Submitted:
10 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
6. Future Directions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
References
- Witchel, S.F.; Teede, H.J.; Peña, A.S. Curtailing PCOS. Pediatr. Res. 2019, 87, 353–361. [Google Scholar] [CrossRef]
- Pundir, C.S.; Deswal, R.; Narwal, V.; Dang, A. The Prevalence of Polycystic Ovary Syndrome: A Brief Systematic Review. J. Hum. Reprod. Sci. 2020, 13, 261–271. [Google Scholar] [CrossRef]
- Chiaffarino, F.; Cipriani, S.; Dalmartello, M.; Ricci, E.; Esposito, G.; Fedele, F.; La Vecchia, C.; Negri, E.; Parazzini, F. Prevalence of polycystic ovary syndrome in European countries and USA: A systematic review and meta-analysis. Eur. J. Obstet. Gynecol. Reprod. Biol. 2022, 279, 159–170. [Google Scholar] [CrossRef]
- Azziz, R.; Carmina, E.; Chen, Z.; Dunaif, A.; Laven, J.S.; Legro, R.S.; Lizneva, D.; Natterson-Horowtiz, B.; Teede, H.J.; Yildiz, B.O. Polycystic ovary syndrome. Nat. Rev. Dis. Primers 2016, 2, 16057. [Google Scholar] [CrossRef]
- Carmina, E. Diagnosis of polycystic ovary syndrome: from NIH criteria to ESHRE-ASRM guidelines. 2004, 56, 1–6. [Google Scholar]
- Blank, S.K.; McCartney, C.R.; Chhabra, S.; Helm, K.D.; Eagleson, C.A.; Chang, R.J.; Marshall, J.C. Modulation of Gonadotropin-Releasing Hormone Pulse Generator Sensitivity to Progesterone Inhibition in Hyperandrogenic Adolescent Girls—Implications for Regulation of Pubertal Maturation. J. Clin. Endocrinol. Metab. 2009, 94, 2360–2366. [Google Scholar] [CrossRef]
- Haqq, L.; McFarlane, J.; Dieberg, G.; Smart, N. Effect of lifestyle intervention on the reproductive endocrine profile in women with polycystic ovarian syndrome: a systematic review and meta-analysis. Endocr. Connect. 2014, 3, 36–46. [Google Scholar] [CrossRef]
- Malini, N.; George, K.R. Evaluation of different ranges of LH:FSH ratios in polycystic ovarian syndrome (PCOS) – Clinical based case control study. Gen. Comp. Endocrinol. 2018, 260, 51–57. [Google Scholar] [CrossRef]
- Xia, Q.; Xie, L.; Wu, Q.; Cong, J.; Ma, H.; Li, J.; Cai, W.; Wu, X. Elevated baseline LH/FSH ratio is associated with poor ovulatory response but better clinical pregnancy and live birth in Chinese women with PCOS after ovulation induction. Heliyon 2023, 9, e13024. [Google Scholar] [CrossRef]
- Kriedt, K.J.; Alchami, A.; Davies, M.C. PCOS: diagnosis and management of related infertility. Obstet. Gynaecol. Reprod. Med. 2018, 29, 1–5. [Google Scholar] [CrossRef]
- Cho, L.W.; Jayagopal, V.; Kilpatrick, E.S.; Holding, S.; Atkin, S.L. The LH/FSH ratio has little use in diagnosing polycystic ovarian syndrome. Ann. Clin. Biochem. Int. J. Biochem. Lab. Med. 2006, 43, 217–219. [Google Scholar] [CrossRef]
- E Joham, A.; Norman, R.J.; Stener-Victorin, E.; Legro, R.S.; Franks, S.; Moran, L.J.; Boyle, J.; Teede, H.J. Polycystic ovary syndrome. Lancet Diabetes Endocrinol. 2022, 10, 668–680. [Google Scholar] [CrossRef]
- Balen, A.H.; Morley, L.C.; Misso, M.; Franks, S.; Legro, R.S.; Wijeyaratne, C.N.; Stener-Victorin, E.; Fauser, B.C.; Norman, R.J.; Teede, H. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum. Reprod. Update 2016, 22, 687–708. [Google Scholar] [CrossRef]
- Melo, A.S.; Ferriani, R.A.; Navarro, P.A. Treatment of infertility in women with polycystic ovary syndrome: approach to clinical practice. Clinics 2015, 70, 765–769. [Google Scholar] [CrossRef]
- Sirmans, S.; Pate, K. Epidemiology, diagnosis, and management of polycystic ovary syndrome. Clin. Epidemiology 2013, 6, 1–13. [Google Scholar] [CrossRef]
- Salley, K.E.S.; Wickham, E.P.; Cheang, K.I.; Essah, P.A.; Karjane, N.W.; Nestler, J.E. POSITION STATEMENT: Glucose Intolerance in Polycystic Ovary Syndrome—A Position Statement of the Androgen Excess Society. J. Clin. Endocrinol. Metab. 2007, 92, 4546–4556. [Google Scholar] [CrossRef]
- Carter, C.S. Oxytocin Pathways and the Evolution of Human Behavior. Annu. Rev. Psychol. 2014, 65, 17–39. [Google Scholar] [CrossRef]
- Freda, S. N. , Priest, M. F., Badong, D., Xiao, L., Liu, Y.; Kozorovitskiy, Y. Brainwide input-output architecture of paraventricular oxytocin and vasopressin neurons. bioRxiv, 2022, 2022-01.
