Submitted:
12 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Study Design and Population:
2.2. Ethical Considerations:
2.3. Study Variables:
2.4. Definitions:
2.5. Calculation of Cardiovascular Risk:
2.6. Statistical Analysis:
3. Results
3.1. Clinical and Demographic Characteristics of the Derivation Cohort
3.2. Cardiovascular Risk in the Derivation Cohort
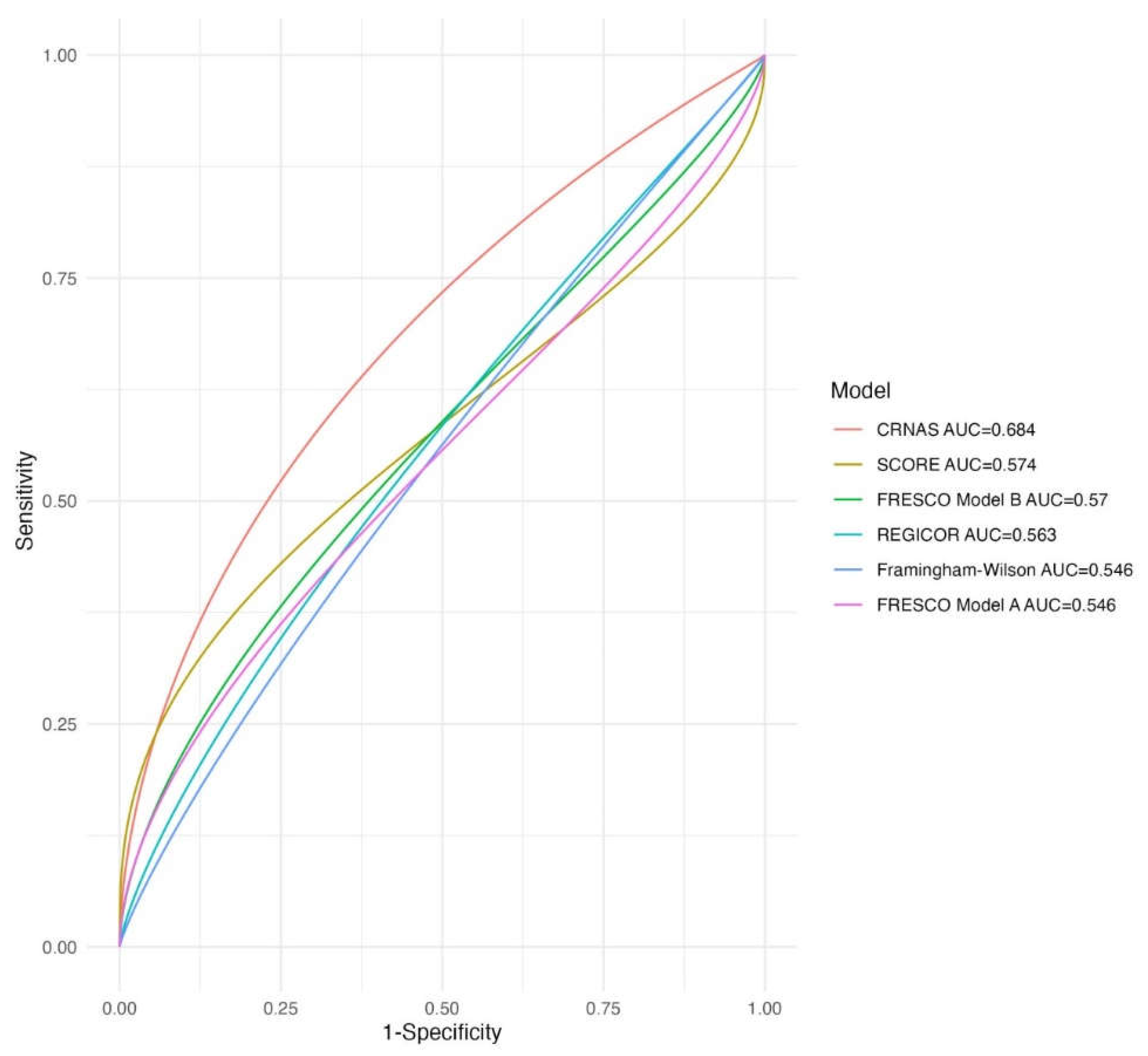
3.3. Predictive Capacity for Advanced CRN of the Cardiovascular Risk Scores
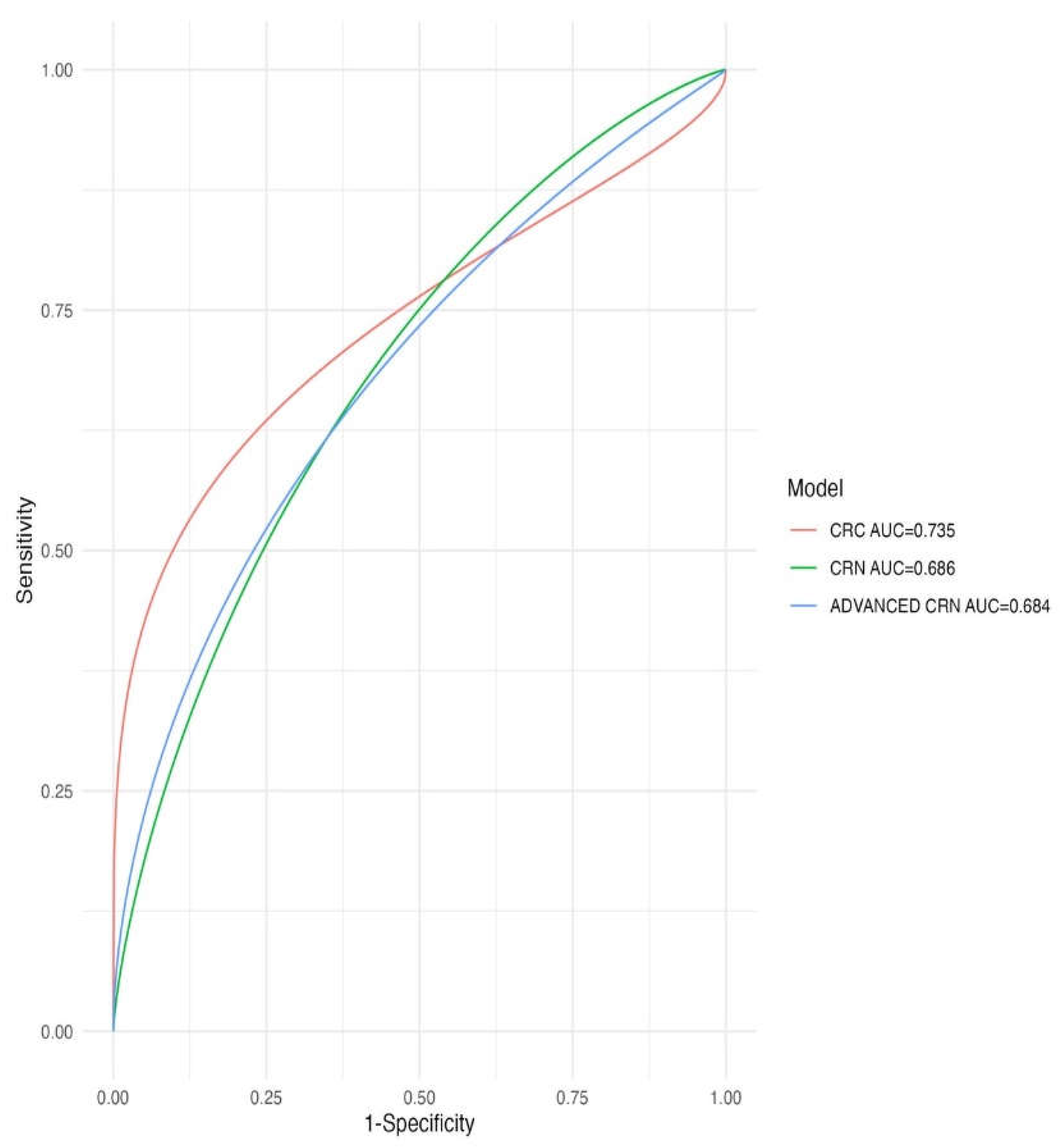
3.4. Predictive Capacity of the New Score
3.5. Internal and External Validation of the CRNAS
4. Discussion
Supplementary Materials
Author Contributions
Acknowledgements
Conflicts of Interest
Ethics Approval and Informed Consent
References
- World Health Organization (WHO). Global Health Estimates 2020: Deaths by Cause, Age, Sex, by Country and by Region, 2000-2019. WHO; 2020. http://who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
- Martínez ME, Baron JA, Lieberman DA, et al. A pooled analysis of advanced colorectal neoplasia diagnoses after colonoscopic polypectomy. Gastroenterology. 2009; 136:832–841. [CrossRef]
- Regula J, Rupinski M, Kraszewska E, et al. Colonoscopy in colorectal- cancer screening for detection of advanced neoplasia. N Engl J Med. 2006; 355:1863–1872. [CrossRef]
- Botteri E, Iodice S, Bagnardi V, Raimondi S, Lowenfels AB, Maisonneuve P. Smoking and colorectal cancer: a meta-analysis. JAMA.2008; 300:2765–2778.
