Submitted:
04 March 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
Cell Culture
Proliferation Assay and Drug Preparation
Protein Extraction and Western Blot
| Primary Antibody | Manufacturer |
|---|---|
| β-actin - HRP conjugated | CellSignaling (Cambridge, UK) |
| phospho-AKT (Ser473) | CellSignaling (Cambridge, UK) |
| AKT | CellSignaling (Cambridge, UK) |
| anti-MLKL (Phospho S358) [ERP9514] | Abcam (Cambridge, UK) |
| anti-MLKL [ERP17514] | Abcam (Cambridge, UK) |
| cleaved caspase-3 (Asp175) | CellSignaling (Cambridge, UK) |
| phospho-p44/42 MAPK (ERK1/2) (Thr202/Tyr204) | CellSignaling (Cambridge, UK) |
| p44/42 MAPK (ERK1/2) | CellSignaling (Cambridge, UK) |
| α-tubulin - HRP conjugated | CellSignaling (Cambridge, UK) |
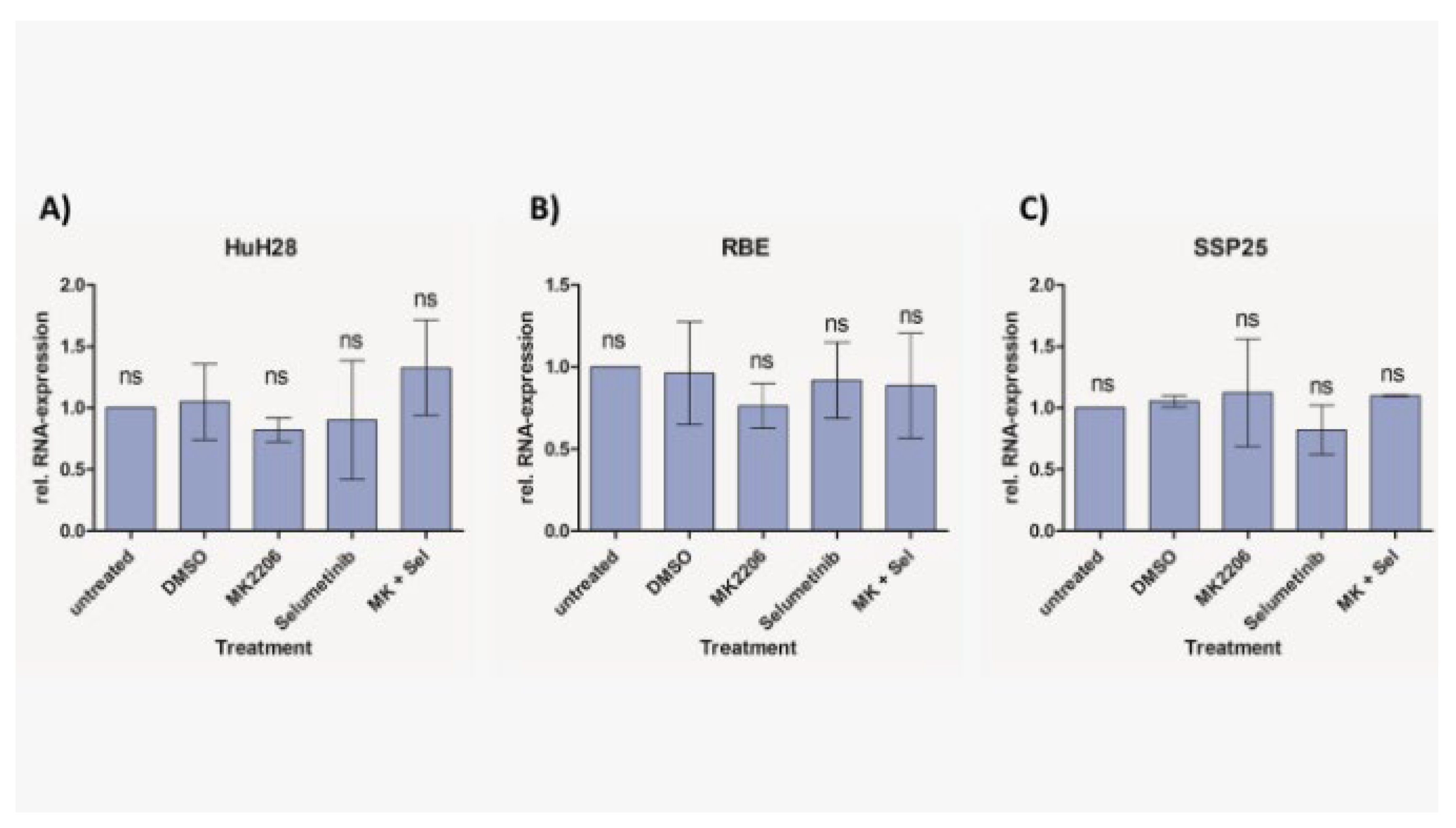
Semiquantitative Real-Time PCR (qPCR)
Flow Cytometry
Statistical Analysis
Results
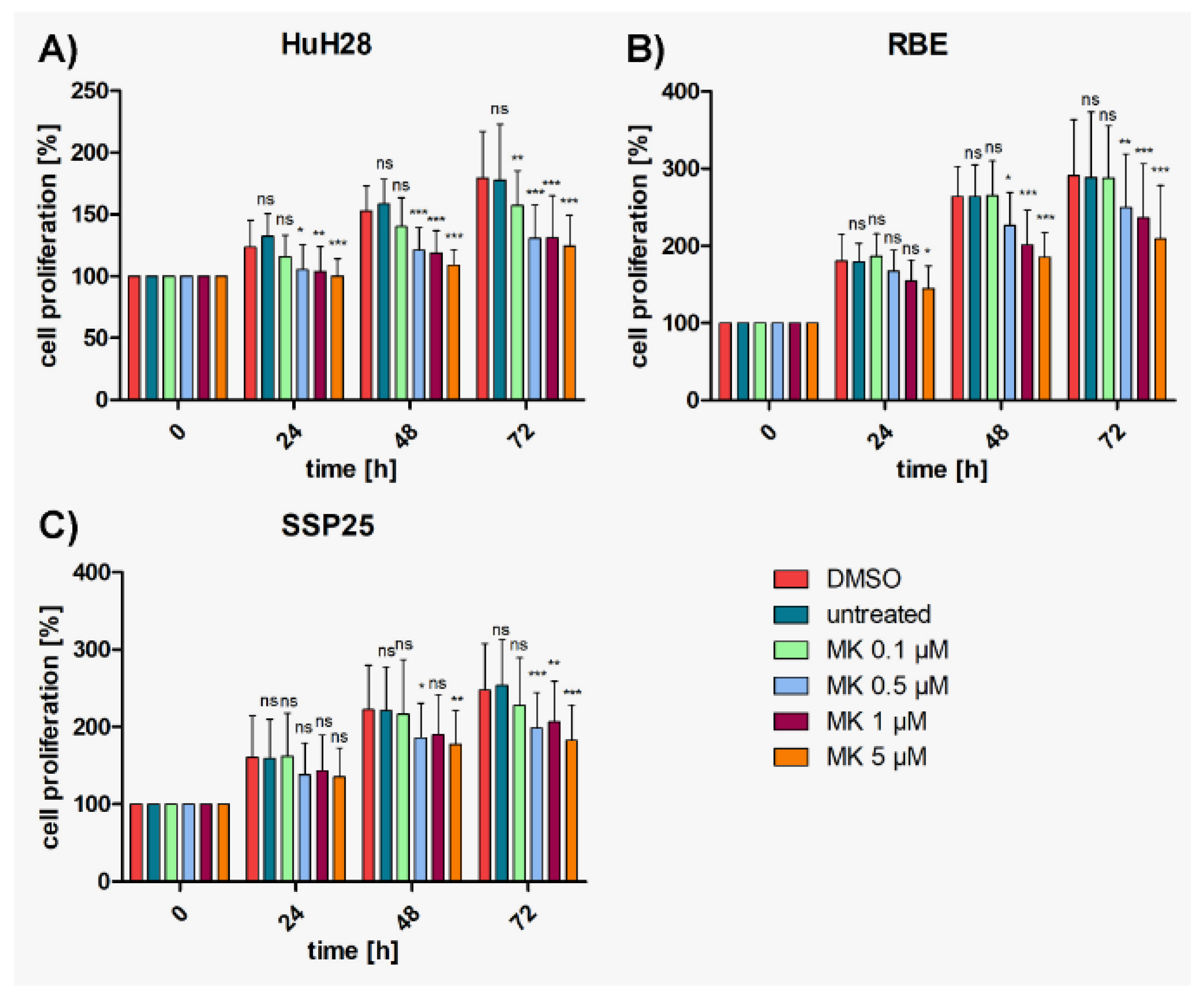
AKT Inhibitor MK2206 Effectively Reduces Proliferation in ICC cell lines
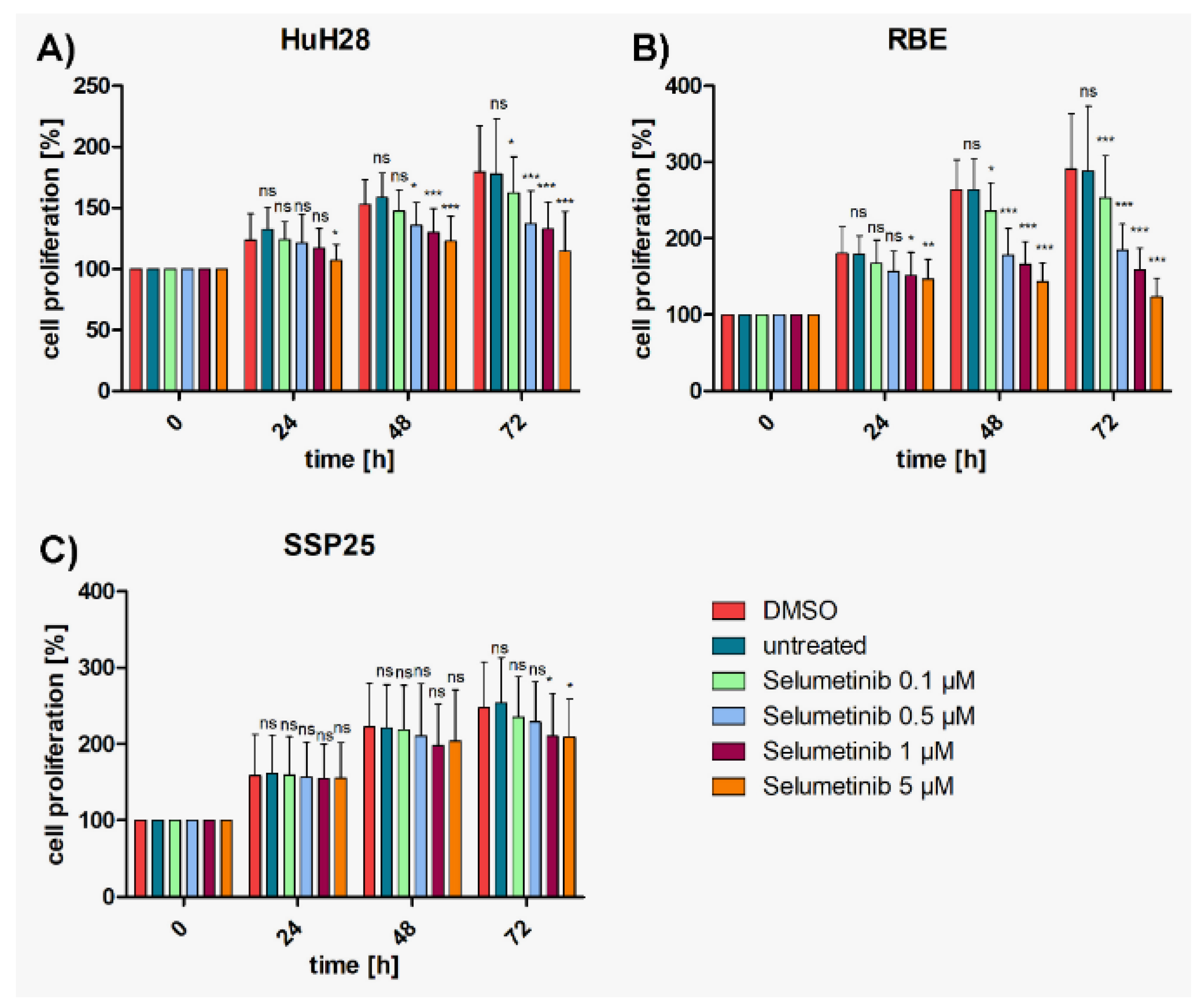
MEK-Inhibitor Selumetinib Significantly Reduces Proliferation of HuH28 and RBE, but Less in SSP25
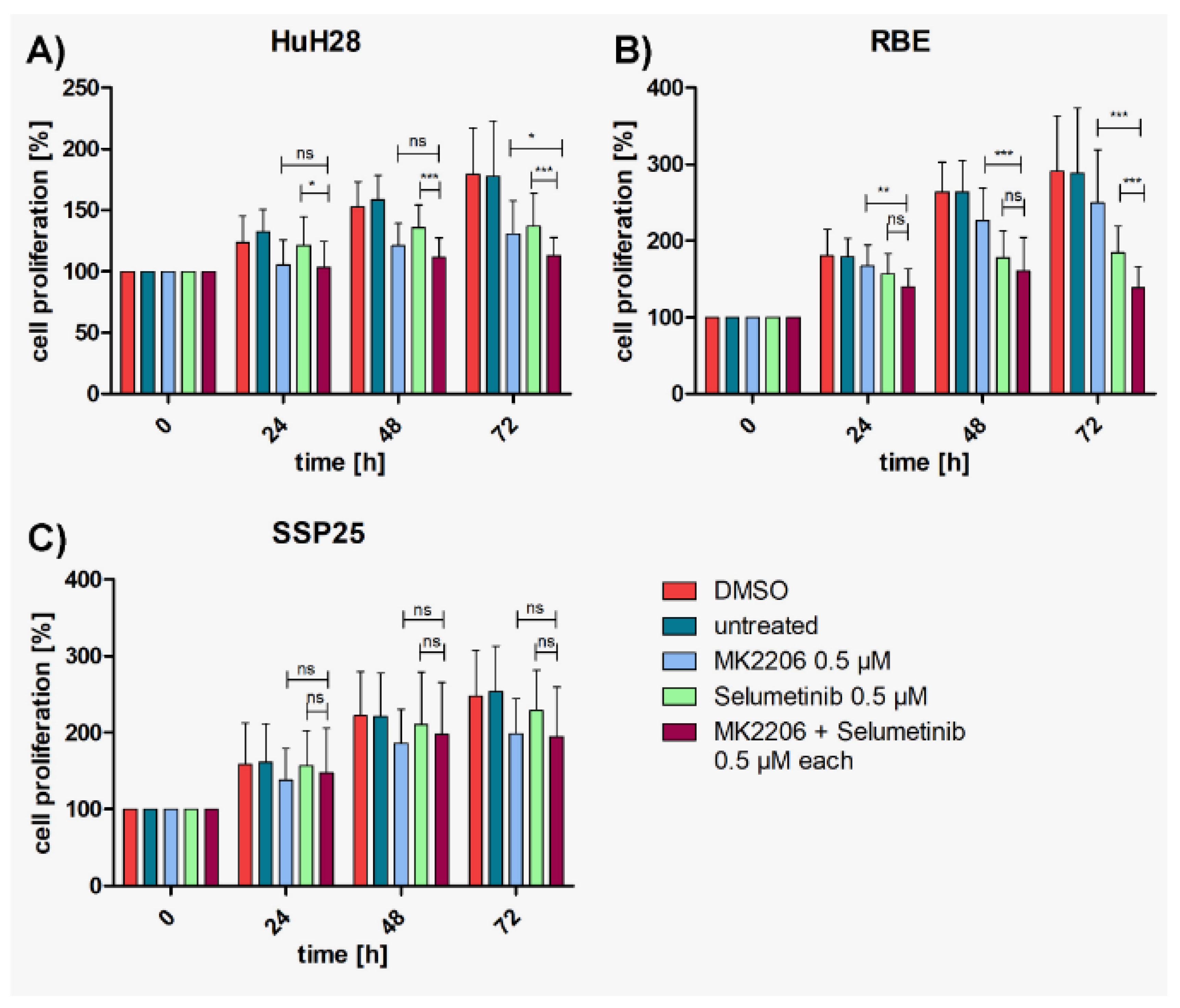
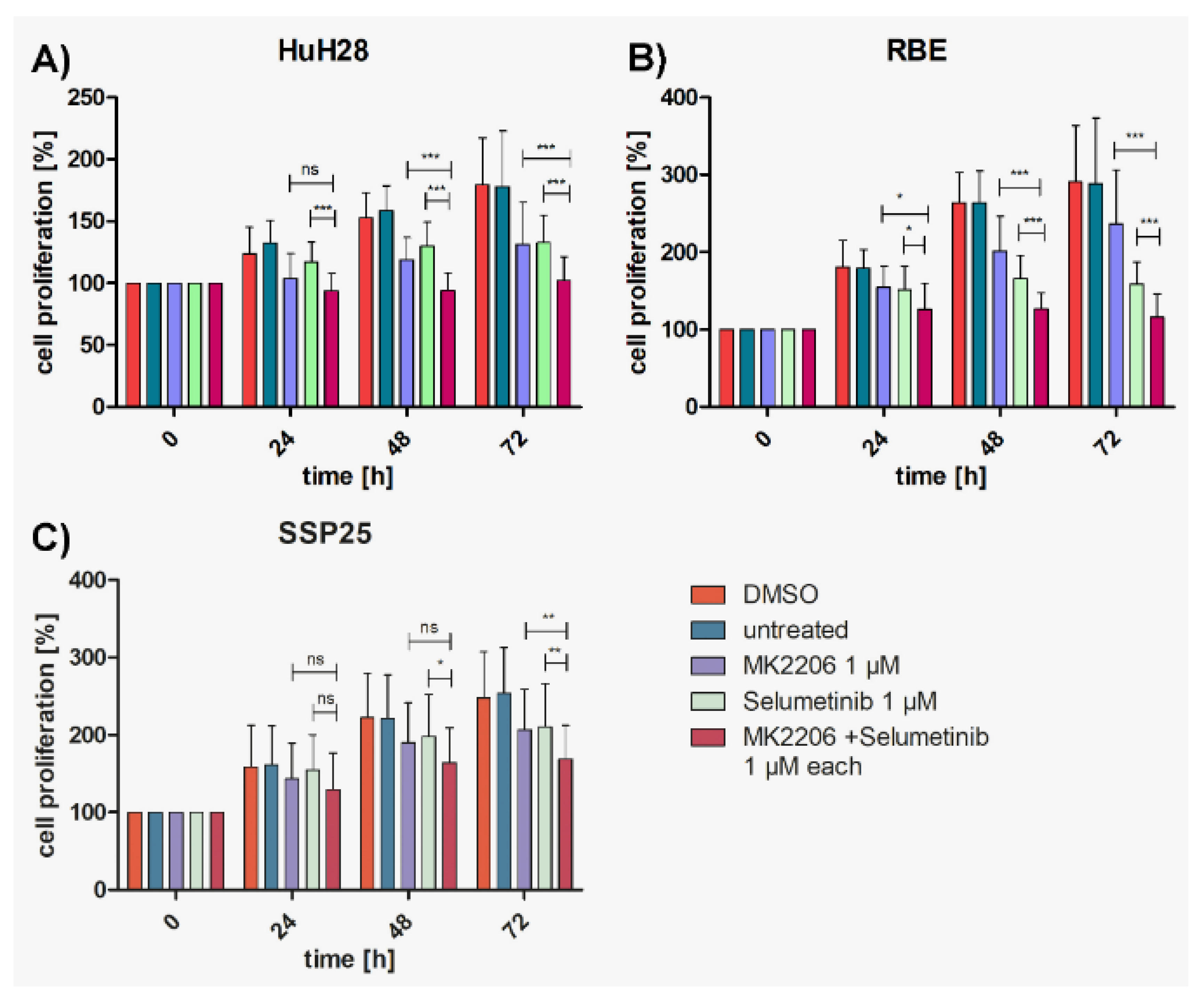
Dual Inhibition Shows Cooperative Effects as Compared with Single Treatments
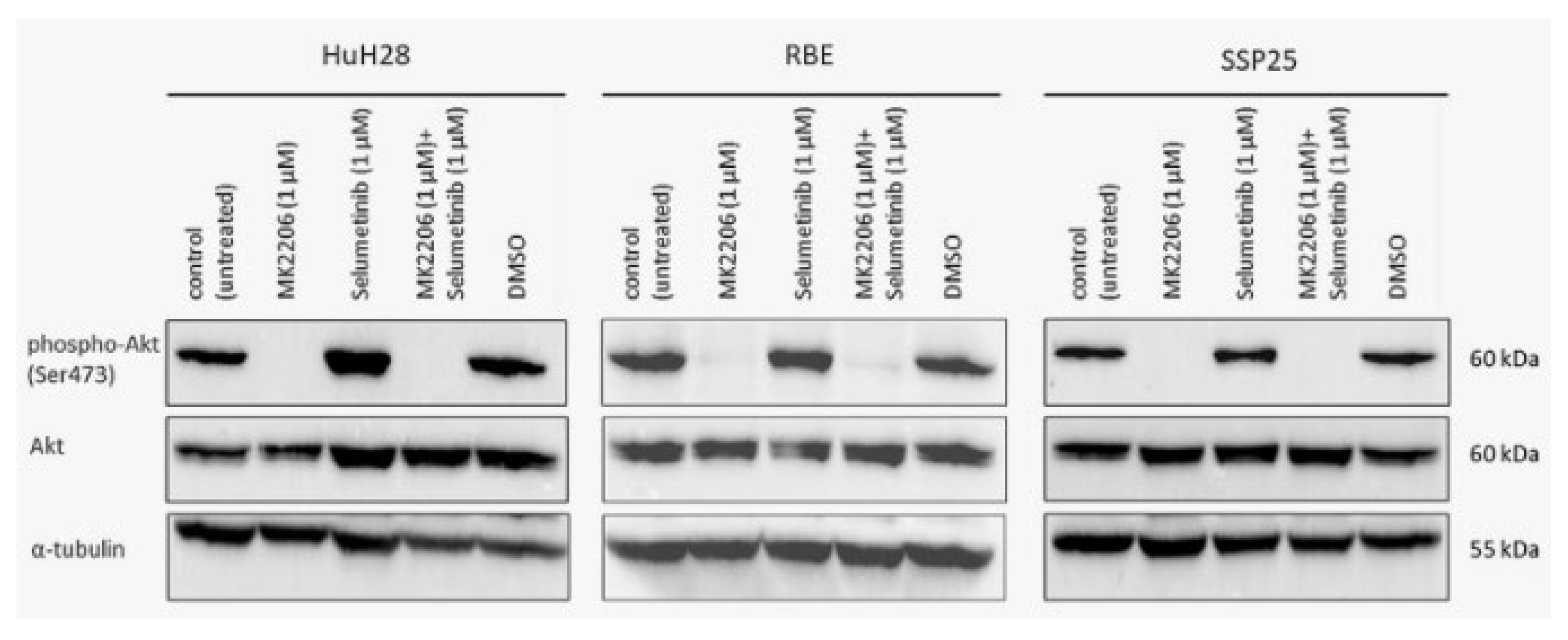
MK2206 Alone or in Combination Effectively Inhibits Phosphorylation of AKT (Ser473)
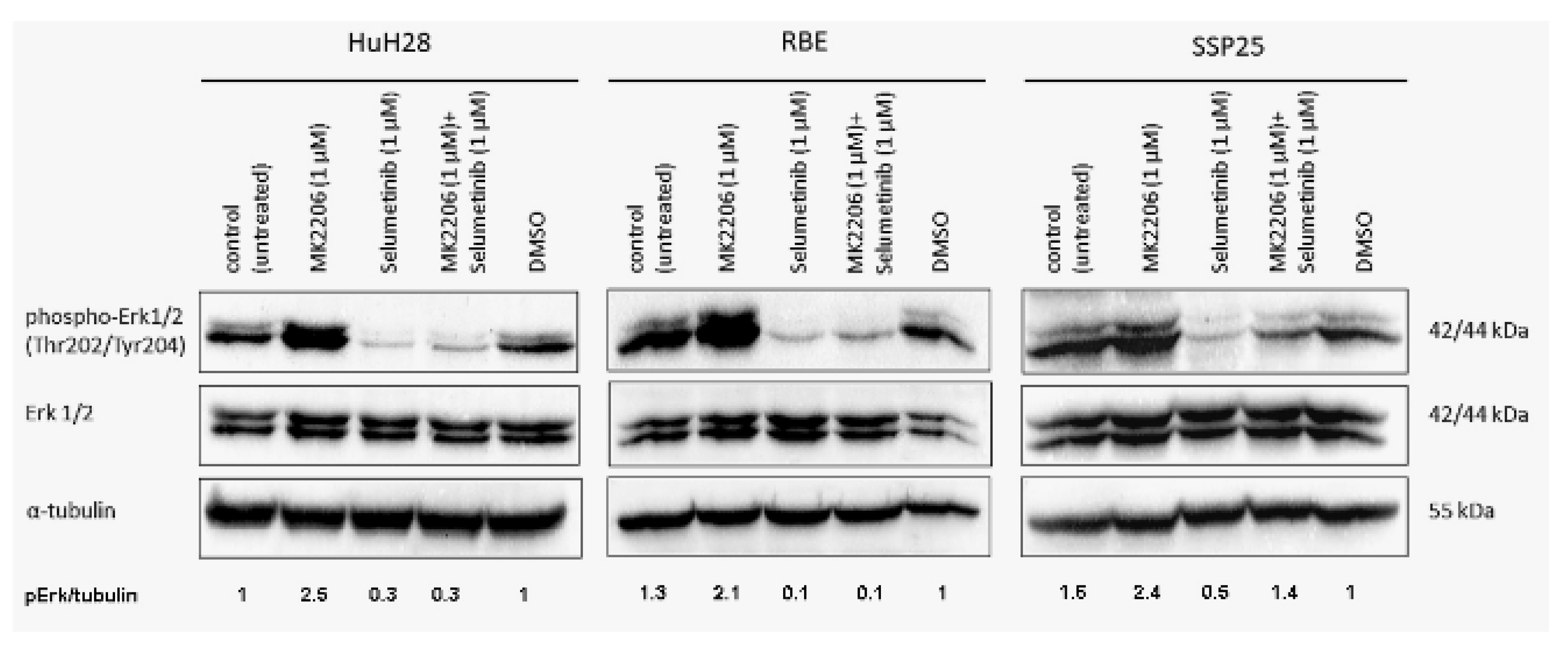
Selumetinib Alone or in Combination Effectively Inhibits ERK1/2 Phosphorylation (Thr202/Tyr204)
