1. Introduction
The tacticity of polymers plays an important role in many industrial processes. Indeed, polymer crystallinity and many macroscopic properties such as the density, glass and melting temperatures, clarity, and stiffness of a polymer depend on it. Furthermore, the determination of polymer tacticity is of crucial interest in the knowledge of polymerization mechanism [
1]. The stereochemistry in polymers has been used to influence and control their physical and mechanical properties, as well as begin to control their function. Several studies were devoted to the effect of stereochemistry on mechanical properties, biodegradation, conductivity and in applications of stereodefined polymers for enantioseparation and as supports for catalysts in asymmetric transformations [
2].
A tactic polymer is defined as ‘a regular polymer, the molecules of which can be described in terms of only one species of configurational repeating unit in a single sequential arrangement [
3]. Tacticity then refers to the relative spatial arrangement of substituents along a polymer chain. At that time, three main types of tacticities were distinguished: isotactic, syndiotactic and atactic [
1].
It is well-known that the atactic polymers have their pendant groups organized randomly around the principal linear chain of the polymers. They lead to form amorphous and soft materials. Whereas, the syndiotactic polymers are characterized by their pendant groups organized in alternating ways around the principal chain by forming crystal structures. The Tacticity of polymers affects the physical and chemical properties of polymeric materials [
4,
5,
6,
7,
8,
9,
10,
11,
12]. The isotactic polymers are characterized by pendant groups spatially located on the same side of the principal chain. The crystallinity of polymers strongly depends on their tacticity. In general, the syndiotactic polymers form the most rigid, crystalline structures, while, isotactic polymers exhibit semi-crystalline structure and an amorphous form for atactic polymers. This affects the thermos-mechanic properties of polymers. For example, the highest melting points are obtained with the syndiotactic polymers.
Izzo et al. [
4] investigated the effect of polymer tacticity on physico-chemical and biological properties relevant to the use of polymer therapeutics using poly(methacrylic acid) (PMA) as a model polymer. They studied the physicochemical behavior in aqueous solution of atactic and syndiotactic PMAs obtained from hydrolysis of PMMAs.
Grigoriadi et al. [
5] studied the effect of tacticity on the ageing kinetics of glassy amorphous polymers at high and low ageing temperatures for atactic, isotactic, and syndiotactic polystyrene using flash-DSC in their glassy state.
The spatial configuration of polymers can be modified by the various parameters of their physicochemical properties. Chat et al. [
6] used the dielectric spectroscopy (DS) to study the effect of the tacticity on the glass-transition dynamics of confined polymer films [
7].
Several studies were interested in the determination of the tacticity effect on the properties of polymers [
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
22,
23,
24,
25,
26]. The polymer tacticity gives information on the arrangement of atoms and/or groups of atoms on the polymer backbone [
6]. For poly(methyl methacrylate) (PMMA) and poly(ethyl methacrylates) (PEMA), it has been observed that different spatial configurations of the polymers are characterized by the various relaxation times and the glass-transition temperatures [
16,
17,
18,
27,
28,
29].
Hasan et al. [
31] investigated the influence of tacticity of the structure formation of Poly(methacrylic acid) by defining an isotactic polymer as consisting only of meso diads (
m), with two adjacent configurational repeating units with identical orientation, whereas a syndiotactic polymer is made up of only racemo diads (
r), with two adjacent configurational repeating units with the opposite orientation, while an atactic polymer contains both
m and
r diads in a random sequence. With this definition, the tacticity can be further described even more precisely with the introduction of higher-order sequences than diads such, tetrads, and pentads [
31].
Zhang et al. [
32] considering that the tacticity of polymers is one of the governing microstructural parameters that determines their material properties, used the tacticity engineering to study the thermo-responsive, adhesion and electrical properties of homopolymers by tuning tacticity without compositional variance.
The physical properties of polymer like solubility, mechanical properties, thermal stability, etc. can be affected by the stereoregularity in polymer. Biswas et al. [
33] investigated the effect of some physical properties of high molecular weight linear poly(
N-isopropylacrylamide)s (PNIPAM) having different isotacticities (
m, meso dyad = 47, 62, 68, 81, and 88 %). The effect of tacticity on the transition temperatures were studied by several scientists [
33,
34,
35,
36,
37,
38,
39,
40,
41,
42,
43].
Nevertheless, we did not find any study in the literature concerning the influence of the tacticity on the surface properties of polymers such as their London dispersive energy, Lewis’s acid-base parameters and acid and base polar surface energies. This paper is interested in filling this gap in this research area by studying the effect of the tacticity of poly(methyl methacrylate) on the dispersive component of the surface energy of polymer, the polar and dispersive free energies as well as the various surface variables relative to Lewis’s acid-base surface properties.
The technique used in this study is the inverse gas chromatography at infinite dilution that was proved to be a very interesting technique capable to examine the surface properties of solid substrates and to quantify the various surface parameters of interaction between polymers and the adsorbed organic solvents by varying the temperature [
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65].
Different chromatographic methods and molecular models were used in the literature to characterize the physicochemical properties of solid surfaces [
39,
45,
46,
47,
48,
49,
50]. We applied the new method recently developed that utilized the thermal model [
51,
53,
58,
59] to determine the London dispersive energy and used the London dispersion equation [
56,
57] based on the deformation polarizability
of the probe and the ionization energies of the solid
and the solvent
with a new chromatographic thermodynamic parameter
. By using the thermal model, we compared between the dispersive surface energy of atactic, isotactic and syndiotactic PMMA and by applying the parameter
, we determined the polar and dispersive free interaction energy of organic solvents adsorbed on the polymer surfaces, and the Lewis’s acid-base surface energy and parameters as a function of the temperature.
2. Methods and Materials
The inverse gas chromatography (IGC) at infinite dilution [
44,
45,
46,
47,
48,
49,
50,
51,
52,
53,
54,
55,
56,
57,
58,
59,
60,
61,
62,
63,
64,
65] was used to characterize the surface physicochemical properties of PMMA at different tacticities. IGC technique allowed obtaining the experimental values of the net retention time
and volume
of polar and non-polar adsorbed solvents on the different PMMAs as a function of the temperature. The free energy of adsorption
of adsorbed molecules on PMMA surfaces was therefore obtained from Equation 1:
Where T is the absolute temperature of the chromatographic column containing the solid material, R the perfect gas constant, and a constant volume depending on the temperature and reference characteristics referred to the two-dimensional state of adsorbed film.
In the case of polar molecules adsorbed on the solid surfaces, two contributions of the free energy of adsorption
were distinguished: the London dispersion component
and the polar or specific component
(Equation 2)
By using our new method recently developed [
56,
57] that consisted in the separation of the London dispersive and polar components of the free surface, it was possible to obtain the London dispersion energy
:
Where and is the Avogadro’s number, the permittivity of vacuum, S denoting the solid particle and X the solvent molecule separated by a distance , and and are the respective ionization energies of the solid and the solvent .
The new method proposed to use the new parameter of interaction
given by
In the case of n-alkanes
adsorbed on PMMA, one obtained:
Where
and B are given by:
The polar free energy
of polar organic solvents adsorbed on PMMA was obtained from the experimental straight-line of n-alkanes (Equation 7] representing the variations of
against
for different temperatures:
The use of Equations 8 and 9 led to the determination of the polar enthalpy
and entropy
of organic solvents adsorbed on PMMA:
The Lewis’s enthalpic acid base constants
and
, and entropic acid base parameters
and
were obtained from the expressions of of
and
:
Where
AN and
DN are respectively the Gutmann electron donor and acceptor numbers of the polar solvents [
66]. The used values were those corrected by Riddle and Fowkes [
67].
Two kinds of probe molecules were used:
- -
Non-polar molecules such as the n-alkanes () such as n-pentane (), n-hexane (), n-heptane (), n-octane (), and n-nonane ()
- -
-
Polar molecules, divided into three groups:
- ➢
Lewis’s acid solvents such as dichloromethane, chloroform, and carbon tetrachloride
- ➢
Basic solvent such as ethyl acetate, diethyl ether, tetrahydrofuran (THF)
- ➢
Amphoteric molecule such as toluene
The isotactic (i), syndiotactic (s) and atactic (a) PMMAs used in this paper, were the same as the polymers previously characterized in other studies by using other classic chromatographic methods and models [
34,
36,
38,
39,
68]. The previous experimental values of
of the various n-alkanes and polar molecules adsorbed on i-PMMa, s-PMMA and a-PMMA, obtained from chromatographic measurements, were used to investigate the tacticity effect on the surface physicochemical properties of the different PMMA surfaces.
3. Experimental Results
3.1. London Dispersive Component of Surface Energy of PMMA Polymers
In previous papers [
51,
54,
55,
56,
57,
58,
69], a new methodology on the determination of the accurate value of the London dispersive surface energy
of materials was proposed. It is based on the thermal effect on the surface areas of organic molecules that are necessary to be know as a function of the temperature to obtain accurate values of
of solid surfaces.
In this section, the same procedure previously developed [
51,
54,
55,
56,
57,
58,
69] was used to determine the
of the different PMMAs. The determination of the net retention volumes of the various organic molecules adsorbed on i-PMMA, s-PMMA and a-PMMA allowed obtaining
of such molecules. The obtained results of
were given in
Table S1 ((
Supplementary Materials).
The Hamieh thermal model [
51,
54,
55,
56,
57,
58,
69] was applied to determine
the various PMMA surfaces.
