1. Introduction
Portions of Florida are dotted with thousands of shallow depression wetlands ranging from meters to kilometers in diameter. Their geologic origins vary, those to the north reflecting karst solution pockets, those in south Florida more likely drainage-impeded dips in the varied sandy-clay substrates [
1]. Inputs and outputs are mostly rain and evaporation, with unclear connections to underlying hydrology. The water is usually acid, nutrient poor if unpolluted, and nutritionally phosphorus-limited [
1]. Depths and hydroperiods vary substantially in time and space. Some depressions are year-round lakes or ponds. The shallowest and best-drained are wet meadows or prairies. The nomenclature applied to Florida shallow wetlands is confusingly inconsistent. The depression marshes in the present project are of intermediate depths, often covering vast areas with water often more or less uniformly deep. With annual variation, the low-rain season extends from late winter to late spring, with peak rain June to September. The marshes of present interest spend time with their surfaces exposed, although with a lag between drying weather and exposure. Submersion often exceeds exposed time, and the subsurface soil typically remains moist during the drier season. Fire may occur some years during dry periods [
1].
The Southeast Florida study area marshes (
Figure 1a) are peppered abundantly by two woody shrubs 0.5-1.5 m tall, generally found sympatrically in our area:
Hypericum fasciculatum (Clusiaceae,
Figure 1b) and
Stillingia aquatica (Euphorbiaceae,
Figure 1c). Freshwater marshlands dominated by this pair define the study habitat, here called “
Hypericum-Stillingia [HS] marshes.” (In wetlands outside the study area additional shrubby
Hypericum species are abundant to dominant.) Appearing in varied proportions, the two dominant herbaceous species are
Rhynchospora harperi (1d, e) and R. tracyi (Figure 1f). As Kral [
2]
observed,” Rhynchospora harperi is most abundant in a very special habitat referred to here as the “
Hypericum pond.” Also sometimes encountered, mostly on exposed wet marsh bottom, is
R. divergens, a usually tiny, grassy species having capillary leaves.
Xyris smalliana is abundant in the study area, and young individuals are often physically adjacent to the
H. fasciculatum trunk. Weakley et al. [
3] noted
X. smalliana as “adjacent” to
Hypericum (and to
Pontederia) in the intermediate zones of basin marshes in peninsular Florida. And long before that, Kral [
4] mentioned
X. smalliana in “
Hypericum ponds.”
The HS marsh is a harsh habitat. Establishment entails all life phases surviving the alternating flooding-drying environmental filter exacerbated by oligotrophy, periphyton blanket, full exposure to wind and subtropical sun, and perhaps occasional fire. For these reasons, the system prompts attention to the question of character trait convergence vs. divergence in the face of a strong filter, discussed below. It also intersects with the stress-gradient-hypothesis (SGH), which suggests a shift in interactive balance from competition to facilitation along increasingly stressful environmental gradients [
5,
6]. Accordingly, facilitation is of special concern in exploring the challenging HS marsh ecology.
The present project is a continuation of the author’s extended interest in the local HS marshes, with prior individual attention to
H. fasciculatum and
S. aquatica, largely their reproductive biology [
7,
8]. The third installment, here based mostly on field measurements, takes a broader look with a special eye to autogenic site alteration and to intra- and interspecific facilitation. Aided by improving technology, there has been recent interest in autogenic environmental heterogeneity [
9,
10]. That such recent work embraces positive feedback (aka “switches”) [
11,
12] in marshes prompted the present research question: beyond stochastic competition and generalized growth and decay, is there detectable plant-mediated habitat alteration? And if so, does that provide intraspecific positive feedback and/or interspecific facilitation?
As the main goal of the present project, this compound question was addressed by field measurements centered chiefly on
Hypericum fasciculatum and its context. Data-acquisition included two components. First, two rounds of microtopographic depth measurements. Second, a series of near-neighbor, nearest-neighbor, photographic, and transect and quadrat studies summarized in
Table 1. The project began with a contextual marsh overview of the local HS marsh community before narrowing to the stated research questions.
2. Materials and Methods
Species are listed in
Appendix A. Authorships and families, given there, are not repeated in the text. Taxonomic details generally follow Wunderlin et al. [
13]. The study sites were two depression marsh systems in Palm Beach County, Florida. The “Mack Dairy Rd.” marsh (N 26.949417, W -80.222182) in the Cypress Creek Natural Area is a small (150 m diam) HS marsh surrounded by wet pinewoods. The “Casuarina” HS marsh (
Figure 1a, named informally for a landmark stand of trees) was the primary study zone. It is multiple kilometers in diameter within the Florida Fish and Wildlife Conservation Commission John C. and Mariana Jones/Hungryland Wildlife and Environmental Area and adjoining Pal-Mar Natural Area, separated by an E-W road. Most of the work was within Casuarina. Data were collected at four subareas referred to here as: Casuarina-South (N 26.929162, W -80.288498), Casuarina-North (26.939811, -80.289019), Casuarina-West (26.941800, -80.308328), and Casuarina-East (26.943650, -80.291711,
Figure 1a). The study period was November through March 2023/2024. Water depths were maximum early in the project to off-peak yet still submerged for almost all of the study. Most of the work was in standing water (8)20(30) cm deep. Weather ranged from heavily rainy in November through much of January to partly drying unevenly through February, and mostly dry in March, with marsh bottom partly exposed in the final days of the study. State and county research permits prohibited destructive sampling. Transect placements were restricted to minimally invasive access by existing vehicular and animal trails from marsh access points. (Transects paralleled trails, and were not within them.) During the study period, the road providing access to Casuarina-North and -South was closed unexpectedly, forcing relocation mostly to Casuarina-East.
