1. Introduction
Metal-organic framework (MOF) compounds, also known as porous coordination polymers (PCPs) are part of the class of crystalline materials,[
1] consisting of organic ligands and inorganic metal centers, frequently denominated as secondary building units (SBUs). These building units consist of metal centers or metallic cluster centers (various metal atoms), mostly coordinated by oxygen or nitrogen atoms and interconnected by organic ligands to originate an infinite network.[
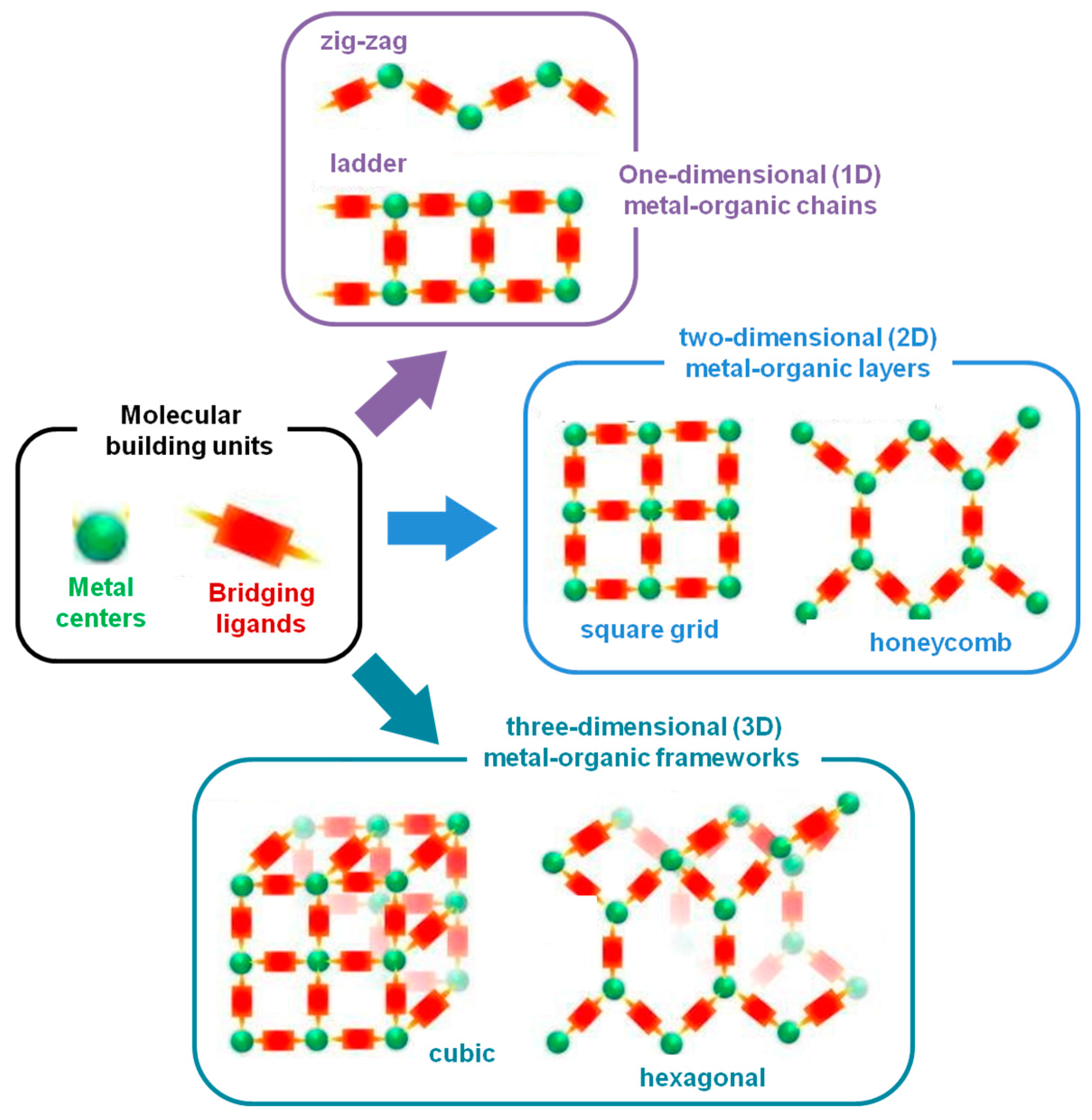
2] The infinite network is fundamentally defined through coordination links, and is possible to obtain one-dimensional (1D), two-dimensional (2D) and three-dimensional (3D) coordination polymers (
Figure 1) [
3]. SBUs are the main components of MOFs, contributing to the construction of porous networks, being essential for determining the underlying topology of MOFs. As there are many possible combinations of SBUs and ligands, this will be reflected in a large number and structural variability of coordination polymers in general, and particularly in porous MOF compounds [
4].
The porosity of the MOFs it is crucial for several properties and potential utilization / application of these families of metal-organic based compounds. The pore structural features (size, shape and others) can be modified and adjusted by judicious changes of the structure of the ligands involved, for example altering their length (
Figure 2). On the other hand, the chemical nature present inside the pores can also be adjusted by the characteristics of the ligands themselves, in particular by the use of distinct functional groups.[
6]
The structural characteristics of the MOFs are mainly influenced by the great possibility of coordination geometries that are adopted by both metal ions and agglomerates, by the structural characteristics and flexibility of the organic ligands, and by the several parameters of the synthesis, for example temperature, metal / ligand ration, solvent and others. The network topology and dimensionality of MOFs are directly related to the different coordination geometries that metals can acquire, which vary depending on the electronic structure of metal ions. Transition metal ions have been widely used because they present a wide diversity of coordination numbers, geometries and oxidation states, thus contributing to a synthetic and structural diversity [
7].
The utilization of rigid or flexible organic ligands plays a relevant role in the preparations of a specific MOF because flexible ligands offer greater degrees of freedom from rigid ones, which can lead to unpredictable crystalline structures [
8]. Organic molecules that have one or more nitrogen (N) or oxygen (O) donor atoms, such as carboxylates, are frequently used as organic ligands to bridge metal ions in MOFs. The solvent of the reaction also have an important role because it can be involved in the crystallization and topology of the network through steric effects, can fill sites of coordination of metal ions, complete pores of the MOF or participate in weak intermolecular interactions, contributing for the structural and thermal stability of the crystalline network [
7]. The three-dimensional structure of MOFs is constructed due to strong coordination bonds between metal ions and organic ligands featuring cavities and internal surfaces, which are occupied by other molecules. Other types of interactions such as hydrogen bonds, metal-metal bonds and π-π interactions may be present, thus contributing to the stability of MOFs [
7]. In fact, this type of materials reveal a high structural diversity due to several factors, such as the coordinative nature of the organic ligands themselves, the metal-ligand interactions and the varied and modifiable configuration of the metal clusters or the metal itself.[
9]
Figure 3.
Examples of MOFs described in the literature to demonstrate the structural variety of already published metal-organic networks. Adapted from the reference [
10].
Figure 3.
Examples of MOFs described in the literature to demonstrate the structural variety of already published metal-organic networks. Adapted from the reference [
10].
2. Preparation Strategies of MOF Compounds
MOFs are prepared by combining the two fundamental constituents, the SBUs and the organic ligands. The process of synthesis of MOFs comprises the crystallization steps during which nucleation and crystal growth occur. These phenomena involve self-assembly between inorganic centers, frequently metal-oxygen or metal-nitrogen clusters and organic binders [
11]. The synthesis of MOFs and their final structure are established by several factors that are related to the reaction time and temperature, the chosen solvent, concentration of reagents, the nature of metal ions and organic ligands, and the kinetics of crystallization, that has influence on nucleation and crystal growth having relevant roles in the morphology and size of the resulting MOFs crystals. It is quite common for the synthesis of MOFs to take place in the liquid phase, where solutions of ligand and metallic salt are mixed. The solvent also plays a crucial role in determining the thermodynamics and activation energy for each reaction and its choice is based on its reactivity, solubility and redox potential [
7,
12].
