1. Introduction
The gram-negative pathogen that causes Q fever,
Coxiella burnetii, is found almost everywhere in the world. It is a zoonotic pathogen that, in addition to sheep, goats, cattle and humans, has been detected in many other animal species, such as various rodents, wild ruminants, cats, dogs, horses, pigs, marsupials, reptiles, birds and arthropods, especially ticks [
1,
2]. Although wild rodents are an important reservoir for the pathogen, domestic ruminants (sheep, goats and cattle) are the most common source of human infection [
3]. The main route of infection for humans and animals is via inhalation of contaminated aerosols [
4,
5]. There is a particular risk of infection of humans, cattle and other susceptible animals at the time of parturition of an infected animal due to the massive shedding of the pathogen with the amniotic fluid and the placental membranes. However,
Coxiella burnetii is also excreted by infected animals in faeces, urine, semen and milk [
6]. In cattle,
C. burnetii can lead to increased abortions, premature or stillbirths and weak calves [
7,
8]. However, there is no solid evidence to support the hypothesis that
C. burnetii causes disorders such as subfertility, endometritis/metritis, or retained fetal membranes. There may be an association between
C. burnetii and subclinical mastitis in dairy cattle [
9]. Since 2010, it is possible to vaccinate cows against Q fever with the vaccine COXEVAC
® (Ceva Santé Animale). Vaccination did not significantly prevent shedding, but was significantly associated with lower bacterial load shed, thus reducing the bacterial load generated in the environment [
10]. Hypothetically, a lower infection rate could diminish the occurence of diseases and subsequently the necessity of antibiotic treatments. It was the aim of this study to examine whether the usage of the vaccine COXEVAC® could reduce the consumption of antibiotics in Q-fever-positive dairy farms. Additionally, the effects of other herd-level factors on the consumption of antibiotics were investigated.
2. Materials and Methods
Farms and animals
In this longitudinal retrospective cohort study, 49 dairy farms from Northern Germany participated. Participation in the study was on a voluntary basis. The study farms were recruited in collaboration with nine veterinary practices that routinely treat all types of animals. Data were collected from the first quarter of 2012 to the last quarter of 2018. All participating farms were located in the federal state of Lower Saxony, north of Bremen and had at least 40 cows in lactation. Samples for Q fever were collected by the local veterinarians because of non-specific illness symptoms or suspicion of Q fever before the beginning of the study. Most of these samples were analysed in the laboratory of the Institute for Animal Health of LUFA Nord-West in Oldenburg. The decision to vaccinate against Q fever was made by the local veterinarians and the owners of the dairy farms independently of participation in the study. Requirements for participation in the study were detection of Coxiella-burnetii-antigen by PCR (in fetus, placenta, cervix swabs or milk) or detection of Coxiella-burnetii-antibodies in serum or in the individual milk sample of at least one cow in the herd. The average herd size (all cattle) was 330 animals and ranged between 105 and 1130 animals. The average number of lactating cows was 149 and ranged between 49 and 520 animals. The average milk yield was 8717 kg and ranged between 5715 and 11363 kg. Forty-five farms (94.4%) used an upgraded mixed ration with separate supplementation of concentrates for the cows. Four farms (5.5%) fed a total mixed ration. All farms with the exception of one operation housed the lactating cows in freestall barns with cubicles. One farm had a tied stall. Twenty-two vaccinated and two non-vaccinated farms allowed lactating cows access to pasture in summer. On 25 farms, the animals were kept indoors all year round (14 vaccinated farms and 11 non-vaccinated farms).