Graphics and calculations were prepared using R [
14] employing the Tidyverse package. The dendrogram was generated using the R dist (binary) and hclust (as.dendrogram) methods applied to
Supplementary Materials “SM” dataset J available in [
15]. All field data tables are in that repository. Species abbreviations used in the graphics are given in the caption to
Figure 2.
A pilot study tabulated the vegetation pattern along the depth gradient at 16 different local HS marsh sites. Diversity was highest near shores, intermediate at medium depths (used for most of the present resulting project), and diminished in permanent or nearly permanent standing water. In the pilot study, non-dominant species varied considerably in presence and abundance, even in sites with generally similar appearances and water levels. The pilot results were not incorporated directly into the present analysis; they are, nonetheless, available as SM dataset G [
15]. Multiple additional datasets for the project were compiled using different combinations of transects and quadrats, depending on specific study questions, and on changing circumstances [
15]. There is no intermixing of datasets, except juxtaposition of the Mack Rd. and Casuarina rank abundance graphs based on the same methodologies. Methods used for compiling each dataset were as follows:
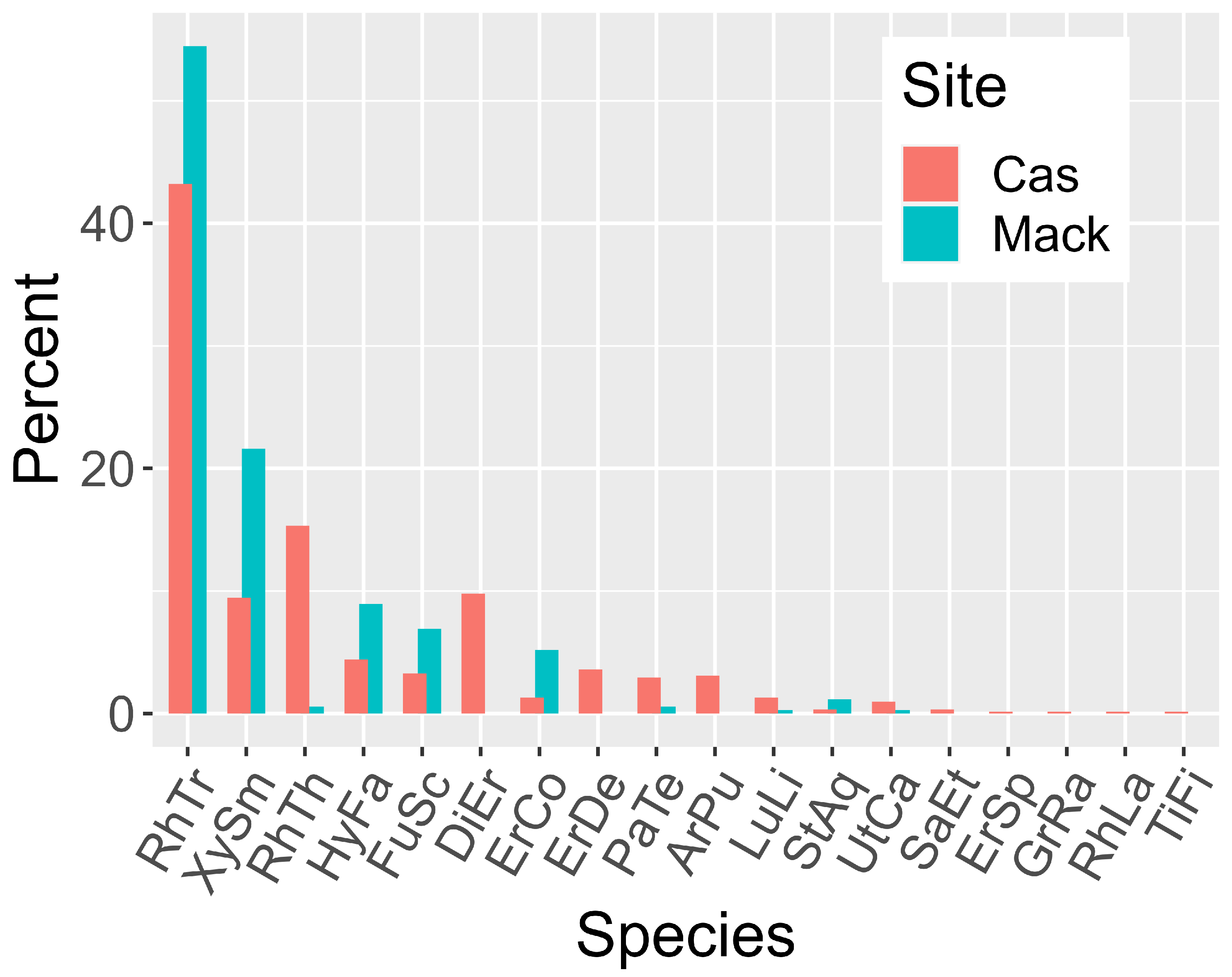
Substudy 1. Species abundances. Species abundances were determined at the Mack Dairy Rd. marsh and at the Casuarina-North site using 30 quadrat placements at both marshes during December and January 2023/2024. The 40 cm x 60 cm quadrat dimensions match the aspect ratio and mounting height of a camera used to photograph quadrats. Quadrat placements in this substudy were blind over-the-shoulder quadrat tosses following a depth contour of ca. 20 cm along a total distance of 60 m. “Individuals” (including ramets) rooted within the frame were counted for each placement, including those with root crowns overlapping the inner frame edge (SM E, F).
