Submitted:
10 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Preparation of Green Coffee, Clove, Cinnamon, Cinnamon and Clove, and Nutmeg Fine Powders
2.2. Determination of Volatile Compounds
2.3. Extraction of Volatile Compounds
2.4. Conditions of Analysis and Instrumentation
2.5. Volatile Compounds Verification
2.6. Statistical Analysis
3. Results
3.1. Volatile Compounds in Green Coffee and Selected Spices
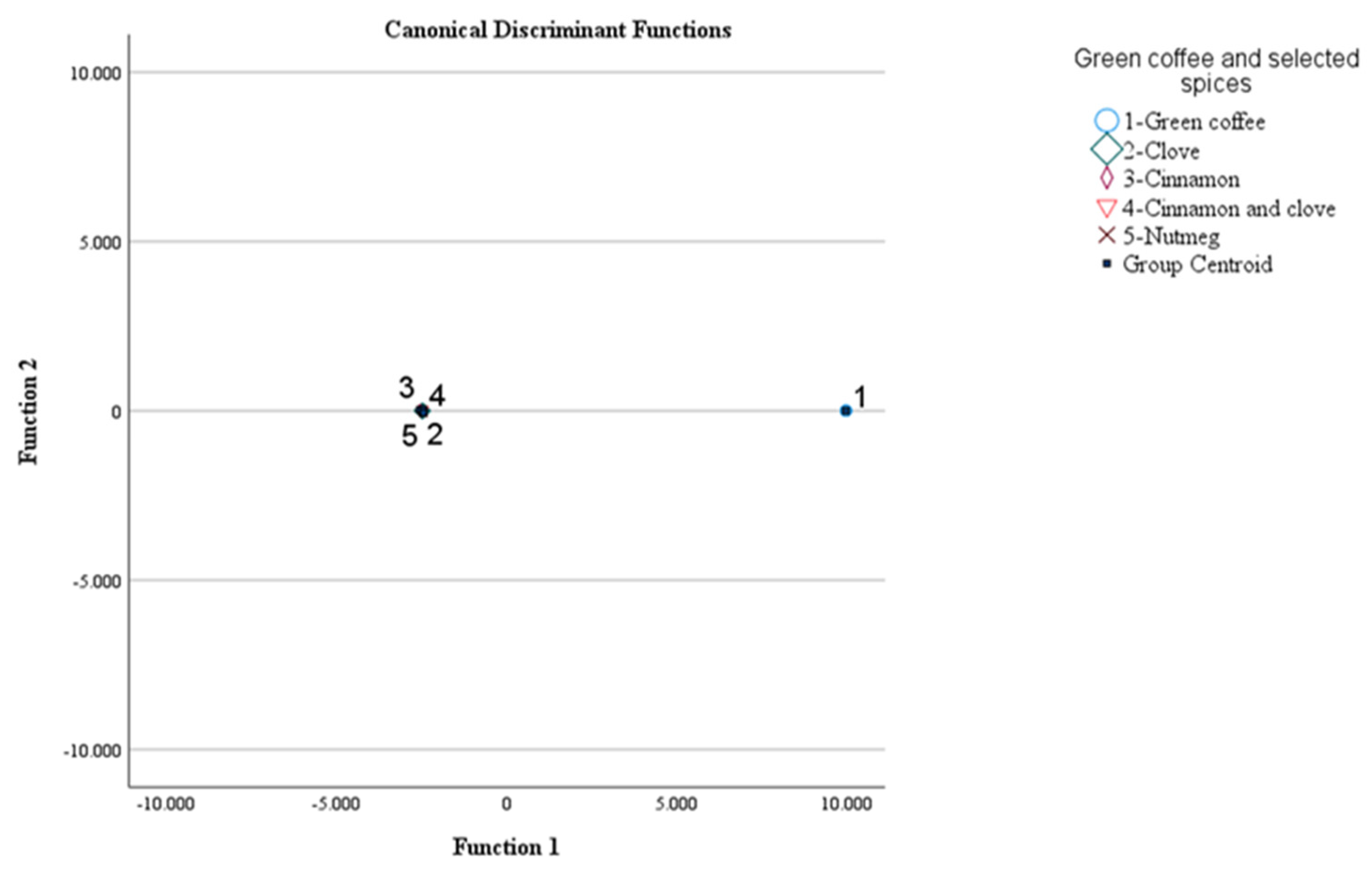
3.2. Typification of green coffee and selected spices using volatile compounds and supervised learning
3.2.1. MANOVA
3.2.2. LDA
| Volatile compounds | Discriminant functions | |||
| 1 | 2 | 3 | 4 | |
| Ethylene | 0.002 | 0.265* | -0.077 | 0.073 |
| Methyl acetate | 0.000 | 0.054 | -0.578* | -0.028 |
| 9-Hexadecen-1-ol | 0.002 | 0.161 | 0.570* | -0.358 |
| 3-Methylpentane | 0.128* | 0.100 | 0.274* | 0.187 |
| Ethyl acetate | 0.023 | -0.003 | -0.321 | 0.602* |
| Methyl alcohol | 0.001 | 0.177 | -0.146 | -0.384* |
| Toluene | 0.000 | -0.002 | -0.160 | 0.339* |
3.2.3. PLS
4. Discussion
5. Conclusions and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lazaridis, D.G. Physicochemical and Phytochemical Characterization of Green Coffee, Cinnamon Clove, and Nutmeg EEGO, and Aroma Evaluation of the Raw Powders. Eur. Food Res. Technol. 2023, 250, 83–96. [CrossRef]
- Muzolf-Panek, M.; Stuper-Szablewska, K. Comprehensive Study on the Antioxidant Capacity and Phenolic Profiles of Black Seed and Other Spices and Herbs: Effect of Solvent and Time of Extraction. Food Measure. 2021, 15, 5, 4561–4574. [CrossRef]
- Velázquez, R. Spice and Herb Frauds: Types, Incidence, and Detection: The State of the Art. Foods 2023 12, 18, 3373. [CrossRef]