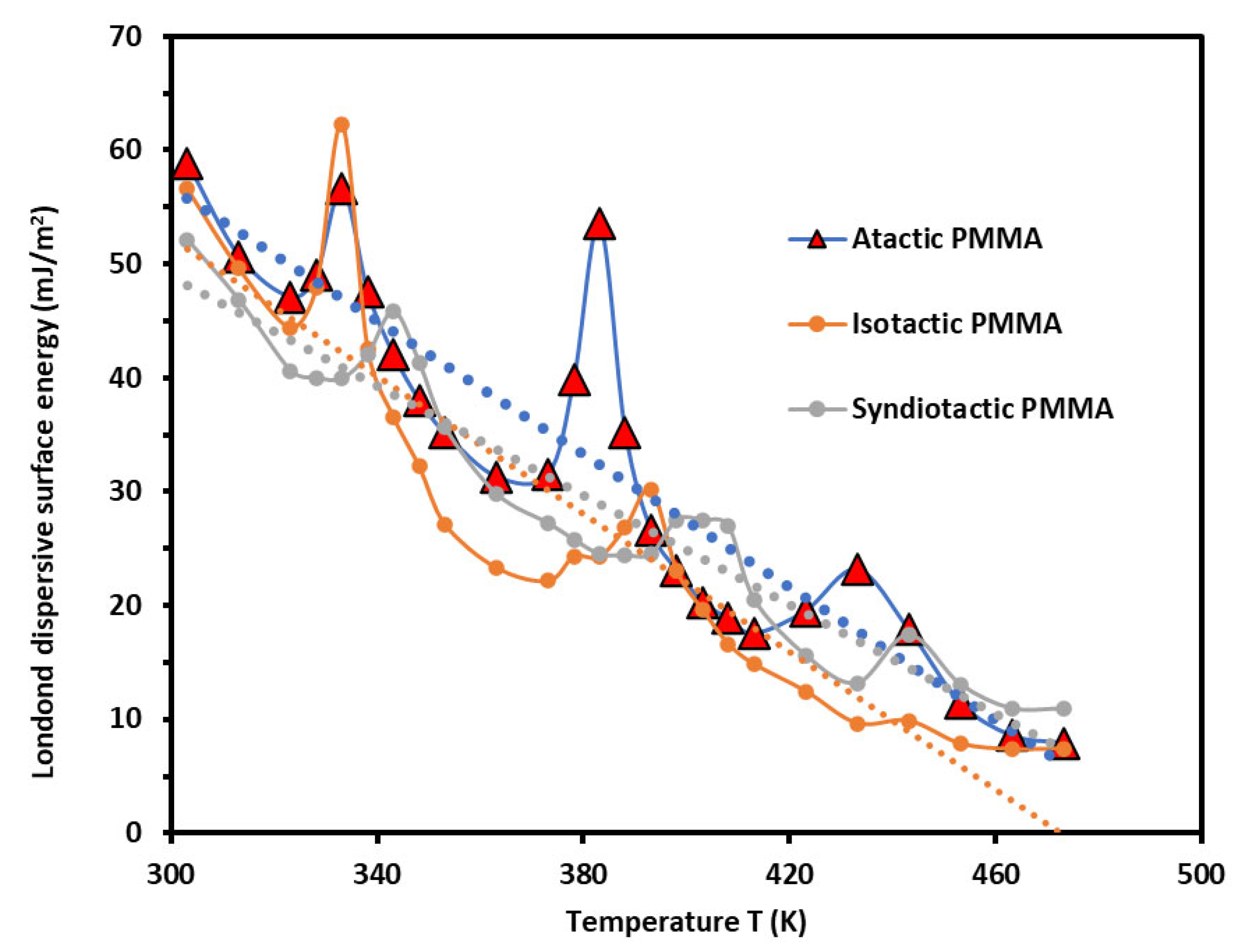
Figure 1 gave the variations of
of PMMA at different tacticities as a function of the temperature was plotted on
Figure 1.
The curves in
Figure 1 showed a non-linear evolution of
, contrary to other cases relative to oxides and other solid materials [
51,
54,
55,
56,
57,
58] where the linearity of
was clearly observed. The precious information collected from
Figure 1 showed not only the large variations of
versus the temperature with permanent change of the slope
, but also it highlighted an important difference in the behavior of PMMA strongly depending on the tacticity of the polymer. Indeed, larger values of
of atactic PMMA were globally observed, respectively followed by those of syndiotactic and isotactic. However, an inversion of this tendency between isotactic and syndiotactic PMMAs was found for temperatures lower than 60°C.
The Curves of
for each of the three examined PMMAs clearly showed three maxima varying from one polymer to another. This interesting observation has to be correlated to the transition phenomena resulting from the influence of the increasing temperature. Indeed, it was showed in previous works [
34,
36,
38,
39,
68] that the PMMA presents three transition temperatures:
- -
The beta-relaxation ,
- -
The glass transition ,
- -
The liquid–liquid transitions .
The above results were perfectly confirmed by the curves of
of atactic PMMA in
Figure 1 and showed the same values obtained in other works. The presence of the maxima in
Figure 1 led to the following
Table 1:
Table 1 showed that a shift of 10°C was observed in the values of
and
when passing from atactic-PMMA to isotactic and this shift increases of additional 10°C in the case of syndiotactic PMMA. This shift in the transition temperatures is certainly due to the geometric configuration and position of methacrylate group relatively to the principal chain of PMMA showing that the tacticity of polymer strongly affects the transition temperatures [
9,
34,
36,
38,
39,
68].
The variations of
of each PMMA are composed by four parts of parabolic arcs represented by parabolic equations as shown in
Table 2 and having the following general form:
Table 2 showed the large difference between the
of the different PMMAs with various tacticities with a sensitive variation as a function of the temperature. The parabolic interpolation was obtained for the various cases with good linear regression coefficients. The variations of
are submitted to the magnitude of the second order transition temperatures and also to the tacticity of PMMA as it was shown in
Figure 1.
The linear interpolation shown in
Table 3 was obtained with a moderate value of the linear regression linear. This bad correlation is due to the non-linearity of the London dispersive energy as a function of the temperature for the different PMMAs.
However,
Table 3 allowed to obtain some useful information on the surface entropy
, the London dispersive energy
at 0K and the intrinsic temperature
of PMMA surfaces. These values were given in
Table 4.
The values of the surface characteristics of the different PMMAs showed an important difference in the behavior of PMMA following its tacticity. It seems that the obtained values of the intrinsic temperatures correspond to degradation temperatures of PMMAs and can be considered as a specific identity of the polymer.
3.1. Polar Free Surface Energy of Atactic, Isotactic and Syndiotactic PMMAs
Using the values of the deformation polarizability
and the ionization energies of the various n-alkanes and polar molecules adsorbed on the different PMMA surfaces obtained from Handbook of Physics and Chemistry [
70], it was possible to study the variations of
as a function of the chromatographic parameter
of the different solvents, and therefore to deduce the values of the free polar surface energy (
) of polar solvents adsorbed on three studied PMMAs. The obtained results were given in
Table 5
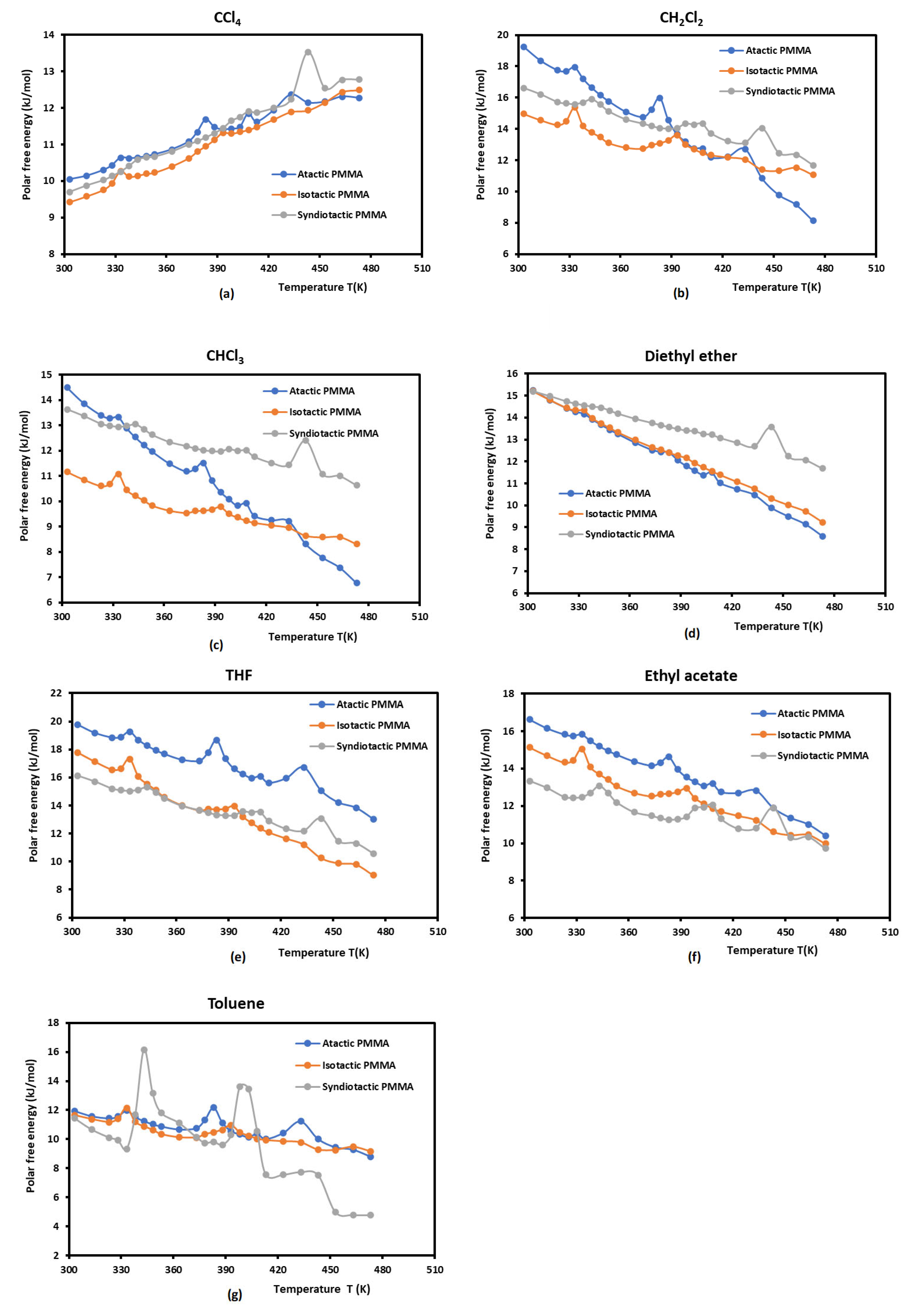
The comparison between the polar interaction free energy of the different PMMAs shown in
Table 5 for the different polar organic molecules globally, highlighted higher values of (
) in the case of atactic PMMA followed by syndiotactic PMMA and isotactic PMMA. A higher basic interaction was observed in all PMMAs showing their higher basicity with an important amphoteric character (
Table 5).