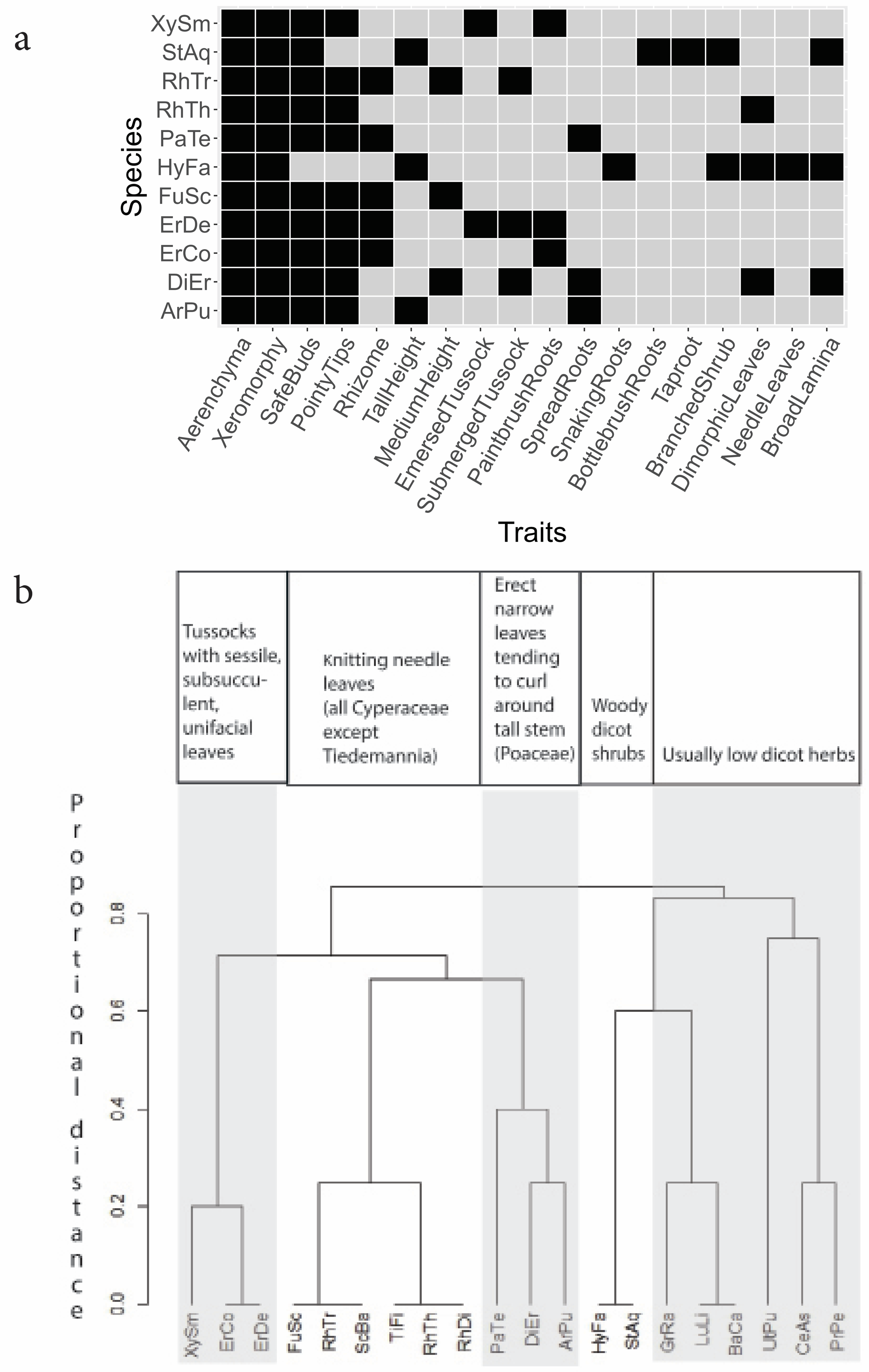
Substudy 2. Qualitative characteristics. In order to discuss functional traits, qualitative information was tabulated for the common species (SM I) by direct field examination, by on-line examination of herbarium specimens at the University of South Florida (USF), and by literature review. To constrain subjectivity, the list of traits designated as key to surviving the marsh environmental filter came from outside sources: xeromorphy in seasonal marsh plants [
16] and from Keddy’s [
17] general characteristics of wetland and marsh species: aerenchyma, rhizomes, pointed shoots, and low protected growing tips. Stillingia aquatica resprouts from latent buds near the highwater line after top damage, and thus was included in the protected buds category. (Such sprouting happens in H. fasciculatum but only rarely, and was not included.) Xeromorphy was here taken to be one or more of the following traits: reduced photosynthetic surfaces, terete or nearly terete (including narrowly channeled) main photosynthetic structures, upright primary photosynthetic surfaces, subsucculence, and thick or waxy covering (e.g., Dichanthelium erectifolium). The last-mentioned grass makes broad leaves at the marsh bottom when submerged, and on its rising culm forms erect narrow leaves. Although Eriocaulon compressum has a soft, lax spreading rosette when submerged, its leaves are upright and relatively stiff on exposed soil. Also with foliar dimorphy, Hypericum fasciculatum has broad leaves on young plants as opposed to the needle leaves on mature individuals. Rhynchospora harperi makes small, grassy, thin leaves when submerged or partly submerged (and can be fertile then), and makes much larger leaves with age and emersion (
