Submitted:
12 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Intermetallic Compounds
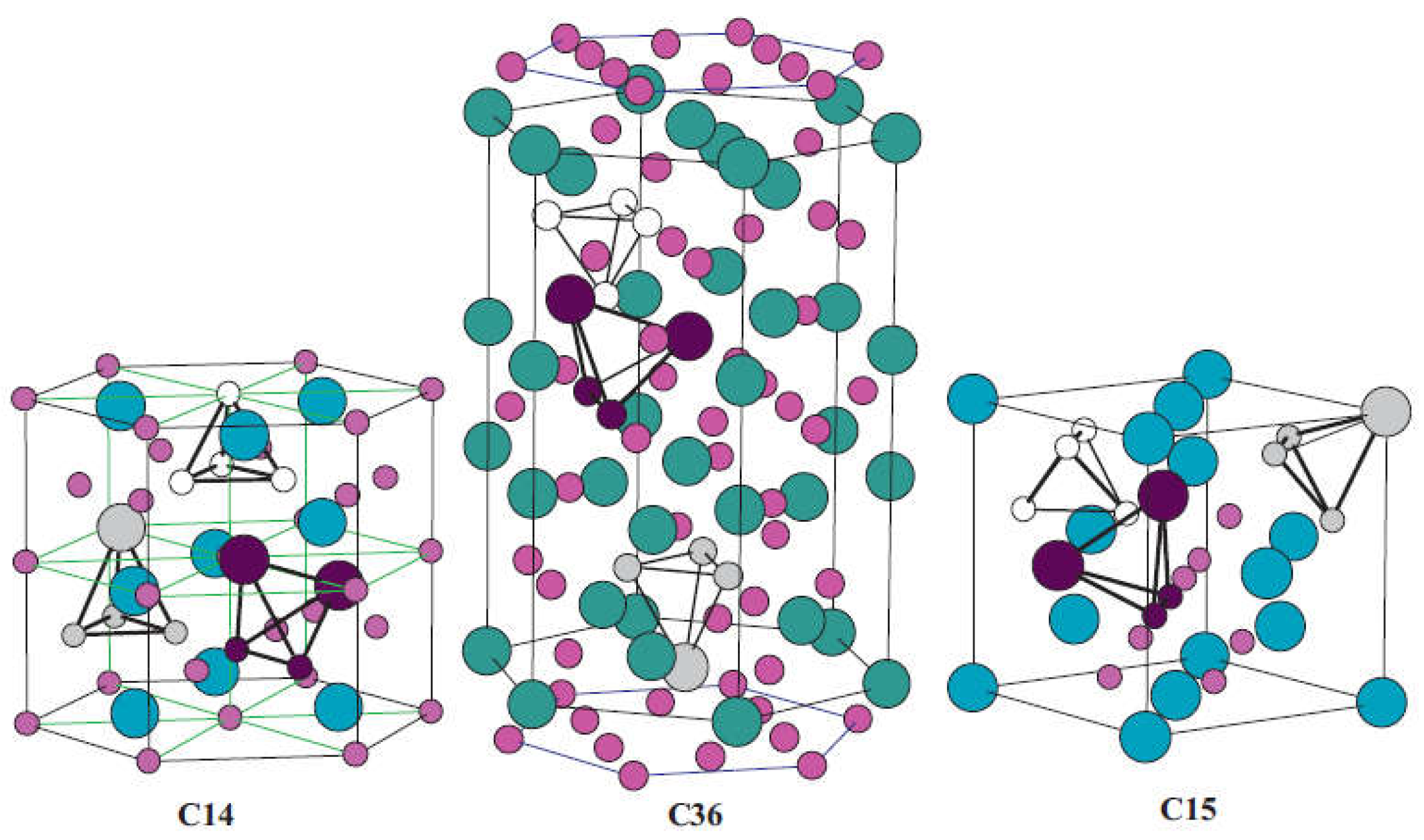
2.1. AB2-Type Laves Phase Alloys
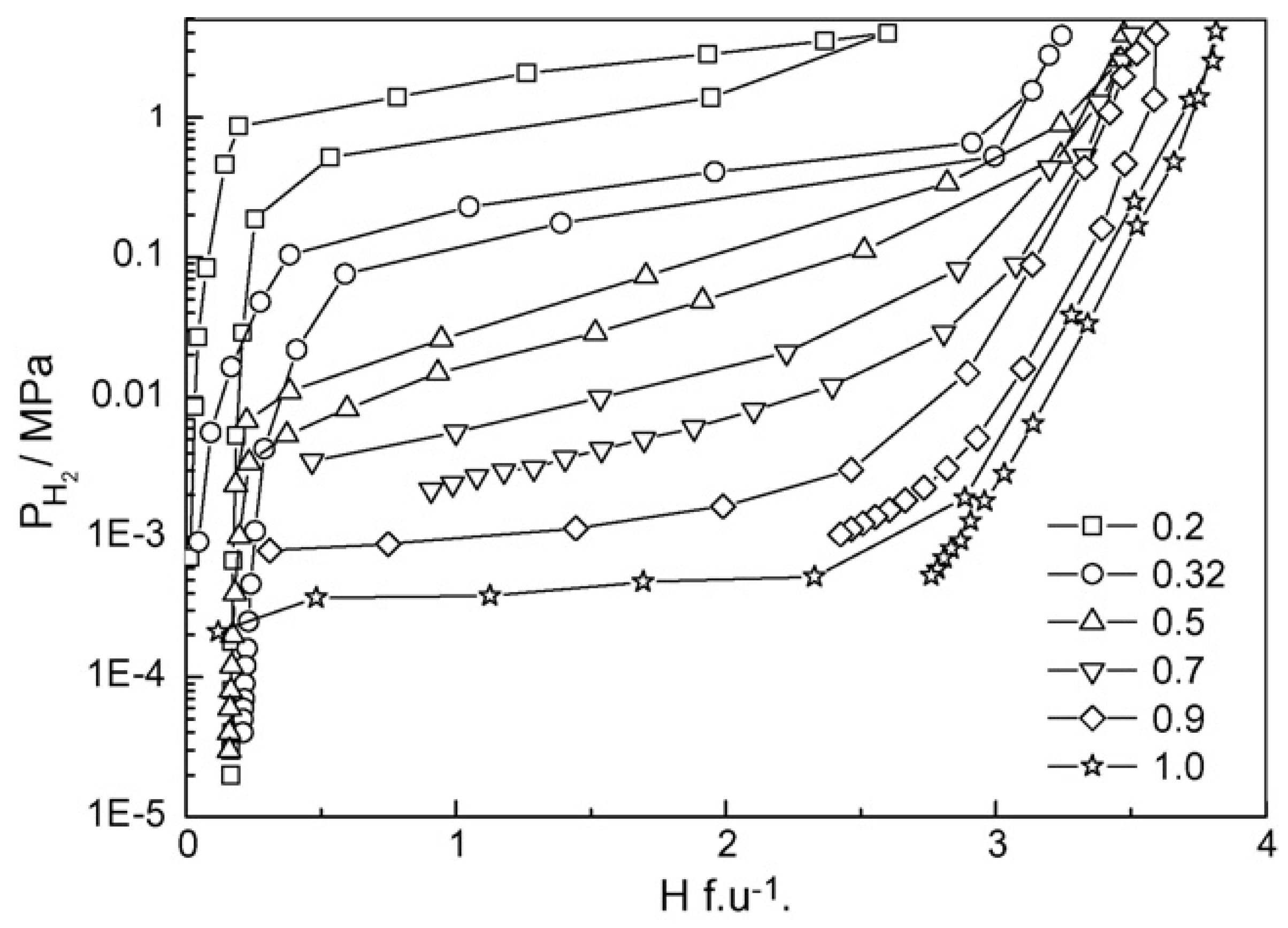
2.1.1. TiCr2-Based Alloys
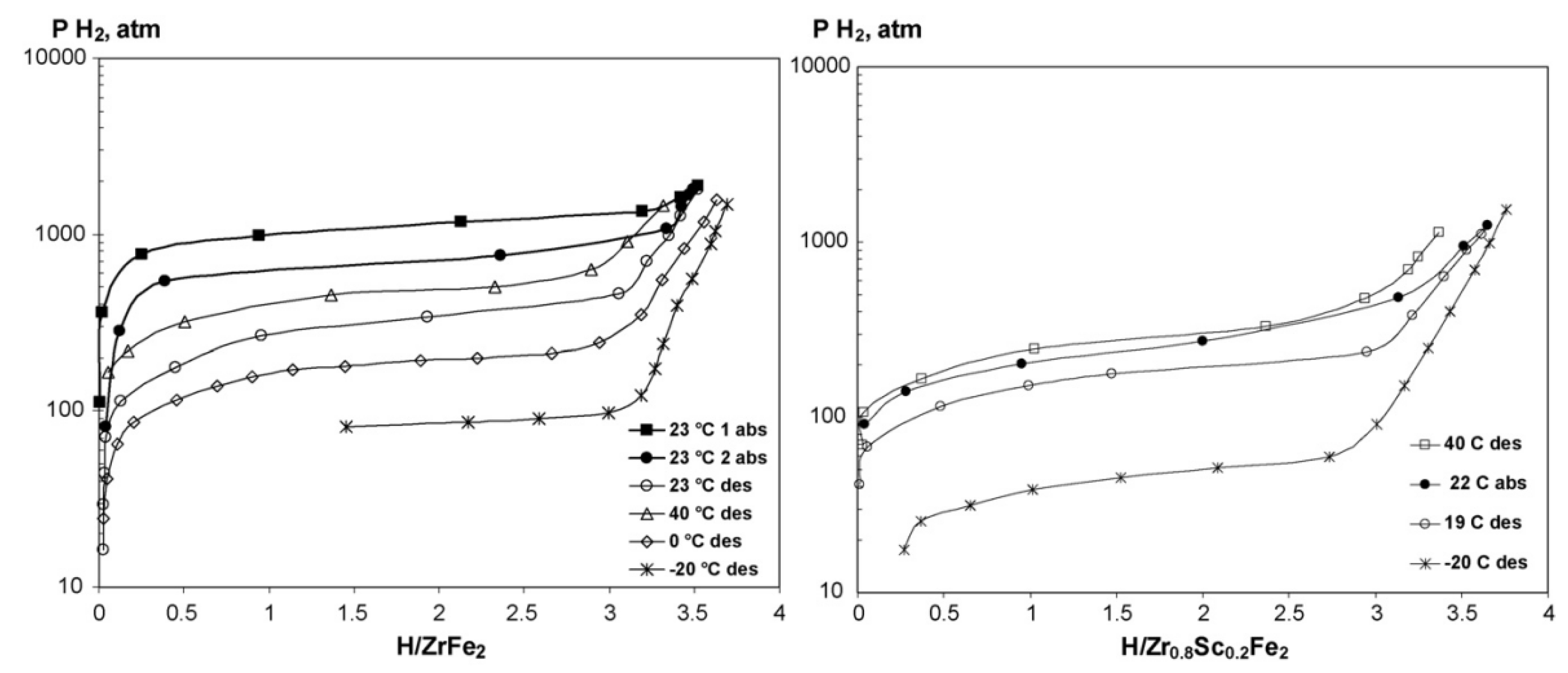
2.1.2. ZrFe2-based alloys
2.2. Solid Solution Alloys
3. Complex Hydrides
3.1. Transition Metal Alanates
3.1.1. Overview of Known Transition Metal Alanates
3.1.2. Synthesis
| MHalx + n | LiAlH4 | → M(AlH4)n + n | LiHal | (R 1) |
| NaAlH4 | NaHal |
| Alanate | wt% H | Td (°C) | Synthesis | Characterization techniques | Ref | |
|---|---|---|---|---|---|---|
| Starting materials | procedure | |||||
| AgAlH4 | 2.9 | -50 | AgClO4, LiAlH4[157] | in solution (diethyl ether) | conductometric titration [157] VDH [158] |
[157,158] |
| Ce(AlH4)3 | 5.2 | -15 | Li3CeBr6, LiAlH4 | in solution (diethyl ether) | VDH, EA | [148] |
| CeAlH6 | 3.5 | 100 | CeCl3, NaAlH4 | high energy ball milling under 1-15 bar hydrogen | DSC, Thermolysis, XRD | [149] |
| CuAlH4 | 4.3 | ≤-80 [148] | CuI, LiAlH4 [161,162] Li2CuBr4, LiAlH4 [148] |
in solution (tetrahydrofuran [161], diethyl ether [148,162]) | VDH, EA [148,161,162] IR [161,162] |
[148,161,162] |
