1. Introduction
While it is true that defining and determining the state of the art of any subject will vary in distinct areas of study, such as education, economy, medicine, biology, among others, the efforts required are always based on analyses of accumulated knowledge (publications) that aim to inventory and systematize production in a particular field [
1]. Determining the state of art is a strict (rule-bound) process that involves contextualizing, searching, classifying, and analyzing existing information (in systematic or systematized reviews) to allow researchers to identify important theories quickly and accurately, key concepts, leading authors, and defining methods most used in a particular topic or field of study [
1,
2].
For macroalgae, such systematized reviews of knowledge are more related with specific topics to specific topics that contextualize, organize, and analyze the information generated for each topic such as invasive species, species of economic importance or with potential for exploitation, ethnobotany, biochemistry, and genetics, among others [
3,
4,
5,
6,
7,
8,
9,
10]. This can also be focused on the historic development of a certain genus of interest. This way, there are reviews about genera like
Caulerpa J. V. Lamouroux,
Dictyota J. V. Lamouroux and
Meristotheca J. Agardh, which provide a widely description of knowledge, regarding varied topics, like taxonomy, ecology, biogeography; but without delving into any subject [
11,
12,
13]. On the other hand, the reviews focused into single subject, like taxonomy, though are scarcer, manage to conduct a detailed analysis and stablished recommendations to future studies, such as the provided about
Sargassum C. Agardh taxonomy [
14]. It is important, then, to elaborate reviews of a greater number of macroalgae taxa, focused on subjects, to facilitate the visualization of primary research (e.g., taxonomic, and nomenclatural reassessment), relevant methods (e.g., molecular techniques), areas of opportunity and topics in this field (e.g., biogeography, phylogeography, ecology), since existing works still are scarce to reflect the true biodiversity of these taxa.
Dictyota species are very common in intertidal and shallow subtidal habitats along rocky coasts worldwide. Although present in cold-temperate waters, the genus reaches its highest diversity in tropical and warm-temperate environments where multiple species often coexist and reach high densities [
15]. Some species constitute a food source or provides substrate and shelter to numerous marine organisms [
16,
17]. But also, the genus is known to exert negative effects on various marine organisms as competitive interactions with corals [
18].
The name
Dictyota was erected by J. V. Lamouroux in 1809 [
19,
20]. Since its description and circumscription, its systematic as result of taxonomic determination (identification) has faced numerous challenges which stem from the appearance of two or more structural forms (polymorphism) in some species of the genus, that can be related to the environmental conditions in which it grows and the morphological changes that occur throughout its life cycle (pleomorphism) [
19,
21,
22]. This has led to the designation of imprecise morphological diagnostic characteristics, and their use in specific and infraspecific ranks, such as color, length of thallus, presence or absence of proliferations, and number of cells in the medulla along the thallus, all of which have contributed to the partial and/or approximate descriptions that exist and, ultimately, incorrect taxonomic and systematic-phylogenetic interpretations [
23]. In contrast, the use of ultrastructural characteristic and gene sequence data have greatly aided species delineation and resulted in the detection of multiple cryptic entities in
Dictyota, leading to a series of taxonomic and nomenclatural changes, descriptions of new species or genera, and even a genus reinterpretation [
15,
21,
24].
Thus, the goal of this study is to elaborate the state of the art of the taxonomy knowledge of Dictyota, taking in account the developed of its nomenclature, taxonomy, and classification based on a systematized review of the knowledge generated since its description.
2. Materials and Methods
The systematized review was divided into two stages: 1) the search for, and examination of taxonomic names corresponding to the genus Dictyota; and 2) the search for, selection, and analysis of literature related to this genus.
For the taxonomic name search Algaebase [
25] was used because it contains nomenclatural, taxonomic (name status, synonyms), and biogeographic information (providing list of species by geographic area as region or country, and a detailed distribution with sources in each species), plus bibliographic data on taxonomic names. For the search, the word “
Dictyota” was used as the only descriptor, but all specific and infraspecific names were also considered. The following information was obtained for each taxon: taxonomic name, taxonomic status (currently accepted, synonyms, uncertain, and preliminary AlgaeBase entry), authority or authorities, year of publication, and related names. All data were captured in a database for subsequent analysis. For the analysis, the taxonomic names found in Algaebase were grouped in three categories: 1) accepted names, i.e., names currently accepted, 2) synonyms, and 3) illegitimate names that included uncertain or preliminary names according with [
25].
After capturing all relevant information, the names were separated by status name. Then a descriptive analysis was conducted to obtain number by status name, authorities by taxon, number of genera related with Dictyota’s name. Additionally, an analysis by century (19th, 20th, 21st) and 20-year periods (bidecadal) was done, that calculated the average of names registered and authorities by bidecade.
The search for publications concerning the genus Dictyota was carried out in 11 databases comprising the period from 1809 to 2023: Academia, Biodiversity Heritage Library (BHL), Cambridge Scientific Abstract (CSA), Google Scholar, Journal Storage (JSTOR), Researchgate, Scientific Electronic Library Online (SciELO), and Science Direct, complemented by three scientific publishers: Blackwell Sinergy, Springer, and Walter de Gruyter.
The descriptors used were: “Dictyota”, “Dictyotaeae”, “Dictyotaceae”, “Dictyotales”, “Taxonomy”, “Phylogeny”, “Biogeography”, “Phylogeography”, “Phylogeographic”, “Systematics”, and “Molecular systematics” in English and Spanish, both individually and combined with the connectors “or, and” (e.g., Dictyota and taxonomy; Dictyotales and phylogeny; Dictyotaceae and phylogeny or taxonomy). The references of the publications found were also reviewed to complete the list of publications related with the genus Dictyota.