In view of the fact that the synthesis of MOFs directly influences the crystallization and structure of the MOF compound, thus crucially determining its properties and its functional performance, an extensive research work in the development of synthesis methods has been carried out over the years [
7]. Furthermore, beyond the chemical nature of the compound, the potential and successful applicability of the synthesized MOFs still depend on their main physical properties, such as morphological characteristics, porosity, particle size and their distribution. Therefore, an in-depth knowledge of the type of synthetic methodology applied for the preparation of MOFs plays a fundamental role in choosing several structures of MOFs with specific physical and chemical properties to meet the needs of the area where the MOF is applied [
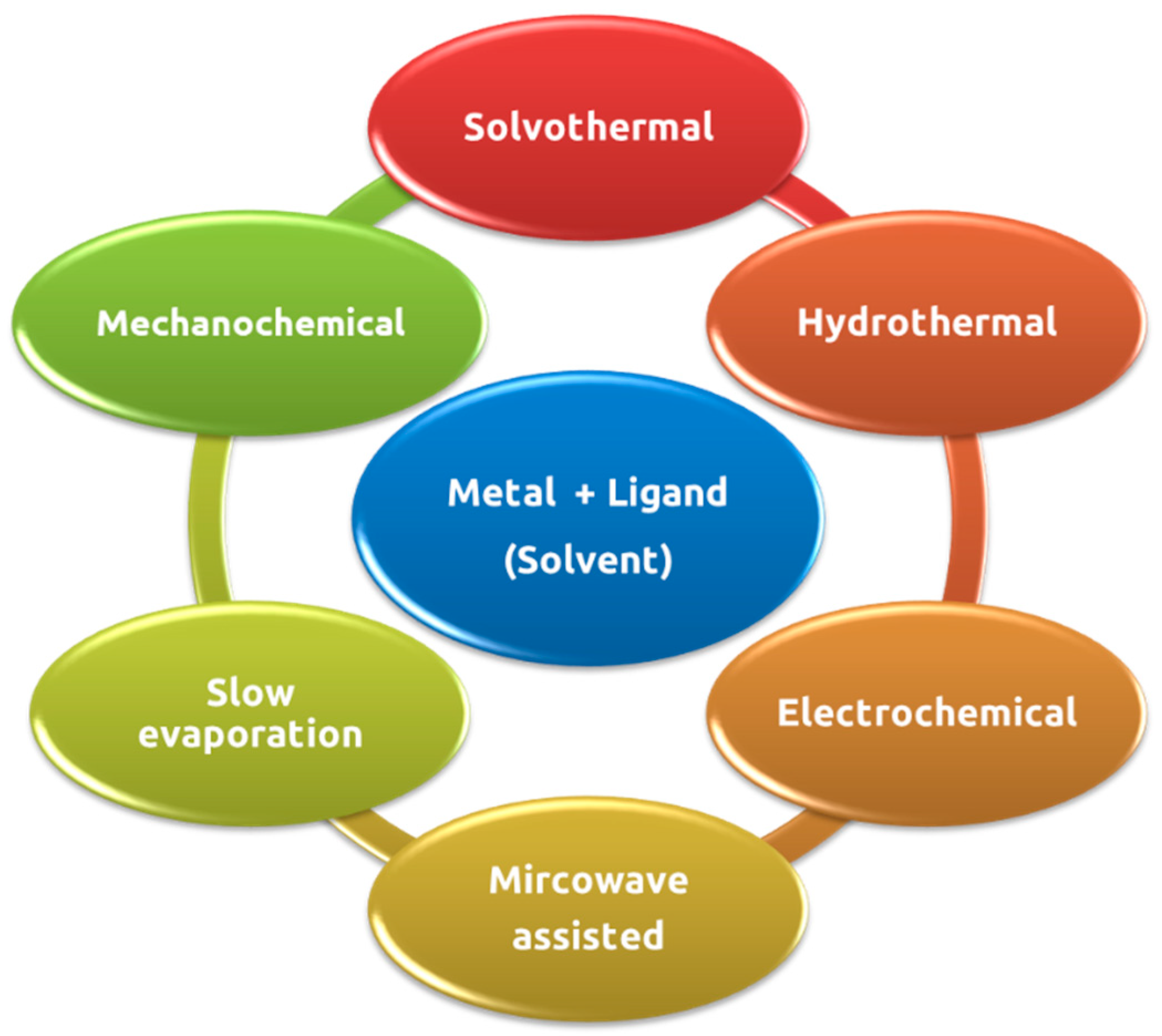
10]. Methods of synthesis of MOFs may include, for example, microwave-assisted methods, electrochemical methods, hydrothermal methods, solvothermal methods, among others (
Figure 4). [
13,
14,
15,
16]
Hydrothermal and solvothermal synthesis are examples of some of the most used techniques in the preparation of MOFs and comprise heating the reaction mixture to a specific temperature. Normally, organic solvents with high solubility such as dimethylformamide (DMF), diethylformamide (DEF), acetone ((CH
3)
2CO), acetonitrile (CH
3CN), ethanol (C
2H
5OH) or methanol (CH
3OH) are widely used in solvothermal processes. However, it is still possible to use solvent mixtures to contribute to the reduction of the problem that is associated with the initial solubility of the reagents in one unique solvent [
10]. Different temperature ranges are usually applied to perform solvothermal and hydrothermal processes, but temperatures are usually in a range of 50-200
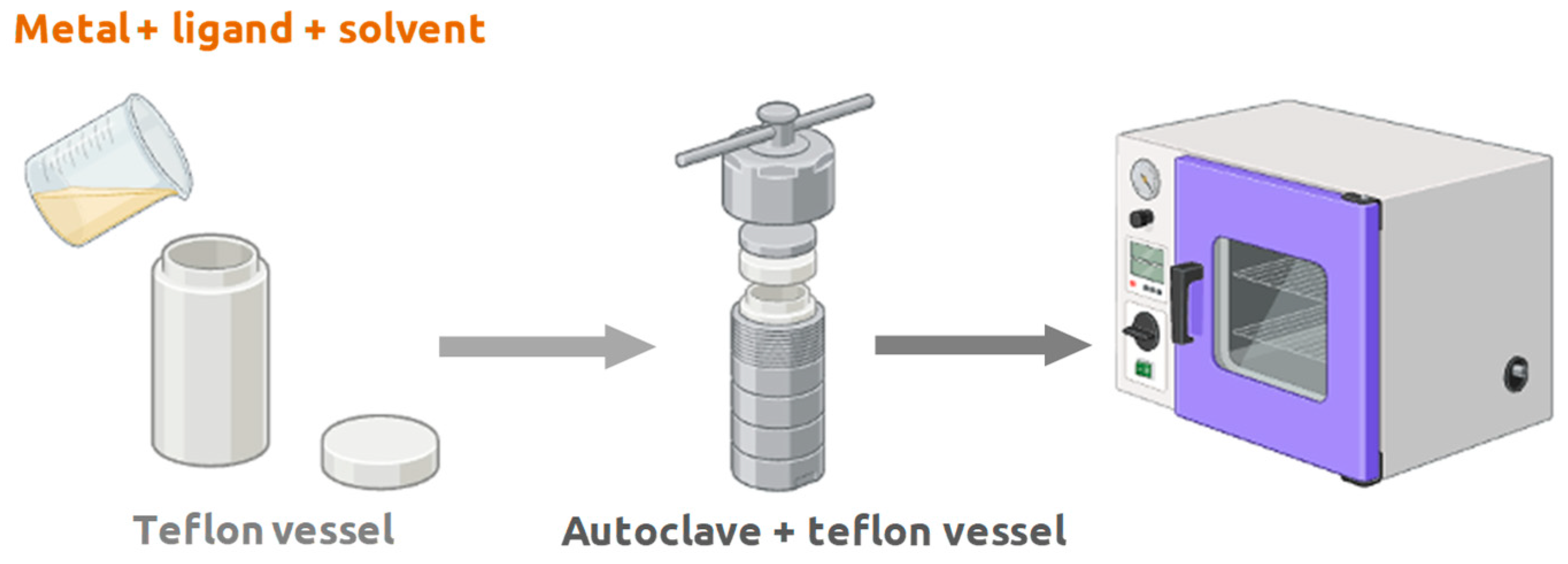
oC, although the crystallization process may take a few hours or even days. The synthesis is frequently made in Teflon reaction vessels, which are subsequently placed inside small volume autoclaves, where the metallic precursor and the organic ligand are dissolved in the chosen solvent, and the preparation is placed in the oven (
Figure 5) [
10].
In the case of hydrothermal synthesis, it is classified as one of the green methods for the synthesis of MOF compounds, because in its production it uses water as solvent instead of other organic solvents, that may be toxic as the case of DMF. In these two methods discussed here, the metallic ion, solvents, the organic ligand, and other materials are mixed to meet the stoichiometry. Briefly, the mixture is placed inside the Teflon coated autoclave at a certain temperature, and after the expected reaction time, the reactor is then allowed to cool to room temperature upon completion of the reaction. Pure MOF can be obtained by washing the product with solvents such as water, ethanol, acetone or other solutions, and is finally vacuum dried [
10]. There is still another process of synthesis of MOFs that must be referred, carried out at room temperature (one-pot synthesis). Some MOFs allow this synthesis, only using a reaction vessel with the solution containing the metal, solvent, and ligand without any use of temperature. Thus, this is the most sustainable method, because in addition to not using solvents that have toxicity as the case of DMF, does not use energy consumption.
The microwave-assisted synthesis (MWAS) method can be about twenty times faster than conventional methods (hydrothermal, solvothermal and one-pot room temperature method). Horcajada et al. reported the synthesis of MOFs whose main metal is chromium and present carboxylate functional groups in aqueous solutions by MWAS [
17]. These materials have advantages in areas of biomedicine, since they can encapsulate drugs with different polarities and pore sizes with various functional groups [
18].
An innovative electrochemical synthesis method applied in the preparation of transition metal-based MOFs was first addressed by Mueller et al. in 2005 [
18]. A few years later (2009), it was reported that electrochemical film growth of metal-organic frameworks (MOFs) enables the self-completing growth of densely packed crystallite layers in a patterned fashion. Interestingly, MOF-based coatings produced by this electrochemical synthesis method demonstrate to have potential synthesis applications in sensors and thin films [
19].