| Eu(AlH4)2 | 3.8 | 100 | EuCl2, NaAlH4 | high energy ball milling under 1-15 bar hydrogen, no separate preparation of EuAlH5 | DSC, Thermolysis, XRD | [163] |
| EuAlH5 | 2.7 | 180 | ||||
| Fe(AlH4)2 | 6.8 | -125 [143] -70 [144] <RT [145] |
FeCl3, LiAlH4 [145,146,164] Li2FeBr4, LiAlH4 [148] |
in solution (diethyl ether) | VDH, EA [144,145,146] IR [144] Thermolysis [145] DTA (not shown), XRD [146] |
[143,144,145,146,148,164] |
| LaAlH6 | 3.5 | 100 | LaCl3, NaAlH4 | high energy ball milling under 1-15 bar hydrogen | DSC, Thermolysis, XRD, 27Al-NMR | [149] |
| Mn(AlH4)2 | 6.9 | -20 | Li2MnBr4, LiAlH4 | in solution (diethyl ether) | VDH, EA | [148] |
| Mo(AlH4)5 | 8.0 | ≤RT | no information | in solution (diethyl ether) | no information stated | [143] |
| Nb2(AlH4)5 | 5.9 | 0 [165] >20 [166] |
NbCl5, LiAlH4 | in solution (diethyl ether) | VDH, EA Thermolysis, DTA (not shown), XRD, IR [165] |
[144,153,165,166] |
| Nb2(AlH4)6 | 6.5 | -50 [165] -40<Td<20 [166] |
VDH, EA | |||
| Nb2(AlH4)7 | 7.0 | -90 [165] -70<Td<-40 [166] |
||||
| NdAlH6 | 3.4 | 100 | NdCl3, NaAlH4 | high energy ball milling under 1-15 bar hydrogen | DSC, Thermolysis, XRD | [149] |
