Submitted:
11 April 2024
Posted:
11 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Step-by-Step Surgical Technique
3.1.1. Step One – Preparation and Positioning
3.1.2. Step Two – Cervical Injection of Indocyanine Green
3.1.3. Step Three – Colpotomy and Access to the Pelvic Retroperitoneal Space
3.1.4. Step Four – vNOTES Port Installation
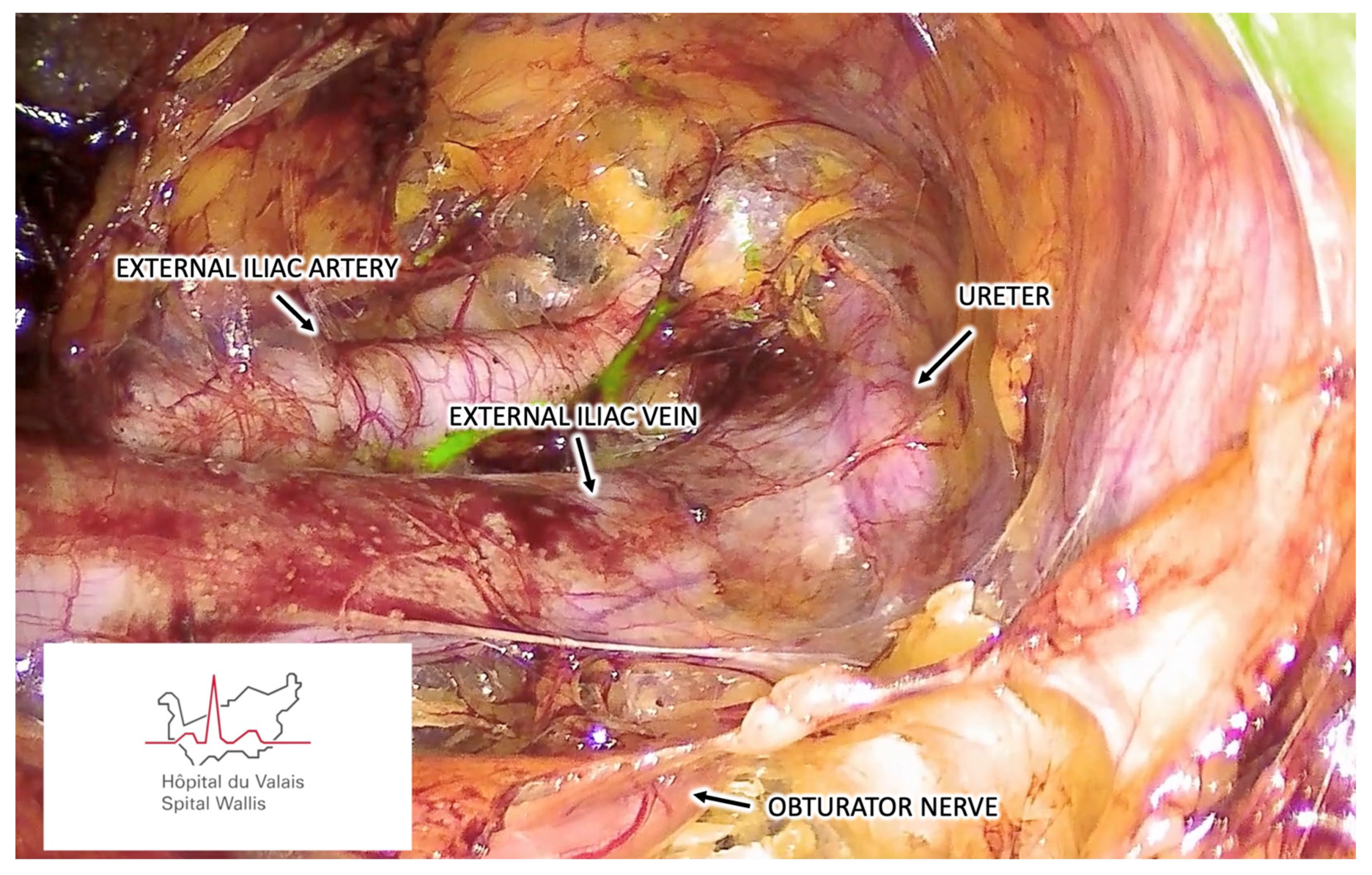
3.1.5. Step Five – Endoscopic Pelvic Retroperitoneal Space Preparation
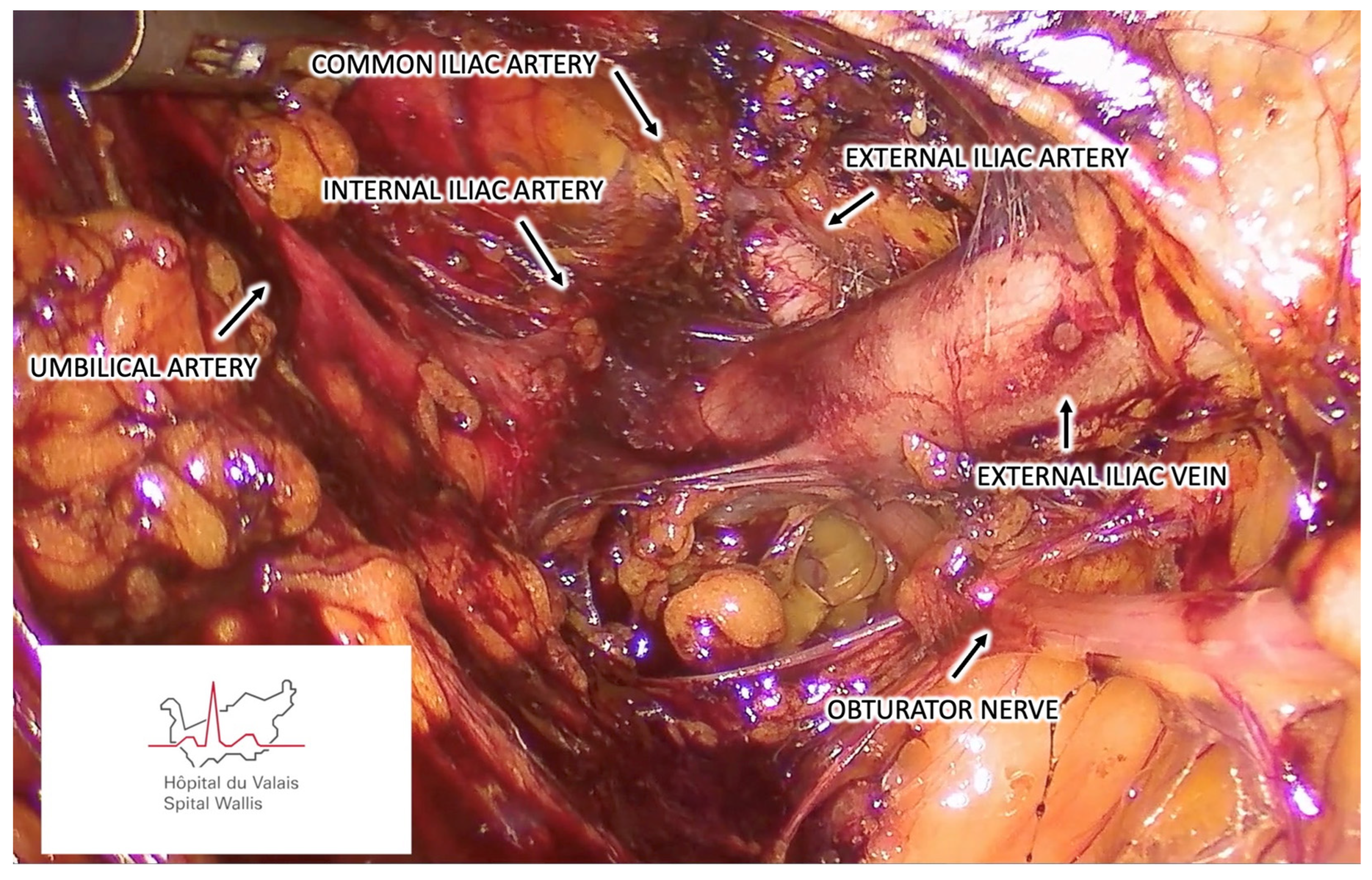
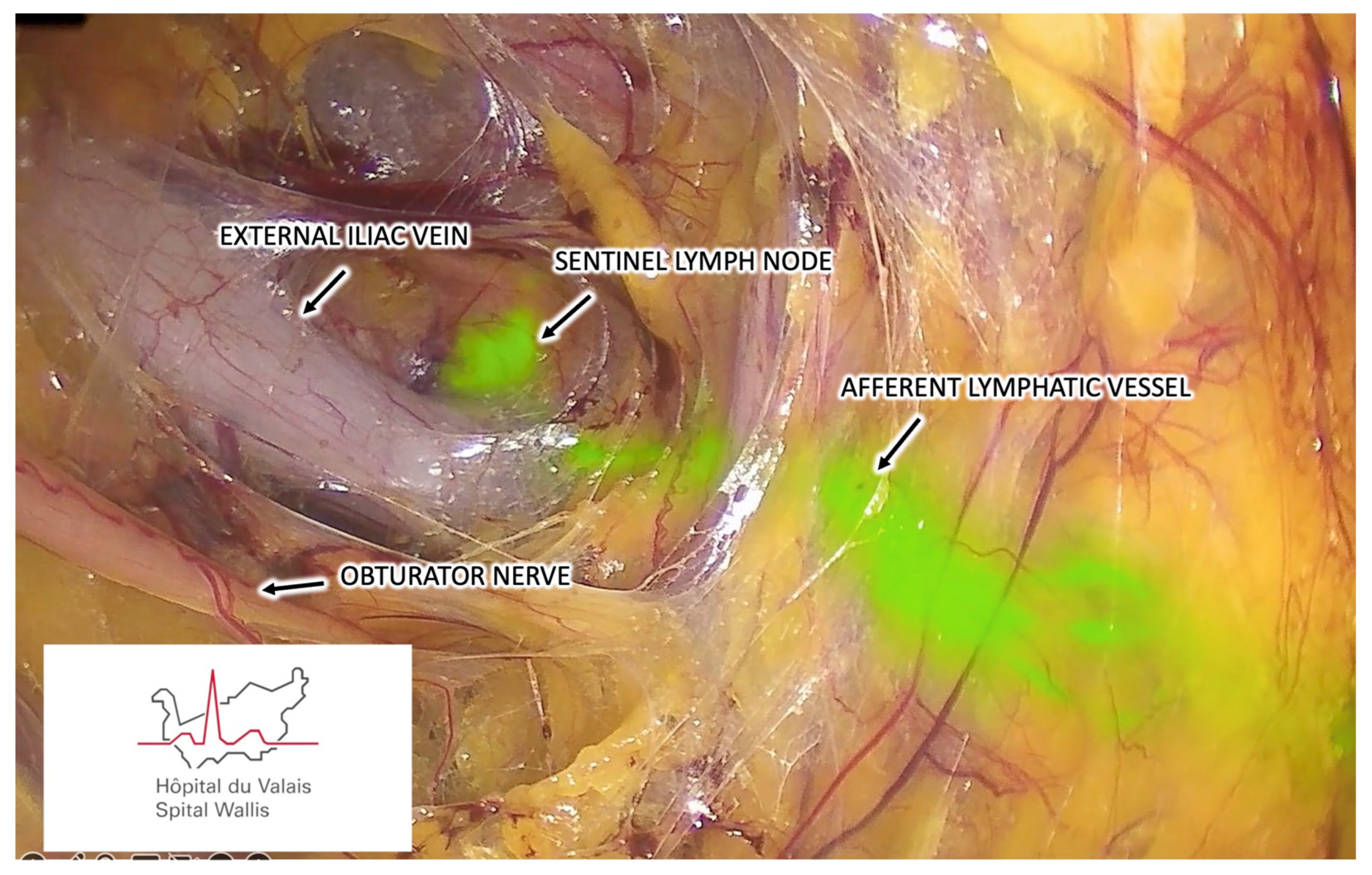
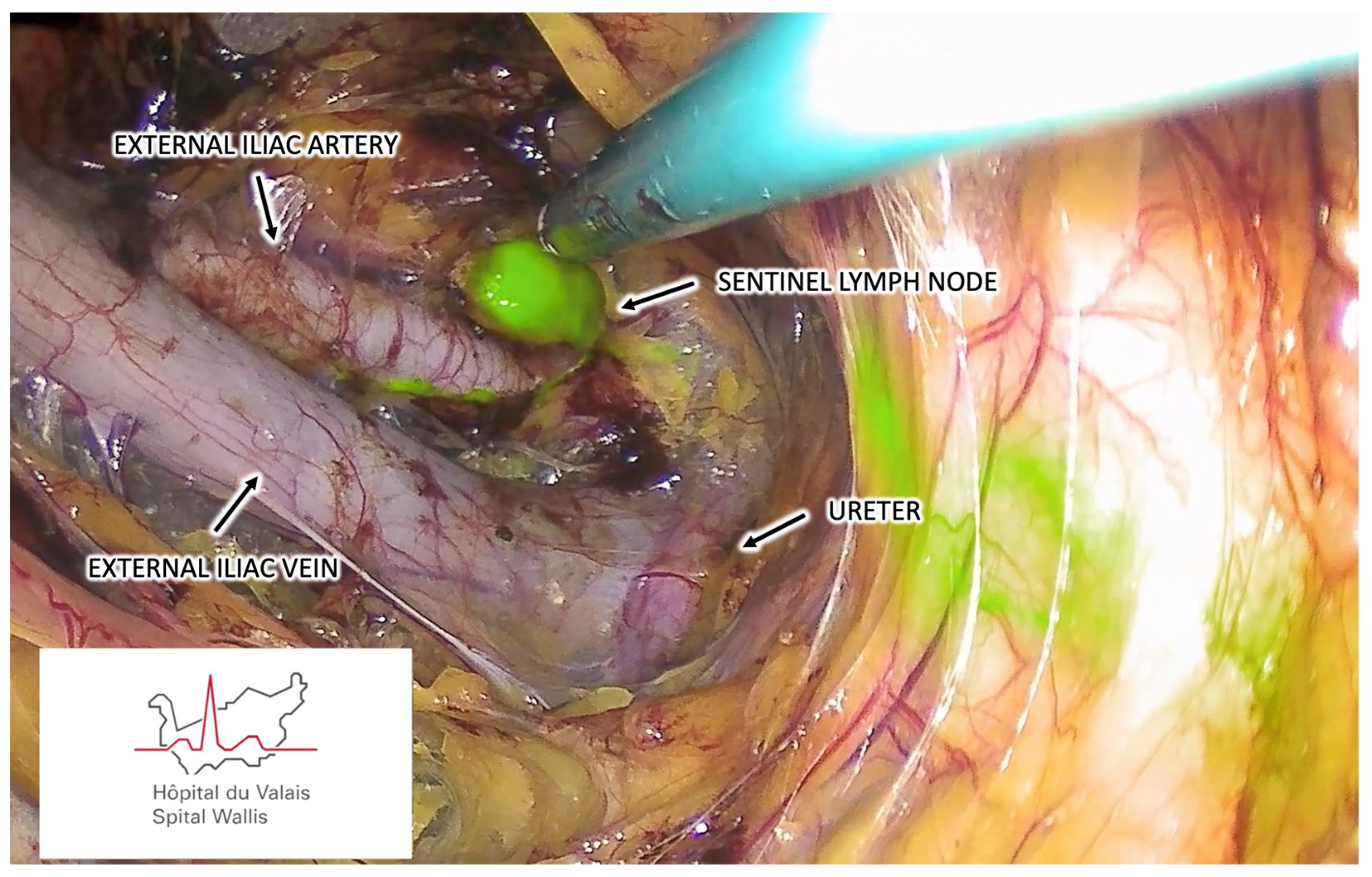
3.1.6. Step Six – Sentinel Lymph Node Identification
3.1.7. Step Seven – Sentinel Lymph Node Harvesting
3.1.8. Step Eight – Vaginal Suture
3.1.9. Step Nine – Additional Interventions
3.1.10. Step Ten – Postoperative Care
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Rossi, E.C.; Tanner, E. Controversies in Sentinel Lymph Node Biopsy for Gynecologic Malignancies. J. Minim. Invasive Gynecol. 2020, 28, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Cibula, D.; Pötter, R.; Planchamp, F.; Avall-Lundqvist, E.; Fischerova, D.; Meder, C.H.; Köhler, C.; Landoni, F.; Lax, S.; Lindegaard, J.C.; et al. The European Society of Gynaecological Oncology/European Society for Radiotherapy and Oncology/European Society of Pathology Guidelines for the Management of Patients With Cervical Cancer. Int. J. Gynecol. Cancer 2018, 28, 641–655. [Google Scholar] [CrossRef] [PubMed]
