Submitted:
10 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
In response to a perceived epidemic of coronary heart disease, Ancel Keys introduced the lipid-heart hypothesis in 1953 which asserted that high intakes of total fat, saturated fat, and cholesterol lead to atherosclerosis and that consuming less fat and cholesterol, and replacing saturated fat with polyunsaturated fat, would reduce serum cholesterol and consequently the risk of heart disease. Keys proposed an equation that would predict the concentration of serum cholesterol (DChol.) from consumption of saturated fat (DS), polyunsaturated fat (DP), and cholesterol (DZ): ΔChol. = 1.2(2ΔS − ΔP) + 1.5ΔZ. However, the Keys equation conflated natural saturated fat and industrial trans-fat into a single parameter and considered only linoleic acid as the polyunsaturated fat. This ignored the widespread consumption of trans-fat and its effects on serum cholesterol and promoted an imbalance of omega-6 to omega-3 fatty acids in the diet. Numerous observational, epidemiological, interventional, and autopsy studies have failed to validate the Keys equation and the lipid-heart hypothesis. Nevertheless, these have been the cornerstone of national and international dietary guidelines which have focused disproportionately on heart disease and much less so on cancer and metabolic disorders, which have steadily increased since the adoption of this hypothesis.
Keywords:
1. Introduction
2. The Ancel Keys Equations and the Diet-Lipid Hypothesis
2.2. Iodine Value: Conflation of Plant-Derived Saturated Fat with Animal Fat
2.3. Solid Fat: Conflation of Plant-Derived Saturated Fat, Animal Fat, and Trans-Fat
2.4. ΔP: High Linoleic Acid Diet
2.5. ΔZ: The Unresolved Role of Dietary Cholesterol
3. The Lipid-Heart Hypothesis Is Not Supported by Observational and Epidemiological Evidence
3.1. Framingham Multi-Generational Study
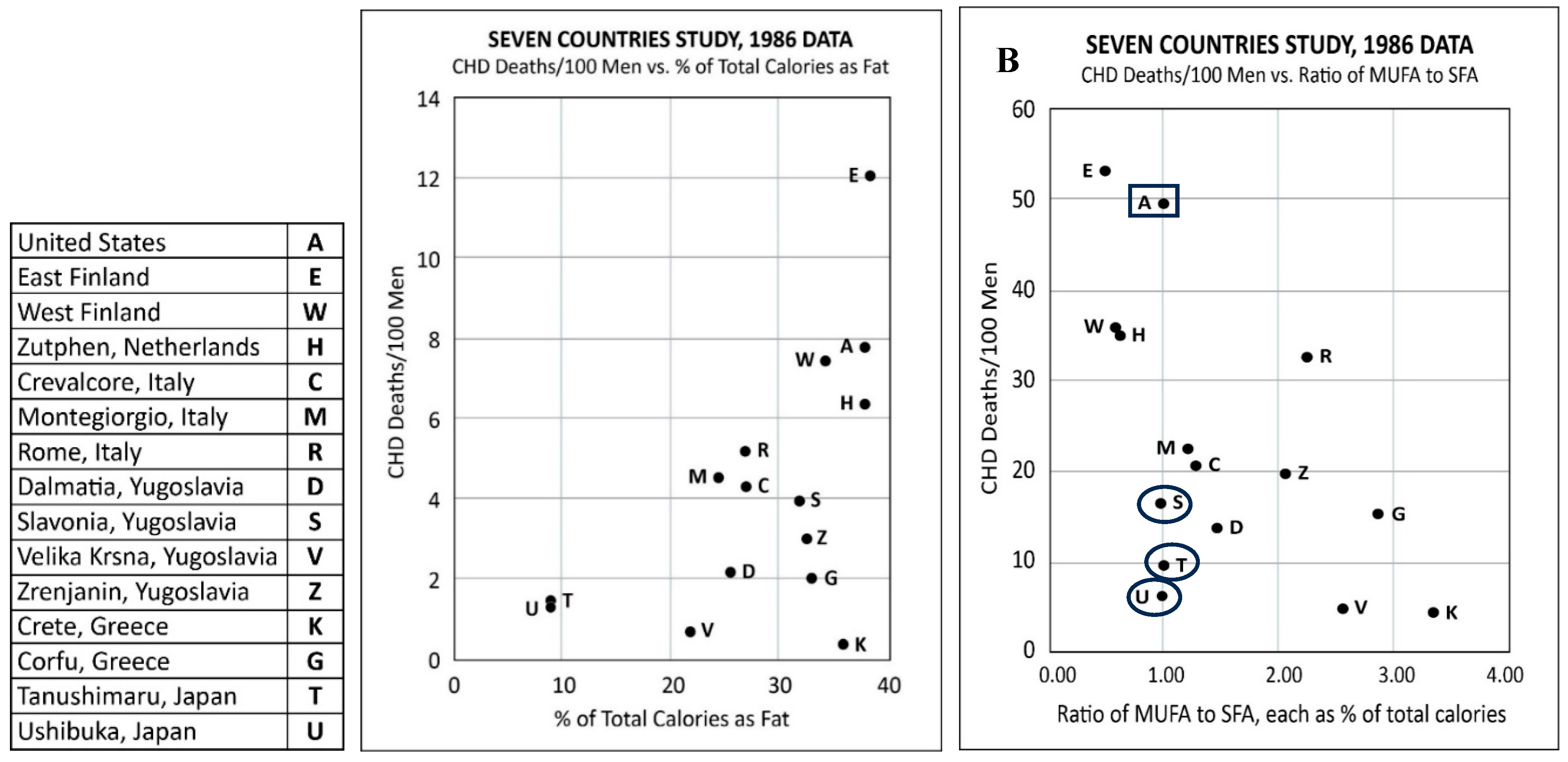
3.2. Seven Countries Study (SCS)
- There was no association of TC levels with CHD deaths.
- High PUFA intake had no association with coronary heart deaths: the cohort with the highest CHD death rate of 12/100 men consumed 2.9% PUFA, which was within the same range of 1.9 to 3.5% as the cohorts with the five lowest coronary heart death rates at < 2/100 men.
- Although Keys ignored oleic acid in his previous studies, the results from SCS showed that all cause and CHD death rates were low in cohorts that consumed olive oil as the main fat.
- There was no association between the percentage of daily calories as total fat and all-cause deaths or CHD deaths (Figure 1A): Crete had the lowest all-cause and CHD deaths but one of the highest fat intakes at 36.1%. However, the East Finland cohort that consumed a comparable amount of fat at 38.5% had the highest coronary heart deaths. The six cohorts with the lowest CHD deaths had total fat intake ranging from 9 to 36.1%.
- Keys claimed that there was an association between CHD deaths and the ratio of the intake of monounsaturated fatty acid to saturated fatty acid (MUFA/SFA). However, the data showed otherwise: two cohorts with the lowest CHD death rates (Tanushimaru and Ushibuka) had the same MUFA/SFA ratio of 1.0 as the cohort which had the second highest number of CHD deaths (US Railroad men) (Figure 1B).
