Submitted:
09 April 2024
Posted:
10 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
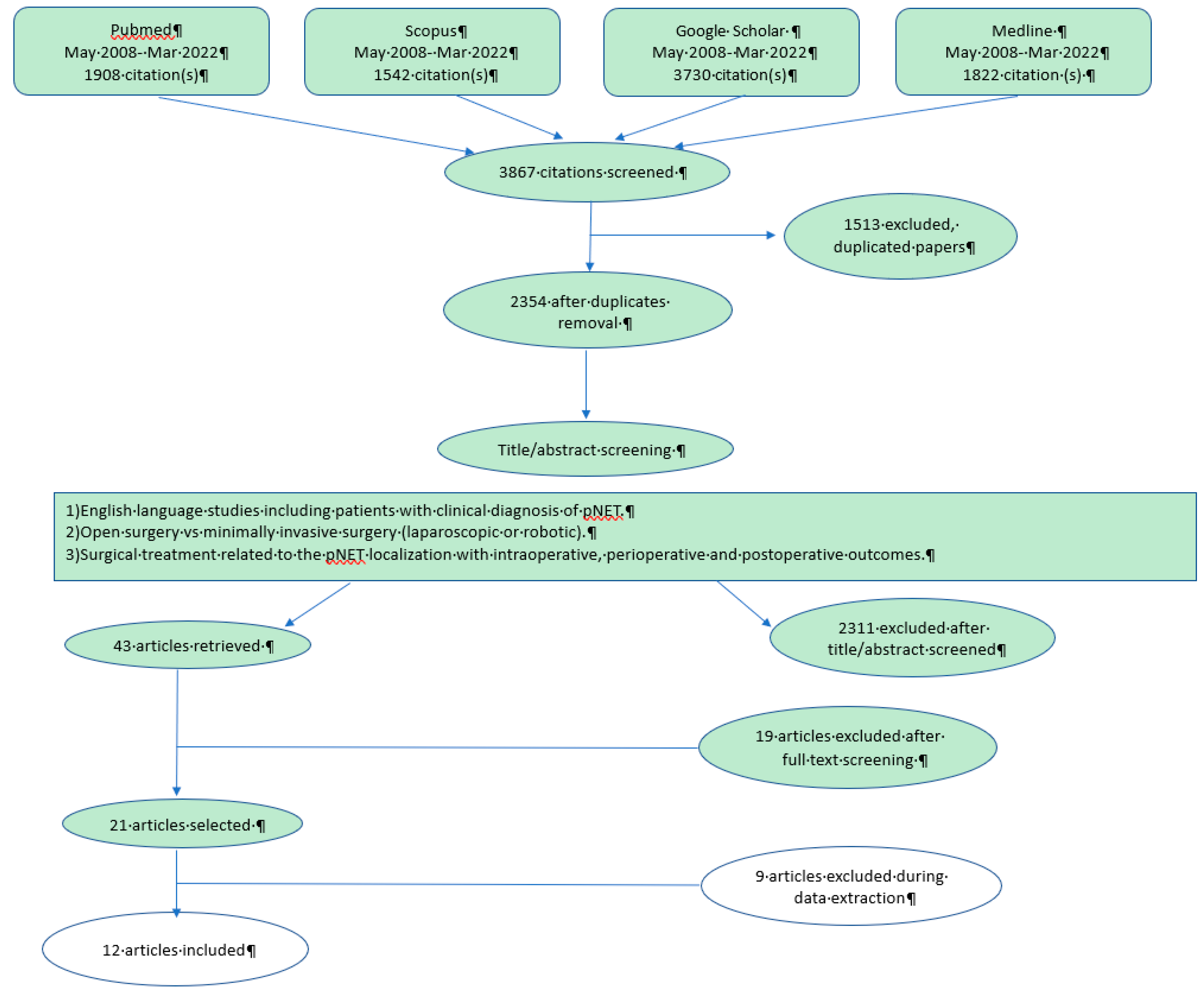
2. Matherial and Methods
3. Results
3.1. Evaluation and Inclusion
3.2. Baseline Characteristics
3.3. Surgical and Post-Operative Results
4. Discussion
5. Conclusions
Funding
Conflicts of Interest
References
- Ma, Z.Y.; Gong, Y.F.; Zhuang, H.K.; et al. Pancreatic neuroendocrine tumors: A review of serum biomarkers, staging, and management. World J Gastroenterol. 2020, 26, 2305–2322. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, B.; Gustafsson, B.I.; Chan, A.; et al. The epidemiology of gastroenteropancreatic neuroendocrinetumors Endocrinol. Metab. Clin. North Am. 2011, 40, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.T.; Howe, J.R. Evaluation and Management of Neuroendocrine Tumors of the Pancreas. Surg Clin North Am. 2019, 99, 793–814. [Google Scholar] [CrossRef] [PubMed]
- Halfdanarson, T.R.; Rabe, K.G.; Rubin, J.; et al. Pancreatic neuroendocrine tumors (PNETs): incidence, prognosis and recent trend toward improved survival. Ann Oncol. 2008, 19, 1727–1733. [Google Scholar] [CrossRef] [PubMed]
- Metz, D.C.; Jensen, R.T. Gastrointestinal neuroendocrine tumors: pancreatic endocrine tumor Gastroenterology, 135 (2008), pp. 1469–1492. Ann. Oncol. 2008, 19, 1727–1733. [Google Scholar]
- <monospace>Vagefi, P.A.; Razo, O.; Deshpande, V.; McGrath, D. J.; et al. Evolving patterns in the detection and outcomes of pancreatic neuroendocrine neoplasms. Arch. Surg. 2007, 142, 347–354. [Google Scholar]
- Souche, R.; Hobeika, C.; Hain, E.; et al. Surgical Management of Neuroendocrine Tumours of the Pancreas. Journal of Clinical Medicine. 2020, 9, 2993. [Google Scholar] [CrossRef] [PubMed]
- Falconi, M.; Eriksson, B.; Kaltsas, G. , et al; Vienna Consensus Conference participants. ENETS Consensus Guidelines Update for the Management of Patients with Functional Pancreatic Neuroendocrine Tumors and Non-Functional Pancreatic Neuroendocrine Tumors. Neuroendocrinology. 2016, 103, 153–171. [Google Scholar] [CrossRef] [PubMed]
- Bettini, R.; Partelli, S.; Boninsegna, L.; et al. Tumor size correlates with malignancy in nonfuctioning pancreatic endocrine tumor. Surgery 2011, 150, 75–82. [Google Scholar] [CrossRef]
- Mehrabi, A.; et al. A systematie review of localization, surgieal treatment options, and outcome of insulinoma. Pancreas Journal 2014.
