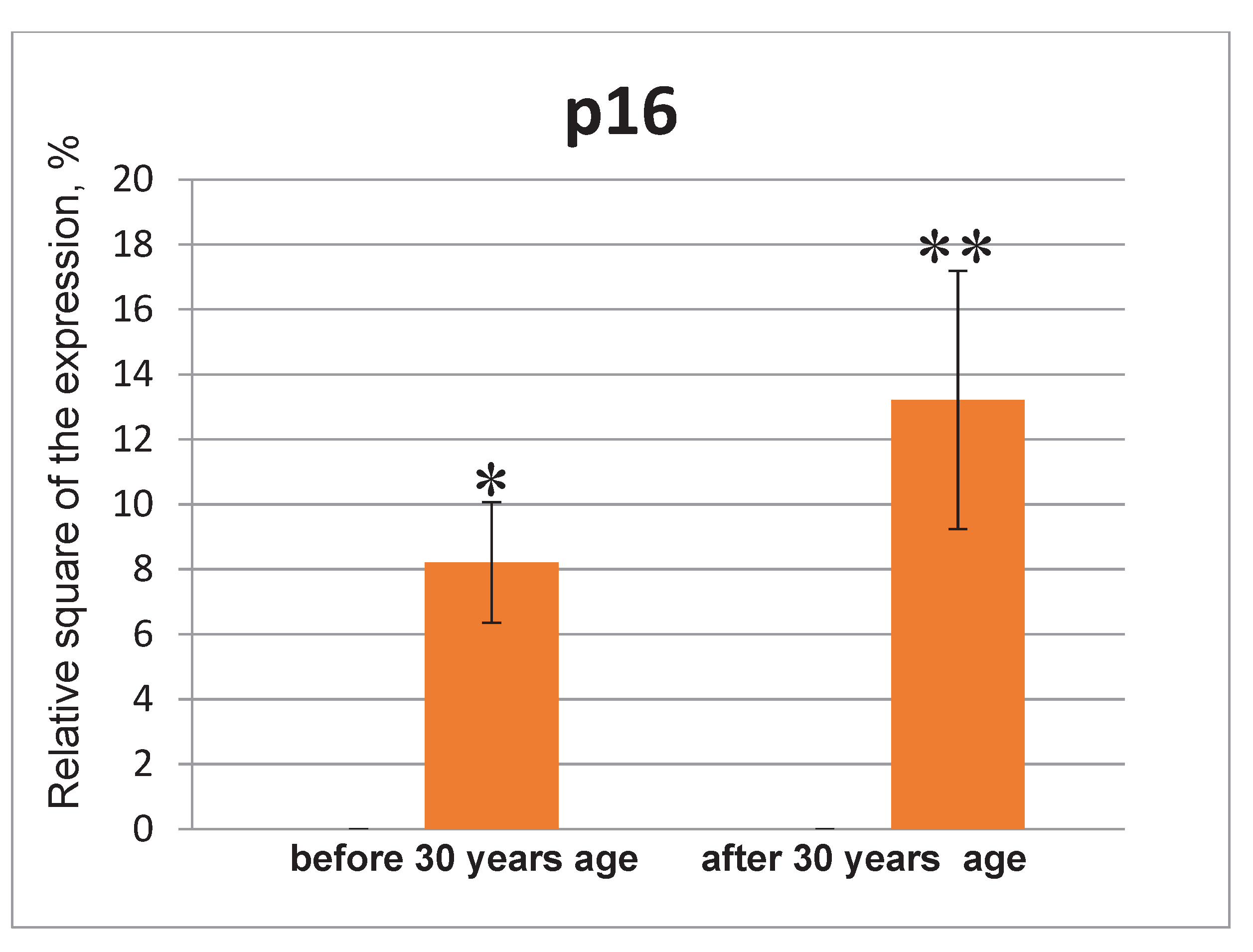1. Introduction
The main feature of cell aging is the inability of cells for the splitting even by the growth factors stimulation. Since aging cells are usually associated with the whole-body aging and age-associated diseases, it is assumed that cellular aging contributes to an age-related decrease of the reproductive function. It is well-known, that cells have a limited ability to replicate - the Hayflick limit, also called "cellular aging" [
1]. As soon as a significant number of proliferating cells in a tissue reaches the limit of cellular aging, the ability of this tissue for the regeneration decreases sharply [
2]. Since the lack of regenerative potential is a hallmark of tissue aging, the population of aging cells is directly related to the whole-body aging and age-associated diseases [
3].
In addition, cellular aging leads to pregnancy complications such as the spontaneous abortion and the premature birth. However, cellular aging is also involved in the maintaining of normal homeostasis during the pregnancy and fetal development, suggesting that a balance between normal and aging cells is necessary in the early stages of the body's development [
4].
Inflammaging (inflammatory aging) is an age-associated, chronic and systemic inflammatory state caused by cells with the acquired SASP phenotype (senescence associated secretory phenotype) [
5]. This type of cell has a pronounced depression of the p16INK4a gene, which supports the viability of aging cells by the apoptosis prevention.
Late reproductive age is associated with infertility and possible complications of the onset and course of pregnancy. Aging cells express proinflammatory cytokines, growth factors, and matrix metalloproteinases, which are collectively referred to as the aging-associated secretory phenotype (SASP). Such cells are viable
in vitro, unlike apoptotic cells, which undergo by the programmed cell death. The number of immunological aging markers increases with the aging [
6].
A feature of cells with the SASP phenotype is its dynamic development over the time. Genetic changes, such as the
p53 loss or an increase in oncogenic RAS, lead to a faster acquisition of the SASP phenotype, which suggest that SASP is a specific program triggered by the genotoxic stress. In the culture, cells acquire full SASP during 3-5 days after the aging induction, and cell growth stops within 24 h after the damage. Not all SASP factors begin to be secreted at the same time. This gradual phenotypic transition is a trait preserved between cell types and aging inducers [
7]. According to the data available, cell cycle arrest in the G1 phase and a decrease in the number of cells in the G2-M phase in all cells are observed in 6 hours after the UV irradiation [
8].
The lack of verified, accurate and reliable biological markers of the inflammaging is the task to be solved for the successful treatment of age-associated diseases. The main characteristics of biological markers of aging are as follows: (1) the marker is associated with the age; (2) the expression of the marker does not change with the disease; (3) the marker does not change with metabolic and nutritional states; (4) the marker is affected by the aging processes; (5) the marker does not change in immortalized cells [
9]. The expansion of the research on biological markers of aging will make it possible to clarify the molecular mechanisms of inflammaging and determine the role of this phenomenon in the process of the body aging.
It was revealed that the SASP cells secretome included the inflammatory factors such as IL-6, IL-8 or MCP-1, growth modulators such as GRO and IGFBP-2, cell survival regulators such as OPG or STNF RI, as well as secreted surface molecules such as uPAR or ICAM-1 [
10].
The recent proteomic studies of the SASP cell phenotype highlighted, that amphoterin (HMGB1), the β-1 subunit of laminins (LAMB1) and tissue metallopeptidase inhibitors (TIMPs) can be considered as the signalling molecules [
11].
SASP cells are capable to secrete an extracellular vesicle - exosomes, microvesicles and apoptotic bodies. After the secretion, these vesicles can be absorbed by recipient cells through endocytosis, phagocytosis, macropinocytosis, or membrane fusion [
12]. All types of extracellular vesicles contain both different types of proteins and mRNAs [
13]. Proteomic analysis of the vesicle contents revealed several known SASP factors [
14].
A large number of molecules and signalling pathways involved in the mechanisms of the aging are known. However, the involvement of these factors in the inflammaging processes and their role in the reproductive aging remains insufficiently investigated. To determine the tissue and age expression of the aging markers, it is necessary to analyse a panel of markers characteristic for SASP: TGFß, IL-6, IL-8, IL-1a, NF-kB, MMP3 [
15]; two classical markers of cellular aging – p16 [
16] and p53 [
17], as well as to conduct studies on the expression of molecules that may be involved in the mechanisms of aging, namely Ki-67 [
18], PCNA [
19], Bcl-2 [
20], SIRT1,6 [
21], TERF-1 [
22], CALR [
23].
In this work, the expression of signalling molecules in mammalian endometrial cells during their aging in vitro was studied to expand the understanding of the SASP phenotype formation and its role in the mechanisms of the human reproductive system inflammaging.
2. Materials and Methods
2.1. The Modelling of Inflammaging
For cell cultures preparation, endometrial material (n=20) was obtained in the gynecological department of St. Petersburg State Medical University by pipel biopsy during an examination by gynaecologists provided by the medical services standard to identify the infertility causes. The material was divided into 2 groups: young reproductive age (under 35 years) (n=10); older reproductive age (after 35 years) (n=10). All patients had normal menstrual cycles. The patients did not receive hormonal therapy. 4 groups of endometrial cell culture (CCE) were studied: 1- from patients of young reproductive age; 2- from patients of older reproductive age; 3- the inflammaging model for patients younger than 35 years; 4- the inflammaging model for patients older than 35 years.
The isolation of cell culture was carried out according to the standard protocol developed in our laboratory [
24].
The modelling of the inflammaging (exposure to genotoxic stress) was carried out as follows: ultraviolet irradiation (UVA+UVB) was performed twice per day for four days at the rate of 0.05 J/cm2 per the treatment, as it is described in the article [
25]. Under such irradiation conditions, no apparent cell death was observed, and the decrease in its proliferation was detected. After the fourth day of the treatment, the cells were allowed to recover and were used for further experiments.
2.2. Immunocytochemistry
The following primary monoclonal antibodies were used for immunocytochemistry: IL-1a (1:100, Abcam), IL-6 (1:500, Abcam), IL-8 (1:2000, Abcam), MMP3 (1:250, Abcam), p16 (1:100, Abcam), SIRT1 (1:250, Abcam), SIRT6 (1:100, Abcam), TERF-1 (1:100, Abcam). Alexa Fluor 488, Alexa Fluor 647 (1:1000, Abcam) and polyclonal antibodies to calreticulin (1:200, Abcam) conjugated with fluorochrome were used as the secondary antibodies. The cell nuclei were stained with Hoechst (dilution of the initial solution 1:100 in distilled water; AppliChem, USA) for 10 min. The preparations were placed under cover glasses in the medium (fluorescent medium for applying Dako, (Dako, USA)) and stored in the dark to protect against fluorochrome fading. Negative control, without the primary antibodies, was used to monitor the quality of the ICR reaction.
2.3. Morphometric Analysis
To analyze the results obtained, the Olympus FluoView 1000 confocal microscope (Japan) and the Videotest Morphology 5.2 software were used. In each case, 5 visual fields were analysed at the 400 magnification.
The expression area was calculated as the ratio of the area occupied by immunopositive cells to the total area of cells in the field of view and expressed as a percentage. This parameter characterizes the number of cells in which the studied marker is expressed.
2.4. Statistical Analysis
Statistical analysis was carried out in the Excel 2016 Microsoft Office 2016 program and in the Statistica 7.0 analytical program.
Statistical analysis of all experimental data included the calculation of the arithmetic mean, standard deviation and confidence interval for each sample. The Shapiro–Wilk criterion (W-test) was used to analyze the distribution type.
