Submitted:
11 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
I. Introduction
II. Theoretical Framework
II.1. Protein Glycosylation
- Attachment through the nitrogen (N) of the amide group of the asparagine (Asn) amino acid (or less commonly, glutamine), resulting in N-glycoproteins (the mechanism known as N-glycosylation).
- Attachment through the oxygen (O) of the hydroxyl group of the serine (Ser) or threonine (Thr) amino acids, forming O-glycoproteins (the mechanism known as O-glycosylation).
II.1.1. N-Glycosylation
- The first stage involves the synthesis of the standard oligosaccharide (or precursor oligosaccharide) and its transfer to the nascent protein.
- The second stage involves the final processing of the oligosaccharide attached to the protein.
II.2. CDG Where N-Glycosylation Is Affected. Current Nomenclature
II.3. Molecular Basis of CDG
II.4. Diagnosis of Congenital Disorders of N-Glycosylation
- Differential composition of the amino acid sequence in the primary structure, resulting from genetic polymorphisms. Among the allelic variants, the most prevalent is Tf C; of these, 16 subtypes have been described, with Tf C1 being present in over 95% of cases and having a pI of 5.4. Variant Tf B has a pI of 5.2 and Tf D, 5.7. This comparison of pI is made considering they have the same Fe3+ content and carbohydrate composition [51,52,53].
- Differential composition of carbohydrate chains (glycoforms). The major isoform, known as tetrasialotransferrin (tetrasialoTf) (pI 5.4), presents two biantennary N-glycan chains, corresponding to four terminations in Neu5Ac residues. However, isoforms can vary from asialotransferrin (asialoTf) to octasialotransferrin (octasialoTf), meaning from no chains to two tetraantennary ones, respectively. Nonetheless, the isoforms following tetrasialoTf in concentration are pentasialotransferrin (pentasialoTf) and trisialotransferrin (trisialoTf). Additionally, very small amounts (less than 2.5%) of isoforms with fewer than three Neu5Ac residues are determined; these isoforms are generally termed carbohydrate deficient transferrin (CDT), corresponding to asialoTf (pI 5.9), monosialotransferrin (monosialoTf) (pI 5.8), and disialotransferrin (disialoTf) (pI 5.7). From a more concrete perspective, the Neu5Ac content can range from 0-8 and determines the microheterogeneity of the transferrin molecule. The variations in the pIs of these isoforms are 0.1 units for each Neu5Ac residue attached [11,40,53].
- Differential Fe3+ content. Each Tf molecule can contain a maximum of two Fe3+ depending on the iron supply to the body. The pI of the Tf molecule decreases by approximately 0.2 units for each Fe3+ bound [53].
III. Scientific Problem and Our Hypothesis
III.1. Scientific Problem
III.2. Hypothesis
IV.1. General Objective