- Moher, D.; Liberati, A.; Tetzlaff, J. , et al; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Slim, K.; Nini, E.; Forestier, D.; et al. Methodological index for non-randomized studies (minors): development and validation of a new instrument. ANZ J Surg 2003, 73, 712–716. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. Available from: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- Mihalache, O.; Doran, H.; Poiană, C.; et al. Pancreatic Neuroendocrine Tumors - Case Series and Literature Review. Chirurgia (Bucur) 2019, 114, 630–638. [Google Scholar] [CrossRef] [PubMed]
- Sutton, T.L.; Pommier, R.F.; Mayo, S.C.; et al. Similar Outcomes in Minimally Invasive versus Open Management of Primary Pancreatic Neuroendocrine Tumors: A Regional, Multi-Institutional Collaborative Analysis. Cancers (Basel). 2022, 14, 1387. [Google Scholar] [CrossRef] [PubMed]
- Zerbi, A.; Capitanio, V.; Boninsegna, L. , et al; AISP Network Study Group. Surgical treatment of pancreatic endocrine tumours in Italy: results of a prospective multicentre study of 262 cases. Langenbecks Arch Surg. 2011, 396, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Lopez-Aguiar, A.G.; Poultsides, G. , et al; United States Neuroendocrine Tumor Study Group. Minimally invasive versus open distal pancreatectomy for pancreatic neuroendocrine tumors: An analysis from the U.S. neuroendocrine tumor study group. J Surg Oncol. 2019, 120, 231–240. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.P.; Zhan, H.X.; Zhang, T.P.; et al. Surgical management of patients with insulinomas: Result of 292 cases in a single institution. J Surg Oncol. 2011, 103, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Haugvik, S.P.; Marangos, I.P.; Røsok, B.I.; et al. Long-term outcome of laparoscopic surgery for pancreatic neuroendocrine tumors. World J Surg. 2013, 37, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Jilesen, A.P.; van Eijck, C.H.; Busch, O.R.; et al. Postoperative Outcomes of Enucleation and Standard Resections in Patients with a Pancreatic Neuroendocrine Tumor. World J Surg. 2016, 40, 715–28. [Google Scholar] [CrossRef]
- Fernández-Cruz, L.; Blanco, L.; Cosa, R.; et al. Is laparoscopic resection adequate in patients with neuroendocrine pancreatic tumors? World J Surg. 2008, 32, 904–917. [Google Scholar] [CrossRef]
- Dimitrios, X.; Tavakkoli, A.; Clancy, T.E.; et al. 2015. Distal pancreatic resection for neuroendocrine tumors: is laparoscopic really better than open? J Gastrointest. Surg. 2015, 19, 831–840. [Google Scholar]
- Han, S.H.; Han, I.W.; Heo, J.S.; et al. Laparoscopic versus open distal pancreatectomy for nonfunctioning pancreatic neuroendocrine tumors: a large single-center study. Surg Endosc. 2018, 32, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Casadei, R.; Ricci, C.; D’Ambra, M.; et al. Laparoscopic versus open distal pancreatectomy in pancreatic tumours: a case–control study. Updates in surgery 2010, 62, 171–174. [Google Scholar] [CrossRef] [PubMed]