The differences between the samples were evaluated using the parametric Student t-test (with a normal distribution of data), the nonparametric Mann-Whitney U-test (in the absence of a normal distribution of data).
For the samples where the variations were significant, multiple comparison procedures using the Mann–Whitney criterion were used. The Student's t-test was used for the groups with a nonsignificant variations. The critical confidence level of the null hypothesis (about the absence of differences) was assumed to be 0.01.
3. Results and Discussion
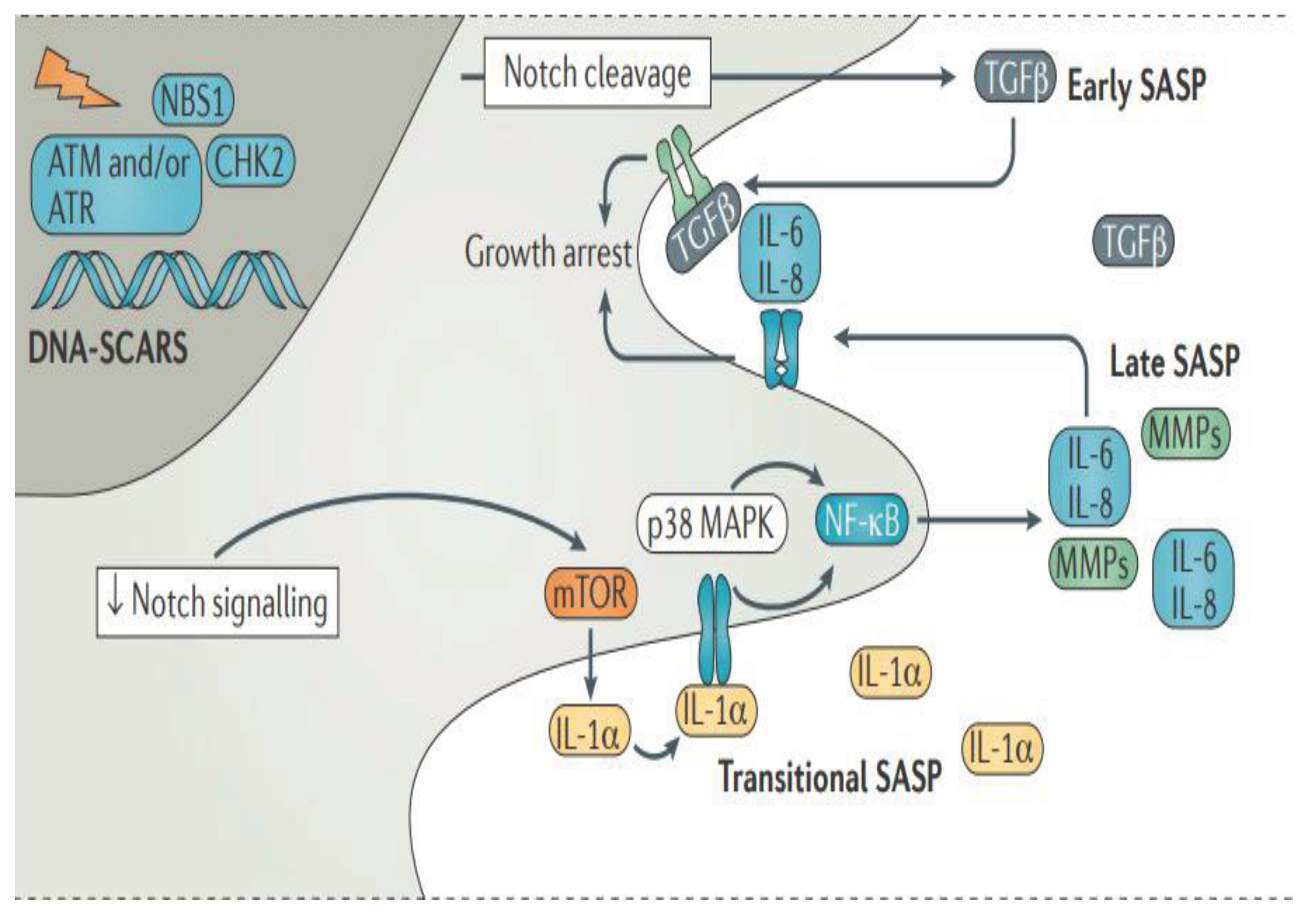
SASP is initiated and maintained by the chronic DNA damage reaction (DDR) - DNA segments with chromatin changes that enhance the aging. Initially, Notch signalling stimulates the transformation of growth factor β (TGFß) secretion ("early SASP"), which acts autonomously for cells, contributing to cell cycle arrest. The subsequent decrease in Notch signalling promotes the transition to DDR-dependent "transient SASP", which is enhanced by the mechanistic target of rapamycin (mTOR), while interleukin-1α (IL-1α) bound to the cell surface binds to the interleukin-1 receptor (IL-1R). Either this cell-autonomous IL-1a signal or the activity of mitogen-activated protein kinase p38 (p38 MAPK) is transmitted through nuclear factor kB (NF-kB), which leads to the secretion of "late SASP", which is characterized by the expression of metalloproteinase (MMPs), IL-6, IL-8 and many others factors (
Figure 1). At this stage, IL-6 and IL-8 can also enhance cell cycle arrest. It is important to note that many specific details of senescent cell growth retardation, cell death resistance, and SASP have not been confirmed
in vivo and are likely to show significant differences.
Based mainly on the in vitro experiments, senescent cells possess several key characteristics, namely, the participation in the permanent arrest of the cell cycle, resistance to cell death signalling, and the production of a biologically active secretome known as the secretory phenotype associated with aging (SASP). The accumulation of SASP cells in tissues contributes to an age-related functional decrease of tissues and organs.
The most widely-used model of the genotoxic stress is the UV irradiation, which triggers a DNA damage reaction (DDR), which is considered as the induction of yH2AX not only in the cells, both in S-, and also in G1- phases [
26].
Here we present the investigation of molecular markers of SASP in the human endometrium cells depending from the health (normal) aging and the aging under the genotoxic stress.
3.1. Interleukins Expression
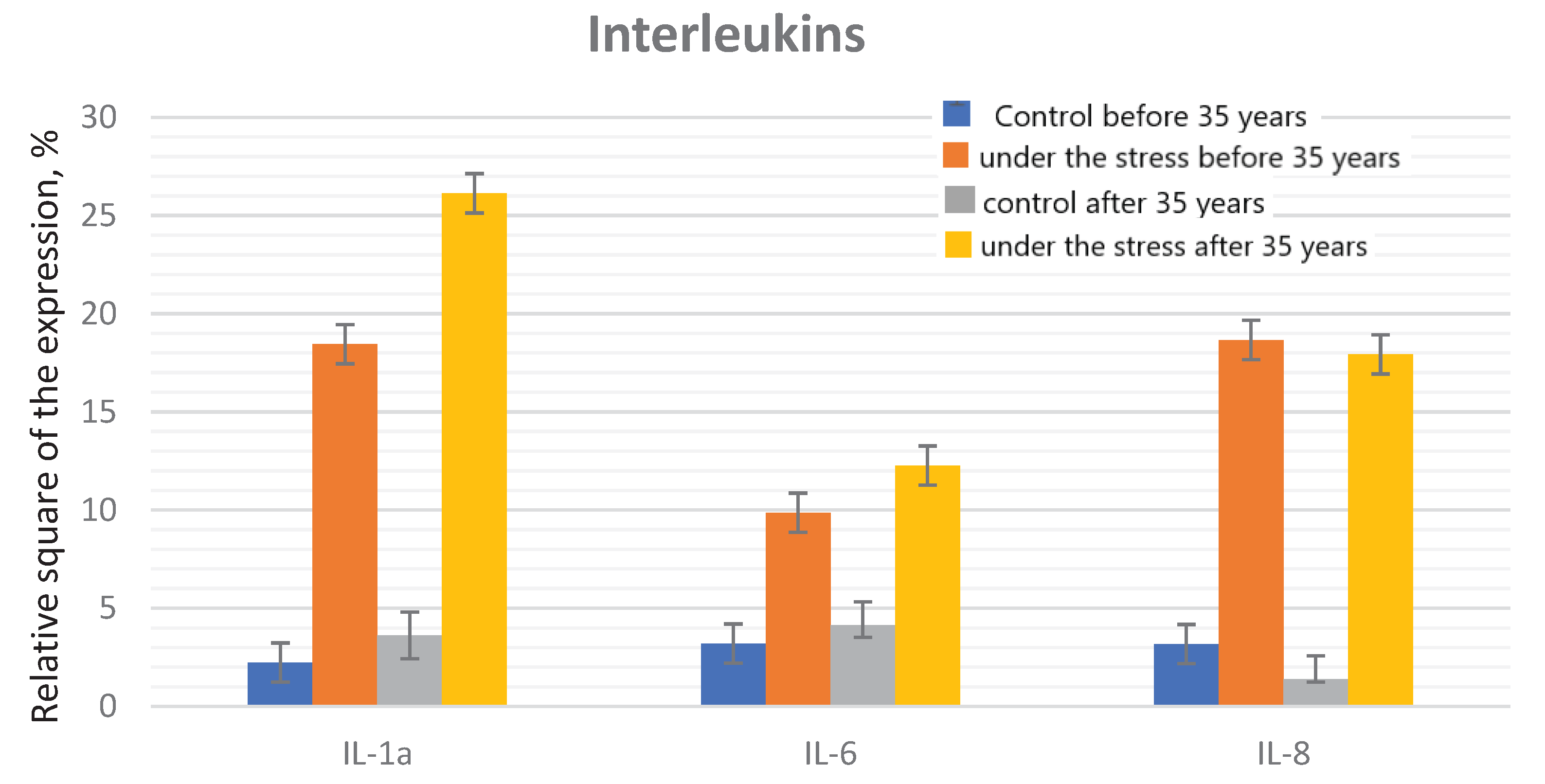
The comparison of the control and under the genotoxic stress groups, the following pattern is observed: in the control groups no significant differences were found between groups under 35 years age and after 35 years age, in the groups under the stress the significant increase in the indicator in the group of older reproductive age was detected (
Figure 2 and
Figure 3).
Under the genotoxic stress, the significant increase in IL-8 levels was found in the groups of young and older reproductive age compared with the control groups: 9 times and 13 times higher in the groups under 30 and after 30 years, respectively.
The significant differences were recorded when comparing with the control groups of different ages. There were no statistically significant differences in the groups under the genotoxic stress of young and older age.
DNA damage stimulates the production of pro-inflammatory cytokines IL-1, IL-6 and IL-8, activating the NF-kB signaling pathway, blocking the cell cycle, inducing and maintaining of the cellular aging phenotype. Interleukins 1-α, 6 and 8 are key markers of the inflammaging [
27].
IL-1a promotes the lymphocytes and macrophages activation, increased adhesion of leukocytes, can cause a fever due to stimulation of the hypothalamus and release of acute phase proteins, cachexia, and is also able to initiate apoptosis in many cell types [
28].
IL1a and IL1β play the role in the ovulation processes, implantation and embryogenesis. IL1a expression is detected during the menstrual cycle and in early pregnancy, where it is localized in the stroma, cells of the cyclic endometrium, and endometrial glands [
29].
