodegeneration in aging [
48]. It is important to consider the multifactorial nature of age-related cognitive decline, highlighting potential avenues for preventative and therapeutic interventions. Aging in the circadian system emerges as a critical consideration for health, disease prevention, and longevity [
49]. The feedback loop between circadian rhythm disruptions and aging processes underscores the importance of maintaining healthy sleep-wake cycles for promoting cognitive health in older adults. An intricate relationship exists between senescence (the process of aging), sleep, and circadian rhythms [
50].
Current research offers valuable insights into the complex interplay between sleep, aging, and cognitive health, highlighting the bidirectional relationship between sleep disturbances and age-related cognitive decline. Understanding the mechanisms underlying this relationship is crucial for developing targeted interventions to promote healthy aging and mitigate the risk of neurodegenerative diseases in older adults. Further research is needed to elucidate the specific pathways linking sleep disturbances to cellular senescence, sleep, brain aging, and cognitive impairment in aging populations, offering opportunities for innovative approaches to improve cognitive health and overall well-being in older adults.
4. Conclusions
Circadian rhythms indeed encompass a wide range of physical, mental, and behavioral changes that occur in organisms over a 24-hour cycle. Light and darkness are indeed the primary cues that synchronize these rhythms. Factors like food intake, stress, physical activity, social environment, and temperature can play a role in modulating them. Circadian rhythms govern various essential functions like sleep Patterns, hormone release including melatonin, appetite and digestion, and body temperature. Overall, circadian rhythms are fundamental to the functioning of the human body, orchestrating a wide range of biological processes to ensure optimal health and well-being. Manipulating the body’s biological clock or circadian rhythm by irregular sleep can have profound consequences on the body’s physiology and can be a leading cause or aggravator for various pathophysiological processes [
51].
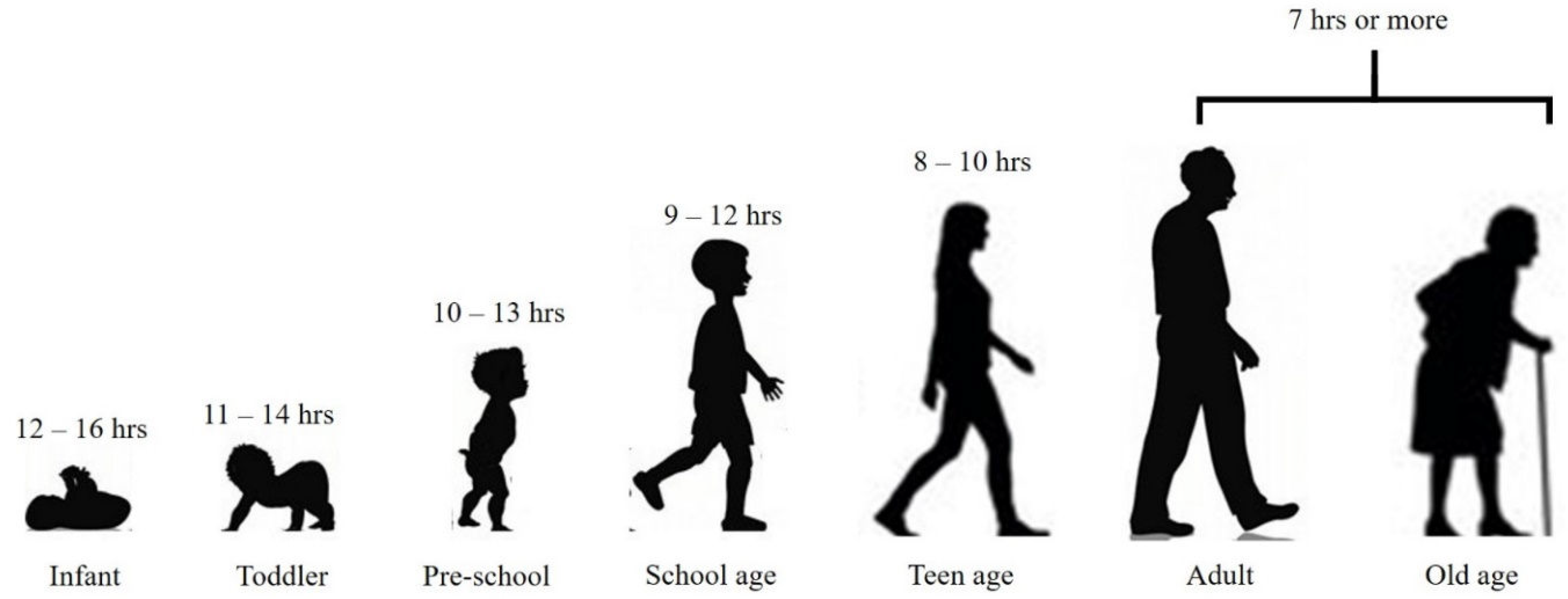
It is essential to note that the recommended sleep hours for each age group are just guidelines (
Figure 2) [modified from 52]. Some individuals may require more or less sleep, depending on their genetics, lifestyle, and health conditions. It is crucial to listen to your body and adjust your sleep hours accordingly. In conclusion, getting enough sleep is crucial for our overall well-being, and the recommended sleep hours vary for different age groups. It is essential to establish a regular sleep routine and prioritize sleep to ensure we are getting the right amount of sleep for our age. Physicians and medical regulatory bodies should consider including suggestions on sleep patterns for patients under standard treatment procedures and prescriptions. With adequate sleep, we can wake up feeling refreshed and ready to take on the day ahead for fulfilling a healthy lifespan.