The effect of the tacticity on the adsorption of polar solvents on PMMA was shown in
Figure 2. The curves of the adsorbed polar molecules plotted on
Figure 2 also showed a specific signature at the various transition temperatures previously highlighted by the variations of the London dispersive surface energy. The shift in the transition temperatures was also observed in
Figure 2 for the different PMMAs. The results presented in
Figure 2 showed an important variation in the behavior of the different polymers. The atactic PMMA exhibited the highest values with the various solvents followed by syndiotactic and isotactic PMMAs.
The variations of (
of adsorbed solvents on PMMAs led to the determination of their polar enthalpy (
and entropy (
as a function of the temperature. The obtained values of (
and (
of the adsorbed solvents on the various PMMAs were given in
Tables S2 and S3, and their variations were plotted on
Figure 3.
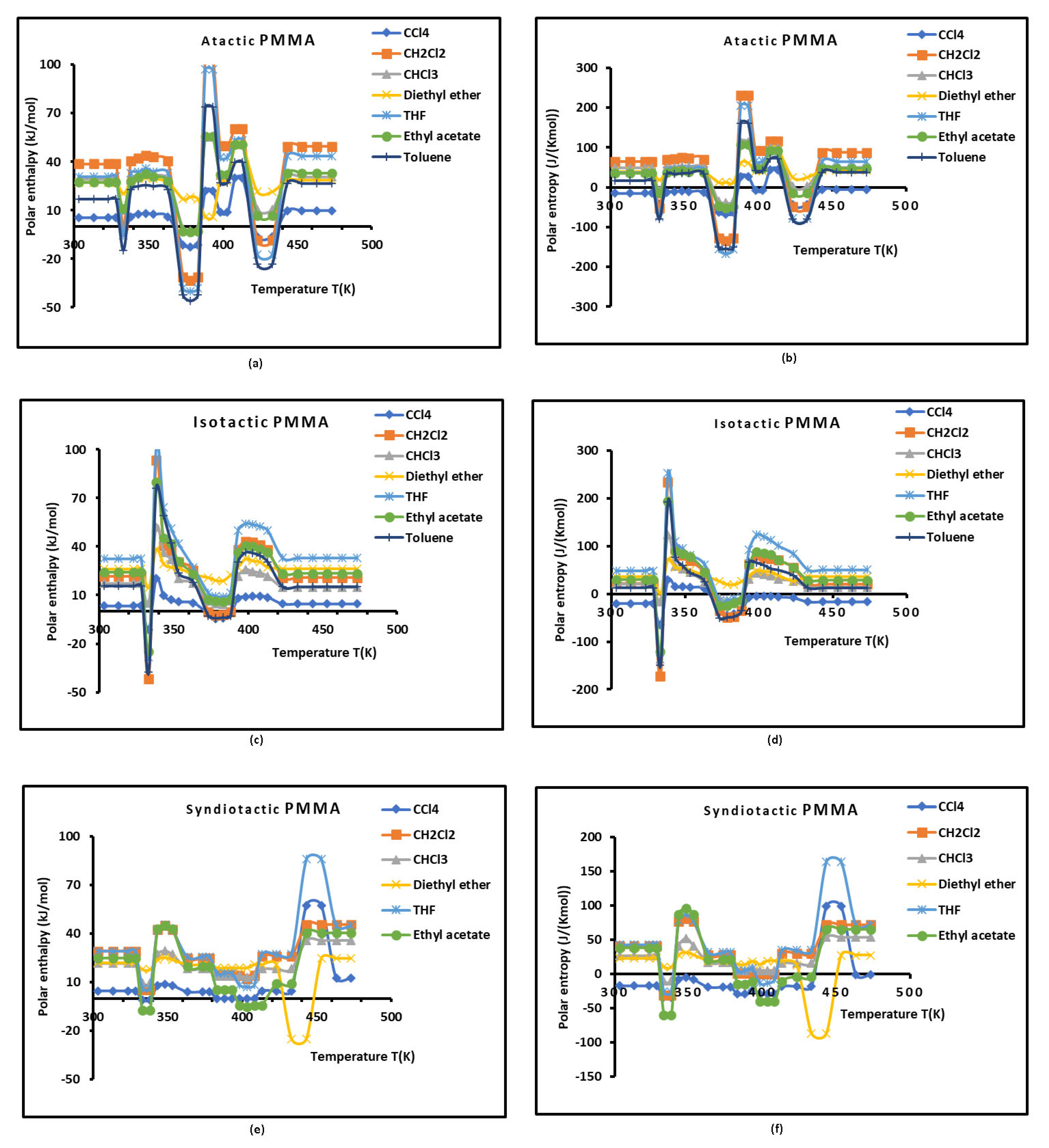
It was observed that the different curves on
Figure 3 clearly reflected the various behaviors of the PMMAs based on their tacticities. Three minima of each curve were observed for all PMMAs. These minima elucidated the presence of the three transition temperatures for the different polymers with a shift of these temperatures for the isotactic and syndiotactic PMMAs.
The positive values of ( evocated a adsorption rather than desorption process, while the negative ones significate a desorption reaction. This led to conclude that the interaction between the polar solvents and PMMAs is repulsive and the transition phases are characterized by smaller polar interactions. The comparison between the polar entropy change ( of the solvents adsorbed on the various polymers in general showed an ordered and organized surface of polymers remaining constant for temperatures lower than the beta-relaxation temperature , whereas, the disorder dramatically increased around , then decreased after this transition temperature and stabilized between the temperature interval .
This process continued by repeating the same evolution with a maximum disorder of the surface groups of the different PMMAs around each transition temperature. These observations were more accentuated with the atactic PMMA, and the more ordered case was obtained with the syndiotactic PMMA justifying its higher crystallinity relative to other PMMAs.
Figure 3 showed the non-linearity variations of (
and (
against the temperature and this will strongly affect the Lewis acid-base properties which was detailed in the next section.
3.3. Lewis’s Acid-Base Properties
The results in
Tables S2 and S3, and
Figure 3 with the thermodynamic relations 10 and 11 led to the determination of the enthalpic acid base parameters
and
and the entropic acid base parameters
and
of atactic, isotactic and syndiotactic PMMAs versus the temperature. The non-linearity of (
and (
of adsorbed solvents necessarily implied the strong variations of the acid-base parameters of PMMAs as a function of the temperature. The values of the different enthalpic and entropic acid-base parameters of PMMAs at different tacticities were presented in
Table 6 as a function of the temperature and led to the corresponding curves in
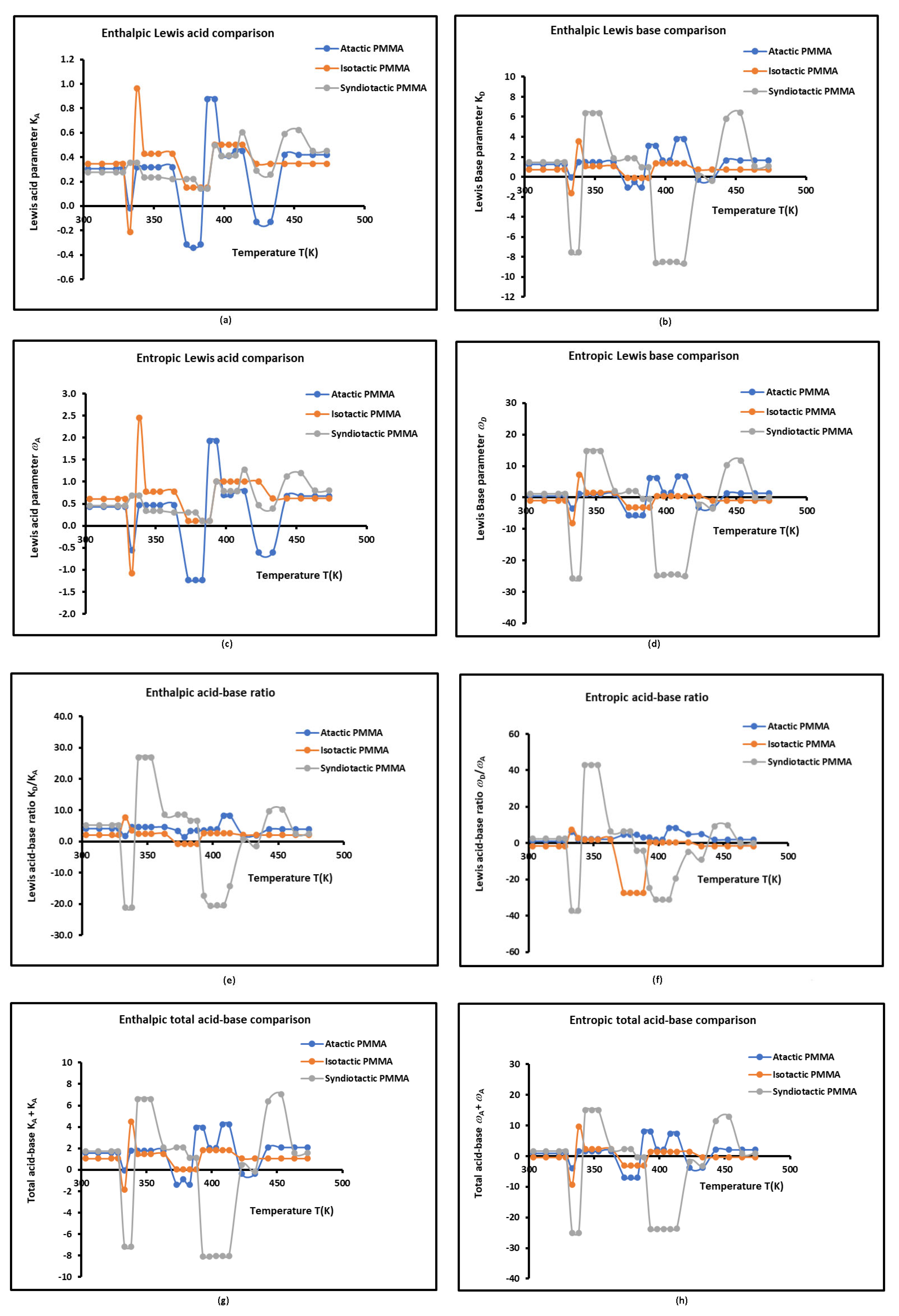
Figure 4. The information collected from
Table 6 and
Figure 4 are very numerous and precious.