- A later analysis revealed that the food consumed in the Seven Countries Study included margarine (trans-fats).[102]
3.3. A Study from the National Cholesterol Education Program (NCEP)
3.4. Observational and Historical Evidence on Coconut Oil, a Saturated Fat
3.5. Prospective Urban Rural Epidemiology (PURE) Study
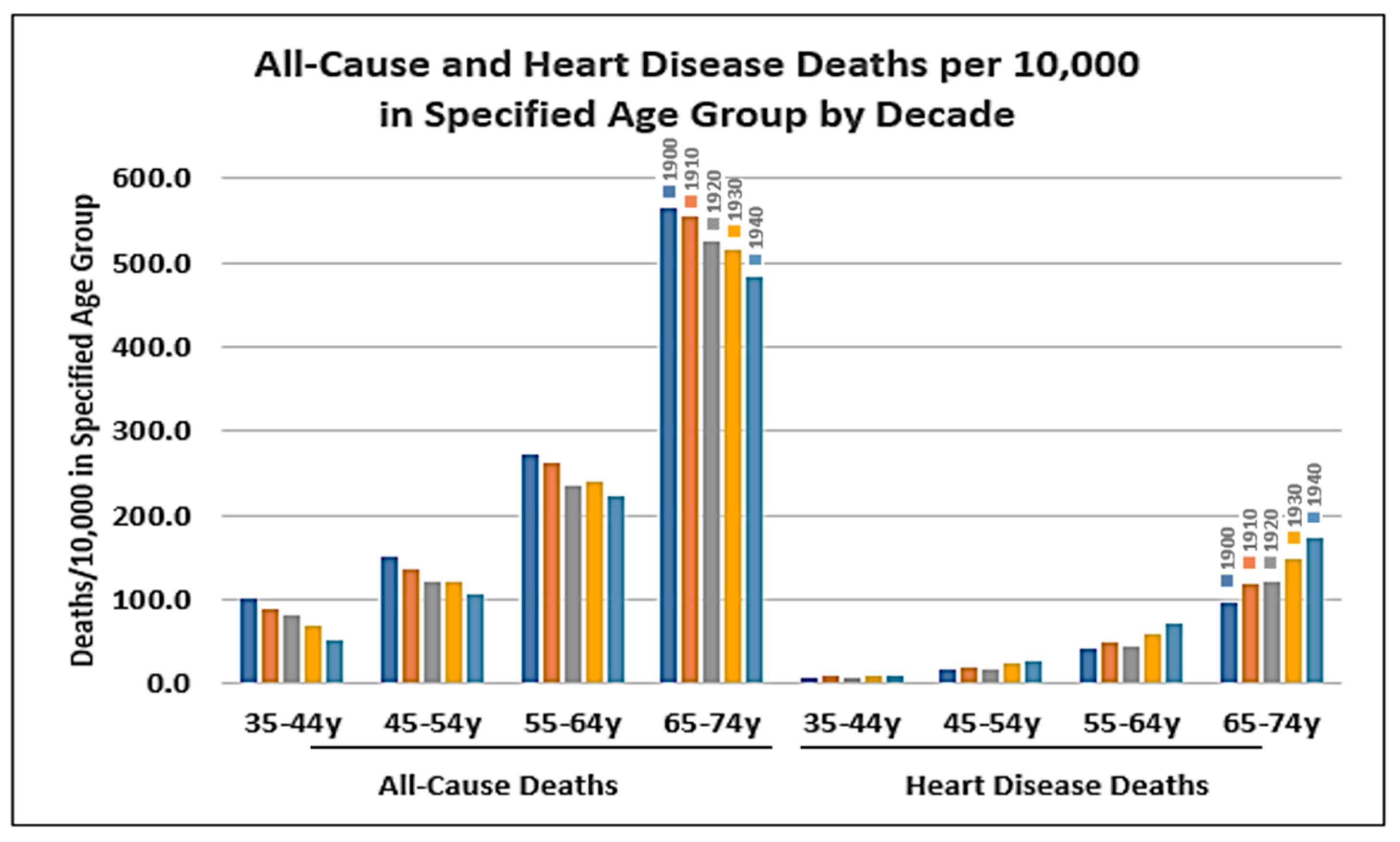
4. Other Factors that Contributed to the Increase in Coronary Heart Disease
4.1. Public Health Measures
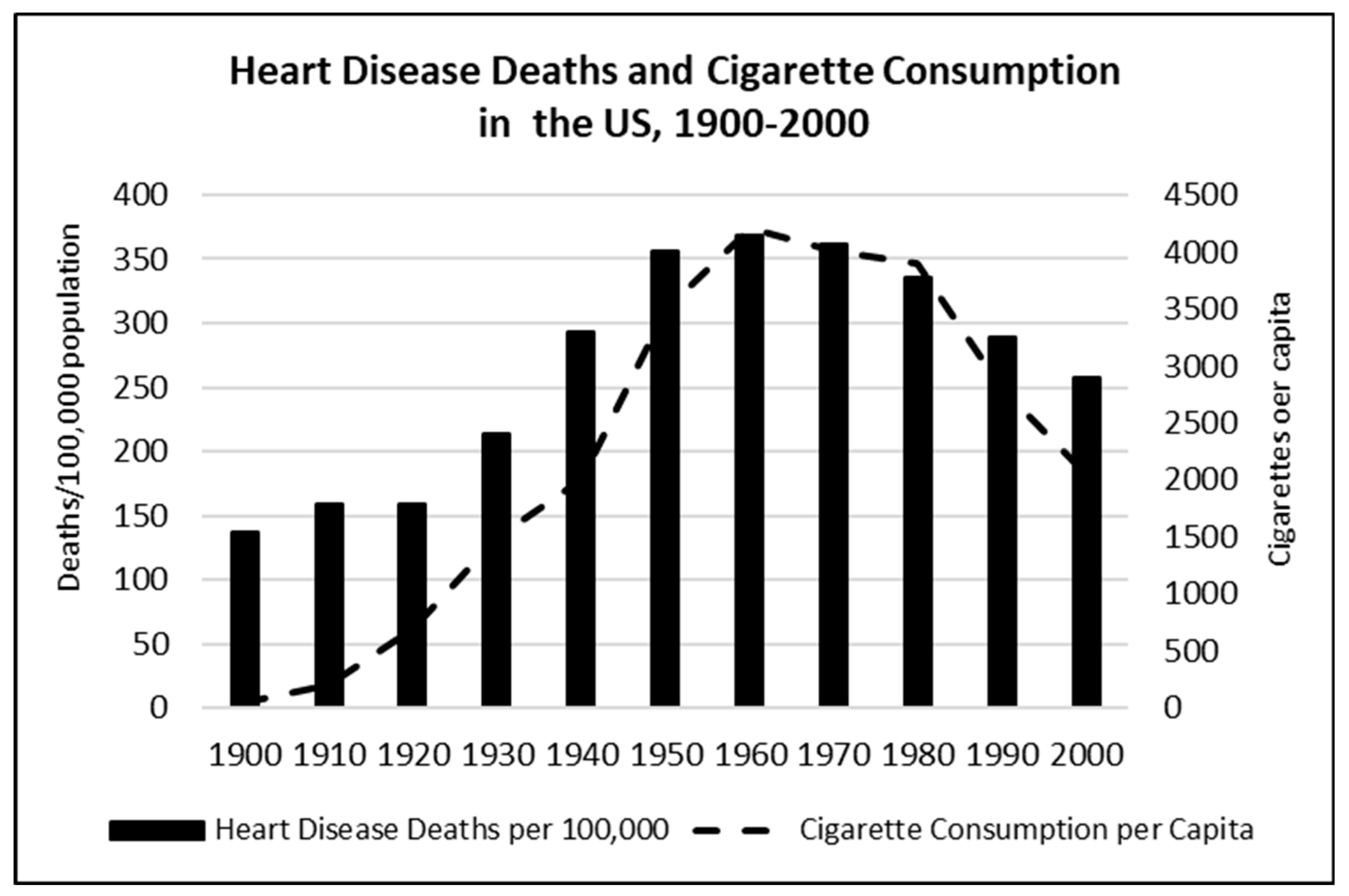
4.2. Behavioural and Environmental Risk Factors: Air Pollution, Smoking, Hypertension, and Diabetes
5. The Lipid-Heart Hypothesis Is Not Supported by Clinical Studies
5.1. The Anti-Coronary Club Study
5.2. National Diet Heart Study
5.3. Multiple Risk Factor Intervention (MRFIT)
5.4. Studies Cited in the 2017 AHA Advisory on Dietary Fats and Cardiovascular Disease
5.5. Sydney Diet Heart Study (SDHS)
5.6. Minnesota Coronary Experiment (MCE)
5.7. Some Autopsy Studies Do Not Support the Lipid-Heart Hypothesis
- In people who die from heart attacks and those who do not.
- In people with high versus low serum cholesterol levels.
- In people who eat higher versus lower percentages of energy as total fat.
- In people who eat higher versus lower percentages of energy as polyunsaturated fat.