We excluded publications like bachelor and masters’ theses, abstract of congress and divulgation notes, as well as literature not related to systematic aspects (e.g., physiology, chemistry, ecology) or publications without mention of
Dictyota’s taxa. To better manage and analyze the selected literature, sources were categorized by type of taxonomy: alpha, responsible for characterizing and naming species, stands out for including processes such as the exploration and description of taxa; beta, a phase of synthesis; extensive and monographic reviews are included, mainly, which may include new species and analysis of the evolutionary relationships between taxa, and floristic works) and gamma, it refers to evolutionary studies, performs a phylogenetic analysis at different levels, incorporating interaction with different areas, e.g. biogeography, genetic, evolution [
26,
27,
28,
29].
The information obtained from each publication was used to create a database that includes type of taxonomic work (alpha, beta, or gamma), author(s), year, title, journal, area of study, geographic coordinates, and the type of molecular marker used; for example, rbcL, cox1, or psbA.
After capturing all relevant information, a descriptive analysis was conducted by century (19th, 20th, 21st) and 20-year periods (bidecade). In this way, it was feasible to detect trends in the different type of taxonomy works, geographic regions where studies were conducted, and tendencies in the use of molecular markers and their application.
3. Results
In the Algaebase platform [
25], 313 infrageneric names in the genus
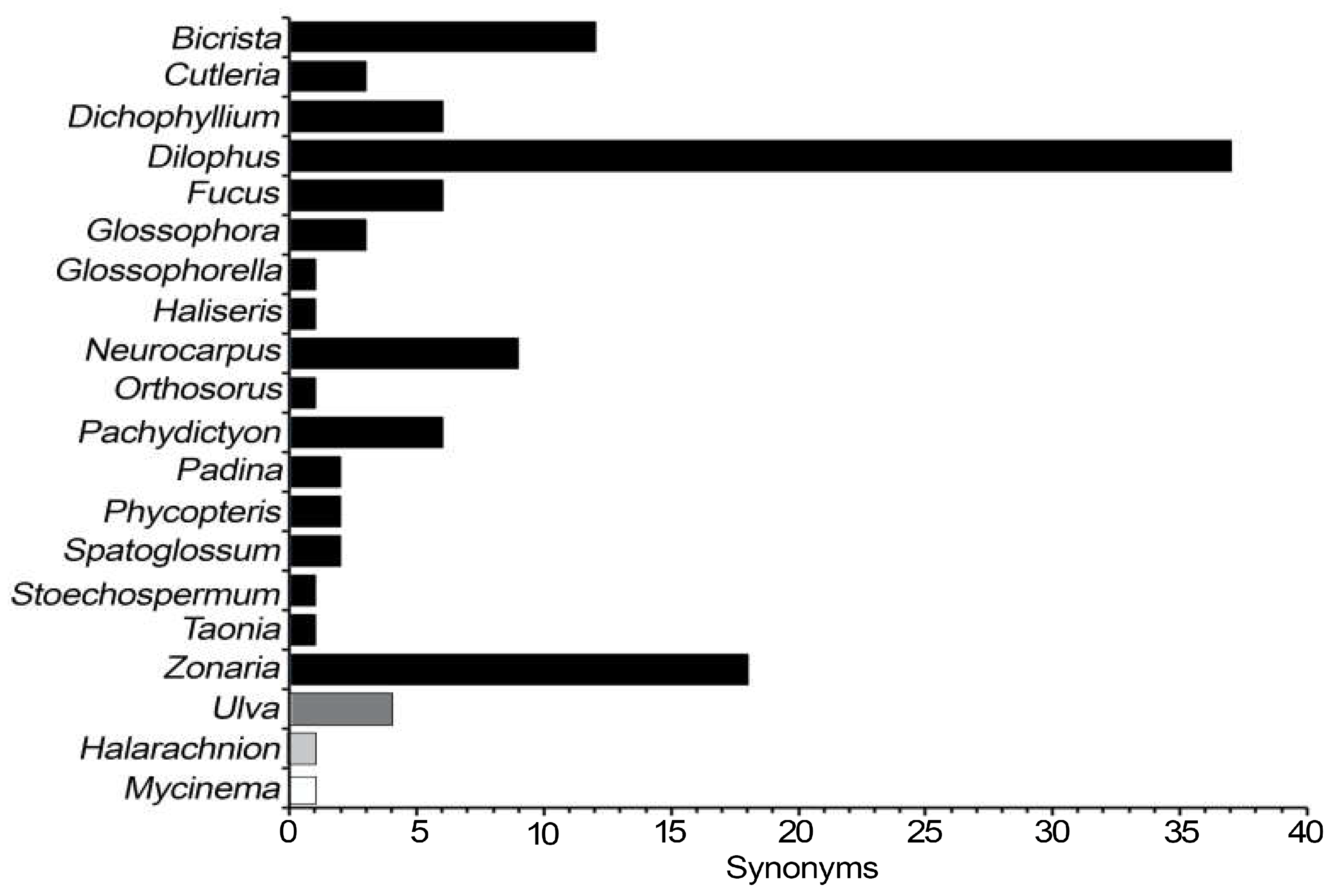
Dictyota were identified, of which 223 belonged at the species category, 62 to variety, and 28 to form. As for the number of names by status, 108 belonged to the category of accepted names (91 species, 10 varieties, seven forms), 177 synonyms (116 species, 44 varieties, 17 forms) and 28 illegitimated (16 species, eight varieties, four forms). Additionally, we found 100 taxa from 20 other genera that are synonyms of
Dictyota, the most common one was
Dilophus J. Agardh with 38 synonyms, followed by
Zonaria C. Agardh (18) and
Bicrista Kuntze (12) (
Figure 1).