- Manjila, S.B.; Betty, R.; Kim, Y. Missing pieces in decoding the brain oxytocin puzzle: Functional insights from mouse brain wiring diagrams. Front. Neurosci. 2022, 16, 1044736. [Google Scholar] [CrossRef]
- Heinrichs, M. , von Dawans, B. ; Domes, G. Oxytocin, vasopressin, and human social behavior. Frontiers in neuroendocrinology, 2009, 30, 548–557. [Google Scholar]
- Yamamoto, K.; Nakano, Y.; Iwata, N.; Soejima, Y.; Suyama, A.; Hasegawa, T.; Otsuka, F. Oxytocin enhances progesterone production with upregulation of BMP-15 activity by granulosa cells. Biochem. Biophys. Res. Commun. 2023, 646, 103–109. [Google Scholar] [CrossRef]
- Saller, S.; Kunz, L.; Dissen, G.; Stouffer, R.; Ojeda, S.; Berg, D.; Berg, U.; Mayerhofer, A. Oxytocin receptors in the primate ovary: molecular identity and link to apoptosis in human granulosa cells. Hum. Reprod. 2010, 25, 969–976. [Google Scholar] [CrossRef]
- Pinto, J.; Cera, N.; Pignatelli, D. Psychological symptoms and brain activity alterations in women with PCOS and their relation to the reduced quality of life: a narrative review. J. Endocrinol. Investig. 2024, 1–22. [Google Scholar] [CrossRef]
- Knobloch, K.; Yoon, U.; Vogt, P.M. Preferred reporting items for systematic reviews and meta-analyses (PRISMA) statement and publication bias. J. Cranio-Maxillofacial Surg. 2010, 39, 91–92. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. An Application of Hierarchical Kappa-type Statistics in the Assessment of Majority Agreement among Multiple Observers. Biometrics 1977, 33, 363–74. [Google Scholar] [CrossRef]
- Ochsenkühn, R.; Pavlik, R.; Hecht, S.; von Schönfeldt, V.; Rogenhofer, N.; Thaler, C.J. The effect of nasal oxytocin on pregnancy rates following intrauterine insemination: double-blind, randomized, clinical pilot study. Arch. Gynecol. Obstet. 2009, 281, 753–759. [Google Scholar] [CrossRef]
- Masrour, M. J.; Azad, Z. A comparison of the effects of human chorionic gonadotropin and oxytocin on ovulation in PCOS patients from 2015 until 2018. Acta Medica Mediterranea, 2018, 34, 1757–1763. [Google Scholar]
- Jahromi, B.N.; Dabbaghmanesh, M.H.; Bakhshaie, P.; Parsanezhad, M.E.; Anvar, Z.; Alborzi, M.; Zarei, A.; Bakhshaei, M. Assessment of oxytocin level, glucose metabolism components and cutoff values for oxytocin and anti-mullerian hormone in infertile PCOS women. Taiwan. J. Obstet. Gynecol. 2018, 57, 555–559. [Google Scholar] [CrossRef]