IL-6 is an interleukin that acts as a pro-inflammatory cytokine and an anti-inflammatory myokine. In the human body, it is encoded by the IL-6 gene [
30].
During the analysis of the IL-6 synthesis in the human endometrium and its relationship with the concentrations in uterine secretions, it was shown that the expression of IL-6 receptor mRNA in the endometrium changes throughout the menstrual cycle [
31]. Low levels of expression of IL-6 and its mRNA were recorded during the proliferative phase, an increase of the concentration was observed in the secretory phase. The increased expression of IL-6 in endometrial epithelial cells in the middle of the secretory phase (the presumed implantation window) and the achievement of maximum expression levels in the late secretory phase (premenstrual period) confirm the role of IL-6 as a multifunctional cytokine in the regulation of endometrial functions [
32].
IL-8 is expressed by endometrial cells, directly stimulating the proliferation of endometrial cells and the growth of other cell types. In addition, changes in IL-8 mRNA that depend on the menstrual cycle have been shown along with protein expression in the endometrium [
33]. An increase in IL-8 mRNA levels was observed in the late secretory and early proliferative phases of the cycle. IL-8 is an important factor involved in inflammatory processes associated with endometriosis [
34]. It can act as a factor of autocrine growth of the endometrium and enhance the invasive properties of endometrial cells, which can lead to a transition from the stage of acute inflammation to chronic [
35].
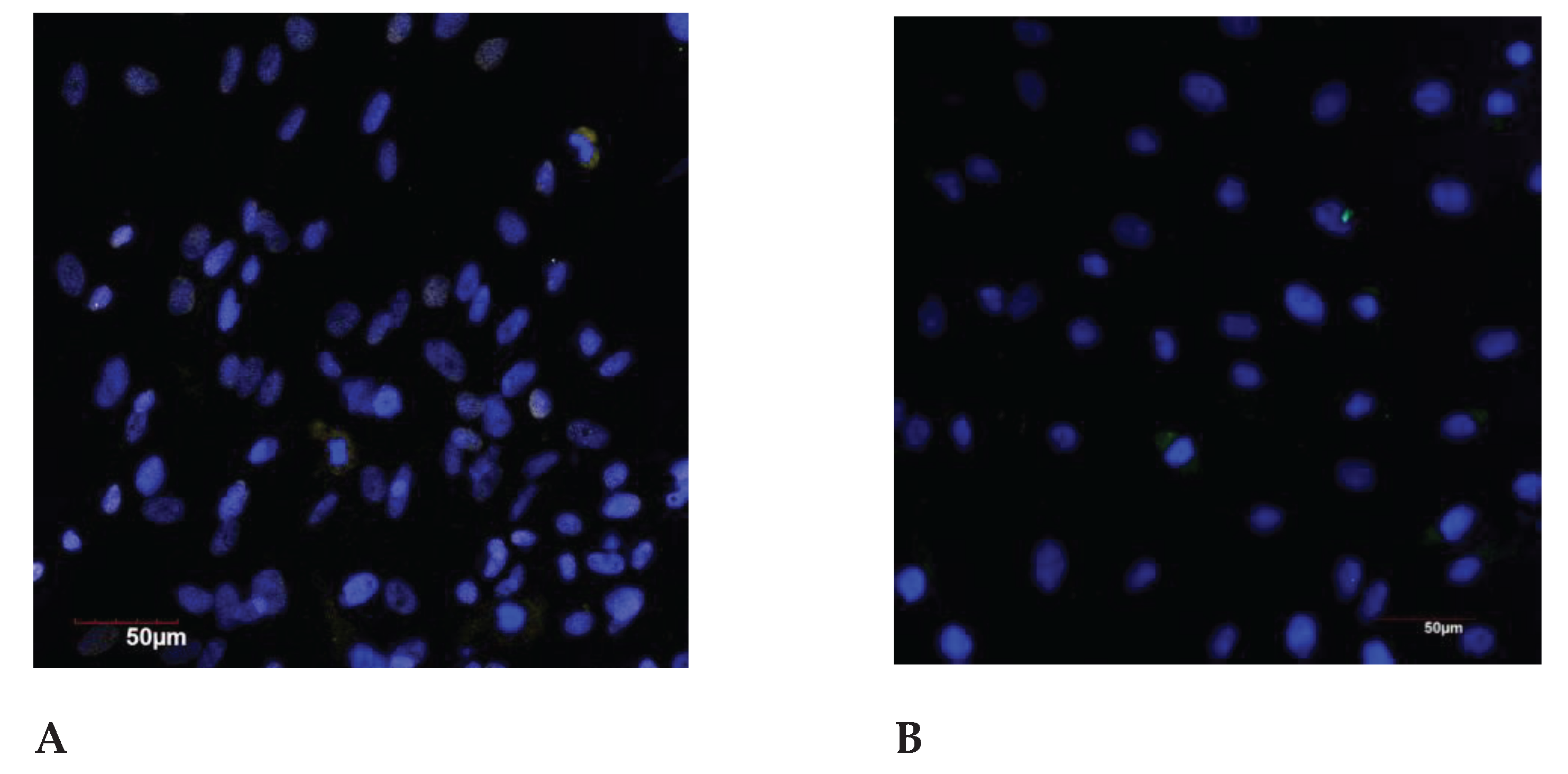
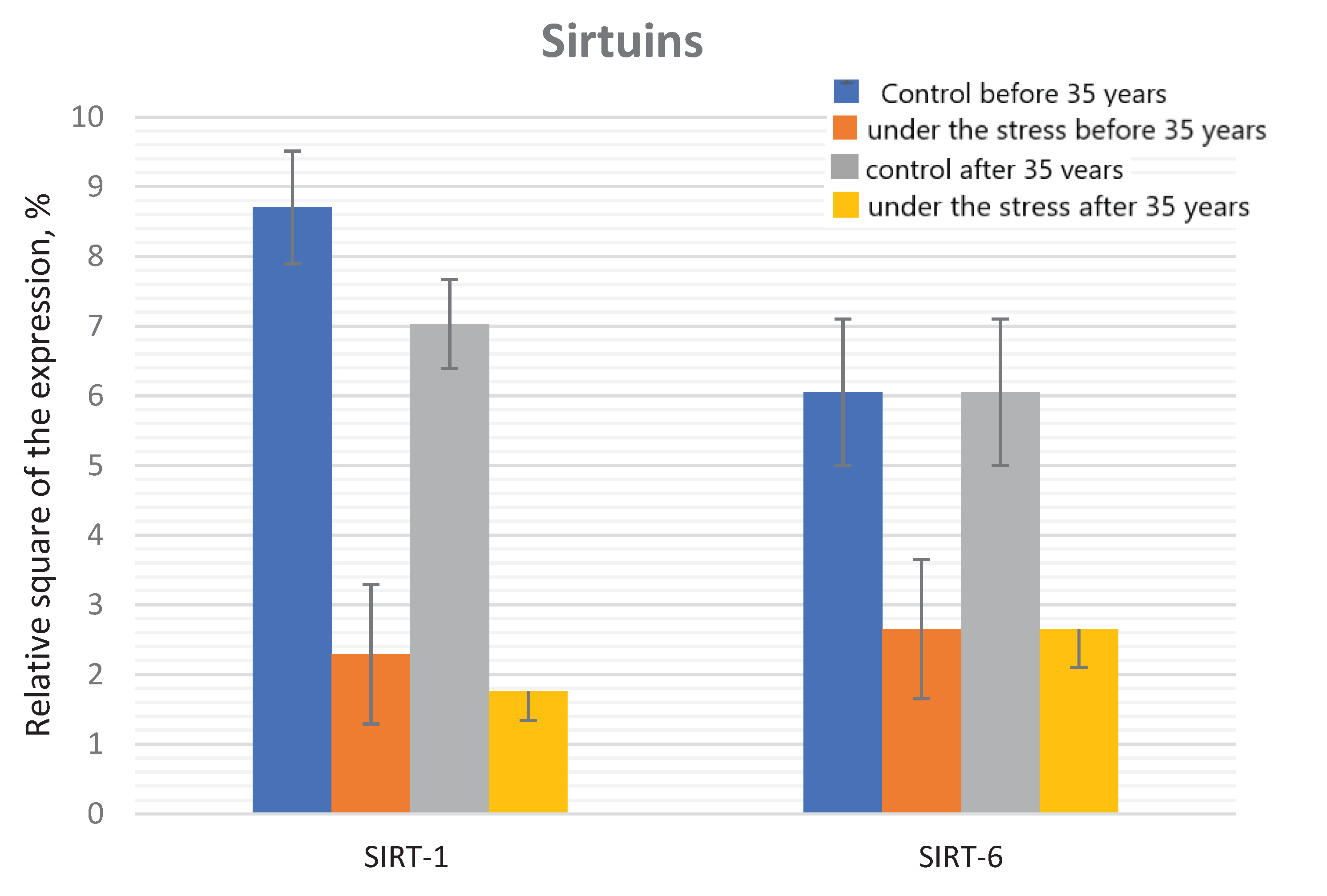
3.2. Sirtuins Expression
In the control group, the significant decrease in the SIRT-1 expression was found in the older reproductive age group compared to the group under 35 years of age. It has also been shown that the relative SIRT1 expression decreases significantly in the both age groups (by 3.8 and 4.0 times, respectively) when exposed to the genotoxic stress.
No any differences were found in the groups of under 35 and before 35 years old groups under the genotoxic stress action (
Figure 4 and
Figure 5).
Significant differences in the SIRT-6 expression under the genotoxic stress were revealed both in the group of young reproductive age and older compared to the corresponding control groups. When comparing the control groups of young and older reproductive age, there is the significant decrease of the SIRT-6 expression with the age, a similar dynamics is also observed when comparing groups under the genotoxic stress. Mammalian sirtuins differ in their localization in the cell. The localization of SIRT1 varies depending from the cell type and the differentiation degree; it can be exclusively nuclear or present in the cell cytoplasm [
36]. In the nucleus, most of SIRT1 is associated with euchromatin, while SIRT6 is associated with heterochromatin, SIRT7 is localized in the nucleolus [
37]. A representative of the sirtuin family located in the cytoplasm is SIRT2 [
38]. SIRT3, SIRT4 and SIRT5 are known as mitochondrial sirtuins [
39].
SIRT1 is expressed in all organs, however, predominates in the most energy-dependent tissues [
37]. Knockout mutations in the SIRT1 gene lead to the gametogenesis disorders, as well as prenatal and perinatal death [
40]. For many years, SIRT1 has attracted the attention, since it is the protein in mammals that has the highest homology with the yeast Sir2 gene associated with longevity. Overexpression of Sir2 leads to an increase in life expectancy by about 30%, while Sir2 knockout contributes to 50% reduction in life expectancy [
41].
Human aging is characterized by a chronic, low level of inflammation [
5], and NF-kB is the main regulator of transcription of genes associated with inflammation [
42]. SIRT1 inhibits the expression of the NF-kB regulated gene by deacetylation of the RelA NF-kB subunit [
43]. The tumor suppressor p53 triggers apoptosis. SIRT1, deacetylating p53, suppresses its transcriptional activity, thereby preventing apoptosis [
44].