- In people who replace saturated fat with polyunsaturated fat.
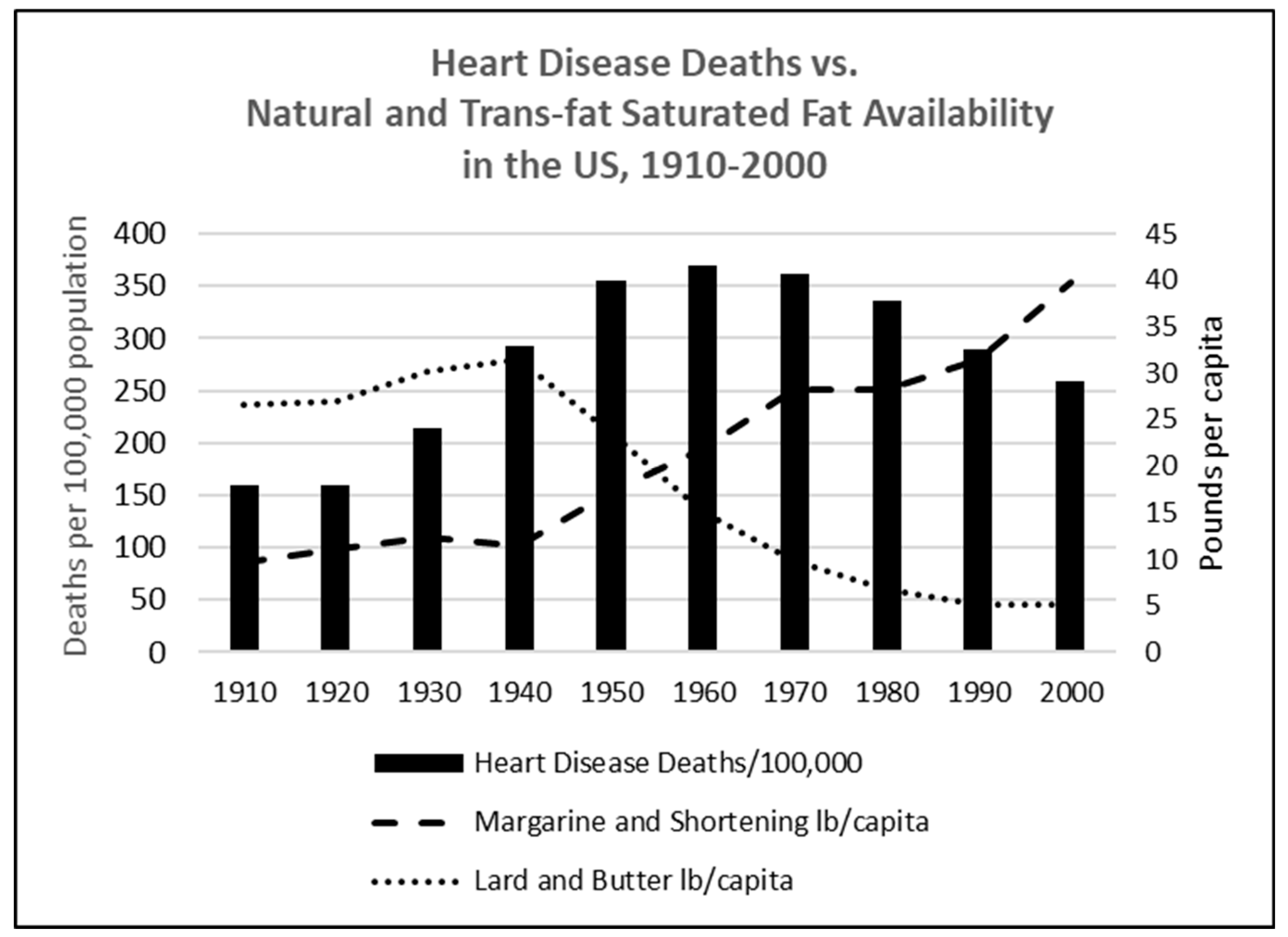
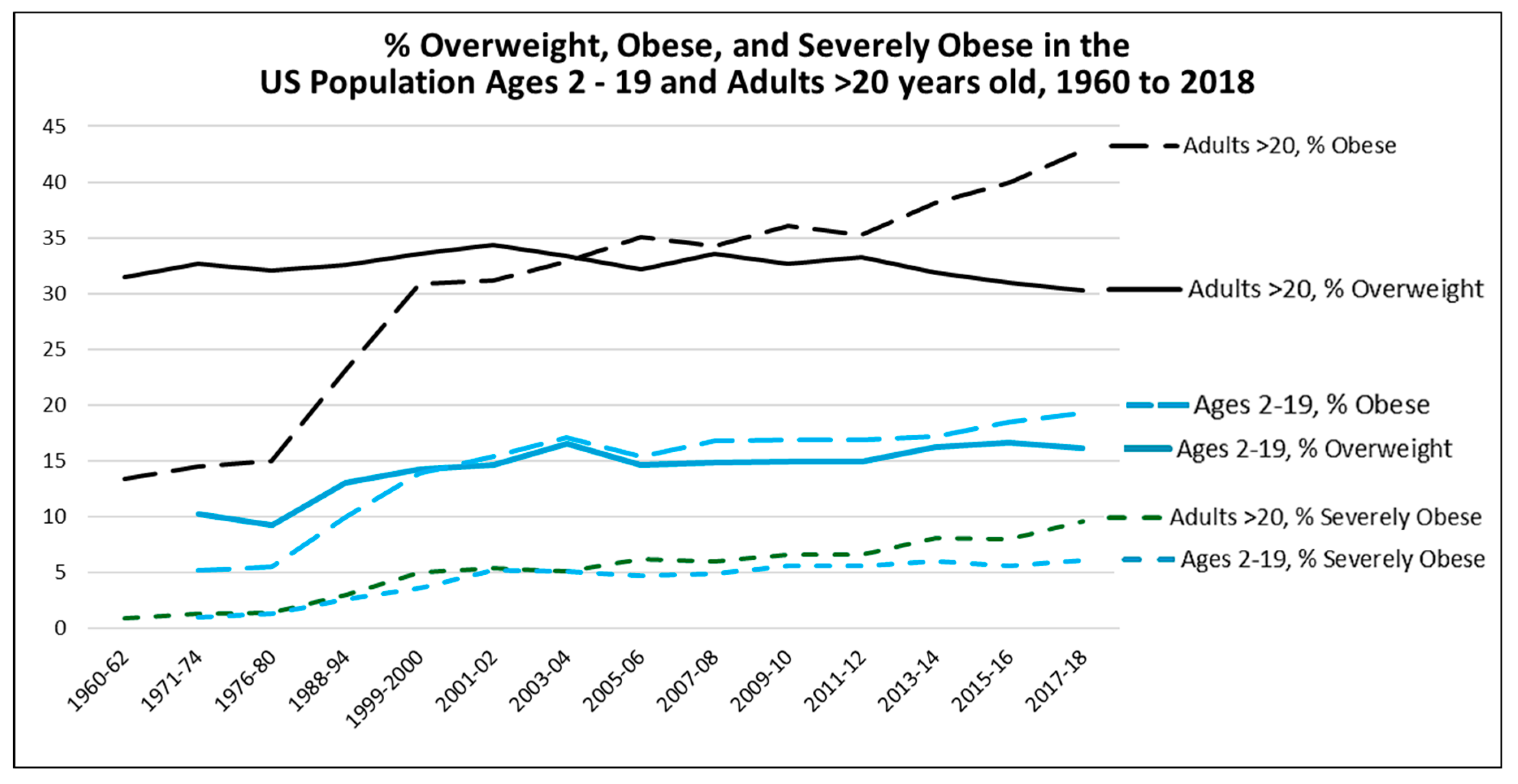
6. The Perceived Epidemic of Heart Disease Has Been Replaced by a Much Larger Epidemic of Metabolic Disorders
7. Conclusions: Implications for Dietary Guidelines
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- U.S. Department of Agriculture and, U.S. U.S. Department of Agriculture and, U.S. Department of Health and Human Services. Nutrition and Your Health. Dietary Guidelines for Americans. 1980.