| PrAlH6 | 3.5 | 100 | PrCl3, NaAlH4 | high energy ball milling under 1-15 bar hydrogen | DSC, Thermolysis, XRD | [149] |
| Sc(AlH4)3 Et2O | 8.8 | 80 | ScBr3, LiAlH4 | in solution (diethyl ether) | VDH, EA, IR, XRD, DTA, DTGA, MS | [147] |
| TaH2(AlH4)2 | 4.1 | 60 [153] 130 [156] |
TaCl5, LiAlH4 [153] TaBr5/TaCl5, LiAlH4 [156] | in solution (diethyl ether) | VDH, EA Thermolysis [153,156] XRD, IR, RAMAN [156] |
[153,156] |
| Ti(AlH4)4 | 9.4 | -70 [146] -85 [167] |
TiBr4/TiCl4, LiAlH4 [146] TiCl4, LiAlH4 [167] |
in solution (diethyl ether) | VDH, EA IR [144] DTA (not shown), XRD [146] |
[144,146,167] |
| Y(AlH4)3 | 6.7 | 50 [168] 80 [169] |
YBr3, LiAlH4 [168] YCl3, LiAlH4 [169] |
in solution (diethyl ether) [168] high energy ball milling under 80 bar hydrogen, no separate preparation of YAlH6 [169] |
VDH, EA IR, DTA (not shown) [168] XRD, IR, TPD,MS, DSC, HP-DSC [169] |
[168,169] |
| YAlH6 | 5.0 | 170 [169] | ||||
| Yb(AlH4)2 | 3.4 | 70 | YbBrx, LiAlH4 | in solution (diethyl ether) | VDH, EA, DTA (not shown), XRD, IR | [144] |
| Yb(AlH4)3 | 4.6 | 100 | YbCl3, LiAlH4 | high energy ball milling under 100 bar hydrogen, no separate preparation of YbAlH6 | XRD, IR, TGA-DSC-MS, HP-DSC | [170] |
| YbAlH6 | 2.9 | 180 | ||||