- Hallet, J.; Law, C.H.; Cukier, M.; et al. Exploring the rising incidence of neuroendocrine tumors: a population-based analysis of epidemiology, metastatic presentation, and outcomes. Cancer 2015, 121, 589–597. [Google Scholar] [CrossRef] [PubMed]
- Capella, C.; Heitz, P.U.; Hofler, H.; et al. Revised classification of neuroendocrine tumours of the lung, pancreas and gut. Virchows Arch 1995, 425. [Google Scholar] [CrossRef]
- Gabriel, M.; Decristoforo, C.; Kendler, D.; et al. 68Ga-DOTA-Tyr3-octreotide PET in neuroendocrine tumors: comparison with somatostatin receptor scintigraphy and CT. J Nucl Med 2007, 48. [Google Scholar] [CrossRef]
- Yao, J.C.; Hassan, M.; Phan, A. One hundred years after "carcinoid": epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. Journal of clinical oncology 2008, 26, 3063–3072. [Google Scholar] [CrossRef]
- Isla, A.; Arbuckle, J.D.; Kekis, P.B.; et al. : Laparoscopic management of insulinomas. Br J Surg 2009, 96, 185–190. [Google Scholar] [CrossRef]
- Benzing, C.; Timmermann, L.; Winklmann, T.; et al. Robotic versus open pancreatic surgery: a propensity score-matched cost-effectiveness analysis. Langenbecks Arch Surg. 2022, 407, 1923–1933. [Google Scholar] [CrossRef]
- Cauley, C.E.; Pitt, H.A.; Ziegler, K.M.; et al. Pancreatic enucleation: improved outcomes compared to resection. J. Gastrointest Surg 2012, 16, 1347–1353. [Google Scholar] [CrossRef]
- Pitt, S.C.; Pitt, H.A.; Baker, M.S.; et al. Small pancreatic and periampullary neuroendocrine tumors: resect or enucleate? J Gastrointest Surg 2009, 13, 1692–1698. [Google Scholar] [CrossRef] [PubMed]
- Bartsch, D.K.; Langer, P.; Wild, A.; et al. Pancreaticoduodenectomy (PD) versus pancreatic segmental resection (PSR) in the surgical treatment of pancreatic ductal adenocarcinoma (PDAC) - a single center experience. Pancreatology. 2007, 7, 440–446. [Google Scholar]
- Zerbi, A.; Capretti, G.; Napoli, N.; et al. The Italian National Registry for minimally invasive pancreatic surgery: an initiative of the Italian Group of Minimally Invasive Pancreas Surgery (IGoMIPS). Updates Surg. 2020, 72, 379–385. [Google Scholar] [CrossRef] [PubMed]
- Asbun, H.J.; Moekotte, A.L.; Vissers, F.L. , et al; International Study Group on Minimally Invasive Pancreas Surgery (I-MIPS). The Miami International Evidence-based Guidelines on Minimally Invasive Pancreas Resection. Ann Surg. 2020, 271, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Isla, A.; Griffith, P.S.; Markogiannakis, H.; et al. A novel laparoscopic approach to lesions related to the posterior aspect of the pancreatic head. Am J Surg 2009, 197, e51–e53. [Google Scholar] [CrossRef] [PubMed]
