1. Introduction
Detection of plant infections prior to the onset of visual symptoms is valuable for executing appropriate management strategies and pest control to prevent the spread of diseases [
1,
2]. However, with many pests and diseases, it is difficult to determine the physical characteristics of infected plants during the asymptomatic or early stages of infestation [
3]. The conventional approaches for the diagnosis of plant diseases are laboratory-based methods, such as polymerase chain reaction (PCR), immunofluorescence (IF), fluorescence in-situ hybridisation (FISH), enzyme-linked immunosorbent assay (ELISA) and flow cytometry (FCM) [
4,
5]. These techniques are expensive, time-consuming, requiring specialist training, and limited application for monitoring large farms where several thousand plants are cultivated. In order to achieve mass monitoring, non-invasive technologies should be used [
6]. A more recent technology is the detection of VOCs released from plants, which has received extensive attention due to its non-invasiveness and rapid results [
7].
Plants emit many VOCs, which deliver functional information related to their growth, health, and disease [
8]. In direct defences, plants emit repellent VOCs to reduce insect attacks, while, in indirect defences, they can attract predators to battle pests. It is clear that VOCs play significant roles in plant communication and present promising functionality for improving crop protection [
9,
10]. The composition of VOCs varies according to the type of damage, such as pathogen infection and herbivore feeding [
11]. In other words, the VOCs profiles, known as VOCs fingerprints, released from plants in different stages indicate their real-time physiological status and can be used as bio-information for early-stage diagnosis [
12].
Generally, VOCs are detected and analysed using a combination of a collection method (e.g. Solid Phase Micro-Extraction – SPME) alongside an analytical chemistry method (e.g. gas chromatography–mass spectrometry - GC-MS) [
3]. SPME is a solvent-free sampling technique that allows non-exhaustive volatile extraction [
13] and requires only a small amount of analyte compared to traditional solvent-based extractions [
14]. Analytes collected from SPME are analysed by GC-MS for the identities and quantities [
15], displaying remarkable potential in plant diagnostic applications. Despite the accuracy of quantitative and qualitative analysis, GC-MS analysis is not always readily available and affordable [
16].
However, GC-MS is time-consuming and requires laboratory-based testing, which limits its application for real-time in-field testing. To take advantage of plant VOC information for disease management, portable, easy-to-operate, real-time detection technology is required. E-noses, or artificial intelligent noses (AI noses), are designed to mimic the functionality of a natural olfactory system. They utilise different sensor arrays, signal conditioning modules, data acquisition units, and pattern recognition algorithms [
18]. E-noses can provide a rapid and real-time approach to detecting VOCs [
17].
The E-nose does not provide quantification or identification of specific VOCs like GC-MS. However, it is an easily operated, fast-turnaround tool for recognising differences among VOCs samples (e.g. healthy vs infected) [
19,
20,
21]. Also, the same device can be used for many different applications, e.g. food freshness, quality, ripeness, and shelf-life [
22].
E-nose devices have been used as a non-invasive technique and have been applied for food safety and quality analysis [
23]. More recently, the devices have been used to detect fungi and oomycetes [
24], and the early detection of Fusarium wilt of banana “Panama disease” [
6] caused by the soil-borne fungus
Fusarium odoratissimum (syn.
F. oxysporum f.sp.
cubense). The strain “Tropical Race 4” was first discovered in Darwin, Northern Territory (NT), in 1999 and in Tully, Queensland, Australia, in 2015 [
26].
Mango Twig Tip Dieback (MTTD) describes an apical necrosis of mango shoots. Severity varies from orchard to orchard across ages, with Kensington Pride (KP) and Nam Doc Mai cultivars being the most susceptible [Mango twig tip dieback | Department of Industry, Tourism and Trade]. Two bacterial pathogens, Pantoea sp. and Staphylococcus sp., have been associated with the disorder, but the exact aetiology of MTTD remains unknown.
2. Materials and Methods
A series of experiments were conducted to compare the capability of three E-nose devices to identify TR4 in banana pseudo-stems and MTTD in mango leaves. Inoculated substrates, samples from field collections, and healthy specimens with no observable symptoms were trialed with both E-noses and GC-MS analysis to confirm VOC fingerprints.
2.1. Electronic Nose Devices
The Cyranose 320 (Sensigent LLC, USA) is equipped with the NoseChipTM with an array of 32 polymer composite chemi-resistor sensors coated with conductive films arranged across electrodes. The Portable Electronic Nose (PEN3) from Win Muster Airsense (WMA) Analytics Inc. (Schwerin, Germany) integrates a sampling apparatus, a detector unit containing an array of sensors, and pattern recognition software (Win Muster v.1.6) for data recording and elaboration. The MSEM 160 (Sensigent LLC, USA) is a multi-sensor and portable instrument for measuring chemicals and odors. This instrument consists of nanocomposite sensors, metal oxide semiconductors (MOS), and electrochemical and sphotoionisation sensors. The MSEM 160 is controlled by an internal computer running the Windows or Linux operating system.
2.2. SPME Coupled with GC-MS
The experiments also utilised sampling with SPME portable Field Sampler (Sigma, coating CAR/PDMS) and GC/MS analysis: an Agilent88890 model with an Agilent 59777B single quadrupole electron ionisation mass selective detector using Agilent MassHunter Workstation GC/MS Data Acquisition (version 10.1.49) software. GC/MS data was analysed using Agilent MassHunter Workstation Qualitative Analysis (version 10.0) software. Compounds were identified based on the comparison with data in NIST/EPA/NIH Mass Spectral Library (Version 3.0).
2.3. Assessing E-Nose Functions and Sampling on Known Volatiles
Isoamyl isovalerate (IAIV) and 3-methyl-2-butanol, 98% (3M2B) were obtained from Thermo Fisher Scientific Pty Ltd, Australia, and a dilution series of five concentrations (0, 1, 10, 100, 1000 ppm) prepared, using n-hexane, 95% as the solvent and the negative control. Mixtures of the two compounds (1 ppm and 100 ppm) were also prepared. Two ml of each was transferred into a 20 ml headspace glass vial and was allowed to saturate for six hrs before being assessed using the three E-nose devices. Five replicates were prepared for each mixture.
2.4. Pure Cultures of Plant Pathogens and Controls for Training
F. odoratissimum (TR4) was isolated from infected cavendish pseudo-stems, and pure cultures were produced on potato dextrose agar (PDA). Pure cultures of F. odoratissimum were then sub-cultured to PDA and hyphal tip cultures were obtained, and identity was confirmed by PCR. Petri dishes were sealed with parafilm and incubated at 18⁰ C for seven days. Uninoculated PDA media in Petri dishes were prepared as the control. Two pathogens of Mango Twig Tip Dieback (MTTD 18 – Pantoea sp. and MTTD 30 – Staphylococcus sp.) were recovered from mango twigs from Colton Road, Northern Territory, Australia. MTTD isolates were obtained on nutrient agar (NA) that were sub-cultured to obtain single colony cultures, and identity was confirmed by PCR. Petri dishes were sealed with parafilm, and incubated at 18⁰ C for seven days. Uninoculated NA media in Petri dishes were prepared as the control. TR4 cultures grown on PDA, Pantoea sp. and Staphylococcus sp. cultures grown on NA in Petri dishes. Petri dishes were half opened and secured with biofilm for rapid collection of VOCs into gas collecting bags. VOCs were collected by inserting the SPME fibers into the bags in duplicates for 17 hrs. VOCs from uninoculated PDA and NA, clean Petri dishes and empty gas-collecting bags were also collected to quantify any background VOCs. Further SPME fibers were subjected to GC-MS analysis.
TR4 cultures on PDA were cut into thin strips, then placed in six septum sealable, 20 ml headspace glass vials and allowed to incubate for 24 hrs at room temperature. Headspace sampling of vials was conducted with three E-nose devices after 24 hrs of saturation. Procedures were repeated with two MTTD bacterial cultures, uninoculated NA and PDA media.
2.5. Sampling of Healthy and Infected Substrates
To infect mango leaves with MTTD pathogens, healthy mango leaves were washed with running tap water and dried. The leaves were trimmed from the edge of the petiole to fit into a petri dish.Inoculation steps were carried out inside the laminar flow cabinet. The surface of each leaf was sterilised with 70% ethanol for one minute, then washed in distilled water for one minute. The leaves were dried with sterilised filter papers and kept inside clean Petri dishes. A surface sterilised, 12 mm cork borer was used to extract MTTD 18 and 30 from the petri dish and pushed into a mango leaf to make a wound in the epidermal tissue. Two punches were made on each leaf, and the leaves were placed in a clean Petri dish and wrapped with biofilm. Fifteen inoculated leaves in individual petri dishes were prepared separately for MTTD 18 and MTTD 30, and incubated at 25⁰ C for five days.
After five days of incubation, each petri dish was half opened and enclosed in gas-collecting bags. Four petri dishes were included in each gas-collecting bag, and two gas-collecting bags were prepared. Bags were placed in the incubator for another 24 hrs at 25⁰ C. Sterilised distilled water was used as the control.