- Page, I.H.; Allen, E.V.; Chamberlain, F.L.; Keys, A.; Stamler, J.; Stare, F.J. Dietary Fat and Its Relation to Heart Attacks and Strokes. Circ. 1961, 23, 133–136. [Google Scholar] [CrossRef]
- U.S. Department of Agriculture and, U.S. U.S. Department of Agriculture and, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th Edition. 20, page 44. 20 December.
- See, for example: Astrup, A. ; Bertram, H.C.S.;, Bonjour, J.-P., et al. WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ 2019, 366: l4137; Achterberg, C.; Astrup, A.; Bier, D.M.; King, J.C.; Krauss, R.M.; Teicholz, N.; Volek, J.S. An analysis of the recent US dietary guidelines process in light of its federal mandate and a National Academies report. PNAS Nexus 2022, 1, 1–12. [CrossRef]
- Keys, A.; Anderson, J.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 270, 959–966. [Google Scholar] [CrossRef]
- Keys, A. Atherosclerosis: a problem in newer public health. J Mt Sinai Hosp N.Y. 1953, 20, 118–39. [Google Scholar] [PubMed]
- Keys, A. Prediction and possible prevention of coronary artery disease. Amer J Public Health 1953, 43, 1399–1407. [Google Scholar] [PubMed]
- Keys, A.; Anderson, J.; Grande, F. Prediction Of Serum-Cholesterol Responses Of Man To Changes In Fats In The Diet. Lancet 1957, 270, 959–966. [Google Scholar] [CrossRef]
- Keys, A. The diet and the development of coronary heart disease. J. Chronic Dis. 1956, 4, 364–380. [Google Scholar] [CrossRef] [PubMed]
- Codex Alimentarius. Standard for Named Vegetable Oils. Codex Stan 210-1999. Food and Agriculture Organization, 2015.
- Kummerow, F.A. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis 2009, 205, 458–465. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 270, 959–966. [Google Scholar] [CrossRef]
- Johnston, P.V.; Johnson, O.C.; Kummerow, F.A. Occurrence of trans Fatty Acids in Human Tissue. Science 1957, 126, 698–699. [Google Scholar] [CrossRef]
- Malmros, H.; Wigand, G. The effect on serum-cholesterol of diets containing different fats. Lancet 1957, 270, 1–8. [Google Scholar] [CrossRef]
- Anderson, J.T.; Grande, F.; Keys, A. Hydrogenated Fats in the Diet and Lipids in the Serum of Man. J. Nutr. 1961, 75, 388–394. [Google Scholar] [CrossRef] [PubMed]
- Page, I.H.; Allen, E.V.; Chamberlain, F.L.; Keys, A.; Stamler, J.; Stare, F.J. Dietary Fat and Its Relation to Heart Attacks and Strokes. Circ. 1961, 23, 133–136. [Google Scholar] [CrossRef]
- Lee, J.H.; Duster, M.; Roberts, T.; Devinsky, O. United States Dietary Trends Since 1800: Lack of Association Between Saturated Fatty Acid Consumption and Non-communicable Diseases. Front. Nutr. 2022, 8, 748847. [Google Scholar] [CrossRef]
- Blasbalg, T.L.; Hibbeln, J.R.; Ramsden, C.E.; Majchrzak, S.F.; Rawlings, R.R. Changes in consumption of omega-3 and omega-6 fatty acids in the United States during the 20th century. Am. J. Clin. Nutr. 2011, 93, 950–962. [Google Scholar] [CrossRef]
- Shurtleff, W.; Aoyagi, A. History of Soy Oil Hydrogenation and of Research on the Safety of Hydrogenated Vegetable Oils. Source: https://www.soyinfocenter.com/HSS/hydrogenation2.php#:~:text=Lightly%20hydrogenated%2C%20winterized%20soy%20oil,cottonseed%20oil%20(Fig.%20%3F%3F).
- Michels, K.; Sacks, F. Trans Fatty Acids in European Margarines. New Engl. J. Med. 1995, 332, 541–542. [Google Scholar] [CrossRef]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. THE DIET AND 15-YEAR DEATH RATE IN THE SEVEN COUNTRIES STUDY. Am. J. Epidemiology 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Seven Countries: A Multivariate Analysis of Death and Coronary Heart Disease; Ancel, K. , Ed.; Harvard University Press: Cambridge, MA, USA, 1980; ISBN 0-674-80237-3. [Google Scholar]
- Menotti, A.; Kromhout, D.; Blackburn, H.; Fidanza, F.; Buzina, R.; Nissinen, A. Food intake patterns and 25-year mortality from coronary heart disease: Cross-cultural correlations in the Seven Countries Study. Eur. J. Epidemiol. 1999, 15, 507–515. [Google Scholar] [CrossRef]
- Jensen, T. The consumption of fats in Denmark 1900-2000. Anthropology of food 2012, S7. Downloaded on April 4, 2024 from: https://journals.openedition.org/aof/7100.
- Hirata, Y. trans-Fatty Acids as an Enhancer of Inflammation and Cell Death: Molecular Basis for Their Pathological Actions. Biol. Pharm. Bull. 2021, 44, 1349–1356. [Google Scholar] [CrossRef]
- Willett, W. Trans fatty acids and cardiovascular disease—epidemiological data. Atheroscler. Suppl. 2006, 7, 5–8. [Google Scholar] [CrossRef]
- Tarrago-Trani, M.T.; Phillips, K.M.; Lemar, L.E.; Holden, J.M. New and Existing Oils and Fats Used in Products with Reduced Trans-Fatty Acid Content. J. Am. Diet. Assoc. 2006, 106, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, L.V.; Krogager, T.P.; Young, C.; Ferreri, C.; Chatgilialoglu, C.; Jensen, O.N.; Enghild, J.J. Effects of Elaidic Acid on Lipid Metabolism in HepG2 Cells, Investigated by an Integrated Approach of Lipidomics, Transcriptomics and Proteomics. PLOS ONE 2013, 8, e74283. [Google Scholar] [CrossRef]
- Kummerow, F.A. The negative effects of hydrogenated trans fats and what to do about them. Atherosclerosis 2009, 205, 458–465. [Google Scholar] [CrossRef] [PubMed]
- Hirata, Y. trans-Fatty Acids as an Enhancer of Inflammation and Cell Death: Molecular Basis for Their Pathological Actions. Biol. Pharm. Bull. 2021, 44, 1349–1356. [Google Scholar] [CrossRef] [PubMed]
- Oteng, A.-B.; Kersten, S. Mechanisms of Action of trans Fatty Acids. Adv. Nutr. Int. Rev. J. 2019, 11, 697–708. [Google Scholar] [CrossRef]
- Guggisberg, D.; Burton-Pimentel, K.J.; Walther, B.; Badertscher, R.; Blaser, C.; Portmann, R.; Schmid, A.; Radtke, T.; Saner, H.; Fournier, N.; et al. Molecular effects of the consumption of margarine and butter varying in trans fat composition: a parallel human intervention study. Lipids Heal. Dis. 2022, 21, 1–19. [Google Scholar] [CrossRef]
- Enig, M.G. Modification of Membrane Lipid Composition and Mixed-Function Oxidases in Mouse Liver Microsomes by Dietary Trans Fatty Acids. Doctoral Dissertation, University of Maryland, 1984.
- Enig, M.G.; Pallansch, L.; Sampugna, J.; Keeney, M. Fatty acid composition of the fat in selected food items with emphasis on trans components1. J. Am. Oil Chem. Soc. 1983, 60, 1788–1795. [Google Scholar] [CrossRef]
- Enig, M.G.; Atal, S.; Keeney, M.; Sampugna, J. Isomeric trans fatty acids in the U.S. diet. J. Am. Coll. Nutr. 1990, 9, 471–486. [Google Scholar] [CrossRef] [PubMed]
- Enig, M.G.; Fallon, S. The Oiling of America. Originally published in Nexus Magazine in two parts Nov/Dec 1998 and Feb/Mar 1999. Republished in 2006 with permission at https://www.westonaprice. org/oiling-of-america-in-new-york/#gsc.tab=0.