Study design
A total of 36 Q fever vaccinated and 13 non-vaccinated dairy farms participated in the study. In 28 farms (77.7%), all animals were vaccinated with COXEVAC
® from the age of 3 months (basic immunization: two vaccinations three weeks apart). Eigth farms (22.3%) vaccinated cows only. 29 farms (80.6%) carried out a booster vaccination every 9 months. The study period for each farm lasted three years. For farms with vaccination, timepoint 0 (t
0) was the day when farms finished the basic immunization with COXEVAC
®. For farms without vaccination, t
0 was four weeks after a positive diagnosis of
C. burnetii. Period one (Period 1 = t
-1 – t
0) was the year before t
0. Period two (Period 2 = t
0 – t
+1) and three (Period 3 = t
+1 – t
+2) spanned the first and second year after t
0. The consumption of antibiotics for each farm was recorded by means of the obligatory veterinary documentation of the application and delivery of drugs. To gather detailed information about herd data, nutrition, milking management, housing and animal health, the farmers were interviewed by means of a questionnaire. The principles of good agricultural practice and findings with scientific consensus served as the basis for the selection of categories and questions for the farm questionnaire [
11]. The mean therapy frequency per cow and farm (MTF) was used to compare the antibiotic consumption of the farms with and without vaccination. The MTF specifies how many days one cow (lactation number ≥ 1) in a herd is treated with one active ingredient on average [