- To propose a method that allows the evaluation of transferrin glycoforms for the diagnosis of congenital defects of N-glycosylation in Cuba.
IV.2. Specific Objectives
- To implement the procedure of serum transferrin glycoform isoelectric focusing for the biochemical diagnosis of congenital defects of N-glycosylation.
- To evaluate the isoelectric focusing patterns of serum transferrin glycoforms in stored patient samples who presented with clinical manifestations suggestive of CDG.
V. Materials and Methods
V.1. Inclusion and Exclusion Criteria
V.1.1. Inclusion Criteria
V.1.2. Exclusion Criteria
V.2. Methodological Design
V.2.1. Variables
-
Variable: Suspected CDG (Qualitative nominal dichotomous).
- -
- CDG Phenotype: Clinical presentation indicative of suspected CDG, characterized by the exhaustion of the diagnostic resources protocolized at the NCMG, along with one or more of the following alterations: mental retardation, severe delay in growth and development, structural and functional abnormalities of the central and peripheral nervous systems (hypotonia, seizures, etc.), cardiac defects, hormonal imbalances, abnormal fat accumulations, inverted nipples, hepatopathy, enteropathies, and coagulopathies.
- -
- Unknown Phenotype: Phenotypic presentation characterized by any symptom and/or sign unrelated to the CDG Phenotype.
-
Variable: Biochemical Diagnosis of CDG (Qualitative nominal dichotomous).
- -
- Positive: Identification of Pattern I or II in IEF of Tf.
- -
- Negative: Absence of abnormal bands characteristic of hyposialylation patterns in the IEF of Tf, indicating a normal result and the absence of CDG.
V.2.2. Algorithm for Standardizing the Conditions of the Manual Polyacrylamide Gel IEF Method for Serum Transferrin Glycoforms
- Saturation and dilution of serum samples,
- Manual preparation of polyacrylamide gel and sample loading,
- Electrophoretic run-in terms of times and voltages,
- Quality of resolution in terms of pH gradients generated by ampholytes,
- Amount of anti-Tf antibody for immunofixation,
- Washing technique,
- Fixation of content to the gel,
- Staining and destaining.
V.2.3. Methods of Data Processing and Analysis
V.3. Ethical Aspects
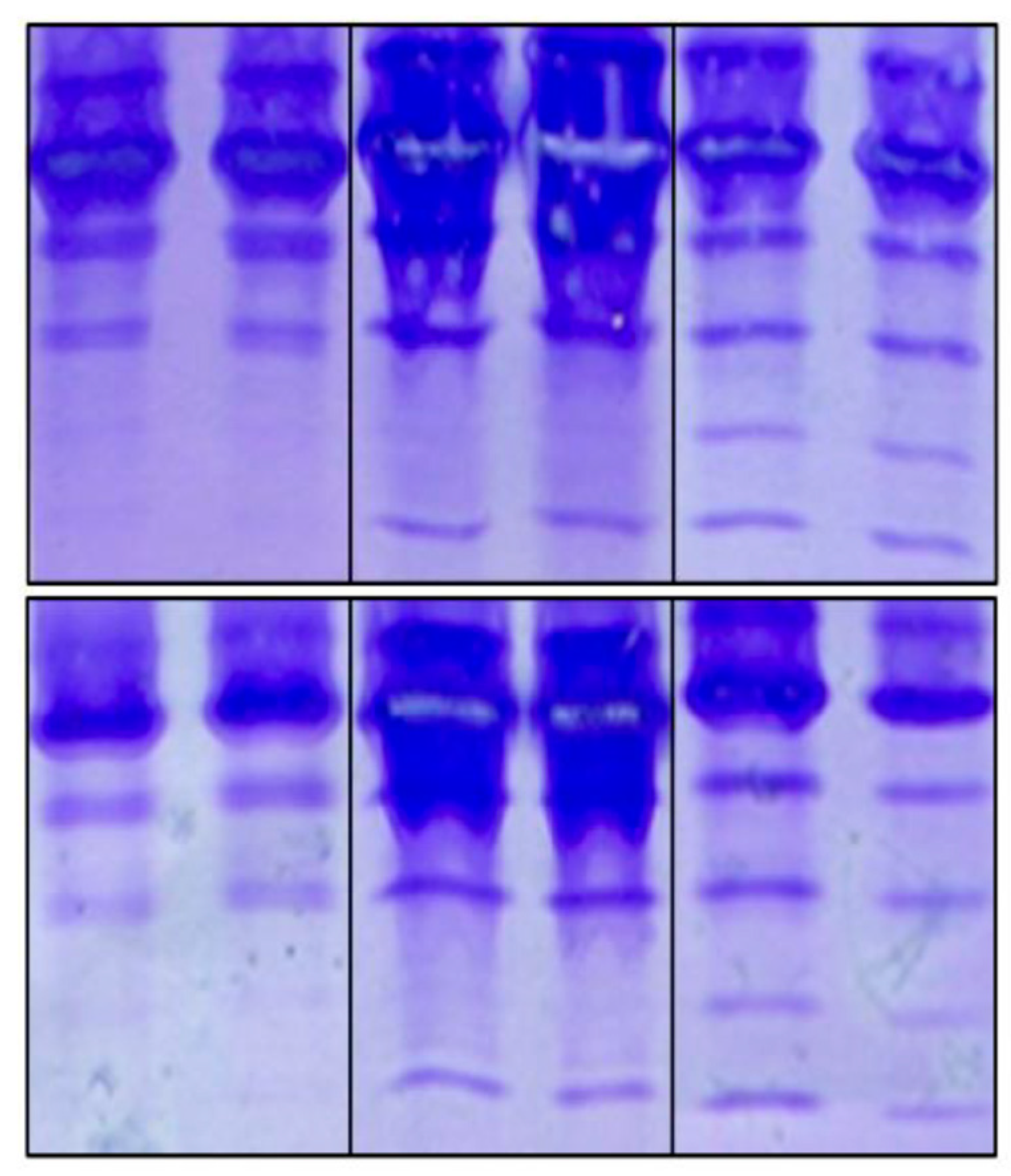
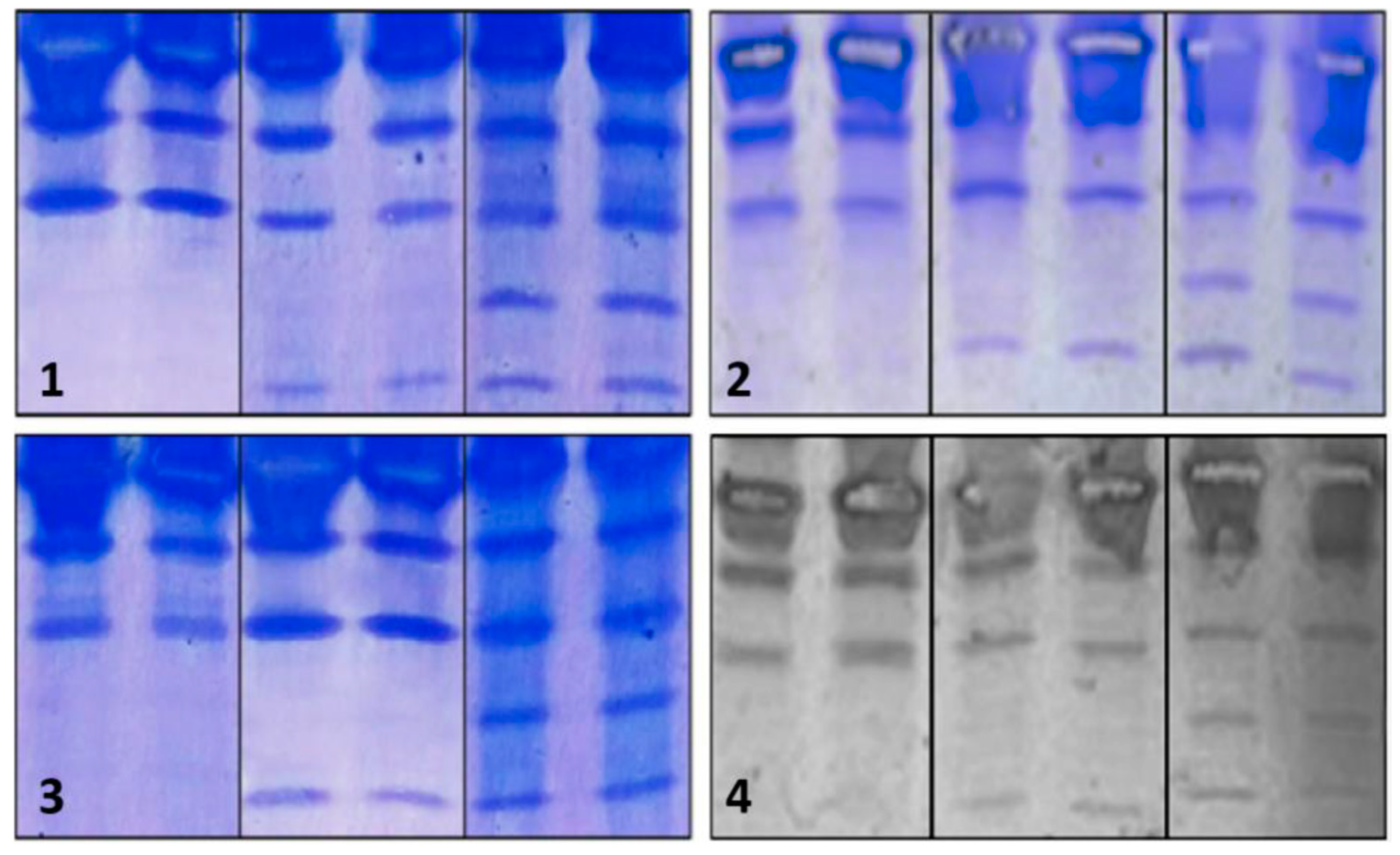
VI. Results
VI.1. Saturation and Dilution of Samples
- 100 μl of serum,
- 5 μl of FeCl3 at 20 mM, and
- 5 μl of NaHCO3.
- 2.75 ml of distilled water,
- 1 ml of 24.25% acrylamide solution / 0.75% bisacrylamide solution,
- 1 ml of 25% glycerol,
- 250 μl of pH 5-7 ampholyte,
- 7.5 μl of 10% APS,
- 25 μl of 0.1% FMN, and
- 2 μl of TEMED.
- 15 minutes at 100 V (for the migration of the ampholytes),
- 15 minutes at 200 V (to align the sample proteins, including Tf), and
- 60 minutes at 450 V (to separate the transferrins according to their isoelectric points).