As mentioned previously, it is not only the composition and crystalline structure of MOFs that are fundamental to most properties and potential applications of this type of compounds. The morphological features, such as particle size, and the number of structural defects can also significantly influence the properties of MOF compounds. The concept of modulation can be attributed to the control of the morphology and size of particles, defects, and crystallinity of MOFs [
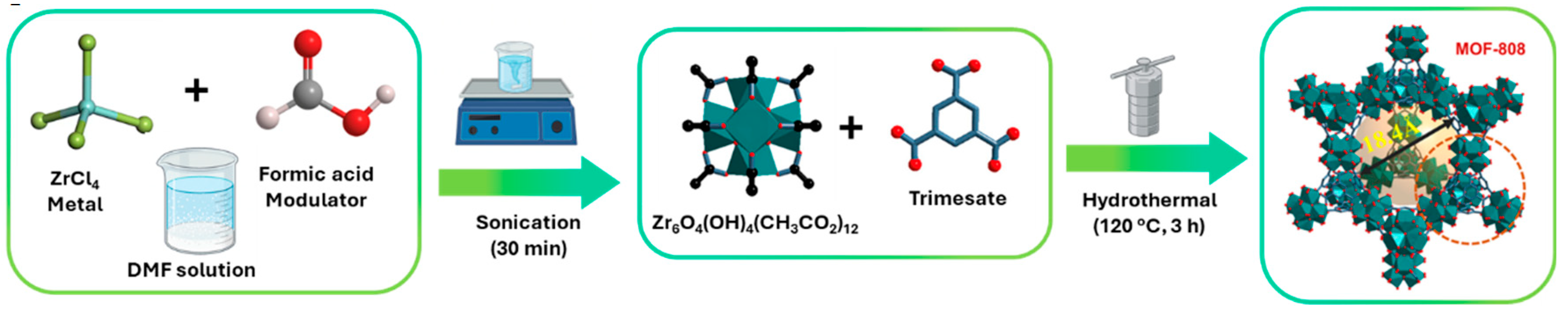
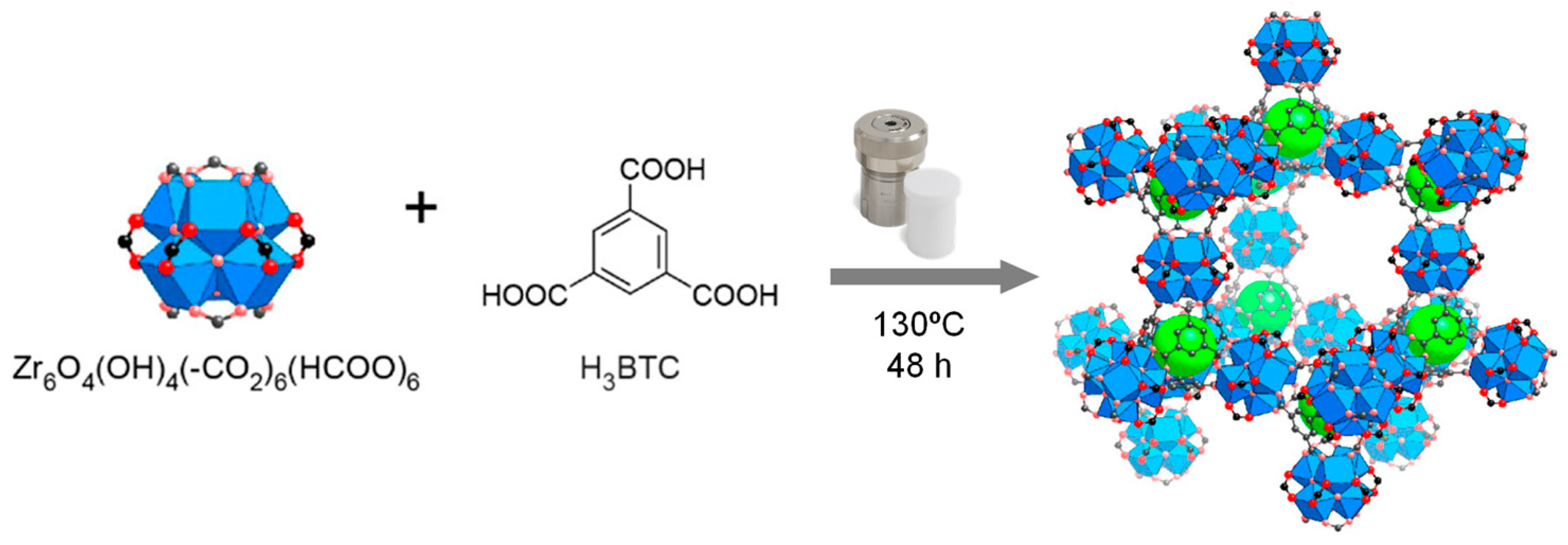
20]. One of the examples of modeling that is frequently explored concerns the introduction of small monocarboxylic acids, which act as modulating agents in the reaction mixture, during the synthesis of MOFs (
Figure 6). These acids will compete with the ligands by coordinating the metal cations, which then results in modulation of the crystallization process [
21]. Using this concept, and the addition of these acids, both the size and morphology of Zr-based MOFs can be modified and controlled. Some examples of these acids are acetic acid, formic acid, and benzoic acid. In the case of MOF-808, showed in the
Figure 6, these acids will thus act as modulators, facilitating the formation of Zr
6O
4(OH)
4 clusters, probably because they can control the nucleation rate of MOFs, competing with ligands by coordination sites in Zr atoms, or Zr clusters, that will become the SBUs of the structure. If this modulation does not exist, the aggregates of Zr MOFs will precipitate or their synthesis originates products with a more disorderly phase and eventually with a smaller specific surface area. However, the concentration of modulating agent must be controlled and moderate, since modulators play a relevant role in controlling the connectivity and topology of the framework, more specifically in MOFs based on Zr [
22].
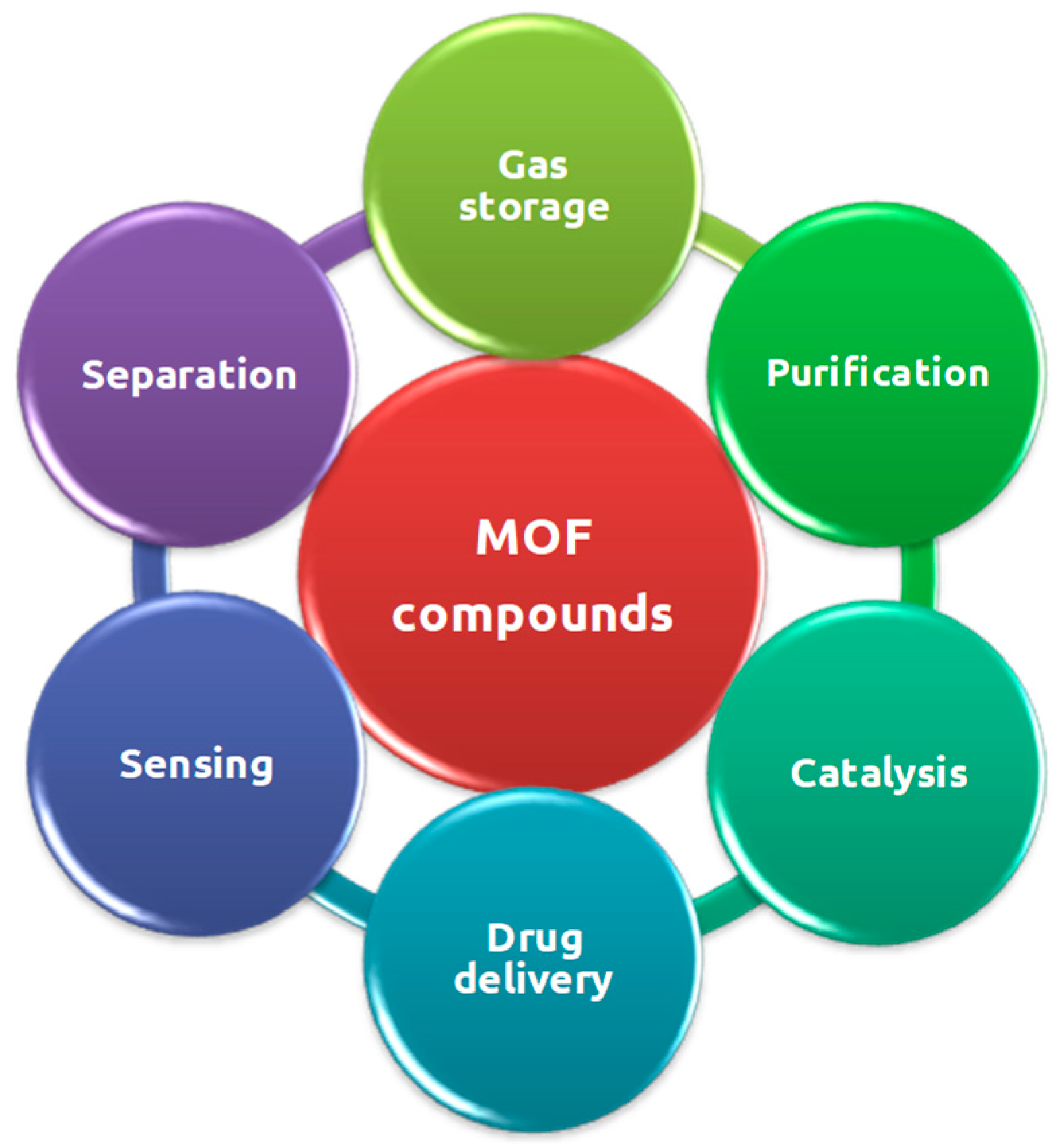
3. Potential of MOF Applications
In the last decade, several studies have demonstrated that MOF compounds can overcome the potential of other known and studied porous solid materials such as zeolites and porous carbon-based materials in a variety of applications [
23]. In fact, the incessant scientific interest for these compounds has been associated not only to their unprecedented structural characteristics (for example, unique topologies, large specific surface area, high porosity and yet an adjustable structure), but also to their several advantageous properties compared to other functional materials, such as absorbance, luminescence, conductivity, magnetism, among others [
24,
25]. These advantages, together with the enormous variety of organic and inorganic components that can be judicious combined for the preparation of MOFs, as well as the relatively ease in the introduction of functional groups in the ligand, make the MOF compounds potentially applied in several areas of interest such as gas storage, adsorption [
26], luminescence, sensors, catalysis, therapy for various diseases such as cancer, water and air purification, and others [
27]. In particular, the heterogeneous catalysis is one of the first demonstrated and published applications and has become one of the most promising applications of MOF compounds, along with its rapid development in the last twenty years [
28].
Figure 7.
Schematic representation of some of the main potential applications of the MOF compounds in several distinct areas.
Figure 7.
Schematic representation of some of the main potential applications of the MOF compounds in several distinct areas.
Even though the enormous potential and advantages of the pristine / original MOF in several applications, most of this type of compounds have a major disadvantage, which is related to their limited stability, more specifically in the presence of moisture/water, acid solvents, bases, solutions or having coordinated anions [
29,
30]. Furthermore, most of the MOFs also have a lower thermal stability relatively to other pure inorganic materials, more specifically is between 350-400
oC [
31]. Thus, the preparation of MOFs with sites that have structural defects, and good stability has been extensively investigated, to improve some properties, in particular gas adsorption and catalytic performance [
32]. This improvement is frequently justified as consequence of an increased accessibility of active places for the metal centre, due to the controlled removal of some structural ligands [
33]. On the other hand, the improvement of catalytic activity can also be achieved by another process, not creating defects, but by preparing a composite called "metal@MOF" by impregnation of metals, polyoxometalates, nanoparticles, and others in the porous structure of the MOF [
34].