Regarding the 177 names of Dictyota with synonyms status, 118 were categorized under accepted names of Dictyota, of those D. dichotoma (Hudson) J. V. Lamouroux and D. dichotoma var. intricata (C. Agardh) Greville had the highest number of synonyms with 23 and 20, respectively. The last 59 synonyms were categorized in other genera, where Canistrocarpus cervicornis (Kützing) De Paula & De Clerck and C. crispatus (J. V. Lamouroux) De Paula & De Clerck had the highest number of synonyms, both with eight.
Concerning the temporal variation in the creation of taxonomic names and their taxonomic status by century, in the 19th, 207 names were created of which only 54 endure as accepted names. In the 20th, 83 names were created, 35 of them which are accepted. In the first 23 years of the 21st century, 23 names have been created, 19 of which are accepted (
Table 1). In contrast, for the total and average number of taxonomic names registered per bidecade by century, the study found that the third bidecade of the 19th century had the highest number of names created (83) with an average of four per year, while the bidecade with the lowest number was the first of the 20th century (five) with an average of just one every four years. In the case of the first bidecade of the 21st century, 22 taxonomic names have been created, having an average of one per year (
Table 1).
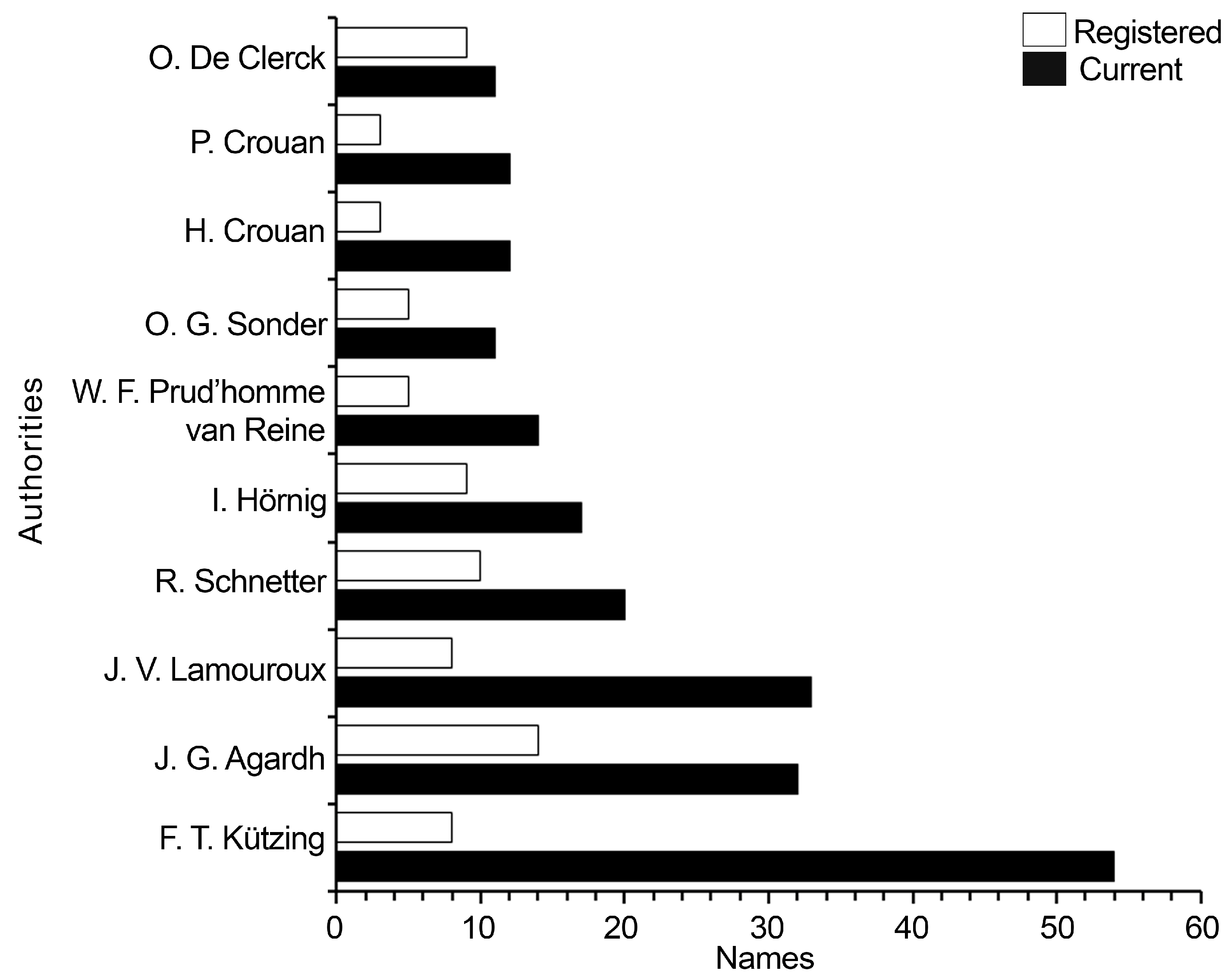
Regarding authorities, it was found that the 313 taxonomic names were registered by 104 authorities. Likewise, each taxon is associated with one to four authorities, 235 taxa with one authority, and only two taxa with four. On the other hand, we found that 55 authorities described only one taxon. The five authorities with the highest number of taxa are F. T. Kützing (54 created, eight accepted), J. G. Agardh (32 created, 14 accepted), J. V. Lamouroux (33 created, eight accepted), R. Schnetter (20 created, 10 accepted) and I. Hörnig (19 created, nine accepted) (
Figure 2).
