1. Introduction
Non-alcoholic fatty liver disease (NAFLD) and its more advanced form (NASH), are the most common liver disorders in industrialized countries, caused by fat and sugar-rich diet, sedentary lifestyle and genetic predisposition [
1,
2,
3]. To date, there are no specific drugs approved for NAFLD [
4,
5], but antioxidant polyphenols, especially in the form of plant-derived extracts are emerging as an important therapeutic option for the management of NAFLD and NASH [
6,
7] in addition to dietary measures and physical activity. In this scenario, Bergamot Polyphenol Fraction (BPF) appears as a particularly promising food supplement. Several preclinical studies documented the efficacy of BPF against hepatic steatosis in rodent models of diet-induced NAFLD and NASH as both a preventive measure or post-induction treatment and clinical studies BPF® obtained from juice and peels of Bergamot (Citrus Bergamia Risso et Poiteau) fruits exceptionally rich in flavonoids, is characterized by a unique profile of flavonoids such as naringin, brutieridin and melitidin and many other flavonoid and non-flavonoid compounds with lipid-lowering, anti-inflammatory, proautophagic and detoxifying activity [
8,
9,
10,
11]. The chemical composition and beneficial effects of BPF antioxidants have been documented in numerous analytical [
8,
9,
12], preclinical [
10,
13,
14,
15] and clinical studies [
16,
17,
18,
19,
20], but little is known about molecular mechanisms underlying these effects.
Hepatic lipid accumulation is a product of an imbalance between fatty acid (FA) synthesis and FA oxidation, but defective lipolysis and lipophagy, esterification of plasma-free FA or increased dietary fat intake also play a role in steatosis pathophysiology [
21]. FA synthesis is the first step of de novo lipogenesis (DNL), by which lipids are endogenously synthesized from acetyl-CoA. The first reaction of endogenous FA synthesis is the conversion of citrate or acetate to acetyl-CoA by the action of citrate lyase (ACLY) or hepatic ACSS2, respectively [
22]. Acetyl-CoA is then converted to malonyl-CoA by acetyl-CoA carboxylase (ACACA). FA synthase (FAS) sequentially utilizes malonyl-CoA to extend the growing fatty acyl chains, which can be elongated and desaturated by stearoyl-CoA desaturase (SCD1). The esterification of FA produces triglycerides and other lipids and its final step is catalyzed by diacylglycerol acyltransferase (DGAT) among other enzymes. Most lipogenic enzymes are upregulated to a different extent in high-fat diet [
23,
24,
25], fructose or sucrose [
22,
26,
27] or Western diet-induced [
28] animal models of NAFLD and NASH, but their differential expression has never been investigated in CAF diet compared to normal diet livers.
The induction of DNL and their catabolism are tightly regulated at the transcription level [
29]. Proteins coded by Sterol regulatory element-binding factor (SREBF1), such as SREB protein 1A (SREB1A) and 1C (SREBP1C), liver X receptors (LXRs), carbohydrate-responsive element-binding protein (ChREBP) and X-box binding protein 1 (XBP-1), which also regulates endoplasmic reticulum (ER) stress, play critical roles in this process. Other transcriptional factors involved in FA synthesis are the peroxisome proliferator-activated receptors (PPARs) that can either enhance genes involved in β-oxidation/translocation of FA (PPAR-α) or promote lipogenesis and glucose uptake and storage (PPAR-γ). SREBP-2 activates genes involved in sterol metabolism and it is the predominant transcriptional factor affecting cholesterol synthesis [
30]. The gene expression of the above transcriptional modulators is also altered in patients with NAFLD/NASH and in animal models of the disease [
29]. Excessive lipogenesis and β-oxidation of FA are opposing processes that strongly contribute to the oxidative imbalance in hepatocytes and other cells [
31].
The vast scientific literature suggests that phenolic phytochemicals may (1) interact with specific proteins in signaling pathways and modulate the activity and/or expression of key antioxidant proteins; (2) regulate the epigenetic mechanisms of gene expression; and (3) modulate the gut microbiota profile and metabolites. For example, flavone- and tyrosol derivatives are implicated in the activation of AMP kinase (AMPK) and as a consequence in the regulation of several metabolic enzymes that play a role in NAFLD [
32,
33]. In addition, AMPK stays at the crossroads of autophagy and energy (lipid and carbohydrate) metabolism and AMPK activation should explain both hypolipemic and proautophagic proprieties of these compounds in hepatic steatosis [
34,
35]. AMPK activation has been shown to be mediated by the direct interaction of certain polyphenols with phosphodiesterases (PDEs) [
36], but it can be also modulated by NRH-quinone oxidoreductase 2 (NQO2) [
32] and possibly by other enzymes among more than 5000 proteins predicted as direct targets of these compounds [
37].
The interaction of flavonoids with their protein targets leads to short- and long-term gene expression changes. The latter are mediated by epigenetic mechanisms such as DNA methylation, histone acetylation and deacetylation, causing spatial reorganization of the chromatin [
38,
39]. In fact, flavones and other dietary polyphenols have been shown to exhibit epigenetic modulatory effects on several gene targets [
40,
41], which are epigenetically altered in NAFLD [
42,
43].
Autophagy is a catabolic pathway that contributes to liver homeostasis through its role in cell quality control, by removing misfolded proteins, damaged organelles and lipid droplets [
44]. Autophagy and energy metabolism gene expression programs are interconnected [
45,
46]. Lipid metabolism and autophagy are orchestrated by several common transcription factors, such as transcriptional factor EB (TFEB), a master regulator of lysosomal biogenesis, autophagy and lipid catabolism. Specifically, TFEB is activated in mouse livers and C. elegans upon starvation, while it is downregulated after the administration of a high-fat diet (HFD) to mice, likely modulating the expression of autophagy-related genes [
47,
48]. Accordingly, autophagy is considered to be an important therapeutic target in the management of NAFLD and NASH [
34,
44,
49]. It is well-known that flavonoids and their phytocomplexes induce autophagy in many cell types including in vivo models of NAFLD [
50,
51]. It is more difficult to study autophagy in vivo, but there is well-documented evidence that NAFLD and NASH-inducing diets suppress hepatic autophagy levels [
48,
52]. In addition, our group has demonstrated that Citrus Bergamia flavonoids induce evident bulk and lipid autophagy (lipophagy), detectable after three months of chronic supplementation of BPF, while efficiently preventing NAFLD in rats treated with CAF diet [
10].
Here, we evaluated the impact of chronic supplementation of BPF in CAF-treated rats on mRNA levels of 84 genes representing different energy metabolism pathways, often altered in hepatic steatosis and 84 genes representing autophagy machinery. Our data clearly show that suppression of BPF hepatic, de-novo lipogenesis at the level gene expression of and is the most important target of bergamot polyphenols, while no b-oxidation most of autophagy genes is not subjected to transcriptional regulation by polyphenols.
2. Materials and Methods
2.1. Animal Procedures and Experimental Design
Male 5-week-old Rcc: Han WISTAR rats (Harlan Laboratories, Indianapolis, IN, USA) were housed two rats/cage in the animal house facility had access to water and standard chow (SC) diet 2016 (“SC,” Harlan Teklad) or SC and CAF diet ad libitum and maintained in standard conditions as previously described [
10]. CAF diet included different sweet or briny foods and condensed milk. The exact composition and feeding protocol has been previously described [
10]. All animal studies were approved by the Italian Health Ministry and by the local ethics committee. At 8 weeks of age, the rats were weighed, marked on the tail for recognition, and randomly assigned to two basic experimental groups: CAF diet group (CAF, n = 10 rats) or SC diet group (SC, n = 5 rats). CAF group was subsequently subdivided into two subgroups, of which one received BPF extract (~50 mg/kg body weight/day) as a supplement in drinking water (CAF+BPF, n = 5) and the other received drinking water without BPF (CAF, n = 5). After a week of adaptation to the new cage mate, the administration of CAF diet started (day “0”) and lasted 91–94 days until the day of sacrifice. Food consumption and body weight gain were monitored weekly for 14 weeks. The animals were sacrificed under Zoletil (80mg/kg) and Dormitor anesthesia for tissue collection.
The blood was collected by cardiac puncture as previously described [
10] and cholesterol and triglycerides analyses were performed using commercial reagents on a Dimension EXL analyzer (Siemens Healthcare Diagnostics s.r.l.,Milano, Italy).