Figure 1d).
Substudy 3. Herbaceous species nearest-neighbors. As a check on interspecific associations among herbaceous plants, at Casuarina-North a 100-m string transect was extended along the ca. 20-cm depth line. The transect was sampled for the six most abundant nondominant herbaceous species at that locality (Aristida palustris, Dichanthelium erectifolium, Eriocaulon compressum, Panicum tenerum, Rhynchospora harperi, Xyris smalliana). Sampling points were determined by the presence within two m of the transect of Panicum tenerum due to it being by far the least abundant species. Each of the other species nearest each P. tenerum transect-encounter had its nearest nonconspecific neighbor recorded (SM A).
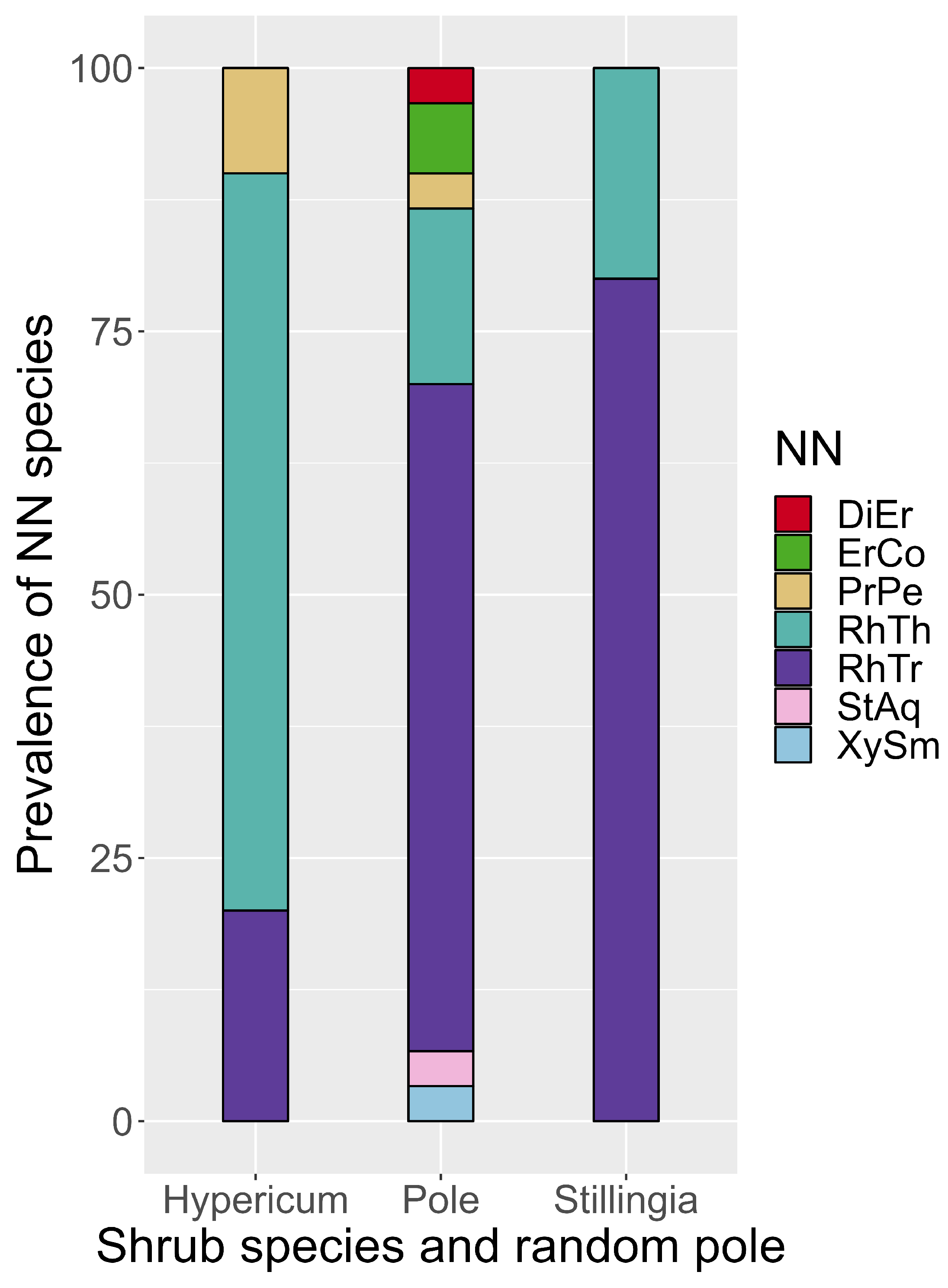
Substudy 4. Woody species neighbors. Seeking interspecific associations for H. fasciculatum and for S. aquatica, nearest neighbors to Hypericum fasciculatum, to Stillingia aquatica, and to a randomly repeatedly placed fiberglass “null” vertical pole were recorded at Casuarina-West. For this a 60-m study strip two m wide extended parallel to shore in standing water. Nearest neighbors were tabulated for each non-seedling Hypericum fasciculatum, Stillingia aquatica, and pole location, 30 samples each. The pole was placed using random X-Y coordinates within the full width and length of the study strip (SM H). In a related separate tabulation to permit reappraisal, at Casuarina-South all neighbors within 10 cm of H. fasciculatum, S. aquatica, and the randomly placed pole, 30 each, were tabulated (SM B1-B3).