- U.S. Department of Agriculture and, U.S. U.S. Department of Agriculture and, U.S. Department of Health and Human Services. Dietary Guidelines for Americans. Fourth Edition, 1995.
- Takeuchi, H.; Sugano, M. IndustrialTransFatty Acid and Serum Cholesterol: The Allowable Dietary Level. J. Lipids 2017, 2017, 1–10. [Google Scholar] [CrossRef]
- Kris-Etherton, P.M. (Ed.) Expert Panel on Trans Fatty Acids and Coronary Heart Disease. Trans fatty acids and coronary heart disease risk. Am J Clin Nutr 1995, 62, 655S–708S. [Google Scholar]
- Katan, M.B. Commentary on the supplement ‘Trans fatty acids and coronary heart disease risk’. Am J Clin Nutr 1995, 62, 518–519. [Google Scholar]
- Willett, W.C.; Ascherio, A. Response to the International Life Sciences Institute report on trans fatty acids. Am J Clin Nutr 1995, 62, 524–526. [Google Scholar]
- AHA Heart Check Food Certification Guide. 2019. Downloaded from: https://www.heart.org/-/media/Files/Healthy-Living/Company-Collaboration/Heart-Check-Certification/Heart-Check-Food-Certification-Guide.pdf.
- Dietary Guidelines for Americans, 2000, 5th edition, page 28.
- Dietary Guidelines for Americans, 2005, 6th edition, page viii.
- FDA. Small Entity Compliance Guide: Trans Fatty Acids in Nutrition Labeling, Nutrient Content Claims, and Health Claims. 2003. Downloaded from: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/small-entity-compliance-guide-trans-fatty-acids-nutrition-labeling-nutrient-content-claims-and.
- FDA. Final Determination Regarding Partially Hydrogenated Oils (Removing Trans Fat). 2015. Downloaded from: https://www.fda.gov/food/food-additives-petitions/final-determination-regarding-partially-hydrogenated-oils-removing-trans-fat#:~:text=In%202015%2C%20FDA%20released%20its,during%20the%20public%20comment%20period.
- Huang, L.; Gao, L.; Chen, C. Role of Medium-Chain Fatty Acids in Healthy Metabolism: A Clinical Perspective. Trends Endocrinol. Metab. 2021, 32, 351–366. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wu, X.; Simonavicius, N.; Tian, H.; Ling, L. Medium-chain Fatty Acids as Ligands for Orphan G Protein-coupled Receptor GPR84. J. Biol. Chem. 2006, 281, 34457–34464. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 270, 959–966. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: I. Iodine value of dietary fat versus 2S-P. Metabolism 1965, 14, 747–758. [Google Scholar] [CrossRef] [PubMed]
- For example, “solid fat” is mentioned over 50 times in the 2015-2020 Dietary Guidelines for Americans, 8th edition.
- Keys, A.; Anderson, J.; Grande, F. Prediction of serum-cholesterol responses of man to changes in fats in the diet. Lancet 1957, 270, 959–966. [Google Scholar] [CrossRef]
- Keys, A. Serum cholesterol response to dietary cholesterol. Am. J. Clin. Nutr. 1984, 40, 351–359. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. THE DIET AND 15-YEAR DEATH RATE IN THE SEVEN COUNTRIES STUDY. Am. J. Epidemiology 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Simopoulos, A.P. The Importance of the Omega-6/Omega-3 Fatty Acid Ratio in Cardiovascular Disease and Other Chronic. Exp Biol Med (Maywood) 2008, 233, 674. [Google Scholar]
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Hear. 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed]
- Maekawa, S.; Takada, S.; Nambu, H.; et al. Linoleic acid improves assembly of the CII subunit and CIII2/CIV complex of the mitochondrial oxidative phosphorylation system in heart failure. Cell Communication and Signaling 2019, 17, 128. [Google Scholar] [CrossRef] [PubMed]
- Zong, G.; Liu, G.; Willett, W.C.; Wanders, A.J.; Alssema, M.; Zock, P.L.; Hu, F.B.; Sun, Q. Associations Between Linoleic Acid Intake and Incident Type 2 Diabetes Among U.S. Men and Women. Diabetes Care 2019, 42, 1406–1413. [Google Scholar] [CrossRef] [PubMed]
- Patterson, E.; Wall, R.; Fitzgerald, G.F.; Ross, R.P. ; Stanton C: Health Implications of High Dietary Omega-6 Polyunsaturated Fatty Acids. J Nutr Metab 2012, 2012, 539426. [Google Scholar] [CrossRef]
- Lands, W.E.M. Fish, Omega-3, and Human Health 2nd ed. Urbana, IL: AOCS Press, 2005.
- Simopoulos, A.P.; DiNicolantonio, J.J. The importance of a balanced ω-6 to ω-3 ratio in the prevention and management of obesity. Open Hear. 2016, 3, e000385. [Google Scholar] [CrossRef] [PubMed]
- Vaskova, H.; Buckova, M. Thermal Degradation of Vegetable Oils: Spectroscopic Measurement and Analysis. Procedia Eng. 2015, 100, 630–635. [Google Scholar] [CrossRef]
- Sébédio, J.L.; Christie, W.W. Metabolism of Trans Polyunsaturated Fatty Acids Formed during Frying. AOCS Lipid Library. 2021. Downloaded from: https://lipidlibrary.aocs.org/chemistry/physics/frying-oils/metabolism-of-trans-polyunsaturated-fatty-acids-formed-during-frying.
- Gadiraju, T.V.; Patel, Y.; Gaziano, J.M.; Djoussé, L. Fried Food Consumption and Cardiovascular Health: A Review of Current Evidence. Nutrients 2015, 7, 8424–8430. [Google Scholar] [CrossRef]
- Steinberg, D. Thematic review series: The Pathogenesis of Atherosclerosis. An interpretive history of the cholesterol controversy: part I. J. Lipid Res. 2004, 45, 1583–1593. [Google Scholar] [CrossRef] [PubMed]
- Page IH, Allen EV, Chamberlain FL, Keys A, Stamler J, Stare FJ (AHA Central Committee). Dietary Fat and Its Relation to Heart Attacks and Strokes. Circ 1961; 23: 133-136.hematic review series: the pathogenesis of atherosclerosis. An interpretive history of the cholesterol controversy: part I. J Lipid Res. 2004;45:1583–1593.