| Zr(AlH4)4 | 7.5 | <RT | Zr(BH4)4, LiAlH4 | in solution (diethyl ether) | EA | [159] |
3.1.3. Dehydrogenation and Hydrogenation Behavior
| Mn(AlH4)n → Mn-m(AlH4)n-m + m AlH3 + 0.5 m H2 | (R 2) |
| M(AlH4)n → M + n AlH3 + 0.5 n H2 | (R 3) |
| M(AlH4)n → M + n Al + 2 n H2 | (R 4) |
| M(AlH4)n → MHx(AlH4)n-x + n AlH3 | (R 5) |
3.2. Transition Metal Boranates
3.2.1. Overview of Known Transition Metal Boranates
3.2.2. Synthesis
| MHaln | n | LiBH4 | → M(BH4)n + n | LiHal | (R 6) |
| NaBH4 | NaHal | ||||
| MHn MHn |
n | Sol*BH3 | → M(BH4)n + n | Solv | ( R 7) |
| 0.5 n | B2H6 | → M(BH4)n | ( R 8) |
| Boranate | wt% H | Td (°C) | Synthesis | Characterization techniques | Ref | |
|---|---|---|---|---|---|---|
| Starting materials | procedure | |||||
| Ag(BH4) | 3.28 | -30 | LiBH4, AgClO4 | in solution (diethyl ether) | VDH | [192] |
| Ce(BH4)2 | 6.55 | 200-251 | CeH3, S(CH3)2*BH3 [195] LiBH4, CeCl3 [203,204,205] |
in solution (dimethyl sulfide-toluene mixture [195], toluene [203]) extraction (dimethyl sulfide [203]) high energy ball milling [204,205] |
in situ-XRD (synchrotron) [195,203], XRD [204,205], FT-IR [195,204,205], PCI [195], TG-DSC-MS [195,205], TG-DSC [203], DSC [204], MP [195] | [195,203,204,205] |