Author Contributions
SPB, JLW and SRP conceptualised the research questions for the review. SPB developed the search strategy and drafted the first version of the manuscript. JLW and SPP critically revised the manuscript drafts and final version of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding.
Acknowledgments
SPB and JLW wish to thankfully acknowledge the support of colleagues who participated in critical discussions while drafting the manuscript and assisted in manuscript revision.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Pandi-Perumal, S. R.; Saravanan, K. M.; Paul, S.; Namasivayam, G. P.; Chidambaram, S. B. Waking Up the Sleep Field: An Overview on the Implications of Genetics and Bioinformatics of Sleep. Mol. Biotechnol. 2024, Jan 10. [CrossRef]
- Borbély, A. The Two-Process Model of Sleep Regulation: Beginnings and Outlook. J. Sleep Res. 2022, 31(4), e13598. [Google Scholar] [CrossRef] [PubMed]
- Saper, C. B.; Scammell, T. E.; Lu, J. Hypothalamic Regulation of Sleep and Circadian Rhythms. Nature 2005, 437(7063), 1257–1263. [Google Scholar] [CrossRef] [PubMed]
- Borbély, A. A.; Achermann, P. Sleep Homeostasis and Models of Sleep Regulation. J. Biol. Rhythms 1999, 14(6), 557–568. [Google Scholar] [CrossRef]
- Pandi-Perumal, S. R.; Trakht, I.; Spence, D. W.; Srinivasan, V.; Dagan, Y.; Cardinali, D. P. The Roles of Melatonin and Light in the Pathophysiology and Treatment of Circadian Rhythm Sleep Disorders. Nat. Clin. Pract. Neurol. 2008, 4(8), 436–447. [Google Scholar] [CrossRef] [PubMed]
- Brinkman, J. E.; Reddy, V.; Sharma, S. Physiology of Sleep. In StatPearls; StatPearls Publishing, 2023.
- Biabani, N.; Birdseye, A.; Higgins, S.; Delogu, A.; Rosenzweig, J.; Cvetkovic, Z.; Nesbitt, A.; Drakatos, P.; Steier, J.; Kumari, V.; et al. The Neurophysiologic Landscape of the Sleep Onset: A Systematic Review. J. Thorac. Dis. 2023, 15(8), 4530–4543. [Google Scholar] [CrossRef] [PubMed]
- Thangaleela, S.; Sivamaruthi, B. S.; Kesika, P.; Mariappan, S.; Rashmi, S.; Choeisoongnern, T.; Sittiprapaporn, P.; Chaiyasut, C. Neurological Insights into Sleep Disorders in Parkinson's Disease. Brain Sci. 2023, 13(8), 1202. [Google Scholar] [CrossRef] [PubMed]
- Futenma, K.; Takaesu, Y.; Komada, Y.; Shimura, A.; Okajima, I.; Matsui, K.; Tanioka, K.; Inoue, Y. Delayed Sleep-Wake Phase Disorder and Its Related Sleep Behaviors in the Young Generation. Front. Psychiatry 2023, 14, 1174719. [Google Scholar] [CrossRef] [PubMed]
- Marin, L.; Guàrdia, A.; González-Rodríguez, A.; Haba-Rubio, J.; Natividad, M.; Bosch, E.; Domínguez, N.; Monreal, J. A. Sleep Disturbances in At-Risk Mental States and First Episode of Psychosis: A Narrative Review on Interventions. Clocks Sleep 2023, 5(2), 249–259. [Google Scholar] [CrossRef] [PubMed]
- Uccella, S.; Cordani, R.; Salfi, F.; Gorgoni, M.; Scarpelli, S.; Gemignani, A.; Geoffroy, P. A.; De Gennaro, L.; Palagini, L.; Ferrara, M.; Nobili, L. Sleep Deprivation and Insomnia in Adolescence: Implications for Mental Health. Brain Sci. 2023, 13(4), 569. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, H. H.; Stubbs, B.; Chen, L. J.; Ku, P. W.; Hsu, T. Y.; Lin, C. W.; Weng, Y. M.; Wu, S. H. The Effect of Physical Activity on Sleep Disturbance in Various Populations: A Scoping Review of Randomized Clinical Trials. Int. J. Behav. Nutr. Phys. Act. 2023, 20(1), 44. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ladyman, C.; Sweeney, B.; Sharkey, K.; Bei, B.; Wright, T.; Mooney, H.; Huthwaite, M.; Cunningham, C.; Firestone, R.; Signal, T. L. A Scoping Review of Non-Pharmacological Perinatal Interventions Impacting Maternal Sleep and Maternal Mental Health. BMC Pregnancy Childbirth 2022, 22(1), 659. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Montero, A.; Stevens, D.; Adams, R.; Drummond, M. Sleep and Mental Health Issues in Current and Former Athletes: A Mini Review. Front. Psychol. 2022, 13, 868614. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Ballard, E. D.; Vande Voort, J. L.; Bernert, R. A.; Luckenbaugh, D. A.; Richards, E. M.; Niciu, M. J.; Furey, M. L.; Duncan, W. C., Jr.; Zarate, C. A., Jr. Nocturnal Wakefulness Is Associated With Next-Day Suicidal Ideation in Major Depressive Disorder and Bipolar Disorder. J. Clin. Psychiatry 2016, 77(6), 825–831. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Killgore, W. D. S.; Grandner, M. A.; Tubbs, A. S.; Fernandez, F. X.; Doty, T. J.; Capaldi, V. F., II; Dailey, N. S. Sleep Loss Suicidal Ideation: The Role of Trait Extraversion. Front. Behav. Neurosci. 2022, 16, 886836. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chasens, E. R.; Imes, C. C.; Kariuki, J. K.; Luyster, F. S.; Morris, J. L.; DiNardo, M. M.; Godzik, C. M.; Jeon, B.; Yang, K. Sleep and Metabolic Syndrome. Nurs. Clin. North Am. 2021, 56(2), 203–217. [Google Scholar] [CrossRef] [PubMed]
- Che, T.; Yan, C.; Tian, D.; Zhang, X.; Liu, X.; Wu, Z. The Association Between Sleep and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Front. Endocrinol. (Lausanne) 2021, 12, 773646. [Google Scholar] [CrossRef] [PubMed]
- Lian, Y.; Yuan, Q.; Wang, G.; Tang, F. Association Between Sleep Quality and Metabolic Syndrome: A Systematic Review and Meta-Analysis. Psychiatry Res. 2019, 274, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Hua, J.; Jiang, H.; Wang, H.; Fang, Q. Sleep Duration and the Risk of Metabolic Syndrome in Adults: A Systematic Review and Meta-Analysis. Front. Neurol. 2021, 12, 635564. [Google Scholar] [CrossRef]
- Ogura, Y.; Koyama, T.; Ozaki, E.; Omichi, C.; Uehara, R. Subjective Irregular Sleep Is Associated with Metabolic Syndrome: A Cross-Sectional Study. Prev. Med. Rep. 2022, 28, 101844. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y. Y.; Chen, J.; Peng, M.; Zhou, J.; Chen, X.; Tan, X.; Wang, N.; Ma, H.; Guo, L.; Zhang, J.; Wing, Y. K.; Geng, Q.; Ai, S. Association between Sleep Duration and Metabolic Syndrome: Linear and Nonlinear Mendelian Randomization Analyses. J. Transl. Med. 2023, 21(1), 90. [Google Scholar] [CrossRef] [PubMed]
- Kim, C. E.; Shin, S.; Lee, H. W.; Lim, J.; Lee, J. K.; Shin, A.; Kang, D. Association between Sleep Duration and Metabolic Syndrome: A Cross-Sectional Study. BMC Public Health 2018, 18(1), 720. [Google Scholar] [CrossRef] [PubMed]
- Depner, C. M.; Stothard, E. R.; Wright, K. P., Jr. Metabolic Consequences of Sleep and Circadian Disorders. Curr. Diab. Rep. 2014, 14(7), 507. [Google Scholar] [CrossRef] [PubMed]
- Chaput, J. P.; Gray, C. E.; Poitras, V. J.; Carson, V.; Gruber, R.; Olds, T.; Weiss, S. K.; Connor Gorber, S.; Kho, M. E.; Sampson, M.; et al. Systematic Review of the Relationships between Sleep Duration and Health Indicators in School-Aged Children and Youth. Appl. Physiol. Nutr. Metab. 2016, 41 (6 Suppl 3), S266–S282. [Google Scholar] [CrossRef]