- Willis, S. Chemopreventive and Anti-Inflammatory Potential of Select Herbal Teas and Cinnamon in an In-Vitro Cell Model. Food and Nutr. Sci. 2019, 10, 9, 1142. [CrossRef]
- Rao, Y.K. Evaluation of the Anti-Inflammatory and Anti-Proliferation Tumoral Cells Activities of Antrodia Camphorata, Cordyceps Sinensis, and Cinnamomum Osmophloeum Bark Extracts. J. Ethnopharmacol. 2007, 114, 1, 78-85. [CrossRef]
- Hossain, M. Antioxidant Activity of Spice Extracts and Phenolics in Comparsion to Synthetic Antioxidants. Rasayan J. Chem. 2008, 1, 751-756. [CrossRef]
- Naeem, N. Nutmeg: A Review on Uses and Biological Properties. IJCBS 2016.
- Khanam, M.; Dar, A.M.; Beg, F.; Khan, S.A.; Nayik, G.A.; Karabagias, I.K. Nutmeg Essential oil. Essential Oils 2023, Chapter 15, 391-399. [CrossRef]
- de Carvalho Couto, C.; dos Santos, D.G.; Oliveira, E.M.M; Freitas-Silva, O. Global Situation of Reference Materials to Assure Coffee, Cocoa, and Tea Quality and Safety. TrAC Trends Anal. Chem. 2021, 143, 116381. [CrossRef]
- Sualeh, A.; Tolessa, K.; Mohammed, A. Biochemical Composition of Green and Roasted Coffee Beans and Their Association with Coffee Quality from Different Districts of Southwest Ethiopia. Heliyon 2020, 6, 12, E05812. [CrossRef]
- Clifford, N.M.; Kerimi, A.; Williamsom G. Bioavailabity and Metabolism of Chlorogenic Acids (Acyl-Quinic Acids) in Humans. Compr. Rev. Food Res. Food Sci. Food Saf. 2020, 19, 4, 1299-1352. [CrossRef]
- Karabagias, I.K. Food Authentication and Adulteration Control Based on Metrics Data of Foods and Chemometrics. Eur. Food Res. Technol. 2024, 250, 1269-1283. [CrossRef]
- Karabagias, I.K. Advances of Spectometric Techniques in Food Analysis and Food Authentication Implemented with Chemometrics. Foods 2020, 9, 11, 1550. [CrossRef]
- Field, A. Discovering statistics using SPSS, 3rd ed.; Sage Publications Ltd, London, UK, 2009.