After 24 hrs of incubation, SPMEs were inserted into gas collecting bags for 17 hrs, from the control and infected mango leaves with MTTD 18 and MTTD 30, and subjected to GC-MS analysis. Six mango leaves in the control set were sliced and placed individually in six 20 ml headspace vials with sealed septa. Before E-nose sampling, these vials were allowed to rest for 24 hrs at room temperature to saturate headspace. Procedures were repeated with infected mango leaves with two MTTD bacterial cultures. E-nose sampling was carried out for eighteen replicates of control and infected samples (
Figure 1).
Thin strips of healthy banana pseudo-stems (received from the field) were placed in six replicates of 20 ml headspace glass vials. Tropical Race 4 positive (according to the external appearance of purple lines along the vascular tissues and confirmed by sterile culturing) pseudo-stems followed the same procedure. All replicates were allowed to incubate for 24 hrs to saturate the headspace, and E-nose sampling was carried out on the three devices. Two pieces (10 cm x 3 cm each) of healthy banana pseudo-stems were placed in collecting gas-collecting bags following a heat seal of the opening in duplicates. The procedureThe procedure was repeated for TR4-infected pseudo-stems. VOCS were collected using SPMEs, as explained in the earlier section, and subjected to GC-MS analysis.
2.6. Data Analysis
Pattern recognition software (Win Muster v.1.6) was used to carry out Principal Component Analysis (PCA) and Linear Discriminant Analysis (LDA) for samples tested with PEN 3 – i.e., healthy, infected, positive and control. PCA is one of the widely used pattern recognitions in E-nose results analysis. It uses an orthogonal transformation to convert the original dataset of correlated variables into a set of values of linearly uncorrelated variables called principal components [
33] and has the property of cutting down the number of dimensions without much loss of information [
34]. LDA assumes that all classes are linearly separable. If there are two classes, then the LDA draws one hyperplane and projects the data onto this hyperplane in such a way as to maximise, then the LDA draws one hyperplane and projects the data onto this hyperplane in such a way as to maximise the separation of the two categories [
35].
Chemometrics data analysis (CDA) uses mathematical, statistical, and other methods employing formal logic to design or select optimal measurement procedures and experiments and to provide maximum relevant chemical information by analysing chemical data [
36]. Chemometric Data analysis software in MSEM 160 instrument monitors changes in the electrical, physical, optical and/or chemical properties of the internal arrays of chemical sensors. Changes in the sensor properties were identified and qualified by the software and translated into metrics during operation according to the explanation in the MSEM 160 user manual.
PC nose software provided by Sensigent LLC, USA, was used to analyse Cyranose 320 data through cross-validation. Cross-validation is a statistical method of evaluating and comparing learning algorithms by dividing data into two segments: one used to learn or train a model and the other used to validate the model. In typical cross-validation, the training and validation sets must cross-over in successive rounds so that each data point can be validated against other data points [
37].
3. Results and Discussion
3.1. Discrimination Capability of Known Volatiles
The Cyranose 320 device was not successful in differentiating between the concentration gradient of 3M2B and IAIV (
Table 1), demonstrating how many replicates were identified successfully out of five samples for each subsequent concentration of both 3M2B and IAIV. Limitations in sensitivity resulted in poor discrimination in each subsequent concentration of the same compound.
A successful differentiation was observed between pure solutions of water, 95% n-hexane and 70% ethanol. These results suggest that Cyranose 320 is more suitable for separating completely different samples than compounds with similar volatile characteristics.
Table 2.
Cross validation results for pure water, 95% n- Hexane and 70% ethanol.
Table 2.
Cross validation results for pure water, 95% n- Hexane and 70% ethanol.
| Trained as |
Water |
95% n-Hexane |
70% Ethanol |
% Accuracy |
| No. of correct identification / 5 (Total no. of replicates) |
5 |
5 |
5 |
100 |
The PEN 3 was capable of differentiating all five classes of concentrations (0, 1, 10, 100, 1000 ppm) of both 3M2B and IAIV [Supplementary Material
Figure S1 A]. Five separated clusters were clearly observed in the LDA plot with a low variance. A standard pattern was created from Winmuster software by merging data for all five subsequent concentrations. This was called “Total Concentration Series”. The two extreme concentrations (0 and 1000 ppm) were located apart from the total series with low percentages of variance. This was observed in both 3M2B and IAIV series. This result identifies the PEN 3 as more efficient than C320 in differentiating consecutive concentrations of the same compound.
PEN 3 device was then trialled to differentiate between two compounds, 3M2B and IAIV. A distinct separation of the two classes was observed with a high variance of 97.11% (Supplementary Material
Figure S2-A). The PEN 3 could discriminate between not only the different compounds but also consecutive concentrations of the same compound. Mixtures of the compounds with optimum concentrations (1ppm and 100ppm) were successfully differentiated from pure compounds with a high variance of 99.15% (Supplementary Material
Figure S2-B).
The MSEM 160 was successful in discriminating consecutive concentrations of compounds (Supplementary Material
Figure S3A). CD analysis was carried out to convert raw data into a score plot in PCA.
Supplementary Figure S3B shows the ability of MSEM 160 to differentiate two compounds in the mixture of 1 ppm 3M2B and IAIV. These results suggest that the MSEM 160 is superior to C320 and PEN 3 in discriminating compounds in a mixture.
3.2. Efficacy of Discriminating Pure Cultures of Plant Pathogens and Controls
3.2.1. Electronic Noses
Twelve replicates of Tropical Race 4 cultures were divided into two groups as “TR4 - G 1” and “TR4 - G 2” with six replicates in each. C 320 successfully identified all 12 samples as TR4, although confused between”G1” and “G 2” occurred (
Table 3). None of the replicates were failed to detect as TR4 and not presented as unknown. Results express that C 320 was able to differentiate the VOCs of
F. odoratissimum cultures from PDA. C 320 was 77.78% successful in detecting eighteen replicates of MTTD cultures and controls.
Table 4 demonstrates that all six controls were identified as NA- control while confusion occurred between two MTTD bacterial verities. Similar volatile characteristics of
Pantoea sp. and
Staphylococcus sp. might affect the indistinct separation of the two sample groups. The suggestion that C 320 is more convenient for cross-validation results indicates that C 320 is more convenient for detecting compounds with entirely different volatile profiles.
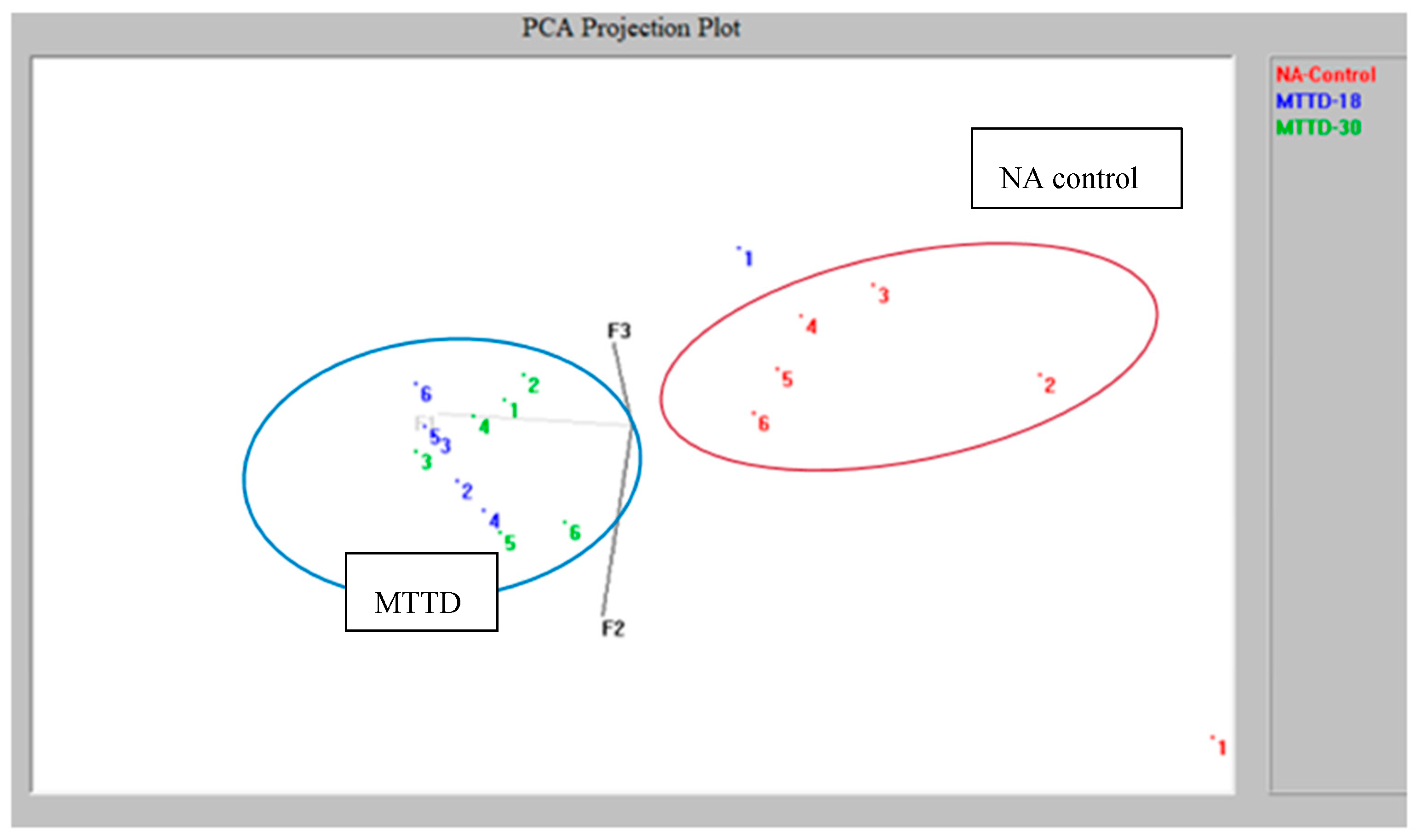
PCA projection plot in
Figure 2 demonstrates that MTTD 18 and MTTD 30 have similar volatile characteristics, which differ from the uninoculated NA control. Therefore, samples of two MTTD varieties were clustered on the same plane opposite to the control samples.