In the last decade, another protein from the sirtuin family, SIRT6, has been actively studied, which, according to the results of numerous studies, is associated with an increase in mammalian life expectancy. SIRT6 is a critical regulator of transcription of genome stability, telomeric integrity, DNA repair and metabolic homeostasis [
45]. Depletion of the SIRT6 pool leads to an abnormal telomere structure and loss of terminal sequences during DNA replication, resulting in genome instability and premature cellular aging [
46].
SIRT6 also plays an important role in the regulation of DNA repair processes. SIRT6 associates with chromatin flanking double-stranded DNA breaks (DSB), thereby stabilizing the repair proteins of double-stranded DNA breaks when connecting non-homologous ends of DSB and contributing to the effective repair of these breaks [
47].
3.3. TERF-1 Expression
The significant difference between TERF-1 expression in the control and under the stress, both in the group under 35 years of age and in the group of older reproductive age were detected.
There is significant decrease in the expression depending from the age: in the control group - by 1.79 times; when comparing groups under the genotoxic stress – by 7.1 times. Telomeric repeat-binding factor 1 (TERF-1) is a DNA–binding protein that is the component of the telomere nucleoprotein complex. The lack or absence of TERF-1 in cells leads to DNA damage and cellular aging. It is assumed that the age-associated decrease of the TERF-1 level leads to serious defects in the later stages of aging, such as telomere damage and chromosome dysfunction [
22]. During the replicative aging of human embryonic fibroblasts, it was shown that the transcription of TERF-1 in cells does not change, and the expression of TERF-1 protein gradually increases at first, and then decreases rapidly. An increase in the TERF-1 protein concentration at the beginning of aging suggests that cells can promote TERF-1 translation in order to improve the stability of telomere DNA [
48].
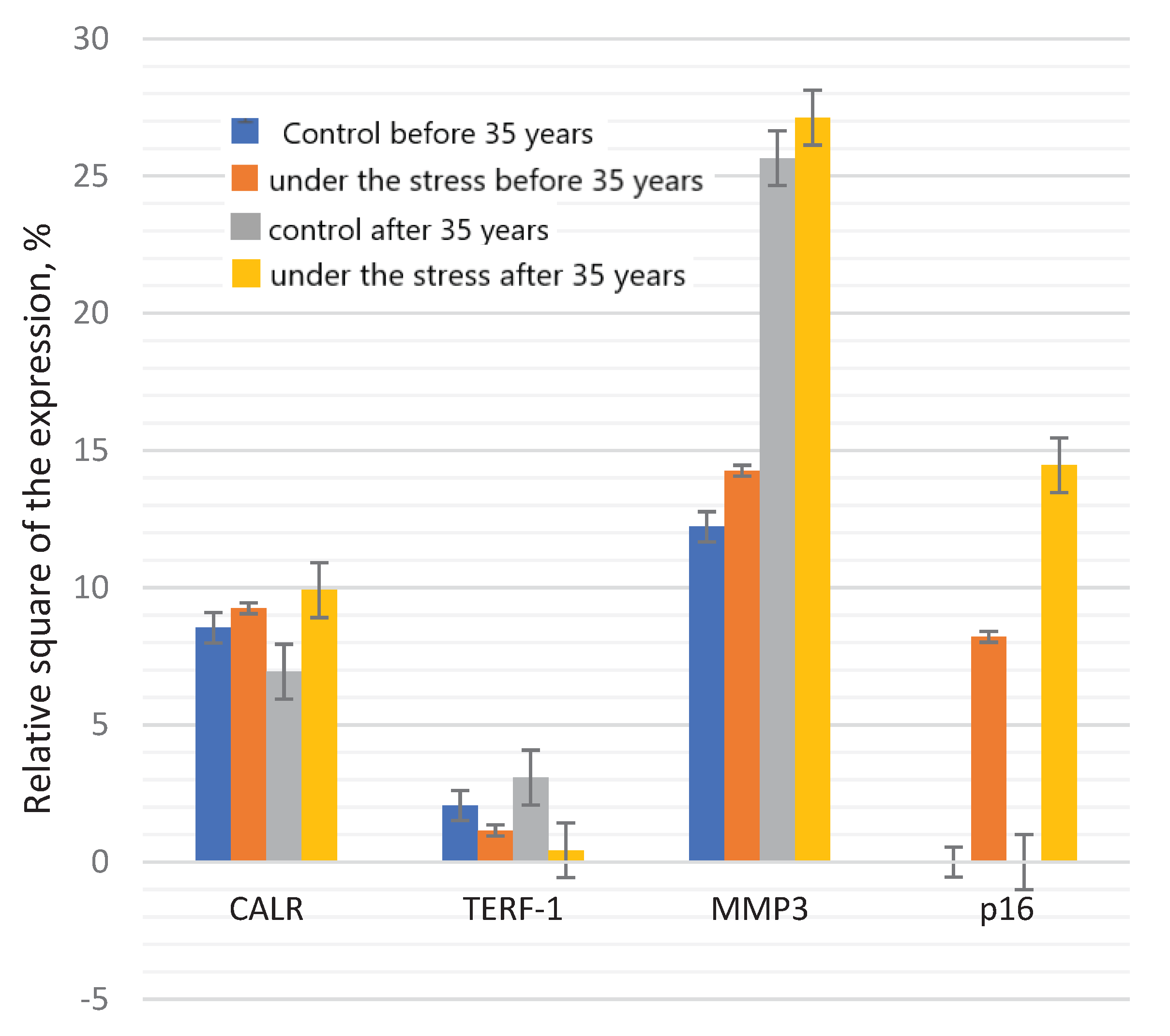
3.4. Calreticulin Expression
The expression of CALR protein was significantly decreased in the control group of older reproductive age, compared to the group under 35 years of age. However, there were no statistically significant differences between these age groups when exposed to the stress. The significant increase of the CALR expression was revealed in the group after 35 years under the genotoxic stress (
Figure 6).
Calreticulin (CALR) also known as calregulin is the protein that in humans is encoded by the CALR gene. Calreticulin is a multifunctional calcium-binding protein of the endoplasmic reticulum (ER) [
49].
In addition to the regulation of intracellular calcium concentration, this protein, together with calnexin, participates in the formation of the tertiary structure of proteins, possessing chaperone functions. Calreticulin binds to improperly folded proteins and glycoproteins and prevents their transport from the endoplasmic reticulum (ER) to the Golgi apparatus. This chaperone attaches to oligosaccharides of partially folded proteins, retaining them in the ER [
50]. The unfolded protein undergoes by the sequential removal (by glucosidase) and by the addition (by glucoside transferase) of glucose. Due to this, the affinity of the protein to calnexin and calreticulin is maintained until the folding is completed. Like other chaperones, calreticulin prevents the irreversible aggregation of the infolded protein [
51].
A decrease in calreticulin level with the age contributes to a decrease in protein quality control, which leads to the destructive changes in the aging process [
52].
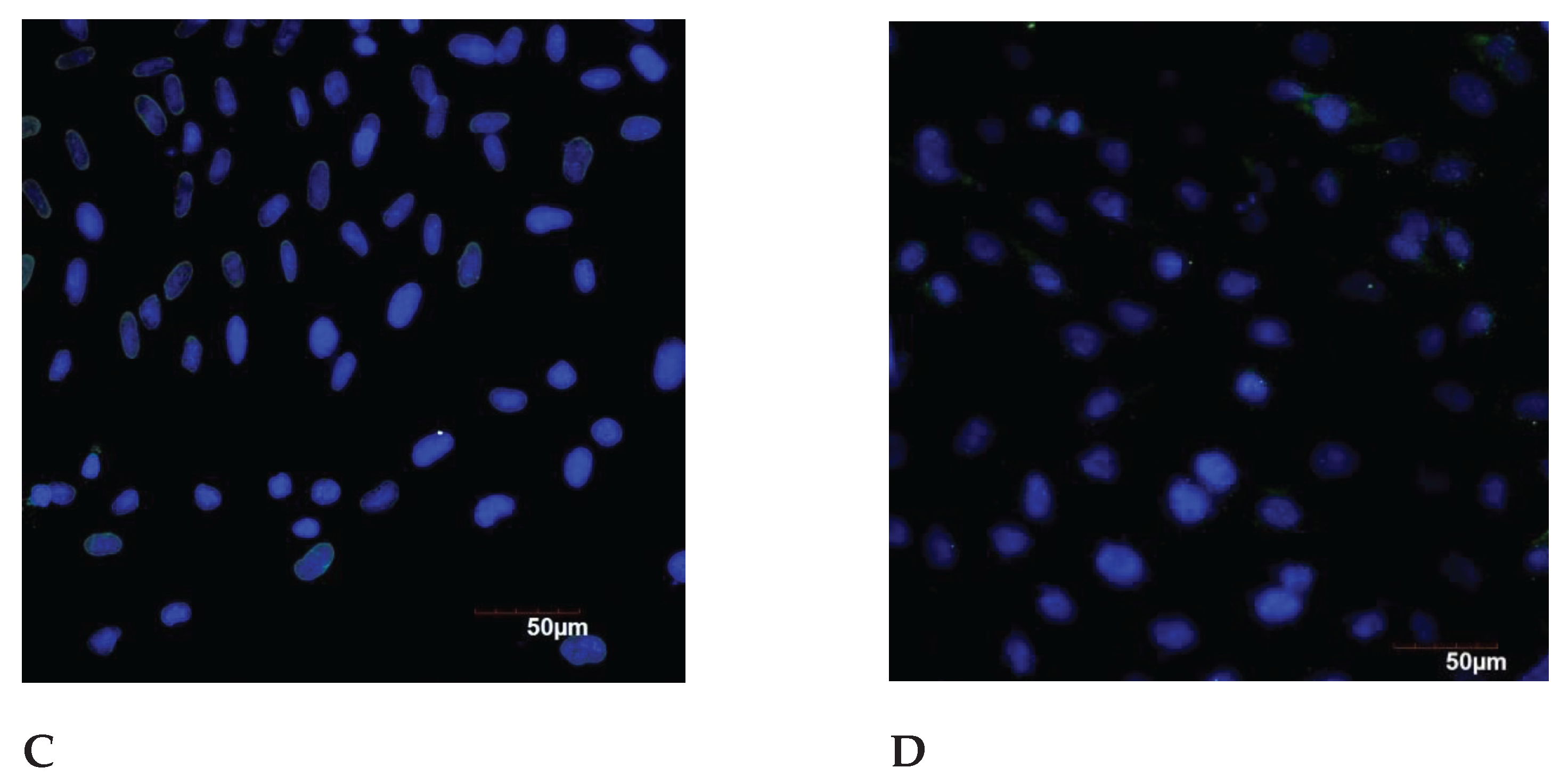
3.5. ММР3. Expression
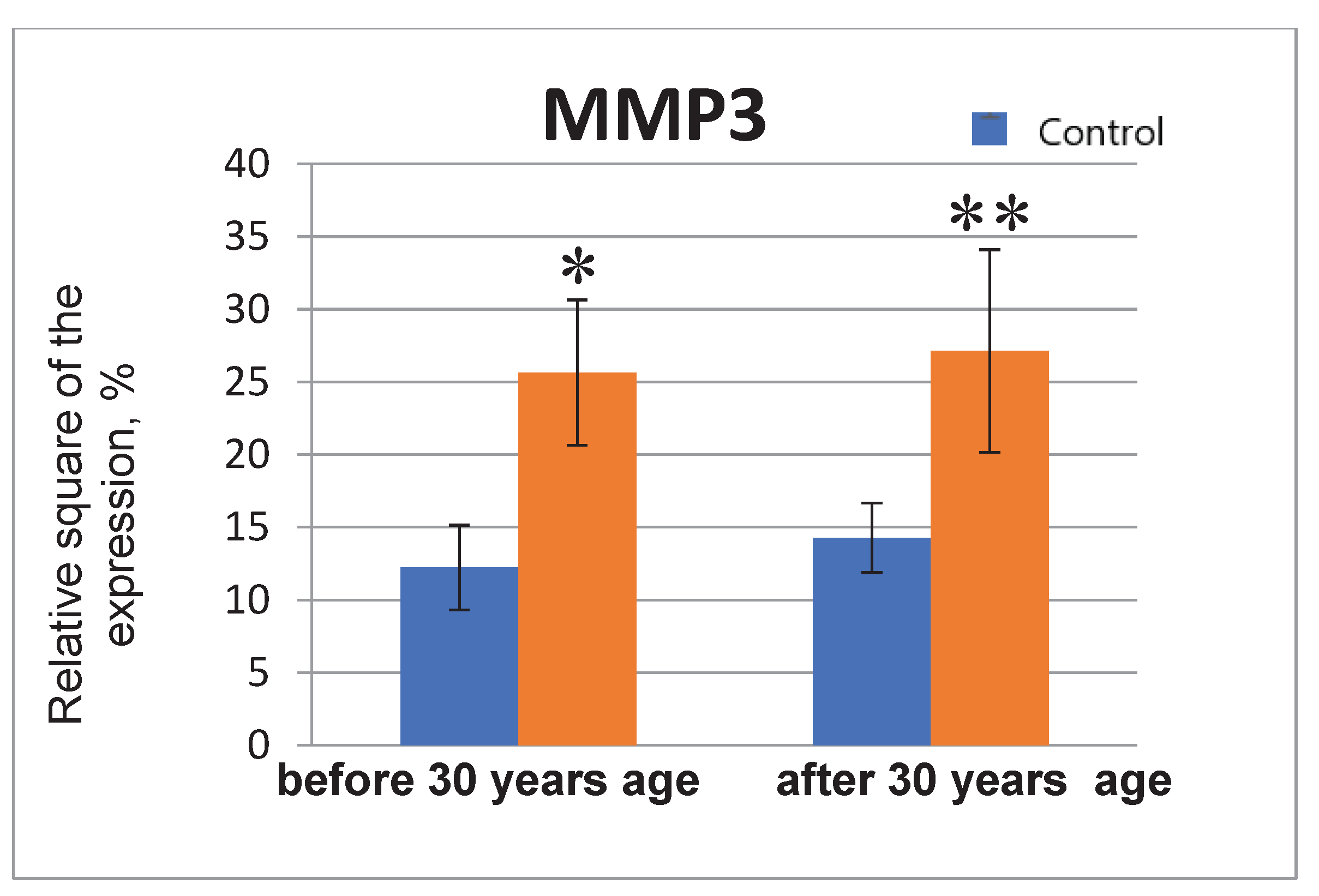
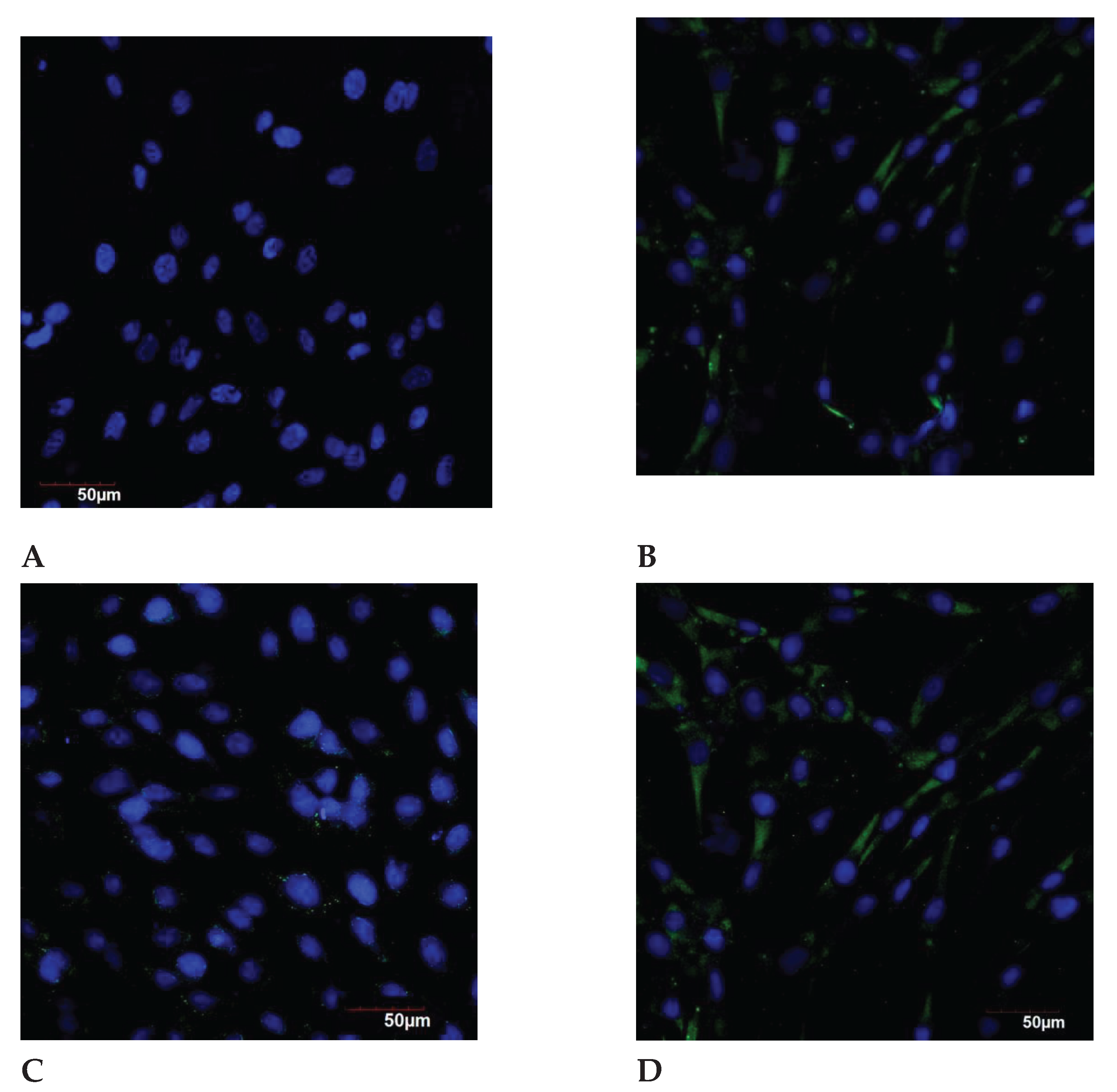
When exposed to the stress, the significant increase in the MMP3 protein expression by 2.1 times in the group under 35 years of age; in the group of older reproductive age, the values of the indicator also increase (by 1.9 times) (
Figure 7 and
Figure 8).
MMP3 (matrix metalloproteinase-3, also known as Stromelysin-1) is the enzyme, encoded by the MMP3 gene in humans.