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: I. Iodine value of dietary fat versus 2S-P. Metabolism 1965, 14, 747–758. [Google Scholar] [CrossRef]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: II. The effect of cholesterol in the diet. Metabolism 1965, 14, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: III. Differences among individuals. Metabolism 1965, 14, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: III. Differences among individuals. Metabolism 1965, 14, 766–775. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Anderson, J.T.; Grande, F. Serum cholesterol response to changes in the diet: II. The effect of cholesterol in the diet. Metabolism 1965, 14, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Mattson, F.H.; Grundy, S.M. Comparison of effects of dietary saturated, monounsaturated, and polyunsaturated fatty acids on plasma lipids and lipoproteins in man. J. Lipid Res. 1985, 26, 194–202. [Google Scholar] [CrossRef] [PubMed]
- Ng, T.K.; Hassan, K.; Lim, J.B.; Lye, M.S.; Ishak, R. Nonhypercholesterolemic effects of a palm-oil diet in Malaysian volunteers. Am. J. Clin. Nutr. 1991, 53, 1015S–1020S. [Google Scholar] [CrossRef] [PubMed]
- McKenney, J.M.; Proctor, J.D.; Wright, J.T.; Kolinski, R.J.; Elswick, R.; Coaker, J.S. The Effect of Supplemental Dietary Fat on Plasma Cholesterol Levels in Lovastatin-Treated Hypercholesterolemic Patients. Pharmacother. J. Hum. Pharmacol. Drug Ther. 1995, 15, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Keys, A. Serum cholesterol response to dietary cholesterol. Am. J. Clin. Nutr. 1984, 40, 351–359. [Google Scholar] [CrossRef]
- Hegsted, D.M. Serum-cholesterol response to dietary cholesterol: a re-evaluation. Am. J. Clin. Nutr. 1986, 44, 299–305. [Google Scholar] [CrossRef] [PubMed]
- Nauck, M.; Warnick, G.R.; Rifai, N. Methods for Measurement of LDL-Cholesterol: A Critical Assessment of Direct Measurement by Homogeneous Assays versus Calculation. Clin. Chem. 2002, 48, 236–254. [Google Scholar] [CrossRef]
- Behbodikhah, J.; Ahmed, S.; Elyasi, A.; Kasselman, L.J.; De Leon, J.; Glass, A.D.; Reiss, A.B. Apolipoprotein B and Cardiovascular Disease: Biomarker and Potential Therapeutic Target. Metabolites 2021, 11, 690. [Google Scholar] [CrossRef] [PubMed]
- Neelakantan, N.; Seah, J.Y.H.; van Dam, R.M. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors. Circ. 2020, 141, 803–814. [Google Scholar] [CrossRef] [PubMed]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Khaw, K.-T.; Sharp, S.J.; Finikarides, L.; Afzal, I.; Lentjes, M.; Luben, R.; Forouhi, N.G. Randomised trial of coconut oil, olive oil or butter on blood lipids and other cardiovascular risk factors in healthy men and women. BMJ Open 2018, 8, e020167. [Google Scholar] [CrossRef] [PubMed]
- Chinwong, S.; Chinwong, D.; Mangklabruks, A. Daily Consumption of Virgin Coconut Oil Increases High-Density Lipoprotein Cholesterol Levels in Healthy Volunteers: A Randomized Crossover Trial. Evidence-Based Complement. Altern. Med. 2017, 2017, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Vijaya Kumar, M.; Vasudevan, D.M.; Sundaram, K.R.; Krishnan, S.; Chandrasekhar, R.; et al. Effect of Virgin Coconut Oil on Lipid Profile and Other CVD Risk Factors. Indian J Nutri 2022, 9, 260. [Google Scholar]
- Fernando, M.G.; Silva, R.; Fernando, W.B.; de Silva, H.A.; Wickremasinghe, A.R.; Dissanayake, A.S.; Sohrabi, H.R.; Martins, R.N.; Williams, S.S. Effect of Virgin Coconut Oil Supplementation on Cognition of Individuals with Mild-to-Moderate Alzheimer’s Disease in Sri Lanka (VCO-AD Study): A Randomized Placebo-Controlled Trial. J. Alzheimer's Dis. 2023, 96, 1195–1206. [Google Scholar] [CrossRef] [PubMed]
- Oliveira-De-Lira, L.; Santos, E.M.C.; De Souza, R.F.; Matos, R.J.B.; da Silva, M.C.; Oliveira, L.D.S.; Nascimento, T.G.D.; Schemly, P.A.d.L.S.; de Souza, S.L. Supplementation-Dependent Effects of Vegetable Oils with Varying Fatty Acid Compositions on Anthropometric and Biochemical Parameters in Obese Women. Nutrients 2018, 10, 932. [Google Scholar] [CrossRef]
- Korrapati, D.; Jeyakumar, S.M.; Putcha, U.K.; Mendu, V.R.; Ponday, L.R.; Acharya, V.; Koppala, S.R.; Vajreswari, A. Coconut oil consumption improves fat-free mass, plasma HDL-cholesterol and insulin sensitivity in healthy men with normal BMI compared to peanut oil. Clin. Nutr. 2018, 38, 2889–2899. [Google Scholar] [CrossRef]
- Neelakantan, N.; Seah, J.Y.H.; van Dam, R.M. The Effect of Coconut Oil Consumption on Cardiovascular Risk Factors. Circ. 2020, 141, 803–814. [Google Scholar] [CrossRef]
- National Diet Heart Study. The National Diet-Heart Study Final Report. Circulation 1968, 37, 1–428.
- Barnard, R.J. Effects of Life-style Modification on Serum Lipids. Arch Intern Med 1991, 151, 1389–1394. [Google Scholar] [CrossRef] [PubMed]
- Okami, Y.; Ueshima, H.; Nakamura, Y.; Okuda, N.; Nakagawa, H.; Sakata, K.; Saitoh, S.; Okayama, A.; Yoshita, K.; Choudhury, S.R.; et al. The Relationship of Dietary Cholesterol with Serum Low-Density Lipoprotein Cholesterol and Confounding by Reverse Causality: The INTERLIPID Study. J. Atheroscler. Thromb. 2019, 26, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Mickelsen, O. ; Miller EvO, Hayes, E. R.; Todd, R.L. The Concentration of Cholesterol in the Blood Serum of Norman Man and its Relation to Age. J Clin Invest 1950, 29, 1347–1353. [Google Scholar]
- Lecerf, J.-M. , de Lorgeril, M. Dietary cholesterol: from physiology to cadiovascular risk. British Journal of Nutrition. 2011, 106, 6–14. [Google Scholar]
- Falomir-Lockhart, L.J.; Cavazzutti, G.F.; Giménez, E.; Toscani, A.M. Fatty Acid Signaling Mechanisms in Neural Cells: Fatty Acid Receptors. Front. Cell. Neurosci. 2019, 13, 162. [Google Scholar] [CrossRef] [PubMed]
- U.S. Department of Agriculture and, U.S. U.S. Department of Agriculture and, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2015-2020. 8th Edition; page 32.
- U.S. Department of Agriculture and, U.S. U.S. Department of Agriculture and, U.S. Department of Health and Human Services. Dietary Guidelines for Americans, 2020-2025. 9th Edition; see for example page 5.
- Carson, J.A.S.; Lichtenstein, A.H.; Anderson, C.A.; Appel, L.J.; Kris-Etherton, P.M.; Meyer, K.A.; Petersen, K.; Polonsky, T.; Van Horn, L.; On behalf of the American Heart Association Nutrition Committee of the Council on Lifestyle; et al. Dietary Cholesterol and Cardiovascular Risk: A Science Advisory From the American Heart Association. Circ. 2020, 141, E39–E53. [Google Scholar] [CrossRef] [PubMed]
- Kannel, W.B.; Gordon, T. “The Framingham Study: an epidemiological investigation of cardiovascular disease.” Unpublished paper. Washington, DC: National Heart, Lung, and Blood Institute (1987):24. Available at: https://www.scribd. com/document/583903774/Kannel-W-Gordon-T-Framingham-dietary-data. Section-24-unpublished.
- Castelli, W.P. Concerning the possibility of a nut. .. Arch Intern Med 1992, 152, 1371–1372. [Google Scholar] [CrossRef] [PubMed]
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. THE DIET AND 15-YEAR DEATH RATE IN THE SEVEN COUNTRIES STUDY. Am. J. Epidemiology 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Keys, A. Seven Countries. Harvard University Press, Cambridge, Massachusetts (1980).
- Keys, A.; Mienotti, A.; Karvonen, M.J.; Aravanis, C.; Blackburn, H.; Buzina, R.; Djordjevic, B.S.; Dontas, A.S.; Fidanza, F.; Keys, M.H.; et al. The diet and 15-year death rate in the seven countries study. Am. J. Epidemiology 1986, 124, 903–915. [Google Scholar] [CrossRef]
- Menotti, A.; Kromhout, D.; Blackburn, H.; Fidanza, F.; Buzina, R.; Nissinen, A. Food intake patterns and 25-year mortality from coronary heart disease: Cross-cultural correlations in the Seven Countries Study. Eur. J. Epidemiol. 1999, 15, 507–515. [Google Scholar] [CrossRef]
- CDC. Leading Causes of Death, 1900-1998. https://www.cdc.gov/nchs/data/dvs/lead1900_98.pdf.
- Keys, A. Seven Countries. Harvard University Press, Cambridge, Massachusetts (1980).
- Blackburn, H. On the Trail of Heart Attacks in Seven Countries. The Country Press, Inc., Minnesota (1995). ISBN 13: 9781887268004.