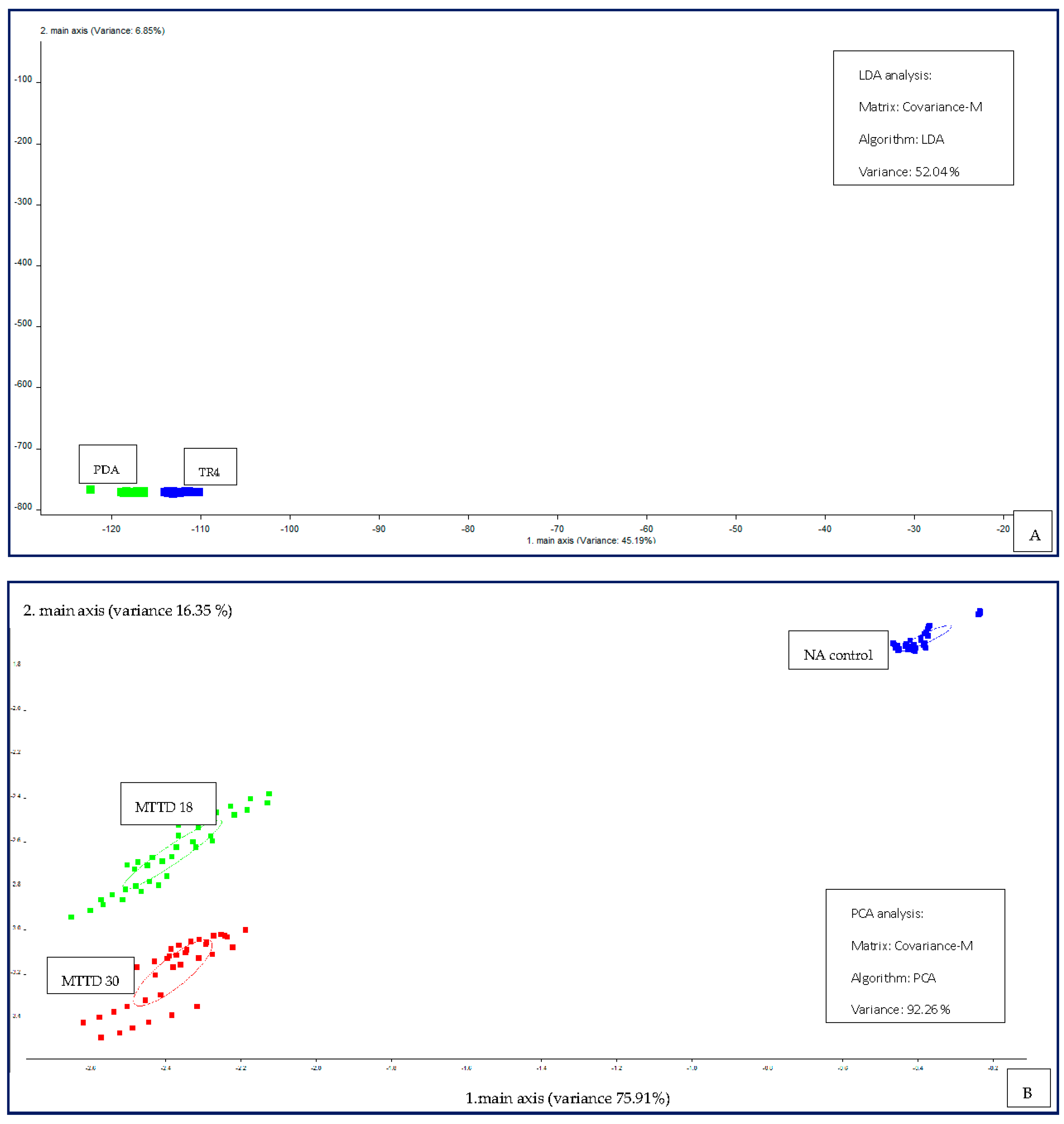
LDA plot for TR4 culture and uninoculated PDA controls using PEN 3 exhibited distinct separation, as shown in
Figure 3A. This demonstrates that PEN 3 can differentiate volatile organic compounds of TR4 cultures from uninoculated PDA. The results demonstrate that PDA volatiles have no impact on TR4 volatile characteristics hence given two distinct clusters. Distinct separation was observed between three classes (NA control, MTTD 18 and MTTD 30) with a high variance of 92.26%, as shown in
Figure 3(B). Samples of the same class have been clustered together, showing similar patterns after analysing raw data through “Winmuster” software. Different volatile compounds between each class would lead to different patterns and hence distinct separation of the classes.
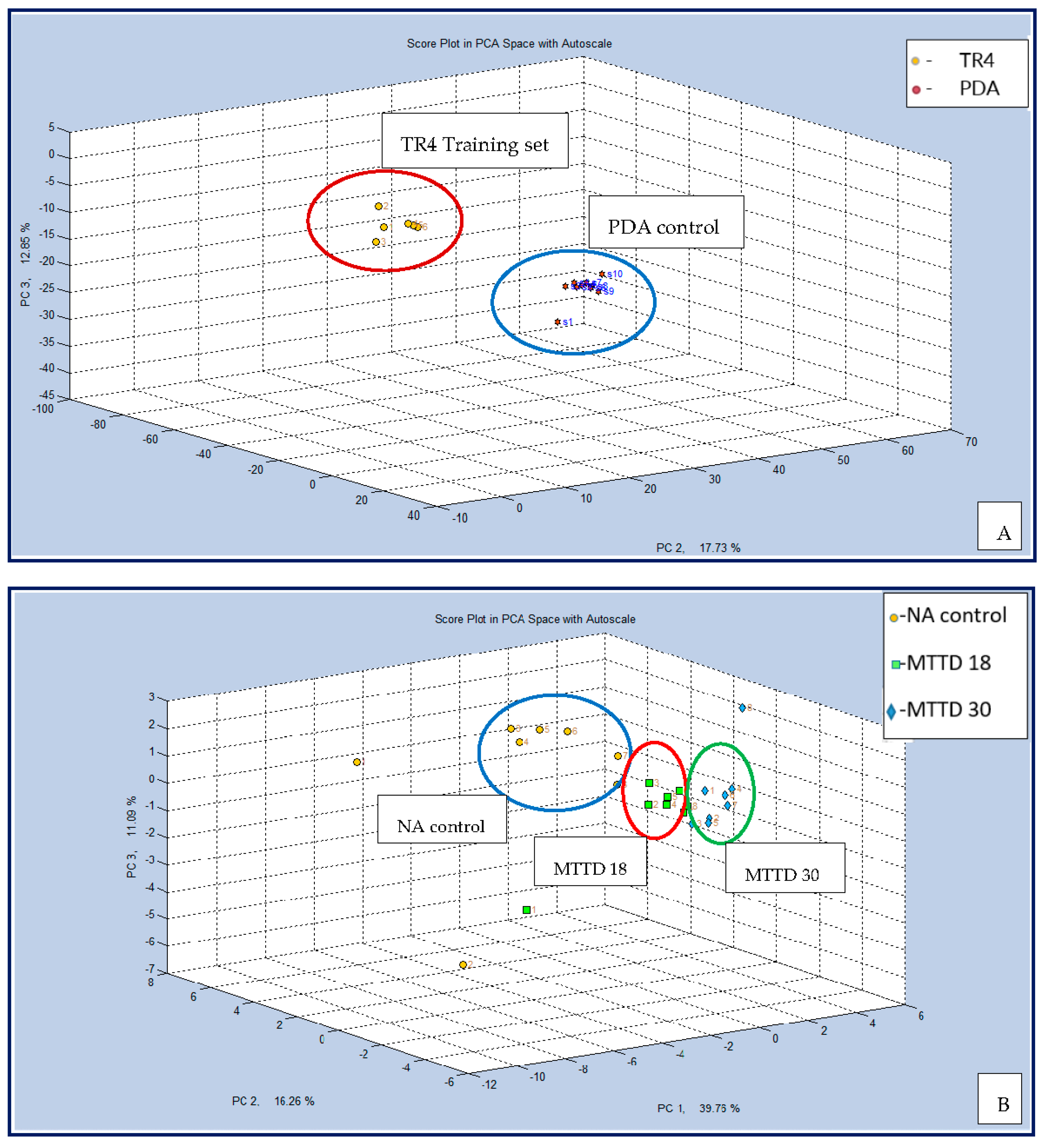
Figure 4A demonstrates that the MSEM 160 distinct separation between TR4 cultures and PDA controls. Two classes were separated and clustered independently, which provides statistical evidence for the high efficacy of MSEM 160.
