Submitted:
10 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Experiment
3.1. Ceramic Cubic-LLZO Nanofiber Preparation [5,14,21,24,28]
3.2. Poly(dioxolane) & c-LLZO Nanofiber Composite Preparation and Battery Assembly [21]
3.3. Battery Test Schedules
4. Results and Discussion
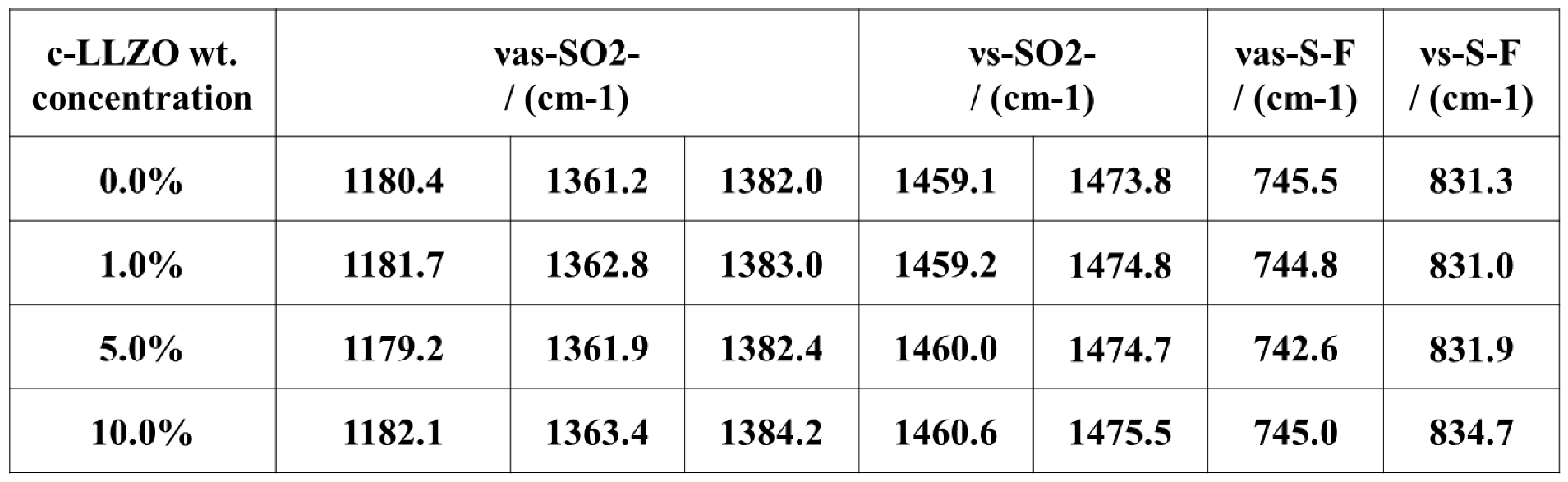
4.1. PDOL Preparation [8,31,32,33,34,35,36]
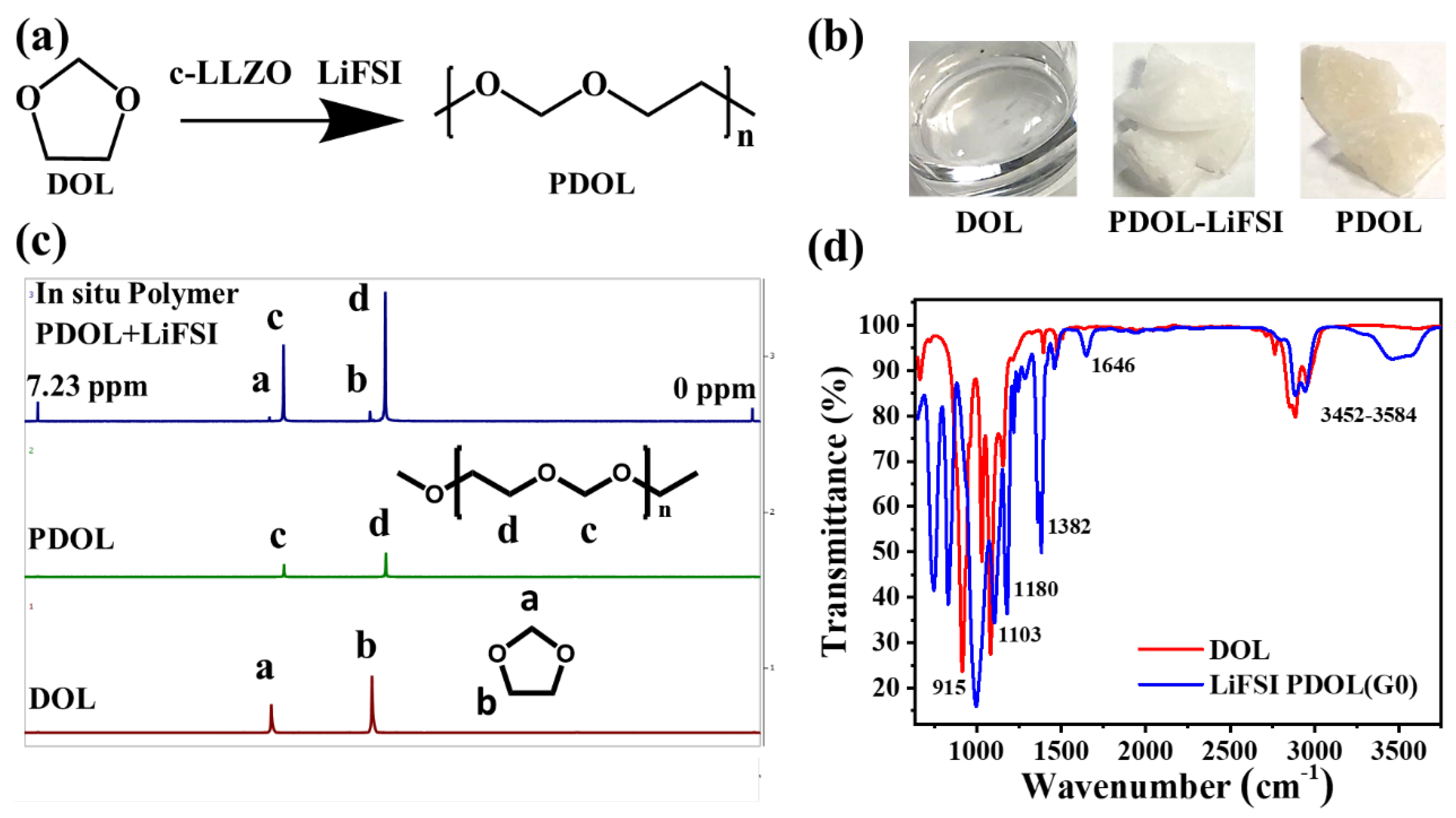
4.2. Cubic-LLZO Ceramic Nanofiber Characterization
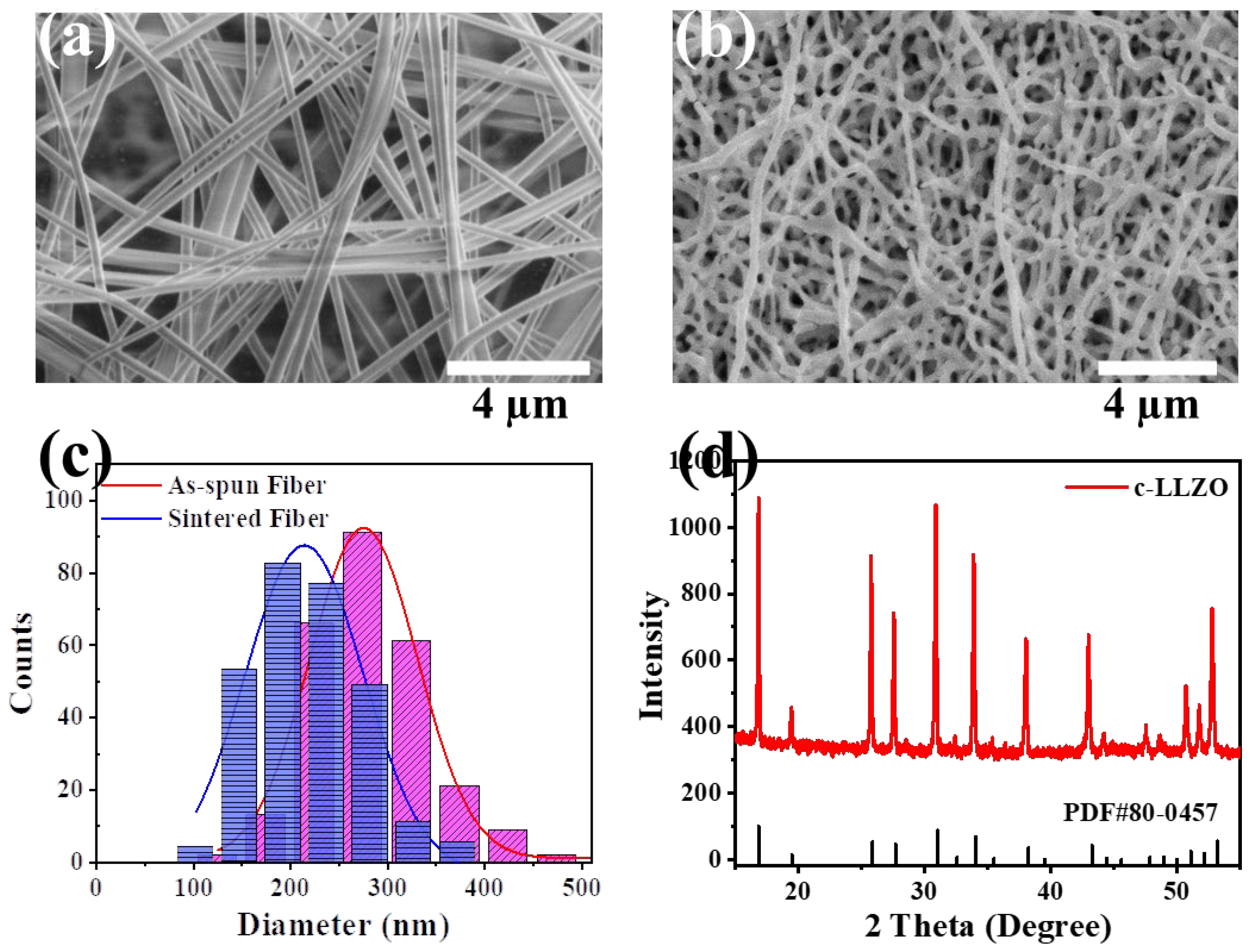
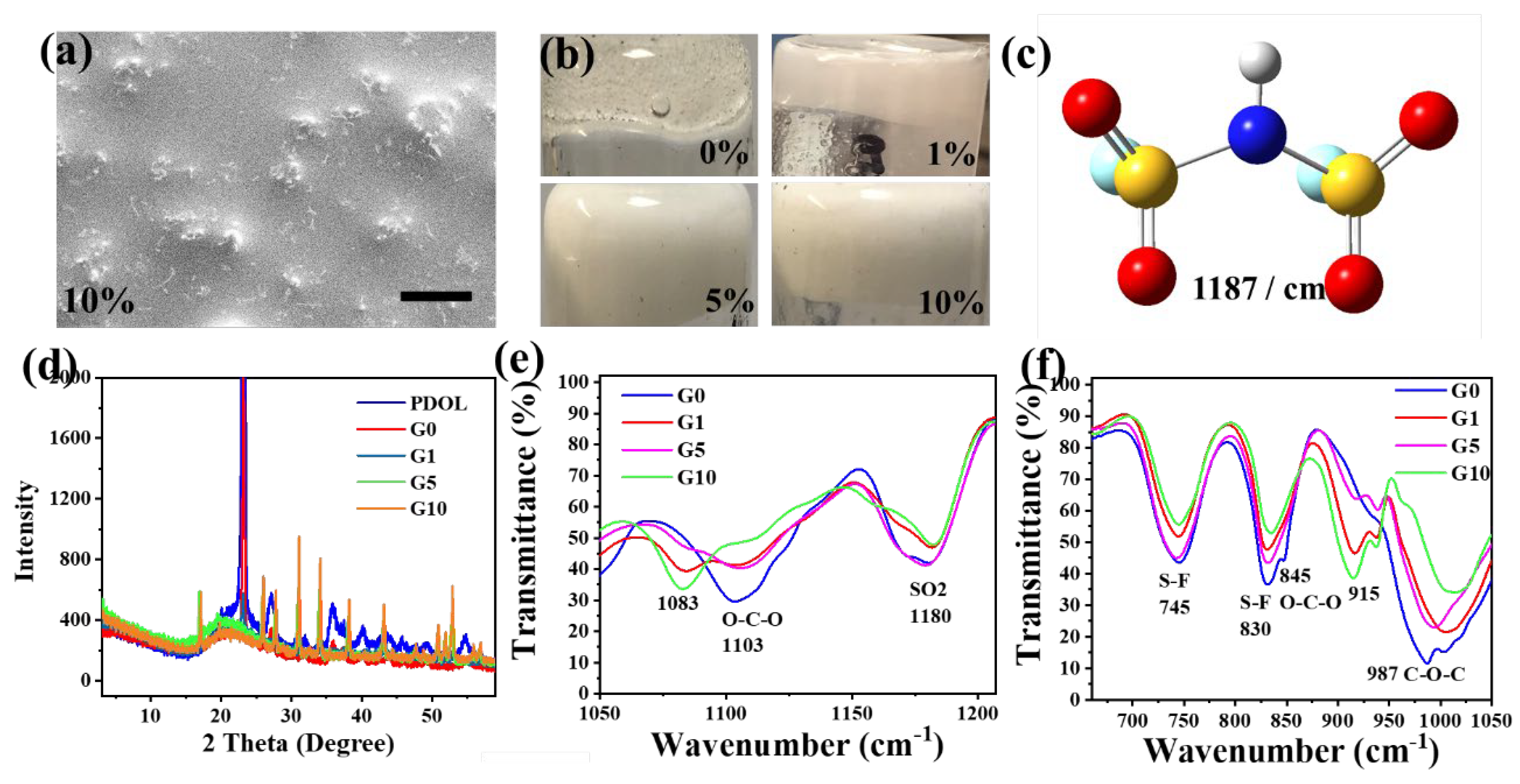
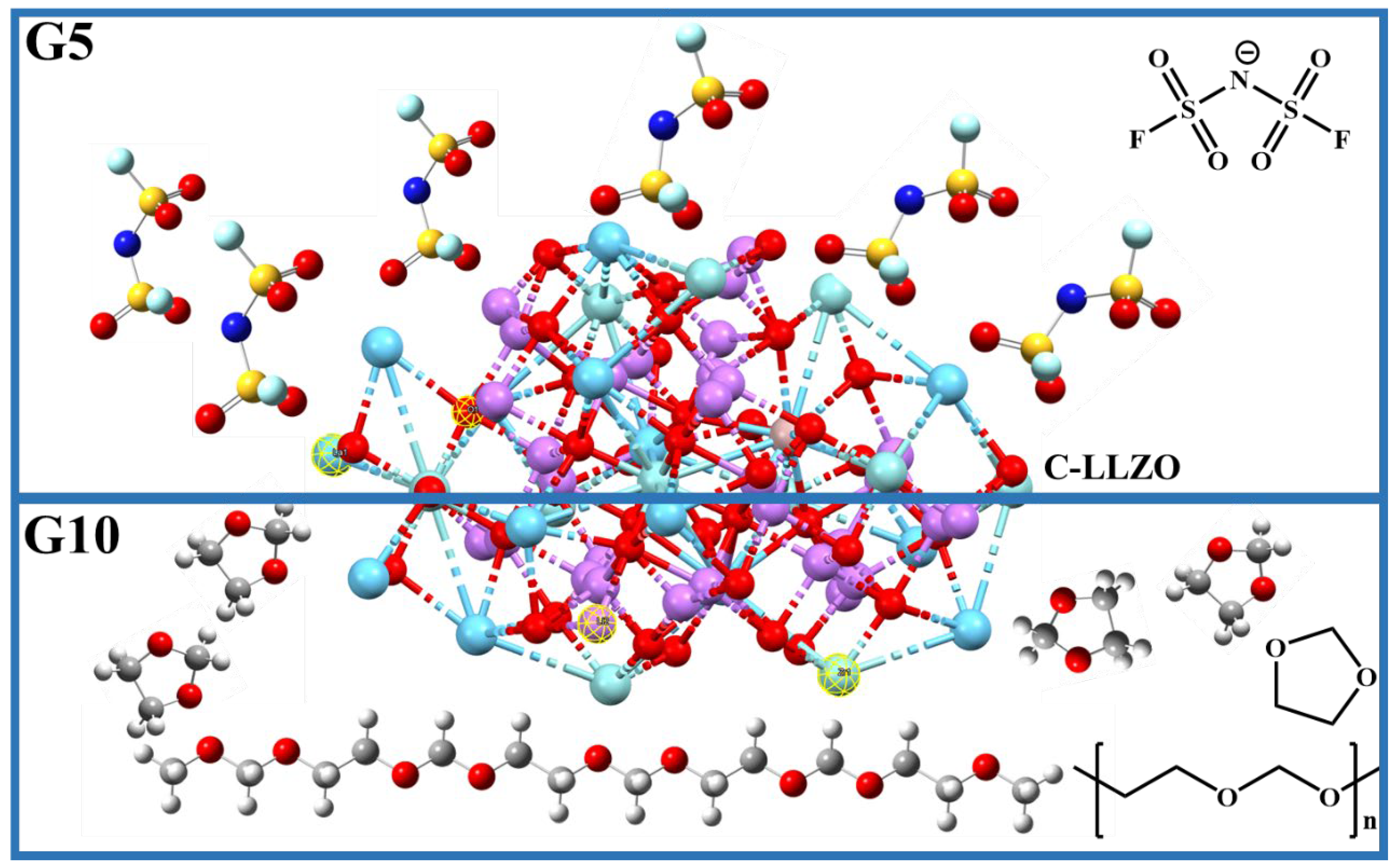
4.3. Composite Electrolytes Characterization
4.4. Thermal Stability
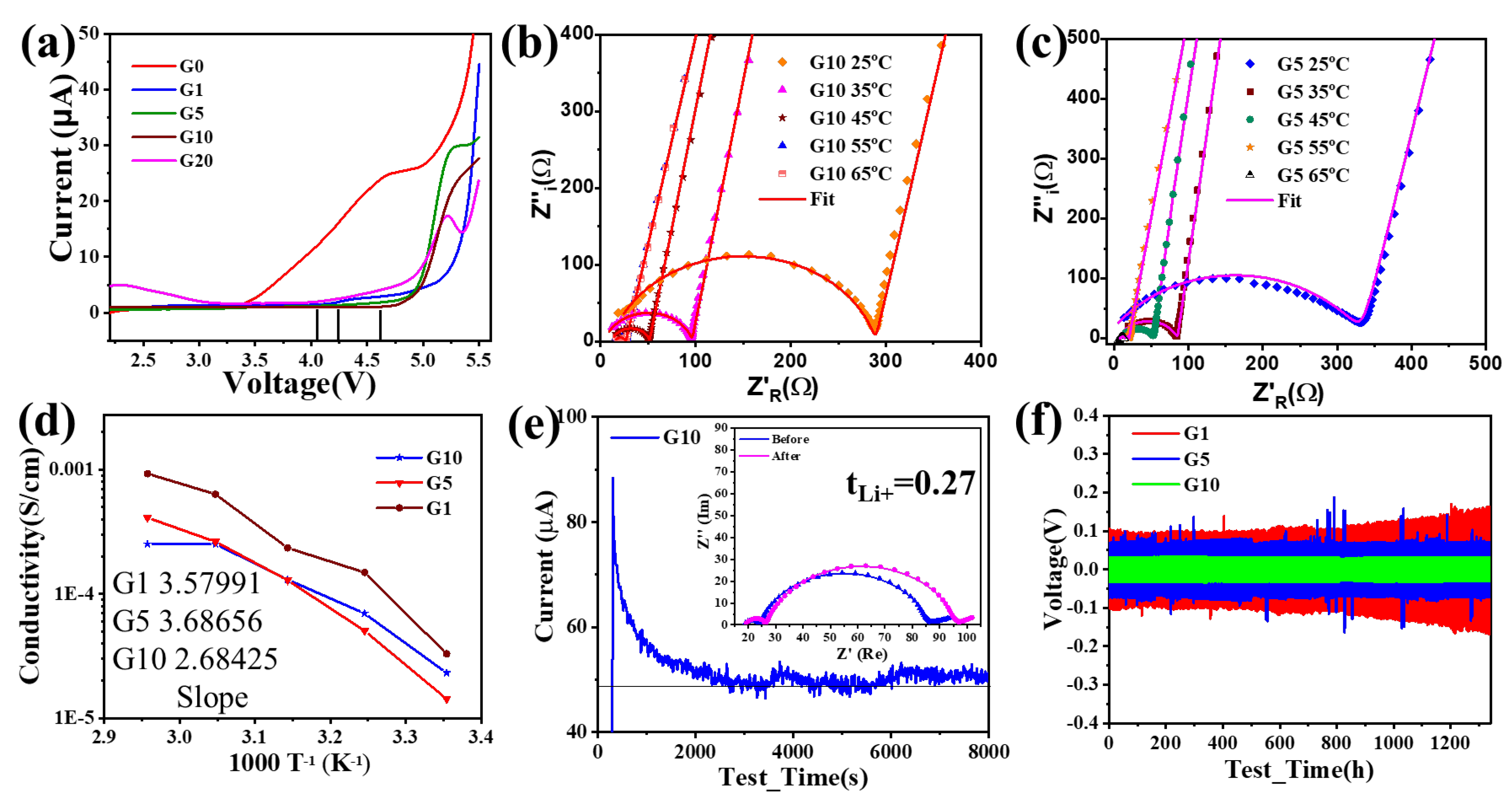
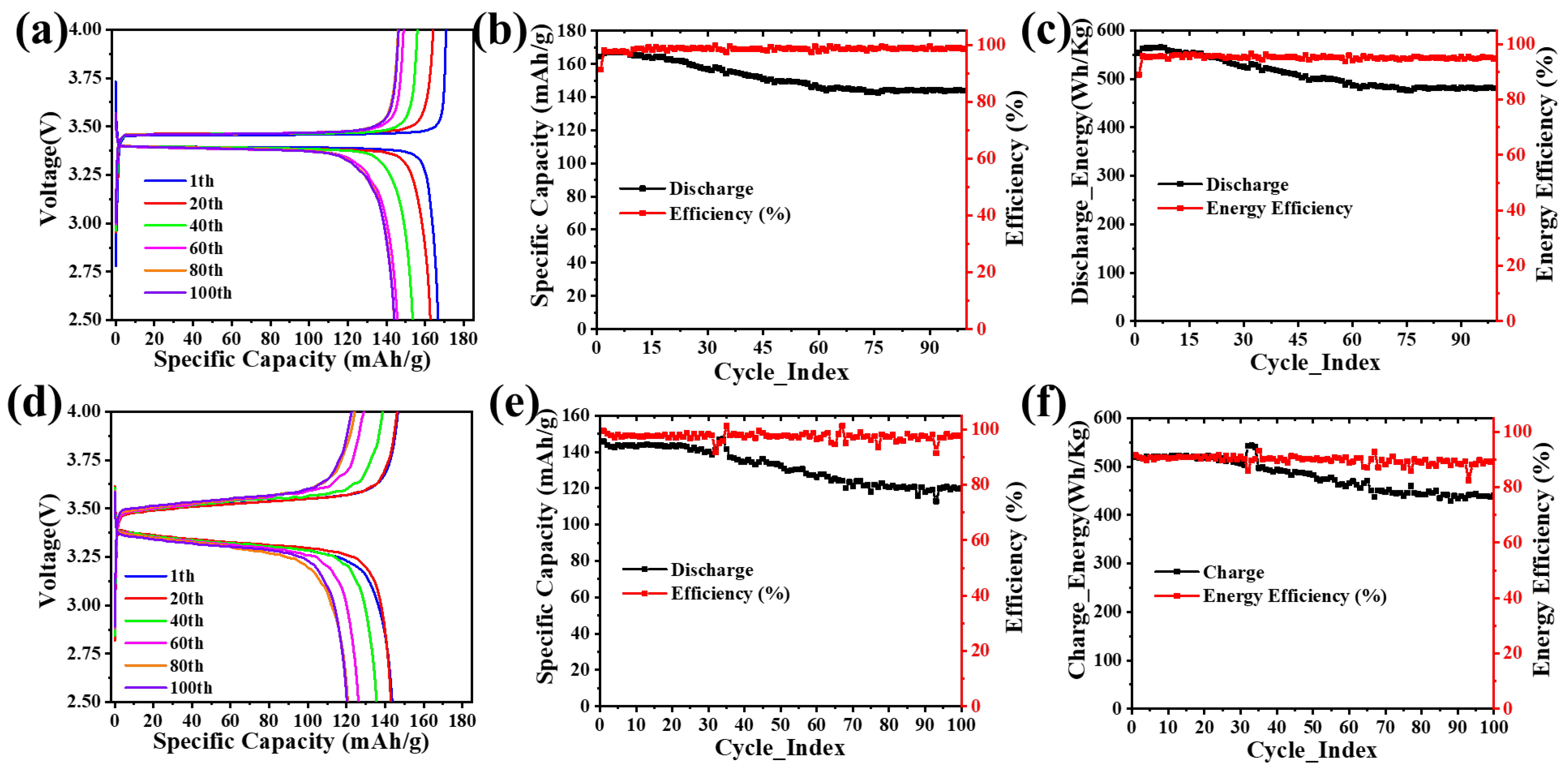