2.2. Gene Expression Analysis
3 representing rats were chosen for each experimental group (SC, CAF, CAF+BPF). Small pieces (0.4 - 1 g) of the central part of the main lobe of rat livers were shock-frozen in liquid nitrogen and stored until needed at -80°C. Frozen tissue was further fragmented and 50-100 mg samples were homogenized with a glass bouncer on ice with 1mL of TRIzol Reagent (Cat. No. 15596026, Invitrogen, Thermo Fisher Scientific, Waltham, MA, USA). Total RNA (totRNA) was extracted using the TRIzol Reagent method followed by DNase treatment (Cat. No. 79254, Qiagen Gmbh, Hilden, Germany). Total RNA was carefully quantified, and its integrity was verified on 0.8% denaturing agarose gel. Equal amounts of totRNA from 3 rats of the same experimental group were pooled and cDNA was synthesized from 500 ng of pooled RNA with RT2 PreAMP cDNA Synthesis Kit (Cat. No. 330451; Qiagen Gmbh) according to manufacturer instructions. The relative gene expression was assayed on 96-well format arrays: Rat Fatty liver (PARN-157Z) and Rat autophagy (PARN-084Z) RT2 Profiler PCR arrays (SABiosciences, Qiagen Gmbh), each containing 84 key genes, likely involved in the pathogenesis of hepatic steatosis and the regulation of autophagy, respectively. Each plate included also six housekeeping genes and control wells for genomic DNA (gDNA) contamination and RT-PCR efficiencies. RT-PCR was performed on an iQ5 real-time PCR (Bio-Rad Laboratoires, Inc., Hercules, CA, USA) using SYBR Green (universal cycling conditions: 95°C, 10 min; 95°C, 15 s; and 60°C, 1 min; repeated for 40 cycles) and subsequent analyses were carried out according to the manufacturer’s recommended protocol (SABioscience, Qiagen Gmbh). Melt curve analysis confirmed the amplification of a single product. Each pooled cDNA was analysed twice on independent plates to calculate the mean. This and further analysis was performed by using a dedicated software available at the Gene Globe Data Analysis Center (
http://www.qiagen.com/it/shop/genes-and-pathways/data-analysis-center-overview-page/). Briefly, two housekeeping (hk) control genes—hypoxanthine phosphoribosyl transferase (
Hprt1) and B2-microglobulin (
B2m) were selected for normalization. Each replicate cycle threshold (CT) was normalized to the average CT of hk controls on a per-plate basis. The comparative CT method was used to calculate the relative quantification of gene expression. The following formula was used to calculate the relative amount of the transcripts in the treated samples (CAF or CAF+BPF) and the control samples (SC or CAF), both of which were normalized to hk controls: ΔΔCT =ΔCT (tt) –ΔCT (con). ΔCT is the log2 difference in CT between the target gene and hk controls by subtracting the average CT of controls from each replicate. The fold change for each treated sample relative to the control sample = 2–ΔΔCT.
2.3. RT-qPCR Analysis of Gene Expression
For standard RT-qPCR analysis, totRNA was isolated as described above from 5 rats for each experimental group, but RNA from each rat was processed separately. cDNA was synthesized from 5 mg of totRNA with TransScript® II First-Strand cDNA Synthesis SuperMix (Cat. No. AH301-02) according to manufacturer instructions (TransGen Biotech Co., Ltd, Haidian District, Beijing, China). RT-qPCR was performed on QuantStudio 3 Real-Time PCR Detection System (Applied Biosystems Europe, Monza (MI), Italy) as previously described [
15]. The list of used primers is shown in
Table 1.
The applied cycling conditions were: 95 °C, 10 min; 95 °C, 15 s; and 61 °C, 1 min; repeated for 40 cycles. Samples were analyzed in triplicate with hypoxanthine phosphoribosyl transferase 1 (Hrpt1) as a HK control. Only results with the amplification of a single product, as verified by melting curve analysis, were considered. Relative gene expression was calculated according to the 2ΔΔCT method using the SC liver tissue cDNA (15 weeks on SC diet) as a starting point control.
2.4. Liver Histology and Lipid Droplets (LDs) Staining and Analysis
10 μm thick frozen sections of the perfused liver from the central portion of the main lobe were prepared as previously described [
10]. For lipid droplets (LDs) staining, the liver sections mounted on slides were rinsed with distilled water and immersed in 60% isopropanol (Sigma-Aldrich, St. Louis, MO, USA) and then LDs were stained with freshly prepared Oil Red O (ORO, Sigma-Aldrich) working solution for 15 min. The sections were then rinsed with 60% isopropanol and washed with de-ionized water three times for 30 sec. Then, half of the sections were counterstained with Mayer’s hematoxylin (05-06002/L; Bio-Optica Milano Spa, Milano, Italy) for 60 sec to visualize the nuclei, rinsed with running tap water for 10 min and then covered with a film of embedding medium
SlowFade Gold (Cat. No. S36936; Life Technologies, Thermo Fischer Scientific, Road Grand Island, NY, USA) and a coverslip. Equivalent ORO and ORO plus hematoxylin-stained liver sections of each experimental group were examined in bright-field with Leica Microscope DM4000B (Leica Microsystems GmbH, Wetzlar, Germany) equipped with 10×, 40×, and 100× objective lenses. For quantitative LDs accumulation analysis, digital images of ORO-stained sections were acquired by Sp2 confocal microscopy (Leica Microsystems GmbH) by using a TRITC wide emission filter (540–580 nm) and accumulation settings. For each liver section, at least five independent images from equivalent central lobe areas were captured at 40× magnification. Within each area, four different regions were chosen randomly and acquired with zoom at 4.65. These images were processed and analyzed using a semiautomatic procedure implemented in Image J2x software.
2.5. Tissue Homogenization and Western Blotting (WB)
Liver fragments were homogenized on Bullet Blender Storm 24 tissue homogenizer (Next Advance, Inc., Averill Park, NY, USA) according to manufacturer indications, using 1 mm diameter Zirconium Oxide Beads (Next Advance) at 4°C in RIPA lysis buffer [Tris-HCl 20 mM (pH 7,5) NaCl 150 mM, Igepal 1%, EDTA 1 mM, SDS 0,1%] supplemented with protease inhibitors (cOmplete Mini, EDTA free, REF 11836170001; Roche Diagnostics Gmbh, Mannheim, Germany), NaF 2 mM and sodium orthovanadate 2 mM. Protein concentration was evaluated and then 40 µg was subjected to gradient 4-12% Bis-Tris electrophoresis NuPAGE (#NP0335BOX) according to manufacturer’s instructions (Invitrogen, Thermo Fisher Scientific) or 12% SDS- PAGE (for anti-LC3 and ATG16 WB). WB was performed on polyacrylamide gel and the primary antibody was usually incubated overnight at 4°C, followed by 1-h incubation with a secondary antibody at RT. Blots were developed with ImmunoBlot ECL reagents (Cat. # 170-5061; Bio-Rad Laboratories, Inc.).
2.6. Antibodies
The antibodies used for WB were as follows: rabbit polyclonal (rp) anti-GCK (H-88) (sc-7908; Santa Cruz Biotechnology Inc., Dallas, TX, USA; 1:1000); rp anti-PCK2/PEPCK (sc-32879; Santa Cruz Biotech. Inc.; 1:400); rp anti-ACLY (Cat. No. 15421-1-AP; Proteintech Group, Inc. Rosemont, IL 60018, USA; 1:1000); mouse monoclonal (mm) anti-ACACA/ACC (Cat. No: 67373-1-Ig, Proteintech Group, Inc.; 1:1000); rp anti-ATG16 (Code No. PM040Y; MBL International., Woburn, MA, USA; 1:1000); rp anti-ADRP/Perilipin 2 (Cat. No. 15294-1-AP, Proteintech Group, Inc.); mm anti-α-tubulin (T6074, Sigma Aldrich, Darmstadt, Germany; 1:1000); rp anti-LC3 (Code No. M186-3 MBL International; 1:1000); rp anti-GAPDH (sc-87752, 1:500; Santa Cruz Biotech. Inc.; 1:500) were used as primary antibodies.
2.7. Data Analysis and Statistical Procedures
WB optical density was analyzed as previously described [
53]. Each liver lysate (from 1 rat) was analyzed at least twice by WB, and the results were expressed as the mean ± standard error (SEM). The data were evaluated using ordinary one-way ANOVA, followed by Tukey’s post-test and occasionally by uncorrected Fisher’s Least Significant Difference (LSD) test as indicated in the legends, for normal distribution of data and Mann-Whitney test when the data were not normally distributed. Data from the experiments are expressed as the mean ± SEM or mean ± standard deviation (SD) for the number n of independent experiments. The between-group differences were considered significant at p < 0.05.
4. Discussion
Citrus Bergamia flavonoids efficiently prevent NAFLD, systemic redox imbalance and other features of metabolic syndrome in rats fed with CAF diet [
10,
14]. These original findings have been subsequently confirmed in other models and extended to NASH [
15,
54,
55,
56] and NAFLD patients with metabolic syndrome patients in subsequent studies [
17,
18,
56]. Accordingly, the rats used in the present study responded to BPF supplementation with a strong reduction of lipid accumulation in CAF-fed livers characterized by a 60% and 80% decrease in fat content and numbers of big lipid droplets, respectively, confirmed by histopathological analysis of liver sections. BPF largely and significantly reduced diabetes and hypertriglyceridemia in CAF rats, while it had no significative impact on the body mass in this experiment; this confirms that BPF is hepatoprotective but has no or little effect on obesity [
10,
15]. In this article, we evaluated the impact of chronic supplementation of BPF on mRNA levels of a battery of 168 genes to address possible mechanisms behind the widely demonstrated efficacy of bergamot polyphenols against NAFLD.
