1. Introductions
Lignin is a kind of natural high polymer compound with abundant reserves [
1], it contains mainly a basic structure of phenylpropane [
2], and thereby, it can be used as an auxiliary raw material for synthesizing adhesives [
3,
4]. However, the character of insoluble in water limits its widespread use in adhesives synthesis, accounting for only 5% -15% of the total synthetic raw materials [
5,
6]. Due to its insolubility in water, lignin exists in the form of solid small particles [
7,
8], making it difficult to achieve a uniform mixing state when mixed with liquid reaction materials such as formaldehyde, urea, melamine, and phenols [
9]. Therefore, to a certain extent, it affects the synthetic reaction process, affects the structural uniformity and adhesion consistency of the adhesive, and thereby, leads to cracking and insufficient smoothness of the adhesive surface, reducing the overall quality of the products [
10,
11]. Therefore, to obtain a kind of water-soluble lignin modified materials that having the ability to uniformly mix with other liquid reaction materials is an important means to increase the amount of lignin raw materials used in the synthesis of adhesives [
12,
13].
In order to obtain lignin modified materials with good water solubility, introducing hydrophilic groups into lignin raw materials is an effective method to improve the water solubility of lignin modified materials [
14]. Konduri et al. adeptly introduced sulfomethyl groups into lignin, marking a notable enhancement in its water solubility and subsequently opening up avenues for its efficient employment as a cement dispersant [
15]. P. Prinsen et al. have successfully incorporated site-specific sulfation techniques to augment the water solubility of lignin, ultimately leading to an improvement in its overall processability [
16]. The introduction of hydroxyl groups into lignin raw materials not only enhances its water solubility but also augments its chemical reactivity, thereby facilitating its utilization in the preparation of phenolic resin adhesives [
17,
18,
19].
However, the incorporation of sulfur into lignin is believed to hinder the activity of enzymes and microbes during the bioconversion process, and lead to the poisoning of metal catalysts during chemo-catalytic treatment [
20,
21]. In addition, the water solubility of introduction of hydroxyl groups is still insufficient. Therefore, and it is necessary to search for lignin modified materials with stronger water solubility without sulfur elements.
Based on the fact of that amino (-NH₂) is a strong hydrophilic group [
22], it proposes an idea of preparing a water-soluble aminated lignin compound through lignin ammonification reaction, and thereby, conducts synthetic experiments and product structure analysis in this paper. The experimental results indicate that the product of lignin ammonification reaction is aminated lignin, which has good water solubility. Therefore, this study breaks through the technical barriers in preparation of lignin modified materials with good water solubility without introducing sulfur-containing groups, enriches theoretical knowledge in the field of materials science, and provides a new approach for the widespread application of lignin raw materials in the synthesis of adhesives.
2. Methods and Materials
2.1. Experimental Materials
Industrial alkali lignin (PR) was obtained from Hunan Taigreen Paper Group Hongjiang Paper Co., Ltd., hydrochloric acid (AR) and ammonia (AR) were purchased from Chengdu Kelong Chemical Co., Ltd.
2.2. Preparation Method of Fine Lignin
Dry industrial alkali lignin in an 80 ℃ blower dryer for 36 hours to obtain absolute dry alkali lignin. Then, treat with a ball mill for 10 minutes to obtain small particles of alkali lignin. Next, dissolve the small particles of alkaline lignin in water to obtain an alkaline lignin solution. Subsequently, diluted hydrochloric acid was added for acidification (adjusted to pH=2), resulting in lignin precipitation. Finally, centrifuge for 0.5 hours, take the lower sediment, dry it to obtain fine lignin particles.
2.3. Synthetic Methods of Aminated Lignin
Add 3.0 g of fine lignin particles and 5.0 ml of ammonia water to a hydrothermal reactor, in where, temperature was raised to 130 ℃ and hold for 1 hour for the reaction to generate an amino lignin solution, which was dried in a drying oven to obtain the solid of aminated lignin.
2.4. Methods of Product Morphology Analysis
Using a scanning electron microscope (SEM; Zeiss Sigma 300) to observe the morphology of the product under an accelerated voltage of 3 kV. In addition, it was compared with the morphology of the lignin raw material before reaction.
2.5. Method for Determining the Molecular Weight of Products
The molecular distribution of synthetic products was determined by gel permeation chromatography (gilent PL-GPC50, Agilent Technologies). Test parameters: The concentration of the test sample is 0.10 mg/mL, the injection volume is 100 microliters, and the distribution coefficient is 14.1000, which describes the parameters of the distribution process or adsorption desorption process of components in fixed and mobile phases.
2.6. Methods for Element Analysis of the Product
Using a matching energy dispersive spectrometer (EDS) to detect and analyze the content of different elements in the sample, while observed the morphology of the product by a scanning electron microscope (SEM; Zeiss Sigma 300) under an accelerated voltage of 10 kV.
For greater accuracy, the content of organic elements (C, H, O, N, S) in the sample was measured through high-temperature combustion and weight adsorption analysis by an organic element analyzer (Elemantar: Vario UNICUB).
2.7. Methods for Structural Analysis
Using X-ray photoelectron spectroscopy (XPS; Thermo Scientific K-Alpha) to test and analyze the composition of the sample. Test parameters: Excitation source Al Kα Ray (hv=1486.6 eV), beam spot 400 um, working voltage 12 kV, filament current 6 mA, full spectrum scanning energy was 100 eV, step size 1 eV, fine spectrum scanning energy was 50 eV, step size 0.1 eV.
2.8. Methods for Thermal Stability Analysis of the Product
Thermogravimetric analysis (TGA, Shimadzu DTG-60(H)) was used to test the thermal stability of the synthetic products and lignin raw materials. The dried sample mass used is about 4-8 mg, which is placed using an alumina crucible to heat from 50 °C to 700 °C at a rate of 10 °C/min. The sample is protected by nitrogen gas with a flow rate of 50 ml/min.
2.9. Methods for Thermal Stability Analysis of the Product
In a typical solubility experiment, precisely 2.0 g of water were introduced into a 25 mL glass flask. The flask was subsequently submerged in a temperature-controlled water bath (model HH-1, manufactured by Changzhou Yuexin Instrument manufacturing Co., LTD), maintaining a temperature fluctuation range of ±1.0 °C. Once the bath temperature attained the target value, approximately 0.01 g of lignin sample were carefully dispensed into the flask. The mixture was continuously stirred under a nitrogen atmosphere and held at a constant temperature of 30 °C. Additional aliquots of lignin (each equivalent to 0.1 wt% of the solvent) were incrementally added after complete solubilization of the previously added lignin. This process was systematically repeated until no further dissolution of lignin was observed within a two-hour timeframe [
23].