- Wu, H.; Zheng, Y.; Liu, D. N.; Liu, X. X.; Yang, Q. D.; Su, Q. Y.; Wang, Y. Q.; Wang, Y. Z.; La, X. N.; Shi, Y.; et al. Association between Sleep Duration and Stroke in Different Status of Metabolic Syndrome: A Cross-Sectional Study in Shanghai Adult Residents. Nat. Sci. Sleep 2023, 15, 639–652. [Google Scholar] [CrossRef] [PubMed]
- Rafati, S.; Isheh, M.; Azarbad, A.; Ghadiri Soufi, F.; Rahimi, A.; Kheirandish, M. The Association of Sleep Duration and Metabolic Syndrome in the Bandare-Kong Cohort Study, a Cross-Sectional Survey (Finding from PERSIAN Cohort Study). Diabetol. Metab. Syndr. 2021, 13(1), 114. [Google Scholar] [CrossRef] [PubMed]
- Smiley, A.; King, D.; Bidulescu, A. The Association between Sleep Duration and Metabolic Syndrome: The NHANES 2013/2014. Nutrients 2019, 11(11), 2582. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tan, F.; Wei, L.; Li, X.; Lyu, Z.; Feng, X.; Wen, Y.; Guo, L.; He, J.; Dai, M.; Li, N. Sleep Duration and the Risk of Cancer: A Systematic Review and Meta-Analysis Including Dose-Response Relationship. BMC Cancer 2018, 18(1), 1149. [Google Scholar] [CrossRef] [PubMed]
- Strøm, L.; Danielsen, J. T.; Amidi, A.; Cardenas Egusquiza, A. L.; Wu, L. M.; Zachariae, R. Sleep during Oncological Treatment - A Systematic Review and Meta-Analysis of Associations with Treatment Response, Time to Progression and Survival. Front. Neurosci. 2022, 16, 817837. [Google Scholar] [CrossRef] [PubMed]
- Garbarino, S.; Lanteri, P.; Bragazzi, N. L.; Magnavita, N.; Scoditti, E. Role of Sleep Deprivation in Immune-Related Disease Risk and Outcomes. Commun. Biol. 2021, 4(1), 1304. [Google Scholar] [CrossRef]
- Besedovsky, L.; Lange, T.; Haack, M. The Sleep-Immune Crosstalk in Health and Disease. Physiol. Rev. 2019, 99(3), 1325–1380. [Google Scholar] [CrossRef] [PubMed]
- Besedovsky, L.; Lange, T.; Born, J. Sleep and Immune Function. Pflugers Arch. 2012, 463(1), 121–137. [Google Scholar] [CrossRef] [PubMed]
- Radmanish, M.; Khalfallah, O.; Glaichenhaus, N.; Forhan, A.; Heude, B.; Charles, M. A.; Davidovic, L.; Plancoulaine, S. Sleep Duration Trajectories Associated with Levels of Specific Serum Cytokines at Age 5: A Longitudinal Study in Preschoolers from the EDEN Birth Cohort. Brain Behav. Immun. Health 2022, 21, 100429. [Google Scholar] [CrossRef] [PubMed]
- Schmitz, N. C. M.; van der Werf, Y. D.; Lammers-van der Holst, H. M. The Importance of Sleep and Circadian Rhythms for Vaccination Success and Susceptibility to Viral Infections. Clocks Sleep 2022, 4(1), 66–79. [Google Scholar] [CrossRef] [PubMed]
- Poluektov, M. G. Sleep and Immunity. Neurosci. Behav. Physi. 2021, 51, 609–615. [Google Scholar] [CrossRef] [PubMed]
- Irwin, M. R.; Opp, M. R. Sleep Health: Reciprocal Regulation of Sleep and Innate Immunity. Neuropsychopharmacology 2017, 42(1), 129–155. [Google Scholar] [CrossRef] [PubMed]
- Dempsey, L. A. Sleep Regulates Innate Immunity. Nat. Immunol. 2024, 25 (3). [CrossRef]
