Submitted:
07 April 2024
Posted:
09 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Global Impact and Evolution of H3N2 Influenza: A Historical Perspective
3. Pathophysiology of H3N2 Influenza: Complex Interactions and Inflammatory Response Mechanisms
4. Evolving Vaccination Strategies
4.1. Current Influenza Vaccines
4.2. Effectiveness and Limitations of Vaccines
4.3. Novel Approaches to Vaccine Design
5. Antiviral Therapies of H3N2 Influenza Virus
5.1. Neuraminidase Inhibitors (Oseltamivir, Zanamivir)
5.2. Polymerase Inhibitors (Baloxavir Marboxil)
6. Advancements in Molecular Detection Methods
6.1. Rapid Diagnostic Methods
6.2. Molecular Detection Techniques
6.3. Point-of-Care Testing Advances
7. Future Directions
8. Conclusion
Funding
Abbreviations
| H3N2 | Hemagglutinin 3 and Neuraminidase 2 |
| CDC | Centres for Disease Control and Prevention |
| WHO | World Health Organization |
| HA | Hemagglutinin |
| RNA | Ribonucleic Acid |
| IBVs | Influenza B Viruses |
| A(H3N2) | Influenza A Subtype H3N2 |
| rRT-PCR | Real-Time Reverse Transcription Polymerase Chain Reaction |
| M | Matrix |
| ILI | influenza-like illness |
| PA | Polymerase acid |
| PB1 | polymerase basic 1 |
| PB2 | polymerase basic 2 |
| TIV | trivalent inactivated vaccine |
| QIV | Quadrivalent influenza vaccine |
| RIDTs | Rapid Influenza Diagnostic Tests |
| HAI | Hemagglutination Inhibition Assay |
| VN | Virus Neutralization Assay |
| SRH | Single Radial Homolysis |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| RT-PCR | Reverse Transcription-Polymerase Chain Reaction |
| LAMP | Loop-Mediated Isothermal Amplification |
| POCT | Point-of-care testing |
| VE | Vaccine Effectiveness |
| H3N2 | Hemagglutinin 3 and Neuraminidase 2 |
| H3N2v | H3N2 variant |
References
- Bonilla-Aldana, D.K.; Aguirre-Florez, M.; Villamizar-Peña, R.; Gutiérrez-Ocampo, E.; Henao-Martínez, J.F.; Cvetkovic-Vega, A.; Dhama, K.; Rabaan, A.; Sah, R.; Rodriguez-Morales, A.J.; et al. After SARS-CoV-2, will H5N6 and other influenza viruses follow the pandemic path? Infez Med 2020, 28, 475–485. [Google Scholar]
- Choudhary, P.; Shafaati, M.; Abu Salah, M.A.H.; Chopra, H.; Choudhary, O.P.; Silva-Cajaleon, K.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. Zoonotic diseases in a changing climate scenario: Revisiting the interplay between environmental variables and infectious disease dynamics. Travel Med Infect Dis 2024, 58, 102694. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Cardona-Ospina, J.A.; Collins, M.H. Editorial: Emerging and Re-emerging Vector-borne and Zoonotic Diseases. Front Med (Lausanne) 2021, 8, 714630. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Bonilla-Aldana, D.K.; Paniz-Mondolfi, A.E. Concerns about influenza H5N8 outbreaks in humans and birds: Facing the next airborne pandemic? Travel Med Infect Dis 2021, 41, 102054. [Google Scholar] [CrossRef]
- Priyanka; Khandia, R.; Chopra, H.; Choudhary, O.P.; Bonilla-Aldana, D.K.; Rodriguez-Morales, A.J. The re-emergence of H3N2 influenza: An update on the risk and containment. New Microbes New Infect 2023, 53, 101147. [Google Scholar] [CrossRef]
- Ahmad, F.; Haque, S.; Tawil, S.; Husni, R.; Bonilla-Aldana, D.K.; Montenegro-Idrogo, J.J.; Rodriguez-Morales, A.J. Avian influenza spillover to humans: Are we prepared to deal with another potential pandemic? Travel Med Infect Dis 2023, 55, 102634. [Google Scholar] [CrossRef]
- Rodriguez-Morales, A.J.; Paniz-Mondolfi, A.E.; Faccini-Martínez Á, A.; Henao-Martínez, A.F.; Ruiz-Saenz, J.; Martinez-Gutierrez, M.; Alvarado-Arnez, L.E.; Gomez-Marin, J.E.; Bueno-Marí, R.; Carrero, Y.; et al. The Constant Threat of Zoonotic and Vector-Borne Emerging Tropical Diseases: Living on the Edge. Front Trop Dis 2021, 2, 676905. [Google Scholar] [CrossRef]
- Yu, J.; Li, F.; Wang, D. The first decade of research advances in influenza D virus. J Gen Virol 2021, 102. [Google Scholar] [CrossRef]
- Forghani, M.; Khachay, M. Feature Extraction Technique for Prediction the Antigenic Variants of the Influenza Virus. International Journal of Medical and Health Sciences 2018, 12, 525–530. [Google Scholar]
- Iuliano, A.D.; Roguski, K.M.; Chang, H.H.; Muscatello, D.J.; Palekar, R.; Tempia, S.; Cohen, C.; Gran, J.M.; Schanzer, D.; Cowling, B.J. Estimates of global seasonal influenza-associated respiratory mortality: a modelling study. The Lancet 2018, 391, 1285–1300. [Google Scholar] [CrossRef] [PubMed]
- Sahu, M.; Singh, N.; Shukla, M.K.; Potdar, V.A.; Sharma, R.K.; Sahare, L.K.; Ukey, M.J.; Barde, P.V. Molecular and epidemiological analysis of pandemic and post-pandemic influenza A (H1N1) pdm09 virus from central India. Journal of Medical Virology 2018, 90, 447–455. [Google Scholar] [CrossRef]
- Somerville, L.K.; Basile, K.; Dwyer, D.E.; Kok, J. The impact of influenza virus infection in pregnancy. Future microbiology 2018, 13, 263–274. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiological reviews 1992, 56, 152–179. [Google Scholar] [CrossRef]
- Vemula, S.V.; Zhao, J.; Liu, J.; Wang, X.; Biswas, S.; Hewlett, I. Current approaches for diagnosis of influenza virus infections in humans. Viruses 2016, 8, 96. [Google Scholar] [CrossRef]
- Riedel, S. Crossing the species barrier: the threat of an avian influenza pandemic. In Proceedings of Baylor University Medical Center Proceedings; pp. 16-20.