3. Results and Discussions
3.1. Product Morphology
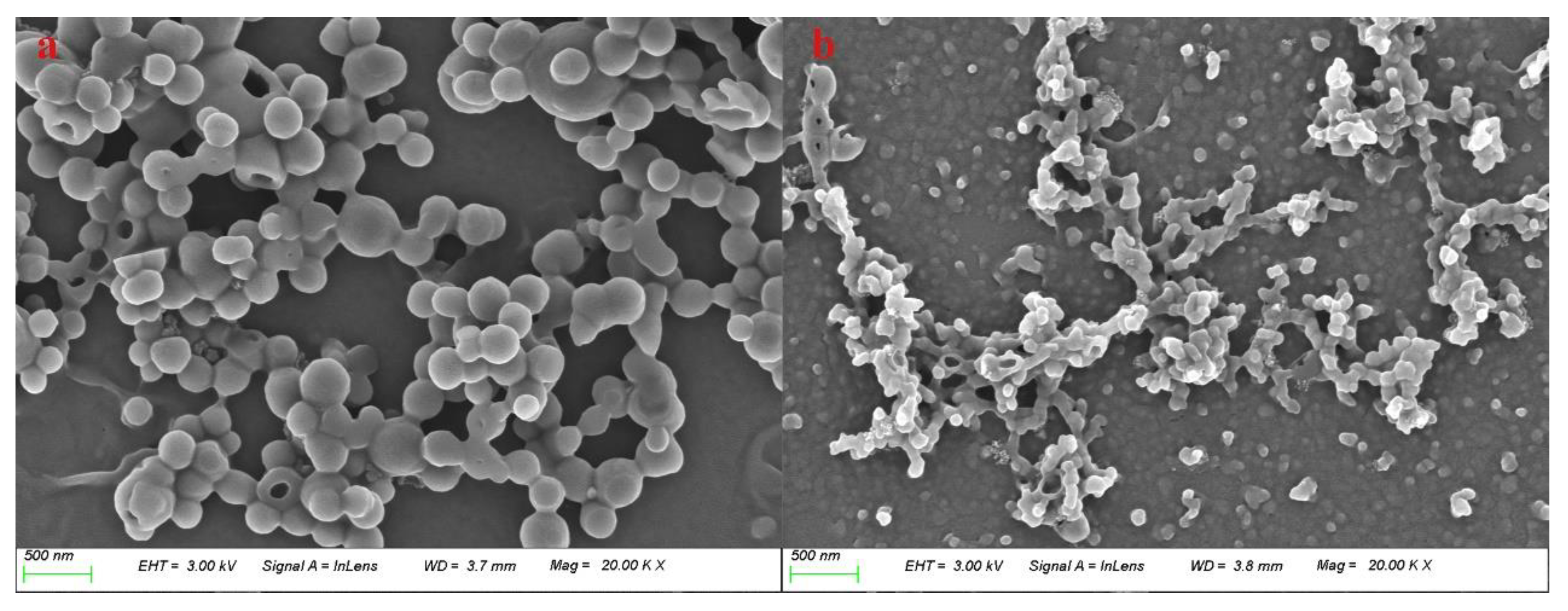
Figure 1 shows a comparison of the morphology of lignin raw materials and ammonia reaction products magnified at 20,000 times. As shown in
Figure 1(a), the lignin raw material is spherical in shape, partially agglomerated, with a particle size about 200 nm. Compared to that, the aminated lignin obtained through ammonification reaction appears as smaller particles, presenting in particle sizes less than 100 nm. In addition, the agglomeration is significantly reduced, leading particles relatively dispersed, as shown in
Figure 1(b).
3.2. The Molecular Weight of the Generated Product
Based on GPC analysis, the molecular weight distribution of lignin raw materials and aminated lignin products are shown in
Figure 2.
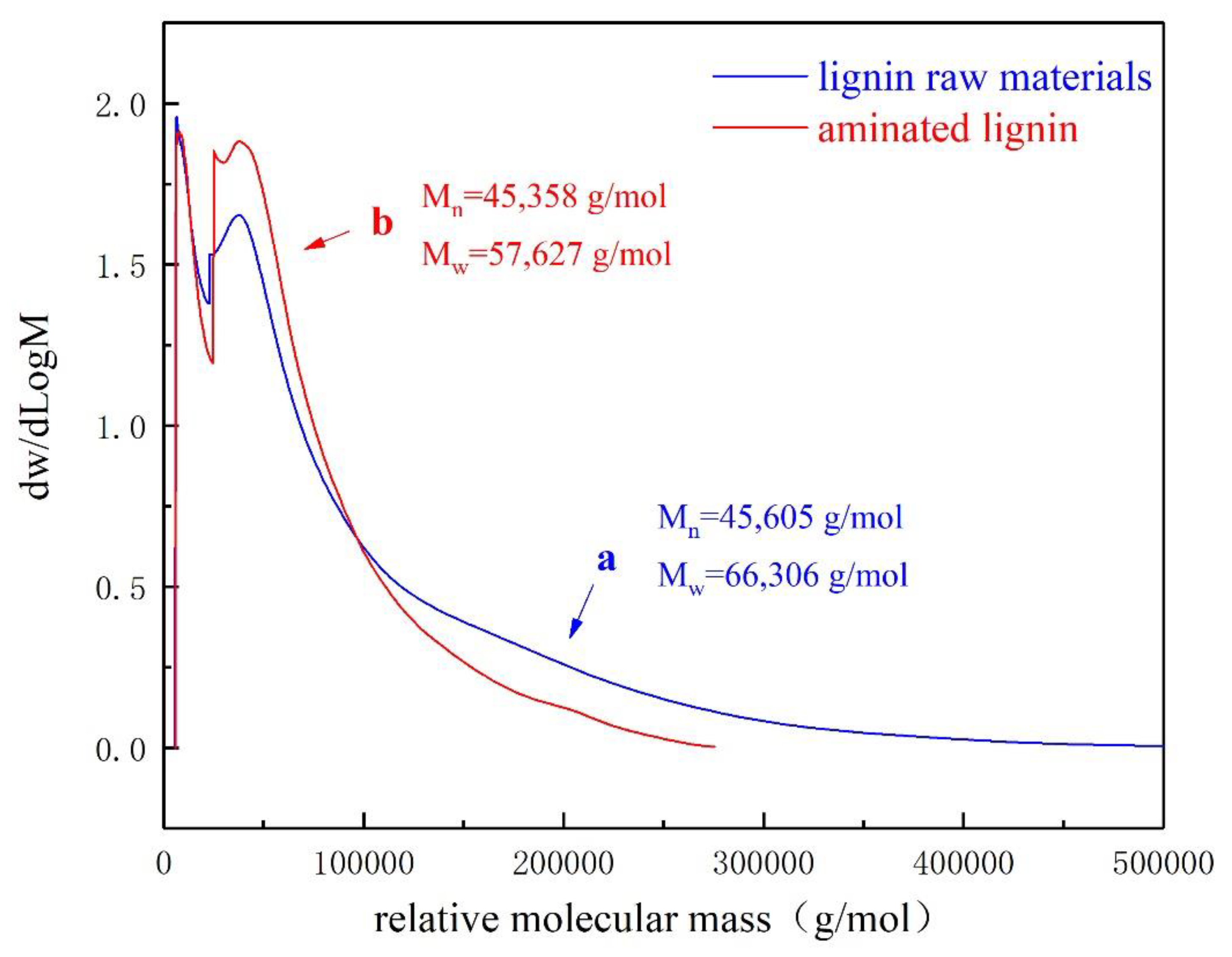
As shown in
Figure 2, the relative molecular weight of the aminated lignin is concentrated in the small molecule absorption peak of 4,000-4,600g/mol, and in the large molecule absorption peak of 40,000-250,000 g/mol. On the other side, the molecular weight of the raw material lignin is also concentrated in the small molecule absorption peak of 4,000-4,600 g/mol, as well as the large molecule absorption peak of 40,000-500,000 g/mol. As a comparison, there was no significant change in the content of small molecular weight substances in lignin after the ammonification reaction. However, the content of large molecular weight substances between 40,000 and 100,000 significantly increased. This test data indicates that the raw material lignin undergoes degradation during the ammonification reaction, producing lower molecular weight aminated lignin.