- Huberty, C.J.; Olejnik, S. Applied MANOVA and Discriminant analysis, 2nd ed.; John Wiley and Sons, New Jersey, USA, 2006.
- Karabagias, I.K.; Karabagias, V.K.; Badeka, A.V. Volatilome of white wines as an indicator of authenticity and adulteration control using statistical analysis. Aust. J. Grape and Wine Res. 2021, 27, 269-279. [CrossRef]
- Pua, A.; Goh, R.M.V.; Huang, Y.; Tang, V.C.Y.; Ee, K-H.; Cornuz, M.; Liu, S.Q.; Lassabliere, B.; Yu, B. Recent advances in analytical strategies for coffee volatile studies: Opportunities and challenges. Food Chem. 2022, 388, 132971. [CrossRef]
- Ribeiro, J. S.; Augusto, F.; Salva, T. J.G.; Thomaziello, R. A.; Ferreira, M. M. C. Prediction of sensory properties of Brazilian Arabica roasted coffees by headspace solid phase microextraction-gas chromatography and partial least squares. Anal. Chim. Acta 2009, 634, 172–179. [CrossRef]
- Karabagias, I.K.; Koutsoumpou, M.; Liakou, V. Characterization and geographical discrimination of saffron from Greece, Spain, Iran, and Morocco based on volatile and bioactivity markers, using chemometrics. Eur Food Res Technol. 2017, 243, 1577–1591. [CrossRef]
- Jeleń, H. H. Sample preparation for the study of flavor compounds in food. Hand-book of Sample Preparation. Pawliszyn, J.; Lord, H. L., Eds.; John Wiley & Sons, New Jersey, USA, 2010; 267–284. [CrossRef]
- Ferreira, T.; Farah, A.; Oliveira, T.C.; Lima I.S.; Vitório, F.; Oliveira, E.M.M. Using Real-Time PCR as a tool for monitoring the authenticity of commercial coffees. Food Chem. 2016, 199, 433-438. [CrossRef]
- Gottstein, V.; Lachenmeier, D.W.; Kuballa, T.; Bunzel, M. 1H NMR-based approach to determine the geographical origin and cultivation method of roasted coffee. Food Chem. 2023, 137278. [CrossRef]
- De Vivo, A.; Balivo, A.; Sarghini, F. Volatile Compound Analysis to Authenticate the Geographical Origin of Arabica and Robusta Espresso Coffee. Appl. Sci. 2023, 13, 5615. [CrossRef]
- Kim, S.Y.; Ko, J.A.; Kang, B.S.; Park, H.J. Prediction of key aroma development in coffees roasted to different degrees by colorimetric sensor array. Food Chem. 2018, 240, 808-816. [CrossRef]
- Karabagias, I.K.; Badeka, A.V. Physicochemical parameters and volatile compounds of herbal teas as indicators of products’ brand name using chemometrics. Eur Food Res Technol. 2021, 247, 961–974. [CrossRef]
- Pages-Rebull, J.; Pérez-Ràfols, C.; Serrano, N.; Manel del Valle; Díaz-Cruz, J.M. Classification and authentication of spices and aronmatic herbs by means of HPLC-UV and chemometrics. Food Biosci 2023, 52, 102401. [CrossRef]
- de Matos, R.C.; de Souza, T.J.T.; Scopel, M. Chemometrics as a Tool for Quality Control of Commercial Samples of Equisetum Teas in Brazil. Rev. Bras. Farmacogn. 2024. [CrossRef]
- Xu, Y.; Zhang, J.; Wang, Y. Recent trends of multi-source and non-destructive information for quality authentication of herbs and spices. Food Chem. 2023, 398, 133939. [CrossRef]
- Alberta Energy Regulator. Hydrocarbon Odour Management Protocol for Upstream Oil and Gas Point Source Venting and Fugitive Emissions, 2014. https://static.aer.ca/prd/documents/directives/D060_OdourManagementProtocol.pdf.