- Concin, N.; Matias-Guiu, X.; Vergote, I.; Cibula, D.; Mirza, M.R.; Marnitz, S.; Ledermann, J.; Bosse, T.; Chargari, C.; Fagotti, A.; et al. ESGO/ESTRO/ESP guidelines for the management of patients with endometrial carcinoma. Int. J. Gynecol. Cancer 2021, 31, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Rossi, E.C.; Kowalski, L.D.; Scalici, J.; Cantrell, L.; Schuler, K.; Hanna, R.K.; Method, M.; Ade, M.; Ivanova, A.; Boggess, J.F. A comparison of sentinel lymph node biopsy to lymphadenectomy for endometrial cancer staging (FIRES trial): a multicentre, prospective, cohort study. Lancet Oncol. 2017, 18, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.J.; A Kennard, J.; Fitzsimmons, C.K.; Manyam, M.; E Kendrick, J.; Singh, C.; McKenzie, N.D.; Ahmad, S.; Holloway, R.W. Robotic sentinel lymph node (SLN) mapping in endometrial cancer: SLN symmetry and implications of mapping failure. Int. J. Gynecol. Cancer 2019, 30, 305–310. [Google Scholar] [CrossRef] [PubMed]
- Baekelandt, J.F. New Retroperitoneal Transvaginal Natural Orifice Transluminal Endoscopic Surgery Approach to Sentinel Node for Endometrial Cancer: A Demonstration Video. J. Minim. Invasive Gynecol. 2019, 26, 1231–1232. [Google Scholar] [CrossRef] [PubMed]
- Leblanc, E.; Narducci, F.; Bresson, L.; Hudry, D. Fluorescence-assisted sentinel (SND) and pelvic node dissections by single-port transvaginal laparoscopic surgery, for the management of an endometrial carcinoma (EC) in an elderly obese patient. Gynecol. Oncol. 2016, 143, 686–687. [Google Scholar] [CrossRef]
