Submitted:
12 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Material and Methods
Study Design
Outcomes and Definitions
Variables of Interest
Study Size
Statistical Analysis
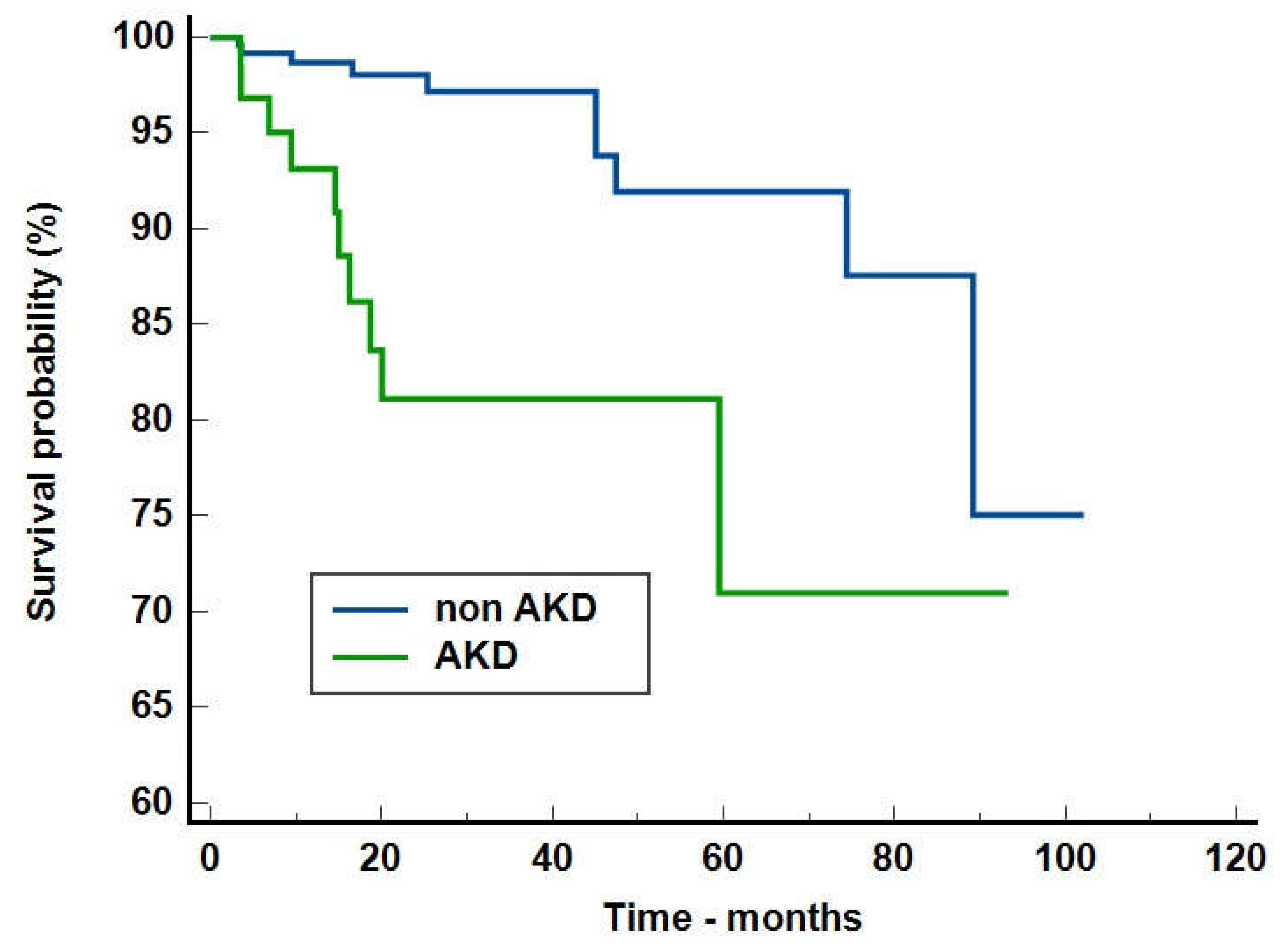
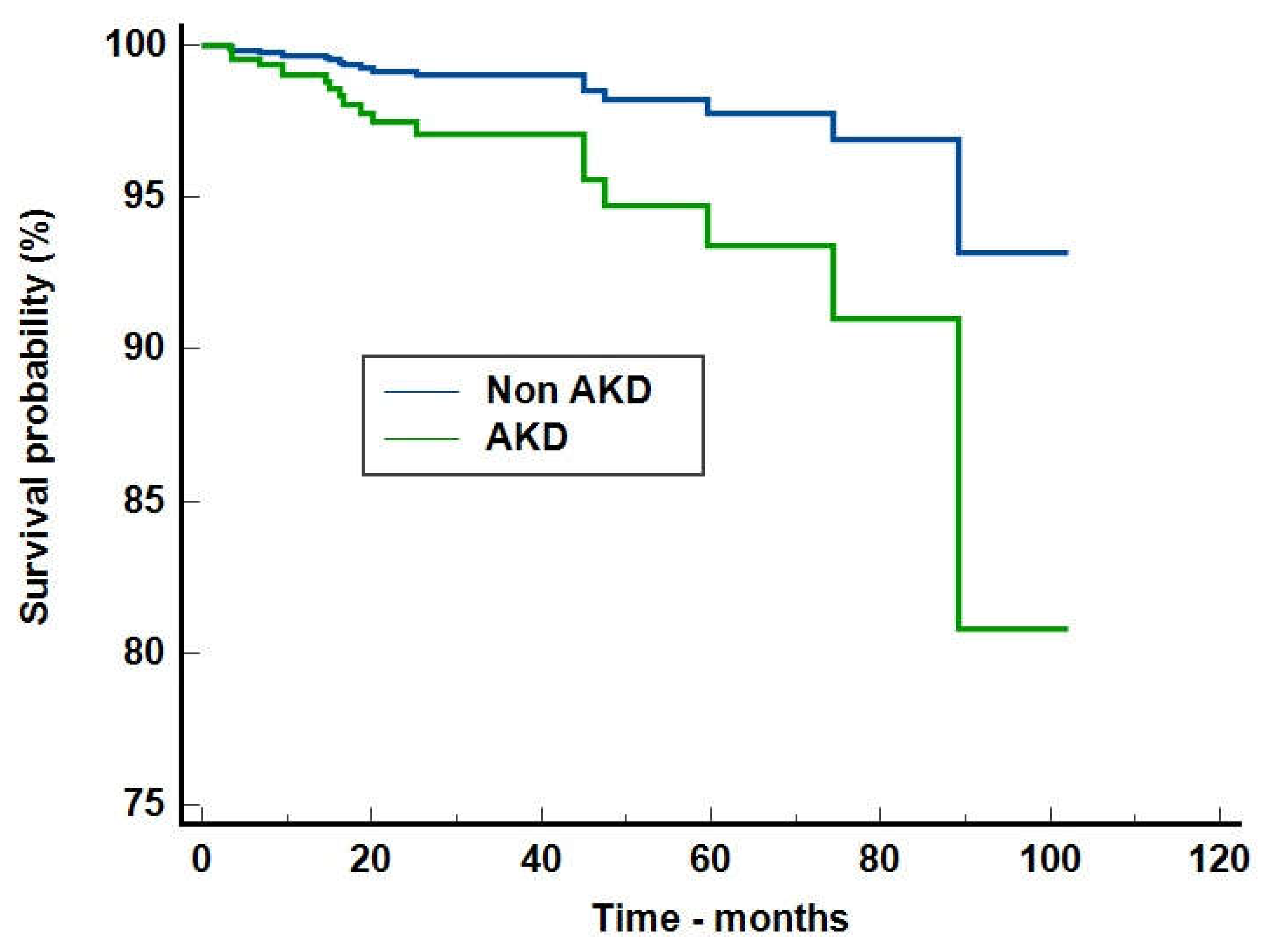
Results
Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgements
Conflicts of Interest
References
- Kellum JA, Romagnani P, Ashuntantang G, Ronco C, Zarbock A, Anders HJ. Acute kidney injury. Nat Rev Dis Primers. 2021 Jul 15;7(1):52. [CrossRef]
- Susantitaphong P, Cruz DN, Cerda J, Abulfaraj M, Alqahtani F, Koulouridis I, Jaber BL; Acute Kidney Injury Advisory Group of the American Society of Nephrology. World incidence of AKI: a meta-analysis. Clin J Am Soc Nephrol. 2013 Sep;8(9):1482-93. Epub 2013 Jun 6. Erratum in: Clin J Am Soc Nephrol. 2014 Jun 6;9(6):1148. [CrossRef]
- Hsu CN, Chen HL, Tain YL. Epidemiology and outcomes of community-acquired and hospital-acquired acute kidney injury in children and adolescents. Pediatr. Res 2018;83:622–629. [CrossRef]
- Kaddourah A, Basu RK, Bagshaw SM, Goldstein SL. AWARE investigators. Epidemiology of acute kidney injury in critically Ill children and young adults. N. Engl. J. Med. 2017;376(1):11–20. [CrossRef]
- Meena J, Mathew G, Kumar J, Chanchlani R. Incidence of Acute Kidney Injury in Hospitalized Children: A Meta–analysis. Pediatrics. 2023; 151(2):e2022058823. [CrossRef]
- Kidney Disease Improving global outcomes (KDIGO) acute kidney injury work group KDIGO clinical practice guideline for acute kidney injury. Kidney Int. 2012;2:1–138. [CrossRef]
- Chawla LS, Bellomo R, Bihorac A, Goldstein SL, Siew ED, Bagshaw SM, et al. Acute Disease Quality Initiative Workgroup 16.. Acute kidney disease and renal recovery: consensus report of the Acute Disease Quality Initiative (ADQI) 16 Workgroup. Nat Rev Nephrol. 2017 Apr;13(4):241-257. Epub 2017 Feb 27. [CrossRef]