The systematic control of the properties of MOFs has enormous scientific importance and has attracted a lot of interest. A recent strategy to try to improve the properties of MOFs is to use defect engineering [
35]. For example, in MOFs whose main metal is Zr, as in the case of UiO-66 [
36] and MOF-808 [
37], the removal of some structural ligands has no drastic implications on their integral structure, but can increase their reactivity and catalytic activity [
38]. The structural defects in the MOFs can be introduced intentionally, to improve some specific property, in this case of catalytic activity. However, these defects can also occur naturally, in an unintentional way. Whether it occurs naturally or by deliberate formation, there are several factors that can be at the origin of these defects. One of them is related to fast crystallization times that usually originate materials with smaller crystals and with more defects in the network. Defects may also occur when a post-synthesis treatment with synthetic acids is performed and when crystallization modulating agents, such as acetic acid and formic acid, are used in the synthesis of MOFs. Furthermore, natural faults that may exist in the coordination between the ligand and the metal during the preparation of the MOF, can also contribute to the formation of defects in the metal center, although these types of defects have been difficult to predict and to evaluate quantitatively. However, it is possible to envisage that the nature and the number of structural defects may have extreme influence on structure stability at medium and long term. In fact, if the structural defects contributes to the decrease of stability in the MOF aging, it will have posterior implications in the potential areas of applicability of the MOFs, particularly in applications such as heterogeneous catalysis.[
39]
4. MOFs with Zirconium Centers
The porous MOF compounds prepared with Zr(IV) metallic centers, corresponds to one of the most investigated family of MOFs, frequently based on Zr−O clusters, which are interconnected by organic ligands. The main characteristics that have motivated the enormous scientific interest in this specific family of MOFs [
40] has associated with their several interesting advantageous properties relatively to other family of MOF with distinct metal centers, such as high porosity, excellent thermal and hydrolytic stability [
41], high specific surface area, [
42] and because they have an high catalytic potential as heterogeneous catalysts, as they can accommodate structural defects [for example UiO-66(Zr) modified to catalyze the CO
2 cycloaddition reaction; a new porphyrin Zr-MOF for heterogeneous catalysis of hetero-Diels–Alder cycloaddition reaction; UiO-66(Zr) encapsulating Pd nanoparticles for effective catalysis of the hydrogenation reaction of benzoic acid; various Zr-MOFs to catalytic conversion of furfural to furfuryl alcohol; MOF-808(Zr) for the acetalization of glycerol; and many others][
43] All the mentioned advantages make MOFs with Zr main metal candidates with a great potential to be applied in catalysis [
42]. In addition, these porous materials still generally have greater stability when compared to MOFs whose main metal is Zn, Cu, Co, or Cd [
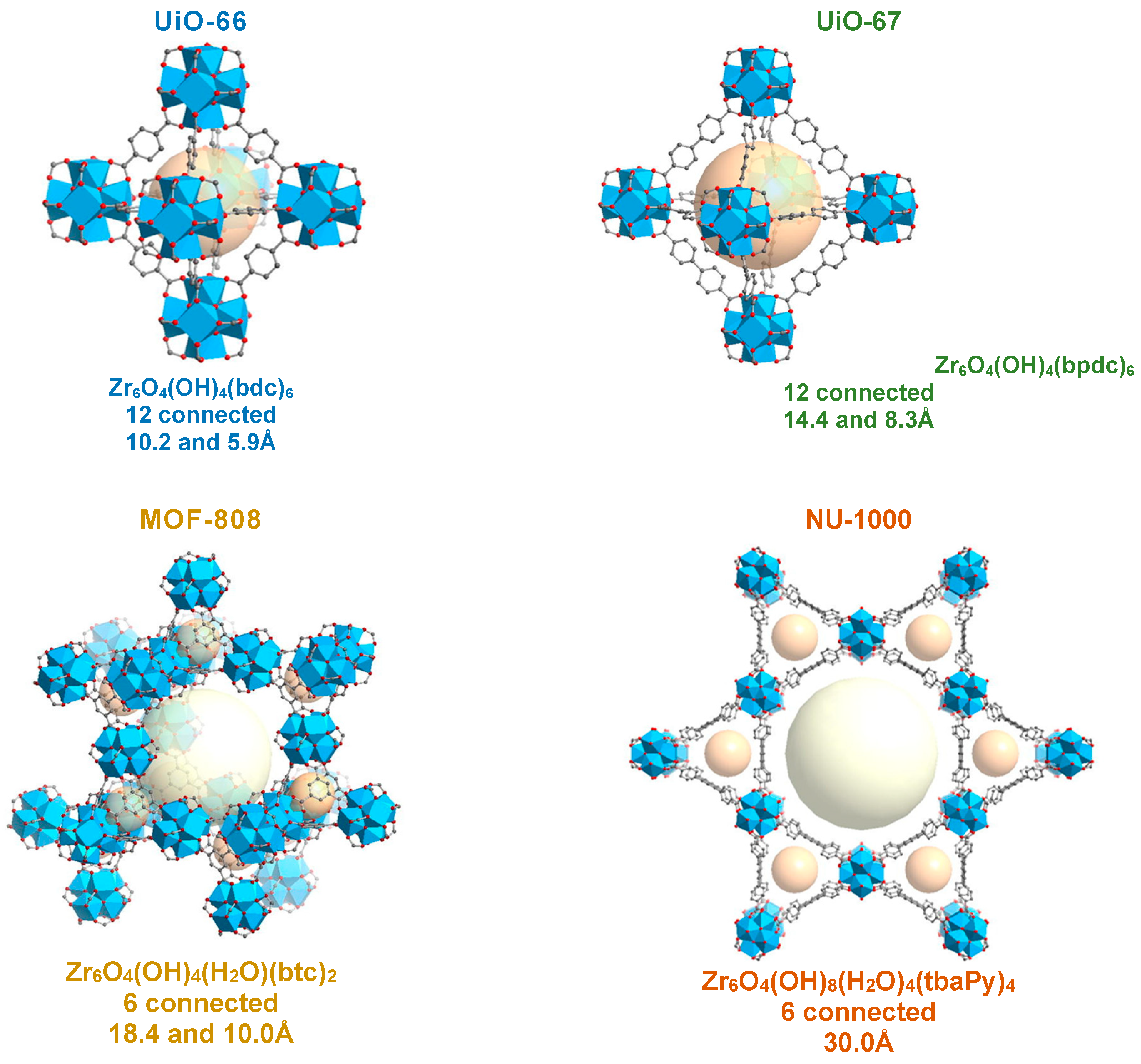
44]. Zr metal is easily found in nature and has a low toxicity, which further favors the development and application of this type of MOFs (a few examples of the most studied Zr-based MOFs are depicted in
Figure 8) [
23].
A considerable number of Zr-based MOF compounds are more stable in water and even in acidic solutions, due to the high oxidation state of Zr(IV), but also, if present, to the existence of strong coordination bonds between the Zr(IV) ions and the carboxylate ligands (Zr-O bonds), relatively to other metal centers [
44]. Considering the carboxylate-based Zr-MOFs, the hexanuclear clusters with the composition [Zr
6O
4(OH)
4]
12+ are the most frequently observed inorganic building units [
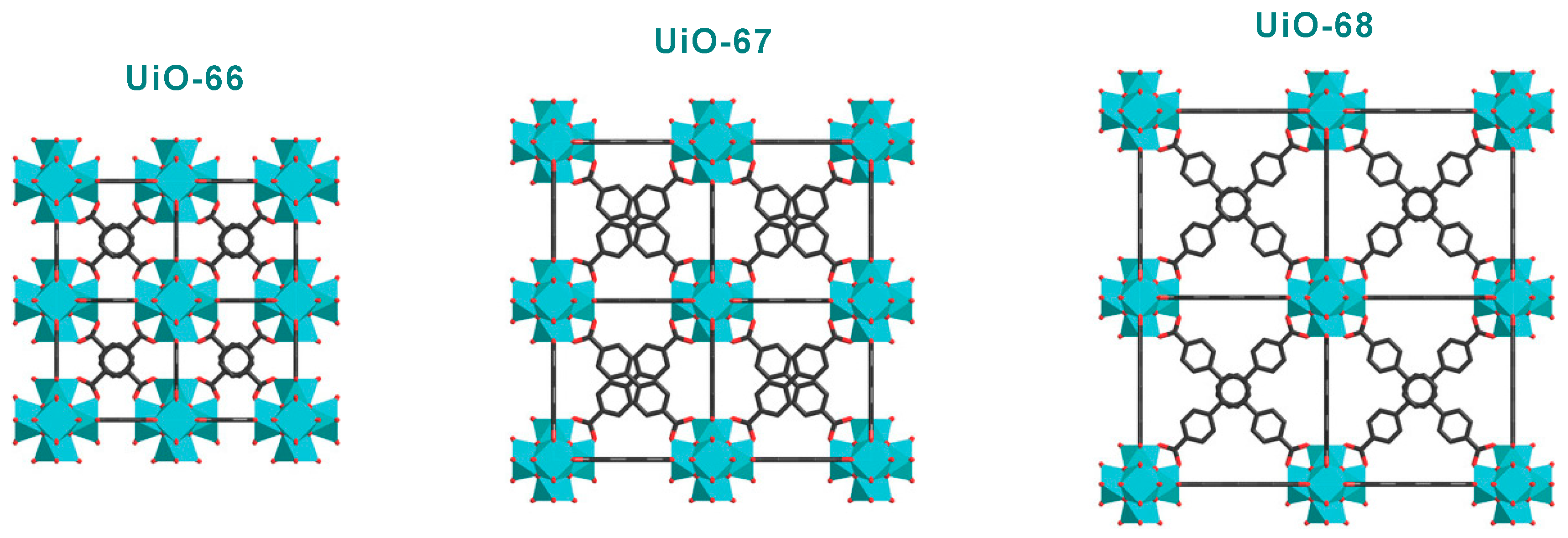
45]. The first members of this class of MOFs were designated by the UiO-66 series (with terephthalate as ligand), UiO-67 (with biphenyl dicarboxylate) and UiO-68 (with terphenyl dicarboxylate), and were reported by Cavka et al. in 2008 (UiO stands for University of Oslo) [
45]. These compounds are isostructural and have an arrangement of SBUs that is topologically like a cubic packaging. Thus, SBUs are twelve times connected to each other by dicarboxylic acids based ligands, giving it cooperative properties of high porosity and thermal, chemical and even mechanical stability (
Figure 9). [
29]
The MOF-808, also a Zr-based compound, has been envisaged as an interesting alternative to the UiO-66 with high potential in terms of applicability, in particular for catalytic processes. This MOF has aggregated Zr
6-oxo like those present in UiO-66, [Zr
6O
4(OH
)4]
12+ clusters, however, in the structure of MOF-808, each cluster is connected only by 6 trimesate ligands, and the other coordination positions of the Zr ions are saturated by ion-shaped molecules (
Figure 10). These molecules can be removed by simple solvent washing or gentle heat treatment (usually 60 – 70
oC), thus leaving two waves of coordination at each metal site [
29].
Comparing the Zr-based MOFs with others, for example those that are based on Zn, the higher stability of the former compounds may be due to the fact that Zr−O connections are stronger than Zn−O connections within SBUs, and to the high degree of interconnection of them [
49]. Nevertheless, it is particularly difficult to obtain single crystals and a regular crystalline morphology of Zr-based MOFs due to the inert coordination bonds between Zr
4+ cations and carboxylate anions, causing ligand exchange reactions quite slow, which has adverse consequences for the improvement of defects during crystal growth [
49]. In the preparation procedures of these Zr-based compounds, it is frequent to utilize of toxic solvents such as dimethylformamide (DMF). However, nowadays, it is already possible to obtain MOFs incorporating Zr centers, by applying "greener" synthesis routes, especially under aqueous conditions, microwave assisted synthesis, mechanochemical processes, among others. Research under new conditions of green synthesis is a promising focus in the area of research of MOFs, especially because the transition to an industrial scale synthesis would not be possible because of the use of hazardous chemicals under adverse reaction conditions [
45].