- Huber, D.; Hurni, Y. Anatomical Distribution of Sentinel Lymph Nodes Harvested by Retroperitoneal vNOTES in 34 Consecutive Patients With Early-Stage Endometrial Cancer: Analysis of 124 Lymph Nodes. J. Minim. Invasive Gynecol. 2024. [Google Scholar] [CrossRef]
- Huber, D.; Hurni, Y. Sentinel Node Biopsy for Endometrial Cancer by Retroperitoneal Transvaginal Natural Orifice Transluminal Endoscopic Surgery: A Preliminary Study. Front. Surg. 2022, 9, 907548. [Google Scholar] [CrossRef]
- Comba, C.; Demirayak, G.; Simsek, C.; Atas, B.S.; Özdemir, I.A. Transvaginal natural orifice transluminal endoscopic surgery (VNOTES) total retroperitoneal sentinel lymph node biopsy for an endometrial cancer patient with prior colon cancer surgery. Int. J. Gynecol. Cancer 2021, 31, 1386–1387. [Google Scholar] [CrossRef]
- Mathey, M.-P.; Romito, F.; Huber, D.E. Retroperitoneal Sentinel Lymph Node Biopsy by Vaginally Assisted Natural Orifices Endoscopic Transluminal Endoscopic Surgery in Early Stage Endometrial Cancer: Description of Technique and Surgeon’s Perspectives after the First Experience. Case Rep. Oncol. 2022, 15, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Matak, L.; Šimičević, M.; Dukić, B.; Matak, M.; Baekelandt, J. vNOTES surgical staging for endometrial carcinoma in overweight patients: a case series. Arch. Gynecol. Obstet. 2024, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Hurni, Y.; Huber, D.E. Sentinel Node Biopsy by Transvaginal Natural Orifice Transluminal Endoscopic Surgery in a Patient with Early-Stage Cervical Cancer: A Case Report. Case Rep. Oncol. 2022, 15, 547–552. [Google Scholar] [CrossRef]