|
Solution A was prepared by mixing 1 g of copper sulphate (CuSO4) and 100 ml of acetic acid, and then making up to 500 ml with distilled water. Solution B was prepared by mixing 600 ml of pure methanol with 400 ml of distilled water. Solution C was prepared by mixing 0.6 g of Coomassie blue, 180 ml of pure methanol, to make up to 300 ml with distilled water. |
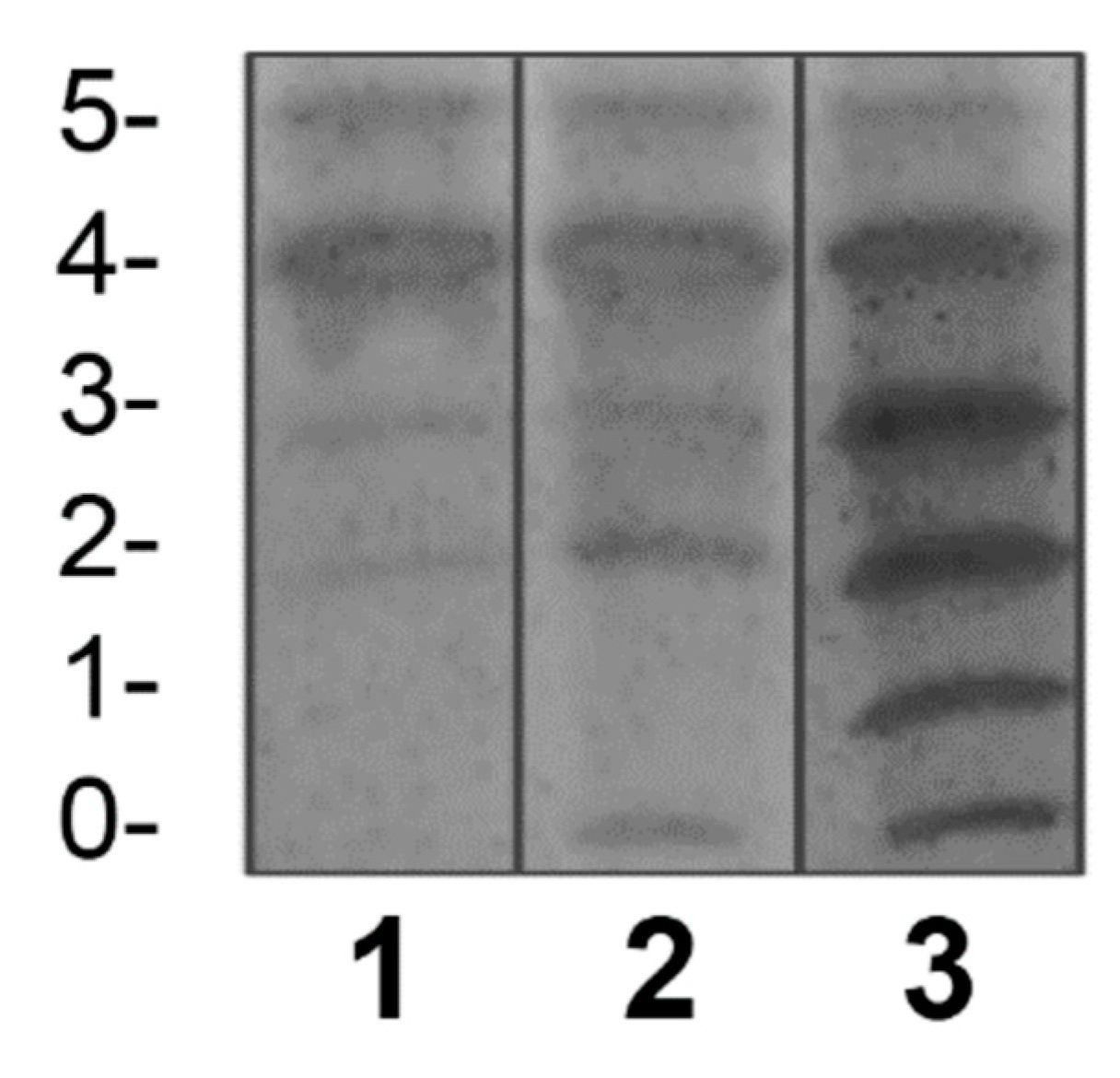
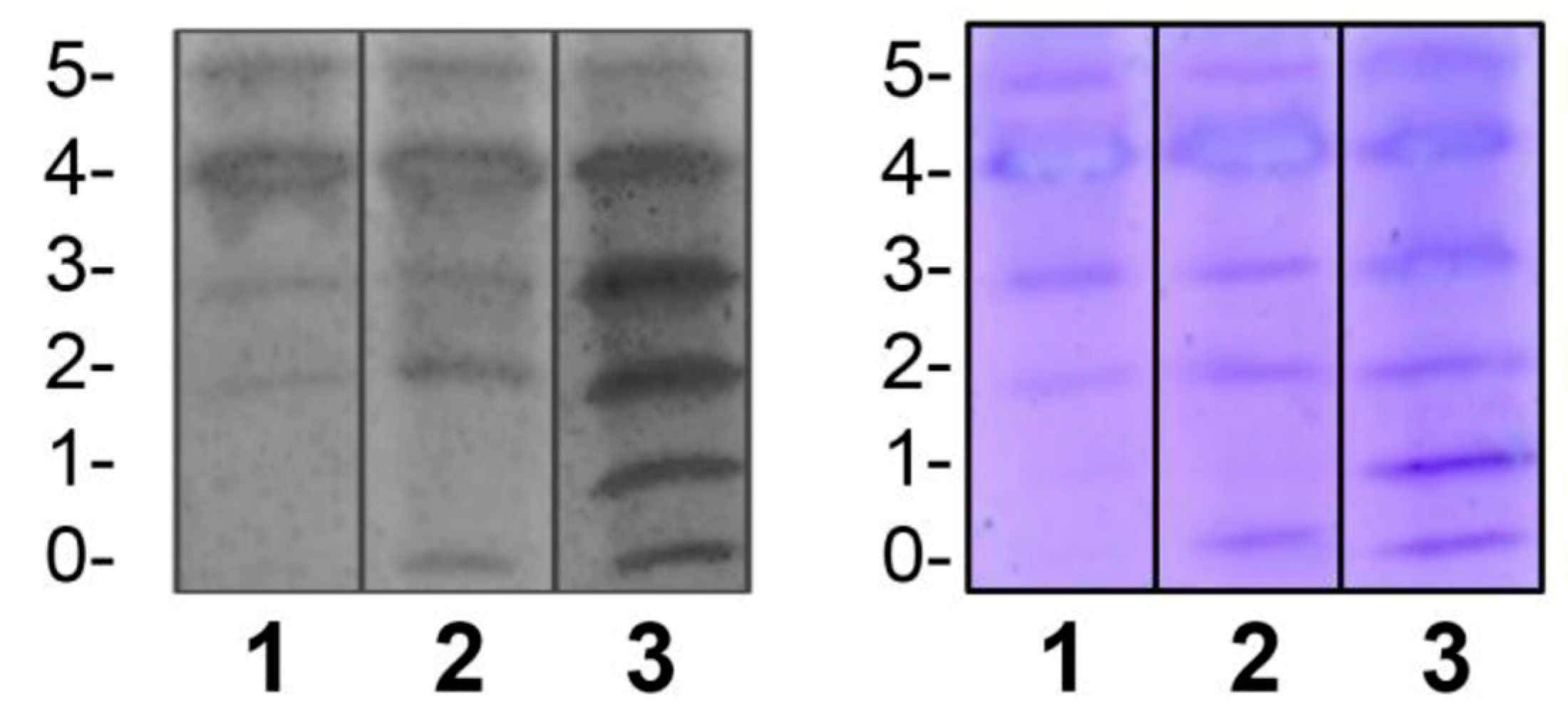
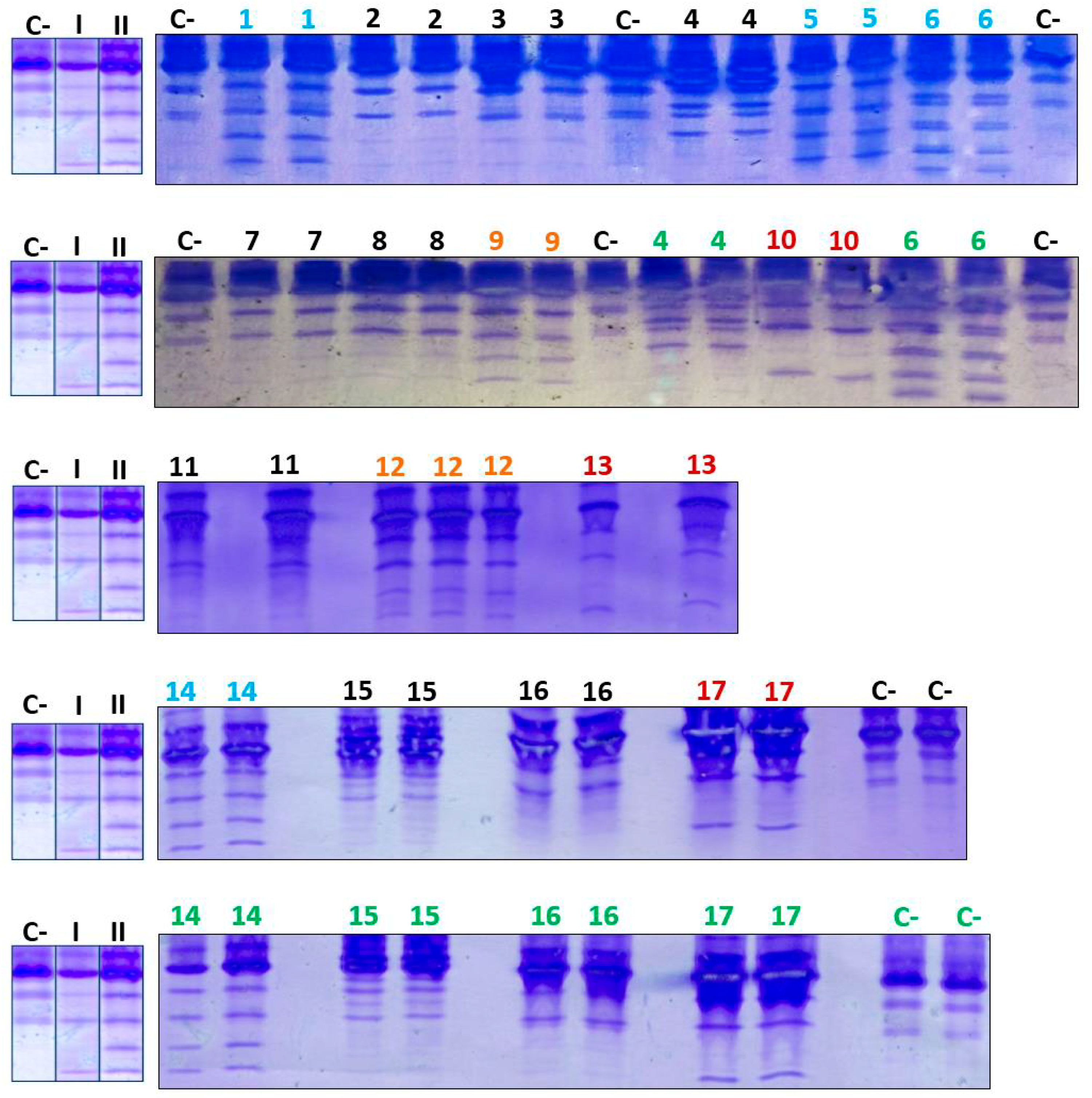
- Pattern-I: samples 10, 13, and 17.
- Pattern-II: samples 1, 5, 6, and 14.
- Dubious case: sample 9.
- Possibly negative cases: samples 2, 3, 4, 7, 8, 11, 12 (previously dubious), 15, and 16.
VII. Discussion
- Sample 1, with pattern-II (bands of asialoTf, monosialoTf, disialoTf, and trisialoTf, homogeneous and with intensity related to control II): patient with a defect in the second stage of N-glycosylation.