- Esposito K, Chiodini P, Colao A, Lenzi A, Giugliano D. Metabolic syndrome and risk of cancer: a systematic review and meta-analysis. Diabetes Care. 2012; 35:2402-11.
- Yang SY, Kim YS, Chung SJ, et al. Association between colorectal adenoma and coronary atherosclerosis detected by CT coronary angiography in Korean men. J Gastroenterol Hepatol. 2010;25: 1795–1799. [CrossRef]
- Hee YJ, Bang CS, Baik GH, Shin IS, Suk KT, Park TY, Kim DJ. Association between ischemic heart disease and colorectal neoplasm: a systematic review and meta-analysis. Springerplus. 2016; 5(1):1664. [CrossRef]
- Hueb W, Lopes N, Gersh BJ, et al. Ten-year follow-up survival of the Medicine, Angioplasty, or Surgery Study (MASS II): a randomized controlled clinical trial of 3 therapeutic strategies for multivessel coronary artery disease. Circulation. 2010; 122:949–957.
- Basyigit S, Ozkan S, Uzman M, et al. Should screening for colorectal neoplasm be recommended in patients at high risk for coronary heart disease: a cross-sectional study. Medicine (Baltimore). 2015; 94: e793.
- Niederseer D, Stadlmayr A, Huber-Schönauer U, et al. Cardiovascular Risk and Known Coronary Artery Disease Are Associated With Colorectal Adenoma and Advanced Neoplasia. J Am Coll Cardiol. 2017; 69: 2348-2350. [CrossRef]
- Wohlfahrt P, Bruthans J, Krajčoviechová A, et al. Systematic COronary Risk Evaluation (SCORE) and 20-year risk of cardiovascular mortality and cancer. Eur J Intern Med. 2020;79:63-69. [CrossRef]
- Cubiella J, Marzo-Castillejo M, Mascort-Roca JJ, et al. Guía de práctica clínica. Diagnóstico y prevención del cáncer colorrectal. Actualización 2018. Gastroenterol Hepatol. 2018; 41:585–96. [CrossRef]
- Betés M, Muñoz-Navas MA, Duque JM, et al. Use of colonoscopy as a primary screening test for colorectal cancer in average risk people. Am J Gastroenterol. 2003; 98(12):2648–2654.
- Cai QC, Yu ED, Xiao Y, et al. Derivation and validation of a prediction rule for estimating advanced colorectal neoplasm risk in average-risk Chinese. Am J Epidemiol. 2012; 175(6):584-93. [CrossRef]
- WHO Classification of Tumours Editorial Board. WHO Classification of Tumours of the Digestive System. 5th ed, vol. 1. Lyon: IARC, 2019. [CrossRef]
- Piepoli MF, Hoes AW, Agewall S, et al; ESC Scientific Document Group. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts)Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur Heart J. 2016;37(29):2315-2381.
- Mostaza JM, Pintó X, Armario P, et al. SEA 2022 Standards for Global Control of Cardiovascular Risk. Clin Investig Arterioscler. 2022; 34(3):130-179.
- Wilson Peter WF, D’agostino R, Levy D, Belanger A, Silbershatz H, Kannel W. Prediction of Coronary Heart Disease Using Risk Factor categories. Circulation. 1998; 97: 1837-47. [CrossRef]
- Marrugat J, D'Agostino R, Sullivan L, et al. An adaptation of the Framingham coronary heart disease risk function to European Mediterranean areas. J Epidemiol Community Health. 2003; 57:634-8. [CrossRef]
- Conroy RM, Pyörälä K, Fitzgerald AP, et al. Estimation of ten-year risk of fatal cardiovascular disease in Europe: the SCORE project. Eur Heart J. 2003; 24:987---1003. [CrossRef]
- Marrugat J, Subirana I, Ramos R, et al FRESCO Investigators. Derivation and validation of a set of 10-year cardiovascular risk predictive functions in Spain: the FRESCO Study. Prev Med. 2014; 61:66-74. [CrossRef]
- Equipo central R. R. Un lenguaje y entorno para la computación estadística. R Fundación para la Computación Estadística. Viena, Austria. 2019. Disponible en: http://www.R-project.org.).