- Ziogou, A.; Ziogos, E.; Giannakodimos, I.; Giannakodimos, A.; Sifakis, S.; Ioannou, P.; Tsiodras, S. Bacterial Vaginosis and Post-Operative Pelvic Infections. Healthcare 2023, 11, 1218. [Google Scholar] [CrossRef]
- Till, S.R.; Morgan, D.M.; Bazzi, A.A.; Pearlman, M.D.; Abdelsattar, Z.; Campbell, D.A.; Uppal, S. Reducing surgical site infections after hysterectomy: metronidazole plus cefazolin compared with cephalosporin alone. Am. J. Obstet. Gynecol. 2017, 217, 187–e1. [Google Scholar] [CrossRef]
- Baekelandt, J.; Stuart, A. A new anterior approach to vNOTES retroperitoneal sentinel node resection for endometrial cancer. Asian J. Surg. 2023, 46, 5491–5492. [Google Scholar] [CrossRef] [PubMed]
- Hurni, Y.; Huber, D. Omentectomy for oncological surgical staging by transvaginal natural orifice transluminal endoscopic surgery (vNOTES): a preliminary study. Front. Surg. 2023, 10, 1224770. [Google Scholar] [CrossRef] [PubMed]
- Guani, B.; Balaya, V.; Ayoubi, J.; Feki, A.; Lecuru, F.R.; Mathevet, P. Laparoscopic vaginal radical trachelectomy in the post-LACC era: step-by-step surgical procedure. Int. J. Gynecol. Cancer 2022, 32, 1617–1618. [Google Scholar] [CrossRef]
- Baekelandt, J.; Chuang, L.; Ortega, J.H.Z.; Burnett, A. A new approach to radical hysterectomy: First report of treatment via vNOTES for cervical cancer. Asian J. Surg. 2023, 46, 1852–1853. [Google Scholar] [CrossRef]