- Piróg, M.; Jach, R.; Ząbczyk, M.; Natorska, J. Increased Serum Levels of Phoenixin-14, Nesfatin-1 and Dopamine Are Associated with Positive Pregnancy Rate after Ovarian Stimulation. J. Clin. Med. 2023, 12, 6991. [Google Scholar] [CrossRef]
- Amin, M.; Horst, N.; Wu, R.; Gragnoli, C. Oxytocin receptor (OXTR) is a risk gene for polycystic ovarian syndrome. 2023, 27, 2634–2638. [CrossRef]
- Gimpl, G.; Fahrenholz, F.; Srinivasa, S.; Aulinas, A.; O’malley, T.; Maehler, P.; Adler, G.K.; Grinspoon, S.K.; Lawson, E.A.; Santoso, P.; et al. The Oxytocin Receptor System: Structure, Function, and Regulation. Physiol. Rev. 2001, 81, 629–683. [Google Scholar] [CrossRef]
- Sajadi, M.; Noroozzadeh, M.; Bagheripour, F.; Tehrani, F.R. Contractions in the Isolated Uterus of a Rat Model of Polycystic Ovary Syndrome Compared to Controls in Adulthood. Int. J. Endocrinol. Metab. 2018, 16, e63135. [Google Scholar] [CrossRef]
- Yamamoto, S.; Noguchi, H.; Takeda, A.; Arakaki, R.; Uchishiba, M.; Imaizumi, J.; Minato, S.; Kamada, S.; Kagawa, T.; Yoshida, A.; et al. Changes in Endogenous Oxytocin Levels and the Effects of Exogenous Oxytocin Administration on Body Weight Changes and Food Intake in Polycystic Ovary Syndrome Model Rats. Int. J. Mol. Sci. 2022, 23, 8207. [Google Scholar] [CrossRef] [PubMed]
- Dinsdale, N.L.; Crespi, B.J. Revisiting the wandering womb: Oxytocin in endometriosis and bipolar disorder. Horm. Behav. 2017, 96, 69–83. [Google Scholar] [CrossRef] [PubMed]
- Burns, P. D. , Mendes Jr, J. O., Yemm, R. S., Clay, C. M., Nelson, S. E., Hayes, S. H.; Silvia, W. J. Cellular mechanisms by which oxytocin mediates ovine endometrial prostaglandin F2α synthesis: role of Gi proteins and mitogen-activated protein kinases. Biology of reproduction, 2001, 65, 1150–1155. [Google Scholar]
- Mechsner, S.; Bartley, J.; Loddenkemper, C.; Salomon, D.S.; Starzinski-Powitz, A.; Ebert, A.D. Oxytocin receptor expression in smooth muscle cells of peritoneal endometriotic lesions and ovarian endometriotic cysts. Fertil. Steril. 2005, 83, 1220–1231. [Google Scholar] [CrossRef] [PubMed]
- Roushangar L, Soleimani Rad J, Nikpou P, Sayahmeli M. Effect of oxytocin injection on folliculogenesis, ovulation and endometrial growth in mice. Int J Reprod Biomed 2009, 7, 91e5.
- Sayyah-Melli M, Ouladsahebmadarek E, Tagavi S, Mostafa-Garabaghi P, Alizadeh M, Ghojazadeh M, et al. Effect of oxytocin (OT) and OT plus human chorionic gonadotropin (hCG), in cycles induced by letrozole or clomiphene citrate (CC). Afr J Pharm 2012, 6, 2112e8.
- Soloff MS, Alexandrova M, Fernstrom MJ. Oxytocin receptors: triggers for parturition and lactation? Science. 1979, 204, 1313–5.