5. Derivatives of MOFs by Heat Treatment
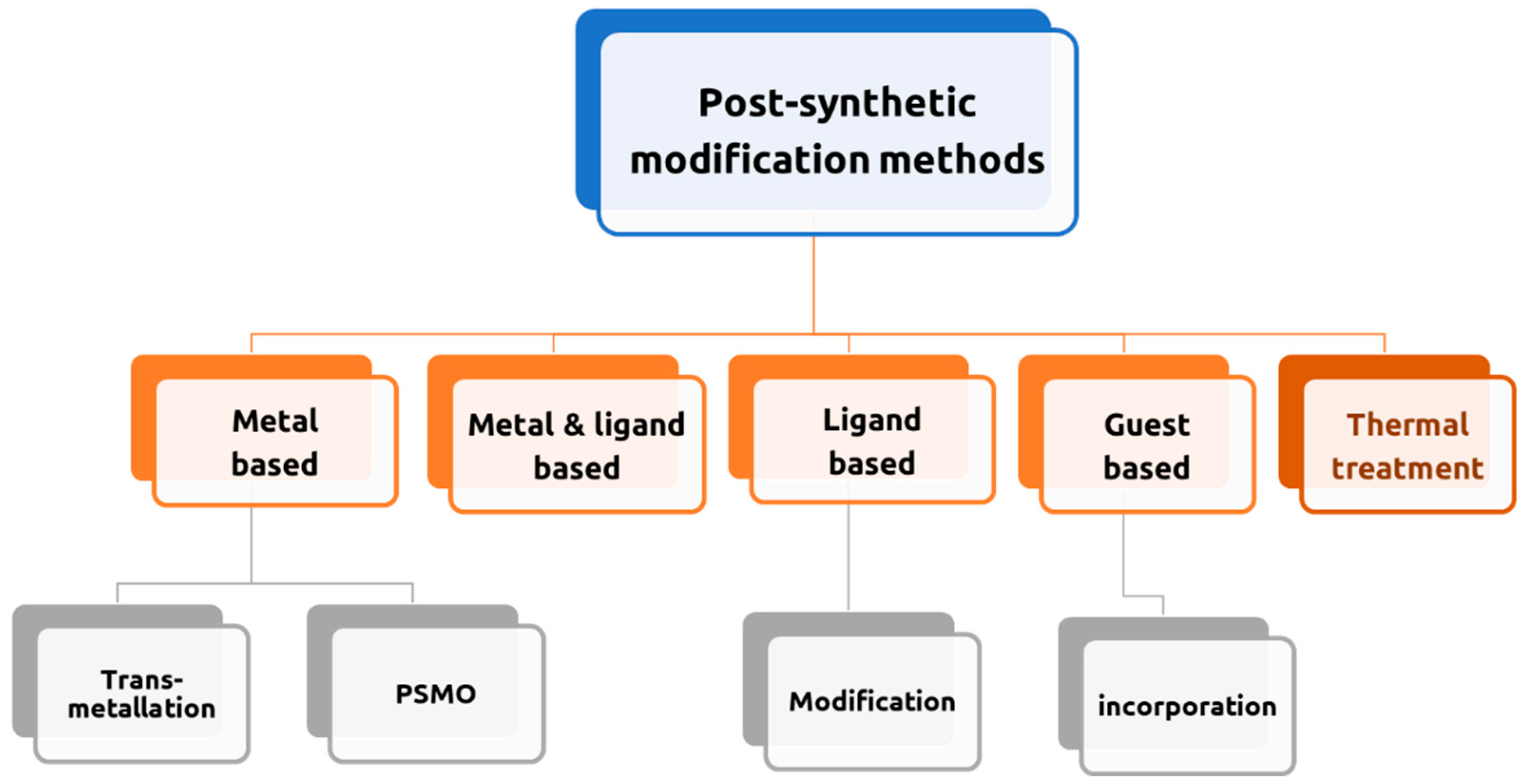
The post-synthetic modification (PSM) of MOFs has offered a workable approach for the creation of unanticipated product structures with a broad range of applications. PSM of MOFs is an important field that necessitates careful consideration in the planning and application of numerous techniques (
Figure 11).[
50] It is a viable and general strategy for creating new scaffolds with superior characteristics over the parent structures. The primary feature of this method is that most of the structures that are generated cannot be obtained via de novo synthesis. Due to the novel chemistry and physics characteristics that MOFs have installed, which have altered their chemical composition, there are now more options to explore a wide range of application areas.[
50]
The different types of stability (thermal, physical and chemical) of the MOF-based compounds need to be distinctively considered relatively to the area of potential applicability of the MOF. For example, chemical and thermal stabilities are particularly important in potential industrial applications, including gas separation, ion exchange, water desalination and energy storage. More specifically, the thermal stability is frequently one of the first to be evaluated, after synthesizing a MOF, because if this stability is high, it is an intended advantage. Interestingly, one of the most thermally stable MOFs is the UiO-66, which was mentioned earlier, which retains its crystallinity (solid state structure) and porosity up to about 500
oC. The thermal degradation of MOFs occurs mainly due to the breakage of the metal-ligand bond, accompanied, or followed by the combustion of the ligand itself. Consequently, thermal stability is generally related to the strength of this bond and the number of ligands that exist in MOF [
51]. Besides the chemical stability, the thermal stability of MOF compounds is another important factor for the success of the area where the MOF is inserted, because complete hybridization of these type of materials is usually achieved by a controlled heat treatment. Thus, understanding the thermal stability of MOFs and choosing a suitable temperature for heat treatment in the synthesis of hybrid nanostructures based on MOF have a fundamental relevance [
52].
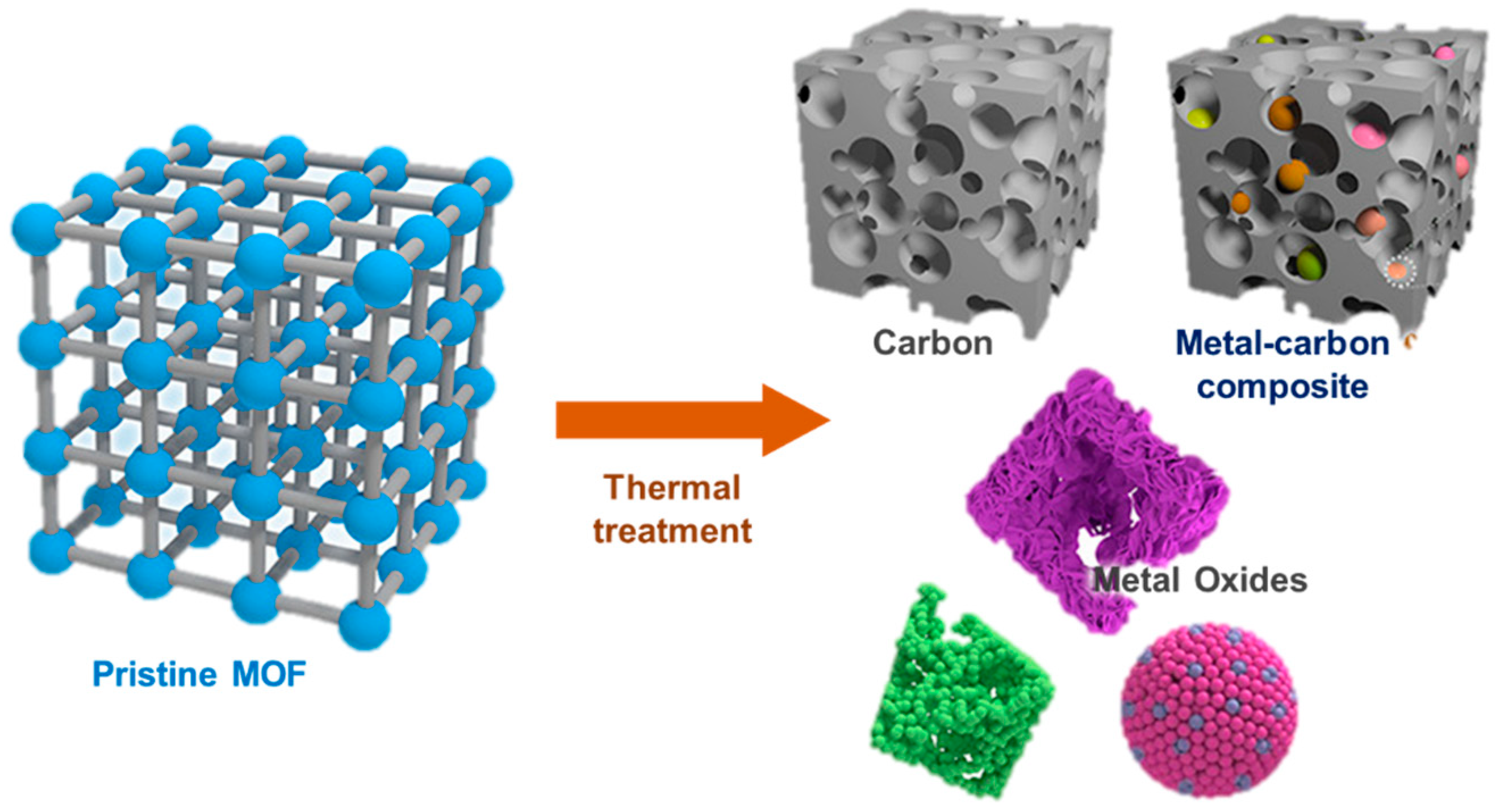
As mentioned before, several forms of post-synthesis treatment are in constant development, to optimize and modify the structure of MOFs, with the objective of enhance and improve the various characteristics of this type of compounds (
Figure 11). A recent and important post-synthesis approach is the controlled heat treatment of materials. MOFs are generally sensitive to heat treatment conditions, including atmosphere, weather and especially temperature. In general, these materials undergo three distinct stages as the temperature increases. In the first phase, the adsorbed water or solvents that may exist in the channels of the MOF compound are removed so that the pores are free (typically, from 60 to 200
oC). Subsequently, the coordination bonds are unstable and partially broken, however the crystallinity and porosity of the MOFs remain (usually, from 200 to 300/400
oC). Finally, in the last step, the structures of the MOFs are completely collapsed and crystallinity as well as porosity are lost (usually at temperatures superiors to 400400
oC). As a consequence, metal agglomerates or metals themselves will generally be transformed into oxides or hydroxides of these metals (
Figure 12) [
51].