- Hurni, Y.; Simonson, C.; Di Serio, M.; Lachat, R.; Bodenmann, P.; Seidler, S.; Huber, D. Feasibility and safety of vNOTES for gynecological procedures in obese patients. J. Gynecol. Obstet. Hum. Reprod. 2023, 52, 102687. [Google Scholar] [CrossRef]
- Moloney, K.; Janda, M.; Frumovitz, M.; Leitao, M.; Abu-Rustum, N.R.; Rossi, E.; Nicklin, J.L.; Plante, M.; Lecuru, F.R.; Buda, A.; et al. Development of a surgical competency assessment tool for sentinel lymph node dissection by minimally invasive surgery for endometrial cancer. Int. J. Gynecol. Cancer 2021, 31, 647–655. [Google Scholar] [CrossRef] [PubMed]
- Bizzarri, N.; Obermair, A.; Hsu, H.-C.; Chacon, E.; Collins, A.; Tsibulak, I.; Mutombo, A.; Abu-Rustum, N.R.; Balaya, V.; Buda, A.; et al. Consensus on surgical technique for sentinel lymph node dissection in cervical cancer. Int. J. Gynecol. Cancer 2024. [Google Scholar] [CrossRef] [PubMed]
- Holloway, R.W.; Abu-Rustum, N.R.; Backes, F.J.; Boggess, J.F.; Gotlieb, W.H.; Lowery, W.J.; Rossi, E.C.; Tanner, E.J.; Wolsky, R.J. Sentinel lymph node mapping and staging in endometrial cancer: A Society of Gynecologic Oncology literature review with consensus recommendations. Gynecol. Oncol. 2017, 146, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Raffone, A.; Raimondo, D.; Raspollini, A.; Oliviero, A.; Travaglino, A.; Renzulli, F.; Rovero, G.; Del Forno, S.; Vullo, G.; Laganà, A.S.; et al. Comparison between Laparoscopic and Robotic Approach for Sentinel Lymph Node Biopsy in Endometrial Carcinoma Women. J. Pers. Med. 2022, 13, 29. [Google Scholar] [CrossRef] [PubMed]