The enzyme MMP3 destroys collagen of types II, III, IV, IX, X, proteoglycans, fibronectin, laminin and elastin [
53]. MMP-3 can also activate other MMPs such as MMP-1, MMP-7 and MMP-9, which indicates MMP-3 as the key factor in connective tissue remodelling [
54]. It is also known that this enzyme is involved in wound healing, atherosclerosis progression and carcinogenesis [
55]. In addition to the classical roles for MMP-3 in the extracellular space, MMP-3 can regulate the expression of certain genes as a transcription factor [
56].
Endometrial expression of MMP-3 occurs during estrogen-mediated proliferation of the endometrial epithelium, however, it is not detected during the secretory phase, since the expression of MMP-3 is suppressed by progesterone. Paracrine factors, including TGF-β and retinoic acid, are also crucial for the regulation of matrix metalloproteinases in the endometrium. In contrast, inflammatory cytokines such as IL-1α can block the steroid-mediated regulation of MMP-3 in ectopic growth sites in the endometriosis [
57].
Tissue remodulation is regulated by the balance of MMP and its inhibitors. The interaction of factors affecting the cell matrix has crucial impact on the cyclic preparation of the endometrium for embryo implantation. A violation of the regulation of matrix remodelling leads to the invasive growth of ectopic endometrial tissue, pathological adhesion, impaired ovulation and decreased fertility [
58].
5.6. р16. Expression
The p16 expression was not detected in the control groups of both young and old age (
Figure 9 and
Figure 10). The significant p16 expression was observed in the groups of under 35 and after 35 years of age under the genotoxic stress, however statistical analysis did not confirm these differences. Whereas, p16 expression was very low or was not detected in young body, its expression was increasing exponentially during the aging [
59]. Both p16 and beta-galactosidase (SA-beta-gal) are considered as biomarkers of cell aging [
16].
Fast and intensive
p16 activation was observed under the mice tissues damage [
60]. When reporter mice were used,
p16INK4a activation of
p16INK4a promoter was detected during 2-3 days after the tissue damage [
61]. In addition to
p16 expression, cells with other markers of the aging, such as NF-kB activation and SASP cytokine expression, are found in tissue damage sites and are apparently important for optimal healing, since the clearance of
p16-expressing cells delays the regeneration [
3]. Since no wound healing defects are found in animals with
p16 deficiency, these observations indicate about some features of
p16-expressing cells, but not about
p16 itself, causing tissue remodeling at the site of injury. SASP components are likely candidates for this effect [
62].
6. Conclusion
During a woman's life and pregnancy, inflammation and cell aging are involved in physiological processes, starting from the ovulation and menstruation and ending with the placental homeostasis and the childbirth.
For the first time, a comparative study of the expression of key signalling molecules involved in the mechanisms of aging in endometrium was carried out: IL-6, IL-8, IL-1a, MMP3, p16, SIRT1,6, TERF-1, CALR in endometrial cells in the normal aging and under the genotoxic stress.
During the investigation of IL-8, SIRT1,6, and CALR levels in the endometrial cell culture, it was found for the first time that their level decreased significantly during the transition from young to older reproductive age. At the same time, the expression of IL-1a and TERF1 molecules increased significantly with the age. The IL-6 and MMP3 levels in the endometrium did not depend from the age. The expression of p16 protein in normal endometrium was insignificant in the both age groups.
For the first time, the significant increase of the expression of IL-6, IL-8, IL-1a, MMP3, p16 proteins and the decrease of the expression of SIRT1,6, TERF-1 proteins in the endometrial cell culture of young and older reproductive age were detected under the genotoxic stress. The significant increase in IL-1a level and the decrease in TERF-1 level in the endometrial cell culture under the genotoxic stress was demonstrated in the group of older reproductive age compared to the group of young reproductive age.