- Potter, C.; Jennings, R.; Clark, A.; Ali, M. Interference following dual inoculation with influenza A (H3N2) and (H1N1) viruses in ferrets and volunteers. Journal of Medical Virology 1983, 11, 77–86. [Google Scholar] [CrossRef]
- Lin, Y.; Wharton, S.A.; Whittaker, L.; Dai, M.; Ermetal, B.; Lo, J.; Pontoriero, A.; Baumeister, E.; Daniels, R.S.; McCauley, J.W. The characteristics and antigenic properties of recently emerged subclade 3C. 3a and 3C. 2a human influenza A (H3N2) viruses passaged in MDCK cells. Influenza and other respiratory viruses 2017, 11, 263–274. [Google Scholar] [CrossRef]
- Xu, R.; Krause, J.C.; McBride, R.; Paulson, J.C.; Crowe Jr, J.E.; Wilson, I.A. A recurring motif for antibody recognition of the receptor-binding site of influenza hemagglutinin. Nature structural & molecular biology 2013, 20, 363–370. [Google Scholar]
- Lin, Y.P.; Xiong, X.; Wharton, S.A.; Martin, S.R.; Coombs, P.J.; Vachieri, S.G.; Christodoulou, E.; Walker, P.A.; Liu, J.; Skehel, J.J. Evolution of the receptor binding properties of the influenza A (H3N2) hemagglutinin. Proceedings of the National Academy of Sciences 2012, 109, 21474–21479. [Google Scholar] [CrossRef]
- Kang, M.; Zanin, M.; Wong, S.-S. Subtype H3N2 influenza A viruses: an unmet challenge in the Western Pacific. Vaccines 2022, 10, 112. [Google Scholar] [CrossRef] [PubMed]
- Russell, C.A.; Jones, T.C.; Barr, I.G.; Cox, N.J.; Garten, R.J.; Gregory, V.; Gust, I.D.; Hampson, A.W.; Hay, A.J.; Hurt, A.C. The global circulation of seasonal influenza A (H3N2) viruses. Science 2008, 320, 340–346. [Google Scholar] [CrossRef] [PubMed]
- Gostic, K.M.; Bridge, R.; Brady, S.; Viboud, C.; Worobey, M.; Lloyd-Smith, J.O. Childhood immune imprinting to influenza A shapes birth year-specific risk during seasonal H1N1 and H3N2 epidemics. PLoS pathogens 2019, 15, e1008109. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.-J.; Ryu, G.; Lee, K.-I. Symptomatic Differences between Influenza A/H3N2 and A/H1N1 in Korea. Journal of Clinical Medicine 2023, 12, 5651. [Google Scholar] [CrossRef] [PubMed]
- Ge, J.; Lin, X.; Guo, J.; Liu, L.; Li, Z.; Lan, Y.; Liu, L.; Guo, J.; Lu, J.; Huang, W. The antibody response against neuraminidase in human influenza A (H3N2) virus infections during 2018/2019 flu season: focusing on the epitopes of 329-N-glycosylation and E344 in N2. Frontiers in Microbiology 2022, 13, 845088. [Google Scholar] [CrossRef]
- Jester, B.J.; Uyeki, T.M.; Jernigan, D.B. Fifty years of influenza A (H3N2) following the pandemic of 1968. American journal of public health 2020, 110, 669–676. [Google Scholar] [CrossRef]
- CDC. 1968 Pandemic (H3N2 virus). Availabe online: https://www.cdc.gov/flu/pandemic-resources/1968-pandemic.html (accessed on.
- Lindstrom, S.; Garten, R.; Balish, A.; Shu, B.; Emery, S.; Berman, L.; Barnes, N.; Sleeman, K.; Gubareva, L.; Villanueva, J. Human infections with novel reassortant influenza A (H3N2) v viruses, United States, 2011. Emerging infectious diseases 2012, 18, 834. [Google Scholar] [CrossRef]
- Galli, C.; Pellegrinelli, L.; Giardina, F.; Ferrari, G.; Renteria, S.C.U.; Novazzi, F.; Masi, E.; Pagani, E.; Piccirilli, G.; Mauro, M.V. On the lookout for influenza viruses in Italy during the 2021-2022 season: Along came A (H3N2) viruses with a new phylogenetic makeup of their hemagglutinin. Virus Research 2023, 324, 199033. [Google Scholar] [CrossRef]
- Viboud, C.; Grais, R.F.; Lafont, B.A.; Miller, M.A.; Simonsen, L. Multinational impact of the 1968 Hong Kong influenza pandemic: evidence for a smoldering pandemic. The Journal of infectious diseases 2005, 192, 233–248. [Google Scholar] [CrossRef]
- Yu, H.; Hua, R.-H.; Zhang, Q.; Liu, T.-Q.; Liu, H.-L.; Li, G.-X.; Tong, G.-Z. Genetic evolution of swine influenza A (H3N2) viruses in China from 1970 to 2006. Journal of Clinical Microbiology 2008, 46, 1067–1075. [Google Scholar] [CrossRef] [PubMed]
- Scholtissek, C.; Rohde, W.v.; Von Hoyningen, V.; Rott, R. On the origin of the human influenza virus subtypes H2N2 and H3N2. Virology 1978, 87, 13–20. [Google Scholar] [CrossRef]
- Wen, F.; Bedford, T.; Cobey, S. Explaining the geographical origins of seasonal influenza A (H3N2). Proceedings of the royal society b: biological sciences 2016, 283, 20161312. [Google Scholar] [CrossRef]
- Pearce, M.B.; Jayaraman, A.; Pappas, C.; Belser, J.A.; Zeng, H.; Gustin, K.M.; Maines, T.R.; Sun, X.; Raman, R.; Cox, N.J. Pathogenesis and transmission of swine origin A (H3N2) v influenza viruses in ferrets. Proceedings of the National Academy of Sciences 2012, 109, 3944–3949. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.I.; Vincent, A.L.; Kitikoon, P.; Holmes, E.C.; Gramer, M.R. Evolution of novel reassortant A/H3N2 influenza viruses in North American swine and humans, 2009–2011. Journal of virology 2012, 86, 8872–8878. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Janjua, N.Z.; De Serres, G.; Purych, D.; Gilca, V.; Scheifele, D.W.; Dionne, M.; Sabaiduc, S.; Gardy, J.L.; Li, G. Cross-reactive and vaccine-induced antibody to an emerging swine-origin variant of influenza A virus subtype H3N2 (H3N2v). The Journal of infectious diseases 2012, 206, 1852–1861. [Google Scholar] [CrossRef]
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: pathophysiology and epidemiology. Critical care 2019, 23, 258. [Google Scholar] [CrossRef]
- Sun, H.; Blackmon, S.; Yang, G.; Waters, K.; Li, T.; Tangwangvivat, R.; Xu, Y.; Shyu, D.; Wen, F.; Cooley, J. Zoonotic risk, pathogenesis, and transmission of avian-origin H3N2 canine influenza virus. Journal of virology 2017, 91, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- McElhaney, J.E.; Kuchel, G.A.; Zhou, X.; Swain, S.L.; Haynes, L. T-cell immunity to influenza in older adults: a pathophysiological framework for development of more effective vaccines. Frontiers in immunology 2016, 7, 180251. [Google Scholar] [CrossRef]
- Lakdawala, S.S.; Jayaraman, A.; Halpin, R.A.; Lamirande, E.W.; Shih, A.R.; Stockwell, T.B.; Lin, X.; Simenauer, A.; Hanson, C.T.; Vogel, L. The soft palate is an important site of adaptation for transmissible influenza viruses. Nature 2015, 526, 122–125. [Google Scholar] [CrossRef]
- Nicholls, J.M.; Bourne, A.J.; Chen, H.; Guan, Y.; Peiris, J.M. Sialic acid receptor detection in the human respiratory tract: evidence for widespread distribution of potential binding sites for human and avian influenza viruses. Respiratory research 2007, 8, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Zangrillo, A.; Biondi-Zoccai, G.; Landoni, G.; Frati, G.; Patroniti, N.; Pesenti, A.; Pappalardo, F. Extracorporeal membrane oxygenation (ECMO) in patients with H1N1 influenza infection: a systematic review and meta-analysis including 8 studies and 266 patients receiving ECMO. Critical Care 2013, 17, 1–8. [Google Scholar] [CrossRef]
- Allen, J.D.; Ross, T.M. H3N2 influenza viruses in humans: Viral mechanisms, evolution, and evaluation. Human vaccines & immunotherapeutics 2018, 14, 1840–1847. [Google Scholar]
- Belongia, E.A.; McLean, H.Q. Influenza vaccine effectiveness: defining the H3N2 problem. Clinical Infectious Diseases 2019, 69, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Barberis, I.; Martini, M.; Iavarone, F.; Orsi, A. Available influenza vaccines: immunization strategies, history and new tools for fighting the disease. Journal of preventive medicine and hygiene 2016, 57, E41. [Google Scholar] [PubMed]
- Flannery, B.; Fry, A.M. Comparing influenza vaccine types: the path toward improved influenza vaccine strategies. Oxford University Press US: 2019; Vol. 220, pp 1237-1239.