- Steidten, T.; Baumbach, P.; May, R.; Gabriel, B.; Herbsleb, M.; Markov, A.; Granacher, U.; Kellmann, M.; Bloch, W.; Gabriel, H. H. W.; Puta, C. Overnight Immune Regulation and Subjective Measures of Sleep: A Three Night Observational Study in Adolescent Track and Field Athletes. Front. Sports Act. Living 2021, 3, 689805. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, M.; Forte, G.; Favieri, F.; Corbo, I. Sleep Quality and Aging: A Systematic Review on Healthy Older People, Mild Cognitive Impairment and Alzheimer's Disease. Int. J. Environ. Res. Public Health 2022, 19(14), 8457. [Google Scholar] [CrossRef] [PubMed]
- Cedernaes, J.; Osorio, R. S.; Varga, A. W.; Kam, K.; Schiöth, H. B.; Benedict, C. Candidate Mechanisms Underlying the Association between Sleep-Wake Disruptions and Alzheimer's Disease. Sleep Med. Rev. 2017, 31, 102–111. [Google Scholar] [CrossRef] [PubMed]
- Romanella, S. M.; Roe, D.; Tatti, E., et al.; et al. The Sleep Side of Aging and Alzheimer's Disease. Sleep Med. 2021, 77, 209–225. [CrossRef] [PubMed]
- Kokošová, V.; Filip, P.; Kec, D.; Baláž, M. Bidirectional Association Between Sleep and Brain Atrophy in Aging. Front. Aging Neurosci. 2021, 13, 726662. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, V.; Spence, W. D.; Pandi-Perumal, S. R.; Zakharia, R.; Bhatnagar, K. P.; Brzezinski, A. Melatonin and Human Reproduction: Shedding Light on the Darkness Hormone. Gynecol. Endocrinol. 2009, 25(12), 779–785. [Google Scholar] [CrossRef] [PubMed]
- Pandi-Perumal, S. R.; Monti, J. M.; Burman, D.; Karthikeyan, R.; BaHammam, A. S.; Spence, D. W.; Brown, G. M.; Narashimhan, M. Clarifying the Role of Sleep in Depression: A Narrative Review. Psychiatry Res. 2020, 291, 113239. [Google Scholar] [CrossRef] [PubMed]
- Tolahunase, M. R.; Gautam, S.; Sagar, R.; Kumar, M.; Dada, R. Yoga in Major Depressive Disorder: Molecular Mechanisms and Clinical Utility. Front. Biosci. (Schol. Ed.) 2021, 13 (1), 56–81. [CrossRef]
- Miller, M. W.; Lin, A. P.; Wolf, E. J.; Miller, D. R. Oxidative Stress, Inflammation, and Neuroprogression in Chronic PTSD. Harv. Rev. Psychiatry 2018, 26(2), 57–69. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, G.; van Heijningen, S.; Reijne, A. C.; Nyakas, C.; van der Zee, E. A.; Eisel, U. L. Integrative Neurobiology of Metabolic Diseases, Neuroinflammation, and Neurodegeneration. Front. Neurosci. 2015, 9, 173. [Google Scholar] [CrossRef] [PubMed]
- Gibson, E. M.; Williams, W. P. 3rd; Kriegsfeld, L. J. Aging in the Circadian System: Considerations for Health, Disease Prevention and Longevity. Exp. Gerontol. 2009, 44 (1–2), 51–56. [CrossRef]
- Pandi-Perumal, S. R.; Seils, L. K.; Kayumov, L.; Ralph, M. R.; Lowe, A.; Moller, H.; Swaab, D. F. Senescence, Sleep, and Circadian Rhythms. Ageing Res. Rev. 2002, 1(3), 559–604. [Google Scholar] [CrossRef] [PubMed]
- Zaki, N. F. W.; Yousif, M.; BaHammam, A. S.; Spence, D. W.; Bharti, V. K.; Subramanian, P.; Pandi-Perumal, S. R. Chronotherapeutics: Recognizing the Importance of Timing Factors in the Treatment of Disease and Sleep Disorders. Clin. Neuropharmacol. 2019, 42(3), 80–87. [Google Scholar] [CrossRef] [PubMed]
- Paruthi, S.; Brooks, L. J.; D’Ambrosio, C.; Hall, W. A.; Kotagal, S.; Lloyd, R. M.; Malow, B. A.; Maski, K.; Nichols, C.; Quan, S. F.; Rosen, C. L.; Troester, M. M.; Wise, M. S. Consensus Statement of the American Academy of Sleep Medicine on the Recommended Amount of Sleep for Healthy Children: Methodology and Discussion. Journal of Clinical Sleep Medicine 2016, 12, 1549–1561. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).