In the last two decades there have been advances in the research of syntheses of metal-based nanomaterials and metal oxides using various chemical and biological methods [
54]. However, the synthesis of non-noble metal nanoparticles in pure form, without oxidation surface, is still quite difficult even with surfactant coatings [
55]. To avoid this problem of surface oxidation, the preparation of various metals and nano metal oxides/microstructures from MOFs were investigated, using various multi-step chemicals oxidation/reduction approaches which often lead to various impurities. For this reason, recently the pyrolysis/thermolysis of MOF compounds was tried to obtain several metal/metal oxide nanoparticles [
56]. Since the MOFs present a unique structure that presents ordered micro/mesopores and abundant organic ligands, these materials can be seen as promising candidates to be used as precursors to derive porous carbon with various morphologies through appropriate treatments [
57]. In addition, as the MOFs in their structure have metal ions or agglomerates, they allow obtaining metal oxides with large surface areas and porous structures under appropriate calcination conditions. These MOF-derived materials can subsequently present the advantages of MOFs, including morphology, high surface area and adjustable porosity, which are suitable for electrochemical, photoelectric, and catalytic applications [
58]. There has been a rapid development of the application of thermal decomposition of MOFs (as precursor materials) to prepare nano-metal oxides. This technique has been used in many sectors and has been used by numerous research groups to synthesis numerous metal oxides. By employing different types of MOFs, metal oxides with unique morphologies can be created. These metal oxides can prevent functional components from clumping together and offer a large number of nanopores, which improves the interaction between the metal oxides and noble metal nanoparticles.[
59]
The synthesis of MOFs derivatives can lead to the dispersion of metal/metal oxide nanocrystallites in the carbon structure derived from the carbonization of MOF organic ligands [
60]. It is interesting to note that the resulting carbon structure can prevent the aggregation of metal/metal oxide nanocrystallites. Through proper and controlled synthesis, a flexible design can be achieved by giving new functionalities to the nanostructure derived from MOF [
61]. The components of the derived MOF can be regulated by changing the temperature of the heat treatment and the heating rate. A high temperature and a rapid heating rate tends to contribute more to metal-carbon composites, and a low temperature and a slow heating rate usually lead to metal-carbon oxide composites [
62]. In this way, multiple carbon-based interfacial magnetic composites can be regulated by adjusting the heat treatment temperature and heating rate.
There is increasing interest in employing MOFs as templates for the production of porous carbons because of their customizable porosity and metal cores [
63]. Usually, direct calcination of MOFs in inert atmospheres such as N
2, Ar, or He yields MOF-derived carbons. The main advantage of carbonization for MOFs is that it increases their hydrolytic stability relative to their parent MOF, which enables them to be used in aqueous environments (typical temperatures of carbonization range from 600-1000ºC).[
64] Because of their adjustable architectures and enhanced stability, MOF-derived carbons have the potential to replace conventional porous carbons in applications like the degradation of chemical warfare agents and water remediation.[
65] The comparatively simple synthesis of graphitic carbon, which is well-known for its electrical conductivity, is another benefit of MOFs' organized structure. Consequently, there are numerous instances of MOF-derived carbons being used in electrochemical processes like the oxygen evolution reaction. Furthermore, to avoid sintering and preserve high chemical activity, it is desirable to scatter metallic nanoparticles throughout a carbon scaffold. MOF-derived carbons are superior at this because of their pre-dispersed metal SBUs.[
66]
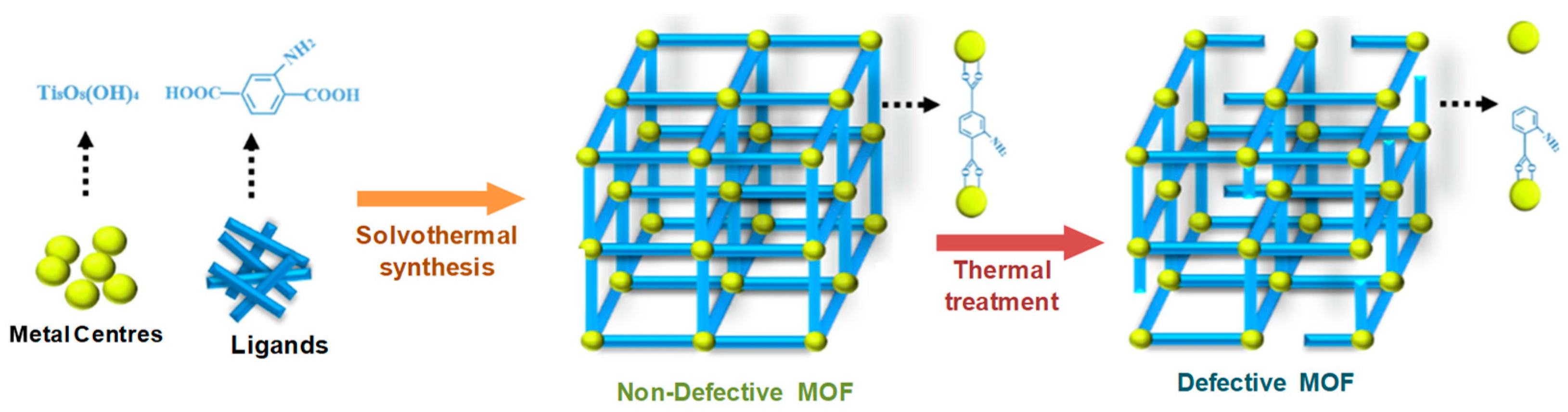
The activation of a MOF by heat treatment may influence the porosity of the material and consequently its properties. Therefore, it is essential to know its behavior at elevated temperatures because a thermal activation leads to high thermal stress, and thus may have a direct influence on its structure, and may even lead to the decomposition of MOF, if it reaches this temperature [
67]. In this way, the heat treatment can be seen as a process of modification of MOFs, by creating structural defects in the MOF (see a schematic representation in
Figure 13)
There are already reported in the literature numerous examples of MOF compounds that were subjected to heat treatment after their synthesis, and have been investigated for several applications, beside the catalysis.
When using pristine MOFs as anodic materials for lithium batteries, often when the MOFs have not undergone any treatment, these materials may have low conductivity and short life cycle. Therefore, these MOFs have been subjected to heat treatment, through pyrolysis in inert gas, and these derivatives MOFs have some advantages to the previous ones, since they present controllable chemical composition, adjustable porosity, and high surface area (thermal treatment at 800ºC). The MOFs most used in this case are MOF-805, ZIF-67 and ZIF-8 which present the 1,4-benzenedicarboxylic acid (H
2BDC) ligands, 1,3,5-benzenetricarbolic acid (H
3BTC) [
69]. Seung et al. demonstrated that ZnO nanoparticles and ZnO composites can be easily prepared by simple MOF-5 heat treatments under a variety of gaseous atmospheric conditions. Hierarchical aggregates of ZnO nanoparticles formed during heat treatment under air atmosphere, and ZnO@C composites with high specific surface areas were produced under nitrogen atmosphere. Interestingly, ZnO nanoparticles derived from MOF exhibited high photocatalytic degradation of Rhodamine-B (RhB) under ultraviolet (UV) irradiation comparable to degradation by P25 (commercial TiO
2). ZnO and ZnO@C composites derived from MOF can potentially be used to remediate organic pollution in aquatic and air environments. [
70]
6. MOF-Derived Compounds as Catalysts
Catalysts are identified as possible entities that can be used to accelerate the reaction rate and alter the path of a chemical reaction without being consumed [
71]. MOFs, nowadays, have a great scientific interest, when they are applied as catalysts, mainly due to their porous 3D structures with large, regular and accessible cages capable of incorporating catalytic active molecules, with suitable shapes and sizes [
72]. On the other hand, the pores of MOFs can also act as individual reactors, since, in addition to the active catalytic species, other molecules involved in catalysis can also be incorporated into their cavities [
73]. Considering and analyzing the structure of the MOF compounds it can be envisaged that there are active sites uniformly dispersed throughout the structure, and the characteristic porosity of this type of chemical materials tends to facilitate the access of active sites and the transport of substrates/catalytic products in MOFs (
Figure 14). Therefore, MOFs can behave as catalysts identical to discrete metallic complexes and still have some advantages of homogeneous catalysis. Furthermore, because they are considerable stable porous solid materials, it is possible to recycle them during several catalytic cycles, a characteristic associated with heterogeneous catalysts. Thus, MOFs are a type of catalysts that have both advantages and characteristics of homogeneous and heterogeneous catalysts [
28]. In fact, they have been explored as potential catalyst in a wide range of different reactions including carbon monoxide oxidation, hydrogen peroxide decomposition, benzyl alcohol oxidation, oxidation of cyclooctane and linear hydrocarbons, Friedel–Crafts alkylation, acetalization of glycerol, catalyst for oxidative desulfurization, and many others. The potential of MOF materials as heterogeneous catalysts is well documented in several recent review publications. [
74,
75,
76,
77]
The pores of MOFs are able to accommodate a wide diversity of additional active species (e.g., gases, liquids, organic molecules and inorganic nanoparticles, metal complexes, enzymes, poms) and thus the MOF behaves as a nanoreactor that can participate in catalytic reactions [
28]. Since MOF compounds reveal advantageous characteristics of heterogeneous and homogeneous catalysts, they have several benefits and improvements compared to existing traditional catalysts, such as: high dispersion and isolation of active sites that increase areas of use; a wide diversity of functional groups and/or active sites that can be integrated into the MOF for applications such as catalysis; high porosity and high surface area help improve access to catalytic sites and substrate concentration, as well as a controllable pore size and a stable internal environment adjusted around the active sites, which is advantageous for the reaction activity and/or its selectivity [
28]. In addition, the possibility of introducing structural defects in MOF structures and the preparation of a wide range of MOF derivatives, such as using thermal treatment, enlarge considerably the potential of these compounds as catalysts.
Defective Zr-Based MOFs for Catalysis
Defect engineering of MOFs is an innovative way to tailor the properties of these materials, offering new opportunities not only for adsorption and catalysis [
79]. There are some MOFs that rely on the formation of defects in the ligand to increase their activity as catalysts, and the Zr-based MOFs have been specially investigated with this strategy [
80].