- Perofsky, A.C.; Nelson, M.I. The challenges of vaccine strain selection. Elife 2020, 9, e62955. [Google Scholar] [CrossRef] [PubMed]
- Mancera Gracia, J.C.; Pearce, D.S.; Masic, A.; Balasch, M. Influenza A virus in swine: epidemiology, challenges and vaccination strategies. Frontiers in Veterinary Science 2020, 7, 647. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Kondor, R.J.G.; Chung, J.R.; Gaglani, M.; Reis, M.; Zimmerman, R.K.; Nowalk, M.P.; Jackson, M.L.; Jackson, L.A.; Monto, A.S. Spread of antigenically drifted influenza A (H3N2) viruses and vaccine effectiveness in the United States during the 2018–2019 season. The Journal of infectious diseases 2020, 221, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Flannery, B.; Chung, J.R.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q.; Gaglani, M.; Murthy, K.; Zimmerman, R.K.; Nowalk, M.P. Influenza vaccine effectiveness in the United States during the 2016–2017 season. Clinical Infectious Diseases 2019, 68, 1798–1806. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; De Serres, G.; Sabaiduc, S.; Winter, A.-L.; Dickinson, J.A.; Gubbay, J.B.; Fonseca, K.; Drews, S.J.; Charest, H. Serial vaccination and the antigenic distance hypothesis: effects on influenza vaccine effectiveness during A (H3N2) epidemics in Canada, 2010–2011 to 2014–2015. The Journal of infectious diseases 2017, 215, 1059–1099. [Google Scholar] [CrossRef]
- Kissling, E.; Pozo, F.; Buda, S.; Vilcu, A.-M.; Rizzo, C.; Gherasim, A.; Horváth, J.K.; Brytting, M.; Domegan, L.; Meijer, A. Effectiveness of influenza vaccine against influenza A in Europe in seasons of different A (H1N1) pdm09 and the same A (H3N2) vaccine components (2016–17 and 2017–18). Vaccine: X 2019, 3, 100042. [Google Scholar] [CrossRef]
- Skowronski, D.M.; Leir, S.; Sabaiduc, S.; Chambers, C.; Zou, M.; Rose, C.; Olsha, R.; Dickinson, J.A.; Winter, A.-L.; Jassem, A. Influenza vaccine effectiveness by A (H3N2) phylogenetic subcluster and prior vaccination history: 2016–2017 and 2017–2018 epidemics in Canada. The Journal of Infectious Diseases 2022, 225, 1387–1398. [Google Scholar] [CrossRef]
- Rose, A.M.; Kissling, E.; Gherasim, A.; Casado, I.; Bella, A.; Launay, O.; Lazăr, M.; Marbus, S.; Kuliese, M.; Syrjänen, R. Vaccine effectiveness against influenza A (H3N2) and B among laboratory-confirmed, hospitalised older adults, Europe, 2017-18: A season of B lineage mismatched to the trivalent vaccine. Influenza and other respiratory viruses 2020, 14, 302–310. [Google Scholar] [CrossRef]
- Trebbien, R.; Fischer, T.K.; Krause, T.G.; Nielsen, L.; Nielsen, X.C.; Weinreich, L.S.; Lis-Tønder, J.; Skov, M.N.; Christiansen, C.B.; Emborg, H.-D. Changes in genetically drifted H3N2 influenza A viruses and vaccine effectiveness in adults 65 years and older during the 2016/17 season in Denmark. Journal of Clinical Virology 2017, 94, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Skowronski, D.M.; Chambers, C.; Sabaiduc, S.; Dickinson, J.A.; Winter, A.-L.; De Serres, G.; Drews, S.J.; Jassem, A.; Gubbay, J.B.; Charest, H. Interim estimates of 2016/17 vaccine effectiveness against influenza A (H3N2), Canada, January 2017. Eurosurveillance 2017, 22, 30460. [Google Scholar] [CrossRef] [PubMed]
- CDC. Frequently Asked Influenza (Flu) Questions: 2022-2023 Season. Availabe online: https://www.cdc.gov/flu/season/faq-flu-season-2022-2023.htm (accessed on.
- FDA, U. Influenza Vaccine for the 2022-2023 Season. Availabe online: https://www.fda.gov/vaccines-bloodbiologics/lot-release/influenza-vaccine-2022-2023-season (accessed on.
- CDC. Vaccine Effectiveness: How Well Do Flu Vaccines Work? Availabe online: https://www.cdc.gov/flu/vaccines-work/vaccineeffect.htm (accessed on.
- Tenforde, M.W.; Patel, M.M.; Lewis, N.M.; Adams, K.; Gaglani, M.; Steingrub, J.S.; Shapiro, N.I.; Duggal, A.; Prekker, M.E.; Peltan, I.D. Vaccine effectiveness against influenza A (H3N2)–associated hospitalized illness: United States, 2022. Clinical Infectious Diseases 2023, 76, 1030–1037. [Google Scholar] [CrossRef] [PubMed]
- WHO. Recommendations announced for influenza vaccine composition for the 2023-2024 northern hemisphere influenza season. Availabe online: https://www.who.int/news/item/24-02-2023-recommendations-announced-for-influenza-vaccine-composition-for-the-2023-2024-northern-hemisphere-influenza-season (accessed on.