- Kofman N, Margolis G, Gal-Oz A, Letourneau-Shesaf S, Keren G, Rozenbaum Z, et al. Long-term renal outcomes and mortality following renal injury among myocardial infarction patients treated by primary percutaneous intervention. Coron Artery Dis. (2019) 30:87–92. [CrossRef]
- Fujii T, Uchino S, Takinami M, Bellomo R. Subacute kidney injury in hospitalized patients. Clin J Am Soc Nephrol. (2014) 9:457–61. [CrossRef]
- Yan P, Duan XJ, Liu Y, Wu X, Zhang NY, Yuan F, et al. Acute kidney disease in hospitalized acute kidney injury patients. PeerJ. (2021) 9:e11400. [CrossRef]
- James MT, Levey AS, Tonelli M, Tan Z, Barry R, Pannu N, et al. Incidence and prognosis of acute kidney diseases and disorders using an integrated approach to laboratory measurements in a universal health care system. JAMA Network Open. (2019) 2:e191795. [CrossRef]
- Su CC, Chen JY, Chen SY, Shiao CC, Neyra JA, Matsuura R, et al. Outcomes associated with acute kidney disease: A systematic review and meta-analysis. EClinicalMedicine. 2022 Dec 13;55:101760. [CrossRef]
- Patel M, Hornik C, Diamantidis C, Selewski DT, Gbadegesin R. Patient level factors increase risk of acute kidney disease in hospitalized children with acute kidney injury. Pediatr Nephrol. 2023 Oct;38(10):3465-3474. [CrossRef]
- Patel M, Heipertz A, Joyce E, Kellum JA, Horvat C, Squires JE, et al. Acute kidney disease predicts chronic kidney disease in pediatric non–kidney solid organ transplant patients. Pediatr Transplant. 2022; 26(6):e14172. [CrossRef]
- LoBasso M, Schneider J, Sanchez-Pinto LN, Del Castillo S, Kim G, Flynn A, et al. Acute kidney injury and kidney recovery after cardiopulmonary bypass in children. Pediatr Nephrol. 2022; 37(3):659–665. [CrossRef]
- Daraskevicius J, Azukaitis K, Dziugeviciute-Tupko J, Peciulyte M, Planciunaite R, Vaitkeviciene G, et al. Phenotypes and Baseline Risk Factors of Acute Kidney Injury in Children After Allogeneic Hematopoietic Stem Cell Transplantation. Front Pediatr. 2020; 8:499. [CrossRef]