Figure 4B clearly shows the efficiency of the MSEM 160 in discriminating between MTTD 18 and MTTD 30 cultures and NA control.
3.2.2. GC-MS Analysis
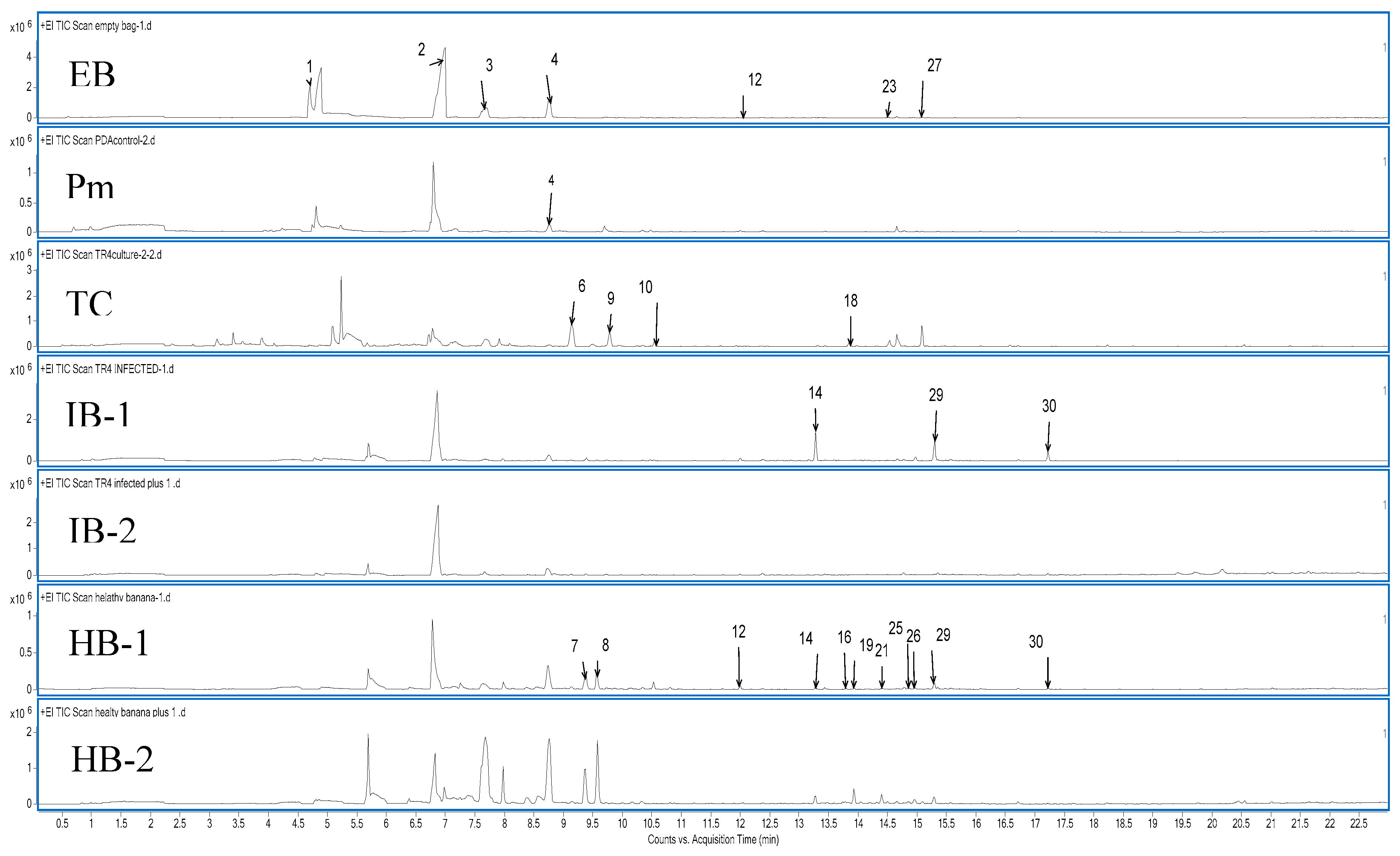
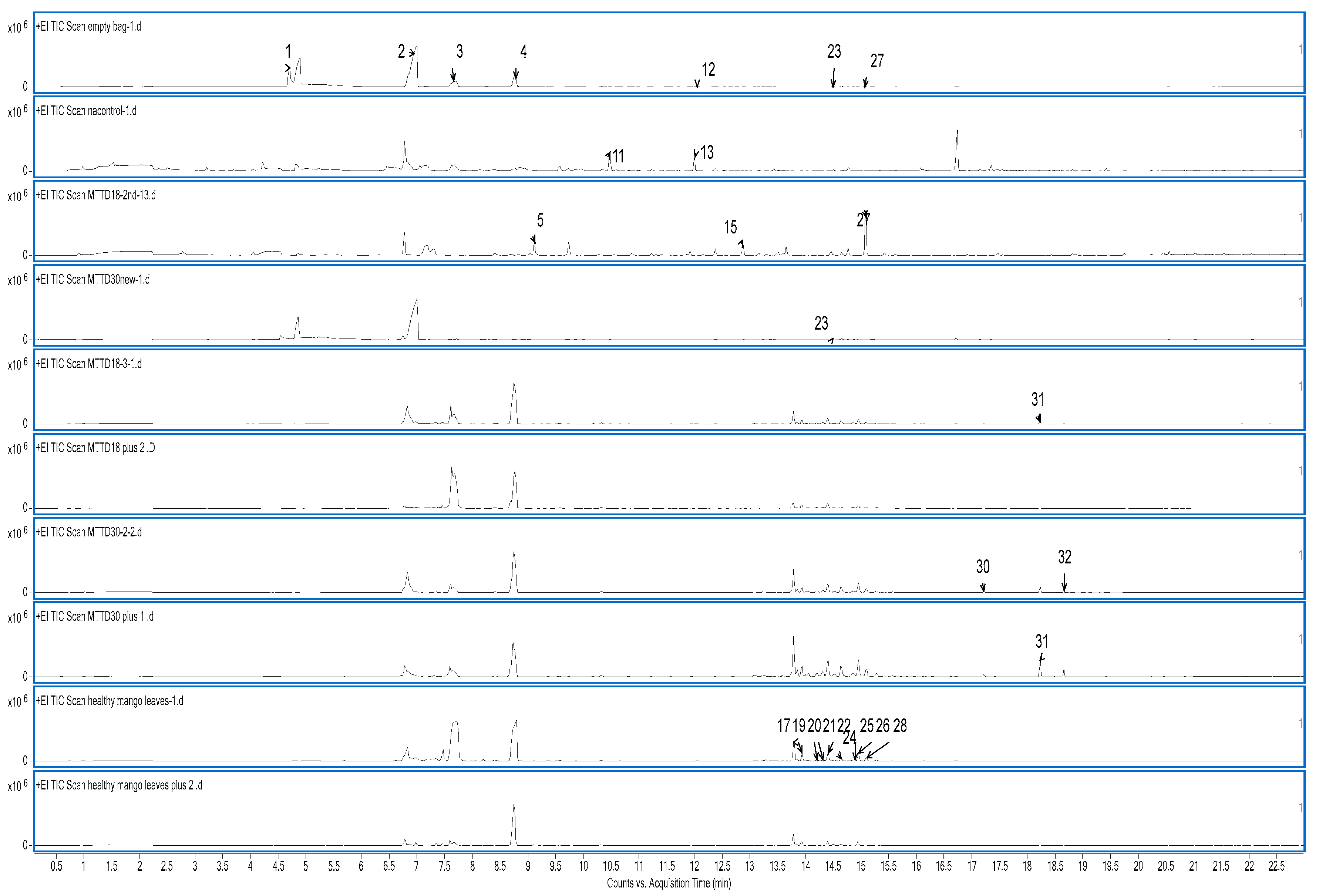
Total ion chromatogram of the VOCs collected using SPMEs from an empty bag, media, bacterial culture
s, infected and healthy banana pseudo-stems and mango leaves are presented in
Figure 5 and
Figure 6. Although all VOCs from all samples were collected over two trials, only the peaks of the healthy and infected samples are presented in the figures. A summary of all the VOCs detected is given in
Table 5.
Compounds 1 to 4 (
Table 5; blue squares) are volatiles collected from empty plastic bags and were one of the most consistently recovered VOC across all samples. Interestingly, none of the VOCs from the media (PDA) and living cultures (Tc or Ms) appear in the infected plant samples. This indicates that the VOCs released by infected plants are very different from those of the bacteria that cause the disease.
Volatile 6 and 18 were unique to the pure culture of TR4 (
Table 5; red squares). Ethylanisole and α-Cedrene were two of six VOCs produced by seven
F. oxysporum strains [
38]. The VOCs in infected and healthy banana pseudo-stems were similar (
Table 5; green squares). However, compounds 14 and 29 (Copaene and Calamenene) were released in higher concentrations by infected samples compared to healthy samples.
Healthy and infected mango leaves also exhibit similar behaviour, with the VOCs released by healthy and infected mango leaves being similar, and the concentrations of compounds 30-32 were detected with high concentrations in the infected mango leaves (
Table 5; brown squares). A group of sesquiterpenes were found to be common between healthy and infected banana pseudo-stems and mango leaves,; for example, compounds 19, 21, 25 and 26 in
Table 5 are present in all four samples (
Table 5; yellow squares).