In addition, calculation by the test system, the number average molecular weight of lignin raw materials is 45,605 g/mol, and the weight average molecular weight is 66,306 g/mol. Where, the molecular weight distribution is rather wide, containing a large amount of macromolecular lignin with a molecular weight of over 300,000 g/mol. On other hand, the number average molecular weight of aminated lignin is 45,605 g/mol, and the weight average molecular weight is 57,627 g/mol.
As a comparison, it finds that the difference in number average molecular weight between aminated lignin and original lignin raw materials is not significant, but the weight average molecular weight is significantly reduced. The fundamental reason is that during the ammonification reaction of lignin, an alkaline catalytic hydrolysis reaction occurs, causing the hydrolysis of high molecular weight lignin, which generates lower molecular weight fragments. And thereby, it results in a decrease in weight average molecular weight. However, due to the relatively large number of these small molecular fragments, their contribution to the number average molecular weight are not significant, leading to the variation in the logarithmic mean molecular weight is not significant [
24].
Therefore, the changes in these two types of molecular weight statistical data further indicate that some high molecular weight chains have broken into lower molecular weight fragments. This analysis results are consistent with the scanning electron microscopy analysis results described ahead.
3.3. Element Compositions
- (1)
Results of energy dispersive spectrometer (EDS) detected analysis.
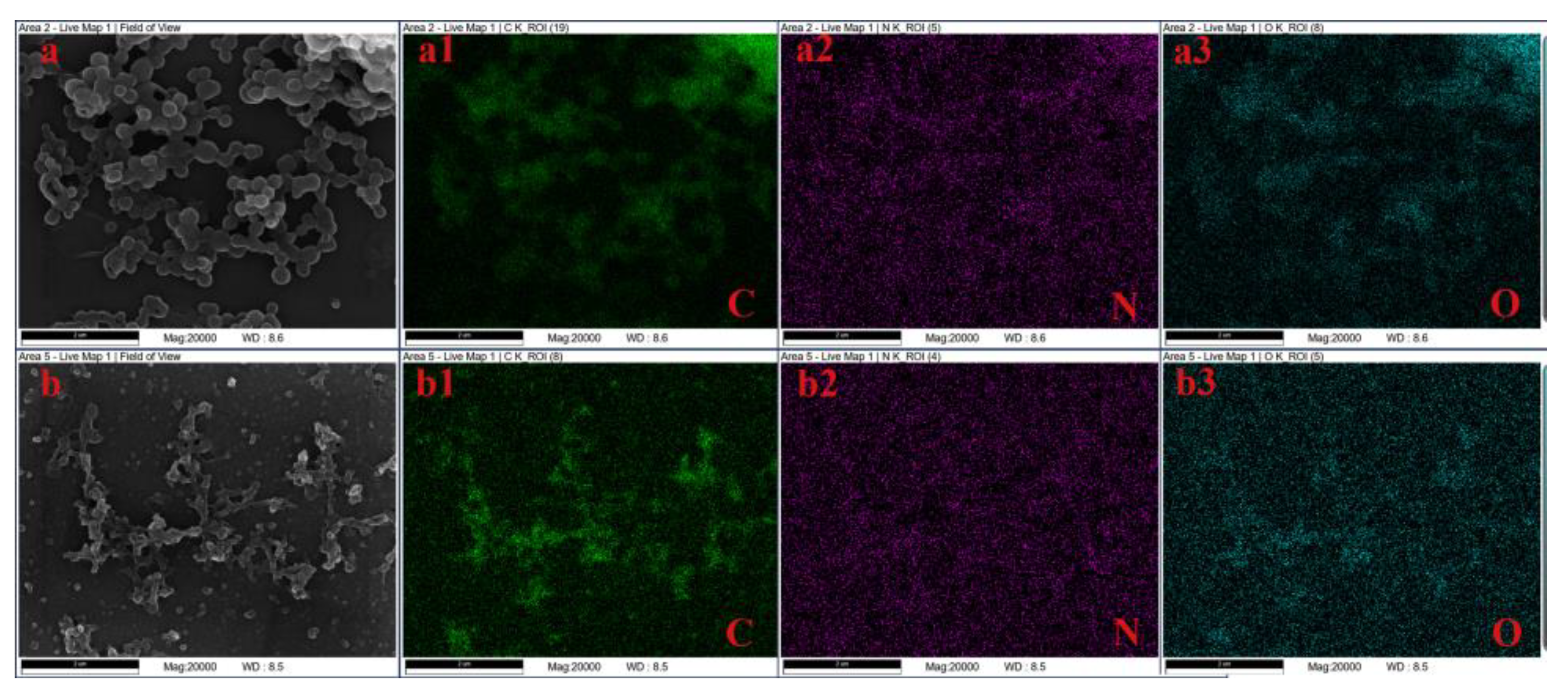
Through a detected analysis by an energy dispersive spectrometer (EDS), it gets the content and elemental distribution of the elements of C, N, O and S, as shown in
Table 1 and
Figure 3. where, the images show that C and O elements are uniformly distributed in lignin raw material and aminated lignin, whereas the lesser percentage of N content is not clearly distributed in the EDS images. Whereas,
Table 1 shows the the decrease of the content of C element after reaction, due to the mass of carbon atoms does not increase, while the product mass increasing by introducing amino groups during the reaction process. More important, the nitrogen content significantly increased after the reaction. Where, it is 0.03% nitrogen content in lignin, which raise to 3.84% nitrogen content in aminated lignin generated by the reaction, due to amino groups being introduced into the raw material lignin by the reaction.
- (1)
Analysis results by an elemental analyzer.
In order to accurately determine the changes in N element content before and after the ammonification reaction, an element analyzer was used to analyze the elemental composition of aminated lignin and lignin raw materials. The analysis results are shown in
Table 2. Where, the nitrogen content in lignin raw materials is very small, only 0.4%. On other side, the nitrogen content in the aminated lignin produced by ammonification reaction is 4.25%. therefore, it shows the content of nitrogen element significantly increases after the reaction. This result indicates that ammonia has reacted with lignin to produce aminated lignin.
In addition, the original unsaturation of lignin molecules is 4.61, while the unsaturation of aminated lignin is 2.96. This data change indicates that during the reaction process, lignin macromolecules undergo hydrolysis, generating smaller molecular fragments, resulting in a decrease in the cyclic unsaturation of aminated lignin compounds. This analysis further confirms that lignin undergoes hydrolysis during the ammonification reaction process.
By calculation, the formula of lignin can be expressed as C9H10.77O4.73N0.06, it means the number of carbon atoms is about 150.0 times of that of nitrogen atoms. While the formula of aminated lignin can be expressed as C9H14.08O4.76N0.67, it means the number of carbon atoms is about 13.43 times of that nitrogen atoms. The ratios between carbon and nitrogen reduced from 150 time to 13.43 time, further more identifies the facts of introduction of the amino groups into the raw lignin materials.