- Deng YH, Yan P, Zhang NY, Luo XQ, Wang XF, Duan SB. Acute Kidney Disease in Hospitalized Pediatric Patients With Acute Kidney Injury in China. Front Pediatr. 2022; 10:885055. [CrossRef]
- Ayesa N. Mian, George J. Schwartz, Measurement and Estimation of Glomerular Filtration Rate in Children, Advances in Chronic Kidney Disease, Volume 24, Issue 6, 2017, Pages 348-356, ISSN 1548-5595. 2017; 24. [CrossRef]
- Heilbron DC, Holliday MA, Al-Dahwi A. et al. Expressing glomerular filtration rate in children. Pediatr Nephrol 5, 5–11 (1991). [CrossRef]
- Schwartz GJ, Muñoz A, Schneider MF, et al. (2009) New equations to estimate GFR in children with CKD. J Am Soc Nephrol 20:629–637. [CrossRef]
- Lameire NH, Levin A, Kellum JA, et al. (2021) Harmonizing acute and chronic kidney disease definition and classification: report of a Kidney Disease: Improving Global Outcomes (KDIGO) Consensus Conference. Kidney Int 100:516–526. [CrossRef]
- KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney inter., Suppl. 2013; 3, 5–14. [CrossRef]
- Stierman B, Afful J, Carroll MD, Chen T, Davy O, Fink S et al. National Health and Nutrition Examination Survey 2017–March 2020 Prepandemic Data Files Development of Files and Prevalence Estimates for Selected Health Outcomes: Corporate Authors(s) : National Center for Health Statistics (U.S.) Published Date : 06/14/2021 Series : NHSR No. 158 Source : National Health Statistics Reports URL : https://stacks.cdc.gov/view/cdc/106273.
- Carullo N, Zicarelli M, Michael A, Faga T, Battaglia Y, Pisani A, et al. Childhood Obesity: Insight into Kidney Involvement. Int J Mol Sci. 2023 Dec 12;24(24):17400. [CrossRef]
- Namazzi R, Batte A, Opoka RO, Bangirana P, Schwaderer AL, Berrens Z, et al. Acute kidney injury, persistent kidney disease, and post-discharge morbidity and mortality in severe malaria in children: A prospective cohort study. EClinicalMedicine. 2022 Feb 12;44:101292. [CrossRef]