- Sample 2, negative for patterns suggestive of N-glycosylation defect (No bands were observed with intensity comparable to positive controls, suggestive of marked hypoglycosylation. These bands are: asialoTf and monosialoTf): presumably, patient negative for N-glycosylation defect.
- Sample 3, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect.
- Sample 4, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect. Possible Tf polymorphism was observed (indicated by potential variation in the pI of glycoforms due to allelic variants).
- Sample 5, with pattern-II: patient with a defect in the second stage of N-glycosylation.
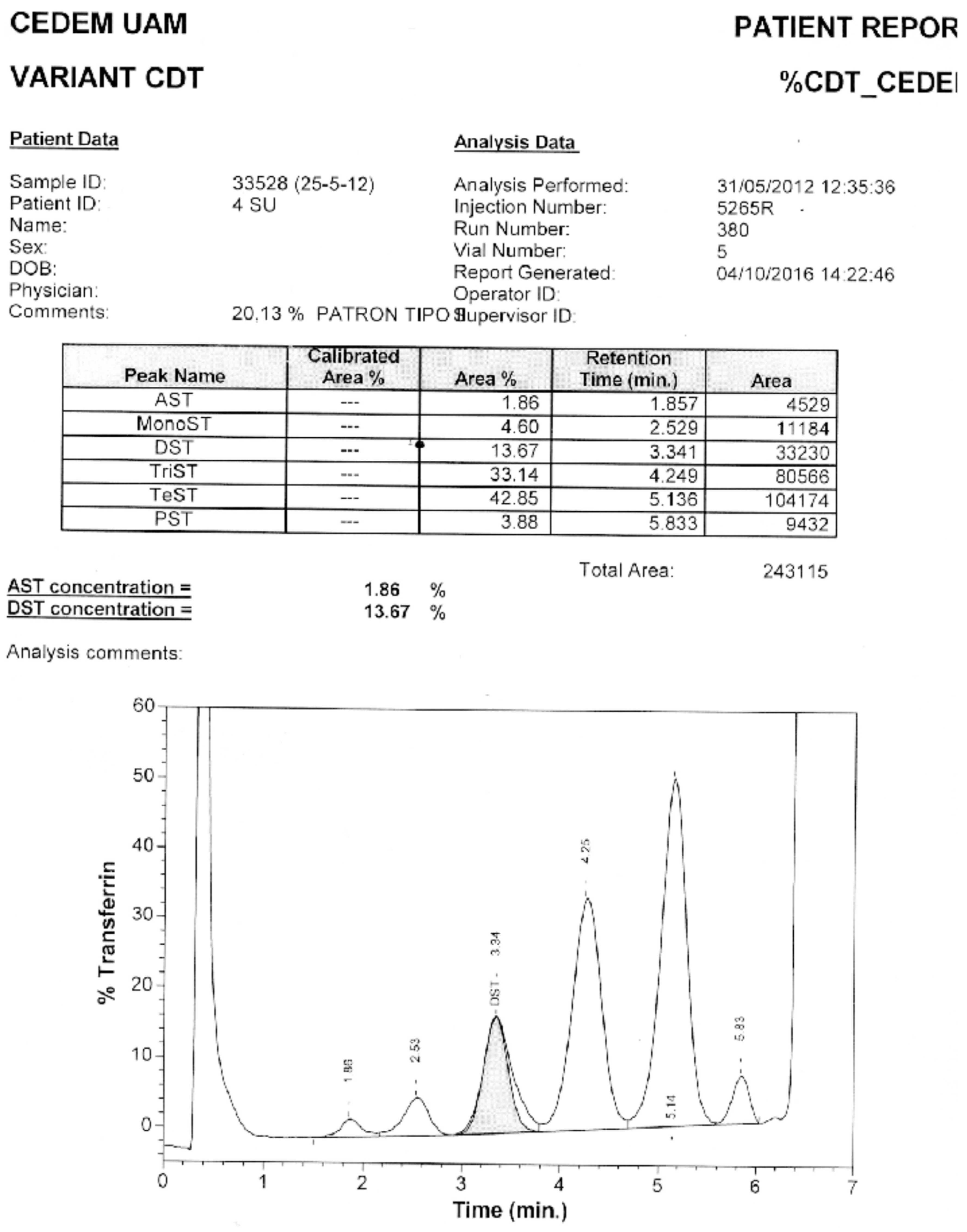
- Sample 6, with pattern-II: patient with a defect in the second stage of N-glycosylation and possible Tf polymorphism.