- Fendrich, V.; Waldmann, J.; Bartsch, D.K.; et al. Surgical management of pancreatic endocrine tumors. Nat Rev 2009, 6, 419–428. [Google Scholar] [CrossRef] [PubMed]
- Rothmund, M.; Angelini, L.; Brunt, L.M.; et al. : Surgery for benign insulinoma: An international review. World J Surg 1990, 14, 393–398. [Google Scholar] [CrossRef]
- Zerbi, A.; Ortolano, E.; Balzano, G.; et al. Pancreatic metastasis from renal cell carcinoma: which patients benefit from surgical resection? Annals of Surgical Oncology 2010, 17, 1631–1638. [Google Scholar] [CrossRef]
- Beane, J.D.; Borrebach, J.D.; Billderback, A.; et al. Small pancreatic neuroendocrine tumors: Resect or enucleate? Am J Surg. 2021, 222, 29–34. [Google Scholar] [CrossRef]
- Ricci, C.; Casadei, R.; Taffurelli, G.; et al. Laparoscopic distal pancreatectomy: what factors are related to the learning curve? Lessons from a single surgical team experience. Updates in Surgery 2019, 71, 91–98. [Google Scholar]
- Zureikat, A.H.; Postlewait, L.M.; Liu, Y.; et al. A multi-institutional comparison of perioperative outcomes of robotic and open pancreaticoduodenectomy. Annals of Surgery 2016, 264, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Croome, K.P.; Farnell, M.B.; Que, F.G.; et al. Total laparoscopic pancreaticoduodenectomy for pancreatic ductal adenocarcinoma: oncologic advantages over open approaches? Annals of Surgery 2014, 260, 633–638. [Google Scholar] [CrossRef] [PubMed]
- Khaled, Y.S.; Ammori, B.J.; Elkhatib, I. Laparoscopic pancreaticoduodenectomy: a systematic review. Surgical Endoscopy 2014, 28, 1783–1792. [Google Scholar]
- Ore, A.S.; Barrows, C.E.; Solis-Velasco, M.; et al. Robotic enucleation of benign pancreatic tumors. J Vis Surg. 2017, 3, 151. [Google Scholar] [CrossRef] [PubMed]
- Chin, K.M.; Goh, B.K.P. Robotic enucleation of a pancreatic uncinate neuroendocrine tumor - a unique parenchyma-saving strategy for uncinate tumors. Ann Hepatobiliary Pancreat Surg. 2020, 24, 97–103. [Google Scholar] [CrossRef] [PubMed]
- Clavien, P.A.; Barkun, J.; de Oliveira, M.L.; et al. The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg. 2009, 250, 187–196. [Google Scholar] [CrossRef]
- Bassi, C.; Dervenis, C.; Butturini, G.; et al. Postoperative pancreatic fistula: an international study group (ISGPF) definition. Surgery 2005, 138, 8–13. [Google Scholar] [CrossRef]
| Sex | Ages (years) | N. Center | |||||
|---|---|---|---|---|---|---|---|