The gene expression analysis of livers after long-term 14 weeks of CAF diet feeding revealed that CAF diet produced both expected and few unexpected effects on the transcriptional profiles of hepatic genes compared to other hypercaloric diets. There is a pattern of insulin and leptin resistance characterized by: upregulation of insulin mRNA 5 to 10 times, downregulation of Insr mRNA but upregulation of leptin receptor Lepr transcript 2 and 2.3-fold, respectively. The key transcription factors of lipid and cholesterol synthesis Srebf1, Xbp1 and Pparg, were also upregulated around 2-fold, which is common to many rodent models of NAFLD and found in patients with histologically diagnosed NAFLD
However, there was only a weak or not statistically significant increase in transcripts coding for lipogenesis enzymes (
Acly, Acaca, Fasn, Scd-1) in CAF livers. This is in contrast to fructose-induced rat and murine models of NAFLD, where the main targets of SREBP-1c mediated transactivation
Acaca and
Fasn, were upregulated 6 to 16-fold compared to control animal [
22,
57]. Yet, such a strong difference between those models might be explained by overnight fasting, but continued fructose supply before sacrifice [
58], while in our study all the animals were deprived for 5 h of all energy sources for blood analysis. Indeed, a study reported that in High-fat diet (HFD)-fed mice
Acaca was more expressed in HFD than in the control group and decreased to baseline levels in fasted control animals. In the same experiment,
Fasn upregulation by 3 to 4-fold in HFD mice did not seem to depend on fasting [
23]. Accordingly, we found consistent, but modest upregulation of
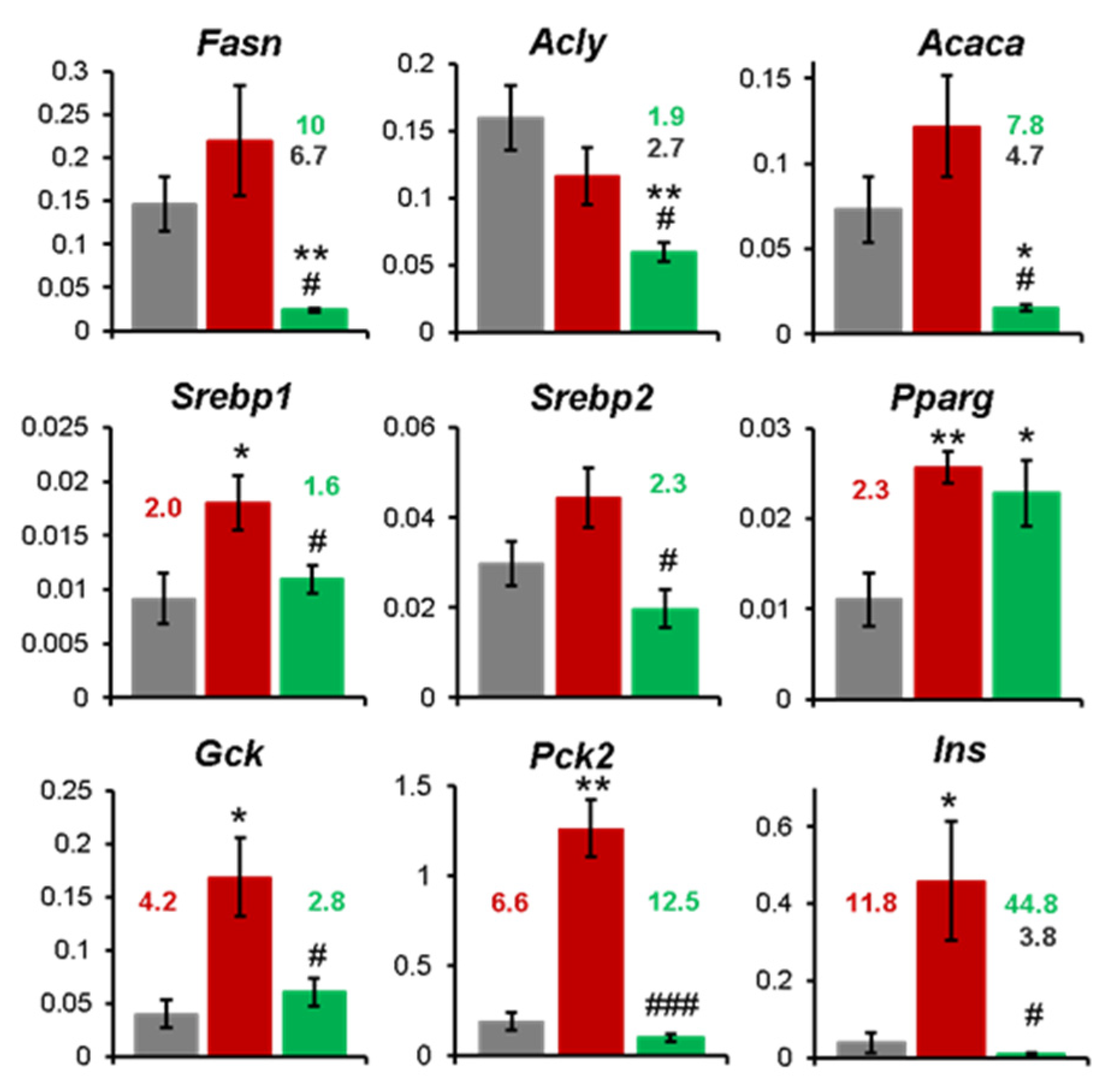
Fasn mRNA in the CAF group by around 1.6, but less consistent data for
Acaca and
Acly in both array pool (
Figure 4) and individual assays (
Figure 7), suggesting that lipogenesis enzymes expression is flexible and quickly responds to nutrient status. In contrast, the upstream transcription factors, such as
Srebf1 and
Pparg maintained the stable 2-fold upregulation, regardless of short-term fasting [
23]. Interestingly,
Acly was potently induced at the protein level.
According to the phenotypic changes in rat livers, BPF was able to suppress several NAFLD-related genes. Among them, mRNA levels of
Acly, Acaca, Fasn, and Scd1 were strongly suppressed in BPF-treated livers far below the mRNA levels of CAF and control livers, as confirmed also by an alternative primer-pair RT2-PCR analysis. For some genes, we could also observe that their mRNA downregulation (
Acaca and
Acly) led to a marked protein decrease in BPF-treated livers. BPF also considerably downregulated liver expression of
Apoa1 in comparison to CAF diet. APOA1 is the major protein component of HDL particles in plasma and it is involved in lipid metabolism and lipid transport from the liver [
59]. It has been proposed that the upregulation of PPARs might promote the expression and molecular transportation of APOA1 to form a PPAR-APOA1 signaling pathway regulating the pathogenesis of NAFLD [
60]. In our study liver expression of PPARgc1a was considerably reduced by supplementation with BPF suggesting that the pathogenic pathway related to APOA1 might be attenuated.
The efficacy of different polyphenols and plant extracts, including Citrus fruit extracts, have been tested in several NAFLD and NASH rodent models, but none of these studies reported such a dramatic transcriptional suppression of lipogenesis as bergamot polyphenols in CAF-induced NAFLD/NASH in rats. This is true for both purified compounds as well as for complex plant extracts [
51,
58,
61,
62,
63,
64,
65]. In another study, the same array of 84 mouse genes related to NAFLD was used to characterize the antisteatotic effect of a complex mixture of natural extracts containing silymarin, curcumin and chlorogenic acid. Yet, even though this extract almost fully reverted NAFLD induced by HFD in mice, only
Scd-1 and
Fabp5 were found downregulated after 16 weeks of treatment [
62].