3.4. Results of XPS Analysis
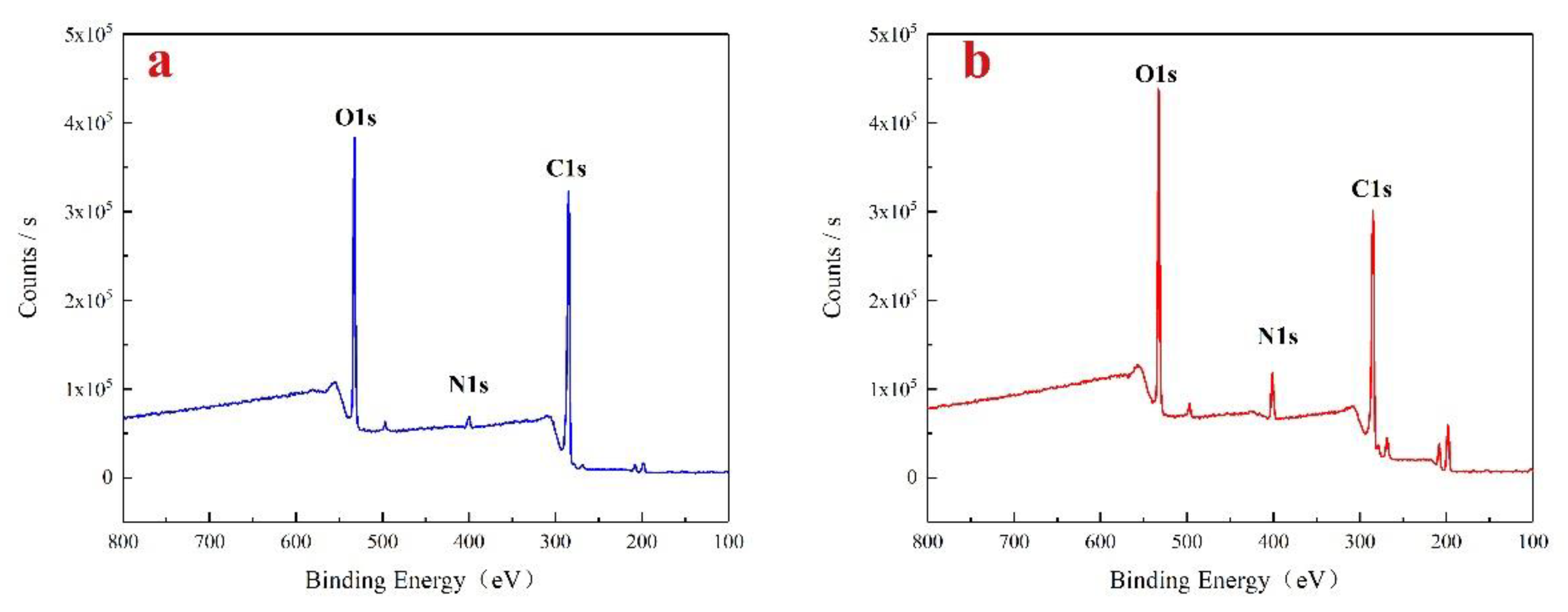
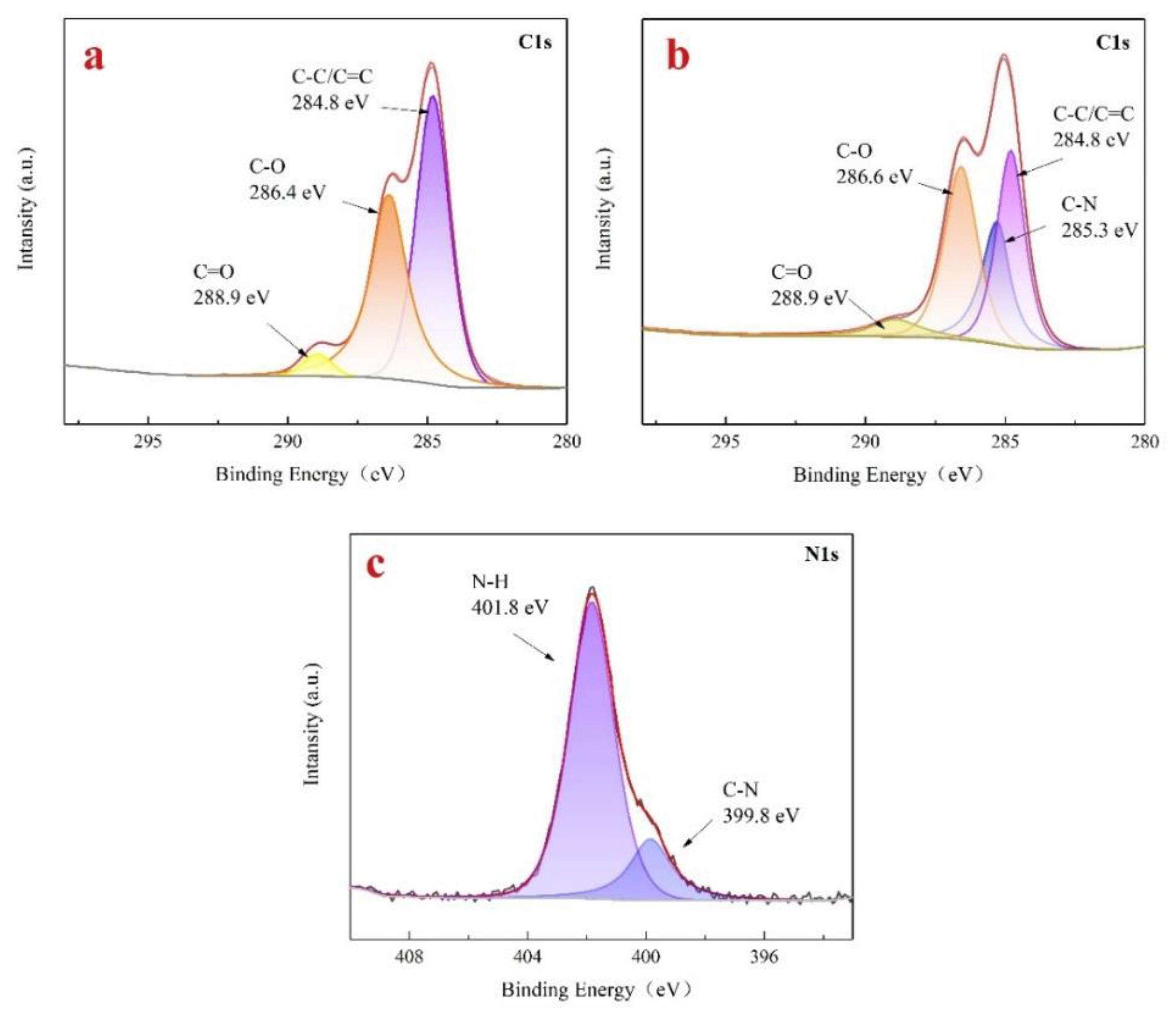
Using XPS analysis method to understand the changes in the content of various elements and chemical bonds during the reaction process.
Figure 4 shows the XPS spectra of lignin raw materials and aminated lignin. Where, both lignin raw materials and aminated lignin contain abundant basic elements such as C and O. However, the absorption peak of nitrogen atom intensity in aminated lignin is significantly increased. Also comparing the ratios of C and N atoms in lignin raw material and aminated lignin, the C/N ratio in lignin raw material was 30.59, whereas in aminated lignin, this ratio decreased to 9.19. This change indicates that amino groups have been successfully introduced into lignin, resulting in an increase in nitrogen content.
Figure 5 shows the fine spectra of C1s of lignin raw material (a) and aminated lignin (b), as well as the N1s of aminated lignin (c). As shown in
Figure 5a, the C1s spectra of lignin raw materials were fitted with three peaks at 284.8 eV, 286.4 eV, and 288.9 eV, respectively belonging to the C=C/C -, C-O and C=O peaks [
25,
26].