The temporal analysis of the authorities revealed that 53 and 46 were registered in the 19th and 20th centuries, respectively, while in the 22 years of the 21st century, 30 authorities have been recorded (
Table 2).
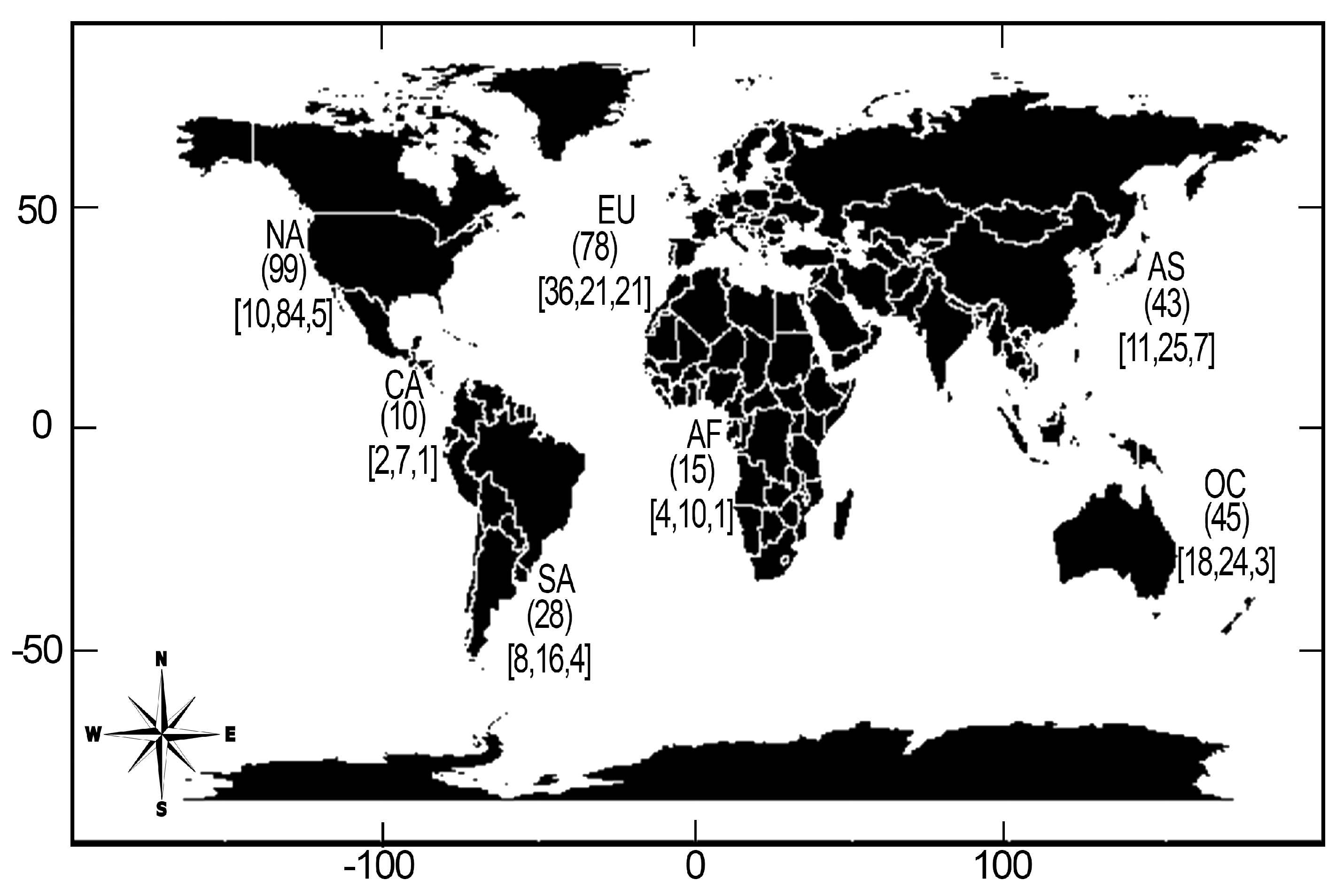
A total of 514 publications were compiled, but after applying the exclusion criteria only 318 remained. Most of them dealt with beta taxonomy (187), followed by alpha (89), and gamma (42). Geographically, the region with the highest number of publications is North America (99), followed by Europe (78), and Oceania (45). By type of taxonomic work for both alpha and gamma, Europe had the highest number of publications, while for beta the highest number of works was found for North America (84), and the lowest for Central America (seven) (
Figure 3).