- Price, A.M.; Flannery, B.; Talbot, H.K.; Grijalva, C.G.; Wernli, K.J.; Phillips, C.H.; Monto, A.S.; Martin, E.T.; Belongia, E.A.; McLean, H.Q. Influenza vaccine effectiveness against influenza A (H3N2)-related illness in the United States during the 2021–2022 influenza season. Clinical Infectious Diseases 2023, 76, 1358–1363. [Google Scholar] [CrossRef] [PubMed]
- Music, N.; Tzeng, W.-P.; Liaini Gross, F.; Levine, M.Z.; Xu, X.; Shieh, W.-J.; Tumpey, T.M.; Katz, J.M.; York, I.A. Repeated vaccination against matched H3N2 influenza virus gives less protection than single vaccination in ferrets. npj Vaccines 2019, 4, 28. [Google Scholar] [CrossRef] [PubMed]
- Gouma, S.; Furey, C.; Santos, J.J.; Parkhouse, K.; Weirick, M.; Muramatsu, H.; Pardi, N.; Fan, S.H.; Weissman, D.; Hensley, S.E. Nucleoside-modified mRNA-based influenza vaccines circumvent problems associated with H3N2 vaccine strain egg adaptation. Journal of Virology 2023, 97, e01723–01722. [Google Scholar] [CrossRef] [PubMed]
- Gouma, S.; Kim, K.; Weirick, M.E.; Gumina, M.E.; Branche, A.; Topham, D.J.; Martin, E.T.; Monto, A.S.; Cobey, S.; Hensley, S.E. Middle-aged individuals may be in a perpetual state of H3N2 influenza virus susceptibility. Nature Communications 2020, 11, 4566. [Google Scholar] [CrossRef]
- CDC. Live Attenuated Influenza Vaccine [LAIV] (The Nasal Spray Flu Vaccine). Availabe online: https://www.cdc.gov/flu/prevent/nasalspray.htm (accessed on.
- Wallis, J.; Shenton, D.; Carlisle, R. Novel approaches for the design, delivery and administration of vaccine technologies. Clinical & Experimental Immunology 2019, 196, 189–204. [Google Scholar]
- Shin, Y.; Kim, J.; Seok, J.H.; Park, H.; Cha, H.-R.; Ko, S.H.; Lee, J.M.; Park, M.-S.; Park, J.-H. Development of the H3N2 influenza microneedle vaccine for cross-protection against antigenic variants. Scientific reports 2022, 12, 12189. [Google Scholar] [CrossRef]
- Dormitzer, P.; Tsai, T.; Del Giudice, G. New technologies for influenza vaccines. Human vaccines & immunotherapeutics 2012, 8, 45–58. [Google Scholar]
- Nguyen, Q.-T.; Choi, Y.-K. Targeting antigens for universal influenza vaccine development. Viruses 2021, 13, 973. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.-J.; Crank, M.C.; Shiver, J.; Graham, B.S.; Mascola, J.R.; Nabel, G.J. Next-generation influenza vaccines: opportunities and challenges. Nature reviews Drug discovery 2020, 19, 239–252. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Chen, C.; Han, X.; Lin, S.; Ao, X.; Han, X.; Wang, J.; Ye, H. Structural insights for anti-influenza vaccine design. Computational and structural biotechnology journal 2019, 17, 475–483. [Google Scholar] [CrossRef] [PubMed]
- CDC. Interim Updated Planning Guidance on Allocating and Targeting Pandemic Influenza Vaccine during an Influenza Pandemic. Availabe online: https://www.cdc.gov/flu/pandemic-resources/nationalstrategy/planning-guidance/index.html (accessed on.
- CDC. Influenza Antiviral Medications: Summary for Clinicians. Availabe online: https://www.cdc.gov/flu/professionals/antivirals/summary-clinicians.htm (accessed on.
- CDC. Variant Influenza Virus Treatment. Availabe online: https://www.cdc.gov/flu/swineflu/variant-treatment.htm (accessed on.
- De Clercq, E. Antiviral agents active against influenza A viruses. Nature reviews Drug discovery 2006, 5, 1015–1025. [Google Scholar] [CrossRef]
- Alchikh, M.; Obermeier, P.E.; Schweiger, B.; Rath, B.A. Successful Treatment of Complicated Influenza A (H3N2) Virus Infection and Rhabdomyolysis with Compassionate Use of IV Zanamivir. Pharmaceuticals 2023, 16, 85. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antiviral Drug Resistance among Influenza Viruses. Availabe online: https://www.cdc.gov/flu/professionals/antivirals/antiviral-drug-resistance.htm (accessed on.
- Jefferson, T.; Jones, M.A.; Doshi, P.; Del Mar, C.B.; Hama, R.; Thompson, M.J.; Spencer, E.A.; Onakpoya, I.J.; Mahtani, K.R.; Nunan, D. Neuraminidase inhibitors for preventing and treating influenza in adults and children. Cochrane database of systematic reviews 2014. [Google Scholar] [CrossRef]
- Mahal, A.; Duan, M.; Zinad, D.S.; Mohapatra, R.K.; Obaidullah, A.J.; Wei, X.; Pradhan, M.K.; Das, D.; Kandi, V.; Zinad, H.S. , et al. Recent progress in chemical approaches for the development of novel neuraminidase inhibitors. RSC Adv 2021, 11, 1804–1840. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.C.; Zagribelnyy, B.; Pascua, P.N.Q.; Bezrukov, D.S.; Barman, S.; Okda, F.; Webby, R.J.; Ivanenkov, Y.A.; Govorkova, E.A. Influenza A virus polymerase acidic protein E23G/K substitutions weaken key baloxavir drug-binding contacts with minimal impact on replication and transmission. PLoS Pathogens 2022, 18, e1010698. [Google Scholar] [CrossRef] [PubMed]
- Takashita, E.; Morita, H.; Ogawa, R.; Nakamura, K.; Fujisaki, S.; Shirakura, M.; Kuwahara, T.; Kishida, N.; Watanabe, S.; Odagiri, T. Susceptibility of influenza viruses to the novel cap-dependent endonuclease inhibitor baloxavir marboxil. Frontiers in microbiology 2018, 9, 3026. [Google Scholar] [CrossRef]
- Mifsud, E.J.; Hayden, F.G.; Hurt, A.C. Antivirals targeting the polymerase complex of influenza viruses. Antiviral research 2019, 169, 104545. [Google Scholar] [CrossRef]
- Takashita, E.; Kawakami, C.; Ogawa, R.; Morita, H.; Fujisaki, S.; Shirakura, M.; Miura, H.; Nakamura, K.; Kishida, N.; Kuwahara, T. Influenza A (H3N2) virus exhibiting reduced susceptibility to baloxavir due to a polymerase acidic subunit I38T substitution detected from a hospitalised child without prior baloxavir treatment, Japan, January 2019. Eurosurveillance 2019, 24, 1900170. [Google Scholar] [CrossRef] [PubMed]
- Checkmahomed, L.; Padey, B.; Pizzorno, A.; Terrier, O.; Rosa-Calatrava, M.; Abed, Y.; Baz, M.; Boivin, G. In vitro combinations of baloxavir acid and other inhibitors against seasonal influenza A viruses. Viruses 2020, 12, 1139. [Google Scholar] [CrossRef]
- CDC. Rapid Influenza Diagnostic Tests. Availabe online: https://www.cdc.gov/flu/professionals/diagnosis/clinician_guidance_ridt.htm (accessed on.