Another important finding in this work is no regulation of fatty acid oxidation genes, such as
Acadl, Acox1, Cpt1a, Cpt2, Fabp1, Irs1, mTOR, and Ppara by long-term BPF supplementation. The only effect was lower Carnitine palmitoyltransferase 1a
(Cpt1a) expression in CAF and CAF+BPF livers with respect to SC group (
Figure 7A). CPT1A is the key enzyme in the carnitine-dependent transport of fatty acids across the external mitochondrial membrane. Its lower expression should limit fatty acids uptake by mitochondria and thus reduce b-oxidation, as an adaptative process to mitochondria-derived oxidative stress, found in fructose- [
26,
27], but not in high-fat diet-induced NAFLD models [
27,
66]. In fact, both mitochondrial as well as peroxisomal fatty acid oxidation are ROS-generating processes and their excessive stimulation is deleterious for liver tissue [
31]. Lack of positive stimulation of fatty-acid oxidation gene expression in BPF-treated livers is in line with a clinical study in which a multifactorial diet (rich in polyphenols and polyunsaturated fatty acids) downregulated lipogenesis, but did not regulate plasma levels of b-hydroxybutyrate and thus b-oxidation [
67]. Remarkably, the stimulation of hepatic b-oxidation is a typical response to different phytochemicals
in vitro in cellular models of NAFLD [
68,
69,
70,
71], but it is only occasionally reported in rodents treated with purified phenolic compounds [
72,
73]. In conclusion, our data demonstrate that transcriptional suppression of lipogenesis, and not stimulation of b-oxidation, is the main mechanism of BPF counteracting fat accumulation in NAFLD and NASH
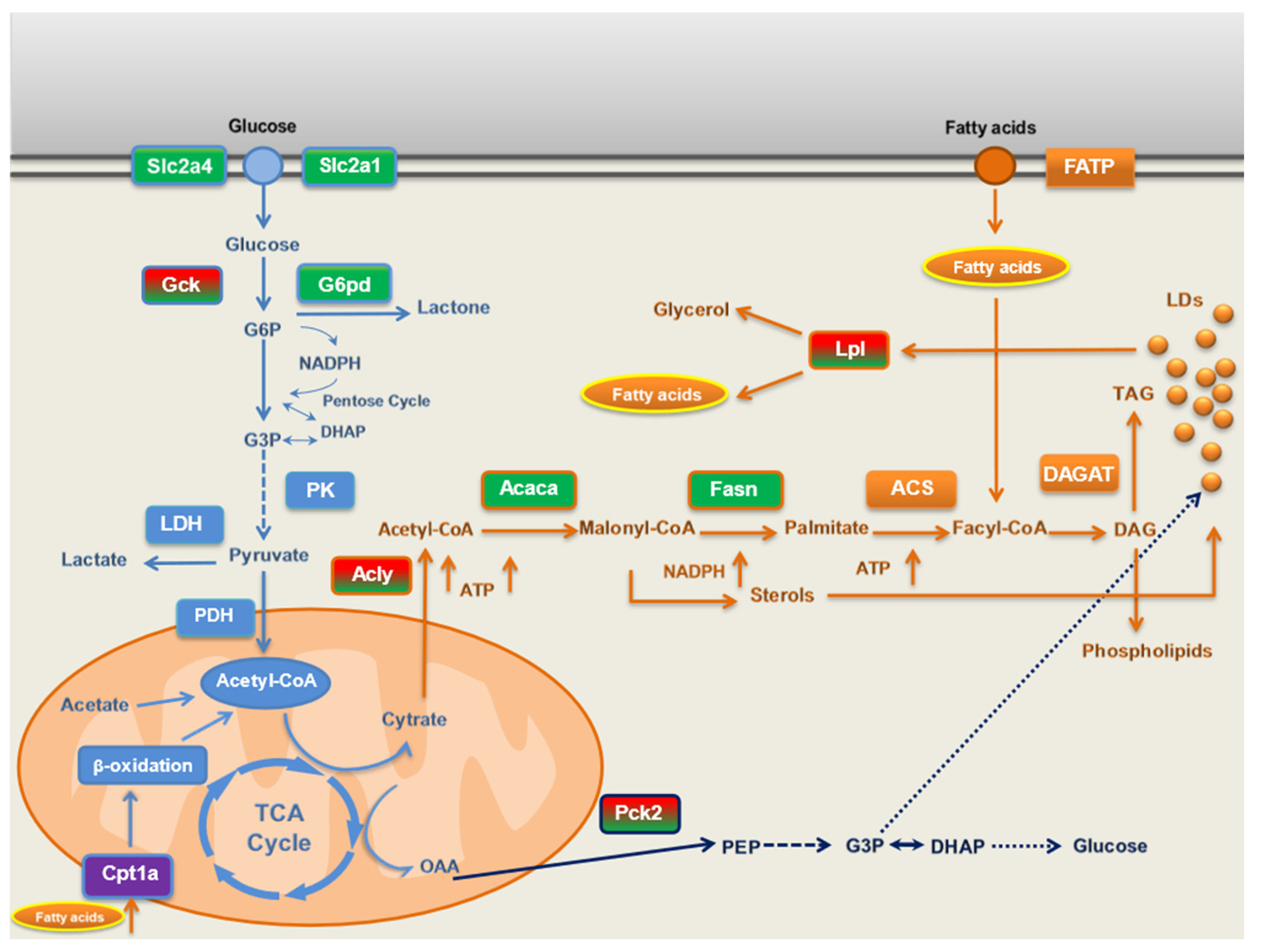
in vivo as depicted in
Figure 9. This finding is consistent with an antioxidant function of flavonoids, mediated by the regulation of expression of genes contributing to healthy redox balance rather than through direct ROS-scavenging.
An unexpected effect of BPF was the induction of Cytochrome 2e1 (CYP2E1) by BPF, a phase I drug metabolism enzyme, but not by CAF diet feeding without BPF. At first glance, it seems surprising since CYP2E1 activity and expression were found elevated in human steatohepatitis and rodent alcohol and methionine-induced liver steatosis [
74]. However, In line with our findings, CYP2E1 expression has not been found increased in most rodent NAFLD models [
71], while it might play a role in polyphenol metabolism and its induction is a hepatic response to flavonoids and related metabolites. In fact, mice treated with flavone-8-acetic acid showed a substantial induction of hepatic CYP2E1 [
75].
Modulation of autophagy to degrade LDs and relieve hepatic inflammation is a potential therapeutic target for NAFLD. Since common transcription factors are believed to regulate lipid metabolism and autophagy, we expected huge transcriptional changes in hepatic autophagy genes, induced by both CAF diet and bergamot polyphenols. Surprisingly, we found that both CAF diet and BPF had limited effects on autophagy-related gene expression. Indeed, it slightly upregulated
Hsp90aa1 and downregulated
Atg16l2, and
Maplc3a, while BPF upregulated only
Maplc3a and
Dapk1. The other two genes
Ins2 and
Tnf, upregulated by CAF diet and downregulated by BPF (
Tnf not significantly) are not typical autophagy-related genes and they play more important roles in diabetes and inflammation. At the protein level, we were able to confirm the downregulation of LC3I and LC3II levels in CAF livers and its upregulation by BPF diet, but not in the case of ATG16L, suggesting that there is a partial correlation between mRNA levels and protein expression. This indicates that polyphenols regulate autophagy mainly by post-translational mechanisms. In fact, Beclin-1 and p62/SQSTM1 were previously found to be regulated by chronic supplementation of BPF in rats [
10], but they are not regulated at mRNA levels in this study. To our knowledge, the data set presented here is the first characterization of autophagy-related gene expression in response to the CAF diet and polyphenols.
Author Contributions
Conceptualization, E.J.; methodology, M.P., D.L., B.F., E.J.; validation, A.L., F.C., and C.R.; formal analysis, E.J., M.P., F.C. and C.R.; investigation, D.L., M.P., A.L and E.J.; resources, E.J.; data curation, E.J., M.P., F.C.; writing—original draft preparation, E.J.; writing—review and editing, E.J. and B.F.; visualization, F.C., C.R and E.J.; supervision, E.J.; funding acquisition, E.J. and V.M. All authors have read and agreed to the published version of the manuscript.
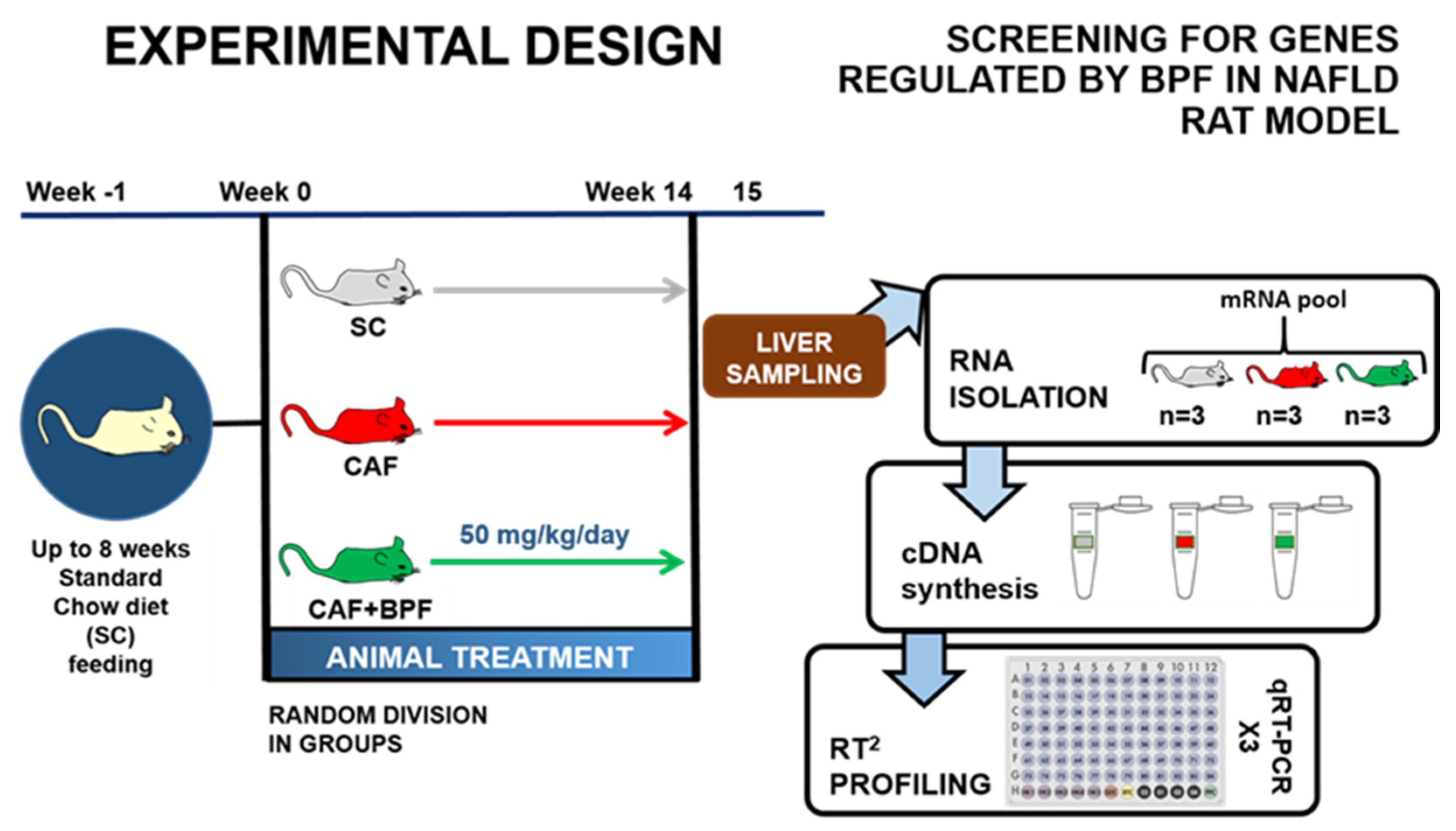
Figure 1.