12]. To calculate the MTF, the number of used daily doses (=number of treated cows x number of treatment days x number of active ingredients) is divided through the number of cows kept per farm per time period. The initial statistical analysis was performed using SAS Enterprise Guide 7.1[
13]. First, descriptive statistics were performed for all questionnaire variables and the MTF. For the categorical variables, a simple frequency determination (FREQ procedure) was performed. For continuous variables, the mean and the standard deviation were calculated (PROC MEANS). The p-values were then calculated using a t-test (PROC TTEST) for normally distributed variables. For non-normally distributed variables non-parametric simple ANOVA (PROC NPAR1WAY) was used. For categorical variables, the Chi-square test or Fisher's exact test (PROC FREQ) was used. A significance level α=0.05 was chosen. Linear mixed models were calculated using R Core Team with the aim of identifying factors that influenced MTF [
14]. In the final model, the number of variables was reduced using stepwise backward variable selection based on the Akaike Information Criterion (AIC).
Selected variables after using AIC:
3. Results
All farms were asked which main problem they had at t
0. 63.8% of the vaccinated farms stated that they had problems with herd fertility, whereas only 38.4% of the control farms complained about this
(p = 0.1123). Udder health problems were reported by 16.6% of the vaccinated farm and 7.6% of the non-vaccinated farms (