- Sample 7, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect.
- Sample 8, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect.
- Sample 9, doubtful pattern: remains doubtful because although bands of marked hypoglycosylation were visualized, they were not homogeneous in intensity (asialoTf and monosialoTf bands were less intense than the others), and HPLC analysis was inconclusive as well.
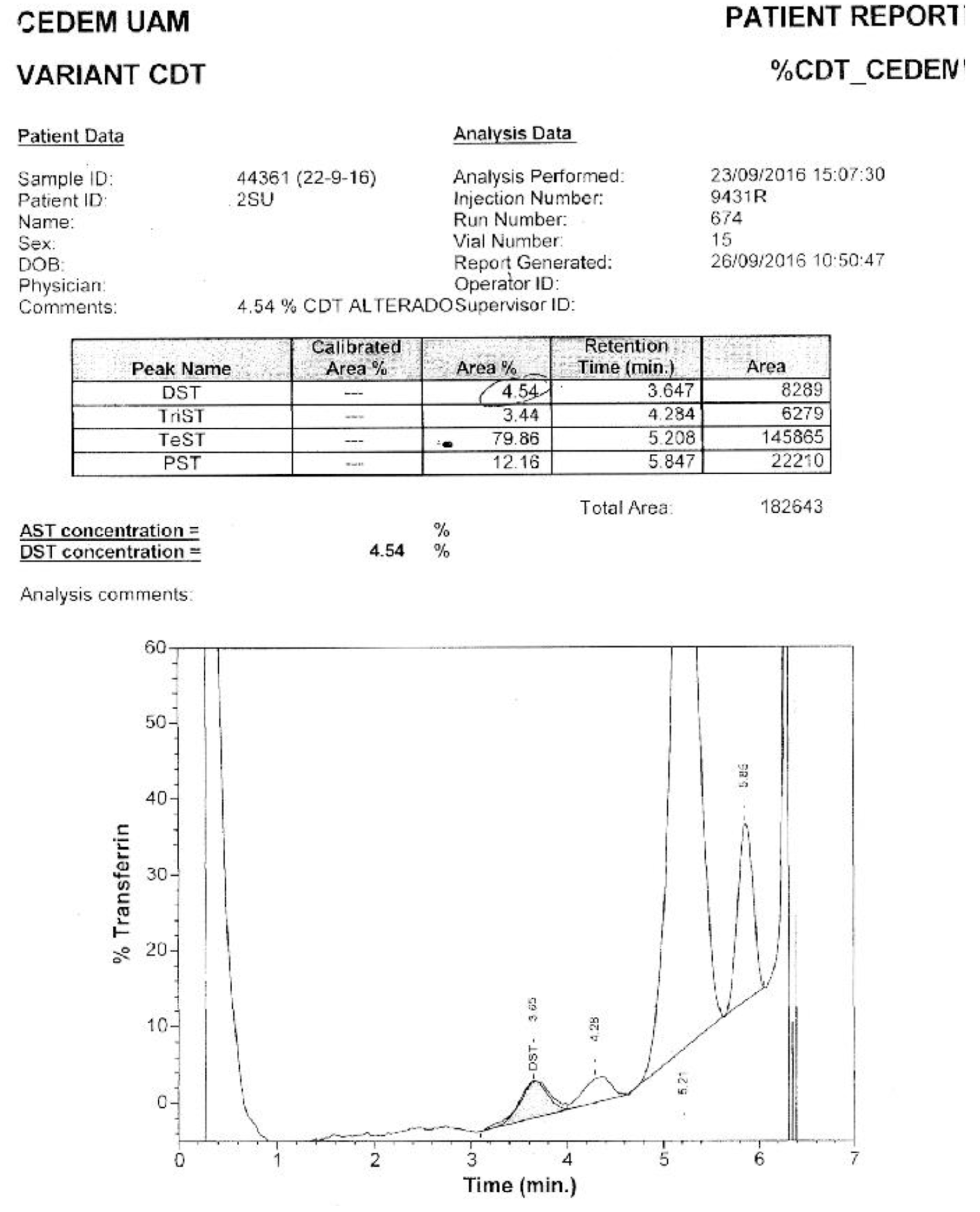
- Sample 10, pattern-I (asialoTf band evident and monosialoTf band slightly visible; in intensities comparable to control I): patient with a defect in the first stage of N-glycosylation.
- Sample 11, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect. Possible Tf polymorphism was observed.
- Sample 12, previously doubtful for the same reasons as case 9, its negativity regarding patterns was confirmed: presumably, patient negative for N-glycosylation defect.
- Sample 13, pattern-I: patient with a defect in the first stage of N-glycosylation.
- Sample 14, with pattern-II: patient with a defect in the second stage of N-glycosylation.
- Sample 15, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect. Possible Tf polymorphism was observed.
- Sample 16, negative for patterns suggestive of N-glycosylation defect: presumably, patient negative for N-glycosylation defect.
- Sample 17, pattern-I: patient with a defect in the first stage of N-glycosylation.
VIII. Conclusions
Acknowledgments
Appendix A. Informed Consent
|
Informed Consent I, ________________________________________[Patient’s Name/Legal Guardian], voluntarily consent to the treatment of stored biological samples at the National Center for Medical Genetics for the detection of congenital metabolic disorders. I acknowledge that these samples will be used solely for diagnostic and screening purposes, and that all information will be treated confidentially. I agree that the samples may be used for anonymous scientific research and I have the right to withdraw this consent at any time. I understand and grant this consent willingly. |
| Signature |
| _____________________________________ |
Appendix B. Confirmation through HPLC. Sample 6. Confirmation of Pattern-II.
Appendix C. Confirmation via HPLC. Sample 10. Confirmation of Pattern-I.
References
- Jaeken, J.; Vanderschueren-Lodewyckx, M.; Caeaer, P.; Snoeck, L.; Corbeel, L.; Eggermont, E. Familial psychomotor retardation with markedly fluctuating serum prolactin, FSH and GH levels, partial TBG deficiency, increased serum arylsulphatase A and increased CSF protein: a new syndrome? Pediatr Res. 1980, 14, 179. https://www.semanticscholar.org/paper/Familial-psychomotor-retardation-with-markedly-FSH-Jaeken-Vanderschueren%E2%80%90Lodeweyckx/080c31d7b117a869c7c545a8ef077ee7f268e25b. [CrossRef]
- Orphanet Report Series [database on the Internet]. Prevalence of rare diseases: Bibliographic data. Number 1. Available from: http://www.orpha.net/orphacom/cahiers/docs/GB/Prevalence_of_rare_diseases_by_alphabetical_list.pdf.