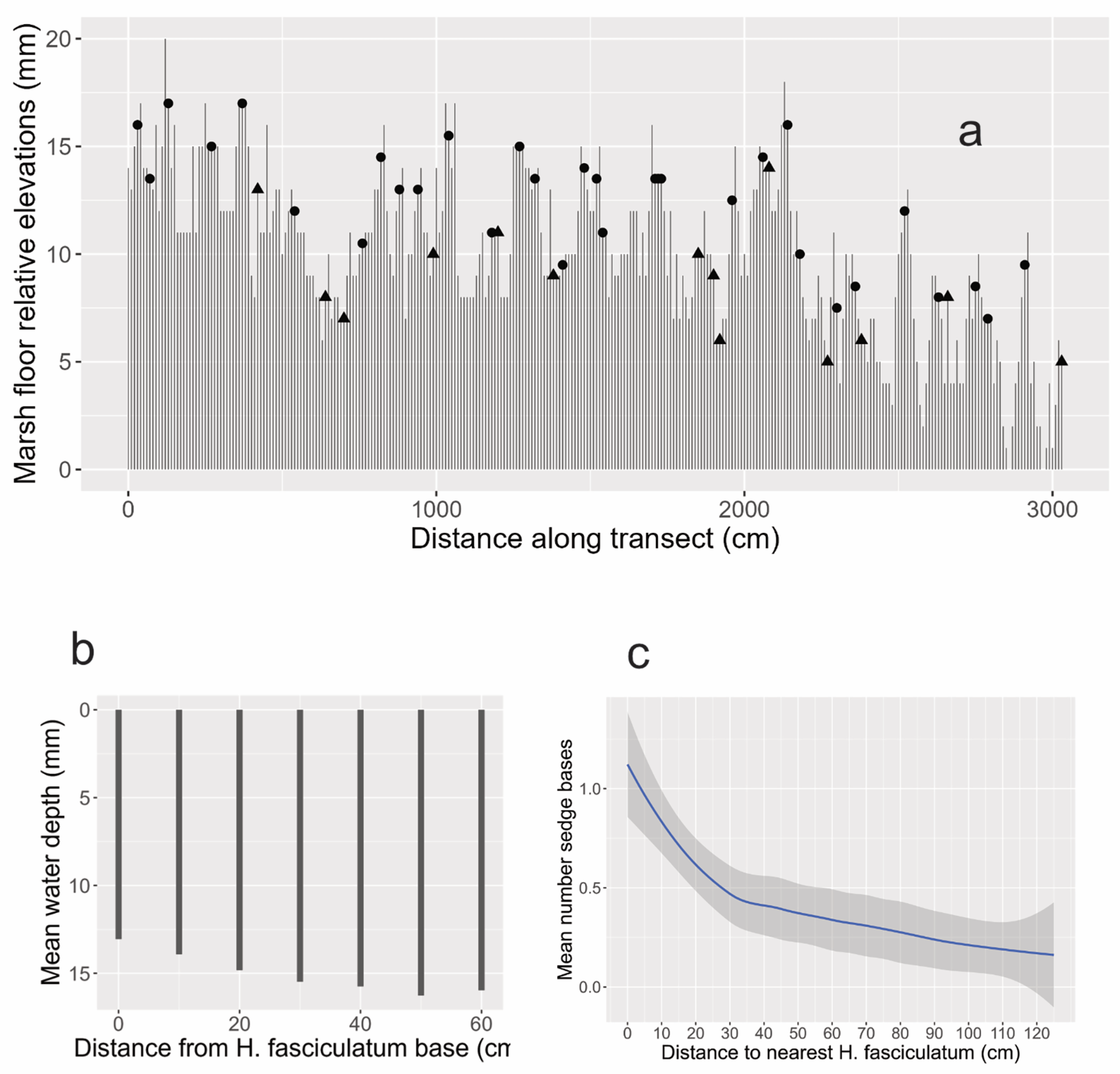
Substudy 5. Hypericum fasciculatum and marsh microtopography. To determine if the H. fasciculatum microdistribution is related to soil elevation beyond the root cone, 30 sequential individuals (trunks > 9 cm diam 20 cm above marsh bottom) were measured along a trail parallel to shore at Casuarina-East. Water depths were recorded at 0, 10, 20, 30, 40, 50, and 60 cm from each H. fasciculatum trunk base along the N,S,E,W radii with deviations when a radius was physically blocked. Individuals were skipped if situated among crowded conspecifics (SM C1).
For a separate appraisal of the same question, and for comparison with Stillingia aquatica, along a new linear transect parallel to shore in standing water at Casuarina-East water depths were recorded at 5 cm intervals, as were the locations of all H. fasciculatum and S. aquatica individuals touching the string. (In spots where a depth reading would occur at a H. fasciculatum root cone, in order to avoid the distortion of measuring depth to the cone top, the two readings on either side of the cone were averaged (SM C2)).
Substudy 6. Hypericum fasciculatum and interspecific facilitation. Association of H. fasciculatum with microtopographical elevations in substudy 5 raised the question of possible facilitation of herbaceous vegetation on the same microelevations. As a first step, the Casuarina-East site was searched for visual patterns of sedge (mostly R. tracyi, R. harperi, Scleria baldwinii) height and/or density association with H. fasciculatum, with examples photographed. To quantify that relationship, a transect was stretched parallel to shore in standing water for approx. 