4.5. Electrochemical Performance
5. Discussion
6. Conclusions
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Chen, S.; Wen, K.; Fan, J.; Bando, Y.; Golberg, D. Progress and future prospects of high-voltage and high-safety electrolytes in advanced lithium batteries: from liquid to solid electrolytes. J. Mater. Chem. A 2018, 6, 11631–11663. [Google Scholar] [CrossRef]
- Perea, A.; Dontigny, M.; Zaghib, K. Safety of solid-state Li metal battery: Solid polymer versus liquid electrolyte. J. Power Sources 2017, 359, 182–185. [Google Scholar] [CrossRef]
- Jaumaux, P.; Wu, J.; Shanmukaraj, D.; Wang, Y.; Zhou, D.; Sun, B.; Kang, F.; Li, B.; Armand, M.; Wang, G. , Non-flammable liquid and quasi-solid electrolytes toward highly-safe alkali metal-based batteries. Advanced Functional Materials 2021, 31(10), 2008644. [Google Scholar] [CrossRef]
- Matsuki, Y.; Noi, K.; Deguchi, M.; Sakuda, A.; Hayashi, A.; Tatsumisago, M. , Lithium dissolution/deposition behavior of Al-doped Li7La3Zr2O12 ceramics with different grain sizes. Journal of The Electrochemical Society 2019, 166(3), A5470. [Google Scholar] [CrossRef]
- Cha, J.H.; Didwal, P.N.; Kim, J.M.; Chang, D.R.; Park, C.-J. Poly(ethylene oxide)-based composite solid polymer electrolyte containing Li7La3Zr2O12 and poly(ethylene glycol) dimethyl ether. J. Membr. Sci. 2019, 595, 117538. [Google Scholar] [CrossRef]