- Freeze, H.H.; Chong, J.X.; Bamshad, M.J.; Ng, B.G. Solving Glycosylation Disorders: Fundamental Approaches Reveal Complicated Pathways. Am J Hum Genet [serial on the Internet]. 2014, 94, 161–175. Available from: Online Mendelian Inheritance in Man (OMIM), http://www.omim.org/. [CrossRef] [PubMed]
- Jaeken, J. Congenital disorders of glycosylation (CDG): it’s (nearly) all in it! J Inherit Metab Dis. 2011, 34, 853–858. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H. Understanding human glycosylation disorders: biochemistry leads the charge. J Biol Chem. 2013, 288, 6936–6945. [Google Scholar] [CrossRef] [PubMed]
- Scott, K.; Gadomski, T.; Kozicz, T.; Morava, E. Congenital disorders of glycosylation: new defects and still counting. J Inherit Metab Dis. 2014, 37, 609–617. [Google Scholar] [CrossRef] [PubMed]
- Higuera, N.; Vázquez, S.; Palencia, R. Diagnóstico y tratamiento de los trastornos congénitos de la glicosilación de las proteínas. Bol Pediatr. 2011, 51, 188–193. [Google Scholar]
- Freeze, H.H.; Aebi, M. Altered glycan structures: the molecular basis of congenital disorders of glycosylation. Curr Opin Struct Biol. 2005, 15, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Apweiler, R.; Hermjakob, H.; Sharon, N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999, 1473, 4–8. [Google Scholar] [CrossRef] [PubMed]
- Yarema, K.J.; Bertozzi, C.R. Characterizing glycosylation pathways. Genome Biol. 2001, 2, 0004.1-.10. https://genomebiology.biomedcentral.com/articles/10.1186/gb-2001-2-5-reviews0004. [CrossRef] [PubMed]
- Supraha, S.; Dabelic, S.; Dumic, J. Insights into complexity of congenital disorders of glycosylation. Biochemia Medica. 2012, 22, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Jaeken, J. Congenital disorders of glycosylation. Ann NY ACAD Sci. 2010, 1214, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Fujita, M.; Kinoshita, T. GPI-anchor remodeling: potential functions of GPI-anchors in intracellular trafficking and membrane dynamics. Biochim Biophys Acta. 2012, 1821, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Furmanek, A.; Hofsteenge, J. Protein C-mannosylation: Facts and questions. ABP. 2000, 47, 781–789. [Google Scholar] [CrossRef] [PubMed]
- Burda, P.; Aebi, M. The dolichol pathway of N-linked glycosylation. Biochim Biophys Acta. 1999, 1426, 239–257. [Google Scholar] [CrossRef] [PubMed]
- Spiro, R.G. Glucose residues as key determinants in the biosynthesis and quality control of glycoproteins with N-linked oligosaccharides. J Biol Chem. 2000, 275, 35657–35660. [Google Scholar] [CrossRef] [PubMed]
- Marquardt, T.; Denecke, J. Congenital disorders of glycosylation: review of their molecular bases, clinical presentations and specific therapies. Eur J Pediatr. 2003, 162, 359–379. [Google Scholar] [CrossRef]
- Mills, P.; Mills, K.; Clayton, P.; Johnson, A.; Whitehouse, D.; Winchester, B. Congenital disorders of glycosylation type I leads to altered processing of N-linked glycans, as well as under glycosylation. Biochem J. 2001, 359, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Helenius, A.; Aebi, M. Roles of N-linked glycans in the endoplasmic reticulum. Annu Rev Biochem. 2004, 73, 1019–1049. [Google Scholar] [CrossRef]
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry, 5th ed.; W.H. Freeman and company New York: New York, 2011; https://search.worldcat.org/es/title/Lehninger-principles-of-biochemistry/oclc/191854286.
- Mohorko, E.; Glockshuber, R.; Aebi, M. Oligosaccharyltransferase: the central enzyme of N-linked protein glycosylation. J Inherit Metab Dis. 2011, 34, 869–878. [Google Scholar] [CrossRef] [PubMed]
- Gavel, Y.; von Heijne, G. Sequence differences between glycosylated and non-glycosylated Asn-X-Thr/Ser acceptor sites: implications for protein engineering. Protein Eng. 1990, 3, 433–442. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.I.; Mollicone, R.; Codogno, P.; Oriol, R. The nucleotide sugar transporter family: a phylogenetic approach. Biochimie. 2003, 85, 245–260. [Google Scholar] [CrossRef] [PubMed]
- Kornfeld, R.; Kornfeld, S. Assembly of asparagine-linked oligosaccharides. Annu Rev Biochem. 1985, 54, 631–664. [Google Scholar] [CrossRef] [PubMed]
- Jaeken J, Hennet T, Matthijs G, Freeze HH. CDG nomenclature: time for a change! Biochim Biophys Acta. 2009, 1792, 825–826. [PubMed]
- Jaeken, J.; Hennet, T.; Freeze, H.H.; Matthijs, G. On the nomenclature of congenital disorders of glycosylation (CDG). J Inherit Metab Dis. 2008, 31, 669–672. [Google Scholar] [CrossRef] [PubMed]
- Cylwik, B.; Naklicki, M.; Chrostek, L.; Gruszewska, E. Congenital disorders of glycosylation. Part I. Defects of protein N-glycosylation. ABP. 2013, 60, 151–161. [Google Scholar] [PubMed]
- Freeze, H.H. Genetic defects in the human glycome. Nature Rev Genet. 2006, 7, 537–551. [Google Scholar] [CrossRef] [PubMed]
- Cantagrel, V.; Lefeber, D.J. From glycosylation disorders to dolichol biosynthesis defects: a new class of metabolic diseases. J Inherit Metab Dis. 2011, 34, 859–867. [Google Scholar] [CrossRef] [PubMed]
- OMIM Online Mendelian Inheritance in Man [database on the Internet]. An Online Catalog of Human Genes and Genetic Disorders. Available from: http://www.omim.org/.