| Author | N. Pts | M | F | Mean Age | max | min | |
| Fernandez-Cruz | 49 | 6 | 43 | 58 | 83 | 22 | 1 |
| Casadei | 44 | 8 | 36 | 60.5 | 77 | 42 | 1 |
| Zerbi | 310 | 166 | 144 | 57.6 | 24 | ||
| Zhao | 328 | 139 | 189 | 42.3 | 57 | 27 | 1 |
| Haugvik | 65 | 27 | 38 | 57 | 87 | 21 | 1 |
| Mehrabi | 6222 | 2551 | 3671 | 53 | 72 | 27 | 114 |
| Xourafas | 171 | 89 | 82 | 61.5 | 95 | 20 | 1 |
| Jilesen | 205 | 93 | 112 | 52.6 | 68 | 34 | 2 |
| Han | 94 | 55 | 39 | 53.5 | 75 | 30 | 1 |
| Mihalache | 18 | 6 | 12 | 53 | 69 | 28 | 1 |
| Zhang | 1020 | 464 | 556 | 58 | 66 | 48 | 9 |
| Sutton | 282 | 142 | 140 | 59 | 77 | 42 | 4 |
| Year | Author | Country | Type of study | N. Center | NOS | MINORS | ||
| Selection | Comparability | Outcame/Exposure | ||||||
| 2008 | Fernandez-Cruz | Spain | ProS - SC | 1 | ** | * | ** | 15 |
| 2010 | Casadei | Italy | ProS - SC | 1 | *** | ** | *** | 22 |
| 2011 | Zerbi | Italy | ProS - MC | 24 | *** | * | ** | 15 |
| 2011 | Zhao | China | RetS - SC | 1 | *** | ** | ** | 18 |
| 2013 | Haugvik | Norway | RetS - SC | 1 | ** | ** | ** | 16 |
| 2014 | Mehrabi | Germany | RetS - DB | 114 | *** | ** | *** | 20 |
| 2015 | Xourafas | USA | RetS - SC | 1 | ** | ** | *** | 18 |
| 2015 | Jilesen | Nederlands | RetS - MC | 2 | *** | ** | *** | 19 |
| 2017 | Han | Korea | RetS - SC | 1 | *** | ** | *** | 20 |
| 2019 | Mihalache | Romania | RetS - SC | 1 | ** | * | ** | 16 |
| 2019 | Zhang | China | RetS - MC | 9 | *** | ** | *** | 19 |
| 2022 | Sutton | USA | RetS - MC | 4 | *** | * | *** | 18 |
| Enucleation | Splenodistalpancreatectomy | Pancreaticoduodenectomies | Central Pancreatectomy | Total Pancreatectomies | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author | Overall | Open | Lap | CR | Overall | Open | Lap | CR | Overall | Open | LAP | CR | Overall | Open | Lap | CR | Overall | Open | Lap | CR |
| Fernandez-Cruz | 22 | 0 | 21 | 1 | 26 | 0 | 23 | 3 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Casadei | \ | \ | \ | \ | 44 | 22 | 22 | 0 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Zerbi | 50 | \ | \ | \ | 114 | \ | \ | \ | 55 | \ | \ | \ | 16 | \ | \ | \ | 12 | \ | \ | \ |
| Zhao | 229 | 199 | 18 | 12 | 53 | 37 | 9 | 7 | 3 | 3 | 0 | 0 | 15 | 15 | 0 | 0 | \ | \ | \ | \ |
| Haugvik | 16 | 0 | 14 | 2 | 53 | 0 | 51 | 2 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Mehrabi | 2866 | 2677 | 189 | 1603 | 1506 | 97 | 140 | 139 | 1 | 0 | 19 | 19 | 0 | 0 | 29 | 29 | 0 | 0 | ||
| Xourafas | \ | \ | \ | \ | 171 | 98 | 73 | 0 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Jilesen | 60 | 49 | 6 | 5 | 72 | 55 | 9 | 8 | 65 | 65 | 0 | 0 | 8 | 8 | 0 | 0 | \ | \ | \ | \ |