The three examined polymers are more basic than acidic in Lewis terms. The basicity of the tactic PMMA is about four times greater than its acidity outside the neighborhood of the transition temperatures of the polymer (
Table 6 and
Figure 4). Whereas, the isotactic PMMA is about 2 to 2.5 times more basic than acid, while the syndiotactic PMMA exhibits the highest basicity by showing a surface 5 to 8 times more basic than acid, of course outside the neighborhood of the transition temperatures of the polymer (
Table 6 and
Figure 4).
The Lewis acid parameter of atactic PMMA is comparable to that of the isotactic PMMA with a slightly higher value in the isotactic PMMA, but greater than that of syndiotactic PMMA. However, the basicity of the latter polymer is higher than those of atactic and isotactic PMMAs. In fact, the alternate acrylate groups present in syndiotactic PMMA principal chain confer the highest basicity of this polymer, whereas, the methyl groups in isotactic and atactic PMMAs give the highest Lewis acidity.
The Lewis amphoteric character given by the values of
and
which are shown in
Table 6 and
Figure 4 led to the conclusion that the highest amphoteric surface is obtained with the syndiotactic PMMA, respectively followed by the atactic PMMA and isotactic PMMA, certainly due to the highest activity of the surface groups of syndiotactic PMMA.
Once again, the curves plotted in
Figure 4 showed the three transition temperatures with sudden changes around each transition temperature with a drop in the values of the different acid-bases parameters at these transition temperatures of the three polymers. The different parameters rapidly increase after reaching the different minima to be stabilized on positive parameter stages between two transition temperatures of PMMA.
The changes in the values of the different acid-base parameters in atactic PMMA are higher than those happened in isotactic and syndiotactic PMMAs. This is due to the random distribution of the acrylate groups in the PMMA principal chain.
An important effect of the temperature on the Lewis acid-base properties of the various PMMAs was highlighted (
Table 6 and
Figure 4).
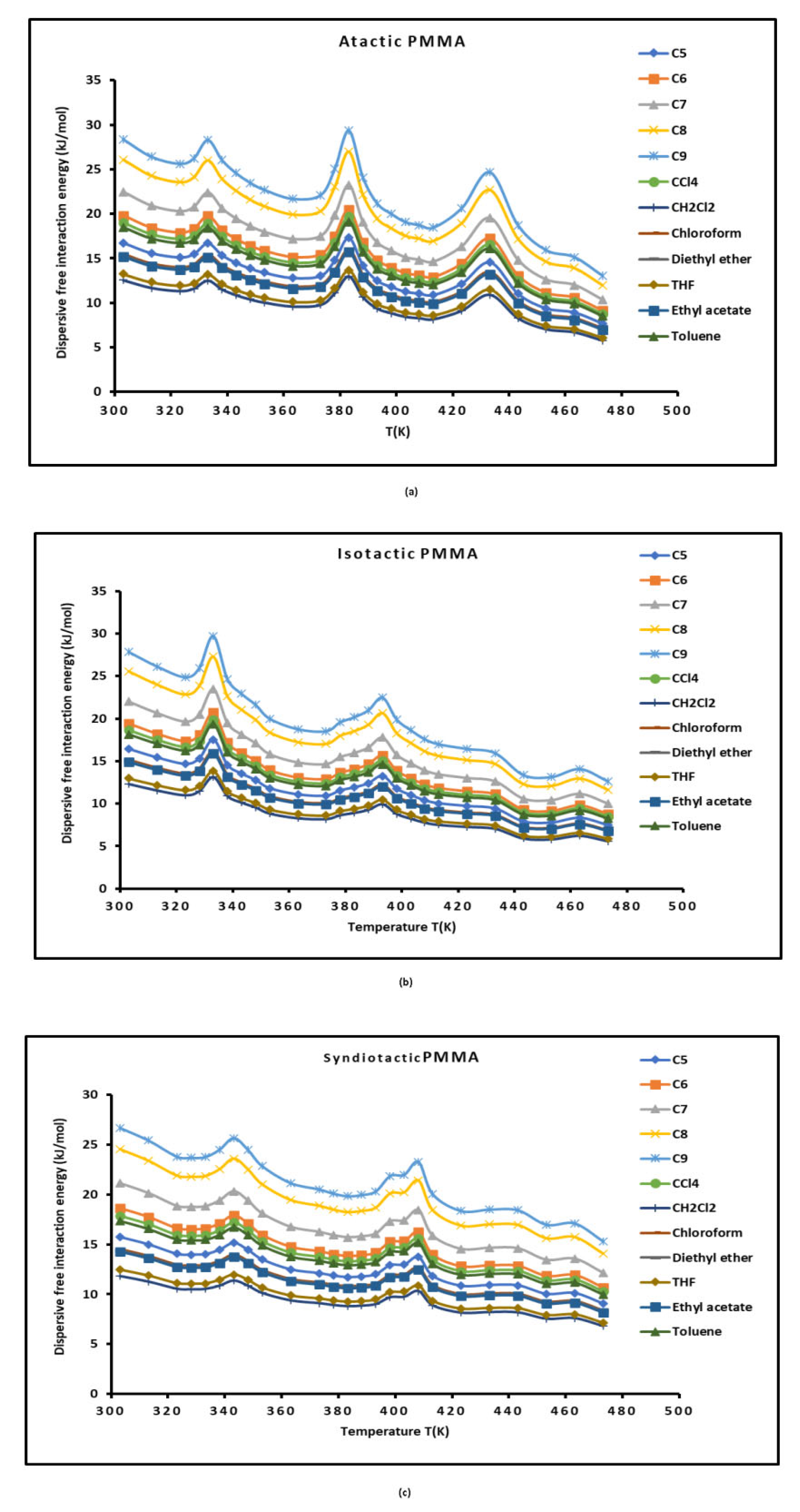
3.4. London Dispersive Free Energies of PMMAs and Dispersion Factor
The values of the dispersion factor
and the London dispersive free energies
of organic solvents adsorbed on the various PMMAS at different temperatures were obtained from Equations 3 and 5. The obtained results of
were given reported in
Table S4 and
Figure 5.
The values of
Table S4 showed a dispersive interaction energy of atactic PMMA stronger than those of isotactic and syndiotactic PMMAs. The curves of
Figure 5 highlighted the presence of the three transition temperatures of PMMAs confirming those previously obtained.
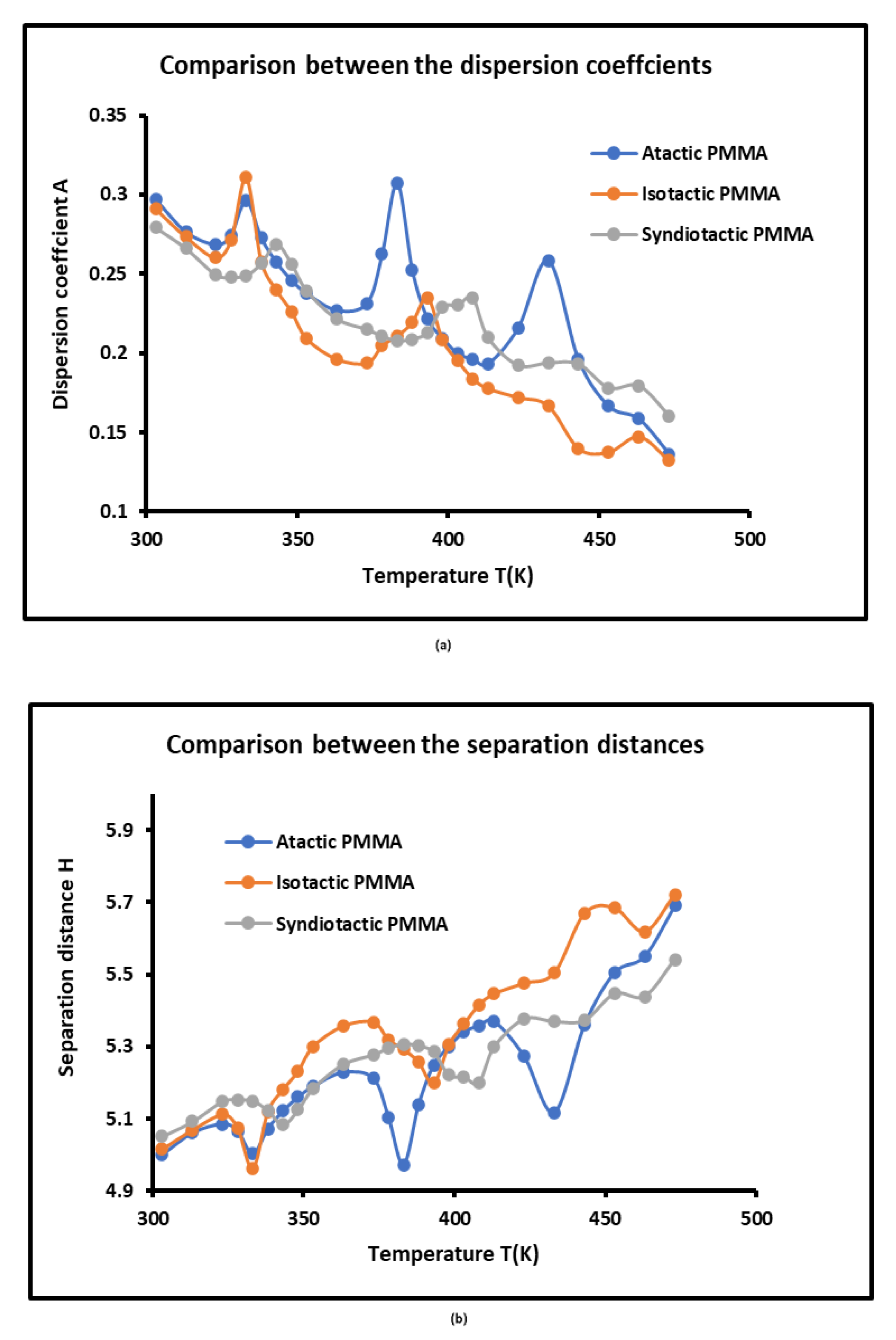
The values of the dispersion factor
and the separation distance
of the various PMMA polymers deduced from equation 5 were given in
Table S5. The curves of
and
also showed the presence of the three transition temperatures of the various PMMAs (
Figure 6) with a shift in their values from tactic PMMA to another. Even if one observed slight variations of the parameters
and
against the temperature, the transition phenomena were clearly elucidated.