- Oka, T.; Krieger, M. Multi-component protein complexes and Golgi membrane trafficking. J Biochem (Tokyo). 2005, 137, 109–114. [Google Scholar] [CrossRef] [PubMed]
- Whyte, J.R.; Munro, S. The Sec34/35 Golgi transport complex is related to the exocyst, defining a family of complexes involved in multiple steps of membrane traffic. Dev Cell. 2001, 1, 527–537. [Google Scholar] [CrossRef] [PubMed]
- Fotso, P.; Koryakina, Y.; Pavliv, O.; Tsiomenko, A.; Lupashin, V. Cog1p plays a central role in the organization of the yeast conserved oligomeric Golgi (COG) complex. J Biol Chem. 2005, 280, 27613–27623. [Google Scholar] [CrossRef] [PubMed]
- Ungar, D.; Oka, T.; Brittle, E.E.; Vasile, E.; Lupashin, V.V.; Chatterton, J.E.; et al. Characterization of a mammalian Golgi-localized protein complex, COG, that is required for normal Golgi morphology and function. J Cell Biol. 2002, 157, 405–415. [Google Scholar] [CrossRef] [PubMed]
- Foulquier, F.; Amyere, M.; Jaeken, J.; Zeevaert, R.; Schollen, E.; Race, V.; et al. TMEM165 deficiency causes a congenital disorder of glycosylation. Am J Hum Genet. 2012, 91, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Brenner, V.; Nyakatura, G.; Rosenthal, A.; Platzer, M. Genomic organization of two novel genes on human Xq28: compact head to head arrangement of IDH-gamma and TRAP-delta is conserved in rat and mouse. Genomics. 1997, 44, 8–14. https://genome.fli-leibniz.de/publications/download/free/Brenner_1997.pdf. [CrossRef] [PubMed]
- Smith, A.N.; Lovering, R.C.; Futai, M.; Takeda, J.; Brown, D.; Karet, F.E. Revised nomenclature for mammalian vacuolar-type H(+)-ATPase subunit genes. Molec Cell. 2003, 12, 801–803. [Google Scholar] [CrossRef] [PubMed]
- Fischer, B.; Dimopoulou, A.; Egerer, J.; Gardeitchik, T.; Kidd, A.; Jost, D.; et al. Further characterization of ATP6V0A2-related autosomal recessive cutis laxa. Hum Genet. 2012, 131, 1761–1773. [Google Scholar] [CrossRef] [PubMed]
- Martínez, D.I.; Palomares, A.L.; Sánchez, F.D.; Mollicone, R.; Ibarra, G.I. Trastornos congénitos de la glicosilación: abordaje clínico y de laboratorio. Acta Pediatr Mex. 2008, 29, 78–88. [Google Scholar]
- Pérez-Cerdá, C.; Ugarte, M. Congenital disorders of glycosylation. Their diagnosis and treatment. Rev Neurol. 2006, 43, 145–156. [Google Scholar]
- Funke, S.; Gardeitchik, T.; Kouwenberg, D.; Mohamed, M.; Wortmann, S.B.; Korsch, E.; Adamowicz, M.; Al-Gazali, L.; Wevers, R.A.; Horvath, A.; Lefeber, D.J.; Morava, E. Perinatal and early infantile symptoms in congenital disorders of glycosylation. Am J Med Genet A. 2013, 161, 578–584, Epub 2013 Feb 7. Erratum in: Am J Med Genet A. 2014 Jun;164A(6):1618. [Google Scholar] [CrossRef] [PubMed]
- Kouwenberg, D.; Gardeitchik, T.; Mohamed, M.; Lefeber, D.; Morava, E. Wrinkled skin and fat pads in patients with ALG8-CDG: Revisiting skin manifestations in congenital disorders of glycosylation. Pediatr Dermatol. 2014, 31, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Wolthuis, D.F.G.J.; Janssen, M.C.; Cassiman, D.; Lefeber, D.J.; Morava-Kozicz, E. Defining the phenotype and diagnostic considerations in adults with congenital disorders of N-linked glycosylation. Expert Rev Mol Diagn. 2014, 14, 217–224. [Google Scholar] [CrossRef]
- Tegtmeyer, L.C.; Rust, S.; van Scherpenzeel, M.; Ng, B.C.; Losfeld, M.E.; Timal, S.; et al. Multiple phenotypes in phosphoglucomutase 1 deficiency. New Engl J Med. 2014, 370, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Freeze, H.H.; Eklund, E.A.; Ng, B.G.; Patterson, M.C. Neurological aspects of human glycosylation disorders. Ann Rev Neurosci. 2015, 38, 105–125. [Google Scholar] [CrossRef]
- Briones, P.; Vilaseca, M.A.; García-Silva, M.T.; Pineda, M.; Colomer, J.; Ferrer, I.; et al. Congenital disorders of glycosylation (CDG) may be underdiagnosed when mimicking mitochondrial disease. Eur J Paediatr Neurol. 2001, 5, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Vilaseca, M.A.; Artuch, R.; Briones, P. Defectos congénitos de la glucosilación: últimos avances y experiencia española. Med Clin (Barc). 2004, 122, 707–716. https://dialnet.unirioja.es/servlet/articulo?codigo=876578. [CrossRef]
- Baker, E.N. Structure and reactivity of transferrins. Adv Inorg Chem. 1994, 41, 389–463. https://www.semanticscholar.org/paper/Structure-and-Reactivity-of-Transferrins-Baker/760f0c009919650618da410e4a04d71ccad067bd.
- Jeffrey, P.D.; Bewley, M.C.; MacGillivray, R.T.; Mason, A.B.; Wood-worth, R.C.; Baker, E.N. Ligand-induced conformational change in transferrins: crystal structure of the open form of the N-terminal half-molecule of human transferrin. Biochemistry. 1998, 37, 13978–13986. [Google Scholar] [CrossRef] [PubMed]
- Ching-Ming Chung, M. Structure and function of transferrin. Biochemical education. 1984, 12, 146–154. [Google Scholar] [CrossRef]
- Fujita, M.; Satoh, C.; Asakawa, J.; Nagahata, Y.; Tanaka, Y.; Hazama, R.; et al. Electrophoretic variants of blood proteins in Japanese, VI. Transferrin. Jinrui Idengaku Zasshi. 1985, 30, 191–200. [Google Scholar] [CrossRef] [PubMed]
- Kamboh, M.I.; Ferrell, R.E. Human transferrin polymorphism. Hum Hered. 1987, 37, 65–81. [Google Scholar] [CrossRef] [PubMed]
- Deschamps, E.M.; Miña, A.; Ciegues, M.A. Isoformas de la transferrina: Utilidad clínica de su determinación. Rev Digan Biol [artículo en Internet]. 2003 Mar [consulta: 2016 Oct];52(1). Disponible en: Http://scielo.isciii.es/scielo.php?script=sci_arttext&pid=S003479732003000100005&lng=es&nrm=iso&tlng=es.
- Voet, D.; Voet, J.G. Biochemistry, 4th ed.; John Wiley & Sons, Inc: United States of America, 2011; https://www.wiley.com/en-us/Biochemistry,+4th+Edition-p-9780470570951.