On other hand, the C1s spectrum of aminated lignin was fitted with four peaks, namely 284.8 eV, 285.3 eV, 286.6 eV, and 288.9 eV, belonging to the C=C/C -, C-N, C-O and C=O peaks respectively, as shown in
Figure 5b. The presence of C-N chemical bonds in aminated lignin indicates it has successfully introducing amino groups into lignin.
As shown in
Figure 5c, the two fitted peaks in the binding energy of 399.8eV and 401.8eV spectra are C-N bond and - NH
2/- NH, respectively [
27]. The existence of these two sets of chemical bonds further proves the successful introduction of amino groups, generating aminated lignin compounds.
3.5. Test Results of Thermal Stability
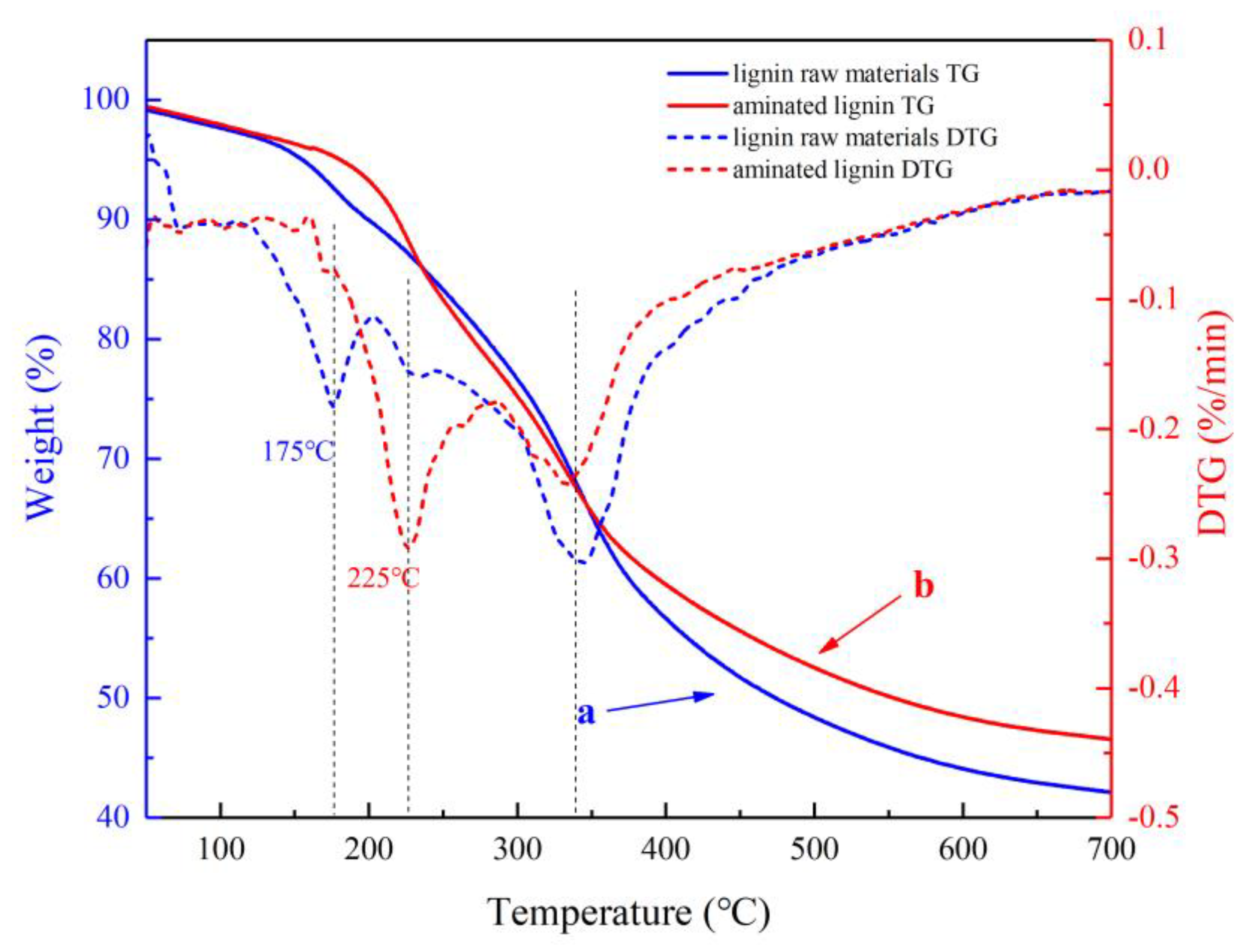
Thermal stability analysis of lignin raw material and aminated lignin was performed by thermogravimetric analysis (TGA) technique. As shown in the TGA curves presented in
Figure 6, it can be observed that the thermal degradation behavior of the two materials showed a similar trend in the temperature between 50°C-700°C. However, there are somewhat different between the two curves. Where, it can see that the lignin raw material experiences significant weight loss in the range of about 140 °C-210 °C, while the weight lose of the sample of aminated lignin is presented in only a slight change between the same temperature. The differentia means the sample of raw lignin material degrades in large-scale at the temperature between 140 °C-210 °C [
28]. In contrast, the sample of aminated lignin degrades in a slow way between the same temperature, clearly indicating that the sample of aminated lignin is more stable than the sample of lignin raw materials under the same temperature.
As shown in the DTG curves presented in
Figure 6, It can be found that the first peak of heat weight loss of the lignin raw material occurs at about 175 °C. it means there is a great amount of degradation reaction take place at the about temperature. As a comparison, the first peak of heat weight loss of the sample of aminated lignin occurs at about 225 °C. it means there is a greate amounts of degradation reaction take place at the about temperature. The differentia of the two first heat weight loss peaks in the two curves indicates the thermal property of the generated aminated lignin product is more stable than that of raw lignin material, consistent with the TGA analysis results metioned above. This significant differentia of the beginning degradation reaction temperatures reconfirms the positive effect of the introduction of amino groups, which enhances the thermal stability of lignin materials. Due to the addition of amino groups facilitates the formation of hydrogen bonds, which augments intermolecular interactions and subsequently elevates the temperature at which thermal degradation occurs [
29].
However, it is worth noting that at the second peak of thermal weight loss, where, the second peak of thermal weight loss of the raw lignin material is at temperature of 342 °C , while that of the generated aminated lignin is at temperatures of 332 °C. The two degradation temperatures at this stage are similar, due to the breaking of the methyl aryl ether bond in the molecule [
30,
31]. The similar temperature of the second peaks of thermal weight lose of the two samples indicates the thermal stability of lignin materials is relatively minor by the effect of amination at the second stage of thermal degradation.
After reaching a temperature of 400°C, the weight loss is attributed to the subsequent degradation of lignin. Where, the C-C bonds within the lignin are cleaved, resulting in the liberation of small molecules such as H
2O, CO, and CO
2 [
28]. Finally, at 700°C, the main component of the residual material is carbon. Where, residual carbon rates of 42.12% and 46.58% for lignin raw material and aminated lignin, respectively. The residual carbon rate from aminated lignin product is greater than that of the raw lignin materials, it further supports the superiority of aminated lignin in terms of thermal stability [
32].
3.6. Test Results of Water Solubility
Introducing amino groups into lignin molecules can significantly improve the water solubility of lignin modified materials. The aminated lignin generated by lignin ammonification reaction can be dissolved in water, presenting in yellow brown in color, as shown in
Figure 7.