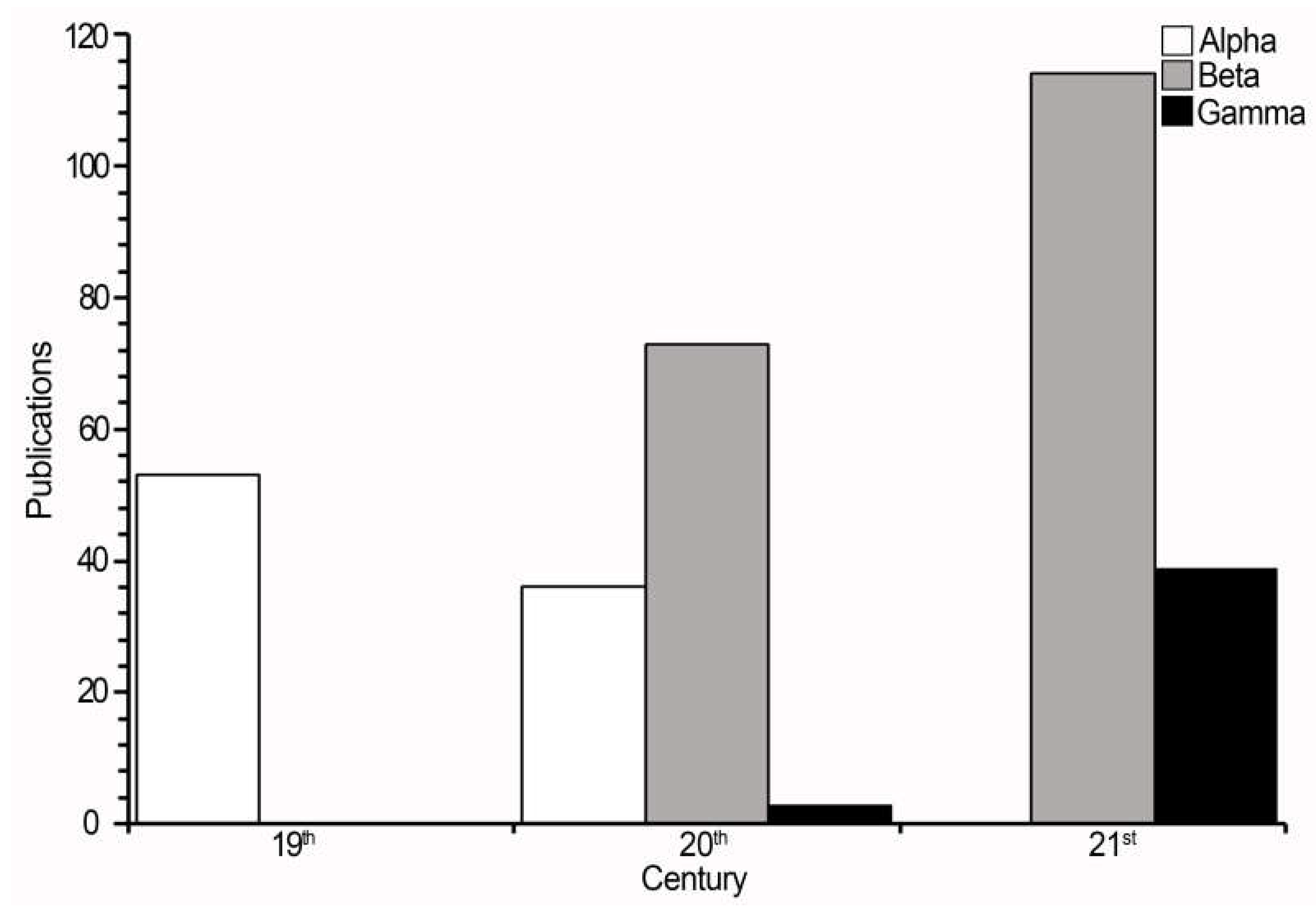
The temporal analysis by century showed, that the type of taxonomic work addressed in the 19th century was exclusively alpha (53), while in the 20th century the three approaches were present, with beta taxonomy having the largest number of publications (73). For the 21st century no publications on alpha taxonomy were compiled, but both beta and gamma taxonomic works have increased (
Figure 4).
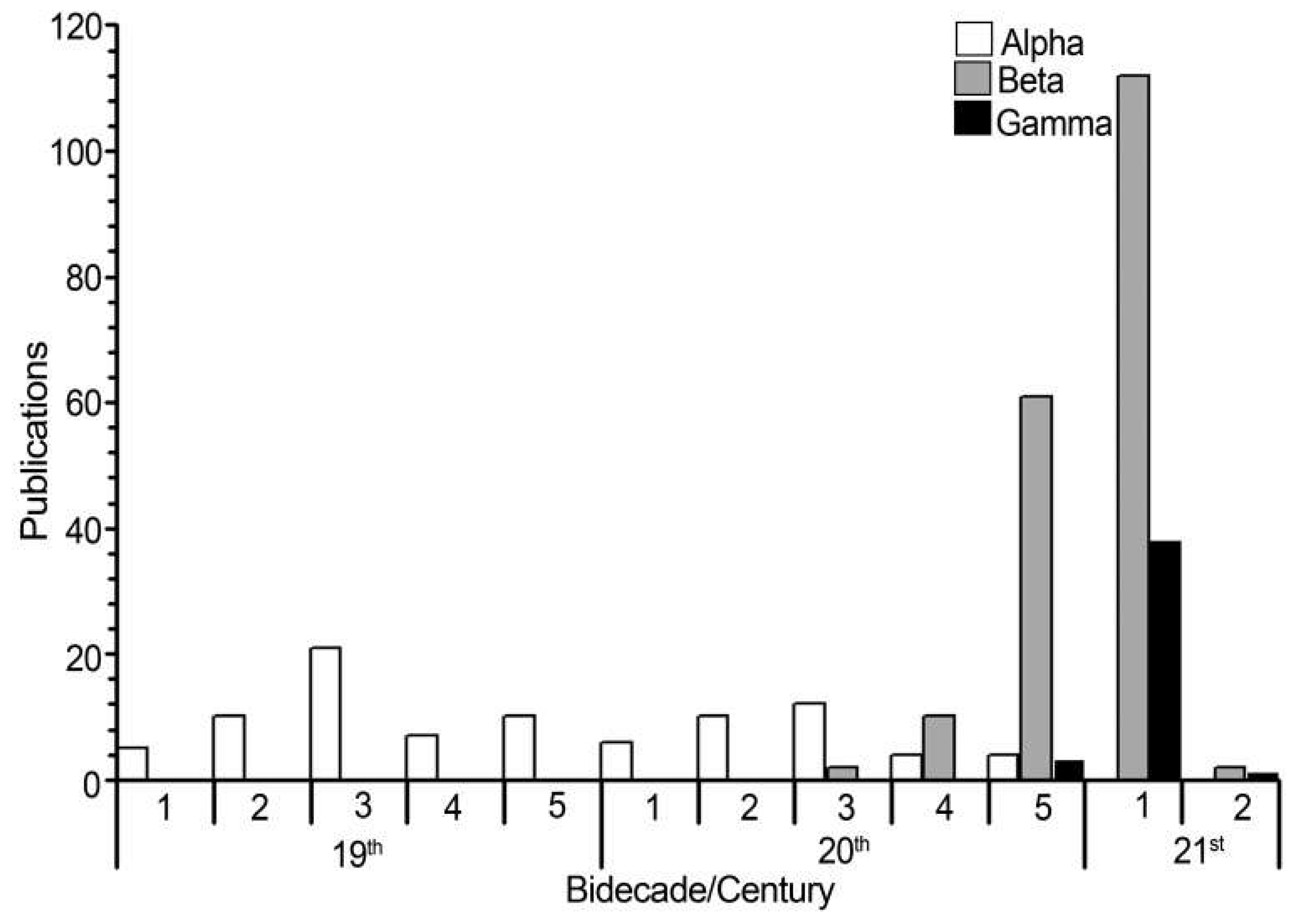
The bidecadal analysis revealed that, the bidecade with the highest number of publications of alpha taxonomy were the third ones in both the 19th and 20th centuries, with 21 and 12 publications, respectively (
Figure 5). Regarding beta taxonomy, it determined in only five bidecades out of the total recorded works of this type. The first bidecade of the 21st century had the highest number of publications (112;
Figure 5). Of the total of publications considered on beta taxonomy, 21 were found to be taxonomic reviews or monographs, while 166 were floristic studies. Of the latter, in terms of region, the highest number of works was found for North America (84), and the lowest for Central America (seven). The six taxa with the highest number of records were
Dictyota dichotoma (82),
D.
bartayresiana J. V. Lamouroux (57),
D.
implexa (Desfontaines) J. V. Lamouroux (55),
D.
friabilis Setchell (45),
D.
ciliolata Sonder ex Kützing (41), and
D.
crenulata J. G. Agardh (33). Geographically,
D.
dichotoma was reported from all regions. From North America region, (
D.
crenulata,
D.
dichotoma, and
D.
implexa) were the most frequently recorded, whereas the species
D.
friabilis and
D.
implexa were the most recorded in Europa and Oceania, respectively. No records of
D.
crenulata from Africa and Asia whereas from South America,
D.
friabilis, and
D.
implexa, from Central America.
Regarding gamma taxonomy, it was recorded in only three bidecades, the last of the 20th century –only three publications– and both 21st century with 39 (
Figure 5). Of the 42 publications collected, 39 used 27 molecular markers, while the other three used biochemical components for species delimitation. The studies with molecular markers can be grouped by origin into three types: nuclear (nine), plastids (seven), and mitochondrial (11). About the number of molecular markers used per publication, we found that between one and nine markers appear. The use of one to three different markers was the most frequent approach (15 publications using two markers, seven with one, six with three).