p = 0.6577). Digital dermatitis was the most common claw disease (vaccinated farms: 63.8%; non-vaccinated farms: 92.3%,
p = 0.2379).
Table 1 shows contact of lactating animals with other animal species.
3.1. Mean Therapy Frequency per Cow and Farm (MTF)
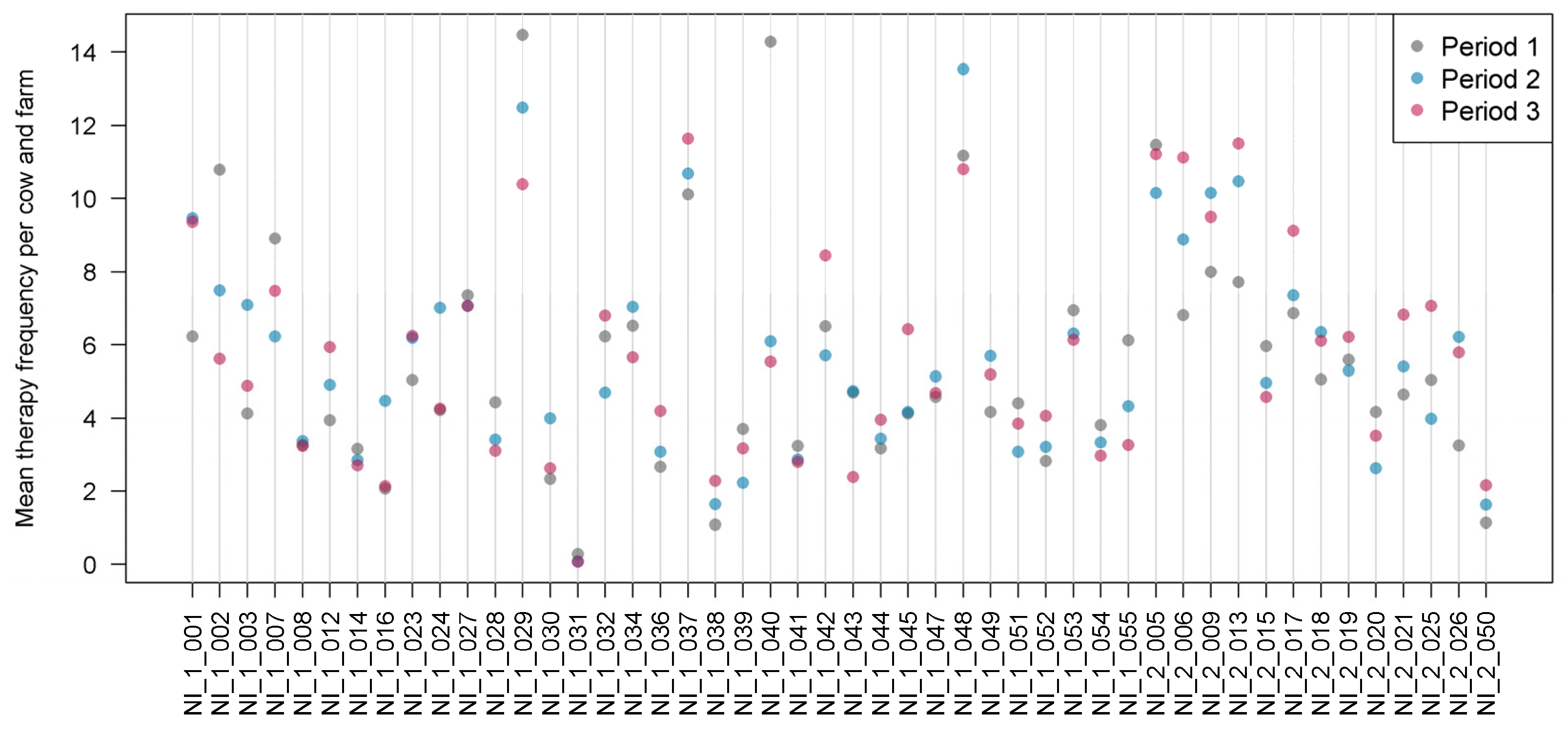
MTF revealed considerable fluctuations between the individual farms and time periods (
Figure 1).
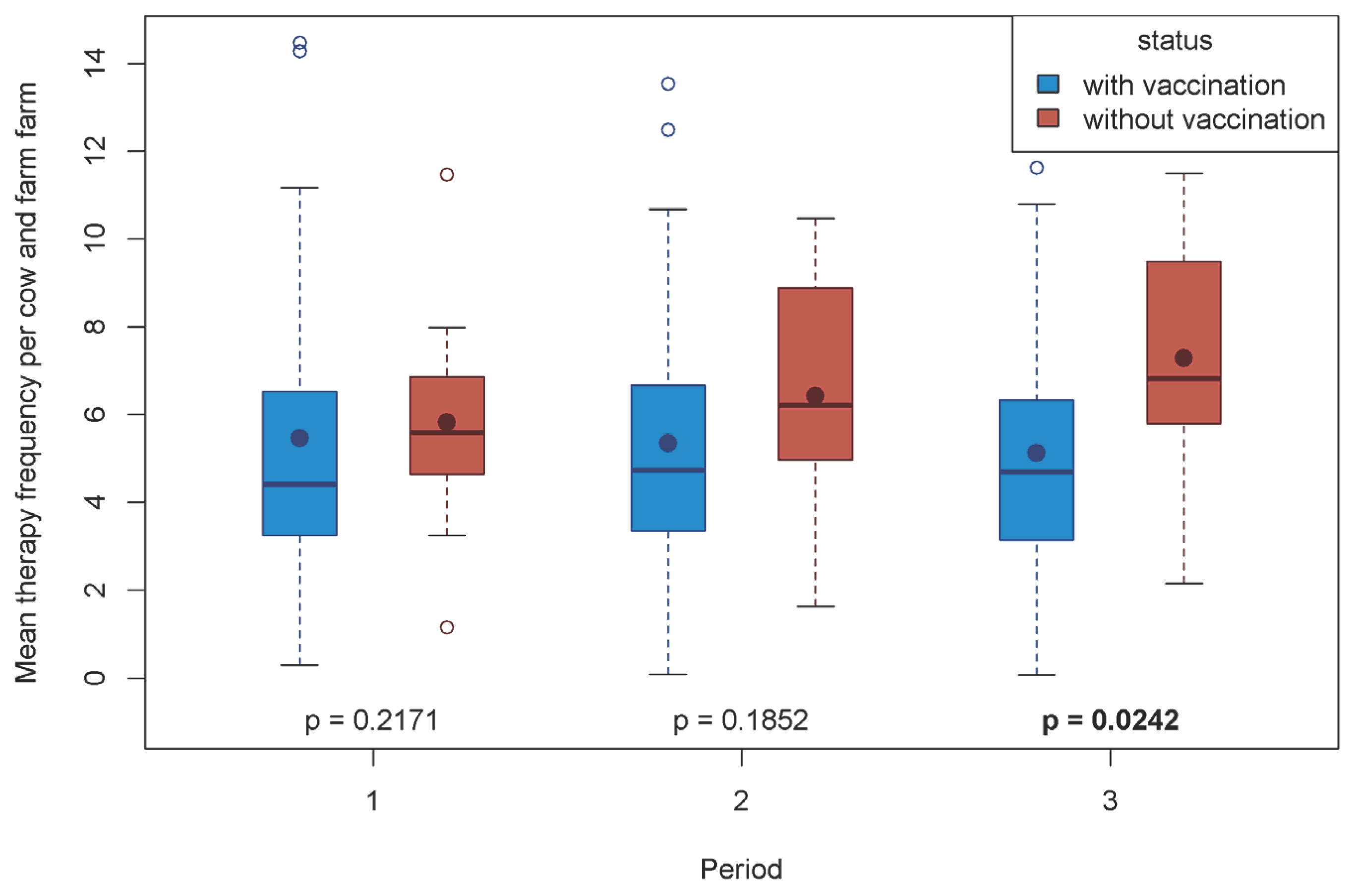
Over all herds and time periods, MTF ranged between 5.1 and 7.3 treatment days per cow and farm. Mean therapy frequency per cow and farm at the three time periods was compared between the Q fever vaccinated and non-vaccinated farms (
Figure 2). In Period 1, MTF did not differ significantly between the study groups. In the non-vaccinated group, MTF continually increased over time, whereas in the vaccinated group MTF slightly decreased. In Period 3, the MTF of the non-vaccinated group was statistically significantly higher than the MTF of the vaccinated group.
3.2. Risk Factors Influencing the Mean Therapy Frequency per Cow and Farm
Table 2 shows the descriptive statistics of the herd-level risk factors selected for the final linear mixed model.
The results of the final linear mixed model (
Table 3) revealed that the annual herd milk yield did not influence the consumption of antibiotics in the study herds. In Period 3, non vaccinated herds had a statistically significantly higher MTF than vaccinated herds. Grooved slatted floor (
p < 0.1) and rubber floor (
p < 0.05) were associated with a lower MTF. Udder cluster disinfection while milking was associated with a higher MTF.