The Experimental Design and screening for genes regulated by BPF in NAFLD rat model in this work: Rat division for dietetic treatment and BPF administration for 15 weeks. On the right side, flow description from the liver sampling to RT2 profiling.
Figure 1.
The Experimental Design and screening for genes regulated by BPF in NAFLD rat model in this work: Rat division for dietetic treatment and BPF administration for 15 weeks. On the right side, flow description from the liver sampling to RT2 profiling.
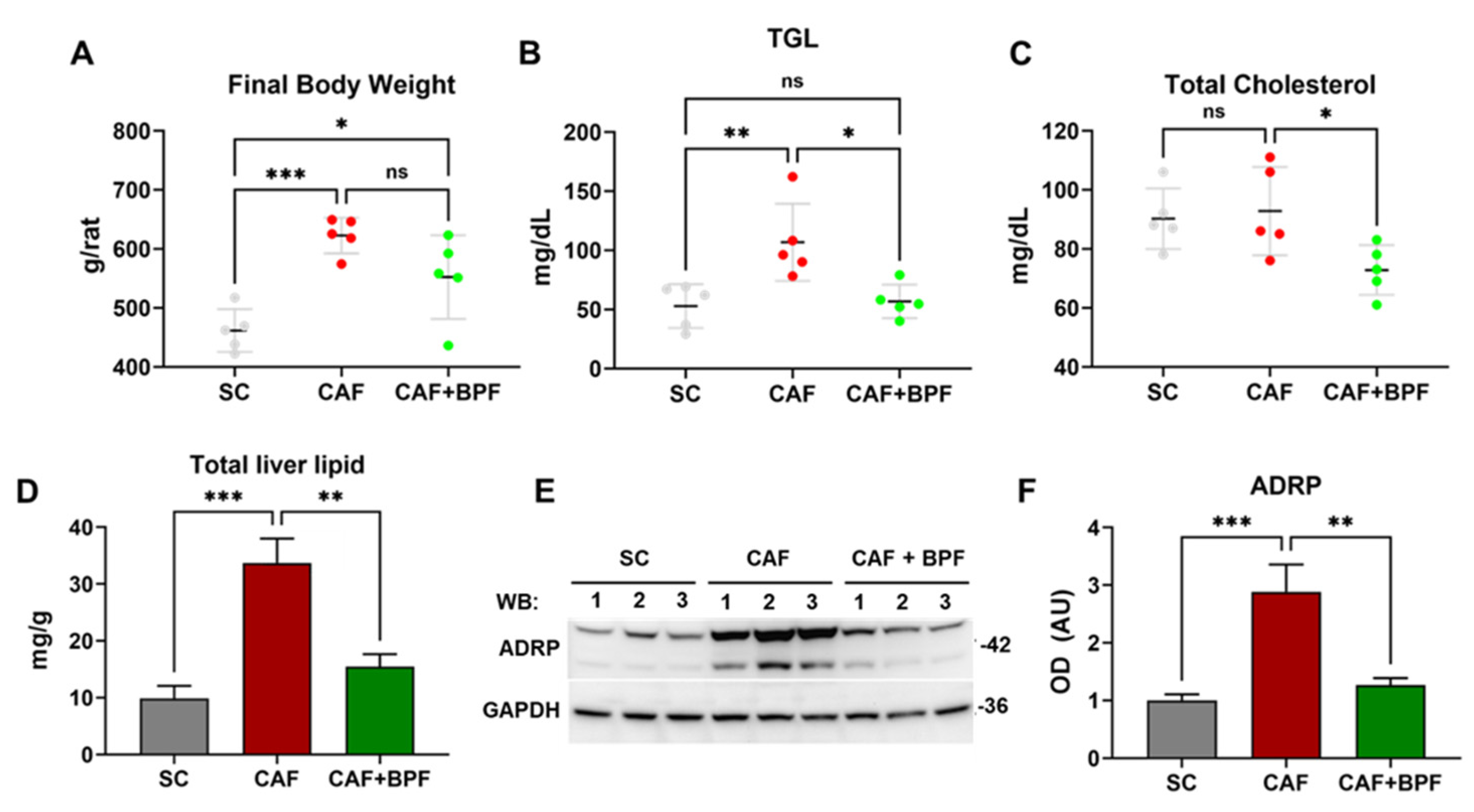
Figure 2.
Bergamot polyphenols prevent CAF diet-induced obesity, hypertriglyceridemia and intracellular fat accumulation in Wistar rats. (A) Final body weight, (B) Blood triglycerides, (TGL) (C) Blood total cholesterol. Data are presented as the mean ± SD of n = 5 rats. Each dot represents a rat. (D) The total lipid content in 400 mg of liver tissue was determined by Folch’s method. Data are presented as the mean ± SD of n = 4 livers for each group. (E) Representative blots for ADRP/Perilipin 2 and GAPDH as a loading control, showing liver lysates from 3 different rats for each group. (F) OD ratio of ADRP to GAPDH expression levels. Data are expressed as the mean ± SEM of n = 6 rat liver lysates for each group. Statistical analysis in A-D and F: One-way ANOVA with Tukey’s post-test. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, ns – not significant change.
Figure 2.
Bergamot polyphenols prevent CAF diet-induced obesity, hypertriglyceridemia and intracellular fat accumulation in Wistar rats. (A) Final body weight, (B) Blood triglycerides, (TGL) (C) Blood total cholesterol. Data are presented as the mean ± SD of n = 5 rats. Each dot represents a rat. (D) The total lipid content in 400 mg of liver tissue was determined by Folch’s method. Data are presented as the mean ± SD of n = 4 livers for each group. (E) Representative blots for ADRP/Perilipin 2 and GAPDH as a loading control, showing liver lysates from 3 different rats for each group. (F) OD ratio of ADRP to GAPDH expression levels. Data are expressed as the mean ± SEM of n = 6 rat liver lysates for each group. Statistical analysis in A-D and F: One-way ANOVA with Tukey’s post-test. * p ≤ 0.05, ** p ≤ 0.01, *** p ≤ 0.001, ns – not significant change.
Figure 3.
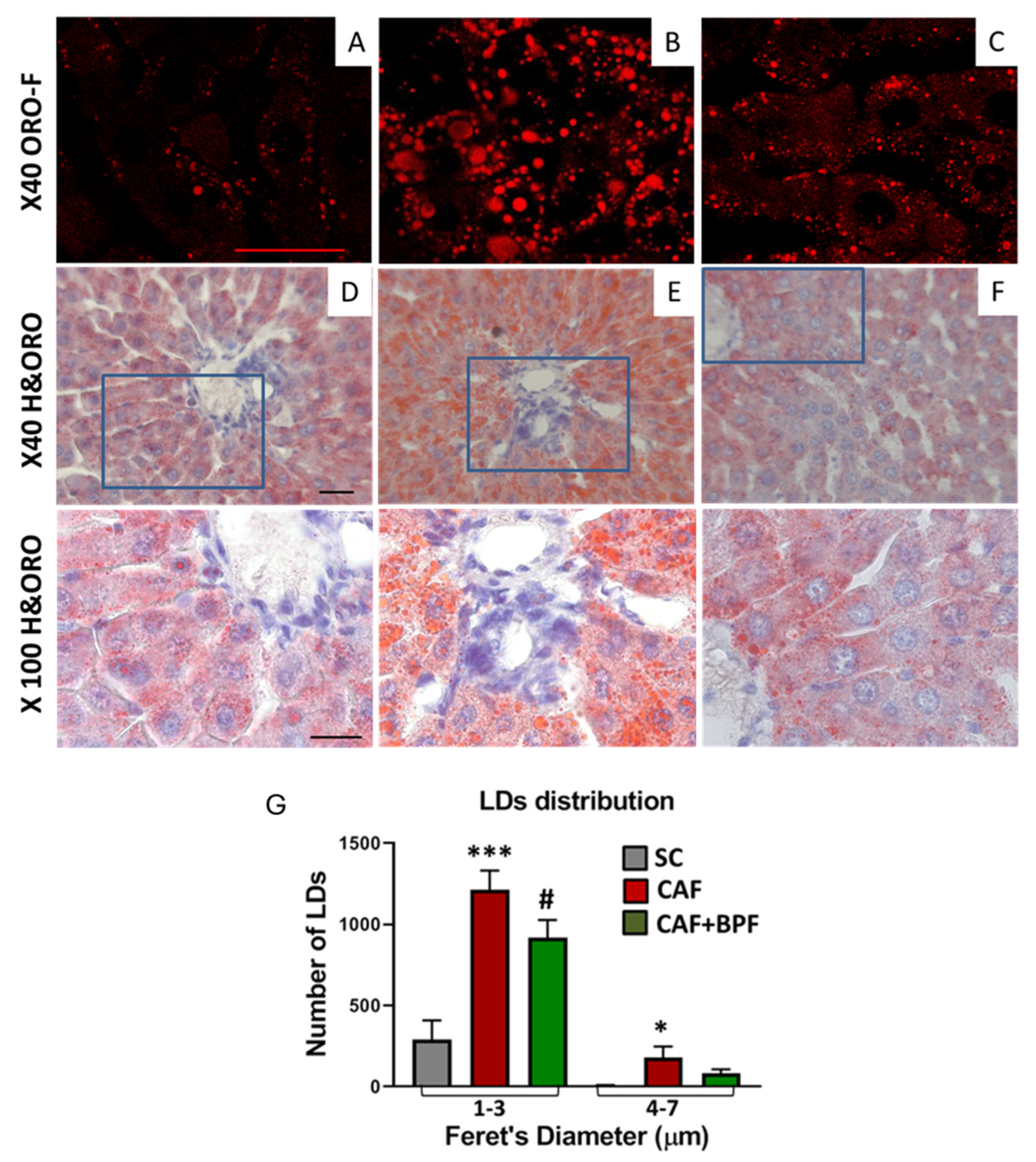
BPF prevents CAF diet-induced hepatic steatosis. Histopathological changes of rat liver tissues between different dietary groups. (A-C) Representative HE and oil-red (ORO) stained liver sections were visualized at high magnification x40, by confocal microscopy bar = 25 μm; (D-F) and by bright-field ×40, bar = 40 μm; Images in the third row show magnified regions of D to F images in indicated by blue boxes, bar = 20 μm to visualize micro- and macro-vesicular steatosis. (G) LDs size and number quantification on confocal sections (as in A-C). LDs sized between 1-3 µm indicate microsteatosis and 4-7 µm macrosteatosis, respectively. Data are presented as the mean ± SEM (n = 3 livers and 9 images for each group). for LDs sized between 1-3 µm and 4-7 µm, respectively. Statistical analysis: Mann-Whitney test. Significant difference in CAF vs SC livers: * p ≤ 0.05, *** p ≤ 0.001. Significant change in CAF+BPF vs CAF group: # p ≤ 0.05.