| Variable | AKD N=125 | Non-AKD N=611 | Total N=736 | P value | |
| Gender - male | 56 (44.8%) | 333 (54.5%) | 389 (52.9%) | 0.047 | |
| Environment - urban | 67 (53.6%) | 331 (54.2%) | 398 (54.1%) | 0.906 | |
| Age – years M+IQR | 12 (8-15) | 8 (4-14) | 9 (4-14) | <0.0001 | |
| Weight – kg (M+IQR) | 44 (25-59.25) | 26 (16-50) | 29 (16-52) | <0.0001 | |
| Height – cm (M+IQR) | 154 (134.75-164.25) | 126 (103-154) | 131 (104-158) | <0.0001 | |
| BMI – kg/sm (M+IQR) | 18.75 (15.48-23.91) | 17.04 (14.12-20.78) | 17.35 (14.31-21.59) | 0.0125 | |
| Maximum SCr mg/dl (M+IQR) | 1.41 (0.9-3.05) | 0.87 (0.61-1.18) | 0.92 (0.63-1.34) | <0.0001 | |
| Baseline SCr mg/dl (M+IQR) | 0.43 (0.31-0.7) | 0.36 (0.27-0.54) | 0.37 (0.28-0.56) | 0.0005 | |
| GFR ml/min/1.73sm (M+IQR) | 133.5 (83.2-174.3) | 138.4 (109.5-174-2) | 137.7 (106.6-174) | 0.087 | |
| Urea mmol/l (M+IQR) | 10.5 (6.4-23.21) | 5.75 (4.34-8.2) | 6.11 (4.45-9.76) | <0.0001 | |
| Uric acid umol/l (M+IQR) | 391 (259.5-618.5) | 284 (203.75-400) | 297 (217-441) | <0.0001 | |
| Serum proteins g/l (M+IQR) | 56 (44.55-65.4) | 60.2 (51.02-65.87) | 59.5 (49.6-65.77) | 0.031 | |
| Procalcitonin ng/ml | 2.13 (0.48-19.12) | 3.08 (0.37-17.86) | 3.03 (0.4-18.21) | 0.866 | |
| C Reactive protein mg/l (M+IQR) | 59.92 (11.41-184.05) | 28.73 (3.74-132.4) | 35.73 (4.58-14.06) | 0.004 | |
| Sodium mmol/l (M+IQR) | 134 (131-138) | 137 (134-139) | 137 (133-139) | 0.001 | |
| Potassium mmol/l (M+IQR) | 4.3 (3.6-5) | 4.3 (3.8-4.8) | 4.3 (3.8-4.8) | 0.71 | |
| Haemoglobin g/dl (M+IQR) | 9.1 (7.6-11.2) | 11.05 (9.2-12.5) | 10.8 (8.8-12.3) | <0.0001 | |
| AKI stage | 1 | 27 (21.6%) | 275 (45%) | 302 (41%) | <0.0001 |
| 2 | 44 (35.2%) | 195 (31.9%) | 239 (32.5%) | ||
| 3 | 54 (43.2%) | 414 (23.1%) | 195 (26.5%) | ||