It was shown in
Figure 6b that the average separation distance
between the solvents adsorbed on PMMA surfaces increased when the temperature increased justifying the effect of the thermal agitation on the separation distance. A larger separation distance was observed with the isotactic PMMA with the respect of the two other PMMAs, certainly due to the lower attractive free interaction energy.
3.5. Lewis Acid-Base Surface Energies of PMMAs and Polar Component of the Surface Energy of Polar Molecules
The Lewis acid
and base
surface energies of atactic, isotactic, and syndiotactic PMMA were determined using the following Van Oss’s relation [
67]:
Where and are the respective acid and base surface energies of the polar molecule adsorbed on polymer surface with the surface area of the adsorbed solvent.
Knowing that the experimental values of Lewis acid-base energies of ethyl acetate (EA) and dichloromethane (CH
2Cl
2) are respectively given by
and
; we were able to determine the values of
and
of PMMAs by using equations (13):
The polar acid-base surface energy
of PMMAs was then determined from equation (14):
The determination of
of PMMAs with the values of the London dispersive surface energy previously discussed in this paper, led to the determination of the Lifshitz – Van der Waals (LW) surface energy
(or total surface energy of the polymer) by using equation (21):
The values of the different polar acid and base surface energies
,
, and
(mJ/m
2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature were given in
Table 7.
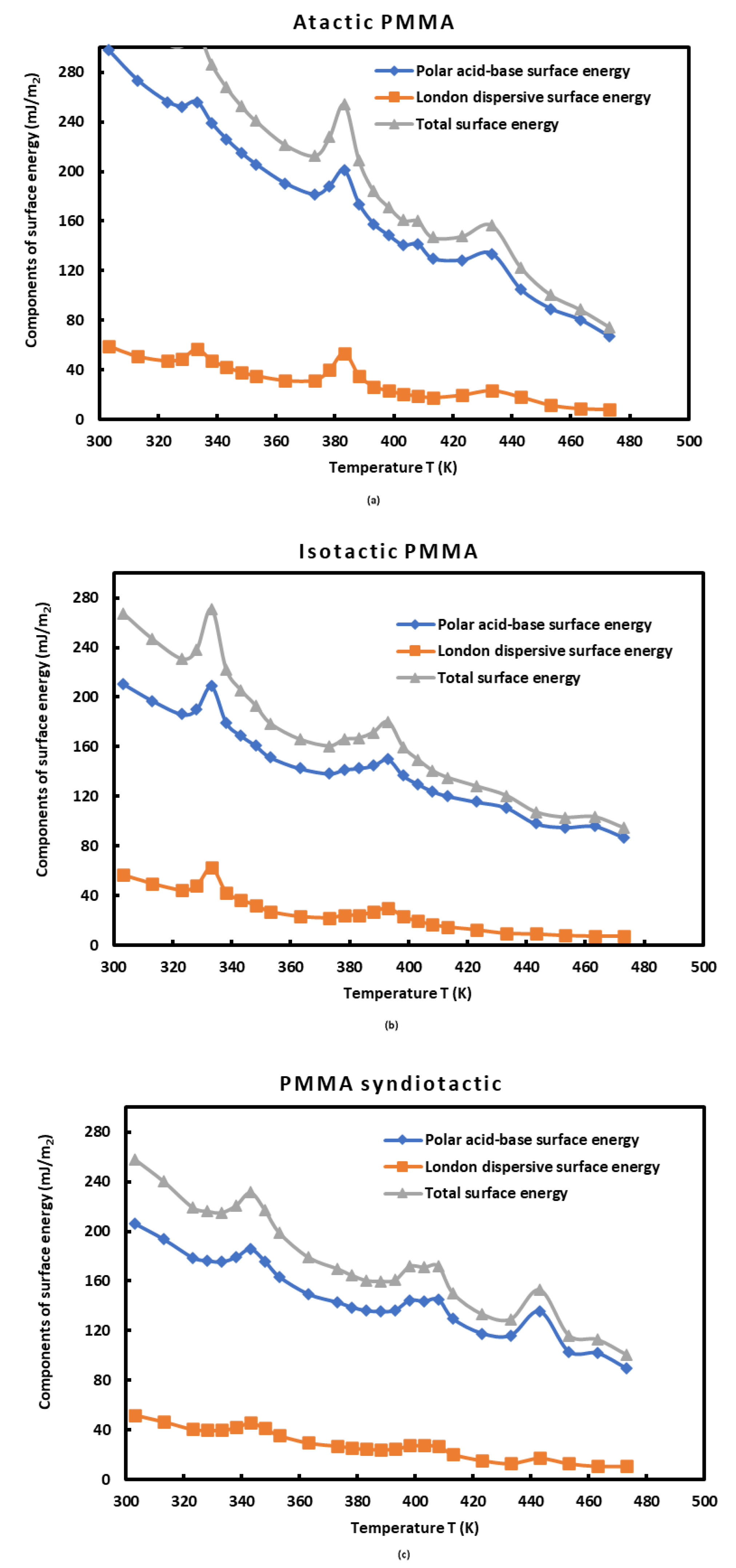
The atactic PMMA exhibited the highest polar basic surface energy , whereas the syndiotactic PMMA presented the highest polar acid surface energy. However, tha atctic PMMA had the highest polar acid-base surface energy due to its highest amphoteric character.
The values in
Table 7 led to draw the curves of
Figure 7 giving the polar acid-base surface energy
(mJ/m
2), the London dispersive surface energy
(mJ/m
2), and the Lifshitz – Van der Waals surface energy
(mJ/m
2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature. It was interesting to notice that the different curves of
Figure 7 gave the same shape of the previous curves obtained in the study of the various surface thermodynamic parameters again highlighting the three transition temperatures of the PMMAs.
These results led to the determination of the polar free energy
of polar molecules adsorbed on the various PMMAs with the help of the polar components of the surface energy of PMMA
using relation 16 and the previous results.
Table S56 reported the values
of the polar solvents adsorbed on the atactic, isotactic, and syndiotactic PMMAs. The results in
Table S6 showed that the highest values of
was obtained with dichloromethane for the three PMMAs, due to their strong Lewis basicity. However, it was noticed that the different values of
, even if their decrease when the temperature increases, are very small relative to their dispersive components.
4. Conclusions
The effect of tacticity on London dispersive surface energy, polar free energy and Lewis’s acid-base surface energies of PMMA was studied. The technique used was the inverse gas chromatography at infinite dilution consisting in the experimental determination of the net retention time and volume of model organic molecules adsorbed on the atactic, isotactic and syndiotactic PMMAs. Our new methodology used in this study was that based on the London dispersion interaction energy applied for the separation of the polar and dispersive energy of the different n-alkanes and polar solvents adsorbed on the different polymer surfaces. The London dispersive component of the surface energy of the different polymers was determined by applying the Hamieh thermal model that took into account the thermal effect on the surface area of molecules adsorbed on the solid surfaces. This study showed non-linear variations of of the different PMMAs with three maxima highlighting the three transition temperatures of PMMA, , and with a shift of 10°C in the values of and when passing from atactic-PMMA to isotactic and an additional shift of 10°C in the case of syndiotactic PMMA. The curves of were assimilated to four parts of perfect parabolic equations with an important difference inf the values of between atactic, isotactic and syndiotactic. The polar interaction free energy of polar solvents adsorbed on PMMAs was calculated as a function of the temperature. The variations of also showed the presence of the three transition temperatures of the polymers. The obtained results showed large differences in the behavior of the different polymers. The determination of the Lewis acid-base properties of PMMAs as a function of the temperature. The non-linearity of the different acid-base parameters was observed in all polymer surfaces proving the highest effect of the temperature and the tacticity on these parameters. The results showed that the basicity of the tactic PMMA is about four times greater than its acidity outside the neighborhood of the transition temperatures of the polymer. Whereas, the isotactic PMMA is about 2 to 2.5 times more basic than acid, while the syndiotactic PMMA exhibits the highest basicity by showing a surface 5 to 8 times more basic than acid. The Lewis acid parameter of atactic PMMA had a value comparable to that of the isotactic PMMA, but greater than that of syndiotactic PMMA. However, the basicity of the latter polymer was higher than those of atactic and isotactic PMMAs.
It was shown that the average separation distance between the organic molecules and the polymers depends on the temperature. It increased when the temperature increased. The slight variations of the separation distance also highlighted the three transition temperatures of the different PMMAs.
This work also determined the values of polar acid-base surface energy (mJ/m2), the London dispersive surface energy (mJ/m2), and the Lifshitz – Van der Waals surface energy (mJ/m2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature. The curves representing the variations of these surface parameters showed the same tendency of the curve shape highlighting the three transition temperatures of the PMMAs.
Figure 1.
Evolution of the London dispersive surface energy of a-PMMA, i-PMMA and s-PMMA as a function of the temperature T (K) by using the Hamieh thermal model. Dashed lines correspond to linear approximation.
Figure 1.
Evolution of the London dispersive surface energy of a-PMMA, i-PMMA and s-PMMA as a function of the temperature T (K) by using the Hamieh thermal model. Dashed lines correspond to linear approximation.
Figure 2.
Variations of the polar free interaction energy (kJ/mol) of different polar solvents adsorbed on atactic, isotactic and syndiotactic PMMAs as a function of the temperature: (a) CCl4; (b) CH2Cl2; (c) CHCl3; (d) diethyl ether; (e) tetrahydrofuran (THF); (f) ethyl acetate; and (g) toluene.
Figure 2.
Variations of the polar free interaction energy (kJ/mol) of different polar solvents adsorbed on atactic, isotactic and syndiotactic PMMAs as a function of the temperature: (a) CCl4; (b) CH2Cl2; (c) CHCl3; (d) diethyl ether; (e) tetrahydrofuran (THF); (f) ethyl acetate; and (g) toluene.