Analysis of the frequency of use of molecular markers according to their origin shows that plastid markers have been used most often (30 publications), while nuclear markers have been used the least (13). Likewise, the most frequent nuclear marker was LSU rDNA, while in plastid and mitochondrial markers were
rbcL and
cox1 (
Table 3).
4. Discussion
The information on Dictyota analyzed in this study comprises a temporal scale of over 200 years, while spatially it considers roughly all regions where this taxon is distributed, both tropical and subtropical. This, besides elaborating on the state of the art of the genus Dictyota, it allows highlighting the dynamics of knowledge on this taxon.
The taxonomic status of the names in
Dictyota was grouped into accepted names, synonyms, and illegitimated names (
Table 1), where the synonyms were 57% of the taxonomic names. It has been mentioned that the overabundance of synonymous and doubtful names is an undeniable failure of past taxonomic practices [
30]. In studies of algae in general, about 50% of the taxonomic names are considered synonyms [
31], because of a series of dynamic, taxonomic, and nomenclatural changes that have affected most algae genera since their description, including the genus
Dictyota, at the specific level [
21,
32,
33,
34,
35].
The difficulty in interpreting the morphological variation of the genus
Dictyota can be appreciated in
Figure 1. Some species present synonyms into other genera because at the beginning of classification processes in the half of 18th century, most phycologists devoted their efforts largely to the delineation of new species within the Linnean system, which recognized six genera of algae defined by their form (
Byssus Linnaeus,
Chara Linnaeus,
Conferva Linnaeus,
Fucus Linnaeus,
Tremella Persoon ex Saint Amans and
Ulva Linnaeus). Most species of
Dictyota were described under
Fucus (e.g.,
F.
fasciola Roth,
F.
implexus Desfontaines) or
Ulva (e.g.,
U.
dichotoma Hudson). But hundreds of new algal species were described so the Linnean system became unmanageable, since then some phycologists started describing new genera based on differences in color of spores and thalli, and on reproductive characters, emerging genus like
Zonaria or
Bicrista, generating synonyms for some species [
32].