- Sachdeva, A.; Cannon, C.P.; Deedwania, P.C.; LaBresh, K.A.; Smith, S.C.; Dai, D.; Hernandez, A.F.; Fonarow, G.C. Lipid levels in patients hospitalized with coronary artery disease: An analysis of 136,905 hospitalizations in Get With The Guidelines. Am. Heart J. 2009, 157, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Ravnskov, U.; de Lorgeril, M.; Diamond, D.M.; Hama, R.; Hamazaki, T.; et al. The LDL paradox: Higher LDL-Cholesterol is Associated with Greater Longevity. Ann Epidemiol Public Health 2020, 3, 1040. [Google Scholar]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef]
- Coconut oil. U.S. Department of Agriculture. Agricultural Research Service. FDC Published: 4/1/2020. Downloaded from: https://ndb.nal.usda.gov/fdc-app.html#/food-details/789034/nutrients.
- Cooke, F.C. The coconut palm as a source of food. Ceylon Coconut Q 1951, 2, 153–156. [Google Scholar]
- Ekanayaka, R.A.I. ; de Silva PGSM, Ekanayaka, M. K.I.; Jayathilake, W.M.M.; Pathirana RPMMR, Amaratunga, Y.N.; De Silva, P.J.D.; Perera, B. Effect of different forms of coconut on the lipid profile in normal free-living healthy subjects: A randomized controlled trial (Phase II). Global Epidemiology 2024, 7, 100138. [Google Scholar]
- Prior, I. Epidemiology of Cardiovascular disease in Asian-Pacific Region. Sing Med J 1973, 14, 223–227. [Google Scholar]
- A Prior, I.; Davidson, F.; E Salmond, C.; Czochanska, Z. Cholesterol, coconuts, and diet on Polynesian atolls: a natural experiment: the Pukapuka and Tokelau Island studies. Am. J. Clin. Nutr. 1981, 34, 1552–1561. [Google Scholar] [CrossRef]
- WHO. Diet, Food Supply and Obesity in the Pacific WHO Regional Office for the Western Pacific. 2003. ISBN 92 9061 044 1.
- Westerdahl, J. Part I: The Traditional Hawaiian Diet: A Paradise of Healthy Foods. Vegetarian Nutrition Update 2006, 14, 1. [Google Scholar]
- Florentino, R.F.; Aguinaldo, A.R. Diet and Cardiovascular Disease in the Philippines. Phil J Coconut Studies 1987, 13, 56–70. [Google Scholar]
- Kumar, P.D. The Role of Coconut and Coconut Oil in Coronary Heart Disease in Kerala, South India. Trop. Dr. 1997, 27, 215–217. [Google Scholar] [CrossRef] [PubMed]
- I Lipoeto, N.; Agus, Z.; Oenzil, F.; Wahlqvist, M.; Wattanapenpaiboon, N. Dietary intake and the risk of coronary heart disease among the coconut-consuming Minangkabau in West Sumatra, Indonesia. J Clin Nutr 2004, 13, 377–84. [Google Scholar]
- Vijayakumar, M.; Vasudevan, D.; Sundaram, K.; Krishnan, S.; Vaidyanathan, K.; Nandakumar, S.; Chandrasekhar, R.; Mathew, N. A randomized study of coconut oil versus sunflower oil on cardiovascular risk factors in patients with stable coronary heart disease. Indian Hear. J. 2016, 68, 498–506. [Google Scholar] [CrossRef] [PubMed]
- Eyres, L.; Eyres, M.F.; Chisholm, A.; Brown, R.C. Coconut oil consumption and cardiovascular risk factors in humans. Nutr. Rev. 2016, 74, 267–280. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Zhang, X.; Swaminathan, S.; Li, W.; Mohan, V.; Iqbal, R.; Kumar, R.; Wentzel-Viljoen, E.; Rosengren, A.; et al. Associations of fats and carbohydrate intake with cardiovascular disease and mortality in 18 countries from five continents (PURE): a prospective cohort study. Lancet 2017, 390, 2050–2062. [Google Scholar] [CrossRef] [PubMed]
- Dehghan, M.; Mente, A.; Rangarajan, S.; Sheridan, P.; Mohan, V.; Iqbal, R.; Gupta, R.; Lear, S.; Wentzel-Viljoen, E.; Avezum, A.; et al. Association of dairy intake with cardiovascular disease and mortality in 21 countries from five continents (PURE): a prospective cohort study. Lancet 2018, 392, 2288–2297. [Google Scholar] [CrossRef] [PubMed]
- CDC. Leading Causes of Death, 1900-1998. https://www.cdc.gov/nchs/data/dvs/lead1900_98.pdf.
- CDC 2016. Table 15. Life expectancy at birth, at age 65, and at age 75, by sex, race, and Hispanic origin: United States, selected years 1900–2016 (cdc.gov) https://www.cdc.gov/nchs/data/hus/2017/015.pd.
- Densen, P.M.; Linder, F.E.; Grove, R.D.; Dunn, H.L. Vital Statistics Rates in the United States: 1900-1940. J. Am. Stat. Assoc. 1944, 39, 524. [Google Scholar] [CrossRef]
- Grove, R.D. ; AM Hetzel. Vital Statistics Rates in the United States, 1940-1960. US Government Printing Office Public Health Service Publication No. 1677. 1968.
- Vaporciyan, A.A.; Kies, M.S.; Stevens, C.W. Factors associated with the development of lung cancer. In: Kufe, D.W.; Pollock, R.E.; Weichselbaum, R.R.; et al., editors. Holland-Frei Cancer Medicine. 6th edition. Hamilton (ON): BC Decker; 2003. Available from: https://www.ncbi.nlm.nih.gov/books/NBK13329/.
- Centers for Disease Control and Prevention, Leading Causes of Death. Availabe online: https://www.cdc.gov/nchs/fastats/leading-causes-of-death.htm.
- MMWR Weekly. Achievements in Public Health, 1900-1999: Tobacco Use -- United States, 1900-1999. 1999, 48, 986–993.
- MMWR Weekly. Achievements in Public Health, 1900-1999: Tobacco Use -- United States, 1900-1999. 1999, 48, 986–993.
- Mahmood, S.S.; Levy, D.; Vasan, R.S.; Wang, T.J. The Framingham Heart Study and the epidemiology of cardiovascular disease: a historical perspective. Lancet 2014, 383, 999–1008. [Google Scholar] [CrossRef]
- Kannel, W.B.; Gordon, T. “The Framingham Study: an epidemiological investigation of cardiovascular disease.” Unpublished paper. Washington, DC: National Heart, Lung, and Blood Institute (1987):24. Available at: https://www.scribd. com/document/583903774/Kannel-W-Gordon-T-Framingham-dietary-data. Section-24-unpublished.
- Singman, H.S.; Berman, S.N.; Cowell, C.; E Maslansky, E.; Archer, M. The Anti-Coronary Club: 1957 to 1972. Am. J. Clin. Nutr. 1980, 33, 1183–1191. [Google Scholar] [CrossRef]
- Christakis, G.; Rinzler, S.H.; Archer, M.; Kraus, A. Effect of the Anti-Coronary Club Program on Coronary Heart Disease Risk-Factor Status. JAMA 1966, 198, 597–604. [Google Scholar] [CrossRef] [PubMed]
- Rinzler, S.H. Primary prevention of coronary heart disease by diet. Bull NY Acad Med 1968, 44, 936–49. [Google Scholar] [CrossRef] [PubMed]
- General Summary, Conclusions and Recommendations. Circulation 1968, 37, Suppl. I: I–1.
- National Diet Heart Study. The National Diet-Heart Study Final Report. Circulation 1968, 37, 1–428.
- National Diet Heart Study. The National Diet-Heart Study Final Report. Circulation 1968, 37 & 38 (Suppl. 1): I64-I71.