Figure 3.
Variations of the polar interaction enthalpy and entropy ( of polar solvents adsorbed on atactic, isotactic and syndiotactic PMMAs as a function of the temperature: (a) of atactic PMMA (b) of atactic PMMA; (c) of isotactic PMMA; (d) of isotactic PMMA; (e) of syndiotactic PMMA; (f) of syndiotactic PMMA.
Figure 3.
Variations of the polar interaction enthalpy and entropy ( of polar solvents adsorbed on atactic, isotactic and syndiotactic PMMAs as a function of the temperature: (a) of atactic PMMA (b) of atactic PMMA; (c) of isotactic PMMA; (d) of isotactic PMMA; (e) of syndiotactic PMMA; (f) of syndiotactic PMMA.
Figure 4.
Comparison between the various enthalpic and entropic acid-base parameters of atactic, isotactic and syndiotactic PMMAs versus the temperature: (a): , (b): , (c): , (d): , (e): /, (f): /, (g): , and (h): .
Figure 4.
Comparison between the various enthalpic and entropic acid-base parameters of atactic, isotactic and syndiotactic PMMAs versus the temperature: (a): , (b): , (c): , (d): , (e): /, (f): /, (g): , and (h): .
Figure 5.
Dispersion free energy of polar solvents adsorbed on atactic, isotactic and syndiotactic PMMAs as a function of the temperature: (a) atactic PMMA, (b) atactic PMMA, and (c) isotactic PMMA.
Figure 5.
Dispersion free energy of polar solvents adsorbed on atactic, isotactic and syndiotactic PMMAs as a function of the temperature: (a) atactic PMMA, (b) atactic PMMA, and (c) isotactic PMMA.
Figure 6.
Variations of the dispersion coefficient A (a) and separation distance H (b) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature.
Figure 6.
Variations of the dispersion coefficient A (a) and separation distance H (b) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature.
Figure 7.
Evolution of the polar acid-base surface energy (mJ/m2), the London dispersive surface energy (mJ/m2), and the Lifshitz – Van der Waals surface energy (mJ/m2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature.
Figure 7.
Evolution of the polar acid-base surface energy (mJ/m2), the London dispersive surface energy (mJ/m2), and the Lifshitz – Van der Waals surface energy (mJ/m2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature.
Table 1.
Values of beta-relaxation, glass transition and liquid-liquid transition temperatures of a-PMMA, i-PMMA and s-PMMA
Table 1.
Values of beta-relaxation, glass transition and liquid-liquid transition temperatures of a-PMMA, i-PMMA and s-PMMA
| Transition temperature |
|
|
|
| Atactic PMMA |
60°C |
110°C |
160°C |
| Isotactic PMMA |
60°C |
120°C |
170°C |
| Syndiotactic PMMA |
70°C |
130°C |
170°C |
Table 2.
Equations of of different PMMAs with the corresponding linear regression coefficients in the case of parabolic interpolation.
Table 2.
Equations of of different PMMAs with the corresponding linear regression coefficients in the case of parabolic interpolation.
| Atactic PMMA |
| Equation of
|
R² |
Temperature interval |
|
0.935 |
303.15K - 333.15K |
|
0.9722 |
333.15K - 378.15K |
|
0.9477 |
378.15K - 423.15K |
|
0.9902 |
423.15K - 473.15K |
| Isotactic PMMA |
| Equation of
|
R² |
Temperature interval |
|
0.9398 |
303.15K - 333.15K |
|
0.9557 |
333.15K - 393.15K |
|
0.9925 |
393.15K - 443.15K |
|
0.9896 |
443.15K - 473.15K |
| Syndiotactic PMMA |
| Equation of
|
R² |
Temperature interval |
|
0.9485 |
303.15K - 343.15K |
|
0.9900 |
343.15K - 403.15K |
|
0.9569 |
403.15K - 443.15K |
|
1.000 |
443.15K - 473.15K |
Table 3.
Equations of of different PMMAs with the corresponding linear regression coefficients with a linear approximation.
Table 3.
Equations of of different PMMAs with the corresponding linear regression coefficients with a linear approximation.
| |
Equation of |
R² |
Temperature interval |
| Atactic PMMA |
|
0.8368 |
303.15K - 473.15K |
| Isotactic PMMA |
|
0.8491 |
303.15K - 473.15K |
| Syndiotactic PMMA |
|
0.8368 |
303.15K - 473.15K |
Table 4.
Values of the surface entropy , the London dispersive energy at 0K and the intrinsic temperature of a-PMMA i-PMMA and s-PMMA.
Table 4.
Values of the surface entropy , the London dispersive energy at 0K and the intrinsic temperature of a-PMMA i-PMMA and s-PMMA.
| |
|
|
|
| Atactic PMMA |
|
144.37 |
494.1 |
| Isotactic PMMA |
|
143.16 |
472.6 |
| Syndiotactic PMMA |
|
120.8 |
503.8 |
Table 5.
Values of ( kJ/mol) of polar molecules adsorbed respectively on atactic, isotactic and syndiotactic PMMAs versus the temperature.
Table 5.
Values of ( kJ/mol) of polar molecules adsorbed respectively on atactic, isotactic and syndiotactic PMMAs versus the temperature.
| Polar free energy of atactic PMMA |
| Temperature T(K) |
CCl4
|
CH2Cl2
|
CHCl3
|
Diethyl ether |
THF |
Ethyl acetate |
Toluene |
| 303.15 |
10.047 |
19.251 |
14.493 |
15.248 |
19.777 |
16.627 |
11.951 |
| 313.15 |
10.142 |
18.357 |
13.862 |
14.794 |
19.179 |
16.162 |
11.585 |
| 323.15 |
10.303 |
17.750 |
13.399 |
14.416 |
18.845 |
15.830 |
11.442 |
| 328.15 |
10.433 |
17.669 |
13.278 |
14.259 |
18.889 |
15.755 |
11.561 |
| 333.15 |
10.642 |
17.926 |
13.323 |
14.166 |
19.257 |
15.828 |
11.961 |
| 338.15 |
10.621 |
17.193 |
12.884 |
13.904 |
18.679 |
15.479 |
11.526 |
| 343.15 |
10.638 |
16.636 |
12.532 |
13.665 |
18.266 |
15.198 |
11.244 |
| 348.15 |
10.676 |
16.168 |
12.229 |
13.447 |
17.938 |
14.959 |
11.033 |
| 353.15 |
10.729 |
15.768 |
11.962 |
13.242 |
17.673 |
14.749 |
10.878 |
| 363.15 |
10.865 |
15.098 |
11.494 |
12.854 |
17.267 |
14.384 |
10.679 |
| 373.15 |
11.081 |
14.759 |
11.178 |
12.521 |
17.185 |
14.163 |
10.758 |
| 378.15 |
11.342 |
15.218 |
11.285 |
12.442 |
17.767 |
14.323 |
11.335 |
| 383.15 |
11.682 |
15.988 |
11.514 |
12.403 |
18.664 |
14.617 |
12.180 |
| 388.15 |
11.479 |
14.558 |
10.824 |
12.058 |
17.371 |
13.963 |
11.140 |
| 393.15 |
11.408 |
13.673 |
10.371 |
11.792 |
16.616 |
13.543 |
10.566 |
| 398.15 |
11.435 |
13.186 |
10.086 |
11.582 |
16.256 |
13.295 |
10.331 |
| 403.15 |
11.476 |
12.754 |
9.827 |
11.382 |
15.950 |
13.070 |
10.144 |
| 408.15 |
11.850 |
12.756 |
9.922 |
11.500 |
16.078 |
13.204 |
10.372 |
| 413.15 |
11.627 |
12.174 |
9.424 |
11.019 |
15.621 |
12.744 |
10.013 |
| 423.15 |
11.940 |
12.230 |
9.255 |
10.729 |
15.944 |
12.693 |
10.431 |
| 433.15 |
12.367 |
12.723 |
9.224 |
10.476 |
16.727 |
12.831 |
11.230 |
| 443.15 |
12.147 |
10.846 |
8.317 |
9.886 |
15.066 |
11.893 |
10.015 |
| 453.15 |