Figure 3.
BPF prevents CAF diet-induced hepatic steatosis. Histopathological changes of rat liver tissues between different dietary groups. (A-C) Representative HE and oil-red (ORO) stained liver sections were visualized at high magnification x40, by confocal microscopy bar = 25 μm; (D-F) and by bright-field ×40, bar = 40 μm; Images in the third row show magnified regions of D to F images in indicated by blue boxes, bar = 20 μm to visualize micro- and macro-vesicular steatosis. (G) LDs size and number quantification on confocal sections (as in A-C). LDs sized between 1-3 µm indicate microsteatosis and 4-7 µm macrosteatosis, respectively. Data are presented as the mean ± SEM (n = 3 livers and 9 images for each group). for LDs sized between 1-3 µm and 4-7 µm, respectively. Statistical analysis: Mann-Whitney test. Significant difference in CAF vs SC livers: * p ≤ 0.05, *** p ≤ 0.001. Significant change in CAF+BPF vs CAF group: # p ≤ 0.05.
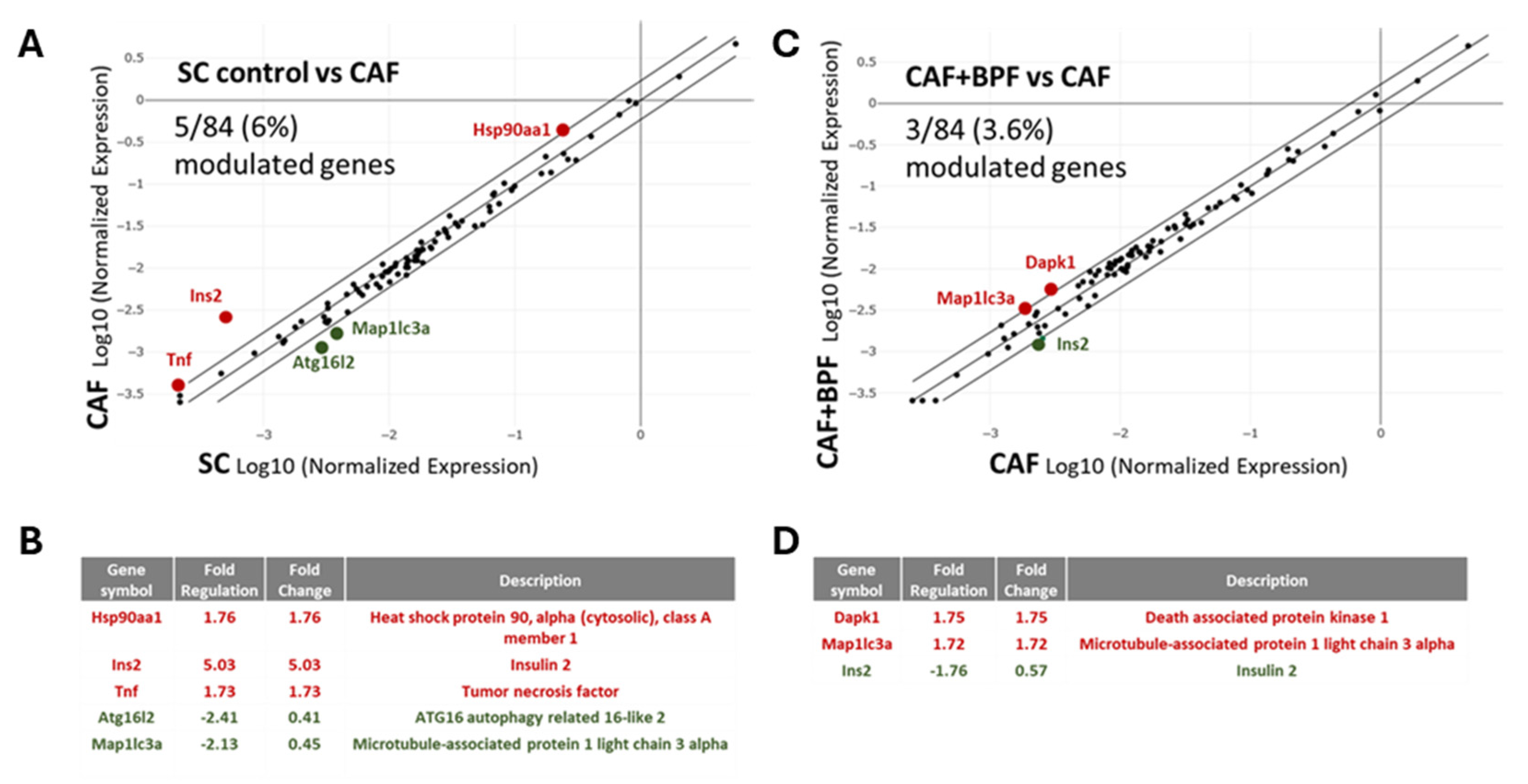
Figure 4.