3.3. Differentiating Healthy and Infected Substrates
3.3.1. Electronic Noses
C320 was only 54% accurate in identifying healthy and MTTD-infected leaves (
Table 6 and
Table 7). None of the classes had obtained the correct total sample identification out of eight samples for each class. This was out of six replicates for each class. The overall result suggests the suitability of the C 320 device for qualitative measurements rather than quantitative analysis.
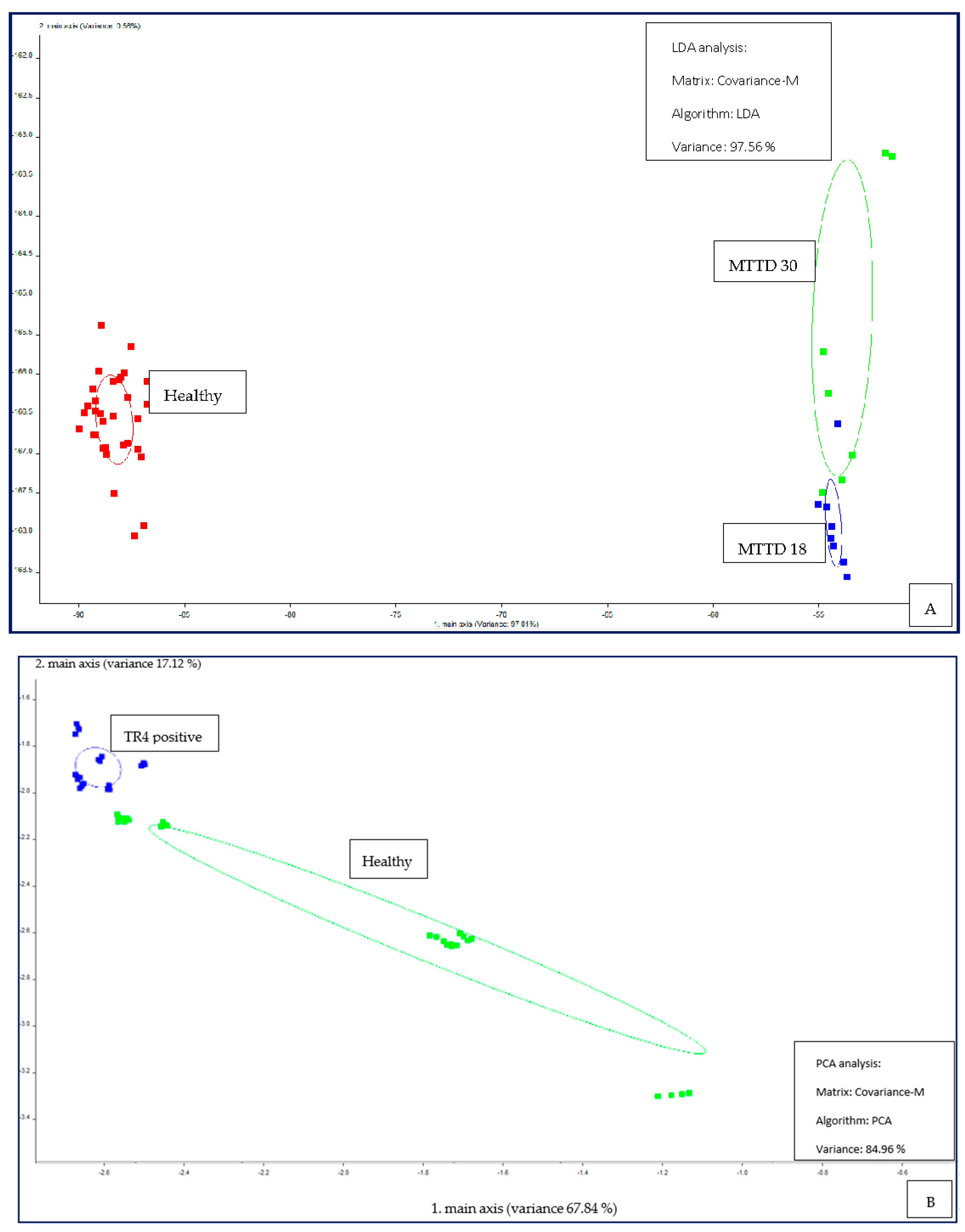
The PEN 3 successfully differentiated three classes of MTTD 18 infected mango leaves, MTTD 30 infected mango leaves, and healthy/control leaves with a variance of 97.56%, which is an excellent value of separation.
Figure 7A shows distinct clustering within three classes. The distance between the control and MTTD-infected samples was higher than the distance between two MTTD-infected varieties. This result suggests that infected samples have similar volatile characteristics, which are different from the volatiles of the control. The distinct separation of two classes, healthy and TR4 infected pseudo-stems, was observed in the PCA plot with a high variance of 84.96%, as shown in
Figure 7B. The result demonstrates the high efficacy of the PEN 3 device in differentiating TR4 and MTTD infections from healthy samples and, hence, the suitability of the device in pathogen detection.
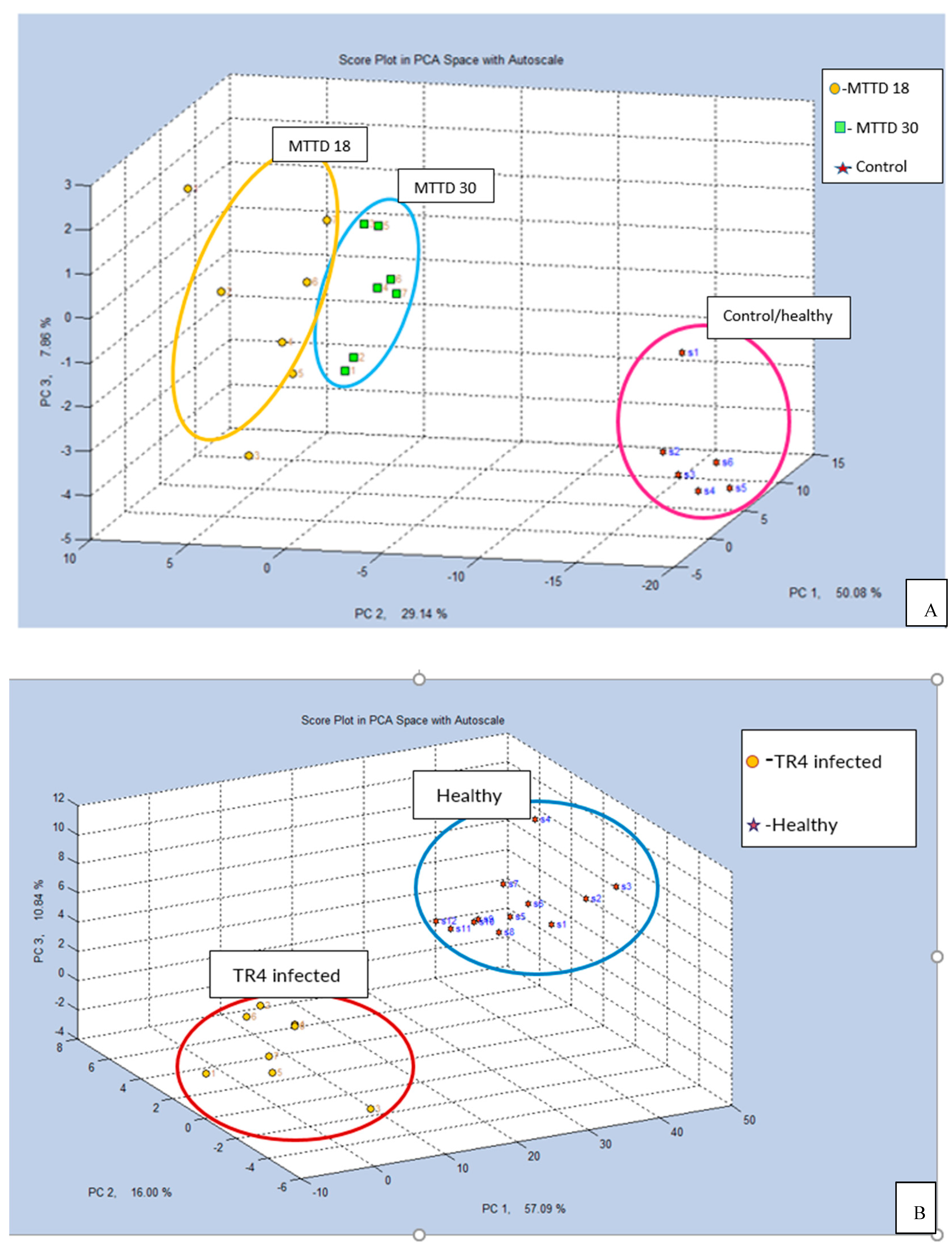
The distinct separation of three classes of leaf samples (control, MTTD 18 and MTTD 30) was observed in the PCA score plot by CD analysis, as shown in
Figure 8A. The results suggest that MSEM 160 can identify and differentiate volatiles of healthy mango leaves from infected samples. Furthermore, the result provides adequate evidence for the high efficacy of the device in differentiating two infected MTTD varieties.
Figure 8B shows significant discrimination of two classes, TR4 infected pseudo stems and healthy pseudo stem samples. CD analysis plot of MSEM 160 thoroughly demonstrates the capability of the device in advanced pathogen identification, hence suitable for further development of the technology to be used in the field.