The SASP phenotype characterizing the phenomenon of inflammaging can be significantly expanded by the including of the studied molecules - SIRT-1, SIRT-6, TERF, CALR, that we demonstrated for the first time. These signalling molecules may be new therapeutic targets for the age-associated female reproductive system diseases treatment.
The investigation of the expression features of the molecules characteristic for the SASP phenotype in the endometrial cell culture significantly enhances the understanding of the mechanisms of inflammaging in the reproductive system depending from the age. Studies of a number of molecules involved in the mechanisms of aging and inflammaging make it possible to expand the concept of inflammatory aging and to identify new signalling molecules characterizing this cell phenotype.
Verification of new signalling molecules characterizing the SASP phenotype (which can be considered as potential therapeutic targets) opens new prospects for optimization and improvement of the effectiveness of preventive and therapeutic geroprotective protocols.
Author Contributions
Conceptualization, D.I and I.K..; formal analysis, V.R., A.D.; Methodology R.N. writing— original draft preparation, A.D. and S.M.; writing—review and editing, D.I. and I.K.; Investigation, V.R., E.M. visualization, Yu.K., T.Z.; supervision and project administration, D.I anf R.N.. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Hayflick, L. The limited in vitro lifetime of human diploid cell strains. Exp. Cell Res. 1965, 37, 614–636. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Gutarra, S.; García-Prat, L.; Rodriguez-Ubreva, J.; Ortet, L.; Ruiz-Bonilla, V.; Jardí, M.; Ballestar, E.; González, S.; Serrano, A.L. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature. 2014, 506(7488), 316–321. [Google Scholar] [CrossRef] [PubMed]
- Baker, D.; Childs, B.; Durik, M.; Wijers, M.; Sieben, C.; Zhong, J.; Saltness, R.; Jeganathan, K.; Verzosa, G.; Pezeshki, A. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature. 2016, 530(7589), 184–189. [Google Scholar] [CrossRef] [PubMed]
- Storer, M.; Mas, A.; Robert-Moreno, A.; Pecoraro, M.; Ortells, M.; Di Giacomo, V.; Yosef, R.; Pilpel, N.; Krizhanovsky, V.; Sharpe, J. Senescence is a developmental mechanism that contributes to embryonic growth and patterning. Cell. 2013, 155(5), 1119–1130. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Campisi, J. Chronic inflammation (Inflammaging) and its potential contribution to age-associated diseases. Biol. Sci. 2014, 1, 4–9. [Google Scholar] [CrossRef] [PubMed]
- Koumei, S.; Iwata, H. Effect of aging on the female reproductive function. Contracept. Reprod. Med. 2017, 23(23), 1–8. [Google Scholar] [CrossRef]
- Gruver, A.; Hudson, L.; Sempowski, G. Immunosenescence of ageing. J Pathol. 2007, 211(2), 144–156. [Google Scholar] [CrossRef] [PubMed]
- Bahrami, E.; Witzel, M.; Racek, T.; Puchałka, J.; Hollizeck, S.; Greif-Kohistani, N.; Kotlarz, D.; Horny, H.P.; Feederle, R.; Schmidt, H.; Sherkat, R.; Steinemann, D.; Göhring, G.; Schlegelbeger, B.; Albert, M.H.; Al-Herz, W.; Klein, C. Myb-like, SWIRM, and MPN domains 1 (MYSM1) deficiency: Genotoxic stress-associated bone marrow failure and developmental aberrations. J Allergy Clin Immunol. 2017, 140(4), 1112–1119. [Google Scholar] [CrossRef] [PubMed]
- Xia, S.; Zhang, X.; Zheng, S.; Khanabdali, R.; Kalionis, B.; Wu, J.; Wan, W.; Tai, X. An Update on Inflamm-Aging: Mechanisms, Prevention, and Treatment. J Immunol Res. 2016, 2016, 8426874. [Google Scholar] [CrossRef] [PubMed]
- Coppé, J.P.; Patil, C.K.; Rodier, F.; Sun, Y.; Muñoz, D.P.; Goldstein, J.; Nelson, P.S.; Desprez, P.Y.; Campisi, J. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008, 6(12), 2853–2868. [Google Scholar] [CrossRef] [PubMed]
- Basisty, N.; Kale, A.; Jeon, O.H.; Kuehnemann, C.; Payne, T.; Rao, C.; Holtz, A.; Shah, S.; Sharma, V.; Ferrucci, L.; Campisi, J.; Schilling, B. A proteomic atlas of senescence-associated secretomes for aging biomarker development. PLoS Biol. 2020, 18(1), e3000599. [Google Scholar] [CrossRef] [PubMed]
- Mulcahy, L.A.; Pink, R.C.; Carter, D.R. Routes and mechanisms of extracellular vesicle uptake. J Extracell Vesicles. 2014, 3. [Google Scholar] [CrossRef] [PubMed]
- Takasugi, M. Emerging roles of extracellular vesicles in cellular senescence and aging. Aging Cell. 2018, 17, e12734. [Google Scholar] [CrossRef] [PubMed]
- Borghesan, M.; Fafián-Labora, J.; Eleftheriadou, O.; Carpintero-Fernández, P.; Paez-Ribes, M.; Vizcay-Barrena, G.; et al. Small Extracellular Vesicles Are Key Regulators of Non-cell Autonomous Intercellular Communication in Senescence via the Interferon Protein IFITM3. Cell Reports. 2019, 27(13), 3956–3971. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Gluscevic, M.; Baker, D.J.; Laberge, R.-M.; Marquess, D.; Dananberg, J.; van Deursen, J.M. Senescent cells: an emerging target for diseases of ageing. Nat. Rev. Drug Discov. 2017, 16(10), 718–735. [Google Scholar] [CrossRef] [PubMed]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.; Leonova, K.; Polinsky, A.; Chernova, O.B.; Gudkov, A.V. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging. 2016, 8(7), 1294–1315. [Google Scholar] [CrossRef] [PubMed]
- Ranjan, A.; Iwakuma, T. Emerging Non-Canonical Functions and Regulation of p53. Int. J. Mol. Sci. 2018, 19(4), 1015. [Google Scholar] [CrossRef] [PubMed]
- Kitson, S.; Sivalingam, V.N.; Bolton, J.; McVey, R.; Nickkho-Amiry, M.; Powell, M.E.; Leary, A.; Nijman, H.W.; Nout, R.A.; Bosse, T.; Renehan, A.G.; Kitchener, H.C.; Edmondson, R.J.; Crosbie, E.J. Ki-67 in endometrial cancer: scoring optimization and prognostic relevance for window studies. Mod Pathol. 2017, 30(3), 459–468. [Google Scholar] [CrossRef]
- Mailand, N.; Gibbs-Seymour, I.; Bekker-Jensen, S. Regulation of PCNA-protein interactions for genome stability. Nature Rev. Mol. Cell Biol. 2013, 14(5), 269–282. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, X.; Mas, A.; Cervelló, I.; Taylor, H.; Simon, C. Uterine stem cells: from basic research to advanced cell therapies. Hum Reprod Update. 2018, 24(6), 673–693. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.A.; Madsen, A.S.; Olsen, C.A.; Hirschey, M.D. Metabolic control by sirtuins and other enzymes that sense NAD+, NADH, or their ratio. Biochim Biophys Acta Bioenerg. 2017, 1858(12), 991–998. [Google Scholar] [CrossRef] [PubMed]
- Hohensinner, P.J.; Kaun, C.; Buchberger, E.; Ebenbauer, B.; Demyanets, S.; Huk, I.; Eppel, W.; Maurer, G.; Huber, K.; Wojta, J. Age intrinsic loss of telomere protection via TRF1 reduction in endothelial cells. Biochim. Biophys. Acta. 2016, 1863(2), 360–367. [Google Scholar] [CrossRef]
- Eggleton, P.; Bremer, E.; Dudek, E.; Michalak, M. Calreticulin, a therapeutic target? Expert Opin Ther Targets. 2016, 20(9), 1137–1147. [Google Scholar] [CrossRef] [PubMed]
- Petrosyan, M.A.; Goryachaya, T.S.; Melezhnikova, N.O.; Domnina, A.P.; Polyakova, V.O. Endometrial cell lime: preparation and characterization. Methodological recommendations. Eco-vector, 2018, 44 p.