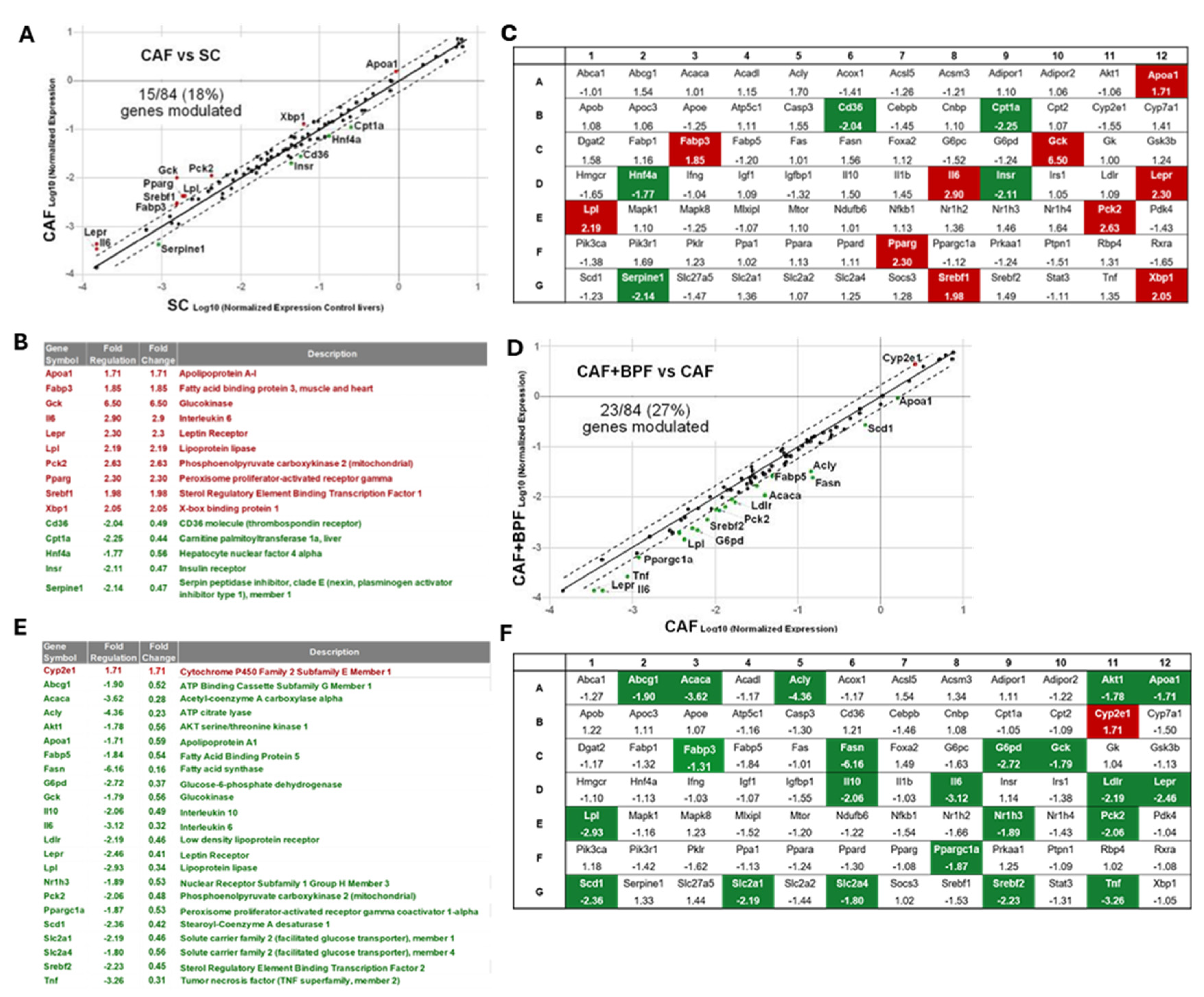
Gene expression changes in steatotic livers exposed to CAF diet for 14 weeks as compared to control SC diet (A, B, C) and the effects of a chronic supplementation of bergamot polyphenols to CAF diet (D, E, F). (A) CAF vs SC and (D) CAF+BPF vs CAF scatter plots for differential RT2 analysis of 84 genes typically modulated in liver steatosis. Below, the lists of genes modulated more than 1.7 fold when CAF are compared to SC livers (B) and CAF+BPF (E) are compared to CAF livers. (B,D) The arrays of all fatty liver-associated genes with respective fold regulation values when (C) CAF vs SC and (F) CAF+BPF vs CAF livers are compared. Note, that bergamot polyphenols strongly suppress lipogenesis-related genes in the liver.
Figure 4.
Gene expression changes in steatotic livers exposed to CAF diet for 14 weeks as compared to control SC diet (A, B, C) and the effects of a chronic supplementation of bergamot polyphenols to CAF diet (D, E, F). (A) CAF vs SC and (D) CAF+BPF vs CAF scatter plots for differential RT2 analysis of 84 genes typically modulated in liver steatosis. Below, the lists of genes modulated more than 1.7 fold when CAF are compared to SC livers (B) and CAF+BPF (E) are compared to CAF livers. (B,D) The arrays of all fatty liver-associated genes with respective fold regulation values when (C) CAF vs SC and (F) CAF+BPF vs CAF livers are compared. Note, that bergamot polyphenols strongly suppress lipogenesis-related genes in the liver.
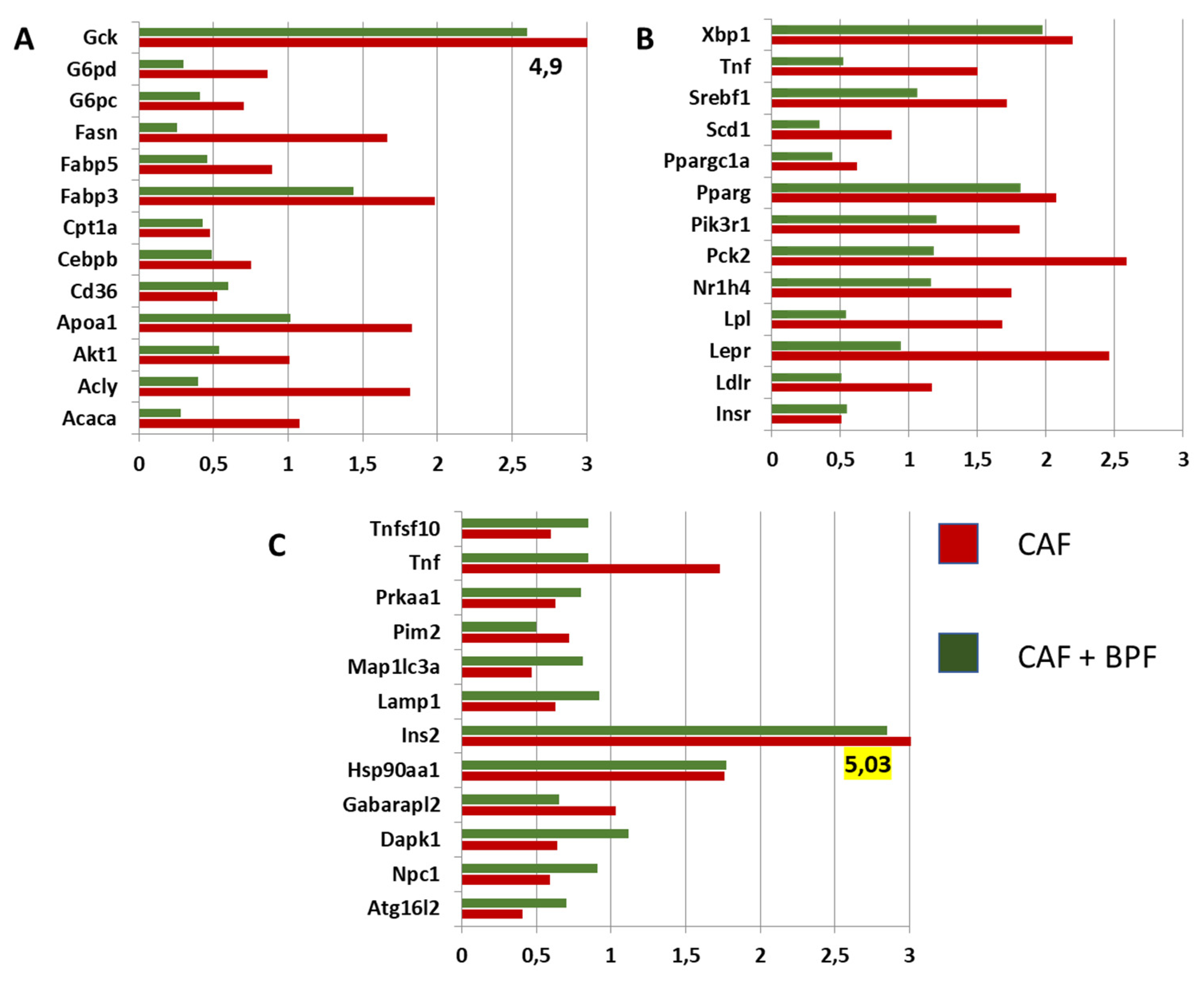
Figure 7.
The expression level of selected genes was analyzed by qRT-PCR with an independent set of primers in each animal separately. The bar represents the mean ± SD of expression data from n = 4 to 5 rat livers relative to the house-keeping control (Hrpt1). *, ** Statistically significant difference compared to the control SC group at p < 0.5 or p < 0.01 respectively. #, ##, ### - Statistically significant difference compared to the control CAF group at p < 0.5, < 0.01 or < 0.001, respectively. Red numbers indicate fold change in CAF compared to SC group. Green and black numbers indicate the fold change in CAF+BPF group compared to CAF and SC groups, respectively.
Figure 7.
The expression level of selected genes was analyzed by qRT-PCR with an independent set of primers in each animal separately. The bar represents the mean ± SD of expression data from n = 4 to 5 rat livers relative to the house-keeping control (Hrpt1). *, ** Statistically significant difference compared to the control SC group at p < 0.5 or p < 0.01 respectively. #, ##, ### - Statistically significant difference compared to the control CAF group at p < 0.5, < 0.01 or < 0.001, respectively. Red numbers indicate fold change in CAF compared to SC group. Green and black numbers indicate the fold change in CAF+BPF group compared to CAF and SC groups, respectively.
Figure 8.
Western blot analysis of protein products of selected genes modulated by CAF diet and BPF. (A) Representative blots showing 3 rat livers, for each group, for ACACA, ACLY, PCK2 and GCK. ADRP/Perilipin 2 has been shown as a marker for steatosis or lipid content and GAPDH as a loading control. (B) OD analysis of expression levels of proteins as in A compared to GAPDH n = 5 to 6 rat livers for each group. (C) Representative blots showing protein lysates from 3 rat livers for each group for autophagy proteins LC3B and ATG16. alpha-tubulin (TUBA) was used as loading control. (D) OD ratio of proteins as in C compared to TUBA in n = 5 to 6 rat livers for each group. Bars show the mean OD ratio ± SEM, normalized to the mean of SC group. Statistical analysis: One-way ANOVA with Tukey’s post-test or with LSD Fisher test for GCK OD analysis. *, **, ***, **** significant difference compared with control SC group at p < 0.5, p < 0.01, p < 0.001 or p < 0.0001, respectively. #, ##, ###, #### significant difference compared with CAF group at p < 0.5, p < 0.01, p < 0.001 or p < 0.0001, respectively. Numbers on the right of blots indicate the approximate position of molecular weights expressed in kDa.