4. Discussion
The aim of the study was to analyse the relationship between Q fever vaccination (COXEVAC®) and the consumption of antibiotics on dairy farms. Therefore, the MTF was compared between farms with and without vaccination. Over all herds and time periods, MTF ranged between 5.1 and 7.3 treatment days per cow and farm. Data of antibiotic consumption quantities are essential to monitor the impact of antimicrobial use reduction strategies in animal health [
15]. Therapy frequency as an assessment parameter for antibiotic consumption on farms is a common measure [
10,
11]. It indicates the average number of days an animal in a flock was treated with antibiotics during a given observation period [
16]. This parameter is also used in the German Medicinal Products Act. The project "VetCAb -Veterinary Consumption of Antibiotics" also recorded treatment frequencies in dairy cows for the years 2011 to 2014 [
17]. The VetCAb-project was initiated by the German Federal Institute for Risk Assessment (BfR). In contrast to the present study with VetCAb, the treatment frequency was assessed every six months rather than 12 months. In addition in VetCAb, it was not the average number of cows kept per period that was taken into account, but rather the number of cubicles of the stables from the farms. In the VetCAb-project, the half-yearly treatment frequency for dairy cows was between 2.2 and 1.3. At 5.1 to 7.3, the MTF in the present study, was higher than in the VetCAb project. This difference is possibly related to the selection of the study farms. In the VetCAb-project, the selected farms took part in the survey on a voluntary basis and it is likely that most of these farms were already aware of the responsible use of antibiotics. On the other hand, farms that expected a high level of use may have been reluctant to participate. As the focus of this study was on the effect of Q fever vaccination, the selection criteria were different from those used in the VetCAb-project. In addition, the current farms already had health problems. This could explain the higher MTF on the participating farms compared to the population of dairy farms in Germany. Furthermore, seasonal differences may have had a greater impact in a 6-month evaluation period than in a 12-month evaluation period.
On the impact of MTF on antibiotic consumption, there was an increase in MTF from 5.82 to 7.28 on non-vaccinated farms and a decrease from 5.42 to 5.11 treatment days on the Q fever vaccinated farms. The difference in MTF between vaccinated and non-vaccinated farms, therefore, suggests that vaccination did indeed have an effect on antibiotic consumption. This is in line with the findings of [
17], who found reduced pathogen shedding and consequently a lower rate of new infections after Q fever vaccination. In the study of [
18], the reactions of the livestock owners to the effects of vaccination with COXEVAC® were almost all positive. In 84 % of the cases, the owners noticed a clear improvement in the health problems that had previously occurred in their herds. The health problems that led to vaccination in the study of [
18] were probably similar to the reasons that led to the decision to test herds for Q fever and the decision to vaccinate herds in the present study. In the study of [
18] the reasons were described in detail. These reasons included increased abortion in 67% of farms and fertility problems in 62% of farms. In addition, there was a sudden drop in milk yield (55%), increased incidence of pneumonia (51%) and non-specific infections or weakness with recurrent fevers (48%). In more than 20% of the farms, there was an increased incidence of births of sick and weak calves. These results support the hypothesis of the present study, namely that the use of antibiotics is reduced due to fewer clinical diseases. It cannot be excluded that, in addition to the pathogen-specific effect, there is also a non-specific immunostimulatory effect of vaccination. In this case, the possible
Coxiella-related immunosuppression, similar to that known in human patients [
19], is less severe. It should be stressed again that the MTF in the period t
-1 to t
0 is equally high in both study groups (5.42 to 5.82). This indicates a similar health status and similar treatment management at time t
0. This factor can be considered positive for the comparability of the farms. In the non-vaccinated farms, the lack of vaccination favours an increasing spread of
C. burnetii and the rate of new infections is increased, thus increasing the MTF in the non-vaccinated farms. As the infection cycle of
C. burnetii tends to take years, as described by [
20], the MTF could also slowly increase as a result.