- Greussing, R.; Hackl, M.; Charoentong, P.; et al. Identification of microRNA-mRNA functional interactions in UVB-induced senescence of human diploid fibroblasts. BMC Genomics. 2013, 14, 224. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Traganos, F.; Darzynkiewicz, Z. Kinetics of the UV-induced DNA damage response in relation to cell cycle phase. Correlation with DNA replication. Cytometry A. 2010, 77(3), 285–293. [Google Scholar] [CrossRef] [PubMed]
- Freund, A.; Orjalo, A.V.; Desprez, P.-Y.; Campisi, J. Inflammatory networks during cellular senescence: causes and consequences. Trends Mol. Med. 2010, 16(5), 238–246. [Google Scholar] [CrossRef] [PubMed]
- Dinarello, C.A. Overview of the IL-1 family in innate inflammation and acquired immunity. Immunol. Rev. 2018, 281(1), 8–27. [Google Scholar] [CrossRef] [PubMed]
- Marquez, C.M.; Ibana, J.A.; Velarde, M.C. The female reproduction and senescence nexus. Am J Reprod Immunol. 2017, 77(5). [CrossRef]
- Luo, Y.; Zheng, S.G. Hall of Fame among Pro-inflammatory Cytokines: Interleukin-6 Gene and Its Transcriptional Regulation Mechanisms. Front Immunol. 2016, 19(7), 604. [Google Scholar] [CrossRef] [PubMed]
- Von Wolff, M.; Thaler, C.J.; Zepf, C.; Becker, V.; Beier, H.M.; Strowitzki, T. Endometrial expression and secretion of interleukin-6 throughout the menstrual cycle. Gynecol Endocrinol. 2002, 16(2), 121–129, PMID: 12012622. [Google Scholar] [CrossRef] [PubMed]
- Critchley, H.O.; Maybin, J.A.; Armstrong, G.M.; Williams, A.R. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020, 100(3), 1149–1179. [Google Scholar] [CrossRef] [PubMed]
- Arici, A.; Seli, E.; Senturk, L.M.; Gutierrez, L.S.; Oral, E.; Taylor, H.S. Interleukin-8 in the human endometrium. J Clin Endocrinol Metab. 1998, 83(5), 1783–1787. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.H.; Monsanto, S.P.; Miller, C.; Singh, S.S.; Thomas, R.; Tayade, C. Pathophysiology and immune dysfunction in endometriosis. Biomed Res Int. 2015, 2015, 795976. [Google Scholar] [CrossRef]
- Sikora, J.; Smycz-Kubanska, M.; Mielczarek-Palacz, A.; Kondera-Anasz, Z. Abnormal peritoneal regulation of chemokine activation-The role of IL-8 in pathogenesis of endometriosis. Am. J. Reprod. Immunol. 2017, 77(4). [CrossRef]
- Chen Wen Yong. SIRT1 (sirtuin (silent mating type information regulation 2 homolog) 1 (S. cerevisiae)). Atlas Genet Cytogenet Oncol Haematol. 2016, 20(1), 26-35. [CrossRef]
- Michishita, E.; Park, J.Y.; Burneskis, J.M.; Barrett, J.C.; Horikawa, I. Evolutionarily conserved and nonconserved cellular localizations and functions of human SIRT proteins. Mol Biol Cell. 2005, 16(10), 4623–4635. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Yang, J.; Hong, T.; Chen, X.; Cui, L. SIRT2: Controversy and multiple roles in disease and physiology. Ageing Res Rev. 2019, 55, 100961. [Google Scholar] [CrossRef] [PubMed]
- Osborne, B.; Bentley, N.L.; Montgomery, M.K.; Turner, N. The role of mitochondrial sirtuins in health and disease. Free Radic Biol Med. 2016, 100, 164–174. [Google Scholar] [CrossRef] [PubMed]
- Tatone, C.; Di Emidio, G.; Barbonetti, A.; Carta, G.; Luciano, A.M.; Falone, S.; Amicarelli, F. Sirtuins in gamete biology and reproductive physiology: emerging roles and therapeutic potential in female and male infertility. Hum Reprod Update. 2018, 24(3), 267–289. [Google Scholar] [CrossRef] [PubMed]
- Chang, K.T.; Min, K.T. Regulation of lifespan by histone deacetylase. Ageing Res. Rev. 2002, 1(3), 313–326. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S.C. NF-κB signaling in inflammation. Signal Transduct Target Ther. 2017, 2. [Google Scholar] [CrossRef] [PubMed]
- Grabowska, W.; Sikora, E.; Bielak-Zmijewska, A. Sirtuins, a promising target in slowing down the ageing process. Biogerontology. 2017, 18(4), 447–476. [Google Scholar] [CrossRef] [PubMed]
- Ong, A.L.; Ramasamy, T.S. Role of Sirtuin1-p53 regulatory axis in aging, cancer and cellular reprogramming. Ageing Res Rev. 2018, 43, 64–80. [Google Scholar] [CrossRef] [PubMed]
- Chang, A.R.; Ferrer, C.M.; Mostoslavsky, R. SIRT6, a Mammalian Deacylase with Multitasking Abilities. Physiol Rev. 2020, 100(1), 145–169. [Google Scholar] [CrossRef] [PubMed]
- Baur, J.A. Resveratrol, sirtuins, and the promise of a DR mimetic. Mech Ageing Dev. 2010, 131(4), 261–269. [Google Scholar] [CrossRef] [PubMed]
- Gorbunova, V.; Seluanov, A. DNA double strand break repair, aging and the chromatin connection. Mutat Res. 2016, 788, 2–6. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Yang, L.; Xu, X.-Y.; Hai, L.; Han, Y.Q.; Shi, Y.X. Telomere-associated factor expression in replicative senescence of human embryonic lung fibroblasts. Genet. Mol. Res. 2015, 14(3), 9269–9276. [Google Scholar] [CrossRef] [PubMed]
- Michalak, M.; Corbett, E.F.; Mesaeli, N.; Nakamura, K.; Opas, M. Calreticulin: one protein, one gene, many functions. Biochem J. 1999, 344(2), 281–292, PMID: 10567207. [Google Scholar] [CrossRef] [PubMed]
- Kozlov, G.; Muñoz-Escobar, J.; Castro, K.; Gehring, K. Mapping the ER Interactome: The P Domains of Calnexin and Calreticulin as Plurivalent Adapters for Foldases and Chaperones. Structure. 2017, 25(9), 1415–1422. [Google Scholar] [CrossRef] [PubMed]
- Adams, B.M.; Oster, M.E.; Hebert, D.N. Protein Quality Control in the Endoplasmic Reticulum. The Protein Journal. 2019, 38(3), 317–329. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Liu, T.; Li, S.; Zhang, X.; Ding, Q.; Que, H.; Yan, X.; Wei, K.; Liu, S. Comparative proteomic analysis of brains of naturally aging mice. Neuroscience. 2008, 154(3), 1107–1120. [Google Scholar] [CrossRef] [PubMed]
- Jing, W.; Jiang, W. MicroRNA-93 regulates collagen loss by targeting MMP3 in human nucleus pulposus cells. Cell Prolif. 2015, 48(3), 284–292. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.C.; Nebert, D.W.; Vasiliou, V. Update of human and mouse matrix metalloproteinase families. Hum Genomics. 2010, 4(3), 194–201. [Google Scholar] [CrossRef] [PubMed]
- Mitschke, J.; Burk, U.C.; Reinheckel, T. The role of proteases in epithelial-to-mesenchymal cell transitions in cancer. Cancer Metastasis Rev. 2019, 38(3), 431–444. [Google Scholar] [CrossRef] [PubMed]
- Eguchi, T.; Calderwood, S. Intra-nuclear MMP-3 controls transcription of HSP70 gene through interaction with heterochromatin protein. FASEB J. 2015, 29(1), 688. [Google Scholar] [CrossRef]