Figure 8.
Western blot analysis of protein products of selected genes modulated by CAF diet and BPF. (A) Representative blots showing 3 rat livers, for each group, for ACACA, ACLY, PCK2 and GCK. ADRP/Perilipin 2 has been shown as a marker for steatosis or lipid content and GAPDH as a loading control. (B) OD analysis of expression levels of proteins as in A compared to GAPDH n = 5 to 6 rat livers for each group. (C) Representative blots showing protein lysates from 3 rat livers for each group for autophagy proteins LC3B and ATG16. alpha-tubulin (TUBA) was used as loading control. (D) OD ratio of proteins as in C compared to TUBA in n = 5 to 6 rat livers for each group. Bars show the mean OD ratio ± SEM, normalized to the mean of SC group. Statistical analysis: One-way ANOVA with Tukey’s post-test or with LSD Fisher test for GCK OD analysis. *, **, ***, **** significant difference compared with control SC group at p < 0.5, p < 0.01, p < 0.001 or p < 0.0001, respectively. #, ##, ###, #### significant difference compared with CAF group at p < 0.5, p < 0.01, p < 0.001 or p < 0.0001, respectively. Numbers on the right of blots indicate the approximate position of molecular weights expressed in kDa.
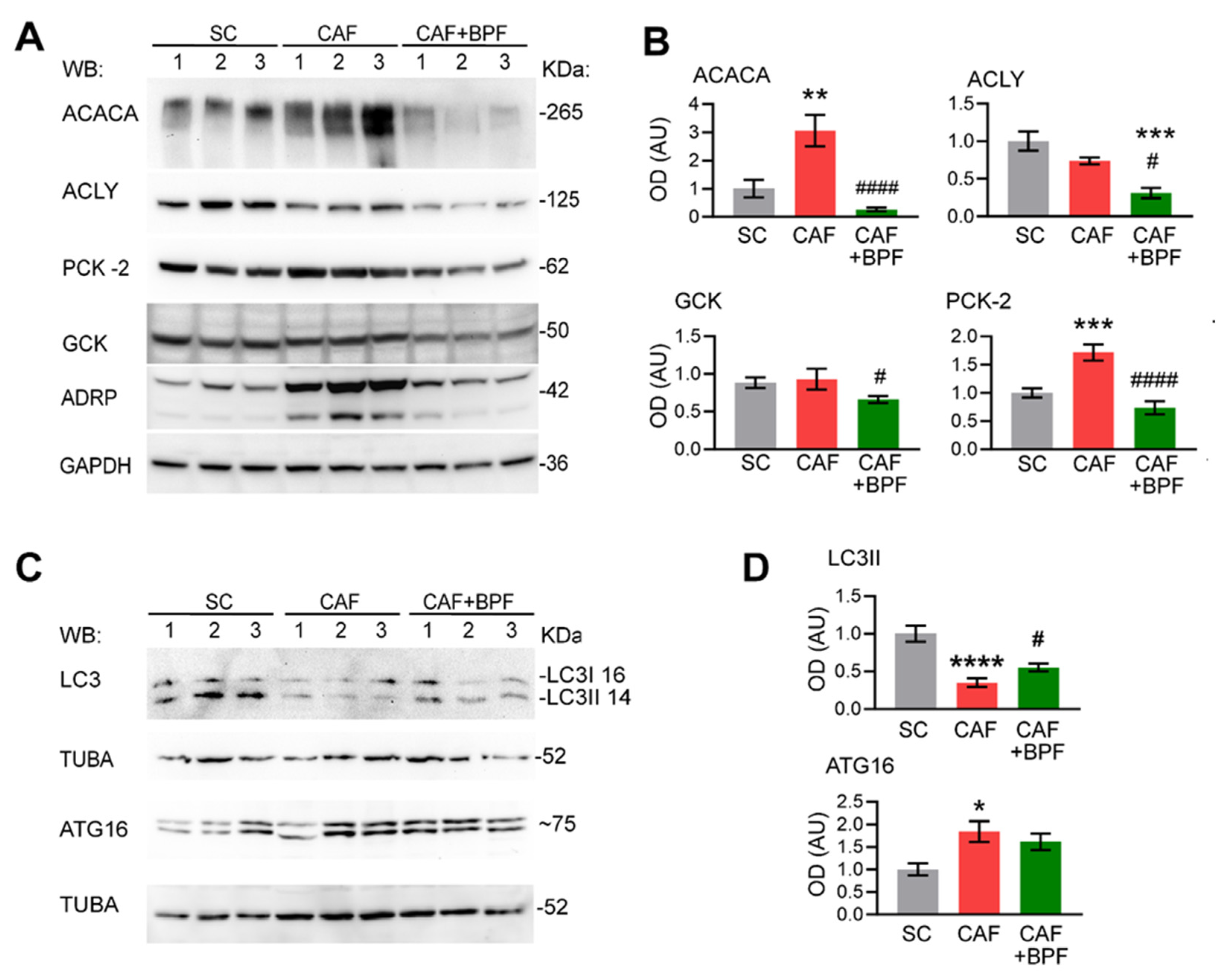
Figure 9.
Schematic representation of main lipid- and glucose-metabolism pathways and their modulation by CAF diet and BPF at mRNA level. Red and green rectangles: genes both upregulated by CAF diet and downregulated in CAF+BPF group, respectively; green rectangles: genes downregulated by BPF; violet rectangles: genes downregulated by CAF diet; light-blue rectangles and outlines: genes and proteins related to glucose metabolism; orange rectangles and outlines: genes and proteins related to lipid metabolism; blue: intermediaries in gluconeogenesis pathway.
Figure 9.
Schematic representation of main lipid- and glucose-metabolism pathways and their modulation by CAF diet and BPF at mRNA level. Red and green rectangles: genes both upregulated by CAF diet and downregulated in CAF+BPF group, respectively; green rectangles: genes downregulated by BPF; violet rectangles: genes downregulated by CAF diet; light-blue rectangles and outlines: genes and proteins related to glucose metabolism; orange rectangles and outlines: genes and proteins related to lipid metabolism; blue: intermediaries in gluconeogenesis pathway.
Table 1.
List of rat-specific primers for RT-qPCR analysis of gene expression. F- Forward, R- reverse.
Table 1.
List of rat-specific primers for RT-qPCR analysis of gene expression. F- Forward, R- reverse.
| ACCESSION |
GENE NAME |
PRIMER |
SEQUENCE 5'-3' |
No.
BASES |
| NM_019130.2 |
Ins2 |
INS2-F |
ATCAGCAAGCAGGTCATTGTTCCA |
24 |
| |
Rattus norvegicus insulin 2 |
INS2-R |
CTTCGCGGCGGGACATGG |
18 |
| NM_016987.2 |
Acly |
ACLY-F |
CGGCTCACACTGCCAACTTC |
20 |
| |
rat ATP citrate lyase |
ACLY-R |
TGGGACTGAATCTTGGGGCA |
20 |
| NM_022193.1 |
Acaca |
ACC1-F |
CTTCGGGGTGGTTCTTGGGT |
20 |
| |
acetyl-CoA carboxylase alpha |
ACC1-R |
TTCCAGAACGGATCCCCTGC |
20 |
| NM_017332.1 |
Fasn |
FASN-F |
ATTGTGGGCGGGATCAACCT |
20 |
| |
fatty acid synthase |
FASN-R |
CGGCAATACCCGTTCCCTGA |
20 |
| NM_013124.3 |
Pparg - peroxisome proliferator |
PPARG-F |
AGCATGGTGCCTTCGCTGAT |
20 |
| |
-activated receptor gamma |
PPARG-R |
GCCCAAACCTGATGGCATTGT |
21 |
| NM_012565.2 |
Gck |
GCK-F |
AGGTGTGGAGCCCAGTTGTTG |
21 |
| |
glucokinase |
GCK-R |
TCCGACTTCTGAGCCTTCTGGG |
22 |
| NM_001108377.2 |
Pck2 |
PCK2-F |
GGTTGAGCATGGAGGGACGA |
20 |
| |
phosphoenolpyruvate carboxykinase 2 |
PCK2-R |
CTAGCACGCGAGCGTTTTCC |
20 |
| NM_001276707.1 |
Srebf1 - sterol regulatory element |
SREBF1-F |
CTCTTGACCGACATCGAAGACAT |
23 |
| |
binding transcription factor 1 |
SREBF1-R |
CCCAGCATAGGGGGCATCAA |
20 |
| NM_001033694.1 |
Srebf2 - sterol regulatory element |
SREBF2-F |
GGCTGTCGGGTGTCATGGG |
19 |
| |
binding transcription factor 2 |
SREBF2-R |
CTGTAGCATCTCGTCGATGTCC |
22 |
| NM_012583.2 |
Hprt1 - hypoxanthine |
HPRT-F |
CTCATGGACTGATTATGGACAGGAC |
25 |
| |
phosphoribosyltransferase 1 |
HPRT-R |
GCAGGTCAGCAAAGAACTTATAGCC |
25 |