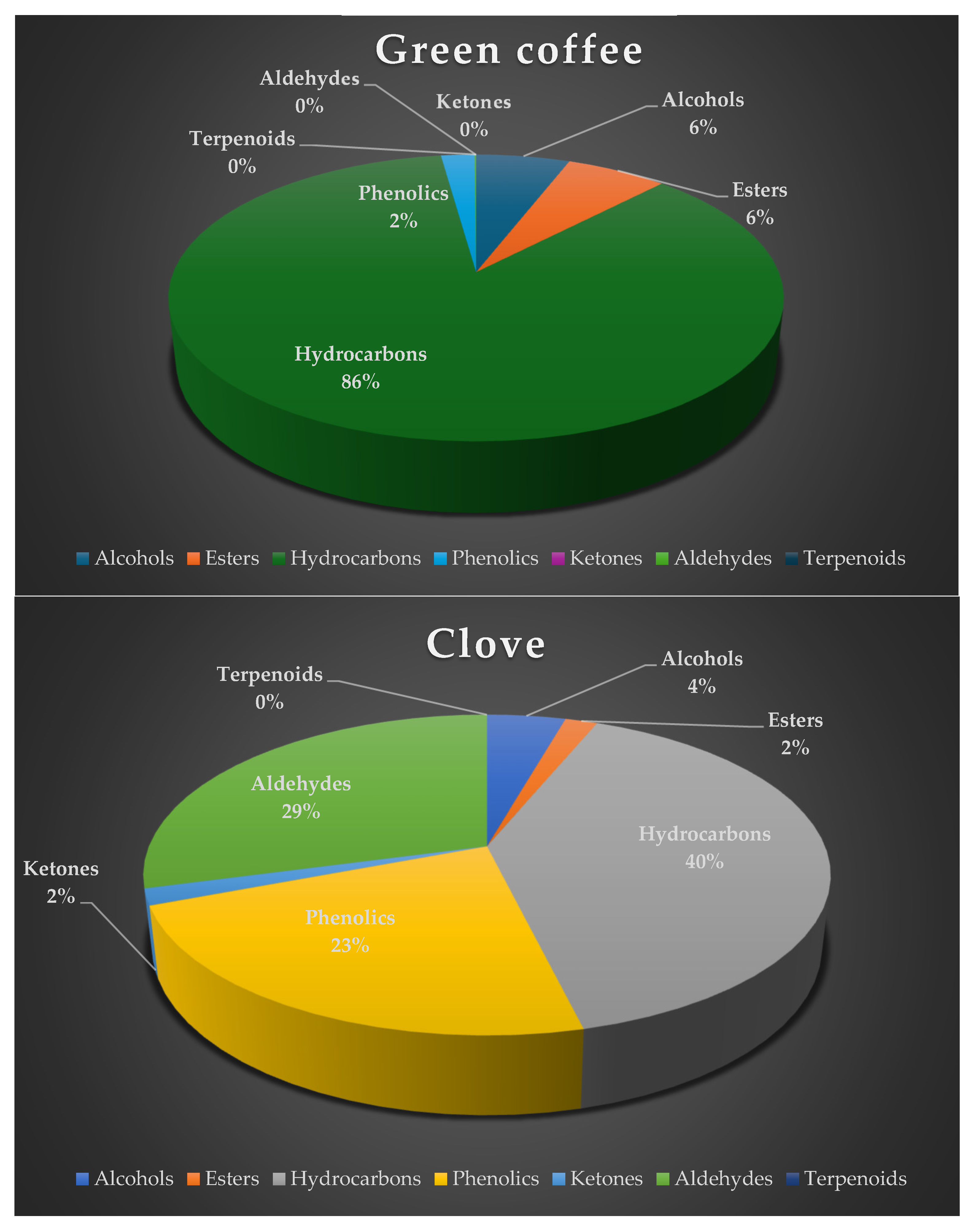
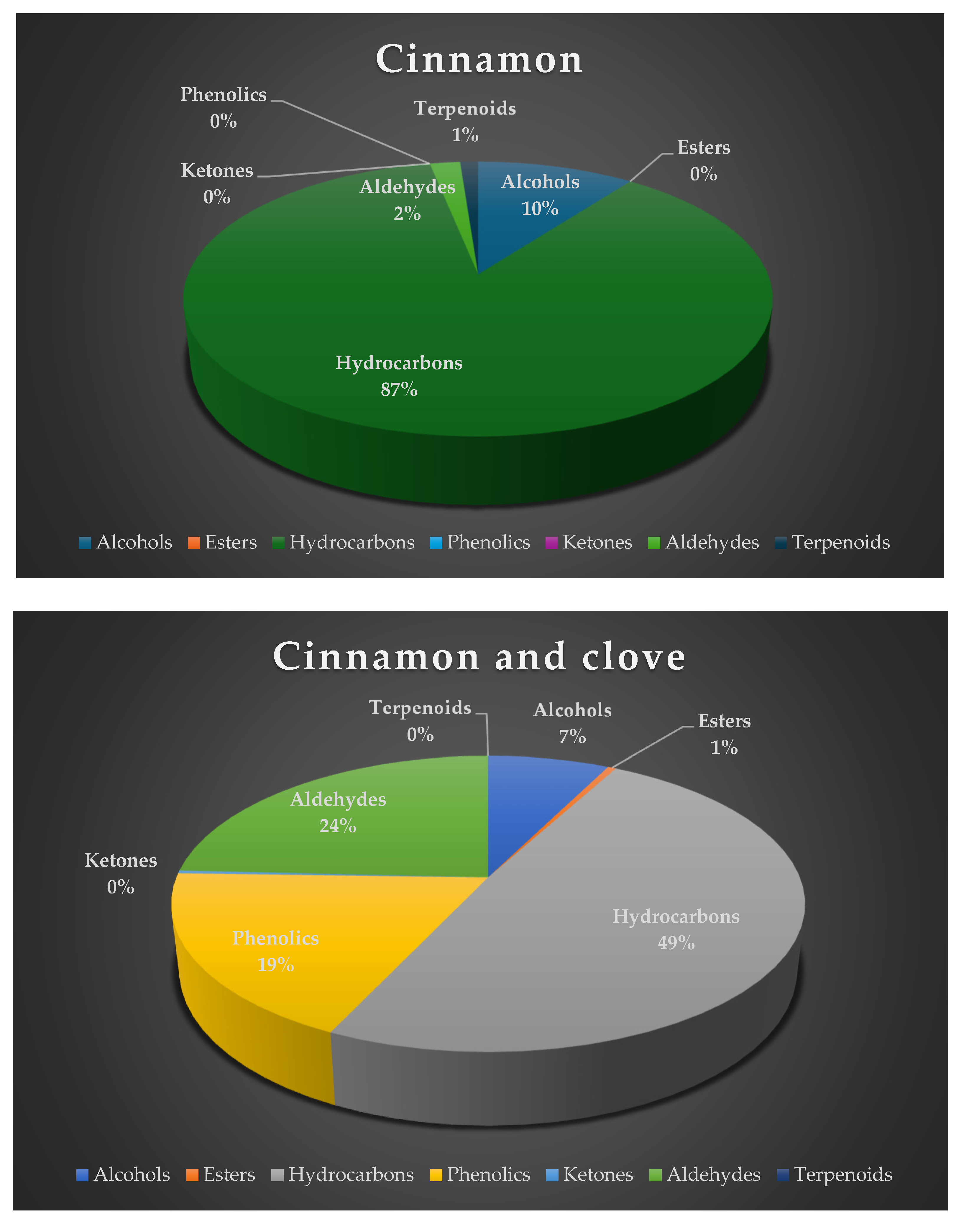
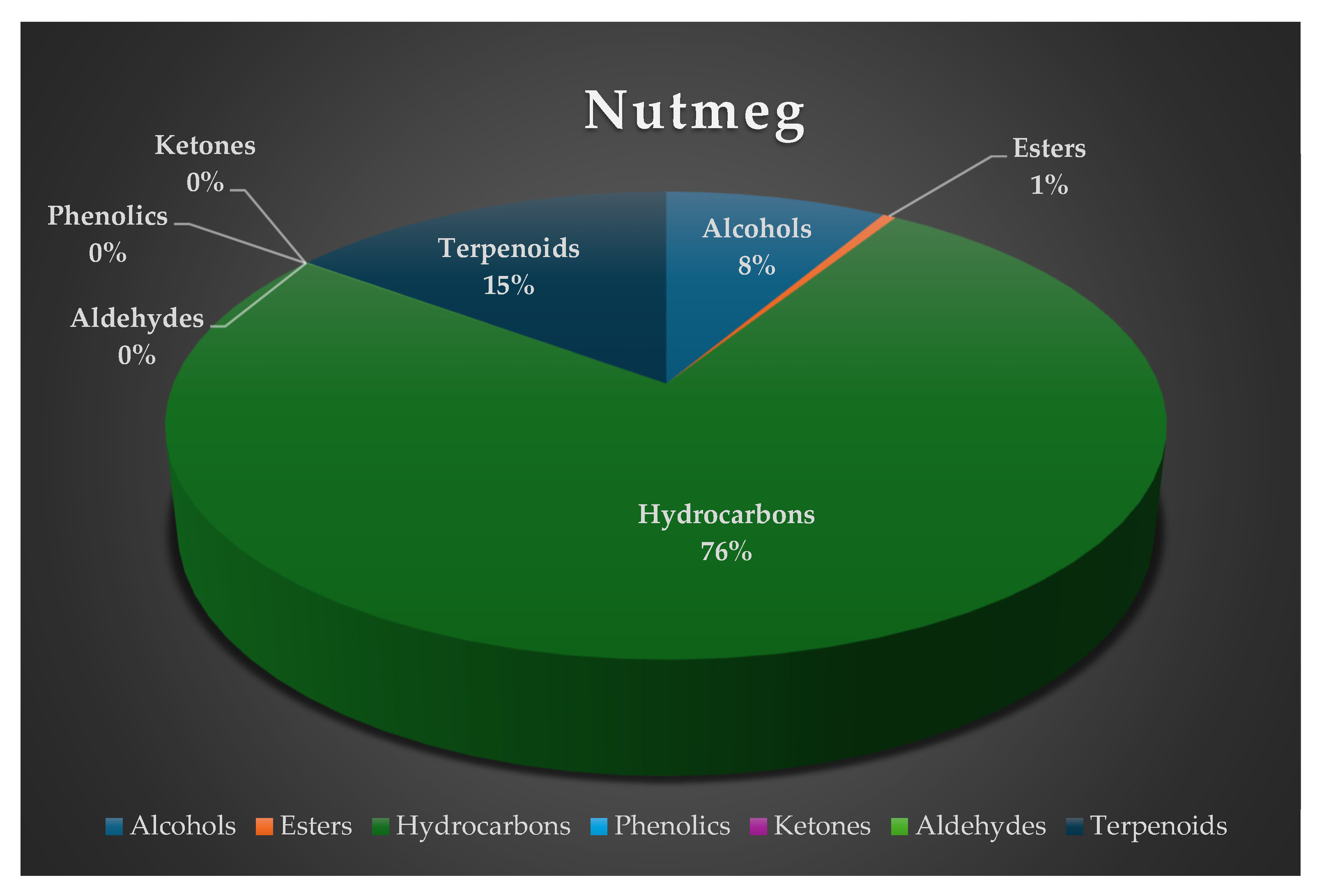
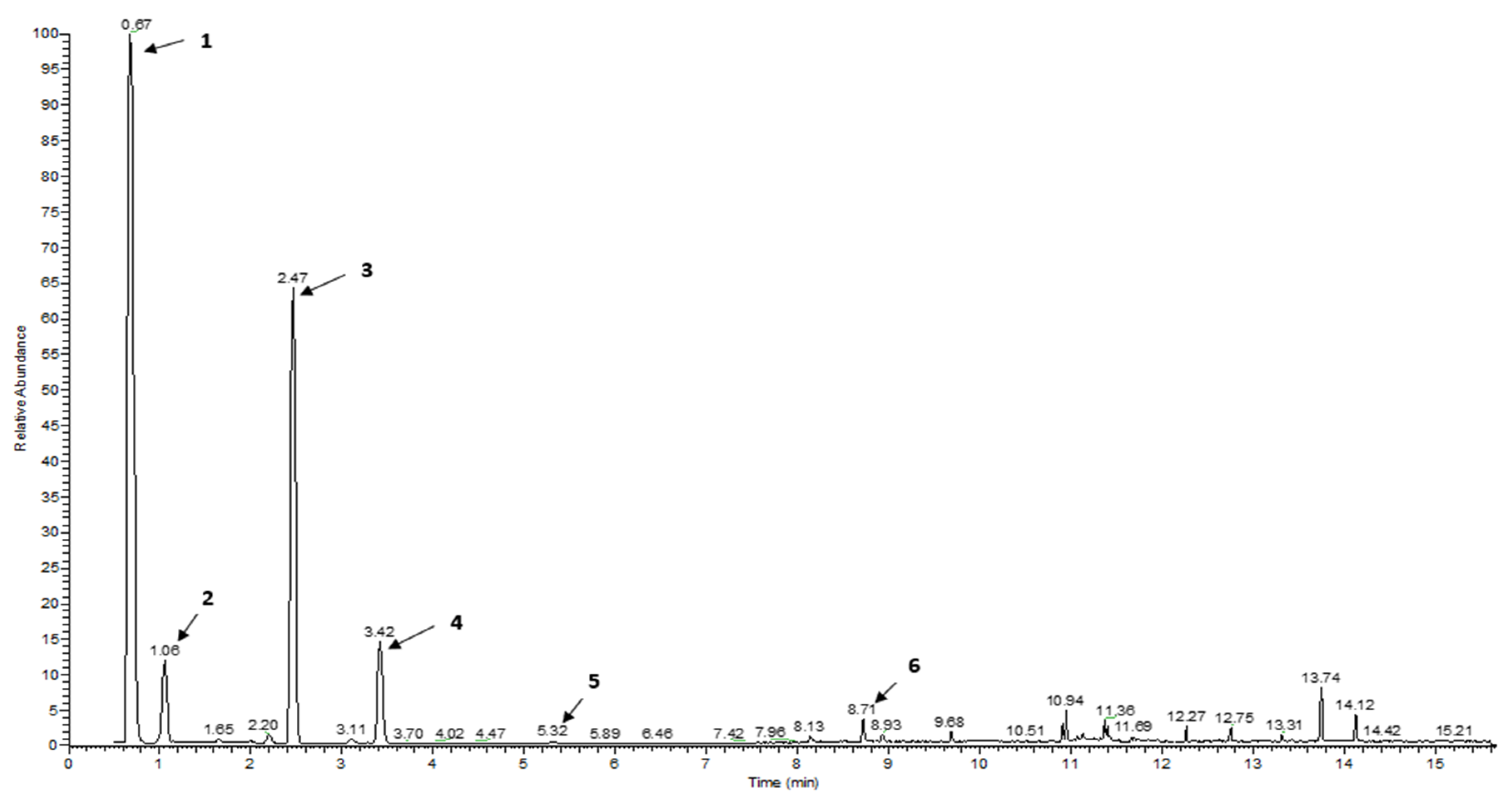
| RT | Volatile compounds | Green coffee (%Area ± SD) | Clove (%Area ±SD) |
Cinnamon (%Area ±SD) | Cinnamon and clove (%Area ±SD) |
Nutmeg (%Area ±SD) | Molecular formula | Odor descripion |
| Alcohols | ||||||||
| 1.06 | Methyl alcohol | 5.19 ± 0.33 | 3.35 ± 0.11 | 4.19 ± 0.39 | 6.00 ± 0.04 | 0.83 ± 0.20 | CH4O | Light alcoholic [13] |