Dual Inhibition with MK2206 and Selumetinib Causes Cell Cycle Arrest in ICC Cell Lines
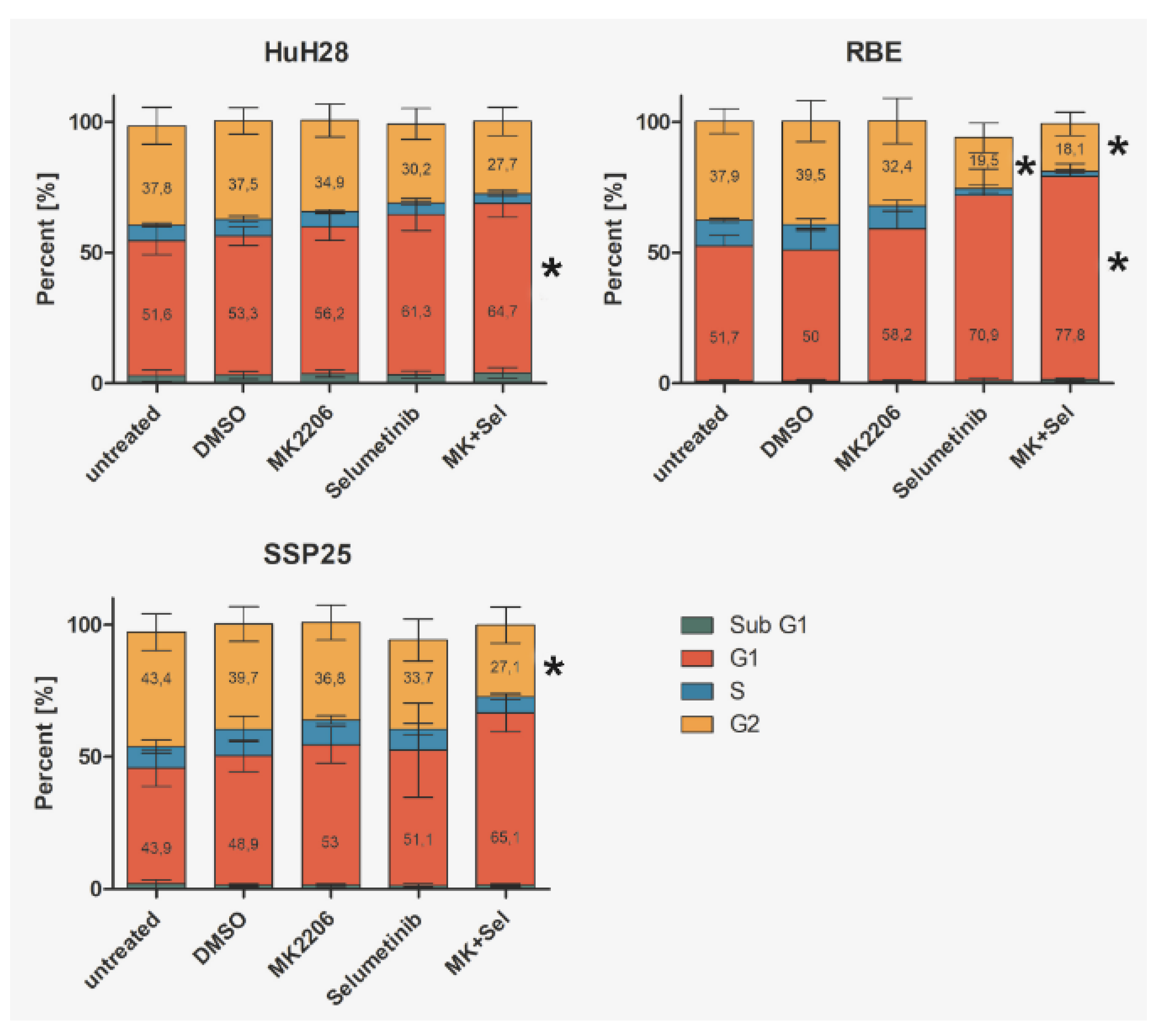
Discussion
Dual Inhibition of MAPK/ERK and PI3K/AKT/mTOR is Highly Effective in ICC
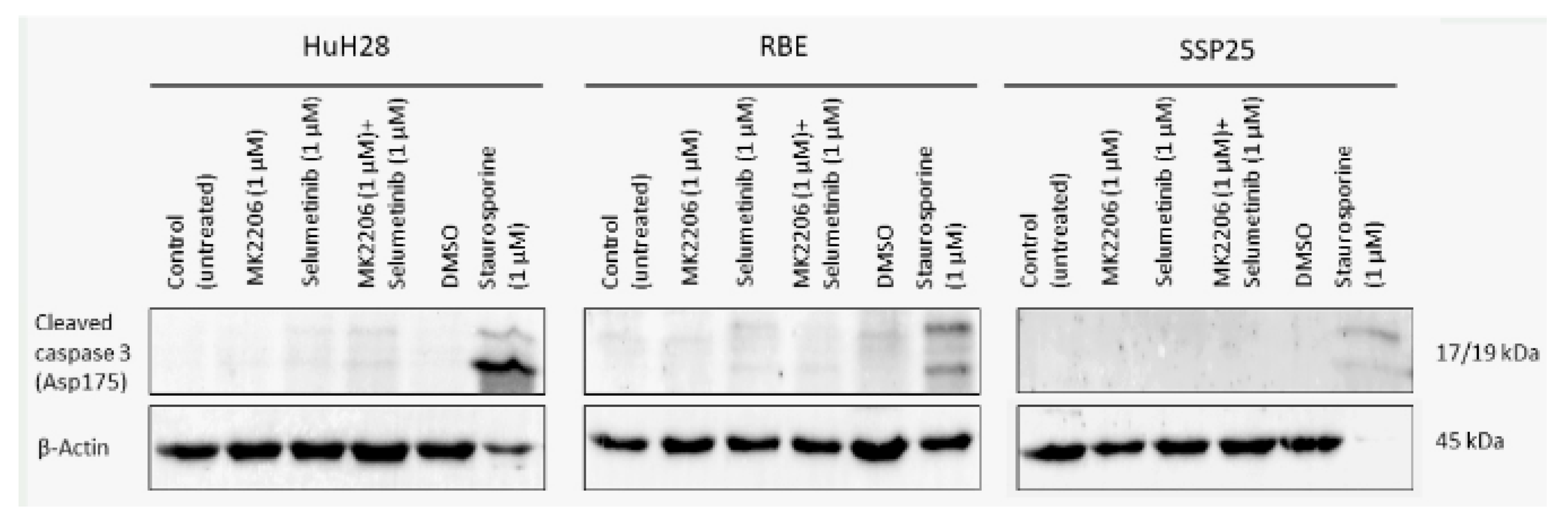
Dual Inhibition of MAPK/ERK and PI3K/AKT/mTOR Does not Induce Apoptosis in ICC
Abbreviations:
| AIFM1 | Apoptosis Inducing Factor Mitochondria Associated 1 |
| AKT | Protein kinase B |
| CCA | Cholangiocellular carcinoma |
| DMSO | Dimethyl sulfoxide |
| ECC | Extrahepatic cholangiocellular carcinoma |
| ERK | Extracellular signal-regulated kinase |
| FGFR2 | Fibroblast growth factor receptor 2 |
| ICC | Intrahepatic cholangiocellular carcinoma |
| MAPK | Mitogen-activated protein kinase |