Be test, the water solubility of the aminated lignin compound is about 0.45g for per 100ml of water. Thereby, it effectively improves the water solubility of lignin raw materials. This water-soluble aminated lignin can reduce its surface tension in the process of preparation of adhesive, and thereby, make various reaction raw materials uniformly mixed. Thus, it will effectively improve the polymerization reaction progress, ensure the product quality of the adhesive. More important, amino groups were introduced into lignin materials to improve the reaction activity of lignin, thereby, it will greatly improve the surface tension in the process of preparation of adhesive.
Therefore, this study breaks through the technical barriers in synthesizing lignin modified materials with good water solubility, additionally introducing amino groups into lignin materials, and thereby provides technical reference for increasing the amount of lignin modified raw materials in the adhesive field.
3.7. Discussion on the Mechanisms of Lignin Ammonification Reaction
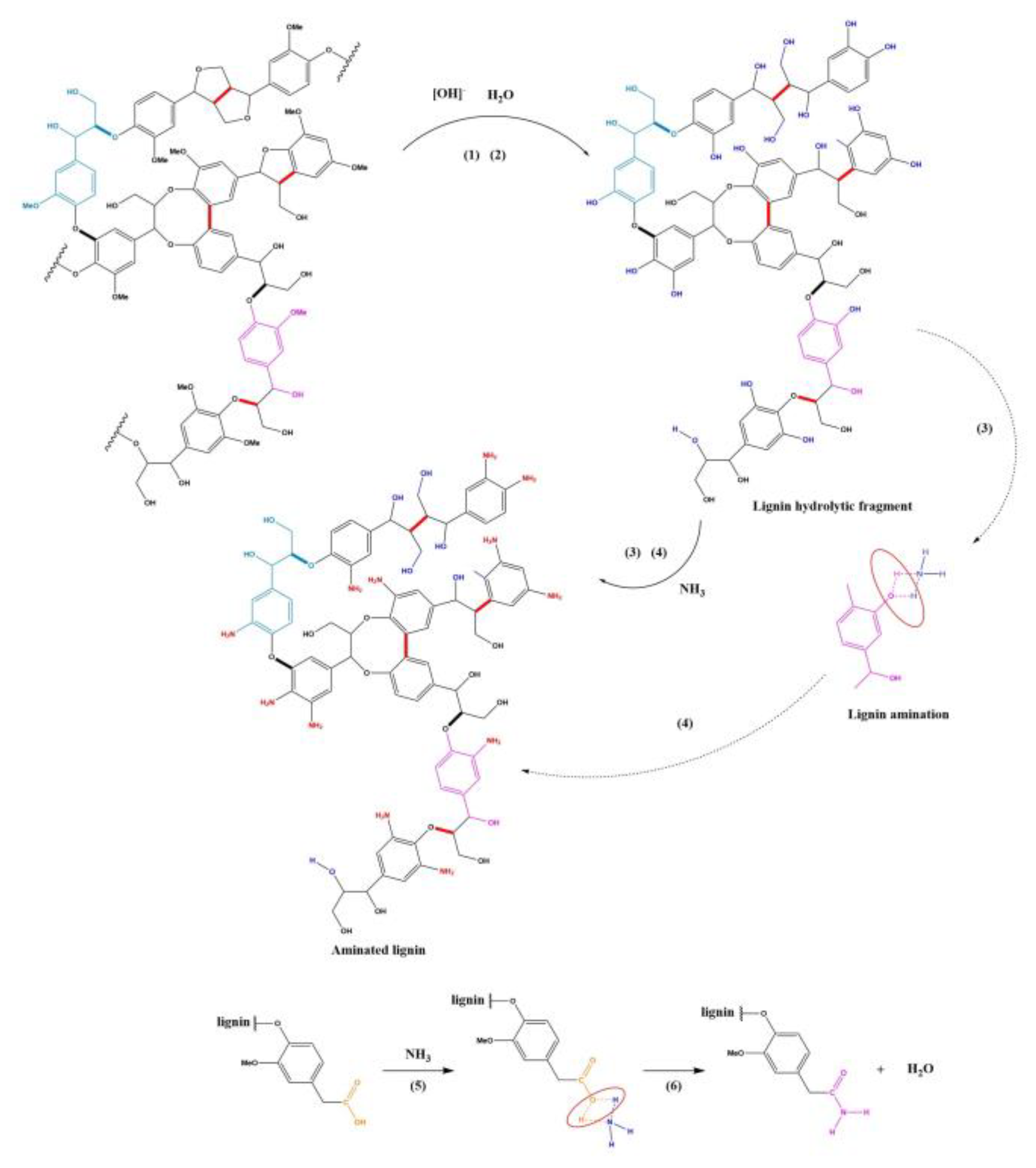
Based on the structural analysis results of the product, combined with the theory of alkaline cooking for lignin removal in pulp and paper making process, alkaline catalyzed ether bond hydrolysis theory, acid-base binding theory, and organic amine generation reaction theory, it is speculated that the lignin ammonification reaction includes six steps, as shown in
Figure 8. This chemical reaction mechanism research enriches the scientific theory of lignin chemical reactions.
In concentrated ammonia aqueous solution, under alkaline and high temperature conditions, β-O-4 Ether bonds undergo hydrolysis, causing lignin to degrade and generate small lignin fragments [
33,
34,
35]. (The principle of lignin degradation under alkaline catalysis)
Subsequently, under alkaline and high temperature conditions, the methoxyether bonds on the side chains of lignin molecules undergo hydrolysis, producing methanol and hydroxylated lignin, which contain a large amount of phenolic and fatty hydroxyl groups [
36]. (The principle of alkaline catalyzed ether bond hydrolysis)
Subsequently, the phenolic hydroxyl group on hydroxylated lignin undergoes an acid-base binding reaction with ammonia molecules, generating a transition state intermediate product between phenolic hydroxyl and ammonia molecules [
37,
38]. (The principle of acid-base combination)
Subsequently, the intermediate product of the phenolic hydroxyl ammonia molecule is unstable and undergoes dehydration reaction, generating aniline, thereby successfully introducing amino groups and generating aminated lignin [
39,
40].(The principles of organic amine generation)
In addition, lignin also contains small amounts of carboxylic acids, and these carboxyl groups combine with ammonia molecules to produce carboxylic acid-ammonia transition state intermediates [
41]. (The principle of acid-base combination)
The transition state intermediate carboxylic acid-ammonia is unstable and under high temperature conditions, a dehydration reaction occurs to produce a structurally stable acid amide group [
42,
43]. (The principle of acid amides generated reaction)
3.8. Discussion on the Advancements and Shortcomings of This Research
The advancements of this research: A kind of aminated lignin was synthesized by lignin ammonification reaction, following with its structural analysis and chemical reaction mechanism study. Especially, the aminated lignin product has a higher thermal stability and a good water solubility property, which was 0.45 g per 100 ml of water by test. Thereby, the generated product of aminated lignin effectively improves the water solubility of lignin raw materials. The generated water-soluble aminated lignin can be used as a fine material to prepare adhesives. It can reduce the surface tension between it and other raw materials, so that it can mix a variety of reaction materials uniformly. And thereby, it can improve the progress of the polymerization reaction. At the same time, the aminated lignin product improves the thermal stability of raw lignin materials, which can also guarantee the quality of the adhesive products. Therefore, this research provides a new way to increase the dosage of lignin-modified raw materials in the field of adhesives and enriches the scientific theory of lignin chemical reaction theory.