- Wang, R.; Liu, F.; Duan, J.; Ren, Y.; Li, M.; Cao, J. Enhanced Electrochemical Performance of Al- and Nb-Codoped LLZO Ceramic Powder and Its Composite Solid Electrolyte. ACS Appl. Energy Mater. 2021, 4, 13912–13921. [Google Scholar] [CrossRef]
- Chang, Z.; Yang, H.; Zhu, X.; He, P.; Zhou, H. A stable quasi-solid electrolyte improves the safe operation of highly efficient lithium-metal pouch cells in harsh environments. Nat. Commun. 2022, 13, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Deng, B.; Jing, M.-X.; Li, R.; Li, L.-X.; Yang, H.; Liu, M.-Q.; Xiang, J.; Yuan, W.-Y.; Shen, X.-Q. Integrating high ionic conductive PDOL solid/gel composite electrolyte for enhancement of interface combination and lithium dentrite inhibition of solid-state lithium battery. J. Colloid Interface Sci. 2022, 620, 199–208. [Google Scholar] [CrossRef]
- Oh, K.; Kim, J.; Kim, S.; Oh, D.; Han, S.; Jung, K.; Wang, Z.; Shi, L.; Su, Y.; Yim, T.; et al. Single-Ion Conducting Soft Electrolytes for Semi-Solid Lithium Metal Batteries Enabling Cell Fabrication and Operation under Ambient Conditions. Adv. Energy Mater. 2021, 11, 2101813. [Google Scholar] [CrossRef]
- Lin, D.; Liu, Y.; Cui, Y. Reviving the lithium metal anode for high-energy batteries. Nat. Nanotechnol. 2017, 12, 194–206. [Google Scholar] [CrossRef]
- Porz, L.; Swamy, T.; Sheldon, B.W.; Rettenwander, D.; Frömling, T.; Thaman, H.L.; Berendts, S.; Uecker, R.; Carter, W.C.; Chiang, Y. Mechanism of Lithium Metal Penetration through Inorganic Solid Electrolytes. Adv. Energy Mater. 2017, 7. [Google Scholar] [CrossRef]
- Wang, C.; Fu, K.; Kammampata, S.P.; McOwen, D.W.; Samson, A.J.; Zhang, L.; Hitz, G.T.; Nolan, A.M.; Wachsman, E.D.; Mo, Y.; et al. Garnet-Type Solid-State Electrolytes: Materials, Interfaces, and Batteries. Chem. Rev. 2020, 120, 4257–4300. [Google Scholar] [CrossRef] [PubMed]
- Biao, J.; Bai, C.; Ma, J.; Liu, M.; Kang, F.; Cao, Y.; He, Y. B. , Perspectives on Li Dendrite Penetration in Li7La3Zr2O12-Based Solid-State Electrolytes and Batteries: Materials, Interfaces, and Charge Transfer. Advanced Energy Materials 2024, 14(10), 2303128. [Google Scholar] [CrossRef]
- Tsai, C.-L.; Yu, S.; Tempel, H.; Kungl, H.; Eichel, R.-A. , All-ceramic Li batteries based on garnet structured Li7La3Zr2O12. Materials Technology 2020, 35 (9-10), 656-674.