- Leonhardt, H.; Gull, B.; Kishimoto, K.; Kataoka, M.; Nilsson, L.; O Janson, P.; Stener-Victorin, E.; Hellström, M. Uterine Morphology and Peristalsis in Women with Polycystic Ovary Syndrome. Acta Radiol. 2012, 53, 1195–1201. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.H.; Chang, W.H.; Chi, M.H.; Peng, Y.C.; Huang, C.-C.; Yang, Y.K.; Chen, P.S. The OXTR Polymorphism Stratified the Correlation of Oxytocin and Glucose Homeostasis in Non-Diabetic Subjects. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2707; 12. [Google Scholar] [CrossRef]
- Malik, A.I.; Zai, C.C.; Abu, Z.; Nowrouzi, B.; Beitchman, J.H. The role of oxytocin and oxytocin receptor gene variants in childhood-onset aggression. Genes, Brain Behav. 2012, 11, 545–551. [Google Scholar] [CrossRef]
- Montag, C.; Brockmann, E.-M.; Bayerl, M.; Rujescu, D.; Müller, D.J.; Gallinat, J. Oxytocin and oxytocin receptor gene polymorphisms and risk for schizophrenia: A case–control study. World J. Biol. Psychiatry 2012, 14, 500–508. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [PubMed]
- Amin, M.; Wu, R.; Gragnoli, C. Novel Risk Variants in the Oxytocin Receptor Gene (OXTR) Possibly Linked to and Associated with Familial Type 2 Diabetes. Int. J. Mol. Sci. 2023, 24, 6282. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Mayila, Y.; Yanagihara, R.; Yamamoto, Y.; Kawakita, T.; Kuwahara, A.; Irahara, M. Oxytocin treatment reduced food intake and body fat and ameliorated obesity in ovariectomized female rats. Neuropeptides 2019, 75, 49–57. [Google Scholar] [CrossRef]
- Carr, D.B.; Utzschneider, K.M.; Hull, R.L.; Kodama, K.; Retzlaff, B.M.; Brunzell, J.D.; Shofer, J.B.; Fish, B.E.; Knopp, R.H.; Kahn, S.E. Intra-Abdominal Fat Is a Major Determinant of the National Cholesterol Education Program Adult Treatment Panel III Criteria for the Metabolic Syndrome. Diabetes 2004, 53, 2087–2094. [Google Scholar] [CrossRef] [PubMed]
- Ferrara, C.M.; Lynch, N.A.; Nicklas, B.J.; Ryan, A.S.; Berman, D.M. Differences in Adipose Tissue Metabolism between Postmenopausal and Perimenopausal Women. J. Clin. Endocrinol. Metab. 2002, 87, 4166–4170. [Google Scholar] [CrossRef] [PubMed]
- Iwasa, T.; Matsuzaki, T.; Yano, K.; Yanagihara, R.; Tungalagsuvd, A.; Munkhzaya, M.; Mayila, Y.; Kuwahara, A.; Irahara, M. The effects of chronic testosterone administration on body weight, food intake, and adipose tissue are changed by estrogen treatment in female rats. Horm. Behav. 2017, 93, 53–61. [Google Scholar] [CrossRef]
- Liang, Y.-Q.; Akishita, M.; Kim, S.; Ako, J.; Hashimoto, M.; Iijima, K.; Ohike, Y.; Watanabe, T.; Sudoh, N.; Toba, K.; et al. Estrogen receptor ß is involved in the anorectic action of estrogen. Int. J. Obes. 2002, 26, 1103–1109. [Google Scholar] [CrossRef]
- Meli, R.; Pacilio, M.; Raso, G.M.; Esposito, E.; Coppola, A.; Nasti, A.; Di Carlo, C.; Nappi, C.; Di Carlo, R. Estrogen and Raloxifene Modulate Leptin and Its Receptor in Hypothalamus and Adipose Tissue from Ovariectomized Rats. Endocrinology 2004, 145, 3115–3121. [Google Scholar] [CrossRef]