However, there are still some shortcoming in this research: It was discovered that a small portion of high molecular weight aminated lignin did not dissolve completely in water. In contrast, they suspense in the water surface in the water solubility experiment. Therefore, an in-depth research on optimizing the preparation process should be carried out to minimize the production of large molecular weight aminated lignin, and thereby, enhance the water solubility of aminated lignin to improve its application performance furtherly. In addition, the molecular weight distribution of ammonified lignin dissolved in water has not been investigated, and thereby, it does not reveal the relationship between the molecular weight and the water solubility of aminated lignin products. On other hand, the synthetic reaction involves high temperature and high pressure, combining with a toxic gas of ammonia. Thereby, it faces the risk of ammonia leakage under high temperature and high pressure conditions in the process of synthetic reaction. To mitigate this potential hazard, the reaction conditions in future experiments should be refined to conduct the ammonification reaction.
4. Conclusions
Through experiments and theoretical analysis, the following conclusion has been drawn: a kind of aminated lignin compound has been successfully synthesized through lignin ammonification reaction. The particle size of the aminated lignin is about 100 nm, with a weight average molecular weight of 57,627 g/mol. The agglomeration of the lignin particle size that is significantly reduced. The aminated lignin has good water solubility of 0.45g per 100ml of water. And the thermal stability of aminated lignin is better than that of raw lignin materials.
The mechanism of lignin ammonification reaction is that: under the action of ammonia water, lignin undergoes degradation reaction and demethoxy reaction, generating hydroxylated lignin. Subsequently, the phenolic hydroxyl and carboxylic acid groups on the hydroxylated lignin undergoes acid-base binding reaction with ammonia molecules, generating a transition state of phenol ammonia and carboxylic acid ammonia intermediates. Finally, Finally, the phenol ammonia and carboxylic acid ammonia intermediates remove one water molecule respectively; and thereby, transformed into amide groups, successfully introducing amino groups into lignin, generating aminated lignin compounds.
Acknowledgments
This project is supported by National Natural Science Foundation of China (Grant #: 202301685) We are grateful for the valuable comments from reviewers and editor, the comments are important to improve the quality of this paper.
References
- Liu, E.; Mercado, M.I.V.; Segato, F.; Wilkins, M.R. A green pathway for lignin valorization: Enzymatic lignin depolymerization in biocompatible ionic liquids and deep eutectic solvents. Enzym. Microb. Technol. 2024, 174, 110392. [Google Scholar] [CrossRef]
- Wang, Y.; Hou, Y.; Wu, W.; Li, H.; Ren, S.; Li, J. Polycyclic Aromatics Observed in Enzymatic Lignin by Spectral Characterization and Ruthenium Ion-Catalyzed Oxidation. J. Agric. Food Chem. 2021, 69, 12148–12155. [Google Scholar] [CrossRef] [PubMed]
- Yang, G.; Gong, Z.; Luo, X.; Chen, L.; Shuai, L. Bonding wood with uncondensed lignins as adhesives. Nature 2023, 621, 511–515. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Peng, Z.; Li, J.; Li, X.; Jiang, X.; Dong, Y. Unlocking the role of lignin for preparing the lignin-based wood adhesive: A review. Ind. Crop. Prod. 2022, 187. [Google Scholar] [CrossRef]
- Balakshin, M.Y.; Capanema, E.A.; Sulaeva, I.; Schlee, P.; Huang, Z.; Feng, M.; Borghei, M.; Rojas, O.J.; Potthast, A.; Rosenau, T. New Opportunities in the Valorization of Technical Lignins. ChemSusChem 2020, 14, 1016–1036. [Google Scholar] [CrossRef]
- Costa, C.A.E.; Casimiro, F.M.; Vega-Aguilar, C.; Rodrigues, A.E. Lignin Valorization for Added-Value Chemicals: Kraft Lignin versus Lignin Fractions. Chemengineering 2023, 7, 42. [Google Scholar] [CrossRef]
- Ghavidel, N.; Konduri, M.K.; Fatehi, P. Chemical reactivity and sulfo-functionalization response of enzymatically produced lignin. Ind. Crop. Prod. 2021, 172, 113950. [Google Scholar] [CrossRef]
- Lievonen, M.; Valle-Delgado, J.J.; Mattinen, M.-L.; Hult, E.-L.; Lintinen, K.; Kostiainen, M.A.; Paananen, A.; Szilvay, G.R.; Setälä, H.; Österberg, M. A simple process for lignin nanoparticle preparation. Green Chem. 2015, 18, 1416–1422. [Google Scholar] [CrossRef]
- Ghosh, T.; Elo, T.; Parihar, V.S.; Maiti, P.; Layek, R. Poly (itaconic acid) functionalized lignin/polyvinyl acetate composite resin with improved sustainability and wood adhesion strength. Ind. Crop. Prod. 2022, 187. [Google Scholar] [CrossRef]
- Vishtal, A. Kraslawski, CHALLENGES IN INDUSTRIAL APPLICATIONS OF TECHNICAL LIGNINS, BioResources 6(3) (2011) 3547-3568.
- Chatel, G.; Rogers, R.D. Review: Oxidation of Lignin Using Ionic Liquids—An Innovative Strategy To Produce Renewable Chemicals. ACS Sustain. Chem. Eng. 2013, 2, 322–339. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, J.; Zong, Q.-J.; Wang, L.; Xu, T.; Gong, J.; Liu, Z.-H.; Li, B.-Z.; Yuan, Y.-J. High-solid ethylenediamine pretreatment to fractionate new lignin streams from lignocellulosic biomass. Chem. Eng. J. 2021, 427, 130962. [Google Scholar] [CrossRef]
- L. Xu, S.J. L. Xu, S.J. Zhang, C. Zhong, B.Z. Li, Y.J. Yuan, Alkali-Based Pretreatment-Facilitated Lignin Valorization: A Review, Industrial & Engineering Chemistry Research 59(39) (2020) 16923-16938.
- Zhao, W.; Xiao, L.-P.; Song, G.; Sun, R.-C.; He, L.; Singh, S.; Simmons, B.A.; Cheng, G. From lignin subunits to aggregates: insights into lignin solubilization. Green Chem. 2017, 19, 3272–3281. [Google Scholar] [CrossRef]
- Konduri, M.K.R.; Fatehi, P. Production of Water-Soluble Hardwood Kraft Lignin via Sulfomethylation Using Formaldehyde and Sodium Sulfite. ACS Sustain. Chem. Eng. 2015, 3, 1172–1182. [Google Scholar] [CrossRef]
- Prinsen, P.; Narani, A.; Hartog, A.F.; Wever, R.; Rothenberg, G. Dissolving Lignin in Water through Enzymatic Sulfation with Aryl Sulfotransferase. ChemSusChem 2017, 10, 2267–2273. [Google Scholar] [CrossRef]
- Ang, A.F.; Ashaari, Z.; Lee, S.H.; Tahir, P.M.; Halis, R. Lignin-based copolymer adhesives for composite wood panels – A review. Int. J. Adhes. Adhes. 2019, 95, 102408. [Google Scholar] [CrossRef]
- Peng, Z.; Jiang, X.; Si, C.; Cárdenas-Oscanoa, A.J.; Huang, C. Advances of Modified Lignin as Substitute to Develop Lignin-Based Phenol-Formaldehyde Resin Adhesives. ChemSusChem 2023, 16, e202300174. [Google Scholar] [CrossRef]
- T. Li, S. T. Li, S. Wu, J. Zhuang, Y. Liu, W. Wei, F. Zhang, Preparation of high-performance lignin-phenolic resin adhesive by oxidative pretreatment of lignin, Applied Chemical Industry 51(4) (2022) 907-911.