Originally, [
36,
37] recognized the genera
Dictyota,
Dilophus,
Glossophora J. Agardh, and
Pachydictyon J. Agardh based on the number of cortical and medullary layers and by the presence or absence of surface proliferations. However, [
33,
34] based in morphological evidence proposed a merger of
Dilophus with
Dictyota. This propose was not recognize by [
35] due to morphological plasticity in the genus is poorly understood. This taxonomic controversy was present until the reinterpretation by [
21] supported by analysis of
rbcL, and partial 26S rDNA sequences. Since then, it has integrated such genera
Dilophus (40% of synonyms),
Pachydictyon,
Glossophora and
Glossophorella Nizamuddin et Campbell.
About the synonyms, we founded that the names under accepted names of
Dictyota, 36% are synonyms of
D.
dichotoma and
D.
dichotoma var.
intricata. In this regard, [
32] points out that the high degree of morphological variation characteristic of the
D.
dichotoma complex led to the description of several new species and combinations, but that those descriptions correspond to minor morphological differences or aberrant growth forms. The variety “intrincata”, for example, is simply a slender growth form of
D.
dichotoma [
22]. Features of this kind may lead to the misidentification of species or descriptions of new ones.
With respect at the 33% of synonyms under other genera, these derived from morphological and molecular revisions that resulted in the conformation of new genera and nomenclatural changes. This is the case of the genera
Canistrocarpus De Paula & De Clerck, and
Rugulopteryx De Clerck & Coppejans which were proposed by [
21] based on molecular markers (
rbcL, 26s rDNA) and morphological characters. In this case, the species
Canistrocarpus cervicornis and
C.
crispatus contain 27% of the synonyms.
Regarding the temporal variation (
Table 1) in the rate of species descriptions for
Dictyota, we observed an erratic pattern, showed that the greatest number occurred in the 19th century. The differences per bidecade and centuries can be explained by a combination of several global events, e.g., great expeditions of the 19th century, World Wars I and II of the 20th century, as well as the technological development (e.g., microscopy technology, molecular technics) [
31,
38].
Along with this, the rules of the current nomenclatural codes (as Algae, Fungi and Plants Nomenclature Code) have been establishing to make sure that all taxonomic names will be stable without causing confusion in the science development to be permanently considered if they are validly published and therefore recognize as taxonomically accepted [
30,
39,
40]. Some names, as
Dictyota harveyana Sonder nom. nud. is considered as illegitimated name due to has never been provided description (Art. 38 Nomenclature Code) and additionally no specimens have been located in the Herbarium Sonder (Art. 38 Nomenclature Code) [
32]. Instead, some species are missing a name verification like
D.
flabellulata Foster and Schiel that appeared in Algaebase [
25] as preliminary, in this case, [
41] (p. 29, 30) not described a new species, mention the presence of the species in a kelp community, for this reason we consider like a typing error, due to that its logical to assume that the authors referring to
D.
flabellata, since several herbaria contain material from California identified as this species. [
32] mentioned that numerous taxa are poorly known and in need of critical re-assessment, which will allow us to validate nomenclatural and taxonomically the names.
In relation to the contribution of the authorities in
Dictyota, it is disproportionate where three of those (
Figure 2), F.T. Kützing, J.G Agardh, and J.V. Lamouroux (19th century), have contributed 38% of the total number of registered names. Out that several pioneers in the 19th century contributed to the knowledge of many of the genera of algae that are currently in use [
38]. J. V. Lamouroux, described
Dictyota and F. T. Kützing and J. G. Agardh are considered the key figures in the taxonomy and nomenclature of algae, and who described more than 80
Dictyota’ species (
Figure 2).
On the other hand, if we compare registered names and number authorities (
Table 1 and
Table 2), it is apparent that the bidecade with the highest registered names was the third of the 19th century. However, it is the bidecade of 21st century with the highest number of authorities, because seven of the 23 names registered have three or four authorities, in contrast to the bidecade of the 19th century, when most of the names were described by just one authority (J. V. Lamouroux), this finding reflects a tendency towards greater collaboration among researchers. At respect, taxonomy will eventually become an integrative discipline, due to the gap in communication among specialists from different disciplines is decreasing, and more and more researchers are already convinced that delimiting species should involve different perspectives, e.g., morphology and phylogeography to delimit some species [
29,
30]. In algae, the taxonomic contribution per capita decreased slightly in the 20th century, but there are some peaks at the end of that century and in the first decade of the 21st [
31], as in the case with
Dictyota (
Table 1).
Overall, the taxonomic works on the genus
Dictyota showed two trends (Figures 4,5). One of these with a decrease of the alpha taxonomy, however, as [
42] points out that this type of taxonomy is central to biology because many fields use species as the central units of their studies. While the increase in the number of works located within the beta and gamma taxonomy (second trend), results from the use of different methods of analysis, current taxonomic studies integrate morphological, ecological, and molecular techniques to answer multiple questions. Some authors have defined this as gamma, integrative, or experimental taxonomy [
26,
27,
43]. However, [
44] mentions that while the emergence of DNA barcoding has spurred significant advances in research on many groups of organisms and provided essential information at different levels, it has limitations, so it is vitally important to maintain the duality of morphological and molecular information at the level of organisms.