- CDC. Overview of Influenza Testing Methods. Availabe online: https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm (accessed on.
- Ravina; Manjeet; Mohan, H.; Narang, J.; Pundir, S.; Pundir, C.S. A changing trend in diagnostic methods of Influenza A (H3N2) virus in human: a review. 3 Biotech 2021, 11, 1–13. [Google Scholar] [CrossRef]
- CDC. Interim Guidance for Enhanced Influenza Surveillance: Additional Specimen Collection for Detection of Influenza A (H3N2) Variant Virus Infections. Availabe online: https://www.cdc.gov/flu/swineflu/variant/h3n2v-surveillance.htm (accessed on.
- CDC. Overview of Influenza Testing Methods. Availabe online: https://www.cdc.gov/flu/professionals/diagnosis/overview-testing-methods.htm (accessed on.
- CDC. Information on Rapid Molecular Assays, RT-PCR, and other Molecular Assays for Diagnosis of Influenza Virus Infection. Availabe online: https://www.cdc.gov/flu/professionals/diagnosis/molecularassays.htm (accessed on.
- Poon, L.L.; Leung, C.S.; Chan, K.H.; Lee, J.H.; Yuen, K.Y.; Guan, Y.; Peiris, J.S. Detection of human influenza A viruses by loop-mediated isothermal amplification. Journal of clinical microbiology 2005, 43, 427–430. [Google Scholar] [CrossRef]
- Kang, J.-S.; Seo, M.-R.; Chung, Y.-J. Development of reverse-transcription loop-mediated isothermal amplification assays for point-of-care testing of human influenza virus subtypes H1N1 and H3N2. Genomics & Informatics 2022, 20. [Google Scholar]
- Heithoff, D.M.; Barnes, L.; Mahan, S.P.; Fox, G.N.; Arn, K.E.; Ettinger, S.J.; Bishop, A.M.; Fitzgibbons, L.N.; Fried, J.C.; Low, D.A. Assessment of a smartphone-based loop-mediated isothermal amplification assay for detection of SARS-CoV-2 and influenza viruses. JAMA network open 2022, 5, e2145669–e2145669. [Google Scholar] [CrossRef] [PubMed]
- Cui, D.; Zhao, D.; Xie, G.; Yang, X.; Huo, Z.; Zheng, S.; Yu, F.; Chen, Y. Simultaneous detection of influenza A subtypes of H3N2 virus, pandemic (H1N1) 2009 virus and reassortant avian H7N9 virus in humans by multiplex one-step real-time RT-PCR assay. Springerplus 2016, 5, 1–8. [Google Scholar] [CrossRef]
- Egilmezer, E.; Walker, G.J.; Bakthavathsalam, P.; Peterson, J.R.; Gooding, J.J.; Rawlinson, W.; Stelzer-Braid, S. Systematic review of the impact of point-of-care testing for influenza on the outcomes of patients with acute respiratory tract infection. Reviews in medical virology 2018, 28, e1995. [Google Scholar] [CrossRef]
- Dickson, E.M.; Zambon, M.; Pebody, R.; de Lusignan, S.; Elliot, A.J.; Ellis, J.; Lackenby, A.; Smith, G.; McMenamin, J. Do point-of-care tests (POCTs) offer a new paradigm for the management of patients with influenza? Eurosurveillance 2020, 25, 1900420. [Google Scholar] [CrossRef]
- Nelson, P.P.; Rath, B.A.; Fragkou, P.C.; Antalis, E.; Tsiodras, S.; Skevaki, C. Current and future point-of-care tests for emerging and new respiratory viruses and future perspectives. Frontiers in cellular and infection microbiology 2020, 10, 181. [Google Scholar] [CrossRef]
- Maignan, M.; Viglino, D.; Hablot, M.; Termoz Masson, N.; Lebeugle, A.; Collomb Muret, R.; Mabiala Makele, P.; Guglielmetti, V.; Morand, P.; Lupo, J. Diagnostic accuracy of a rapid RT-PCR assay for point-of-care detection of influenza A/B virus at emergency department admission: A prospective evaluation during the 2017/2018 influenza season. PloS one 2019, 14, e0216308. [Google Scholar] [CrossRef] [PubMed]
- Melhuish, A.; Vargas-Palacios, A.; Yaziji, N.; Selfridge, J.; Pisavadia, M.; Sagoo, G.S.; Minton, J. Cost evaluation of point-of-care testing for community-acquired influenza in adults presenting to the emergency department. Journal of Clinical Virology 2020, 129, 104533. [Google Scholar] [CrossRef] [PubMed]