| 5.32 | 9-Hexadecen-1-ol | 0.13 ± 0.06 | ni | 0.07 ± 0.01 | 0.05 ± 0.00 | ni | C16H32O | - |
| Esters | ||||||||
| 1.89 | Methyl acetate | ni | 0.28 ± 0.02 | ni | 0.27 ± 0.06 | ni | C16H32O | Sweet and fruity [14] |
| 3.42 | Ethyl acetate | 5.73 ± 0.00 | 0.20 ± 0.02 | ni | 0.06 ± 0.01 | 0.05 ± 0.04 | C4H8O2 | Sweet and fruity [15] |
| 12.66 | Methyl salicylate | ni | 0.90 ± 0.13 | ni | 0.09 ± 0.02 | ni | C8H8O3 | Sweet, fruity, rooty, beer-like [16] |
| Hydrocarbons | ||||||||
| 0.68 | Ethylene | 49.78 ± 0.02 | 27.96 ± 1.52 | 35.72 ± 1.91 | 40.02 ± 0.39 | 7.43 ± 0.70 | C2H4 | Fruity, pineapple, pungent, varnish [17] |
| 2.45 | 3-Methylpentane | 25.40 ± 0.03 | ni | ni | 0.13 ± 0.03 | ni | C6H14 | Oil-like, gasoline-like, asphalt-like [29] |
| 8.13 | Toluene | 0.73 ± 0.36 | 0.84 ± 0.25 | ni | ni | ni | C7H8 | Sweet, pungent [19] |
| 8.71 | 2-Chloro-2nitropropane | 0.71 ± 0.02 | 0.37 ± 0.06 | 0.27 ± 0.05 | 0.06 ± 0.00 | ni | C3H6ClNO2 | Oil-like, gasoline-like, asphalt-like [29] |