- Masoudkabir F, Sarrafzadegan N, Krahn A, et al. Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention Cardiovascular disease and cancer: evidence for shared disease pathways and pharmacologic prevention HHS Public Access. Atherosclerosis. 2017; 263:343–351. [CrossRef]
- Quintero E, Carrillo M, Leoz ML, et al. Oncology Group of the Asociación Española de Gastroenterología (AEG). Risk of advanced neoplasia in first-degree relatives with colorectal cancer: A large multicenter cross-sectional study. PLoS Med. 2016; 13(5):e1002008. [CrossRef]
- Butterworth AS, Higgins JPT, Pharoah P. Relative and absolute risk of colorectal cancer for individuals with a family history: a meta-analysis. Eur J Cancer. 2006; 42:216-27. [CrossRef]
- Peng L, Weigl K, Boakye D, Brenner H. Risk Scores for Predicting Advanced Colorectal Neoplasia in the Average-risk Population: A Systematic Review and Meta-analysis. Am J Gastroenterol. 2018; 113(12):1788-1800. [CrossRef]
- Chen Y, Chen X, Wang X, Liu Z, Zhou H, Xu S. Association of Cardiovascular Risk Assessment with Early Colorectal Neoplasia Detection in Asymptomatic Population: A Systematic Review and Meta-Analysis. Clin Epidemiol. 2020; 12: 865-873. [CrossRef]
- Wernly S, Semmler G, Völkerer A, et al. Cardiovascular Risk Assessment by SCORE2 Predicts Risk for Colorectal Neoplasia and Tumor-Related Mortality. J Pers Med. 2022;12(5):848. [CrossRef]
- Kaminski MF, Polkowski M , Kraszewska E , Rupinski M , Butruk E , Regula J . A score to estimate the likelihood of detecting advanced colorectal neoplasia at colonoscopy. Gut. 2014; 63:1112–19. [CrossRef]
- Jeon J, Du M, Schoen RE, et al. Determining risk of colorectal cancer and starting age of screening based on lifestyle, environmental, and genetic factors. Gastroenterology. 2018; 154(8): 2152–64. [CrossRef]
- Ibáñez-Sanz G, Díez-Villanueva A, Alonso MH, et al. Risk Model for Colorectal Cancer in Spanish Population Using Environmental and Genetic Factors: Results from the MCC-Spain study. Sci Rep. 2017; 7:43263.
- Gargallo-Puyuelo CJ, Aznar-Gimeno R, Carrera-Lasfuentes P, et al. Predictive Value of Genetic Risk Scores in the Development of Colorectal Adenomas. Dig Dis Sci. 2022; 67(8):4049-4058. [CrossRef]
- Wong MCS, Leung EY, Chun SCC, Wang HH, Huang J. Prediction of advanced colorectal neoplasia based on metabolic parameters among symptomatic patients. J Gastroenterol Hepatol. 2023; 38(9):1576-1586. [CrossRef]
- Bertagnolli MM, Eagle CJ, Zauber AG, et al. APC Study Investigators. Celecoxib for the prevention of sporadic colorectal adenomas. N Engl J Med. 2006; 355:873–884. [CrossRef]
- Arber N, Eagle CJ, Spicak J, et al. A randomized controlled trial of celecoxib for prevention of colorectal sporadic adenomatous polyps (PreSAP). N Engl J Med. 2006; 355 (9):885–895.
| CHARACTERISTICS | TOTAL COHORT (n=1049) |
NO NEOPLASIA (n=670) |
NON-ADVANCED CRN (n=151) | ADVANCED CRN (n=228) |
p-value |
|---|---|---|---|---|---|
| Age ( years) Median (Q1-Q3) |
58 ( 51.3-64.5) | 56 (49.2 - 63.7) | 59.5 (54.1 - 63.8) | 60.9 (54.7 - 66.4) | <0.001 |
| Sex-Male | 463 (44%) | 238 (36%) | 82 (54%) | 143 (63%) | <0.001 |
| Tobacco Current smoker Former smoker |
246(33%) 216(21%) |
150(22%) 125(19%) |