3.3.2. GC-MS Analysis
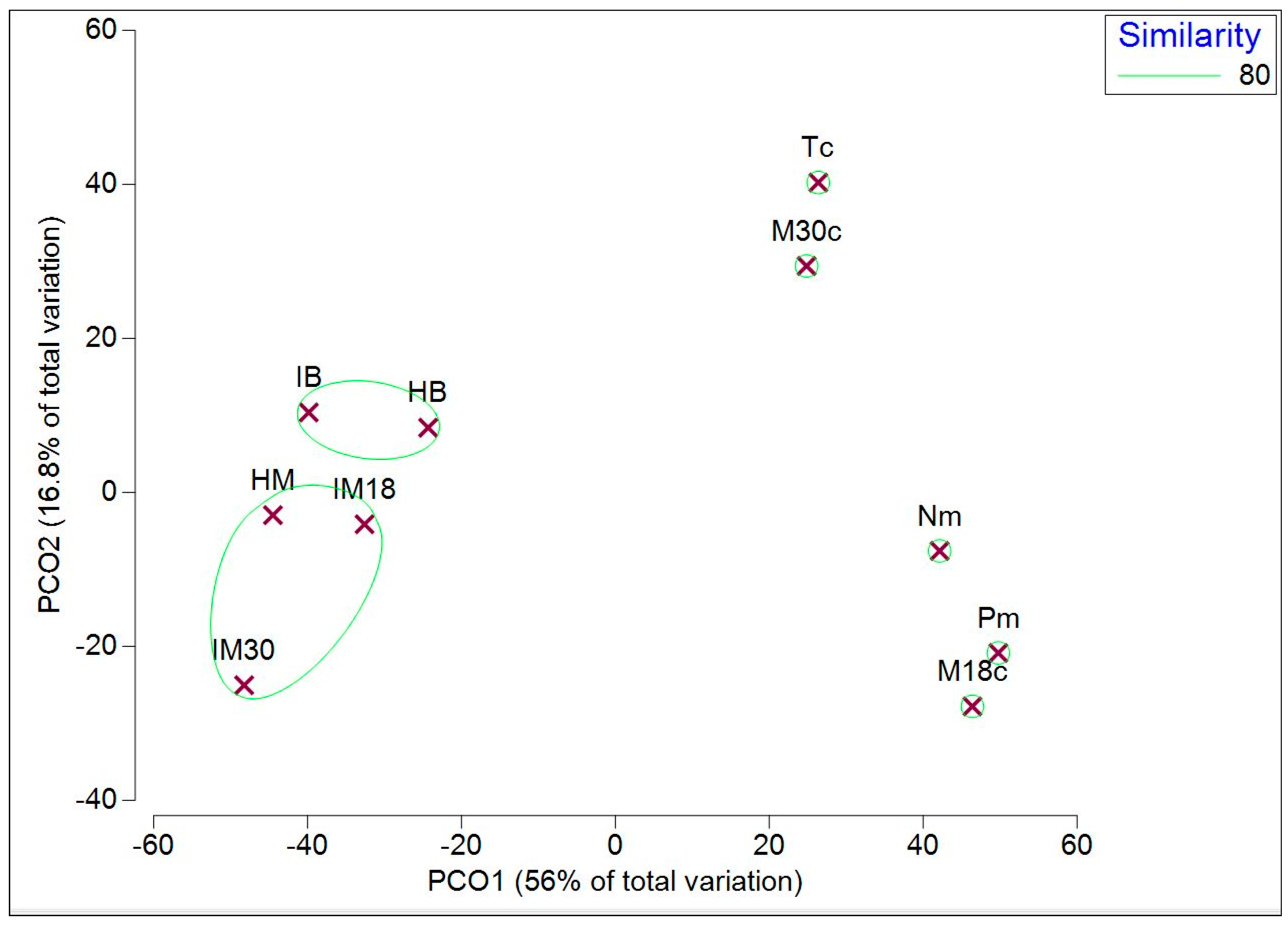
The PCA resemblance plot of the VOCs identified by GC-MS analysis is shown in Figure 9. The VOCs were collected from two types of media and the M18c bacterial culture, segregated from the Tc-and M30c-cultures. For the infected plant substrates, GC-MS analysis clustered the health and infected plant samples of both mango and banana pseudo-stem together.
Figure 10.
PCA score plot of GC-MS VOCs identified in all the samples. Pm: PDA media, Tc: TR4 culture, HB: Health banana pseudo-stems, IB: Infected banana pseudo-stems, Nm: NA media, M18c: MTTD18 culture, M30c: MTTD30 culture, HM: Health mango leaves, IM18: Infected mango leaves with MTTD18, IM30: Infected mango leaves with MTTD30.
Figure 10.
PCA score plot of GC-MS VOCs identified in all the samples. Pm: PDA media, Tc: TR4 culture, HB: Health banana pseudo-stems, IB: Infected banana pseudo-stems, Nm: NA media, M18c: MTTD18 culture, M30c: MTTD30 culture, HM: Health mango leaves, IM18: Infected mango leaves with MTTD18, IM30: Infected mango leaves with MTTD30.
5. Conclusions
Results from this project highlighted statistical evidence confirming the three devices' efficacy in detecting specific volatiles and plant diseases. Of the three devices, the Cyranose 320 would be considered unsuitable for quantitative studies. However, it is a hand-held, portable unit that can be used for in-field applications. The PEN 3 and MSEM 160 have quantitative capabilities to differentiate between synthetic and natural volatiles associated with pure plant pathogens and infected plant material. The limitation of these units is that they are not portable, are suited to a laboratory set-up, and require a separate computer to process and visualise data by a trained specialist. GC-MS analysis confirmed unique VOC fingerprints of pure pathogen cultures and infected plant materials, but the VOCs from infected and healthy plant material were found to be the same but varied in concentration.
Our results demonstrate that E-nose technology was more efficient than GC-MS in differentiating between healthy and diseased plant substrates. The PEN 3 and MSEM 160 E-nose devices could also distinguish between two different bacterial species, as well as a pure TR4. The e-Nose devices appear to be more sensitive to pathogen and plant disease volatiles. Therefore, this technology has the potential to increase the efficiency of pathogen and plant disease diagnosis.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Figure S1. Linear Discriminant Analysis plot for individual classes of concentration and total concentration series data obtained from PEN 3 device . (A) 3-Methyl-2-butanol, (B) Isoamyl isovalerate. Figure S2. Linear Discriminant Analysis Plots of data obtained from the PEN 3 device. (A) 3M2B total series vs IAIV total series, (B) Mixture of 1 ppm 3M2B and IAIV against pure 3M2B and IAIV of 1 ppm. Figure S3. CD analysis score plots in PCA of data obtained from MSEM 160 device. (A) 3-Methyl-2-butanol total concentration series, (B) Mixture of 1ppm 3M2B and IAIV.
Author Contributions
Conceptualisation, S.E.B., W.L.R., V.M. and H.W.; methodology, S.E.B., W.L.R., V.M., and H.W.; software, W.L.R., and H.W.; validation, W.L.R.,and H.W.; formal analysis, W.L.R., H.W., and V.M.; investigation, W.L.R., H.W., and V.M.; resources, S.E.B., and V.M.; data curation, W.L..R and V.M.; writing—original draft preparation, W.L.R.; writing—review and editing, S.E.B. and V.M.; visualisation, W.L.R. and V.M. and H.W; supervision, S.E.B., and V.M.; project administration, S.E.B., and V.M.; funding acquisition, S.E.B. All authors have read and agreed to the published version of the manuscript.
Funding
This project is supported by the Northern Hub, through funding from the Australian Government’s Agricultural Innovation Hubs Program.
Acknowledgments
the authors are grateful to Dr S. Mintoff and Dr C. Asis of Department of Industry, Tourism and Trade, Northern Territory Government, Australia for preparation of pure pathogen cultures and infected substrate samples and C.N. Morton for project advocacy.
Conflicts of Interest
The authors declare there is no conflict of interest in this work.
References
- Sankaran, S.; Mishra, A.; Ehsani, R.; Davis, C. A Review of Advanced Techniques for Detecting Plant Diseases. Computers and electronics in agriculture 2010, 72, 1–13.
- Zee, F.; Judy, J.W. Micromachined Polymer-Based Chemical Gas Sensor Array. Sensors and Actuators B: Chemical 2001, 72, 120–128. [CrossRef]
- Cui, S.; Ling, P.; Zhu, H.; Keener, H. Plant Pest Detection Using an Artificial Nose System: A Review. Sensors 2018, 18, 378. [CrossRef]
- Webster CG, Wylie SJ and Jones GK (2004) Diagnosis of plant viral pathogens. Current Science 86(12): 1604-1607.