- Palmisano, B.T.; Zhu, L.; Stafford, J.M. Role of Estrogens in the Regulation of Liver Lipid Metabolism. Sex and Gender Factors Affecting Metabolic Homeostasis, Diabetes and Obesity, 2017, 1043, 227–256. [Google Scholar] [CrossRef]
- Cera, N.; Vargas-Cáceres, S.; Oliveira, C.; Monteiro, J.; Branco, D.; Pignatelli, D.; Rebelo, S. How Relevant is the Systemic Oxytocin Concentration for Human Sexual Behavior? A Systematic Review. Sex. Med. 2021, 9, 100370–100370. [Google Scholar] [CrossRef]
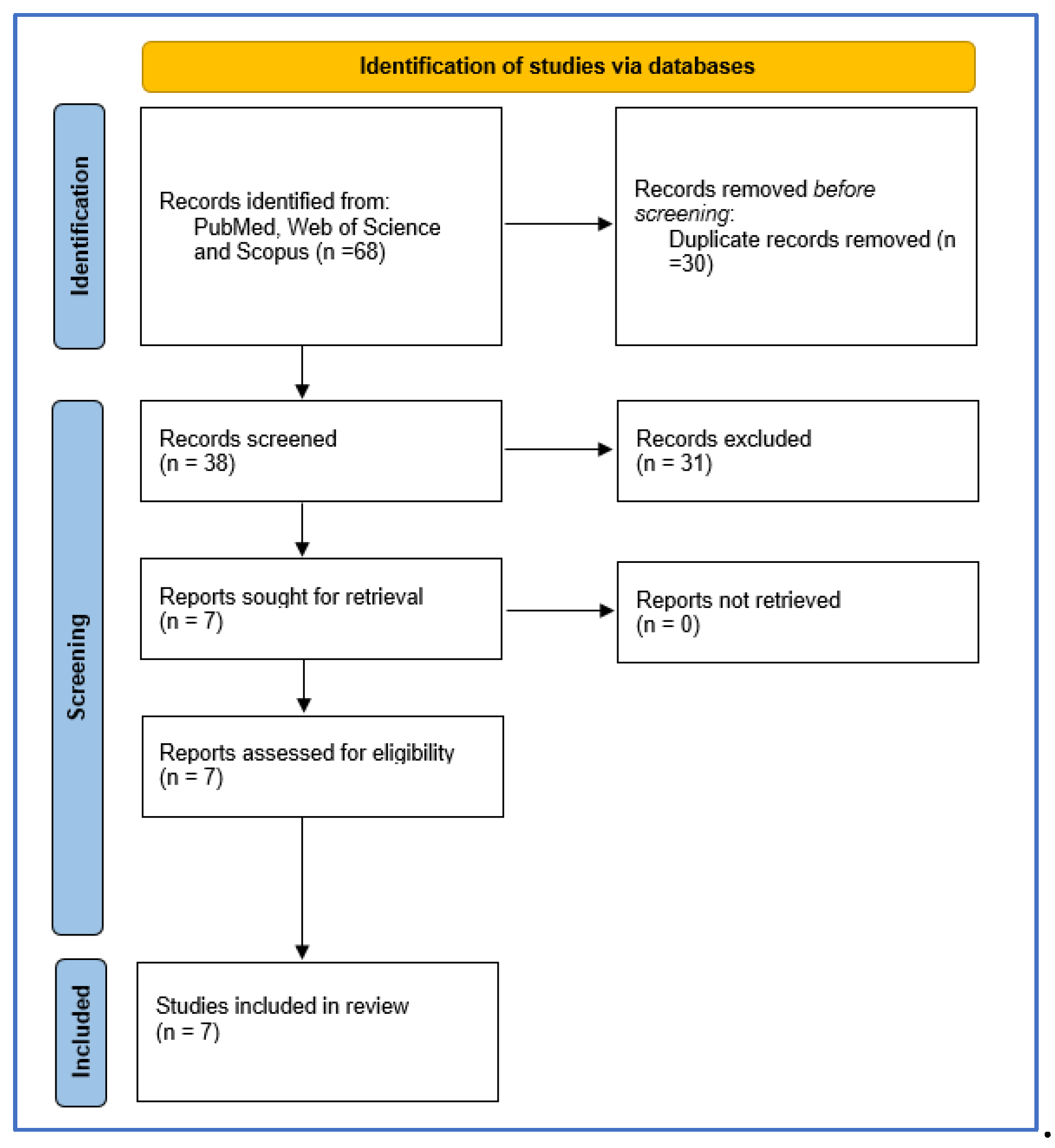
|
Polycystic ovary syndrome 1. Polycystic ovary syndrome [MeSHTerms] 2. Polycystic [All Fields] 3. Ovary [All Fields] 4. Syndrome [All Fields] 5. Polycystic ovary syndrome [All Fields] OR/ 1-2; 4-1 AND/ 3-4; 1-10 |
|
Oxytocin 6. Oxytocin [MeSH Terms] 7. Oxytocin [All Fields] 8. Oxytocin s [All Fields] 9. Oxytocin [All Fields] 10. Oxytocins [All Fields] OR/ 6-10 |
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Source | Country | Subjects | Age | Design | Assessment | Treatment | Results |
|---|---|---|---|---|---|---|---|
| Amin et al., 2023 | Italy | 212 women | - | Population genetics study | Single nucleotide polymorphisms (SNPs) within OXTR | - | Out of 22 OXTR-risk variants tested, 5 independent variants were significantly linked to/in LD with PCOS. Three intronic variants were linked to PCOS. One intronic variant and a synonymous variant were both linked and associated with PCOS. All variants are novel and have not been previously associated with PCOS or any PCOS-related phenotype. Three of the variants were found to confer risk for PCOS intersected with a repressed chromatin state in the ovaries. |
| Jahromi et al., 2018 | Iran | 161 women (PCOS = 80; Non-PCOS = 81) |
20-35 years | Case-control | OT, AMH, BMI, LH, T, FSH, TSH, Prolactin, DHEAS. Fasting Blood Sugar, Fasting Insulin, Blood sugar 2 h after 75 gr glucose, Insulin 2h after 75g glucose, HOMA-IR | - | The mean OT level was lower in the case group. The mean BMI, AMH, HOMA-IR, fasting insulin and insulin 2-h after 75-g glucose were higher in the PCOS group. OT was negatively correlated to AMH when evaluated for all participants or only among controls. OT was also negatively correlated to HOMA-IR among all participants. There was not a significant relationship between OT and BMI. The calculated cutoff value for OT was 125 ng/L and for AMH was 3.6 ng/mL in the PCOS group. |
| Piróg et al., 2023 | Poland | 56 infertile women with PCOS18 pregnant | 31.89 ±4.59 years | Case-control | Assessment before ovarian stimulation (OS) and before hCG administration. Assessments of PNX-14, NES-1, DA, and OT serum levels were performed. Other Tests: LH, FSH, Estradiol, PRL, AMH, BMI |