- Sano, H.; Sakaebe, H.; Senoh, H.; Matsumoto, H. Effect of Current Density on Morphology of Lithium Electrodeposited in Ionic Liquid-Based Electrolytes. J. Electrochem. Soc. 2014, 161, A1236–A1240. [Google Scholar] [CrossRef]
- Sagane, F.; Ikeda, K.-I.; Okita, K.; Sano, H.; Sakaebe, H.; Iriyama, Y. Effects of current densities on the lithium plating morphology at a lithium phosphorus oxynitride glass electrolyte/copper thin film interface. J. Power Sources 2013, 233, 34–42. [Google Scholar] [CrossRef]
- Pei, A.; Zheng, G.; Shi, F.; Li, Y.; Cui, Y. Nanoscale Nucleation and Growth of Electrodeposited Lithium Metal. Nano Lett. 2017, 17, 1132–1139. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Gastol, D.; Marshall, J.; Sommerville, R.; Goodship, V.; Kendrick, E. A review of current collectors for lithium-ion batteries. J. Power Sources 2020, 485, 229321. [Google Scholar] [CrossRef]
- Fu, A.; Wang, C.; Peng, J.; Su, M.; Pei, F.; Cui, J.; Fang, X.; Li, J.; Zheng, N. Lithiophilic and Antioxidative Copper Current Collectors for Highly Stable Lithium Metal Batteries. Adv. Funct. Mater. 2021, 31, 2009805. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, D.; Xiang, H.; Feng, X.; Yu, Y. Research Progress on Copper-Based Current Collector for Lithium Metal Batteries. Energy Fuels 2021, 35, 12921–12937. [Google Scholar] [CrossRef]
- Chen, L.-H.; Huang, Z.-Y.; Chen, S.-L.; Tong, R.-A.; Wang, H.-L.; Shao, G.; Wang, C.-A. In situ polymerization of 1,3-dioxolane infiltrating 3D garnet framework with high ionic conductivity and excellent interfacial stability for integrated solid-state Li metal battery. Rare Met. 2022, 41, 3694–3705. [Google Scholar] [CrossRef]
- Bahmani, F.; Rodmyre, C.; Ly, K.; Mack, P.; Smirnova, A.W. In Situ/Operando Techniques for Unraveling Mechanisms of Ionic Transport in Solid-State Lithium Indium Halide Electrolyte. Batteries 2024, 10, 21. [Google Scholar] [CrossRef]
- Zhai, Y.; Yang, G.; Zeng, Z.; Song, S.; Li, S.; Hu, N.; Tang, W.; Wen, Z.; Lu, L.; Molenda, J. Composite Hybrid Quasi-Solid Electrolyte for High-Energy Lithium Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 7973–7982. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, W.; Dou, Q.; Wong, K. W.; Ng, K. M. , Li 7 La 3 Zr 2 O 12 ceramic nanofiber-incorporated composite polymer electrolytes for lithium metal batteries. Journal of Materials Chemistry A 2019, 7(7), 3391–3398. [Google Scholar] [CrossRef]
- Chen, L.; Li, Y.; Li, S.-P.; Fan, L.-Z.; Nan, C.-W.; Goodenough, J. B. , PEO/garnet composite electrolytes for solid-state lithium batteries: From “ceramic-in-polymer” to “polymer-in-ceramic”. Nano Energy 2018, 46, 176–184. [Google Scholar] [CrossRef]
- Li, Z.; Huang, H.-M.; Zhu, J.-K.; Wu, J.-F.; Yang, H.; Wei, L.; Guo, X. Ionic Conduction in Composite Polymer Electrolytes: Case of PEO:Ga-LLZO Composites. ACS Appl. Mater. Interfaces 2018, 11, 784–791. [Google Scholar] [CrossRef]
- Li, Z.; Zhou, X.-Y.; Guo, X. High-performance lithium metal batteries with ultraconformal interfacial contacts of quasi-solid electrolyte to electrodes. Energy Storage Mater. 2020, 29, 149–155. [Google Scholar] [CrossRef]
- Zhang, H.; An, X.; Lu, Z.; Liu, L.; Cao, H.; Xu, Q.; Liu, H.; Ni, Y. A three dimensional interconnected Li7La3Zr2O12 framework composite solid electrolyte utilizing lignosulfonate/ cellulose nanofiber bio-template for high performance lithium ion batteries. J. Power Sources 2020, 477, 228752. [Google Scholar] [CrossRef]
- Xue, J.; Wu, T.; Dai, Y.; Xia, Y. Electrospinning and Electrospun Nanofibers: Methods, Materials, and Applications. Chem. Rev. 2019, 119, 5298–5415. [Google Scholar] [CrossRef]
- Mengesha, T.H.; Beshahwured, S.L.; Wu, S.-H.; Wu, Y.-S.; Jose, R.; Lue, S.J.; Yang, C.-C. Freestanding Trilayer Hybrid Solid Electrolyte with Electrospun Interconnected Al-LLZO Nanofibers for Solid-State Lithium-Metal Batteries. ACS Appl. Energy Mater. 2021, 4, 14554–14574. [Google Scholar] [CrossRef]
- Yang, H.; Jing, M.; Wang, L.; Xu, H.; Yan, X.; He, X. PDOL-Based Solid Electrolyte Toward Practical Application: Opportunities and Challenges. Nano-Micro Lett. 2024, 16, 1–33. [Google Scholar] [CrossRef]