In addition to vaccination, two other factors influencing MTF were identified in the linear mixed model: the type of floor in the alleyways and the use of udder cluster disinfection while milking. It was found that the presence of a rubber coating on the slatted floor significantly decreased MTF. However, as only four farms used this type of flooring, it was not possible to calculate an interaction estimate and the result should be treated with caution. Nevertheless, the slatted floor also showed a reduced treatment frequency compared to the reference of a concrete floor. On a grooved slatted floor the effect was even greater. In conclusion, the type of floor is an important factor influencing MTF. This is in line with studies by [
21,
22,
23], who found better claw health in animals housed on rubber floors. A concrete floor is considered to be more difficult to keep clean. Frequently used scraping systems create a wave of manure that cows often have to wade through. This causes constant stress to the horn and skin of the foot and allows infectious claw diseases to establish [
23]. One of the most common infectious claw diseases is digital dermatitis, which is often treated with antibiotic sprays [
23]. Poor udder cleanliness and probably also poor leg cleanliness were associated with lameness [
24]. It is likely that slatted floor can improve the leg cleanliness. As a result, the risk of infectious claw diseases was reduced. This could lead to a lower MTF compared to concrete floors.
On the other hand, the parameter udder cluster disinfection while milking had a strong increasing influence on the MTF. This management tool to reduce udder infections while milking has been studied for many years [
25]. Recent studies show that the use of peracetic acid based udder cluster disinfection can lead to a reduction in teat canal colonization [
26]. In automatic milking systems (AMS), interim disinfection is state of the art and is performed fully automatically after each milking. The cleaning and disinfection methods used in AMS are mainly steam disinfection or the use of peracetic acid [
27]. Contrary to expectations, the final model revealed that farms using intermediate disinfection had an increased MTF. It is assumed that these farms have a general udder health problem and that the intermediate udder cluster disinfection was introduced as a prophylactic measure to minimise new infections during milking. Conversely, farmers with good udder health do not go to the extra trouble of intermediate disinfection. Therefore, the higher mean therapy frequency per cow on farms using udder cluster disinfection while milking may be related to a mastitis problem.
In conclusion, it seems likely that vaccination has a positive effect on herd health. Limitations to the generalisability of the study results arise from the sample size, in particular the overrepresentation of vaccinated farms. A larger sample size would be desirable for further studies, especially for the control group. In addition, it would be interesting to follow the farms with ongoing booster vaccination for a longer period of time. Another way to determine the effect of vaccination is to divide a whole herd into vaccinated and control animals. All farm-specific factors would then be standardised. However, a persistent circulation of the pathogen through the control animals would possibly have a confounding effect, so that there would be a difference to a fully vaccinated herd.
5. Conclusions
The present results suggest that there may be an association between the vaccination against Q fever (COXEVAC®) and a reduced consumption of antibiotics. Further studies are needed to clarify the cause-effect relationship between vaccination and the consumption of antibiotics on dairy farms. The variables floor type and udder cluster disinfection while milking were shown to be important factors influencing the therapy frequency in Q-fever-positive dairy farms in Northern Germany.
Author Contributions
Conceptualization, N.H. and M.H.; methodology, N.H. and M.H.; software, N.H.,N.G., M.T. and U.L.; validation, N.H., N.G. and M.H.; formal analysis, N.H., M.T. and U.L.; investigation, N.H..; resources, M.H.; data curation, N.H., M.T. and U.L.; writing—original draft preparation, N.H. and N.G.; writing—review and editing, N.H., N.G., M.T., U.L. and M.H.; visualization, N.H., M.T. and U.L.; supervision, M.H.; project administration, N.G. and M.H.; funding acquisition, N.G. All authors have read and agreed to the published version of the manuscript.
Funding
This Open Access publication was funded by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation)—491094227 “Open Access Publication Costs” and the University of Veterinary Medicine Hannover, Foundation.
Institutional Review Board Statement
Ethical review and approval was not required for this animal study. The reason was that this study did not contain animal experiments, which required any approval by the animal health and welfare authorities.