- Meola, J.; Rosa e Silva, J.C.; Dentillo, D.B.; da Silva, W.A.; Veiga-Castelli, L.C.; de Souza Bernardes, L.A.; Ferriani, R.A.; de Paz, C.C.; Giuliatti, S.; Martelli, L. Differentially expressed genes in eutopic and ectopic endometrium of women with endometriosis. Fertil. Steril. 2010, 93(6), 1750–1773. [Google Scholar] [CrossRef] [PubMed]
- Bałkowiec, M.; Maksym, R.B.; Włodarski, P.K. The bimodal role of matrix metalloproteinases and their inhibitors in etiology and pathogenesis of endometriosis (Review). Mol Med Rep. 2018, 18(3), 3123–3136. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Sharpless, N.E. Senescence in health and disease. Cell. 2017, 169(6), 1000–1011. [Google Scholar] [CrossRef] [PubMed]
- Demaria, M., Ohtani, N., Youssef, S.A., Rodier, F., Toussaint, W., Mitchell, J.R., Laberge, R.M., Vijg, J., Van Steeg, H., Dollé, M.E., Hoeijmakers, J.H., de Bruin, A. An essential role for senescent cells in optimal wound healing through secretion of PDGF-AA. Dev Cell. 2014, 31(6), 722-733. [CrossRef]
- Sorrentino, J.A.; Krishnamurthy, J.; Tilley, S.; Alb, J.G.; Burd, C.E.; Sharpless, N.E. p16INK4a reporter mice reveal age-promoting effects of environmental toxicants. J. Clin. Invest. 2014, 124(1), 169–173. [Google Scholar] [CrossRef] [PubMed]
- Ritschka, B.; Storer, M.; Mas, A.; Heinzmann, F.; Ortells, M.C.; Morton, J.P.; Sansom, O.J.; Zender, L.; Keyes, W.M. The senescence-associated secretory phenotype induces cellular plasticity and tissue regeneration. Genes Dev. 2017, 31(2), 172–183. [Google Scholar] [CrossRef]
Figure 1.
The intracellular signalling of SASP [
15].
Figure 1.
The intracellular signalling of SASP [
15].
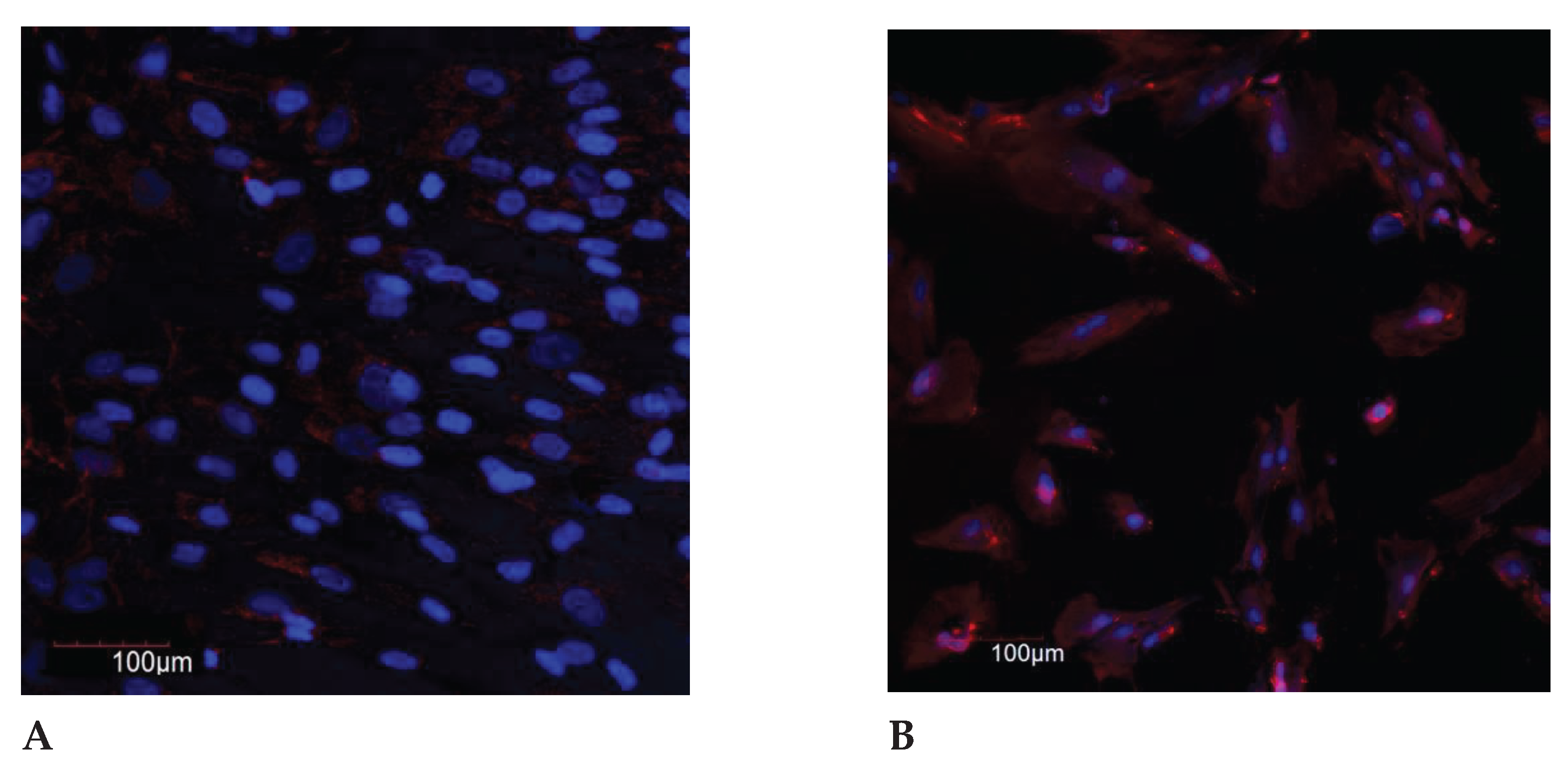
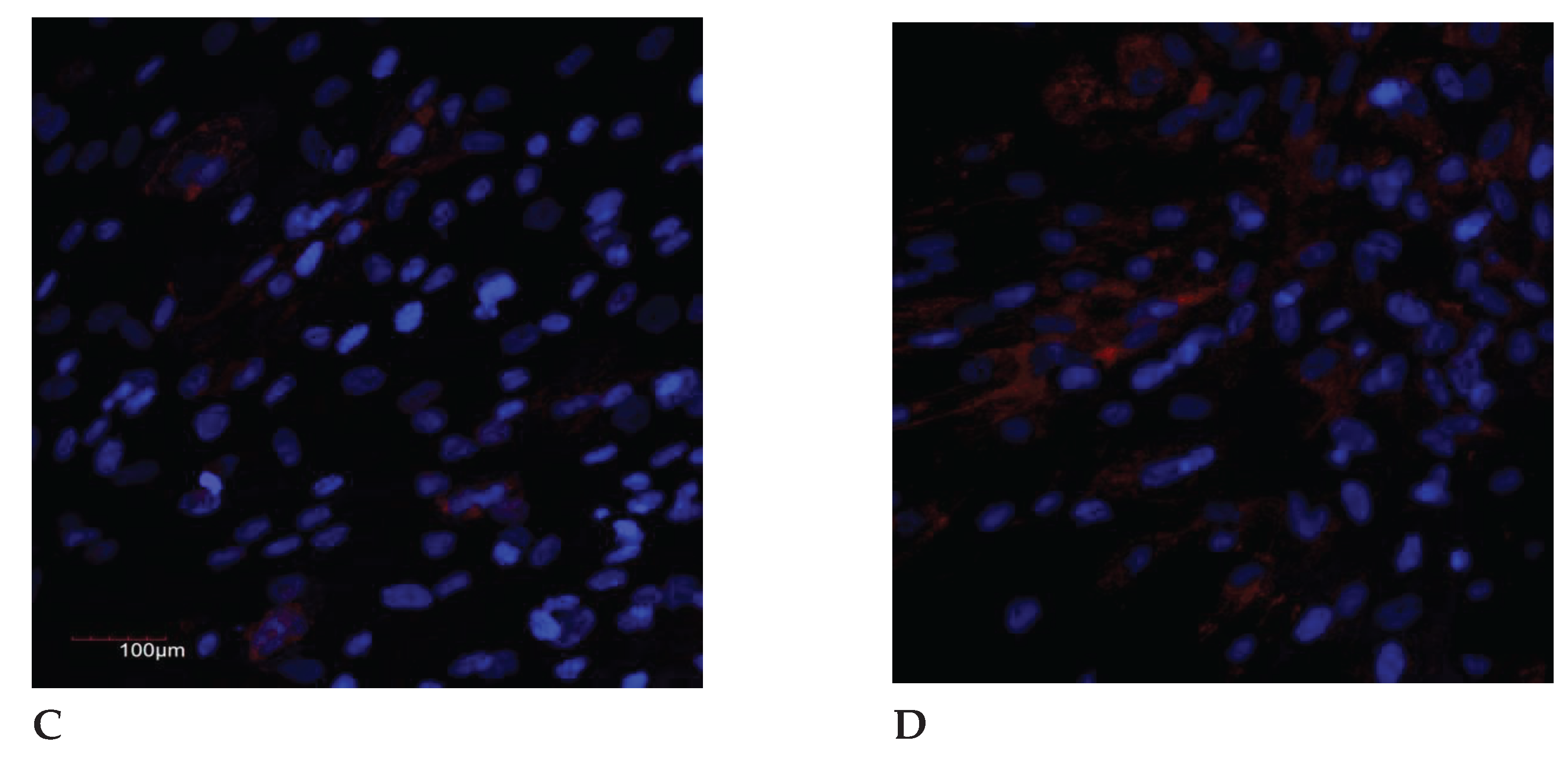
Figure 2.
The IL-8 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400, Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with Alexa Fluor 647 (red fluorescence): A – control (<35 years); B – exposure to the genotoxic stress (<30 years); C – control (>35 years); D – exposure to the genotoxic stress (>30 years).
Figure 2.
The IL-8 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400, Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with Alexa Fluor 647 (red fluorescence): A – control (<35 years); B – exposure to the genotoxic stress (<30 years); C – control (>35 years); D – exposure to the genotoxic stress (>30 years).
Figure 3.
The interleukin expression in the endometrial cell culture in the normal and under the influence of the genotoxic stress groups.
Figure 3.
The interleukin expression in the endometrial cell culture in the normal and under the influence of the genotoxic stress groups.
Figure 4.
The SIRT6 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400, Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with AlexaFluor 488 (green fluorescence): A – control (<35 years); B – exposure to the genotoxic stress (<35 years); C – control (>35 years); D – exposure to the genotoxic stress (>35 years).
Figure 4.
The SIRT6 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400, Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with AlexaFluor 488 (green fluorescence): A – control (<35 years); B – exposure to the genotoxic stress (<35 years); C – control (>35 years); D – exposure to the genotoxic stress (>35 years).
Figure 5.
The sirtuin expression in the endometrial cell culture in the normal and under the genotoxic stress groups.
Figure 5.
The sirtuin expression in the endometrial cell culture in the normal and under the genotoxic stress groups.
Figure 6.
The expression of calreticulin, TERF-1, MMP3 and p16 in the endometrial cell culture in the normal and under the genotoxic stress groups.
Figure 6.
The expression of calreticulin, TERF-1, MMP3 and p16 in the endometrial cell culture in the normal and under the genotoxic stress groups.
Figure 7.
The MP3 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400; Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with AlexaFluor 488 (green fluorescence). A – control (<30 years); B – exposure to the genotoxic stress (<30 years); C – control (>30 years); D – exposure to the genotoxic stress (>30 years).
Figure 7.
The MP3 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400; Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with AlexaFluor 488 (green fluorescence). A – control (<30 years); B – exposure to the genotoxic stress (<30 years); C – control (>30 years); D – exposure to the genotoxic stress (>30 years).
Figure 8.
The MMP3 expression in the endometrial cell culture in the normal and under the genotoxic stress groups. * - p<0.05 compared to the control group under 30 years of age. ** - p<0.05 compared to the control group after 30 years.
Figure 8.
The MMP3 expression in the endometrial cell culture in the normal and under the genotoxic stress groups. * - p<0.05 compared to the control group under 30 years of age. ** - p<0.05 compared to the control group after 30 years.
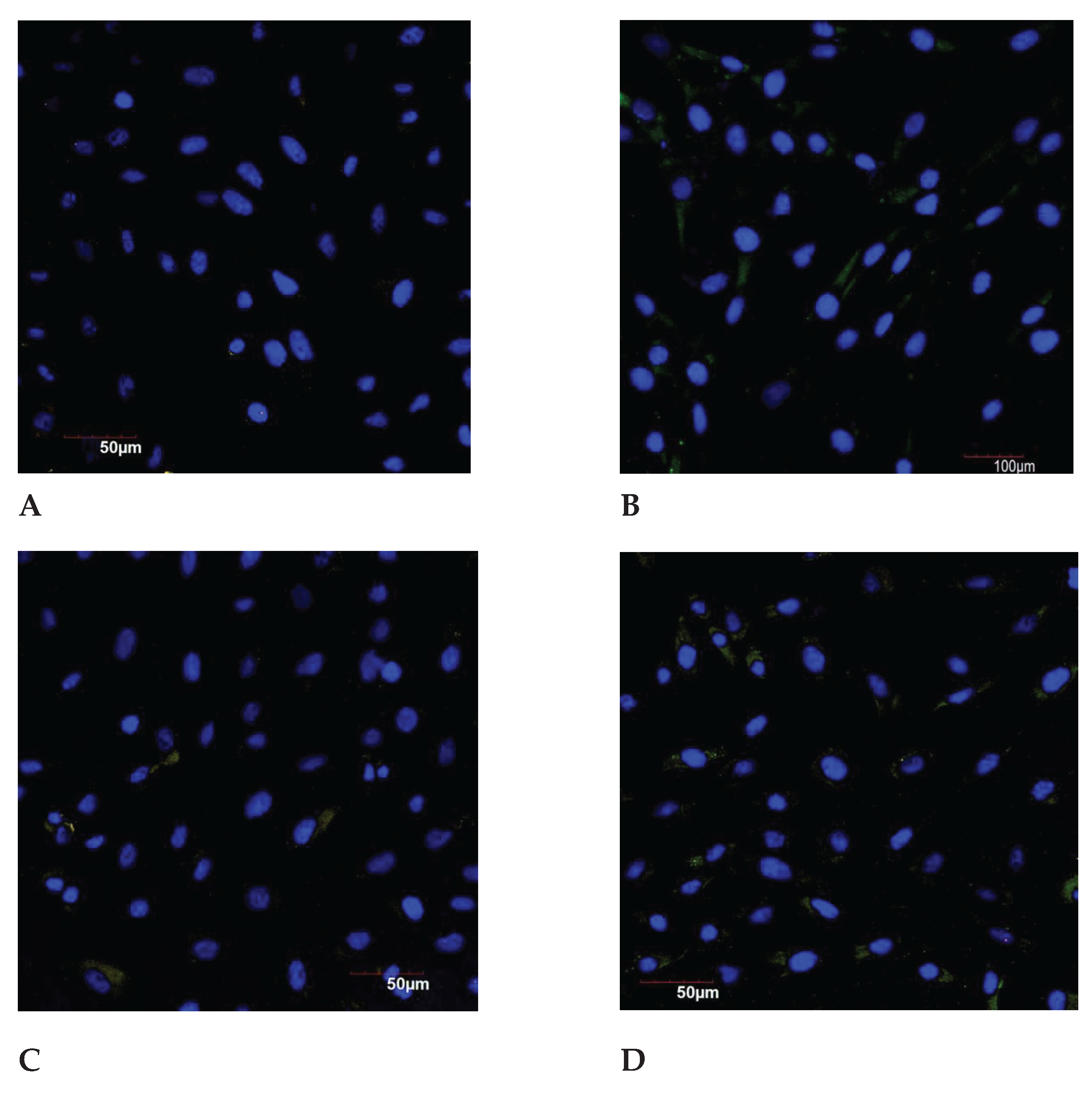
Figure 9.
The p16 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400; Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with Alexa Fluor 488 (green fluorescence). A – control (<30 years); B – exposure to the genotoxic stress (<30 years); C – control (>30 years); D – exposure to the genotoxic stress (>30 years).
Figure 9.
The p16 expression in the endometrial cell culture, immunofluorescence confocal microscopy, x400; Hoechst 33258 (blue fluorescence) was used to stain the nuclei. Protein visualization was performed using the secondary antibodies conjugated with Alexa Fluor 488 (green fluorescence). A – control (<30 years); B – exposure to the genotoxic stress (<30 years); C – control (>30 years); D – exposure to the genotoxic stress (>30 years).
Figure 10.
The p16 expression in the endometrial cell culture in the normal and under the influence of the genotoxic stress groups. * - p<0.05 compared to the control group under 30 years age. ** - p<0.05 compared to the control group after 30 years age.
Figure 10.
The p16 expression in the endometrial cell culture in the normal and under the influence of the genotoxic stress groups. * - p<0.05 compared to the control group under 30 years age. ** - p<0.05 compared to the control group after 30 years age.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).