- Yang, H.; Zhang, B.; Jing, M.; Shen, X.; Wang, L.; Xu, H.; Yan, X.; He, X. In Situ Catalytic Polymerization of a Highly Homogeneous PDOL Composite Electrolyte for Long-Cycle High-Voltage Solid-State Lithium Batteries. Adv. Energy Mater. 2022, 12. [Google Scholar] [CrossRef]
- Liu, F.-Q.; Wang, W.-P.; Yin, Y.-X.; Zhang, S.-F.; Shi, J.-L.; Wang, L.; Zhang, X.-D.; Zheng, Y.; Zhou, J.-J.; Li, L.; et al. Upgrading traditional liquid electrolyte via in situ gelation for future lithium metal batteries. Sci. Adv. 2018, 4, eaat5383. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Wu, H.; Wan, J.; Li, M.; Zhang, Y.; Sun, L.; Liu, Y.; Chen, L.; Wen, R.; Wang, C. Synthetic poly-dioxolane as universal solid electrolyte interphase for stable lithium metal anodes. J. Energy Chem. 2021, 62, 172–178. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, M.; Kang, P.; Zhu, T.; Yuan, H.; Lan, J.; Yang, X.; Sui, G. Self-Enhancing Gel Polymer Electrolyte by In Situ Construction for Enabling Safe Lithium Metal Battery. Adv. Sci. 2021, 9, 2103663. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Tang, W.; Deng, Y.; Zhou, M.; Wang, X.; Liu, R.; Wang, C.-A. Enabling highly stable lithium metal batteries by using dual-function additive catalyzed in-built quasi-solid-state polymer electrolytes. J. Mater. Chem. A 2022, 10, 23047–23057. [Google Scholar] [CrossRef]
- Cheng, H.; Zhu, J.; Jin, H.; Gao, C.; Liu, H.; Cai, N.; Liu, Y.; Zhang, P.; Wang, M. , In situ initiator-free gelation of highly concentrated lithium bis (fluorosulfonyl) imide-1, 3-dioxolane solid polymer electrolyte for high performance lithium-metal batteries. Materials Today Energy 2021, 20, 100623. [Google Scholar] [CrossRef]
- Ma, J.; Wang, Z.; Wu, J.; Gu, Z.; Xin, X.; Yao, X. In Situ Solidified Gel Polymer Electrolytes for Stable Solid−State Lithium Batteries at High Temperatures. Batteries 2022, 9, 28. [Google Scholar] [CrossRef]
- Zhao, Y.; Yan, J.; Cai, W.; Lai, Y.; Song, J.; Yu, J.; Ding, B. Elastic and well-aligned ceramic LLZO nanofiber based electrolytes for solid-state lithium batteries. Energy Storage Mater. 2019, 23, 306–313. [Google Scholar] [CrossRef]
- Kerner, M.; Plylahan, N.; Scheers, J.; Johansson, P. Thermal stability and decomposition of lithium bis(fluorosulfonyl)imide (LiFSI) salts. RSC Adv. 2016, 6, 23327–23334. [Google Scholar] [CrossRef]
- Huang, J.; Hollenkamp, A.F. Thermal Behavior of Ionic Liquids Containing the FSI Anion and the Li+ Cation. J. Phys. Chem. C 2010, 114, 21840–21847. [Google Scholar] [CrossRef]
- Li, L.; Zhou, S.; Han, H.; Li, H.; Nie, J.; Armand, M.; Zhou, Z.; Huang, X. Transport and Electrochemical Properties and Spectral Features of Non-Aqueous Electrolytes Containing LiFSI in Linear Carbonate Solvents. J. Electrochem. Soc. 2011, 158, A74–A82. [Google Scholar] [CrossRef]
- Frisch, M. J.; Trucks, G. W.; Schlegel, H. B.; Scuseria, G. E.; Robb, M. A.; Cheeseman, J. R.; Scalmani, G.; Barone, V.; Petersson, G. A.; Nakatsuji, H.; Li, X.; Caricato, M.; Marenich, A. V.; Bloino, J.; Janesko, B. G.; Gomperts, R.; Mennucci, B.; Hratchian, H. P.; Ortiz, J. V.; Izmaylov, A. F.; Sonnenberg, J. L. ; Williams; Ding, F.; Lipparini, F.; Egidi, F.; Goings, J.; Peng, B.; Petrone, A.; Henderson, T.; Ranasinghe, D.; Zakrzewski, V. G.; Gao, J.; Rega, N.; Zheng, G.; Liang, W.; Hada, M.; Ehara, M.; Toyota, K.; Fukuda, R.; Hasegawa, J.; Ishida, M.; Nakajima, T.; Honda, Y.; Kitao, O.; Nakai, H.; Vreven, T.; Throssell, K.; Montgomery Jr., J. A.; Peralta, J. E.; Ogliaro, F.; Bearpark, M. J.; Heyd, J. J.; Brothers, E. N.; Kudin, K. N.; Staroverov, V. N.; Keith, T. A.; Kobayashi, R.; Normand, J.; Raghavachari, K.; Rendell, A. P.; Burant, J. C.; Iyengar, S. S.; Tomasi, J.; Cossi, M.; Millam, J. M.; Klene, M.; Adamo, C.; Cammi, R.; Ochterski, J. W.; Martin, R. L.; Morokuma, K.; Farkas, O.; Foresman, J. B.; Fox, D. J. Gaussian 16 Rev. C.01, Wallingford, CT, 2016.
- Li, G.; Liang, K.; Li, Y.; Duan, X.; Fu, L.; Cai, Z.; Zhang, Z.; Dai, J.; Sun, Y. Catalytic anode surface enabling in situ polymerization of gel polymer electrolyte for stable Li metal batteries. Nano Res. 2024, 1–8. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).