One of the examples portrayed in the literature consisted of preparing MOF-808 materials through defect engineering by a mixed ligand approach. Thus, tritopic trimesic acid ligands were combined with a small amount (about 10%) of a ditopic ligand, such as isophthalic acid, pyridine-3,5-dicarboxylic acid or 5-aminoisophthalic acid so that it is possible to prepare a series of mixed ligand MOFs. It was demonstrated that this strategy increases the availability of open metal sites in Cu and Ru trimesate compounds, which translated into a considerable improvement in the catalytic properties of MOFs with defects compared to the original compound [
80,
81,
82].
In another example, with the aim of further increase the availability of open Zr sites in MOF-808, a new competitive coordination removal strategy was presented in an attempt to increase ligand defects in the MOF-808 structure. One type of defective MOF-808 (Dx-MOF-808) samples were prepared by introducing benzoic acid (HBC) to compete with trimesic acid (H
3BTC) to coordinate with metal clusters and then removing the HBC by washing with methanol at room temperature. Different ratios of HBC/H
3BTC could adjust the amounts of exposed Zr sites as well as the degree of ligand deficiency in Dx-MOF-808 samples. The results showed that the catalytic activity is closely correlated with the number of exposed Zr sites, the degree of ligand deficiency, and the specific surface areas of the Dx-MOF-808 samples [
83].
MOF-808(Zr) is generally synthesized by a solvothermal route, where formic acid is required and usually requires a post-treatment process to remove formate ligands and create defect sites [
84]. Recently, MOF-808(Zr) was confirmed as a promising catalyst in oxidative desulfurization (ODS) which is considered as a supplementary or alternative method for industrial hydrodesulfurization due to its superior ability to eliminate refractory compounds containing aromatic sulfur with simple and economical operation. Most processes require an acid-treated process to expose the active sites, although high reaction temperature or organic oxidant is still required to obtain considerable ODS efficiency or can only remove dibenzothiophene (DBT) in the model oil [
85]. In this way, an in situ green route was developed to synthesize defective MOF-808(Zr) with rich open metal sites and hierarchical porosity without the help of formic acid and solvent. This MOF could exhibit good thermal stability and provide superior ODS activity to remove 1000 ppm sulfur from DBT and 4,6-dimethyldibenzothiophene (4,6-DMDBT) in 20 min at room temperature [
86].
The defects in the UiO-66 structure are mainly of two types, ligand defects and missing cluster defects, which are respectively, omission of some ligands and clusters from the perfect crystallographic structure [
87]. Lejaeghere et al. reported that, in addition to changing the ligand, the defects were able to provide an alternative path to transform UiO-66 in the field of photocatalysis by theoretical calculations [
88]. Recently, Jiang et al. experimentally verified that the structural defects in UiO-66-NH
2 were able to improve the production of photocatalyzed hydrogen by Pt@UiO-66-NH
2 in CH
3CN-H
2O solution [
89].
Thermally Modified MOFs for Catalysis
The post-synthesis treatment of MOFs has been investigated over the last years and has been scientifically considered as a versatile method either to functionalize the MOFs, adjusting their structure and morphologies, or optimizing the active sites and improving the catalytic performance. The methods of post-synthesis treatment can be chemical, where there is metal exchange or ligand, or physical by controlled thermal treatment where MOF compounds are exposed at elevated temperatures. This physical method tends to modify the properties of materials, such as hydrophilicity and hydrophobicity, can introduce active structural defects, and regulate the size of MOF particles. All these changes can introduce improvements in the catalytic performance of the MOF compound, as demonstrated by some examples reported below.
It is reported that the post-synthetic thermal treatment of UiO-66(Zr) structures is typically used to activate the material by removing the solvent remaining in the porous structure. At temperatures between 250–300 °C, the structure is dehydroxylated, and the central cluster is changed to Zr
6O
6 as consequence of the loss of two water molecules. However, Vandichel et al. claimed that increasing the temperature of the framework to the dehydroxylation temperature (around 300 °C) not only causes the loss of a water molecule but also originates the formation of defective oxygen sites and changes the Zr atom’s coordination number from 8 to 7.[
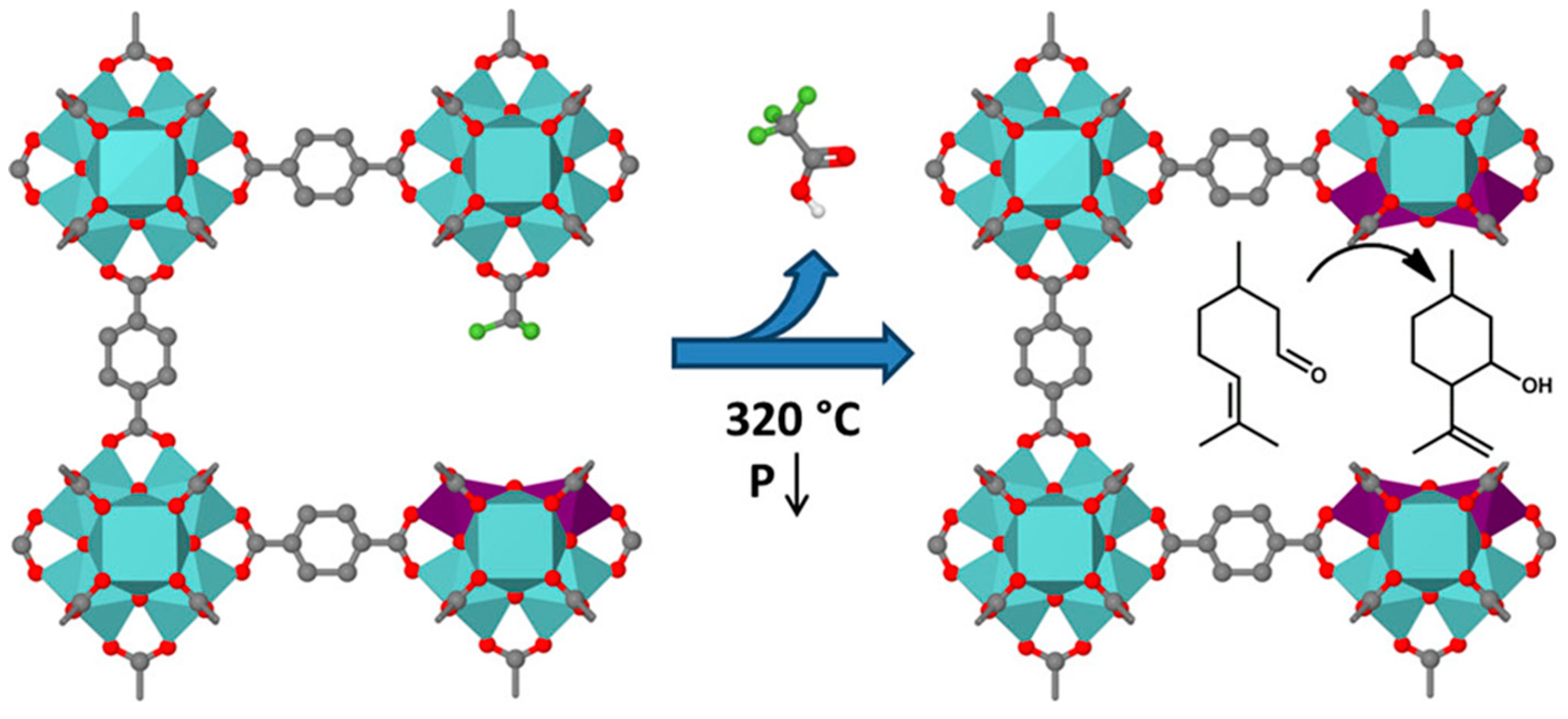
90] In fact, thermal treatment / activation can be more effective as a defect engineering method when coupled with modulation synthesis. Vermoortele et al. have reported this approach by combining HCl and trifluoroacetic acid as modulators in the synthesis of UiO-66(Zr), that resulted in a highly crystalline material, with partial substitution of terephthalates by trifluoroacetate. The subsequent thermal treatment of the material leads not only to dehydroxylation of the hexanuclear Zr cluster but also to post-synthetic removal of the trifluoroacetate groups, creating a highly porous and open structure rich in open Zr metal sites (
Figure 15), drastically improving the catalytic activity of UiO-66(Zr) in a couple of Lewis acid catalyzed reactions: citronellal cyclization and Meerwein-Ponndorf-Verley reduction.[
79,
91]
In 2014, Gadipelli and co-workers submitted MOF-5 to 380 C, which induced structural defects through partial decarboxylation of the ligands. This structure modification was achieved, by application of a well-careful route of thermal annealing of MOF structures over the intermediate temperature window slightly above that for normal outgassing of as-synthesized MOFs to remove pore-occluded guest solvent molecules, but below that for complete framework decomposition/carbonization.[
92] It was later proved that the thermal activation of UiO-66 led not only to dehydroxylation of Zr-O clusters, but also to the removal of the modifiers of terephthalate ligands. The Lewis acid sites that were created made UiO-66 with structural defects much more active for several catalytic Lewis acid reactions [
93].