| MEK | Mitogen-activated protein kinase kinase |
| MLKL | Mixed Lineage Kinase Domain Like |
| mTOR | Mammalian target of rapamycin |
| PBS | Phosphate-buffered saline |
| PI3K | Phosphatidylinositol-3-kinase |
Author Contributions
Funding
Ethics Approval and Consent to Participate
Consent for Publication
Data Availability Statement
Acknowledgments
Conflicts of Interest Statement
References
- Turati, F.; Bertuccio, P.; Negri, E.; La Vecchia, C. Epidemiology of cholangiocarcinoma. Hepatoma Res. 2022, 8, 19. [CrossRef]
- Poultsides GA, Zhu AX, Choti MA, Pawlik TM. Intrahepatic cholangiocarcinoma. Surg Clin North Am. 2010 Aug;90(4):817–37.
- Banales, J.M.; Marin, J.J.G.; Lamarca, A.; Rodrigues, P.M.; Khan, S.A.; Roberts, L.R.; Cardinale, V.; Carpino, G.; Andersen, J.B.; Braconi, C.; et al. Cholangiocarcinoma 2020: The next horizon in mechanisms and management. Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 557–588. [CrossRef]
- Vogel, A.; Wege, H.; Caca, K.; Nashan, B.; Neumann, U. The Diagnosis and Treatment of Cholangiocarcinoma. Dtsch. Aerzteblatt Online 2014, 111, 748–54. [CrossRef]
- Sia, D.; Tovar, V.; Moeini, A.; Llovet, J.M. Intrahepatic cholangiocarcinoma: pathogenesis and rationale for molecular therapies. Oncogene 2013, 32, 4861–4870. [CrossRef]
- Rizvi S, Gores GJ. Pathogenesis, diagnosis, and management of cholangiocarcinoma. Gastroenterology. 2013 Dec;145(6):1215–29.
- Gupta, A.; Dixon, E. Epidemiology and risk factors: intrahepatic cholangiocarcinoma. HepatoBiliary Surg. Nutr. 2017, 6, 101–104. [CrossRef]