12.172 |
9.778 |
7.776 |
9.494 |
14.219 |
11.360 |
9.451 |
| 463.15 |
12.310 |
9.164 |
7.383 |
9.138 |
13.845 |
11.019 |
9.285 |
| 473.15 |
12.276 |
8.131 |
6.767 |
8.597 |
13.044 |
10.414 |
8.814 |
| Polar free energy of isotactic PMMA |
| Temperature T(K) |
CCl4
|
CH2Cl2
|
CHCl3
|
Diethyl ether |
THF |
Ethyl acetate |
Toluene |
| 303.15 |
9.422 |
14.973 |
11.160 |
15.214 |
17.783 |
15.139 |
11.652 |
| 313.15 |
9.579 |
14.564 |
10.848 |
14.812 |
17.121 |
14.694 |
11.374 |
| 323.15 |
9.760 |
14.259 |
10.605 |
14.441 |
16.553 |
14.331 |
11.174 |
| 328.15 |
9.943 |
14.504 |
10.689 |
14.334 |
16.646 |
14.457 |
11.401 |
| 333.15 |
10.269 |
15.363 |
11.071 |
14.330 |
17.328 |
15.059 |
12.148 |
| 338.15 |
10.120 |
14.192 |
10.454 |
13.969 |
16.068 |
14.088 |
11.188 |
| 343.15 |
10.147 |
13.788 |
10.220 |
13.741 |
15.539 |
13.712 |
10.874 |
| 348.15 |
10.198 |
13.485 |
10.041 |
13.539 |
15.103 |
13.414 |
10.639 |
| 353.15 |
10.231 |
13.105 |
9.833 |
13.329 |
14.592 |
13.058 |
10.338 |
| 363.15 |
10.401 |
12.806 |
9.635 |
12.975 |
14.011 |
12.701 |
10.129 |
| 373.15 |
10.625 |
12.735 |
9.544 |
12.655 |
13.652 |
12.521 |
10.117 |
| 378.15 |
10.804 |
12.970 |
9.615 |
12.537 |
13.740 |
12.637 |
10.338 |
| 383.15 |
10.955 |
13.096 |
9.636 |
12.400 |
13.722 |
12.671 |
10.471 |
| 388.15 |
11.121 |
13.277 |
9.678 |
12.268 |
13.759 |
12.745 |
10.649 |
| 393.15 |
11.326 |
13.611 |
9.781 |
12.161 |
13.952 |
12.936 |
10.956 |
| 398.15 |
11.297 |
13.014 |
9.509 |
11.928 |
13.210 |
12.416 |
10.465 |
| 403.15 |
11.344 |
12.718 |
9.363 |
11.737 |
12.768 |
12.127 |
10.232 |
| 408.15 |
11.400 |
12.466 |
9.238 |
11.556 |
12.367 |
11.872 |
10.034 |
| 413.15 |
11.482 |
12.320 |
9.155 |
11.384 |
12.074 |
11.698 |
9.930 |
| 423.15 |
11.687 |
12.183 |
9.055 |
11.068 |
11.642 |
11.467 |
9.850 |
| 433.15 |
11.891 |
12.051 |
8.958 |
10.751 |
11.213 |
11.241 |
9.776 |
| 443.15 |
11.933 |
11.393 |
8.645 |
10.315 |
10.245 |
10.615 |
9.277 |
| 453.15 |
12.152 |
11.328 |
8.582 |
10.009 |
9.880 |
10.440 |
9.259 |
| 463.15 |
12.437 |
11.521 |
8.595 |
9.719 |
9.789 |
10.457 |
9.468 |
| 473.15 |
12.492 |
11.059 |
8.311 |
9.232 |
9.025 |
9.972 |
9.163 |
| Polar free energy of syndiotactic PMMA |
| Temperature T(K) |
CCl4
|
CH2Cl2
|
CHCl3
|
Diethyl ether |
THF |
Ethyl acetate |
Toluene |
| 303.15 |
9.704 |
16.617 |
13.631 |
15.200 |
16.129 |
13.339 |
11.448 |
| 313.15 |
9.879 |
16.212 |
13.363 |
14.978 |
15.709 |
12.965 |
10.664 |
| 323.15 |
10.028 |
15.714 |
13.055 |
14.733 |
15.199 |
12.476 |
10.121 |
| 328.15 |
10.138 |
15.625 |
12.986 |
14.642 |
15.093 |
12.442 |
9.935 |
| 333.15 |
10.259 |
15.581 |
12.939 |
14.559 |
15.031 |
12.470 |
9.343 |
| 338.15 |
10.416 |
15.691 |
12.969 |
14.506 |
15.116 |
12.701 |
11.685 |
| 343.15 |
10.594 |
15.893 |
13.038 |
14.458 |
15.293 |
13.068 |
16.158 |
| 348.15 |
10.646 |
15.560 |
12.853 |
14.326 |
14.953 |
12.704 |
13.145 |
| 353.15 |
10.673 |
15.123 |
12.624 |
14.180 |
14.510 |
12.198 |
11.800 |
| 363.15 |
10.809 |
14.608 |
12.344 |
13.950 |
13.968 |
11.670 |
11.126 |
| 373.15 |
11.001 |
14.337 |
12.182 |
13.759 |
13.661 |
11.473 |
10.155 |
| 378.15 |
11.091 |
14.178 |
12.092 |
13.660 |
13.483 |
11.342 |
9.749 |
| 383.15 |
11.190 |
14.056 |
12.021 |
13.570 |
13.341 |
11.259 |
9.829 |
| 388.15 |
11.309 |
14.017 |
11.982 |
13.488 |
13.283 |
11.293 |
9.622 |
| 393.15 |
11.444 |
14.041 |
11.971 |
13.417 |
13.287 |
11.408 |
10.293 |
| 398.15 |
11.646 |
14.333 |
12.065 |
13.380 |
13.561 |
11.891 |
13.616 |
| 403.15 |
11.755 |
14.278 |
11.999 |
13.262 |
13.493 |
11.937 |
13.468 |
| 408.15 |
11.905 |
14.333 |
12.013 |
13.226 |
13.527 |
12.062 |
10.553 |
| 413.15 |
11.879 |
13.723 |
11.757 |
13.076 |
12.890 |
11.309 |
7.576 |
| 423.15 |
12.004 |
13.210 |
11.520 |
12.858 |
12.328 |
10.781 |
7.563 |
| 433.15 |
12.235 |
13.114 |
11.441 |
12.694 |
12.192 |
10.819 |
7.746 |
| 443.15 |
13.534 |
14.036 |
12.398 |
13.561 |
13.081 |
11.903 |
7.539 |
| 453.15 |
12.543 |
12.452 |
11.078 |
12.240 |
11.439 |
10.311 |
4.992 |
| 463.15 |
12.772 |
12.352 |
10.999 |
12.073 |
11.299 |
10.345 |
4.800 |
| 473.15 |
12.779 |
11.679 |
10.632 |
11.688 |
10.574 |
9.720 |
4.785 |
Table 6.
Values of the enthalpic acid base parameters , , / and , and the entropic acid base parameters , , / and of PMMA as a function of the temperature.
Table 6.
Values of the enthalpic acid base parameters , , / and , and the entropic acid base parameters , , / and of PMMA as a function of the temperature.
| Atactic PMMA |
| T(K) |
|
|
|
/
|
|
|
|
/
|
| 303.15 |
0.304 |
1.260 |
1.565 |
4.142 |
0.43 |
0.52 |
0.95 |
1.22 |
| 313.15 |
0.304 |
1.260 |
1.565 |
4.142 |
0.43 |
0.52 |
0.95 |
1.22 |
| 323.15 |
0.304 |
1.260 |
1.565 |
4.142 |
0.43 |
0.52 |
0.95 |
1.22 |
| 328.15 |
0.304 |
1.260 |
1.565 |
4.142 |
0.43 |
0.52 |
0.95 |
1.22 |
| 333.15 |
-0.018 |
-0.033 |
-0.051 |
1.847 |
-0.56 |
-3.43 |
-3.99 |
6.16 |
| 338.15 |
0.317 |
1.459 |
1.775 |
4.602 |
0.46 |
1.09 |
1.56 |
2.36 |
| 343.15 |
0.317 |
1.459 |
1.775 |
4.602 |
0.46 |
1.09 |
1.56 |
2.36 |
| 348.15 |
0.317 |
1.459 |
1.775 |
4.602 |
0.46 |
1.09 |
1.56 |
2.36 |
| 353.15 |
0.317 |
1.459 |
1.775 |
4.602 |
0.46 |
1.09 |
1.56 |
2.36 |
| 363.15 |
0.317 |
1.459 |
1.775 |
4.602 |
0.46 |
1.09 |
1.56 |
2.36 |
| 373.15 |
-0.313 |
-1.063 |
-1.376 |
3.397 |
-1.23 |
-5.71 |
-6.93 |
4.65 |
| 378.15 |
-0.343 |
-0.508 |
-0.851 |
1.478 |
-1.23 |
-5.71 |
-6.93 |
4.65 |
| 383.15 |
-0.313 |
-1.063 |
-1.376 |
3.397 |
-1.23 |
-5.71 |
-6.93 |
4.65 |
| 388.15 |
0.876 |
3.081 |
3.957 |
3.518 |
1.93 |
6.11 |
8.04 |
3.17 |
| 393.15 |
0.876 |
3.081 |
3.957 |
3.518 |
1.93 |
6.11 |
8.04 |
3.17 |
| 398.15 |
0.411 |
1.630 |
2.042 |
3.966 |
0.69 |
1.44 |
2.13 |
2.07 |
| 403.15 |
0.411 |
1.630 |
2.042 |
3.966 |
0.69 |
1.44 |
2.13 |
2.07 |
| 408.15 |
0.453 |
3.784 |
4.237 |
8.350 |
0.79 |
6.62 |
7.41 |
8.40 |
| 413.15 |
0.453 |
3.784 |
4.237 |
8.350 |
0.79 |
6.62 |
7.41 |
8.40 |
| 423.15 |
-0.128 |
-0.259 |
-0.388 |
2.016 |
-0.61 |
-3.12 |
-3.73 |
5.13 |
| 433.15 |
-0.128 |
-0.259 |
-0.388 |
2.016 |
-0.61 |
-3.12 |
-3.73 |
5.13 |
| 443.15 |
0.418 |
1.667 |
2.085 |
3.989 |
0.67 |
1.40 |
2.08 |
2.08 |
| 453.15 |
0.418 |
1.667 |
2.085 |
3.989 |
0.67 |
1.40 |
2.08 |
2.08 |
| 463.15 |
0.418 |
1.667 |
2.085 |
3.989 |
0.67 |
1.40 |
2.08 |
2.08 |
| 473.15 |
0.418 |
1.667 |
2.085 |
3.989 |
0.67 |
1.40 |
2.08 |
2.08 |
| Isotactic PMMA |
| T(K) |
|
|
|
/