Zhao et al. performed the synthesis of MIL-101(Cr) free of HF and used it for the removal of Hg. These altered methods did not use hydrofluoric acid, leading to a less dangerous synthesis protocol and the thermal treatment of the MIL-101(Cr) improved the catalytic performance.[
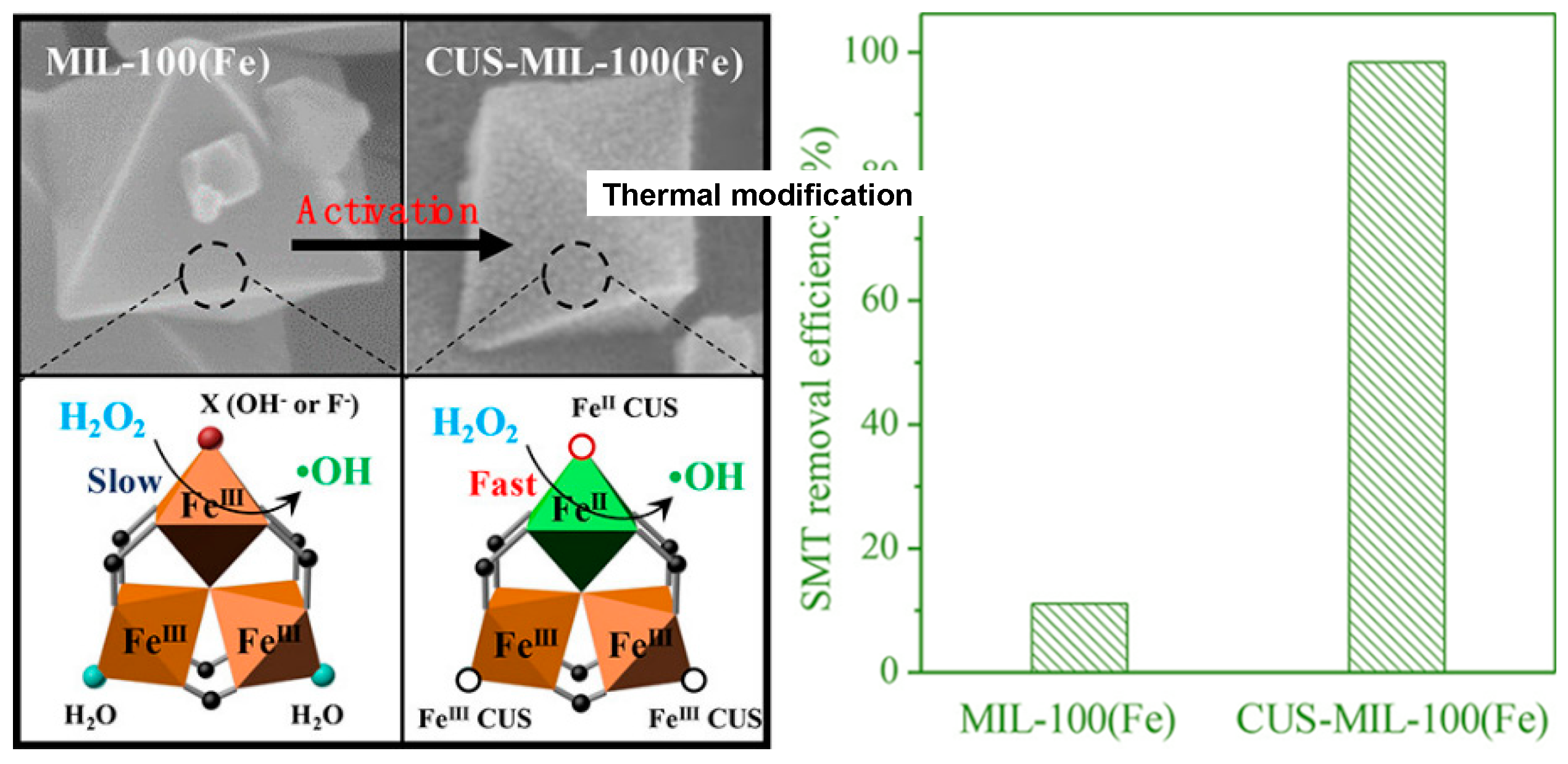
94] Interestingly, the controlled post-synthetic thermal treatment of the metal-organic framework MIL-100(Fe
III) (under vacuum conditions at 230 °C for 12 hours) originated Fe
II/Fe
III mixed-valence coordinatively unsaturated iron center (CUS-MIL-100(Fe)) (
Figure 16).[
95] This thermal modified MOF-based material revealed notable catalytic activity in the degradation of sulfamethazine. It could effectively degrade sulfamethazine, with almost 100% efficiency within 180 min, contrasting with only the 10% obtained with the pristine MIL-100(Fe
III). The enhanced catalytic activity can be ascribed to the incorporation of Fe
II and Fe
III sites, the large surface area, as well as the formation of mesopores, induced by the thermal treatment. Furthermore, CUS-MIL-100(Fe) exhibited a good stability and reusability.[
95]
The morphology of the metallic oxide prepared by pyrolysis (extreme thermal treatment) of MOFs tends to acquire the morphology and specific surface area of the pristine MOF. Through thermal decomposition, MnOx mesoporous spherical nanoparticles were prepared using Mn-MOF as precursor [
96]. The structure of MnOx particles was modified, changing the conditions of thermal decomposition. By thermal decomposition of three different coordinates: Cu-MOFs, porous heterostructures CuO / Cu
2O, pure phase CuO and Cu
2O were synthesized respectively. According to the study, the CuO/Cu
2O heterostructure had a larger pore volume, a larger BET specific surface area, stronger acidity, and more Lewis acid sites than CuO or pure Cu
2O. Peng et al. compared the catalytic effect of CuO prepared by the thermal decomposition method and co-precipitation method [
97]. The specific surface area of CuO-p (15.4m2/g) obtained by pyrolysis of HKUST-1 was much larger than that of CuO-c (3.4m2/g) produced by coprecipitation. Ce-BTC was used as a precursor to synthesize three-dimensional penetrating mesoporous CeO
2 for combustion of toluene [
98]. Similarly, Li et al. reported a porous structure Co
3O
4 polyhedron by direct pyrolysis of the ZIF-67 crystal in air. [
99]
Karam et al. prepared an alumina-based MOF (MIL-53) as a model to produce a nickel-alumina catalyst for DRM reactions. A two-step heat treatment was implemented in this material after its synthesis; calcination at 500
oC to remove the organic ligands with subsequent reduction at 800
oC to produce an active nickel phase. It was subsequently verified with this material derived from MOF by heat treatment that this catalyst is quite stable and active at a reaction temperature of 650
oC, without loss in activity after 100 h in flow. The use of the MOF model created a catalyst with strong metal-support interaction (SMSI) between nickel and alumina and presents a relatively high surface area [
100]. In addition, Chin et al. prepared a catalyst derived from nickel-ceria MOFs grown on NH
2-MIL-88 alumina using a solvothermal method. Unlike Karam et al. [
25], to activate the metallic sites of the catalyst, the calcination phase was bypassed, and the catalyst was reduced to 500
oC after synthesis, in a single step. The results showed a highly dispersed metallic nanoparticle [
101]. On the other hand, Khan et al. prepared bimetallic Ni-Co catalysts using CPO-27/MOF-74 MOFs but using a two-step reduction aproach. After the synthesis, the MOFs were treated at to 750
oC for 8 h under an uninterrupted flow of N
2 to remove the organic binder and promote carbonization. Subsequently, the sample was passivated under 5% oxygen in N
2 flow. The bimetallic Ni-Co@CMOF-74 catalyst derived from MOFs showed better catalytic activity (reaction of dry reforming of methane) in comparison with monometallic materials due to the synergistic effect of Ni and Co that impedes the coke formation. To be more specific, stable performance for at least 10 h at 700 °C, 5 bar and 33 L·h
−1·g
−1 was found for the Ni-Co@CMOF-74 catalyst in contrast with the fast deactivation observed for the monometallic counterparts.[
102]. The reaction of CO
2 fixation using epoxides has been thoroughly studied using composites produced from post-synthetic thermal treatment of MOFs. Bifunctional acid–base catalysts were successfully created by Toyao et al. through direct pyrolysis of ZIFs (ZIF-7, -8, -9, and -67) at various temperatures.[
103] The materials containing Co NPs and N species have the maximum catalytic activity when it comes to converting CO
2 and epoxides into cyclic carbonates at 80°C under 0.6 MPa of CO
2. These materials can function as acid and base sites independently.
The pyrolysis of MOFs uses these structures as a model and can be also an interesting and promising way to synthesize catalysts for the carbon-based oxygen reduction reaction (ORR). Some factors such as size, shape and composition of the pyrolysis product can be controlled by choosing, for example, precursors based on MOFs, and still trying to adjust some parameters of the pyrolysis. Some MOFs that are usually used in ORR catalysis have been prepared by thermal treatment of ZIF-8 and ZIF-67 [
104].
7. Concluding Remarks and Future Perspectives
The design, preparation and application of MOF compound as potential catalysts have been extremely vigorous in the last two decades, resulting in many interesting results in different catalytic processes, including heterogeneous catalysis, electrocatalysis and photocatalysis. This family of compounds has been investigated as potential hetero catalysts in a large number of different reactions including carbon monoxide oxidation, hydrogen peroxide decomposition, benzyl alcohol oxidation, hydrogenations, oxidation of cyclooctane and linear hydrocarbons, Friedel–Crafts alkylation, acetalization of glycerol, catalyst for oxidative desulfurization, oxygen evolution reaction, oxygen reduction reactions, among many others. In fact, despite the considerable advance of knowledge in the subject of MOF compounds for catalysis, there are many questions that remain unclear concerning the detailed role and specify function of the MOF as catalysts. Even though most of the MOF compounds have well-defined bulk structures, their catalytically active sites frequently remain to be clearly recognized, in particular the defect sites. In the next future, it will be essential to deepen studies in order to unequivocally recognize such sites and to improve the control of the synthesis procedures enhancing the reproducibility of the defect’s sites in the MOF compounds. The precise quantification of these defected sites it will be very important for quantitative evaluation of defective MOF catalyst performance, both in terms of activity, selectivity, and reutilization / recyclability. Furthermore, a considerable number of MOF compounds reveal a special challenge in terms of stability in catalysis, particularly in more severe reaction conditions. Even though some MOFs have been shown to maintain their structural integrity to temperatures up to as 350-400 °C, it is frequently difficult to apply these compounds as efficient catalysts at extreme conditions (such high temperatures and high pressures), as consequence of their stability limitations and regeneration associated to the organic components (ligand or linkers). As reported in this review article a post-synthesis thermal treatment strategy has been successfully applied to the pristine MOF compounds to prepare MOF-derivatives with modified and optimized structures, improving their structural stability and catalytic performance, relatively to the pristine MOF. As example, the controlled post-synthetic thermal treatment of Zr-based MOF UiO-66 structures is typically used to activate the material by removing the solvent remaining in the porous structure; at temperatures between 250–300 °C, the structure is dehydroxylated, and the central cluster is changed to as consequence of the loss of coordinated water molecules; furthermore, the increasing of the temperature slightly above 300 °C also originates the formation of defective oxygen sites and changes the Zr-center coordination number from 8 to 7. In fact, there are numerous issues related with the “modus operandi” of the thermal treatment of MOFs that need to be better clarified and understood, namely the influence of the thermal treatment parameters (temperature, treatment time, type of atmosphere and others) in the structure of MOF-derived materials and their catalytic performance. Such fundamental understanding will be the driving force for the next step toward the industrial and technological applications of MOF compounds and MOF-derived materials.