- M. Li, M. M. Li, M. Wilkins, Lignin bioconversion into valuable products: fractionation, depolymerization, aromatic compound conversion, and bioproduct formation, Systems Microbiology and Biomanufacturing 1(2) (2021) 166-185.
- Limarta, S.O.; Kim, H.; Ha, J.-M.; Park, Y.-K.; Jae, J. High-quality and phenolic monomer-rich bio-oil production from lignin in supercritical ethanol over synergistic Ru and Mg-Zr-oxide catalysts. Chem. Eng. J. 2020, 396, 125175. [Google Scholar] [CrossRef]
- Chen, J.; An, L.; Bae, J.H.; Heo, J.W.; Han, S.Y.; Kim, Y.S. Green and facile synthesis of aminated lignin-silver complex and its antibacterial activity. Ind. Crop. Prod. 2021, 173, 114102. [Google Scholar] [CrossRef]
- Xue, Z.; Zhao, X.; Sun, R.-C.; Mu, T. Biomass-Derived γ-Valerolactone-Based Solvent Systems for Highly Efficient Dissolution of Various Lignins: Dissolution Behavior and Mechanism Study. ACS Sustain. Chem. Eng. 2016, 4, 3864–3870. [Google Scholar] [CrossRef]
- Wu, W.; Jiang, B.; Yang, L.; Jin, Y. Isolation of Lignin from Masson Pine by Liquid-Liquid Extraction Based on Complete Dissolution in NaOH Aqueous Solution. BioResources 2017, 13, 231–240. [Google Scholar] [CrossRef]
- Chen, R.; Hu, K.; Tang, H.; Wang, J.; Zhu, F.; Zhou, H. A novel flame retardant derived from DOPO and piperazine and its application in epoxy resin: Flame retardance, thermal stability and pyrolysis behavior. Polym. Degrad. Stab. 2019, 166, 334–343. [Google Scholar] [CrossRef]
- Y.P. Shang, G.Z. Y.P. Shang, G.Z. Zhu, D.X. Yan, Q.Z. Liu, T.T. Gao, G.W. Zhou, Tannin cross-linked polyethyleneimine for highly ef fi cient removal of hexavalent chromium, J. Taiwan Inst. Chem. Eng. 119 (2021) 52-59.
- Zhang, Y.; Liu, Q.; Ma, W.; Liu, H.; Zhu, J.; Wang, L.; Pei, H.; Liu, Q.; Yao, J. Insight into the synergistic adsorption-reduction character of chromium(VI) onto poly(pyrogallol-tetraethylene pentamine) microsphere in synthetic wastewater. J. Colloid Interface Sci. 2021, 609, 825–837. [Google Scholar] [CrossRef] [PubMed]
- Sahoo, S.; Seydibeyoğlu, M.; Mohanty, A.; Misra, M. Characterization of industrial lignins for their utilization in future value added applications. Biomass- Bioenergy 2011, 35, 4230–4237. [Google Scholar] [CrossRef]
- Zhang, J.; Cao, D.; Wang, S.; Feng, X.; Zhu, J.; Lu, X.; Mu, L. Valorization of industrial lignin as lubricating additives by C–C Bond Cleavage and doping heteroelement-rich groups. Biomass- Bioenergy 2022, 161. [Google Scholar] [CrossRef]
- Majeke, B.; Collard, F.-X.; Tyhoda, L.; Görgens, J. The synergistic application of quinone reductase and lignin peroxidase for the deconstruction of industrial (technical) lignins and analysis of the degraded lignin products. Bioresour. Technol. 2020, 319, 124152. [Google Scholar] [CrossRef]
- Naron, D.; Collard, F.-X.; Tyhoda, L.; Görgens, J. Production of phenols from pyrolysis of sugarcane bagasse lignin: Catalyst screening using thermogravimetric analysis – Thermal desorption – Gas chromatography – Mass spectroscopy. J. Anal. Appl. Pyrolysis 2018, 138, 120–131. [Google Scholar] [CrossRef]
- Wang, C.; Feng, X.; Shang, S.; Liu, H.; Song, Z.; Zhang, H. Lignin/sodium alginate hydrogel for efficient removal of methylene blue. Int. J. Biol. Macromol. 2023, 237, 124200. [Google Scholar] [CrossRef]
- Liao, J.J.; Latif, N.H.A.; Trache, D.; Brosse, N.; Hussin, M.H. Current advancement on the isolation, characterization and application of lignin. Int. J. Biol. Macromol. 2020, 162, 985–1024. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; He, C.; Liu, C.; Tong, H.; Wu, L.; Wu, S. A computational study on thermal decomposition mechanism of β-1 linkage lignin dimer. Comput. Theor. Chem. 2015, 1054, 80–87. [Google Scholar] [CrossRef]
- Roberts, V.M.; Stein, V.; Reiner, T.; Lemonidou, A.; Li, X.; Lercher, J.A. Towards Quantitative Catalytic Lignin Depolymerization. Chem. – A Eur. J. 2011, 17, 5939–5948. [Google Scholar] [CrossRef]
- Schutyser, W.; Renders, T.; Van Den Bosch, S.; Koelewijn, S.-F.; Beckham, G.T.; Sels, B.F. Chemicals from lignin: an interplay of lignocellulose fractionation, depolymerisation, and upgrading. Chem. Soc. Rev. 2018, 47, 852–908. [Google Scholar] [CrossRef] [PubMed]
- F. Braghiroli, V. F. Braghiroli, V. Fierro, A. Pizzi, K. Rode, W. Radke, L. Delmotte, J. Parmentier, A. Celzard, Reaction of condensed tannins with ammonia, Industrial Crops and Products 44 (2013) 330-335.
- Hashida, K.; Makino, R.; Ohara, S. Amination of pyrogallol nucleus of condensed tannins and related polyphenols by ammonia water treatment. Holzforschung 2008, 63, 319–326. [Google Scholar] [CrossRef]
- Dai, L.; Wang, Y.; Liu, Y.; He, C.; Ruan, R.; Yu, Z.; Jiang, L.; Zeng, Z.; Wu, Q. A review on selective production of value-added chemicals via catalytic pyrolysis of lignocellulosic biomass. Sci. Total. Environ. 2020, 749, 142386. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Yao, Q.; Zhang, Y.; Fu, Y. Integrated Production of Aromatic Amines and N-Doped Carbon from Lignin via Catalytic Fast Pyrolysis in the Presence of Ammonia over Zeolites. ACS Sustain. Chem. Eng. 2017, 5, 2960–2969. [Google Scholar] [CrossRef]
- Jursic, B.S.; Zdravkovski, Z. A SIMPLE PREPARATION OF AMIDES FROM ACIDS AND AMINES BY HEATING OF THEIR MIXTURE. Synth. Commun. 1993, 23, 2761–2770. [Google Scholar] [CrossRef]
- D'Amaral, M.C.; Jamkhou, N.; Adler, M.J. Efficient and accessible silane-mediated direct amide coupling of carboxylic acids and amines. Green Chem. 2020, 23, 288–295. [Google Scholar] [CrossRef]
- Stoll, E.L.; Tongue, T.; Andrews, K.G.; Valette, D.; Hirst, D.J.; Denton, R.M. A practical catalytic reductive amination of carboxylic acids. Chem. Sci. 2020, 11, 9494–9500. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).