| Han | \ | \ | \ | \ | 94 | 52 | 42 | 0 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Mihalache | 10 | 7 | 3 | 0 | 8 | 8 | 0 | 0 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Zhang | 107 | 85 | 22 | 0 | 576 | 362 | 214 | 0 | 288 | 286 | 2 | 0 | 32 | 31 | 1 | 0 | 17 | 17 | 0 | 0 |
| Sutton | 13 | 3 | 10 | 0 | 184 | 75 | 98 | 11 | 77 | 54 | 14 | 9 | \ | \ | \ | \ | 8 | 7 | 1 | 1 |
| Pancreatic fistula | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Author Ref. | OS | CR | Lap | EN | DP | PD | ||||||
| Overall | A | B | C | Overall | A | B | C | Overall | ||||
| Fernandez-Cruz | \ | \ | \ | 8 | 4 | 3 | 1 | 2 | 2 | \ | \ | \ |
| Casadei | 4 | 0 | 2 | \ | \ | \ | \ | 6 | \ | \ | \ | \ |
| Zerbi | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Zhao | 112 | 9 | 11 | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Haugvik | \ | \ | \ | 7 | 1 | 6 | 0 | 7 | 0 | 7 | 0 | \ |
| Mehrabi | 698 | 0 | 21 | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Xourafas | 32 | 16 | 0 | \ | \ | \ | \ | 48 | 30 | 16 | 2 | \ |
| Jilesen | \ | \ | \ | 19 | \ | \ | \ | 7 | \ | \ | \ | 9 |
| Han | 26 | 0 | 29 | \ | \ | \ | \ | 2 | 43 | 11 | 1 | \ |
| Mihalache | \ | \ | \ | 2 | \ | \ | \ | 2 | \ | \ | \ | \ |
| Zhang | 97 | 0 | 52 | \ | \ | \ | \ | 149 | 88 | 57 | 4 | \ |
| Sutton | 20 | 0 | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| LHS | BLOOD LOOSES | ||||||||||||||
| Author | Open | CR | Laparoscopy | EN | DP | PD | Open | Laparoscopy | EN | DP | PD | ||||
| Median | Max | Min | Median | Max | Min | Median | Median | Median | Median mL | Median mL | Median mL | Median mL | Overall | ||
| Fernandez-Cruz | \ | \ | \ | \ | \ | \ | \ | 5.5 | 6.7 | \ | \ | < 220 | < 220 | \ | \ |
| Casadei | 11 | 14 | 8 | \ | 8 | 9.3 | 6.7 | \ | 9.5 | \ | \ | \ | \ | \ | \ |
| Zerbi | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Zhao | 21.2 | 38.5 | 3.9 | 27.8 | 15.1 | 22.9 | 7.9 | \ | \ | \ | 163.6 | 124.8 | \ | \ | \ |
| Haugvik | \ | \ | \ | \ | 7 | 27 | 2 | 8 | 6.5 | \ | \ | 300 | 500 | 300 | \ |
| Mehrabi | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Xourafas | 7 | 39 | 4 | \ | 5 | 18 | 3 | \ | 6 | \ | \ | \ | \ | \ | \ |
| Jilesen | \ | \ | \ | \ | \ | \ | \ | 21 | 11 | 15 | \ | \ | \ | \ | 7 |
| Han | 9 | 66 | 7 | \ | 7 | 18 | 4 | \ | 8 | \ | \ | \ | \ | \ | \ |
| Mihalache | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
| Zhang | 7 | 9 | 5 | \ | 4 | 6 | 4 | \ | 6 | \ | 300 | 100 | \ | 200 | \ |
| Sutton | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ | \ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