Informed Consent Statement
Informed consent statement was obtained from the owners of the farms to use data of their farm.
Data Availability Statement
Data available on request to authors.
Acknowledgments
We would like to thank the participating farmers and their families, without whom this study would not have been possible.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Angelakis, E.; Raoult, D. Emergence of Q Fever. Iran J Public Health 2011, 40, 1–18. [Google Scholar] [PubMed]
- Schleenvoigt, B. T.; Sprague, L. D.; Mertens, K.; Moog, U.; Schmoock, G.; Wolf, G.; Neumann, M.; Pletz, M. W.; Neubauer, H. Acute Q Fever Infection in Thuringia, Germany, after Burial of Roe Deer Fawn Cadavers (Capreolus Capreolus): A Case Report. New Microbes New Infect 2015, 8, 19–20. [Google Scholar] [CrossRef] [PubMed]
- Maurin, M.; Raoult, D. Q Fever. Clin Microbiol Rev 1999, 12, 518–53. [Google Scholar] [CrossRef]
- Brooke, R. J.; Kretzschmar, M. E.; Mutters, N. T.; Teunis, P. F. Human Dose Response Relation for Airborne Exposure to Coxiella Burnetii. BMC Infect Dis 2013, 13, 488. [Google Scholar] [CrossRef]
- Todkill, D.; Fowler, T.; Hawker, J. I. Estimating the Incubation Period of Acute Q Fever, a Systematic Review. Epidemiol Infect 2018, 146, 665–72. [Google Scholar] [CrossRef] [PubMed]
- Fournier, P. E.; Marrie, T. J.; Raoult, D. Diagnosis of Q Fever. J Clin Microbiol 1998, 36, 1823–34. [Google Scholar] [CrossRef]
- Marrie, T. J. Q Fever - a Review. Can Vet J 1990, 31, 555–63. [Google Scholar] [PubMed]
- Barlow, J.; Rauch, B.; Welcome, F.; Kim, S. G.; Dubovi, E.; Schukken, Y. Association between Coxiella Burnetii Shedding in Milk and Subclinical Mastitis in Dairy Cattle. Vet Res 2008, 39, 23. [Google Scholar] [CrossRef]
- Agerholm, J. S. Coxiella Burnetii Associated Reproductive Disorders in Domestic Animals-a Critical Review. Acta Vet Scand 2013, 55, 13. [Google Scholar] [CrossRef]
- Taurel, A. F.; Guatteo, R.; Lehebel, A.; Joly, A.; Beaudeau, F. Vaccination Using Phase I Vaccine Is Effective to Control Coxiella Burnetii Shedding in Infected Dairy Cattle Herds. Comp Immunol Microbiol Infect Dis 2014, 37, 1–9. [Google Scholar] [CrossRef]
- De Kruif, A.; Mansfeld, R.; Hoedemaker, M. Tierärztliche Bestandsbetreuung Beim Milchrind., 3rd ed.; Enke Verlag: Stuttgart, Germany, 2014. [Google Scholar]
- van Rennings, L.; Merle, R.; von Münchhausen, C.; Stahl, J.; Honscha, W.; Käsbohrer, A.; Kreienbrock, L. Variables Describing the Use of Antibiotics in Food-Producing Animals. Berl. Munch Tierarztl. Wochenschr. 2013, 126, 297–309. [Google Scholar] [PubMed]
- 13. Statistical Analysis Software (Sas Enterprise Guide©, Version 7.1).
-
R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/, 2023.
- Martin, H.; Gribben, L.; Regan, A.; Manzanilla, E. G.; McAloon, C. G.; Burrell, A. M. Recording Antimicrobial Use on Irish Dairy Farms: Barriers and Facilitators to Using Technology and Sharing Data. J Dairy Sci 2024, TBC. in press. [Google Scholar] [CrossRef] [PubMed]
- Merle, R.; Mollenhauer, Y.; Hajek, P.; Robanus, M.; Hegger-Gravenhorst, C.; Honscha, W.; Käsbohrer, A.; Kreienbrock, L. "Verbrauchsmengenerfassung Von Antibiotika Beim Rind in Landwirtschaftlichen Betrieben." In Berl. Munch. Tierarztl. Wochenschr., 318-25, 2013.