23 m at Casuarina-East. The full length of the transect was tallied for all sedge clump bases (or isolated ramets) in 463 5 X 5 cm grid squares arranged serially along the string with no gaps except the wire grid margins (SM D).
As a separate reappraisal of the relationship between sedge density and distance from H. fasciculatum, a second study was conducted at Casuarina-East. Rather than follow a transect, the reappraisal study tabulated 30 individuals of H. fasciculatum (over 9 cm diam at 20 cm above the base). The H. fasciculatum shrubs were selected using a spinner. A single radius leading outward from each selected H. fasciculatum trunk was then chosen, again by spinner (redone if the radius was physically blocked or within ca. 1 m of a different H. fasciculatum). For each H. fasciculatum the radial samples consisted of 15 serially adjacent 5 X 5 cm wire grid squares proceeding outward from the trunk base, the numbers of sedge bases or isolated ramets in each square recorded. Because the result echoed closely the transect study, the second data table and resulting plot are not included here, and are in the SM as “D reappraisal.”
Table 1.
Summary of the substudies. Cas = Casuarina site Mack = Mack Dairy Rd. site. SM = Supplementary Materials. NN = nearest neighbor.
Table 1.
Summary of the substudies. Cas = Casuarina site Mack = Mack Dairy Rd. site. SM = Supplementary Materials. NN = nearest neighbor.