- Fjelltveit, E.B.; Cox, R.J.; Østensjø, J.; Blomberg, B.; Ebbesen, M.H.; Langeland, N.; Mohn, K.G. Point-of-Care Influenza Testing Impacts Clinical Decision, Patient Flow, and Length of Stay in Hospitalized Adults. The Journal of Infectious Diseases 2022, 226, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Weitzel, T.; Schnabel, E.; Dieckmann, S.; Börner, U.; Schweiger, B. Evaluation of a new point-of-care test for influenza A and B virus in travellers with influenza-like symptoms. Clinical microbiology and infection 2007, 13, 665–669. [Google Scholar] [CrossRef] [PubMed]
- Ravina; Dalal, A.; Mohan, H.; Prasad, M.; Pundir, C. Detection methods for influenza A H1N1 virus with special reference to biosensors: a review. Bioscience reports 2020, 40, BSR20193852. [Google Scholar] [CrossRef] [PubMed]
- Storms, S.M.; Shisler, J.; Nguyen, T.H.; Zuckermann, F.A.; Lowe, J.F. RT-LAMP as Diagnostic Tool for Influenza—A Virus Detection in Swine. Veterinary Sciences 2023, 10, 220. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, N.N.; McCarthy, C.; Lantigua, D.; Camci-Unal, G. Development of diagnostic tests for detection of SARS-CoV-2. Diagnostics 2020, 10, 905. [Google Scholar] [CrossRef]
- Stockton, J.; Ellis, J.; Saville, M.; Clewley, J.; Zambon, M. Multiplex PCR for typing and subtyping influenza and respiratory syncytial viruses. Journal of clinical microbiology 1998, 36, 2990–2995. [Google Scholar] [CrossRef]
- Zhou, B.; Deng, Y.-M.; Barnes, J.R.; Sessions, O.M.; Chou, T.-W.; Wilson, M.; Stark, T.J.; Volk, M.; Spirason, N.; Halpin, R.A. Multiplex reverse transcription-PCR for simultaneous surveillance of influenza A and B viruses. Journal of Clinical Microbiology 2017, 55, 3492–3501. [Google Scholar] [CrossRef]
- Dziąbowska, K.; Czaczyk, E.; Nidzworski, D. Detection methods of human and animal influenza virus—current trends. Biosensors 2018, 8, 94. [Google Scholar] [CrossRef]
- Lin, X.; Liu, X.-Y.; Zhang, B.; Qin, A.-Q.; Hui, K.-M.; Shi, K.; Liu, Y.; Gabriel, D.; Li, X.J. A rapid influenza diagnostic test based on detection of viral neuraminidase activity. Scientific Reports 2022, 12, 505. [Google Scholar] [CrossRef] [PubMed]
- Hľasová, Z.; Košík, I.; Ondrejovič, M.; Miertuš, S.; Katrlík, J. Methods and current trends in determination of neuraminidase activity and evaluation of neuraminidase inhibitors. Critical Reviews in Analytical Chemistry 2019, 49, 350–367. [Google Scholar] [CrossRef] [PubMed]
- Faye, O.; Faye, O.; Dupressoir, A.; Weidmann, M.; Ndiaye, M.; Sall, A.A. One-step RT-PCR for detection of Zika virus. Journal of Clinical Virology 2008, 43, 96–101. [Google Scholar] [CrossRef] [PubMed]
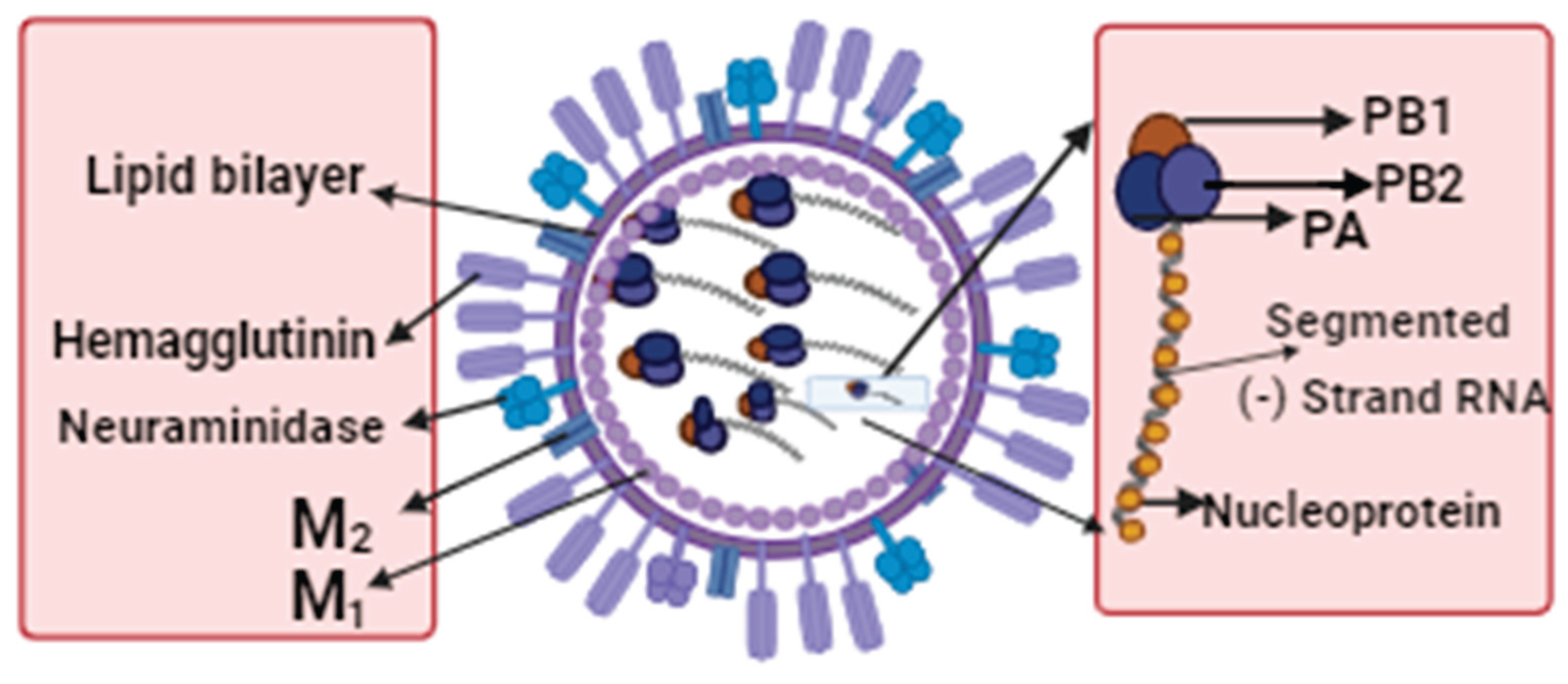
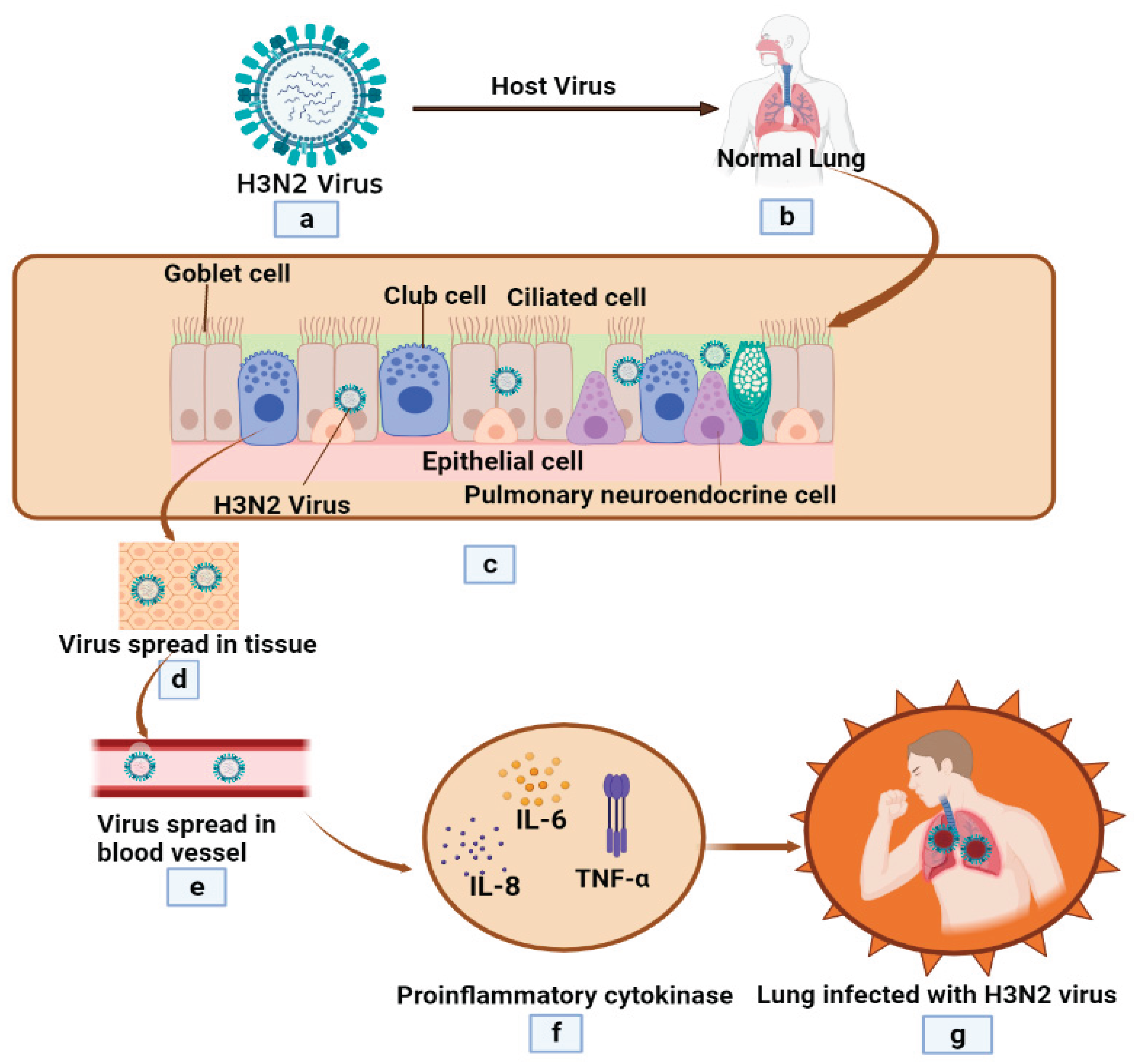
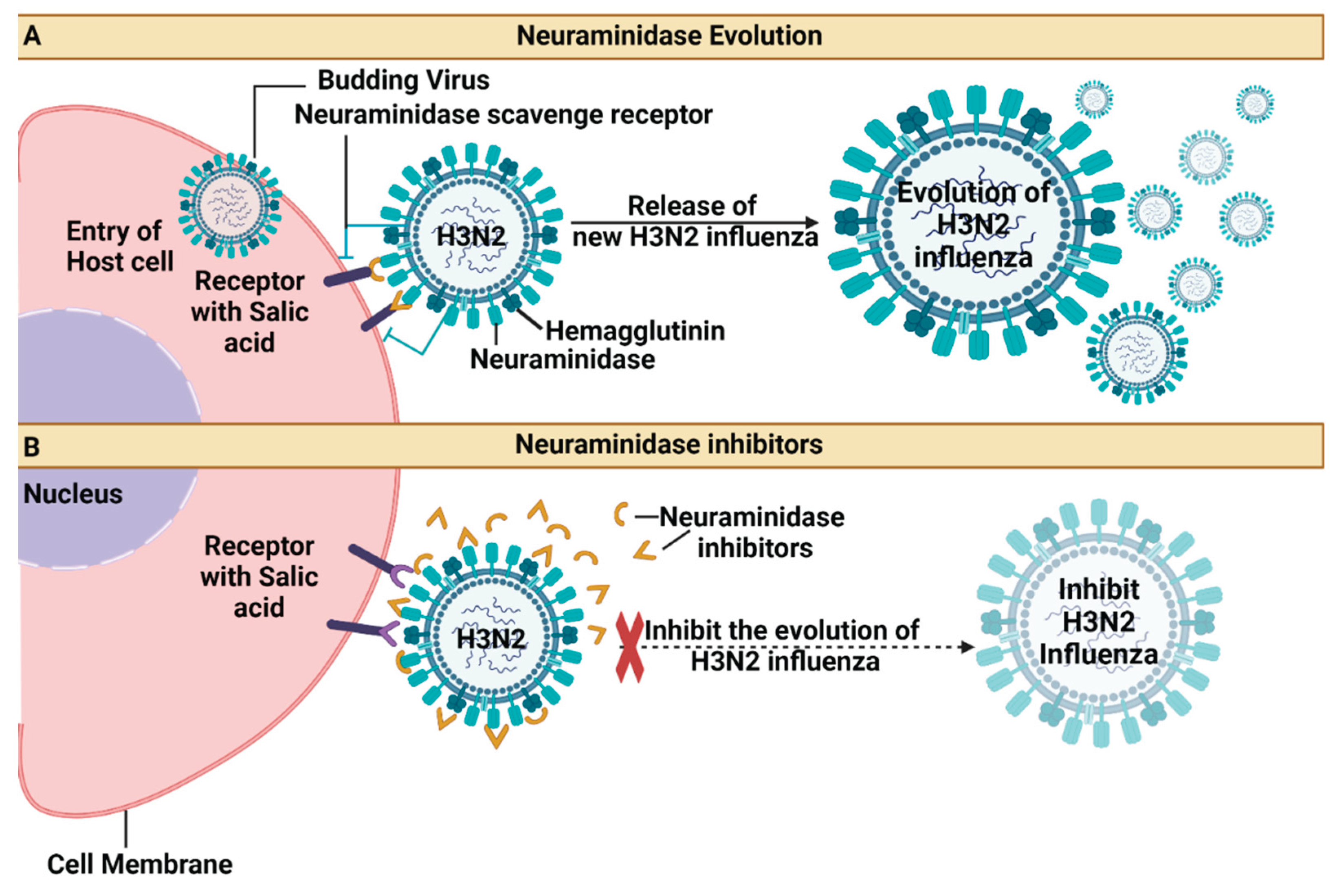
| # | Year | Event Description | Impact | Ref. |
|---|---|---|---|---|
| 1 | 1968-1970 | The first influenza A/H3N2 pandemic season (1968/1969) it led to significant US mortality, while the second (1969/1970) caused most deaths in England. This reveals a global mortality pattern. | Mortality patterns in Europe and Asia were delayed until the second pandemic season due to higher neuraminidase immunity and a drift in the antigen during 1969/1970. | [29] |
| 2 | 1970-2006 | It has been discovered that pandemic influenza viruses persist in Chinese pigs as intermediate hosts, with triple-, double-, and entirely human-like H3N2 viruses coexisting. |
This study analyses eight H3N2 virus genes from 1970 to 2006, revealing that pigs serve as mixing vessels for the virus generation. The coexistence of these viruses underscores the importance of reinforcing swine influenza virus surveillance in China. | [30] |