| 10.94 | Decane | 0.94 ± 0.05 | 1.26 ± 0.16 | ni | ni | ni | C10H22 | Oil-like, gasoline-like, asphalt-like [29] |
| Phenolics | ||||||||
| 6.07 | 4-(2-aminopropyl)-Phenol | 0.02 ± 0.00 | 0.01 ± 0.01 | ni | 0.01 ± 0.01 | ni | C9H13NO | - |
| 13.77 | Eugenol | 1.19±0.09 | 17.5 ± 0.38 | ni | 14.03 ± 0.15 | ni | C10H12O2 | Clove, pungent and spicy [20] |
| 15.22 | 2-Methoxy-4-(1-propenyl)-phenol acetate | 0.69±0.18 | ni | ni | 1.13±0.38 | ni | C12H14O3 | Weak rose-carnation [21] |
| Ketones | ||||||||
| 11.90 | 2-Nonanone | ni | 1.26 ± 0.86 | ni | 0.25 ± 0.01 | ni | C9H18O | Sweet, earthy and fishy [22] |
| Aldehydes | ||||||||
| 14.14 | Vitamin A aldehyde | 0.09 ± 0.006 | 22.19 ± 0.17 | 0.82 ± 0.11 | 19.85 ± 0.93 | 0.01 ± 0.01 | C20H28O | - |
| Terpenoids | ||||||||
| 12.51 | 4-Methyl-1-(1-methylethyl) 3-cyclohexen-1-ol | ni | ni | 0.49 ± 0.13 | ni | 1.46 ± 0.41 | C10H18O | Musty [23] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