| Substudies |
Sites and dates |
Methods |
Data access |
Questions
addressed |
Summarized results |
Related
graphics |
| 1 |
Cas-N
and Mack
12/9/23-1/15/24 |
Quadrats along 20-cm depth contours |
SM: E, F |
Inventory |
Species compositions differ between similar sites |
Figure 2 |
| 2 |
All sites, plus literature &
online herbarium |
Qualitative trait assessments |
SM: I, J |
Convergence and divergence of plant traits in relation to
alpha and beta niches |
Convergence in beta niche, divergence in alpha niche |
Figure 3b
(Figure 3c based on
SM J) |
| 3 |
Cas-N
1/3-1/11/24 |
Transect along ca. 20-cm depth contour |
SM: A |
Exploratory, seeking herbaceous species NN patterns |
Inconsequential. |
|
| 4 |
Data B1-B3:
Cas -S
2/1-2/3 2024
Data H: Cas-W
2/6-2/8 2024
|
60 m X 2 m study strip
100 m X 2 m
study strip |
SM:
B1-B3
SM: H |
B1-B3: all neighbors within 10 cm of H. fasciculatum, of S. aquatica, and of random pole
H: NN to H. fasciculatum, to S. aquatica, and to random pole
|
Hypericum
fasciculatum had more close neighbor species than S. aquatica or random pole, with R. harperi disproportionately as NN, at high water |
Figures 4b,
6 |
| 5 |
Cas-E
2/19-2/25 2024 |
Transect parallel to shore
for depths around bases (C1), same transect measured at 5 cm intervals for depth profile and shrub placements (C2) |
SM:
C1, C2 |
Does H. fasciculatum placement reflect microtopography? |
On average H. fasciculatum had soil raised around its base to ca. 50 cm radius (C1), and H. fasciculatum tended to be on raised marsh bottom (C2) |
Figure 7a , b |
| 6 |
Cas-E
2/26-3/4 2024 |
For Cyperaceae, transect with 5 cm square micro-
quadrats
|
SM: D |
Does H. fasciculatum influence vegetation beyond its adjacent small neighbors? |
H. fasciculatum appears to influence neighbors out to over 30 cm |
Figure 7c,
also “D reappraisal” data and plot in SM
|
4. Discussion
Keddy and Laughlin [
19] observed that competition is often between individual species and a single dominant species, as opposed to chiefly with each other. In the present study rhizomatous
R. tracyi is usually the dominant herbaceous species, although sometimes there is abundant (nonrhizomatous)
R. harperi. The additional herbaceous species are intermixed in gaps among shrubs and among the large rhynchosporas, as Boutin and Keddy [
20] described as typical for other marshes.
The harsh HS marsh environmental filter suggests a balanced expression of two opposing trends in plant traits: As several authors have discussed [
12,
19,
21,
22,
23], on one hand the abiotic filter hypothetically selects largely for convergence in traits pertaining mostly to surviving harsh physical conditions. On the other hand, the filtered survivors must then compete, promoting limiting similarity and functional trait divergence especially in traits relating chiefly to competitive architecture and to spatial extension [
21]. As suggested by
Figure 3a, the present HS marsh species met expectations of convergence with respect to the physical environmental filter (aerenchyma, xeromorphy, protected buds, rhizomes) and divergence with respect to short-distance competitive foliar and root diversity.
Nearest-neighbor data among the herbaceous species revealed very little non-stochastic community structure. The particularly low-frequency nearest-neighbor relationship of Aristida palustris with R. tracyi occurred probably because A. palustris has a broad spreading root pattern (or conceivably a preference for shallower microsite). The particularly close proximity of Panicum tenerum (Coleataenia tenera) to its varied nearest neighbors may result from being a thin wispy grass having a tightly knotted rhizome suited to gaps. That species resembles its congener “Panicum longifolium”(
Coleataenia longifolia (Torr.) Soreng), which conforms to the “interstitial reed” guild in Boutin and Keddy [
20]. Both species have a similar knotty rhizome.
Probably promoting coexistence in the HS marsh are trade-offs between competitive dominance vs. colonization, dispersal, and reproductive pattern [
17,
24]. Three rhynchosporas may exemplify such tradeoffs. Rhynchospora tracyi is relatively tall and thick, tough, rhizomatous, and nearly dormant aboveground when flooded, becoming green and increasingly fertile as waters recede. By contrast, R. harperi regenerates with small grassy leaves (
Figure 4b) and produces flowers even during elevated water levels, both submerged and on relatively elevated spots. Rhynchospora harperi graduates from thin grassy leaves to more substantial foliage (note the mixed leaf sizes in
Figure 1d taken as water levels were dropping). While R. tracyi elongates unidimensionally by elongating rhizome growth, R. harperi wanders two-dimensionally by basal budding. Typically much smaller than the other two, R. divergens, aka “spreading beaksedge,” is perennial but inconspicuous during high water, and rises quickly from newly exposed wet substrate.