| 3 | 1976 | Eight segments of the Singapore (H2N2) strain's RNA have been tagged, divided, and associated with proteins and gene functions. The base sequence homology between the H2N2 virus and several influenza A strains was discovered using molecular hybridisation. | The Singapore strain exhibits a base sequence homology of about 160% compared to the FM1 strain (HlNl). Still, the H3N2 strain is likely descended from an H2N2 subtype, as evidenced by its retention of four segments and the HA gene from a different strain. | [31] |
| 4 | 2000-2010 |
This work recreated the ecological and evolutionary dynamics of influenza using a host metapopulation representative of the tropical, temperate, and southern regions. |
Results showed that a region's primary reproductive number significantly impacts the antigenic evolution of its viral population and the probability of its strains spreading globally. Seasonality increases the probability of tropical populations exporting evolutionarily successful strains but doesn't predict their antigenically advanced status alone. | [32] |
| 5 | 2009-2011 | Concerns have been raised about the possibility of a pandemic brought on by four A(H3N2) v influenza viruses that were isolated from US people and examined in a study. It was discovered that the viruses may effectively propagate among ferrets living together and infect newly acquired ferrets via respiratory droplets. |
The study found that A(H3N2) v viruses replicated in Calu-3 cells at considerably higher levels than the usual seasonal H3N2 influenza viruses, highlighting the significance of continuous public health surveillance. |
[33] |
| 6 | 2009-2011 | During a phylogenetic analysis of influenza viruses from swine and humans in North America, thirty-four rH3N2p viruses with identical H3, N2, and pM segments to the human-identified H3N2v viruses were found. |
Combination events between H3N2 viruses and the pM segment have produced these viruses about four to ten times since 2009. All H3N2v viruses recovered from humans have an N2 segment originating from a genetically unique N2 lineage, which may affect the development of influenza vaccines and the possibility of pandemics. | [34] |
| 7 | 2010-2012 | Recent US cases of H3N2v influenza infection, primarily among children, are being studied to determine cross-reactive antibody levels and whether seasonal TIV may increase seroprotection. | While teenagers and young adults have cross-reactive H3N2v antibodies, children and older individuals are susceptible. The lack of seroprotection in recent TIV formulations makes a particular vaccine necessary to spread the epidemic. |
[35] |
| # | Population | Study Design | Vaccine formulation | Vaccine effectiveness % | Key finding | Ref. |
|---|---|---|---|---|---|---|
| 1 | Paediatric (2-17 Years) | Observational studies | Trivalent inactivated vaccine | Efficacy of vaccination, 5%; 95% confidence interval, -47 to 39 | Between 2015 and 2016, influenza vaccinations dramatically decreased the likelihood of contracting the illness. | [49] |
| 2 | Adults in the 20–364 age range |
Meta-analysis of TND studies | Trivalent influenza | VE of 65% | According to the ADH, the impact of recurrent influenza vaccination may have contributed to the low VE in recent A(H3N2) epidemics in Canada since 2010. | [50] |
| 3 | General Population | test-negative design and observational studies | trivalent vaccine | VE was 59% | According to the study, the recently developed A(H1N1)pdm09 vaccine offered reasonable defence against circulating strains. However, VE against A(H3N2) was less than 35% in 2016–17 and 2017–18, presumably due to the antigenic mismatch obtained from egg multiplication. | [51] |