| Hospital stay days (M+IQR) | 17 (9.75-31.5) | 8 (4-15) | 9 (5-18) | <0.0001 | |
| IH AKI | 67 (53.6%) | 353 (57.8%) | 420 (57.1%) | 0.39 | |
| ICU admission | 55 (44%) | 187 (30.6%) | 242 (32.9%) | 0.003 | |
| RRT necessity | 12 (9.6%) | 4 (0.7%) | 16 (2.2%) | <0.0001 | |
| Parameter | AKD N=125 | Non-AKD N=611 | Total N=736 | OR and 95%CI | P value | |
| Exposures | Mechanical ventilation | 36 (28.8%) | 136 (22.3%) | 172 (23.4%) | 1.41 (0.91-2.17) | 0.115 |
| Sepsis | 57 (45.6%) | 178 (29.1%) | 235 (31.9%) | 2.03 (1.37-3.02) | 0.0003 | |
| Critical illness | 17 (13.6%) | 45 (7.4%) | 62 (8.4%) | 1.97 (1.09-2.65) | 0.022 | |
| Hypovolemic shock | 3 (2.4%) | 18 (2.9%) | 21 (2.9%) | 0.81 (0.23-2.79) | 0.738 | |
| Trauma | 2 (1.6%) | 35 (5.7%) | 37 (5%) | 0.26 (0.06-1.12) | 0.054 | |
| Major non-cardiac surgery | 10 (8%) | 62 (10.1%) | 72 (9.8%) | 0.77 (0.38-1.54) | 0.461 | |
| Nephrotoxins | 49 (39.2%) | 122 (20%) | 171 (23.2%) | 2.58 (1.71-3.89) | <0.0001 | |
| Poisonous plants | 3 (2.4%) | 12 (2%) | 15 (2%) | 1.22 (0.34-4.41) | 0.735 | |
| Susceptibilities | Dehydration/Volume depletion | 115 (92%) | 584 (95.6%) | 699 (95%) | 0.53 (0.25-1.12) | 0.095 |
| Chronic kidney disease | 15 (12%) | 39 (6.4%) | 54 (7.3%) | 2 (1.06-2.73) | 0.028 | |
| Chronic disease (heart, liver and lung) | 19 (15.2%) | 68 (11.1%) | 87 (11.8%) | 1.43 (0.83-2.47) | 0.199 | |
| Diabetes mellitus | 3 (2.4%) | 25 (4.1%) | 28 (3.8%) | 0.57 (0.17-1.93) | 0.368 | |
| Neoplasia | 41 (32.8%) | 93 (15.2%) | 134 (18.2%) | 2.71 (1.76-4.19) | <0.0001 | |
| Anaemia | 92 (73.6%) | 295 (48.3%) | 387 (52.6%) | 2.98 (1.94-4.58) | <0.0001 | |
| Heart failure | 27 (21.6%) | 39 (6.4%) | 66 (9%) | 4.04 (2.36-6.9) | <0.0001 | |
| Arterial hypertension | 26 (20.8%) | 41 (6.7%) | 67 (9.1%) | 3.65 (2.13-6.23) | <0.0001 | |
| Stem cell transplant | 8 (6.4%) | 15 (2.5%) | 23 (3.1%) | 2.71 (1.12-6.55) | 0.021 | |