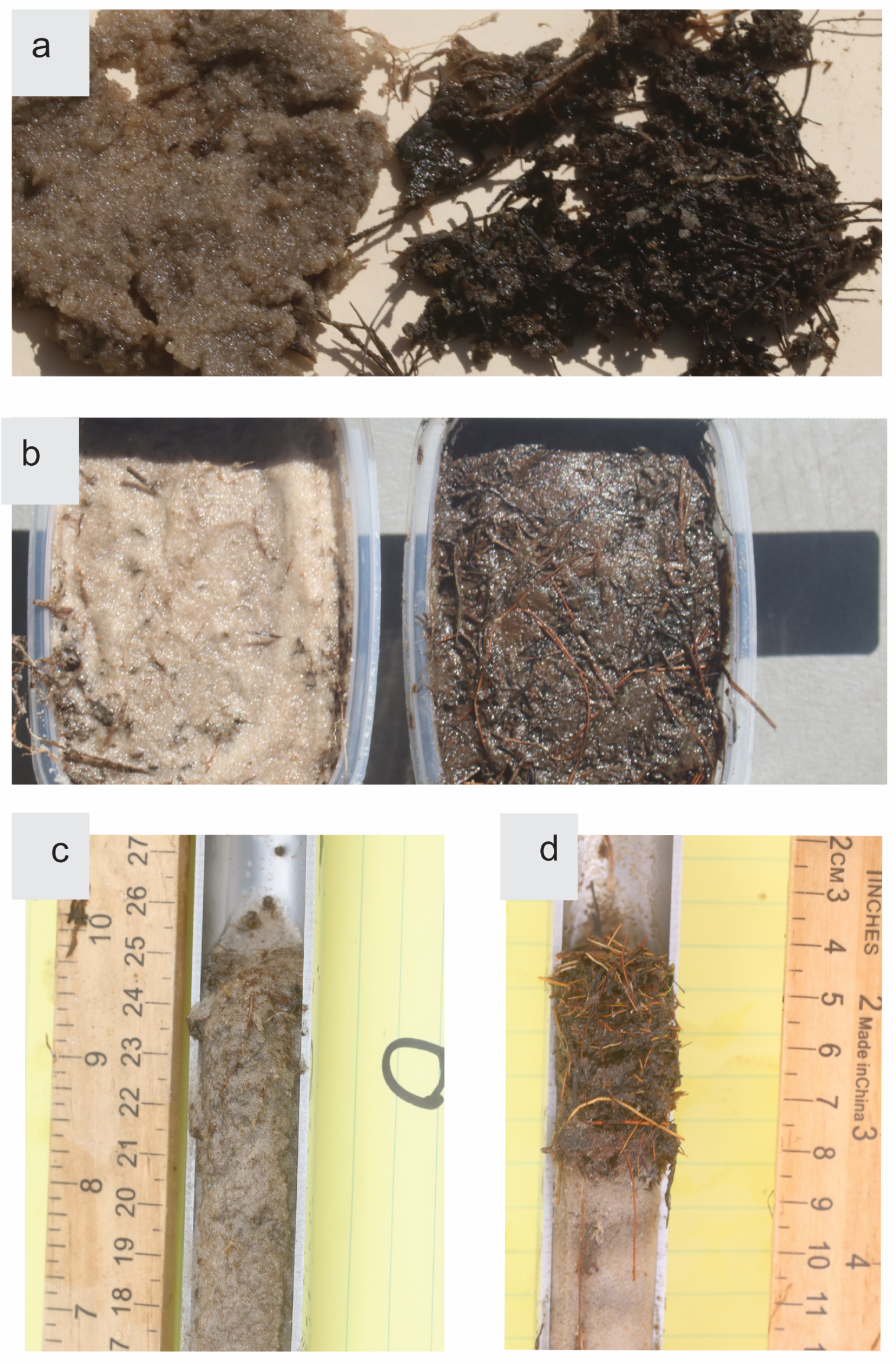
That large Hypericum fasciculatum accumulates rich, peaty, aerated soil penetrated by its own roots is an example of an autogenic intraspecific positive feedback. Turning to interspecific facilitation, although the stress-gradient-hypothesis has generated controversy and inconsistent data [
25], a metastudy by Adams et al. [
6] generally supported it for plants, also see [
26]. That Hypericum fasciculatum in multimonth standing water selectively facilitates small plants at its elevated base (
Figure 4b, 6) is consistent with SGH.
Expanding the perspective now to increasing distance from the base, Figures 7a and 7b indicate soil elevation to extend outward from the root cone. As
Figure 7c shows, sedges concentrate to some extent in more or less the same radius. The reappraisal study in the
Supplementary Materials echoed nearly identical sedge concentration pattern. (It should be noted that Hypericum fasciculatum occurs in varied wetlands. (It is not contended here that an elevated root cone surrounded by raised soil and concentrated sedge associates is universal for the species, especially in view of large variation in hydroperiods and substrates at non-studied sites.)
The situational associations of H. fasciculatum with elevated microtopography radiating to 30-60 cm and with associated enhanced sedge growth in the same perimeter poses a “cause and effect” concern. Does the shrub raise the marsh floor, or do the H. fasciculatum and the sedges around it merely fare best on higher ground? (Less likely, do the plants all benefit mutually from some additional underlying feature? Does H. fasciculatum cast protective shade, or suppresses non-sedge competitors allelopathically?)
That H. fasciculatum and the sedges benefit from raised marsh bottom regardless of the elevational cause is probably true. As Keddy [
17] pointed out, “a few centimeters” wetland elevation can substantially affect species composition. Accordingly, relatively high spots will favor certain species, including it seems H. fasciculatum and associates. That H. fasciculatum is at least a contributor to the elevation is a visible fact, given the peaty substrate collecting at large root cones. Root penetration into that enriched soil, and influence on near neighbor relative frequencies are likewise visible (Figs. 4b, 6) at the cones. The question thus becomes the radial extent and broad ecological significance of the H. fasciculatum makes to soil building and to other species. The shrub is abundant throughout the HM marshes with obvious high turnover, and its collected soil has to go somewhere. In addition to the root cone, the shrub’s long radiating shallow roots would fortify soft loose sediments and, upon decay would add to the substrate beyond the canopy.