- Kwon, J.Y.; Ryu, K.H.; Choi, S.H. Reverse Transcription Polymerase Chain Reaction-Based System for Simultaneous Detection of Multiple Lily-Infecting Viruses. The Plant Pathology Journal 2013, 29, 338–343. [CrossRef]
- Sanjay, M.; Kalpana, B. Early Mass Diagnosis of Fusarium Wilt in Banana Cultivations Using an E-Nose Integrated Autonomous Rover System. Int J Appl Sci Biotechnol 2017, 5, 261–266. [CrossRef]
- Raza, W.; Wang, J.; Wu, Y.; Ling, N.; Wei, Z.; Huang, Q.; Shen, Q. Effects of Volatile Organic Compounds Produced by Bacillus Amyloliquefaciens on the Growth and Virulence Traits of Tomato Bacterial Wilt Pathogen Ralstonia Solanacearum. Appl Microbiol Biotechnol 2016, 100, 7639–7650. [CrossRef]
- Baldwin, I.T.; Halitschke, R.; Paschold, A.; Von Dahl, C.C.; Preston, C.A. Volatile Signaling in Plant-Plant Interactions: “Talking Trees” in the Genomics Era. Science 2006, 311, 812–815. [CrossRef]
- Zou, H.-Q.; Li, S.; Huang, Y.-H.; Liu, Y.; Bauer, R.; Peng, L.; Tao, O.; Yan, S.-R.; Yan, Y.-H. Rapid Identification of Asteraceae Plants with Improved RBF-ANN Classification Models Based on MOS Sensor E-Nose. Evidence-Based Complementary and Alternative Medicine 2014, 2014, 1–6. [CrossRef]
- Paré, P.W.; Tumlinson, J.H. Plant Volatiles as a Defense against Insect Herbivores. Plant Physiology 1999, 121, 325–332.
- Maffei, M.E. Sites of Synthesis, Biochemistry and Functional Role of Plant Volatiles. South African Journal of Botany 2010, 76, 612–631.
- Cui, S.; Inocente, E.A.A.; Acosta, N.; Keener, Harold.M.; Zhu, H.; Ling, P.P. Development of Fast E-Nose System for Early-Stage Diagnosis of Aphid-Stressed Tomato Plants. Sensors 2019, 19, 3480. [CrossRef]
- Gherghel, S.; Morgan, R.M.; Arrebola-Liébanas, J.; Romero-González, R.; Blackman, C.S.; Garrido-Frenich, A.; Parkin, I.P. Development of a HS-SPME/GC–MS Method for the Analysis of Volatile Organic Compounds from Fabrics for Forensic Reconstruction Applications. Forensic Science International 2018, 290, 207–218. [CrossRef]
- Ouyang, G.; Pawliszyn, J. A Critical Review in Calibration Methods for Solid-Phase Microextraction. Analytica chimica acta 2008, 627, 184–197.
- Wu, Z.; Wang, H.; Wang, X.; Zheng, H.; Chen, Z.; Meng, C. Development of Electronic Nose for Qualitative and Quantitative Monitoring of Volatile Flammable Liquids. Sensors 2020, 20, 1817.
- Murray, J.; Delahunty, C.; Baxter, I. Descriptive Sensory Analysis: Past, Present and Future. Food research international 2001, 34, 461–471.
- Xu, X.; Tian, F.; Yang, S.; Li, Q.; Yan, J.; Machacek, J. A Solid Trap and Thermal Desorption System with Application to a Medical Electronic Nose. Sensors 2008, 8, 6885–6898. [CrossRef]
- Alphus Dan Wilson, A.D. Recent Progress in the Design and Clinical Development of Electronic-Nose Technologies. NDD 2016, 15. [CrossRef]
- Lee, S.-J.; Noble, A.C. Characterization of Odor-Active Compounds in Californian Chardonnay Wines Using GC-Olfactometry and GC-Mass Spectrometry. J. Agric. Food Chem. 2003, 51, 8036–8044. [CrossRef]
- Vandenvelde, S.; Nevens, F.; Vanhee, P.; Vansteenberghe, D.; Quirynen, M. GC–MS Analysis of Breath Odor Compounds in Liver Patients. Journal of Chromatography B 2008, 875, 344–348. [CrossRef]
- Mota, I.; Teixeira-Santos, R.; Cavaleiro Rufo, J. Detection and Identification of Fungal Species by Electronic Nose Technology: A Systematic Review. Fungal Biology Reviews 2021, 37, 59–70. [CrossRef]
- Cellini, A.; Biondi, E.; Blasioli, S.; Rocchi, L.; Farneti, B.; Braschi, I.; Savioli, S.; Rodriguez-Estrada, M.T.; Biasioli, F.; Spinelli, F. Early Detection of Bacterial Diseases in Apple Plants by Analysis of Volatile Organic Compounds Profiles and Use of Electronic Nose: Early Detection of Bacterial Diseases by Volatile Organic Compounds Profiling. Ann Appl Biol 2016, 168, 409–420. [CrossRef]
- Bonah, E.; Huang, X.; Aheto, J.H.; Osae, R. Application of Electronic Nose as a Non-Invasive Technique for Odor Fingerprinting and Detection of Bacterial Foodborne Pathogens: A Review. J Food Sci Technol 2020, 57, 1977–1990. [CrossRef]
- Loulier, J.; Lefort, F.; Stocki, M.; Asztemborska, M.; Szmigielski, R.; Siwek, K.; Grzywacz, T.; Hsiang, T.; Ślusarski, S.; Oszako, T.; et al. Detection of Fungi and Oomycetes by Volatiles Using E-Nose and SPME-GC/MS Platforms. Molecules 2020, 25, 5749. [CrossRef]
- Zhang, A.; Hartung, J.S. Phenylacetaldehyde O -Methyloxime: A Volatile Compound Produced by Grapefruit Leaves Infected with the Citrus Canker Pathogen, Xanthomonas Axonopodis Pv. Citri. J. Agric. Food Chem. 2005, 53, 5134–5137. [CrossRef]
- Daly A and Walduck G. 2006. Fusarium Wilt of Bananas (Panama Disease)(Fusarium oxysporum f. sp. cubense) Agnote 2006, 151.
- Delétoile, A.; Decré, D.; Courant, S.; Passet, V.; Audo, J.; Grimont, P.; Arlet, G.; Brisse, S. Phylogeny and Identification of Pantoea Species and Typing of Pantoea Agglomerans Strains by Multilocus Gene Sequencing. J Clin Microbiol 2009, 47, 300–310. [CrossRef]
- Manulis, S.; Barash, I. Pantoea Agglomerans Pvs. Gypsophilae and Betae , Recently Evolved Pathogens? Molecular Plant Pathology 2003, 4, 307–314. [CrossRef]
- Gutiérrez-Barranquero, J.A.; Cazorla, F.M.; Torés, J.A.; De Vicente, A. First Report of Pantoea Ananatis Causing Necrotic Symptoms in Mango Trees in the Canary Islands, Spain. Plant Disease 2019, 103, 1017. [CrossRef]
- Gutiérrez-Barranquero, J.A.; Cazorla, F.M.; Torés, J.A.; De Vicente, A. Pantoea Agglomerans as a New Etiological Agent of a Bacterial Necrotic Disease of Mango Trees. Phytopathology® 2019, 109, 17–26. [CrossRef]
- Plotnikova, J.M.; Rahme, L.G.; Ausubel, F.M. Pathogenesis of the Human Opportunistic Pathogen Pseudomonas Aeruginosa PA14 in Arabidopsis. Plant Physiology 2000, 124, 1766–1774. [CrossRef]
- Prithiviraj, B.; Bais, H.P.; Jha, A.K.; Vivanco, J.M. Staphylococcus Aureus Pathogenicity on Arabidopsis Thaliana Is Mediated Either by a Direct Effect of Salicylic Acid on the Pathogen or by SA-dependent, NPR1-independent Host Responses. The Plant Journal 2005, 42, 417–432. [CrossRef]
- Jolliffe, I. Principal Component Analysis. 2nd ed,; Springer: New York, USA, 2002; pp. 111-149.
- Sun, Y.; Zheng, Y. Prediction of tomato plants infected by fungal pathogens at different disease severities using E-nose and GC–MS. Journal of Plant Diseases and Protection 2024.. [CrossRef]
- Vaibhaw, Sarraf, J.; Pattnaik, P. K. Brain–computer interfaces and their applications. In An Industrial IoT Approach for Pharmaceutical Industry Growth. Elsevier 2020, 31–54. [CrossRef]
- Héberger, K. Chemoinformatics—Multivariate mathematical–statistical methods for data evaluation In Medical Applications of Mass Spectrometry. Elsevier 2008. 141–169. [CrossRef]
- Refaeilzadeh, P.; Tang, L.; Liu, H. Cross-Validation. In: LIU, L., ÖZSU, M.T. (eds) Encyclopedia of Database Systems. Springer. Boston, MA, 2009; pp.532-538. [CrossRef]
- Li, N., Alfiky, A., Wang, W., Islam, M., Nourollahi, K., Liu, X., Kang, S. 2018. Volatile compound-mediated recognition and inhibition between Trichoderma biocontrol agents and Fusarium oxysporum. Frontiers in Microbiology . [CrossRef]
Figure 1.
Thin strips of substrates were placed in 20 ml headspace glass vials prior to E-nose sampling. (A) Healthy and Tropical Race 4 infected banana pseudo stems, (B) Healthy and infected mango leaf samples.
Figure 1.
Thin strips of substrates were placed in 20 ml headspace glass vials prior to E-nose sampling. (A) Healthy and Tropical Race 4 infected banana pseudo stems, (B) Healthy and infected mango leaf samples.
Figure 2.
Principal Component Analysis projection plot for two Mango Twig Tip Dieback bacterial varieties and Nutrient Agar control.
Figure 2.