- Hemme, M.; van Rennings, L.; Hartmann, M.; von Münchhausen, C.; Käsbohrer, A.; Kreienbrock, L. Antibiotikaeinsatz in Der Nutztierhaltung in Deutschland: Erste Ergebnisse Zu Zeitlichen Trends Im Wissenschaftlichen Projekt „Vetcab-Sentinel“. Dtsch. Tierärztebl. 2016, 4, 516–20. [Google Scholar]
- Lehner, S.; Lohan, K.; Dieckhoff, H. J.; Gerdes, U. Effects of Vaccination against Q-Fever in Lower Saxony Dairy Cattle Farms. Tierarztl Prax Ausg G Grosstiere Nutztiere 2017, 45, 141–49. [Google Scholar] [CrossRef] [PubMed]
- Eldin, C.; Mélenotte, C.; Mediannikov, O.; Ghigo, E.; Million, M.; Edouard, S.; Mege, J. L.; Maurin, M.; Raoult, D. From Q Fever to Infection: A Paradigm Change. Clin. Microbiol. Rev. 2017, 30, 115–90. [Google Scholar] [CrossRef] [PubMed]
- Motsch, B.; Alves, C.; Janowetz, B.; Werth, S.; Deckinger, E.; Böttcher, J. Der Coxiellen-Kreislauf in Milchviehbeständen. Paper presented at the 11. Berlin-Brandenburgischer Rindertag, 07.-08. Oktober, Berlin, Germany; 2016. [Google Scholar]
- Blowey, R. Factors Associated with Lameness in Dairy Cattle. In Practice 2005, 27, 154–62. [Google Scholar] [CrossRef]
- Vanegas, J.; Overton, M.; Berry, S. L.; Sischo, W. M. Effect of Rubber Flooring on Claw Health in Lactating Dairy Cows Housed in Free-Stall Barns. J. Dairy Sci. 2006, 89, 4251–58. [Google Scholar] [CrossRef]
- Ahrens, F.; Platz, S.; Link, C.; Mahling, M.; Meyer, H. H.; Erhard, M. H. Changes in Hoof Health and Animal Hygiene in a Dairy Herd after Covering Concrete Slatted Floor with Slatted Rubber Mats: A Case Study. J Dairy Sci 2011, 94, 2341–50. [Google Scholar] [CrossRef]
- Oehm, A. W.; Merle, R.; Tautenhahn, A.; Jensen, K. C.; Mueller, K. E.; Feist, M.; Zablotski, Y. Identifying Cow - Level Factors and Farm Characteristics Associated with Locomotion Scores in Dairy Cows Using Cumulative Link Mixed Models. PLoS One 2022, 17, e0263294. [Google Scholar] [CrossRef]
- Scheib, S.; Leimbach, S.; Avramidis, G.; Bellmann, M.; Nitz, J.; Ochs, C.; Tellen, A.; Wente, N.; Zhang, Y.; Viol, W.; Krömker, V. Intermediate Cluster Disinfection: Which Disinfection Solution Is Most Effective on Milking Liners? A Comparison of Microorganism Reduction on Liner Inner Surfaces Using Quantitative Swab Sampling Technique. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Tenhagen, B. A.; Heuwieser, W. Cluster Disinfection to Reduce New Intramammary Infections in Lactating Dairy Cattle. Tierarztl. Umsch. 2007, 62, 364–68. [Google Scholar]
- Hovinen, M.; Pyorala, S. Invited Review: Udder Health of Dairy Cows in Automatic Milking. J Dairy Sci 2011, 94, 547–62. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).