- Kaewpitoon N, Kaewpitoon SJ, Pengsaa P, Sripa B. Opisthorchis viverrini: The carcinogenic human liver fluke. World J Gastroenterol. 2008 Feb 7;14(5):666–74.
- Vogel A, Saborowski A. Cholangiocellular Carcinoma. Digestion. 2017;95(3):181–5.
- Bridgewater, J.; Galle, P.R.; Khan, S.A.; Llovet, J.M.; Park, J.-W.; Patel, T.; Pawlik, T.M.; Gores, G.J. Guidelines for the diagnosis and management of intrahepatic cholangiocarcinoma. J. Hepatol. 2014, 60, 1268–1289. [CrossRef]
- Wang, M.; Chen, Z.; Guo, P.; Wang, Y.; Chen, G. Therapy for advanced cholangiocarcinoma: Current knowledge and future potential. J. Cell. Mol. Med. 2020, 25, 618–628. [CrossRef]
- Lee, S.; Rauch, J.; Kolch, W. Targeting MAPK Signaling in Cancer: Mechanisms of Drug Resistance and Sensitivity. Int. J. Mol. Sci. 2020, 21, 1102. [CrossRef]
- Glaviano, A.; Foo, A.S.C.; Lam, H.Y.; Yap, K.C.H.; Jacot, W.; Jones, R.H.; Eng, H.; Nair, M.G.; Makvandi, P.; Geoerger, B.; et al. PI3K/AKT/mTOR signaling transduction pathway and targeted therapies in cancer. Mol. Cancer 2023, 22, 1–37. [CrossRef]
- Schmitz KJ, Lang H, Wohlschlaeger J, Sotiropoulos GC, Reis H, Schmid KW, et al. AKT and ERK1/2 signaling in intrahepatic cholangiocarcinoma. World J Gastroenterol. 2007 Dec 28;13(48):6470–7.
- Ewald F, Nörz D, Grottke A, Hofmann BT, Nashan B, Jücker M. Dual Inhibition of PI3K-AKT-mTOR- and RAF-MEK-ERK-signaling is synergistic in cholangiocarcinoma and reverses acquired resistance to MEK-inhibitors. Invest New Drugs. 2014 Dec;32(6):1144–54.