34 (23%) 34 (23%) |
62 (27%) 57(25%) |
0.056 |
| Alcohol consumption | 368(35%) | 200(30%) | 67 (44%) | 101 (44%) | <0.001 |
| Obesity | 243(25%) | 141(22%) | 37 (28%) | 65 (30%) | 0.044 |
| Hypertension | 260(25%) | 156(23%) | 37 (25%) | 67 (29%) | 0.182 |
| Diabetes | 92 (9%) | 45 (7%) | 13 (9%) | 34 (15%) | <0.001 |
| Hypercholesterolemia | 567(54%) | 367(55%) | 91 (60%) | 109 (48%) | 0.048 |
| Hypertriglyceridemia | 115(11%) | 70 (11%) | 11 (8%) | 34 (15%) | 0.058 |
| NSAID use | 484(46%) | 323(48%) | 71 (47%) | 90 (39%) | 0.071 |
| Antiplatelet use | 82 (8%) | 49 (7%) | 11 (7%) | 22 (10%) | 0.507 |
| First-degree family history of CRC | 375 (36%) |
259(39%) | 43 (28%) | 73 (32%) | 0.026 |
| Second-degree family history of CRC | 133(13%) | 90 (13%) | 17 (11%) | 26 (11%) | 0.621 |
| CHARACTERISTICS | OR | 95%CI | p-value |
|---|---|---|---|
| Sex-Female | 0.40 | 0.29-0.56 | <0.001 |
| Age Total Cholesterol HDL-cholesterol BMI Systolic blood pressure Diastolic blood pressure |
1.05 1 1 0.99 1 1 |
1.03-1.06 1-1 0.99-1.01 0.95-1.03 0.99-1.02 0.99-1.03 |
<0.001 0.7 0.7 0.5 0.6 0.3 |
| Diabetes | 1.66 | 1-2.71 | 0.047 |
| Current smokers Antihypertensive treatment First-degree family history of CRC |
1.55 0.82 0.98 |
1.07-2.24 0.55-1.21 0.7-1.37 |
0.019 0.3 >0.9 |
| CARDIOVASCULAR RISK SCORES | TOTAL COHORT (n =1049) |
NO NEOPLASIA (n =670) |
NON-ADVANCED CRN (n =151) |
ADVANCED CRN (n=228) |
p-value |
|---|---|---|---|---|---|
| FRAMINGHAM-Wilson | 8.5 (5.1 - 12.5) | 7.9 (4.7 - 12) | 9.2 (6.6 - 13.4) | 9.3 (5.5 - 13.1) | <0.001 |
| REGICOR | 3.1 (1.9 - 4.7) | 2.8 (1.7 - 4.4) | 3.6 (2.4 - 5.2) | 3.6 (2.2 - 5.2) | <0.001 |
| SCORE | 0.7 (0.2 - 1.5) | 0.6 (0.2 - 1.4) | 0.6 (0.3 - 1.3) | 0.8 (0.3 - 1.7) | <0.001 |
| FRESCO-Model A | 3.1 (1.6 - 4.7) | 3.1 (1.6 - 4.7) | 3 (1.6 - 4.2) | 3 (1.7 - 5.2) | 0.353 |
| FRESCO-Model B | 3.2 (1.7 - 5.8) | 3(1.5 - 5.5) | 3 (2.1 - 5.4) | 3.7 (2.2 - 7.2) | <0.001 |
| Predictive capacity of advanced CRN. | ||
| MODEL |
AUC derivation cohort (n=1049) |
AUC validation cohort (n=308) |
| FRAMINGHAM-Wilson | 0.55(0.50-0.59) | 0.51(0.41-0.61) |
| REGICOR | 0.56(0.52-0.61) | 0.51(0.42-0.61) |
| SCORE | 0.57(0.53-0.62) | 0.58(0.48-0.67) |
| FRESCO Model A | 0.55(0.50-0.59) | 0.55(0.45-0.64) |
| FRESCO Model B | 0.57(0.53-0.61) | 0.58(0.48-0.68) |
| CRNAS | 0.68(0.64-0.73) | 0.67(0.57-0.76) |
| Predictive capacity of CRN | ||
| MODEL |
AUC derivation cohort (n=1049) |
AUC validation cohort (n=308) |
| FRAMINGHAM-Wilson | 0.56(0.52-0.6) | 0.59(0.52-0.66) |
| REGICOR | 0.58(0.54-0.62) | 0.59(0.52-0.66) |
| SCORE | 0.55(0.52-0.59) | 0.59(0.52-0.66) |
| FRESCO Model A | 0.52(0.48-0.56) | 0.58(0.51-0.65) |
| FRESCO Model B | 0.55(0.52-0.6) | 0.62(0.55-0.69) |
| CRNAS | 0.69(0.65-0.72) | 0.62(0.55-0.69) |
| Predictive capacity of CRC | ||
| MODEL |
AUC derivation cohort (n=1049) |
AUC validation cohort (n=308) |
| FRAMINGHAM-Wilson | 0.56(0.45-0.66) | 0.55(0.4-0.7) |
| REGICOR | 0.57(0.46-0.67) | 0.52(0.38-0.67) |
| SCORE | 0.61(0.5-0.71) | 0.63(0.5-0.78) |
| FRESCO Model A | 0.64(0.53-0.74) | 0.55(0.4-0.7) |
| FRESCO Model B | 0.61(0.51-0.72) | 0.56(0.42-0.71) |
| CRNAS | 0.74(0.63-0.84) | 0.74(0.59-0.88) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