| 4 | Aged one year and above. | Test-negative design | vaccine strain uses egg-adaptation mutations | In 2016–17 and even lower in 2017–18, VE against A(H3N2) was below 40%. |
The study suggests that VE, influenced by phylogenetic sub-clusters and vaccination history, exhibits informative heterogeneity. However, it requires larger sample sizes and may be linked to pivotal mutations. | [52] |
| 5 | Age group is greater than 65-79 years. | test-negative design | trivalent vaccine | Influenza A(H3N2) IVE was 24%, while B IVE was 30%, 37%, and 19%. | IVE against influenza B in hospitalised older adults is similar to A(H3N2), highlighting the importance of influenza vaccination. | [53] |
| 6 | Aged 65 and above. | test-negative case-control design | trivalent influenza vaccine | The adjusted VE for inpatients was 7.4%, while outpatients had 19.3%. | Denmark experienced multiple genetically drifted H3N2 viruses during the 2016-17 influenza season, with low estimated VE and varying VEs across four main virus clusters. | [54] |
| 7 | Aged one year and older. | test-negative design | - | The vector error (VE) for Canada's influenza A(H3N2) outbreak in 2016–17 is over forty per cent higher than in 2014–15. | The intermediate vector error (VE) is approximately 40% higher in Canada's 2016–17 influenza A(H3N2) epidemic than in the 2014–15 pandemic. To reduce morbidity and death, particularly in high-risk individuals, further steps are required. |
[55] |
| # | Method | Description | Ref. |
|---|---|---|---|
| 1. | Biosensors | Advanced methods to detect H3N2 influenza viruses based on different parameters, aiming to improve specificity and sensitivity. The human influenza virus binds to α, 2–6 glycosidic bonds, while the avian influenza virus binds to α, 2-3 glycosidic bonds. Viruses detect distinct receptors on host cells. Pigs show both genetic re-assortment and antigenic shift since they have both types. | [87,102] |
| 2. | RT-LAMP stands for Reverse Transcription Loop-Mediated Isothermal Amplification. | Molecular diagnostic tool for influenza A viruses, including H3N2. It is quick, easy, cost-effective, sensitive, and specific, suitable for point-of-care testing during outbreaks. The process involves amplifying nucleic acid using reverse transcriptase, DNA polymerase, and oligos, resulting in double-stranded looped DNA structures that can be detected using pH sensitivity, fluorescent response, and turbidity. | [103,104] |
| 3. | Multiplex PCR (Polymerase Chain Reaction). |
The diagnostic tool uses several primer pairs in the same reaction, amplifying different specific amplicons for various targets. Increasingly used for the diagnosis of infectious diseases, including RNA-containing viruses like H3N2 influenza. Using this technique, different influenza viruses' HA, NA, and M gene segments can be amplified simultaneously from clinical specimens or isolates to be sequenced. |
[105,106] |
| 4. | Rapid Influenza Diagnostic Tests, or RIDTs | Rapid influenza tests can detect viral nucleoprotein antigens in respiratory specimens in less than 15 minutes; commercial and laboratory-developed RT-PCR assays are suitable reference tests. | [85,107] |
| 5. | Neuraminidase Activity-Based Assay | A chemiluminescent assay for detecting influenza viral neuraminidase (NA) activity utilises a unique substrate related to NA, a target for newer-generation influenza therapeutic drugs. The main goal of mechanism-based drug design is to locate and create target enzyme competitive inhibitors. | [108,109] |
| 6. | one- step RT-PCR | The one-step RT-PCR assay proved quicker and easier than virus isolation and serological methods. | [110] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