- Anderson, J.T.; Lawler, A.; Keys, A. Weight Gain from Simple Overeating. II. Serum Lipids and Blood Volume 1. J. Clin. Investig. 1957, 36, 81–88. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.L. Diet, physical activity and the serum cholesterol concentration. Minn Med. 1958, 41, 149–53. [Google Scholar]
- E Olson, R. Obesity as a nutritional disorder. Fed Proc 1959, 18, 58–67. [Google Scholar]
- Caldwell, A.B.; Watson, P.; Green, D.P.; Florin, A.; Braun, P.; Bierenbaum, M.L. Weight Reduction and Serum Cholesterol Levels. Am. J. Clin. Nutr. 1963, 12, 401–405. [Google Scholar] [CrossRef]
- National Diet Heart Study. The National Diet-Heart Study Final Report. Circulation 1968, 37 & 38 (Suppl. 1): I-212.
- MRFIT Research Group. Multiple risk factor intervention trial. Risk factor changes and mortality results. J Am Med Assoc 1982, 248, 1465–1477.
- Gorder, D.D.; Dolecek, T.A.; Coleman, G.G.; Tillotson, J.L.; Brown, H.B.; Lenz-Litzow, K.; Bartsch, G.E.; Grandits, G. Dietary intake in the Multiple Risk Factor Intervention Trial (MRFIT): Nutrient and food group changes over 6 years, J. Am. Diet. Assoc. 1986, 86, 744–751. [Google Scholar] [CrossRef] [PubMed]
- Sacks, F.M.; Lichtenstein, A.H.; Wu, J.H.Y.; Appel, L.J.; Creager, M.A.; Kris-Etherton, P.M.; Miller, M.; Rimm, E.B.; Rudel, L.L.; Robinson, J.G.; et al. Dietary Fats and Cardiovascular Disease: A Presidential Advisory From the American Heart Association. Circulation 2017, 136, e1–e23. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.N.; Ball, K.P.; Antonis, A.; et al. Controlled Trial of Soya-Bean Oil in Myocardial Infarction: Report of a Research Committee to the Medical Research Council. Lancet 1968, 292, 693–700. [Google Scholar]
- Dayton, S.; Pearce, M.L.; Hashimoto, S.; Dixon, W.J.; Tomiyasu, U. A Controlled Clinical Trial of a Diet High in Unsaturated Fat in Preventing Complications of Atherosclerosis. Circ. 1969, 40, 1237–42. [Google Scholar] [CrossRef]
- Leren, P. The Oslo Diet-Heart Study. Circ. 1970, 42, 935–942. [Google Scholar] [CrossRef] [PubMed]
- Turpeinen, O.; J, M.; Pekkarinen, M.; Miettinen, M.; Elosuo, R.; Paavilainen, E. Dietary Prevention of Coronary Heart Disease: The Finnish Mental Hospital Study. Leuk. Res. 1979, 8, 99–118. [Google Scholar] [CrossRef]
- Hooper, L.; Martin, N.; Abdelhamid, A.; Davey Smith, G. Reduction in saturated fat intake for cardiovascular disease. Cochrane Database of Systematic Reviews 2015, Issue 6. Art. No.: CD011737, page 23.
- Ramsden, C.E.; Zamora, D.; Leelarthaepin, B.; Majchrzak-Hong, S.F.; Faurot, K.; Suchindran, C.M.; Ringel, A.; Davis, J.M.; Hibbeln, J.R. Use of dietary linoleic acid for secondary prevention of coronary heart disease and death: Evaluation of recovered data from the Sydney Diet Heart Study and updated meta-analysis. BMJ 2013, 346, e8707. [Google Scholar] [CrossRef] [PubMed]
- Frantz, I.D.; A Dawson, E.; Ashman, P.L.; Gatewood, L.C.; E Bartsch, G.; Kuba, K.; Brewer, E.R. Test of effect of lipid lowering by diet on cardiovascular risk. The Minnesota Coronary Survey. Arter. Off. J. Am. Hear. Assoc. Inc. 1989, 9, 129–135. [Google Scholar] [CrossRef]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.; Broste, S.K.; Frantz, R.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef]
- Stehbens, W.E. Coronary heart disease, hypercholesterolemia, and atherosclerosis. I. False premises. Exp Mol Pathol 2001, 70, 103–119. [Google Scholar] [CrossRef]
- Henein, M.Y.; Vancheri, S.; Longo, G.; Vancheri, F. The Role of Inflammation in Cardiovascular Disease. Int. J. Mol. Sci. 2022, 23, 12906. [Google Scholar] [CrossRef] [PubMed]
- Ramsden, C.E.; Zamora, D.; Majchrzak-Hong, S.; Faurot, K.; Broste, S.K.; Frantz, R.; Davis, J.M.; Ringel, A.; Suchindran, C.M.; Hibbeln, J.R. Re-evaluation of the traditional diet-heart hypothesis: analysis of recovered data from Minnesota Coronary Experiment (1968–73). BMJ 2016, 353, i1246. [Google Scholar] [CrossRef] [PubMed]
- Dayton, S.; S Hashimoto, ML Pearce. Influence of a diet high in unsaturated fat upon composition of arterial tissue and atheromata in man. Circulation 1965, 32, 911–924; Dayton, S.; S Hashimoto, W Dixon, ML Pearce. Composition of lipids in human serum and adipose tissue during prolonged feeding of a diet high in unsaturated fat. J Lipid Res 1966, 7, 103–111; Dayton, S.; ML Pearce, S Hashimoto, LJ Fakler, E. Hiscock, WJ Dixon. A controlled clinical trial of a diet high in unsaturated fat. Preliminary observations. N Engl J Med 1968, 266, 1060–1062; Dayton, S.; ML Pearce, S Hashimoto, WJ Dixon, U Tomiyasu. A controlled clinical trial of a diet high in unsaturated fat in preventing complications of atherosclerosis. Circulation 1969, 40(Suppl. II): II1-63. [CrossRef]
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of overweight, obesity, and severe obesity among adults aged 20 and over: United States, 1960–1962 through 2017–2018. NCHS Health E-Stats. 2020. PDF available at: https://www.cdc.gov/nchs/data/hestat/obesity-adult-17-18/obesity-adult.htm#1.
- Fryar, C.D.; Carroll, M.D.; Afful, J. Prevalence of overweight, obesity, and severe obesity among children and adolescents aged 2–19 years: United States, 1963–1965 through 2017–2018. NCHS Health E-Stats. 2020: https://www.cdc.gov/nchs/data/hus/2019/027-508.pdf.
- Obesity Among Young Children Enrolled in WIC: Overweight & Obesity. Table 2. https://www.cdc.gov/obesity/data/obesity-among-WIC-enrolled-young-children.html%23trends.
- Selvin, E.; Parrinello, C.M.; Sacks, D.B.; Coresh, J. Trends in Prevalence and Control of Diabetes in the United States, 1988–1994 and 1999–2010. Ann. Intern. Med. 2014, 160, 517–525. [Google Scholar] [CrossRef]
- Centers for Disease Control and Prevention. National Diabetes Statistics Report website. Table 1a. https://www.cdc.gov/diabetes/data/statistics-report/index.html.
- Hebert, L.E.; Scherr, P.A.; Beinias, J.L.; Bennett, D.; Evans, D.A. Alzheimer Disease in the US population: Estimates using the 2000 census. Arch Neurol 2003, 60, 1119–1122. [Google Scholar] [CrossRef]
- From Alzheimer’s Association Facts and Figures Report, 2023. https://www.alz.org/alzheimers-dementia/facts-figures.
- Data Statistics on Autism Spectrum Disorder. CDC. https://www.cdc.gov/ncbddd/autism/data.html.
- Kris-Etherton, P.M.; ed. Expert Panel on Trans Fatty Acids and Coronary Heart Disease. Trans fatty acids and coronary heart disease risk. Am J Clin Nutr 1995, 62, 655S–708S.
- Boffey, P.M. Cholesterol: Debate Flares Over Wisdom In Widespread Reductions, New York Times, July 14, 1987, Section C:1.
- Astrup, A.; Bertram, H.C.S.; Bonjour, J.-P. , et al. WHO draft guidelines on dietary saturated and trans fatty acids: time for a new approach? BMJ 2019, 366, l4137. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