Publications on beta taxonomy represent 59% of the total collected, this type of taxonomy represents a synthesis phase, where concern focused on contextualizing information on species to generate robust classification proposals based on the distribution of characteristics present in distinct groups. This was achieved through extensive, monographic revisions and floristic inventories, many of which include new species and analyses of evolutionary relationships between taxa [
26,
27,
28]. In publications addressing this type of taxonomy, we found 21 papers on taxonomic revisions for specific regions (e.g., [
23,
32,
45,
46]), and 166 floristic papers (89% of the total for the beta taxonomy) from different geographic regions (
Figure 3). This finding suggests that extensive reviews and monographic approaches are still required to achieve a better understanding of this genus.
In the case of the gamma taxonomy, a positive trend can also be observed in the works published towards the beginning of the present century (Figures 4,5). Their increase is due to developments in molecular biology that have manifested the use of methods and their interpretations in the results on systematics, biogeography, and evolution of terrestrial and aquatic organisms [
47]. [
48] point out that the major challenge of taxonomies based on DNA sequences is to provide the sequence for the species that have Linnean (binomial) names, as this contributes to the baseline of knowledge on diversity especially in groups such as algae, where cryptic diversity, morphological simplicity, phenotypic plasticity, and evolutionary convergence have been widely observed [
49,
50]. In
Dictyota, the gamma taxonomy gives a support and a better understanding of species delimitation, as well as the systematic with the higher taxonomic levels.
In this regard, 51% of these works were focused on delimiting or describing new species (e.g., [
51,
52]) and even enabled the identification of new species within groups in which small chromatic, morphological, or behavioral variations were once attributed to environmental or distributional factors (e.g., [
21,
53]), originating more than 50% of the names registered in the bidecade of the 21st century (
Table 2). This agrees with [
54] who mention that at least 50% of the papers on algae published in one year (2012–2013) dealt with species delimitation or descriptions of new species, based on a combination of molecular data with at least some information on the morphology, ecology, or physiology of the organisms.
A total of 27 molecular markers were considered in the gamma taxonomy papers, but the number most often used per paper was two, equivalent to 58%. Although the
rbcL marker (Table 3) is the one most frequently used (78% of publications), yet there is no universal marker for
Dictyota. [
54] affirm that because algae form a phylogenetically heterogeneous group, the application of a universal marker for species delimitation is unfeasible and different markers (multi-locus) are applied in different algal groups.
With regards to the type of molecular markers, plastids were the first to be employed [
55], in species delimitation studies, followed later by nuclear ones [
56], which ceased to be used in 2012 [
57]. [
54] note that species delimitation studies in many algal groups (macroalgae in particular) have relied on plastid and mitochondrial markers. In addition to the fact that several practical aspects make the amplification and sequencing of organellar loci relatively easy, their popularity for species delineation can be attributed to faster coalescence within species lineages compared to nuclear loci, that produces clearer discontinuities between interspecific divergence and intraspecific variation [
58], two factors that have often been used to determine the phylogenetic relationships, evolution, or phylogeography of species or genera [
57,
59,
60].
The markers
rbcL,
psbA and
cox1 (Table 3) were found most often in molecular studies of
Dictyota; a result that concurs with [
54] and [
11], who identify them among the ones most used for species delimitation in brown algae and particularly in
Dictyota.
This analysis of the information highlights that the state of the art in the systematic of
Dictyota is dynamic in terms of both taxonomic criteria and related studies, with consequent nomenclatural and phylogenetic relationships interpretations. This is due to the evolution of our knowledge of the genus, which leads to taxonomic and nomenclatural changes in existing names and, consequently, changes of category. Likewise, the current trend in taxonomic studies on
Dictyota is integrative (gamma taxonomy), since they include taxonomic, systematic, and biogeographical aspects in both phenotypic and molecular terms. An integrative approach to taxonomy is necessary because the complexity of species biology requires that species boundaries be studied from multiple and complementary perspectives [
29,
30]. However, epistemological reflection must adhere to preestablished criteria, such as the methodological and technical processes employed in the research as it was observed in this review. This condition makes it possible to construct the state of the art of the object of study [
61], in as much it is a basic, essential element for defining and structuring research.
Although the genus
Dictyota has been studied taxonomically along the centuries, taxonomic revisions of poorly known species, such as
D.
alternifida J. Agardh,
D.
masonii Setchell & Gardner, and
D.
vivesii Howe, are still lacking, while for others we have only a few records. In other cases, studies have detected possible taxonomic problems, as in
D.
concrescens W. R. Taylor [
53], or names that could be considered like illegitimate such as
D.
anastomosans Steen, Vieira, Leliaert, Payri, & De Clerck, and
D.
adnata f.
nana Post, what makes a nomenclatural revision and further investigation necessary. In addition, though beta and gamma taxonomic works had been development in specific areas e.g., [
22,
23,
62], some geographical areas remain little explored (
Figure 3), like the coasts of Africa or Central America where our findings show that the number of publications on the genus is significantly lower than in other regions. Considering the foregoing, it is to be expected that the number of species recognized within this genus will change, they may decrease as happened in other genera e.g.,
Caulerpa [
63], or that new species will be discovered masked among poorly studied “species” present in geographic areas not yet studied or where molecular techniques have not been applied.
The challenge of systematic classification of organisms stays in force, in the case of Dictyota, the effort in integrative taxonomy must be intensified, providing morphological and anatomical descriptions of the species, multiloci molecular support or genomes, which allow determining the species, that can be used in supporting other investigations. Since species are used as fundamental units of studies such as ecological, of conservation biology and biogeography, an incorrect delimitation can pose serious difficulties for example to detect biodiversity loose, alien species arrive or flora tropicalization.