In the whole cohort of patients, OT levels were weakly associated with BMI (r = 0.26, p = 0.048), and FSH (r = 0.47, p = 0.0002). Pregnant group: positive correlations between baseline OT and PRL (r = 0.47; p = 0.04), as well as OT and NES-1 (r = 0.55; p = 0.02). The OT level increase was associated with positive pregnancy rates. In the post-OS, in pregnant PCOS, OT was 2.7 times lower than non-pregnant. |
|
| Masrour et al., 2018 | Iran | 150 women | 19-39 (29 ± 4.48) years |
Clinical Trial | OT, HCG, FSH, Prolactin, Follicle number, Progesterone. | The three groups at random received: 100 mg clomiphene-citrate + 8 units of OT; 100mg clomiphene-citrate + 10000 units of HCG; 100 mg clomiphene citrate + 8 units of OT + 10000 units of HCG. |
There was no major difference among the groups regarding the ovulation rate or the number of follicles, nor were there any significant side effects observed in any groups. |
| Ochsenkühn et al., 2010 | Germany | 86 women | 18-42 (34.2 ± 4.3) years | Randomized, Double-blind, Placebo-controlled Clinical Pilot-Study | Follicle number, Double endometrial width, Estradiol, LH, Progesterone, To assess male fertility: Semen parameters (native sperm concentration, progressive motility, normal sperm morphology, semen volume, total progressive motile sperm count). | 132 homologous IUI cycles with nasal application of placebo or 8 IU OT following IUI | In 132 IUI cycles of 86 women, 17 pregnancies were achieved, accounting for a pregnancy rate of 12.9% per IUI cycle. The pregnancy rates were 13.4% per IUI cycle in the placebo group, and 12.3% per IUI cycle in the OT group. So, the difference was not statistically significant. No relevant side effects were observed in both groups. |
| Sajadi et al., 2018 | Iran | 14 female rats (PCOS = 7; Control = 7) | 75-95 days | Randomized Clinical Trial | CCh; OT. | Rats in the experimental group were subcutaneously injected with 5m/g of free testosterone on gestational day 20; controls received solvent. The contractions of isolated uterus in offspring of both groups were recorded by the power lab system, after exposure to CCh and OT. | Uterine contractions were more irregular in PCOS rats than controls, after exposure to both contractile agonists. |
| Yamamoto et al., 2022 | Japan | 16 female rats (PCOSChronic = 8; ControlCronic = 8; PCOSAcute = 8; ControlAcute =8) |
28 days | Randomized Clinical Trial | OT. | At 10 weeks after the surgical day, all rats were injected with saline for 7 consecutive days, then injected with OT (1200 µg/kg, 0.4 to 0.5 mL injection volume) for the following 7 consecutive days. | The serum OT level was lower in PCOS model rats than in control rats, whereas the hypothalamic OT mRNA expression level did not differ between them. Acute intraperitoneal administration of OT during the dark phase reduced the body weight gain and food intake in PCOS model rats. However, these effects were not observed in control rats. In contrast, chronic administration of OT decreased the food intake in both the PCOS model rats and control rats. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