- Baekelandt, J.; Stuart, A. Old meets new: Vnotes retroperitoneal promontory fixation in conjunction with the uterus preserving manchester procedure(✰,✰✰). J Gynecol Obstet Hum Reprod. 2023, 52, 102628. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.J. Primary Management of Early Stage Cervical Cancer (IA1-IB) and Appropriate Selection of Adjuvant Therapy. J. Natl. Compr. Cancer Netw. 2008, 6, 47–52. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, L.; Tang, S.; Dou, Y.; Yao, Y.; Li, Y.; Deng, Y.; Chen, Y.; Liang, Z. vNOTES Hysterectomy with Sentinel Lymph Node Mapping for Endometrial Cancer: Description of Technique and Perioperative Outcomes. J. Minim. Invasive Gynecol. 2021, 28, 1254–1261. [Google Scholar] [CrossRef] [PubMed]
- Deng, L.; Liu, Y.; Yao, Y.; Deng, Y.; Tang, S.; Sun, L.; Wang, Y. Efficacy of vaginal natural orifice transluminal endoscopic sentinel lymph node biopsy for endometrial cancer: a prospective multicenter cohort study. Int. J. Surg. 2023, 109, 2996–3002. [Google Scholar] [CrossRef]
- Baekelandt, J.F.; De Mulder, P.; Le Roy, I.; Mathieu, C.; Laenen, A.; Enzlin, P.; Weyers, S.; Mol, B.W.J.; Bosteels, J. Hysterectomy by transvaginal natural orifice transluminal endoscopic surgery versus laparoscopy as a day-care procedure: a randomised controlled trial. BJOG: Int. J. Obstet. Gynaecol. 2018, 126, 105–113. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