- Spencer, K.; Pappas, L.; Baiev, I.; Maurer, J.; Bocobo, A.G.; Zhang, K.; Jain, A.; De Armas, A.D.; Reyes, S.; Le, T.M.; et al. Molecular profiling and treatment pattern differences between intrahepatic and extrahepatic cholangiocarcinoma. JNCI J. Natl. Cancer Inst. 2023, 115, 870–880. [CrossRef]
- Strazzabosco, M.; Fabris, L. Development of the bile ducts: Essentials for the clinical hepatologist. J. Hepatol. 2012, 56, 1159–1170. [CrossRef]
- Muntean, A.; Davenport, M. Biliary atresia & choledochal malformation–—Embryological and anatomical considerations. Semin. Pediatr. Surg. 2022, 31, 151235. [CrossRef]
- EMA. Pemazyre [Internet]. European Medicines Agency. 2021 [cited 2023 Nov 22]. Available from: https://www.ema.europa.eu/en/medicines/human/EPAR/pemazyre.
- Yuan, J.; Dong, X.; Yap, J.; Hu, J. The MAPK and AMPK signalings: interplay and implication in targeted cancer therapy. J. Hematol. Oncol. 2020, 13, 113. [CrossRef]
- Rychahou PG, Jackson LN, Silva SR, Rajaraman S, Evers BM. Targeted Molecular Therapy of the PI3K Pathway: Therapeutic Significance of PI3K Subunit Targeting in Colorectal Carcinoma. Ann Surg. 2006 Jun;243(6):833.
- Becker, J.; Erdlenbruch, B.; Noskova, I.; Schramm, A.; Aumailley, M.; Schorderet, D.F.; Schweigerer, L. Keratoepithelin Suppresses the Progression of Experimental Human Neuroblastomas. Cancer Res 2006, 66, 5314–5321. [CrossRef]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods San Diego Calif. 2001 Dec;25(4):402–8.
- Malik, I.A.; Rajput, M.; Werner, R.; Fey, D.; Salehzadeh, N.; von Arnim, C.A.F.; Wilting, J. Differential in vitro effects of targeted therapeutics in primary human liver cancer: importance for combined liver cancer. BMC Cancer 2022, 22, 1–11. [CrossRef]
- Belmokhtar, C.A.; Hillion, J.; Ségal-Bendirdjian, E. Staurosporine induces apoptosis through both caspase-dependent and caspase-independent mechanisms. Oncogene 2001, 20, 3354–3362. [CrossRef]
- Liu H, Liu C, Wang M, Sun D, Zhu P, Zhang P, et al. Tanshinone IIA affects the malignant growth of Cholangiocarcinoma cells by inhibiting the PI3K-Akt-mTOR pathway. Sci Rep. 2021 Sep 29;11(1):19268.
- Zhang, Y.; Yang, Y.; Liu, R.; Meng, Y.; Tian, G.; Cao, Q. Downregulation of microRNA-425-5p suppresses cervical cancer tumorigenesis by targeting AIFM1. Exp. Ther. Med. 2019, 17, 4032–4038. [CrossRef]
- Wenzel, U.; Kuntz, S.; Brendel, M.D.; Daniel, H. Dietary flavone is a potent apoptosis inducer in human colon carcinoma cells.. 2000, 60, 3823–31.
- Silvestri, M.; Vu, T.N.; Nichetti, F.; Niger, M.; Di Cosimo, S.; De Braud, F.; Pruneri, G.; Pawitan, Y.; Calza, S.; Cappelletti, V. Comprehensive transcriptomic analysis to identify biological and clinical differences in cholangiocarcinoma. Cancer Med. 2023, 12, 10156–10168. [CrossRef]
- Mendoza MC, Er EE, Blenis J. The Ras-ERK and PI3K-mTOR pathways: cross-talk and compensation. Trends Biochem Sci. 2011 Jun;36(6):320–8.
- Britten, C.D. PI3K and MEK inhibitor combinations: examining the evidence in selected tumor types. Cancer Chemother. Pharmacol. 2013, 71, 1395–1409. [CrossRef]
- Manning, B.D.; Toker, A. AKT/PKB Signaling: Navigating the Network. Cell 2017, 169, 381–405. [CrossRef]
- Cao Z, Liao Q, Su M, Huang K, Jin J, Cao D. AKT and ERK dual inhibitors: The way forward? Cancer Lett. 2019 Sep 10;459:30–40.
- Nicholson, D.W.; Ali, A.; Thornberry, N.A.; Vaillancourt, J.P.; Ding, C.K.; Gallant, M.; Gareau, Y.; Griffin, P.R.; Labelle, M.; Lazebnik, Y.A.; et al. Identification and inhibition of the ICE/CED-3 protease necessary for mammalian apoptosis. Nature 1995, 376, 37–43. [CrossRef]
- Slee, E.A.; Adrain, C.; Martin, S.J. Executioner Caspase-3, -6, and -7 Perform Distinct, Non-redundant Roles during the Demolition Phase of Apoptosis. J. Biol. Chem. 2001, 276, 7320–7326. [CrossRef]
- Yoon, S.; Kovalenko, A.; Bogdanov, K.; Wallach, D. MLKL, the Protein that Mediates Necroptosis, Also Regulates Endosomal Trafficking and Extracellular Vesicle Generation. Immunity 2017, 47, 51–65.e7. [CrossRef]
- Tolcher AW, Baird RD, Patnaik A, Moreno Garcia V, Papadopoulos KP, Garrett CR, et al. A phase I dose-escalation study of oral MK-2206 (allosteric AKT inhibitor) with oral selumetinib (AZD6244; MEK inhibitor) in patients with advanced or metastatic solid tumors. J Clin Oncol. 2011 May 20;29(15_suppl):3004–3004.
- Do, K.; Speranza, G.; Bishop, R.; Khin, S.; Rubinstein, L.; Kinders, R.J.; Datiles, M.; Eugeni, M.; Lam, M.H.; Doyle, L.A.; et al. Biomarker-driven phase 2 study of MK-2206 and selumetinib (AZD6244, ARRY-142886) in patients with colorectal cancer. Investig. New Drugs 2015, 33, 720–728. [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