|
|
|
|
/
|
| 303.15 |
0.344 |
0.717 |
1.060 |
2.084 |
0.61 |
-1.03 |
-0.42 |
-1.68 |
| 313.15 |
0.344 |
0.717 |
1.060 |
2.084 |
0.61 |
-1.03 |
-0.42 |
-1.68 |
| 323.15 |
0.344 |
0.717 |
1.060 |
2.084 |
0.61 |
-1.03 |
-0.42 |
-1.68 |
| 328.15 |
0.344 |
0.717 |
1.060 |
2.084 |
0.61 |
-1.03 |
-0.42 |
-1.68 |
| 333.15 |
-0.211 |
-1.628 |
-1.838 |
7.730 |
-1.08 |
-8.20 |
-9.28 |
7.59 |
| 338.15 |
0.964 |
3.531 |
4.494 |
3.664 |
2.45 |
7.28 |
9.72 |
2.98 |
| 343.15 |
0.427 |
1.066 |
1.493 |
2.498 |
0.78 |
1.52 |
2.30 |
1.95 |
| 348.15 |
0.427 |
1.066 |
1.493 |
2.498 |
0.78 |
1.52 |
2.30 |
1.95 |
| 353.15 |
0.427 |
1.066 |
1.493 |
2.498 |
0.78 |
1.52 |
2.30 |
1.95 |
| 363.15 |
0.427 |
1.066 |
1.493 |
2.498 |
0.78 |
1.52 |
2.30 |
1.95 |
| 373.15 |
0.153 |
-0.119 |
0.034 |
-0.778 |
0.12 |
-3.21 |
-3.10 |
-27.41 |
| 378.15 |
0.153 |
-0.119 |
0.034 |
-0.778 |
0.12 |
-3.21 |
-3.10 |
-27.41 |
| 383.15 |
0.153 |
-0.119 |
0.034 |
-0.778 |
0.12 |
-3.21 |
-3.10 |
-27.41 |
| 388.15 |
0.153 |
-0.119 |
0.034 |
-0.778 |
0.12 |
-3.21 |
-3.10 |
-27.41 |
| 393.15 |
0.501 |
1.338 |
1.839 |
2.668 |
1.00 |
0.49 |
1.50 |
0.49 |
| 398.15 |
0.501 |
1.338 |
1.839 |
2.668 |
1.00 |
0.49 |
1.50 |
0.49 |
| 403.15 |
0.501 |
1.338 |
1.839 |
2.668 |
1.00 |
0.49 |
1.50 |
0.49 |
| 408.15 |
0.501 |
1.338 |
1.839 |
2.668 |
1.00 |
0.49 |
1.50 |
0.49 |
| 413.15 |
0.501 |
1.338 |
1.839 |
2.668 |
1.00 |
0.49 |
1.50 |
0.49 |
| 423.15 |
0.347 |
0.728 |
1.076 |
2.097 |
1.00 |
0.49 |
1.50 |
0.49 |
| 433.15 |
0.347 |
0.728 |
1.076 |
2.097 |
0.63 |
-0.99 |
-0.37 |
-1.58 |
| 443.15 |
0.347 |
0.728 |
1.076 |
2.097 |
0.63 |
-0.99 |
-0.37 |
-1.58 |
| 453.15 |
0.347 |
0.728 |
1.076 |
2.097 |
0.63 |
-0.99 |
-0.37 |
-1.58 |
| 463.15 |
0.347 |
0.728 |
1.076 |
2.097 |
0.63 |
-0.99 |
-0.37 |
-1.58 |
| 473.15 |
0.347 |
0.728 |
1.076 |
2.097 |
0.63 |
-0.99 |
-0.37 |
-1.58 |
| Syndiotactic PMMA |
| T(K) |
|
|
|
/
|
|
|
|
/
|
| 303.15 |
0.276 |
1.452 |
1.728 |
5.262 |
0.46 |
1.14 |
1.60 |
2.47 |
| 313.15 |
0.276 |
1.452 |
1.728 |
5.262 |
0.46 |
1.14 |
1.60 |
2.47 |
| 323.15 |
0.276 |
1.452 |
1.728 |
5.262 |
0.46 |
1.14 |
1.60 |
2.47 |
| 328.15 |
0.276 |
1.452 |
1.728 |
5.262 |
0.46 |
1.14 |
1.60 |
2.47 |
| 333.15 |
0.355 |
-7.527 |
-7.172 |
-21.189 |
0.69 |
-25.75 |
-25.06 |
-37.30 |
| 338.15 |
0.355 |
-7.527 |
-7.172 |
-21.189 |
0.69 |
-25.75 |
-25.06 |
-37.30 |
| 343.15 |
0.237 |
6.367 |
6.603 |
26.907 |
0.34 |
14.74 |
15.08 |
42.88 |
| 348.15 |
0.237 |
6.367 |
6.603 |
26.907 |
0.34 |
14.74 |
15.08 |
42.88 |
| 353.15 |
0.237 |
6.367 |
6.603 |
26.907 |
0.34 |
14.74 |
15.08 |
42.88 |
| 363.15 |
0.220 |
1.888 |
2.108 |
8.591 |
0.30 |
2.01 |
2.32 |
6.60 |
| 373.15 |
0.220 |
1.888 |
2.108 |
8.591 |
0.30 |
2.01 |
2.32 |
6.60 |
| 378.15 |
0.220 |
1.888 |
2.108 |
8.591 |
0.30 |
2.01 |
2.32 |
6.60 |
| 383.15 |
0.143 |
0.961 |
1.104 |
6.703 |
0.11 |
-0.46 |
-0.35 |
-4.25 |
| 388.15 |
0.143 |
0.961 |
1.104 |
6.703 |
0.11 |
-0.46 |
-0.35 |
-4.25 |
| 393.15 |
0.496 |
-8.578 |
-8.082 |
-17.310 |
1.01 |
-24.79 |
-23.79 |
-24.65 |
| 398.15 |
0.411 |
-8.490 |
-8.079 |
-20.645 |
0.79 |
-24.58 |
-23.79 |
-31.17 |
| 403.15 |
0.413 |
-8.464 |
-8.052 |
-20.514 |
0.79 |
-24.51 |
-23.72 |
-30.94 |
| 408.15 |
0.413 |
-8.464 |
-8.052 |
-20.514 |
0.79 |
-24.51 |
-23.72 |
-30.94 |
| 413.15 |
0.605 |
-8.652 |
-8.047 |
-14.291 |
1.28 |
-24.99 |
-23.71 |
-19.53 |
| 423.15 |
0.290 |
0.185 |
0.475 |
0.639 |
0.47 |
-2.19 |
-1.72 |
-4.69 |
| 433.15 |
0.259 |
-0.401 |
-0.143 |
-1.552 |
0.39 |
-3.54 |
-3.15 |
-8.98 |
| 443.15 |
0.590 |
5.780 |
6.369 |
9.796 |
1.12 |
10.32 |
11.44 |
9.24 |
| 453.15 |
0.624 |
6.415 |
7.039 |
10.283 |
1.19 |
11.79 |
12.98 |
9.86 |
| 463.15 |
0.451 |
1.105 |
1.556 |
2.453 |
0.80 |
0.10 |
0.90 |
0.12 |
| 473.15 |
0.451 |
1.105 |
1.556 |
2.453 |
0.80 |
0.10 |
0.90 |
0.12 |
Table 7.
Values of the polar acid and base surface energies , , and (mJ/m2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature.
Table 7.
Values of the polar acid and base surface energies , , and (mJ/m2) of atactic, isotactic and syndiotactic PMMAs as a function of the temperature.
| |
Atactic PMMA |
Isotactic PMMA |
Syndiotactic PMMA |
| T(K) |
|
|
|
|
|
|
|
|
|
| 303.15 |
203.75 |
108.73 |
297.68 |
123.27 |
90.13 |
210.82 |
151.82 |
69.97 |
206.14 |
| 313.15 |
183.42 |
101.70 |
273.16 |
115.44 |
84.06 |
197.02 |
143.04 |
65.44 |
193.50 |
| 323.15 |
169.77 |
96.59 |
256.11 |
109.56 |
79.16 |
186.25 |
133.06 |
59.99 |
178.69 |
| 328.15 |
167.38 |
95.20 |
252.46 |
112.78 |
80.16 |
190.16 |
130.89 |
59.37 |
176.30 |
| 333.15 |
171.41 |
95.61 |
256.03 |
125.91 |
86.53 |
208.76 |
129.50 |
59.34 |
175.32 |
| 338.15 |
156.91 |
90.97 |
238.96 |
106.91 |
75.36 |
179.52 |
130.68 |
61.25 |
178.94 |
| 343.15 |
146.17 |
87.28 |
225.89 |
100.41 |
71.04 |
168.91 |
133.41 |
64.53 |
185.56 |
| 348.15 |
137.38 |
84.13 |
215.02 |
95.56 |
67.65 |
160.81 |
127.25 |
60.68 |
175.74 |
| 353.15 |
130.01 |
81.38 |
205.72 |
89.82 |
63.79 |
151.39 |
119.60 |
55.66 |
163.18 |
| 363.15 |
118.03 |
76.64 |
190.22 |
84.91 |
59.76 |
142.47 |
110.49 |
50.45 |
149.32 |
| 373.15 |
111.70 |
73.58 |
181.32 |
83.16 |
57.51 |
138.31 |
105.40 |
48.28 |
142.68 |
| 378.15 |
118.18 |
74.88 |
188.14 |
85.83 |
58.30 |
141.48 |
102.57 |
46.95 |
138.79 |
| 383.15 |
129.80 |
77.62 |
200.74 |
87.09 |
58.32 |
142.53 |
100.32 |
46.05 |
135.93 |
| 388.15 |
107.09 |
70.48 |
173.76 |
89.07 |
58.72 |
144.64 |
99.29 |
46.10 |
135.31 |
| 393.15 |
94.01 |
65.98 |
157.52 |
93.16 |
60.20 |
149.77 |
99.14 |
46.82 |
136.26 |
| 398.15 |
87.01 |
63.28 |
148.40 |
84.75 |
55.19 |
136.79 |
102.80 |
50.62 |
144.27 |
| 403.15 |
81.01 |
60.87 |
140.44 |
80.55 |
52.40 |
129.94 |
101.53 |
50.77 |
143.59 |
| 408.15 |
80.65 |
61.82 |
141.22 |
77.02 |
49.97 |
124.08 |
101.82 |
51.59 |
144.95 |
| 413.15 |
73.10 |
57.31 |
129.46 |
74.87 |
48.29 |
120.25 |
92.89 |
45.13 |
129.50 |
| 423.15 |
73.07 |
56.31 |
128.29 |
72.51 |
45.96 |
115.46 |
85.25 |
40.62 |
117.70 |
| 433.15 |
78.33 |
57.00 |
133.64 |
70.27 |
43.74 |
110.89 |
83.23 |
40.53 |
116.15 |
| 443.15 |
56.38 |
48.50 |
104.59 |
62.22 |
38.64 |
98.07 |
94.44 |
48.59 |
135.48 |
| 453.15 |
45.40 |
43.84 |
89.22 |
60.93 |
37.02 |
94.99 |
73.62 |
36.11 |
103.13 |
| 463.15 |
39.51 |
40.86 |
80.36 |
62.44 |
36.80 |
95.87 |
71.77 |
36.02 |
101.68 |
| 473.15 |
30.81 |
36.16 |
66.75 |
56.99 |
33.15 |
86.93 |
63.57 |
31.50 |
89.50 |