Principal Component Analysis projection plot for two Mango Twig Tip Dieback bacterial varieties and Nutrient Agar control.
Figure 3.
(A) LDA plot for TR4 culture and PDA control, (B) PCA plot for MTTD 18, MTTD 30 pure cultures and uninoculated NA control.
Figure 3.
(A) LDA plot for TR4 culture and PDA control, (B) PCA plot for MTTD 18, MTTD 30 pure cultures and uninoculated NA control.
Figure 4.
MSEM 160 CD analysis plot for (A) TR4 cultures and empty PDA control, (B) MTTD 18, MTTD 30 cultures and NA control.
Figure 4.
MSEM 160 CD analysis plot for (A) TR4 cultures and empty PDA control, (B) MTTD 18, MTTD 30 cultures and NA control.
Figure 5.
Chromatogram of samples related to Panama disease (Tropical Race 4) in banana pseudo-stems EB: Empty bag, Pm: PDA media, Tc: TR4 culture, IB: Infected banana pseudo-stems and HB: Health banana pseudo-stems.
Figure 5.
Chromatogram of samples related to Panama disease (Tropical Race 4) in banana pseudo-stems EB: Empty bag, Pm: PDA media, Tc: TR4 culture, IB: Infected banana pseudo-stems and HB: Health banana pseudo-stems.
Figure 6.
Chromatogram of samples related to Mango Twig Tip Dieback disease (MTTD) in mango leaves. EB: Empty bag, Nm: NA media, M18c: MTTD18 culture, M30c: MTTD30 culture, HM: Health mango leaves, IM18: Infected mango leaves with MTTD18, IM30: Infected mango leaves with MTTD30.
Figure 6.
Chromatogram of samples related to Mango Twig Tip Dieback disease (MTTD) in mango leaves. EB: Empty bag, Nm: NA media, M18c: MTTD18 culture, M30c: MTTD30 culture, HM: Health mango leaves, IM18: Infected mango leaves with MTTD18, IM30: Infected mango leaves with MTTD30.
Figure 7.
LDA analysis plot for (A) MTTD infected samples and healthy mango leaves, (B) PCA plot for TR4 positive banana pseudo-stem samples and healthy pseudo-stem samples.
Figure 7.
LDA analysis plot for (A) MTTD infected samples and healthy mango leaves, (B) PCA plot for TR4 positive banana pseudo-stem samples and healthy pseudo-stem samples.
Figure 8.
CD analysis plot for MSEM 160 measurements, (A) MTTD 18, MTTD 30 and control/healthy mango leaves, (B) TR4 infected and healthy banana pseudo-stems. .
Figure 8.
CD analysis plot for MSEM 160 measurements, (A) MTTD 18, MTTD 30 and control/healthy mango leaves, (B) TR4 infected and healthy banana pseudo-stems. .
Table 1.
Cross-validation results for each of the five replicates of different concentrations of 3M2B and IAIV.
Table 1.
Cross-validation results for each of the five replicates of different concentrations of 3M2B and IAIV.
| Trained as |
0 ppm |
1 ppm |
10 ppm |
100 ppm |
1000 ppm |
% Accuracy |
| No. of correct identification / 5 (Total no. of replicates) |
3M2B |
3 |
1 |
1 |
4 |
4 |
52 |
| IAIV |
0 |
4 |
0 |
3 |
3 |
40 |
Table 3.
Cyranose 320 cross-validation results for sampling two sets of TR4 cultures.
Table 3.
Cyranose 320 cross-validation results for sampling two sets of TR4 cultures.
| Trained as |
TR4 – G 1 |
TR4 – G 2 |
% Accuracy |
| No. of correct identification / 6 (Total no. of replicates) |
TR4 – G 1 |
5 |
|
83.33 |
| TR4 – G 2 |
|
5 |
Table 4.
Cyranose 320 cross validation results for sampling uninoculated Nutrient Agar, MTTD 18 and MTTD 30 cultures.
Table 4.
Cyranose 320 cross validation results for sampling uninoculated Nutrient Agar, MTTD 18 and MTTD 30 cultures.
| Trained as |
NA-Control |
MTTD - 18 |
MTTD-30 |
% Accuracy |
| No. of correct identification / 6 (Total no. of replicates) |
6 |
4 |
4 |
77.78 |
Table 5.
Summary of VOCs identified from samples by using SPME-GC-MS.
Table 5.
Summary of VOCs identified from samples by using SPME-GC-MS.
| RT(min) |
No. |
ID |
EB |
Pm |
Tc |
HB |
IB |
Nm |
M18c |
M30c |
HM |
IM18 |
IM30 |
| 4.7 |
1 |
Dimethylacetamide |
+ |
|
|
|
|
|
|
|
|
|
|
| 6.9 |
2 |
phenol |
+ |
+ |
+ |
+ |
|
+ |
+ |
+ |
|
+ |
|
| 7.7 |
3 |
2-Ethyl-p-xylene |
+ |
|
+ |
+ |
+ |
|
|
+ |
+ |
+ |
|
| 8.8 |
4 |
p-(1-Propenyl)-toluene |
+ |
|
|
|
|
|
|
+ |
+ |
+ |
|
| 9.1 |
5 |
β-Phenylethanol |
|
|
|
|
|
|
+ |
|
|
|
|
| 9.2 |
6 |
p-Ethylanisole |
|
|
+ |
|
|
|
|
|
|
|
|
| 9.4 |
7 |
Cosmene |
|
|
|
+ |
|
|
|
|
|
|
|
| 9.6 |
8 |
Neo-allo-ocimene |
|
|
|
+ |
|
|
|
|
|
|
|
| 9.8 |
9 |
p-Vinylanisole |
|
|
+ |
|
|
|
|
|
|
|
|
| 10.5 |
10 |
6-Methyl-3,5-heptadiene-2-one |
|
|
+ |
|
|
|
|
|
|
|
|
| 10.5 |
11 |
Dodecane |
|
|
|
|
|
+ |
|
|
|
|
|
| 12.0 |
12 |
indole |
+ |
|
|
+ |
|
|
|
|
|
|
|
| 12.0 |
13 |
Tridecane |
|
|
|
|
|
+ |
|
|
|
|
|
| 12.3 |
14 |
Copaene |
|
|
|
+ |
++ |
|
|
|
|
|
|
| 12.9 |
15 |
2,4-Toluene diisocyanate |
|
|
|
|
|
|
+ |
|
|
|
|
| 13.8 |
16 |
Chrysanthenone |
|
|
|
+ |
+ |
|
|
|
|
|
|
| 13.8 |
17 |
α-Gurjunene |
|
|
|
|
|
|
|
|
+ |
+ |
+ |
| 13.9 |
18 |
α-Cedrene |
|
|
+ |
|
|
|
|
|
|
|
|
| 13.9 |
19 |
Caryophyllene |
|
|
|
+ |
+ |
|
|
|
+ |
+ |
+ |
| 14.2 |
20 |
γ-Gurjunene |
|
|
|
|
|
|
|
|
+ |
+ |
+ |
| 14.3 |
21 |
Humulene |
|
|
|
+ |
+ |
|
|
|
+ |
+ |
+ |
| 14.3 |
22 |
Isoledene |
|
|
|
|
|
|
|
|
+ |
+ |
+ |
| 14.5 |
23 |
2,6-Di-tert-butylbenzoquinone |
+ |
|
|
|
|
|
|
+ |
|
|
|
| 14.7 |
24 |
Bulnesene |
|
|
|
|
|
|
|
|
+ |
+ |
+ |
| 14.9 |
25 |
Aromadendrene |
|
|
|
+ |
+ |
|
|
|
+ |
+ |
+ |
| 15.0 |
26 |
Viridiflorene |
|
|
|
+ |
+ |
|
|
|
+ |
+ |
+ |
| 15.1 |
27 |
BHT |
+ |
+ |
|
|
|
|
+ |
|
|
|
|
| 15.1 |
28 |
Guaiene |
|
|
|
|
|
|
|
|
+ |
+ |
+ |
| 15.3 |
29 |
Calamenene |
|
|
|
+ |
++ |
|
|
|
|
|
|
| 17.2 |
30 |
Cadalene |
|
|
|
+ |
+ |
|
|
|
+ |
++ |
++ |
| 18.2 |
31 |
Guaiazulene |
|
|
|
|
|
|
|
|
+ |
++ |
++ |
| 18.7 |
32 |
3-Isopropyl-1,1'-biphenyl |
|
|
|
|
|
|
|
|
+ |
++ |
++ |
Table 6.
Cross-validation results of Healthy, MTTD 18 and MTTD 30 infected mango leaves using C320.
Table 6.
Cross-validation results of Healthy, MTTD 18 and MTTD 30 infected mango leaves using C320.
| Trained as |
Healthy |
MTTD 18 |
MTTD 30 |
% Accuracy |
| No. of correct identification / 6 (Total no. of replicates) |
4 |
4 |
5 |
54.17 |
Table 7.
Cross-validation results of Healthy banana pseudo-stem and TR4 infected pseudo-stem samples.
Table 7.
Cross-validation results of Healthy banana pseudo-stem and TR4 infected pseudo-stem samples.
| Trained as |
Healthy |
TR4 positive |
% Accuracy |
| No. of correct identification / 6 (Total no. of replicates) |
4 |
5 |
75 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).