- de Jong, G.; van Eijk, H.G. Microheterogeneity of human serum transferrin: a biological phenomenon studied by isoelectric focusing in immobilized pH gradients. Electrophoresis. 1988, 9, 589–598. [Google Scholar] [CrossRef] [PubMed]
- Helander, A.; Eriksson, G.; Stibler, H.; Jeppsson, J.O. Interference of transferrin isoform types with carbohydrate-deficient transferrin quantification in the identification of alcohol abuse. Clin Chem. 2001, 47, 1225–1233. [Google Scholar] [CrossRef] [PubMed]
- Carchon, H.A.; Chevigne, R.; Falmagne, J.B.; Jaeken, J. Diagnosis of congenital disorders of glycosylation by capillary zone electrophoresis of serum transferrin. Clin Chem. 2004, 50, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Quintana, E.; Montero, R.; Casado, M.; Navarro-Sastre, A.; Vilaseca, M.A.; Briones, P.; et al. Comparison between high performance liquid chromatography and capillary zone electrophoresis for the diagnosis of congenital disorders of glycosylation. J Chromatogr B Analyt Technol Biomed Life Sci. 2009, 877, 2513–2518. [Google Scholar] [CrossRef] [PubMed]
- Sturiale, L.; Barone, R.; Garozzo, D. The impact of mass spectrometry in the diagnosis of congenital disorders of glycosylation. J Inherit Metab Dis. 2011, 34, 891–899. [Google Scholar] [CrossRef] [PubMed]
- Barone, R.; Sturiale, L.; Garozzo, D. Mass spectrometry in the characterization of human genetic N-glycosylation defects. Mass Spectrom Rev. 2009, 28, 517–542. [Google Scholar] [CrossRef] [PubMed]
- Wada, Y.; Nishikawa, A.; Okamoto, N.; Inui, K.; Tsukamoto, H.; Okada, S.; et al. Structure of serum transferrin in carbohydrate-deficient glycoprotein syndrome. Biochem Biophys Res Commun. 1992, 189, 832–836. [Google Scholar] [CrossRef] [PubMed]
- Knopf, C.; Rod, R.; Jaeken, J.; Berant, M.; van Schaftingen, E.; Fryns, J.P.; et al. Transferrin protein variant mimicking carbohydrate-deficient glycoprotein syndrome in trisomy 7 mosaicism. J Inherit Metab Dis. 2000, 23, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Artuch, R.; Ferrer, I.; Pineda, J.; Moreno, J.; Busquets, C.; Briones, P.; et al. Western blotting with diaminobenzidine detection for the diagnosis of congenital disorders of glycosylation. J Neurosci Methods. 2003, 125, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.; Peters, V.; Korner, C.; Hoffmann, G.F. Improvement of CDG diagnosis by combined examination of several glycoproteins. J Inherit Metab Dis. 2004, 27, 581–590. [Google Scholar] [CrossRef]
- Körner, C.; Linnebank, M.; Koch, H.G.; Harms, E.; Von Figura, K.; Marquardt, T. Decreased availability of GDP-L-fucose in a patient with LAD II with normal GDP-D-mannose dehydratase and FX protein activities. J Leukoc Biol. 1999, 66, 95–98. [Google Scholar] [CrossRef] [PubMed]
- De Praeter, C.M.; Gerwig, G.J.; Bause, E.; Nuytinck, L.K.; Vliegenthart, J.F.; Breuer, W.; et al. A novel disorder caused by defective biosynthesis of Nlinked oligosaccharides due to glucosidase I deficiency. Am J Hum Genet. 2000, 66, 1744–1756. [Google Scholar] [CrossRef] [PubMed]
- Fletcher, M.; Matthijs, G.; Jaeken, J.; van Schaftingen, E.; Nelson, P.V. Carbohydrate- deficient glycoprotein syndrome: beyond the screen. J Inherit Metab Dis. 2000, 23, 396–398. [Google Scholar] [CrossRef]
- Stibler, H.; Von Dobeln, U.; Kristiansson, B.; Guthenberg, C. Carbohydratedeficient transferrin in galactosaemia. Acta Paediatr. 1997, 86, 1377–1378. [Google Scholar] [CrossRef] [PubMed]
- Jaeken, J.; Pirard, M.; Adamowicz, M.; Pronicka, E.; van Schaftingen, E. Inhibition of phosphomannose isomerase by fructose 1-phosphate: an explanation for defective N-glycosylation in hereditary fructose intolerance. Pediatr Res. 1996, 40, 764–766. [Google Scholar] [CrossRef] [PubMed]
- Adamowicz, M.; Pronicka, E. Carbohydrate deficient glycoprotein syndrome-like transferrin isoelectric focusing pattern in untreated fructosaemia. Eur J Pediatr. 1996, 155, 347–348. [Google Scholar] [PubMed]
- Charlwood, J.; Clayton, P.; Keir, G.; Mian, N.; Winchester, B. Defective galactosylation of serum transferrin in galactosemia. Glycobiology. 1998, 8, 351–357. [Google Scholar] [CrossRef] [PubMed]
- Landberg, E.; Pahlsson, P.; Lundblad, A.; Arnetorp, A.; Jeppsson, J.O. Carbohydrate composition of serum transferrin isoforms from patients with high alcohol consumption. Biochem Biophys Res Comm. 1995, 210, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Yamashita, K.; Ideo, H.; Ohkura, T.; Fukushima, K.; Yuasa, I.; Ohno, K.; et al. Sugar chains of serum. J Biol Chem. 1993, 268, 5783–5789. [Google Scholar] [CrossRef] [PubMed]
- Norma ISO 15189:2007. Laboratorios clínicos. Requisitos particulares relativos a la calidad y competencia. https://www.une.org/encuentra-tu-norma/busca-tu-norma/norma?
- Quality Management Systems in the Medical Laboratory, D.M. Quality Management Systems in the Medical Laboratory, D.M. Browning. Mar 2004. [PubMed]
- Briones, P.; Vilaseca, M.A.; Schollen, E.; Ferrer, I.; Maties, M.; Busquets, C.; et al. Biochemical and molecular studies in 26 Spanish patients with congenital disorder of glycosylation type Ia. J Inherit Metab Dis. 2002, 25, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Andrews, A.T. Electrophoresis: theory, techniques, and biochemical and clinical applications, 2th ed.; Oxford University Press: Oxford, 1986; https://books.google.es/books/about/Electrophoresis.html?id=eZMRAQAAIAAJ&redir_esc=y.
- Westermeier, R. Electrophoresis in practice: a guide to methods and applications of DNA and protein separations, 2th ed.; VCH Press: Weinheim, 1997; https://beckassets.blob.core.windows.net/product/readingsample/16142615/3527338802_c01.pdf.
- Righetti, P.G. Isoelectric focusing: theory, methodology, and applications; Elsevier: Amsterdam, 1983; https://shop.elsevier.com/books/isoelectric-focusing-theory-methodology-and-application/righetti/978-0-444-80498-3.
- van Eijk, H.G.; van Noort, W.L.; Dubelaar, M.L.; van der Heul, C. The microheterogeneity of human transferrins in biological fluids. Clin Chim Acta. 1983, 132, 167–171. [Google Scholar] [CrossRef] [PubMed]
- Cantín, M. Declaración de Helsinki de la Asociación Médica Mundial: Principios éticos para las investigaciones médicas en seres humanos. Revisando su última versión. Int J Med Surg Sci. 2014, 1, 339–346. https://www.wma.net/es/policies-post/declaracion-de-helsinki-de-la-amm-principios-eticos-para-las-investigaciones-medicas-en-seres-humanos/. [CrossRef]
- Estrella, L.; Castañeda, C.; Sánchez, J.; Zaharia, M. New version of the Declaration of Helsinki: shortcomings to resolve. Rev Peru Med Exp Salud Publica. 2014, 31, 803–804. [Google Scholar] [PubMed]
- World Medical Association. World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. JAMA. 2013, 310, 2191–2194. https://jamanetwork.com/journals/jama/fullarticle/1760318.
- Lefeber, D.J.; Morava, E.; Jaeken, J. How to find and diagnose a CDG due to defective N-glycosylation. J Inherit Metab Dis. 2011, 34, 849–852. [Google Scholar] [CrossRef] [PubMed]
- Denecke, J. Biomarkers and diagnosis of congenital disorders of glycosilation. Expert Opin Med Diagn. 2009, 3, 395–409. [Google Scholar] [CrossRef] [PubMed]
- van Eijk, H.G.; van Noort, W.L. The analysis of human serum transferrins with the PhastSystem: Quantitation of microheterogeneity. Electrophoresis 1992, 13, 354–358. [Google Scholar]
- Grunewald, S.; Matthijs, G.; Jaeken, J. Congenital disorders of glycosylation: a review. Pediatr Res. 2002, 52, 618–24. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).