| Female gender | 69 (55.2%) | 278 (45.5%) | 347 (47.1%) | 1.47 (1-2.17) | 0.047 |
| AKI cause | OR (95%CI) | P value | |
| Prerenal | Hypovolemia/ Dehydration | 0.11 (0.06-0.19) | <0.0001 |
| Systemic vasodilatation | 1.25 (0.76-2.06) | 0.361 | |
| Hypoxia/ Ischemia | 0.74 (0.25-2.17) | 0.588 | |
| Renal | Renal microvasculature alterations | 6.89 (2.34-20.23) | 0.0004 |
| Glomerulonephritis | 4.74 (1.96-11.42) | 0.0005 | |
| Acute tubular necrosis | 5.01 (1.23-20.33) | 0.023 | |
| Acute tubule-interstitial nephritis | 3.39 (2.26-5.08) | <0.0001 | |
| Postrenal | Urinary tract malformation/ obstruction | 0.97 (0.32-2.9) | 0.966 |
| Non AKD N=611 (83%) | AKD stage 1 N=55 (7.5%) | AKD stage 2 N=26 (3.5%) | AKD stage 3 N=44 (6%) | P value | ||
| AKI | AKI stage 1 N=302 (41%) | 275 (45%) | 22 (40%) | 1 (3.8%) | 4 (9.1%) | <0.0001 |
| AKI stage 2 N=239 (32.5%) | 195 (31.9%) | 23 (41.8%) | 16 (61.5%) | 5 (11.4%) | ||
| AKI stage 3 N=195 (26.5%) | 141 (23.1%) | 10 (18.2%) | 9 (34.6%) | 35 (79.5%) | ||
| ICU admission | 187 (30.6%) | 17 (30.9%) | 9 (34.6%) | 29 (65.9%) | <0.0001 | |
| Hospital stay | 8 (4-15) | 12 (8-24.2) | 18 (10-33) | 24 (14.5-39.5) | <0.0001 | |
| Baseline creatinine | 0.36 (0.27-0.54) | 0.5 (0.36-0.71) | 0.34 (0.27-0.5) | 0.39 (0.32-0.77) | 0.0005 | |
| Baseline GFR | 138.4 (109.5-174.2) | 125.5 (88.3-173.2) | 140.2 (95.3-214.3) | 132.7 (69.3-170.9) | 0.207 | |
| AKD duration | - | 6 (3-19.75) | 8.5 (3-20) | 11 (5-23) | 0.354 | |
| Subsequent AKI events | 54 (8.8%) | 3 (5.5%) | 3 (11.5%) | 3 (6.8%) | 0.752 | |
| Crude analysis OR and 95%CI | Adjusted analysis OR and 95%CI | |
| Mortality risk during hospitalization | 4.22 (2.11-8.4), p<0.0001 | 3.13 (1.5-6.51), p=0.0022 |
| Mortality risk after discharge | 2.73 (1.003-7.44), p=0.049 | 2.73 (1.003-7.44), p=0.049 |
| Overall mortality | 3.75 (2.09-6.72), p<0.0001 | 2.98 (1.63-5.45), p=0.0004 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