| Co(BH4)2 | 9.10 | -20 [206] | CoCl2/CoLi2Br4, LiBH4 [206] CoBr2, LiBH4 [207] |
in solution (diethyl ether) | EA [207] | [206,207] |
| CuBH4 | 5.14 | 0 | LiBH4, CuCl2 [208,209] LiBH4,CuCl [210,211] |
in solution (diethyl ether [208,209,210], THF/diethyl ether [211]) | VDH, iodometric (Cu) [211] | [208,209,210,211] |
| Cd(BH4)2 | 5,67 | 75 | LiBH4, CdCl2 [212] LiBH4/NaBH4/KBH4, CdCl2 [213] |
high energy ball milling | in situ-XRD (synchrotron) [213], XRD [212,213], PCI [212], MS [212], DSC [212], TG-DSC [213] | [212,213] |
| Dy(BH4)3 | 5.84 | 250 | DyH3, S(CH3)2*BH3 | in solution (dimethyl sulfide-toluene mixture) | in situ-XRD (synchrotron), FT-IR, PCI, TG-DSC-MS, MP | [195] |
| Eu(BH4)2 | 4.44 | 290-395 | EuH2, N(C2H5)2*BH3 [197] EuH2, S(CH3)2*BH3 [195] EuCl3, LiBH4 [214] EuCl2, LiBH4 [214] |
in solution (N(C2H5)2*BH3 [197], dimethyl sulfide-toluene mixture [195], diethyl ether [214]) extraction (dimethyl sulfide [214]) high energy ball milling [214] annealing [214] |
in situ-XRD (synchrotron) [195,197,214], XRD [197,214], FT-IR [195,197,214], Raman [197], PCI [195,214], TG-DSC-MS [195,214], DSC [197], MP [195] | [195,197,214] |
| Eu(BH4)3 | 6.16 | < 100 [215] 168 [216] |
EuCl3, LiBH4 | high energy ball milling | in situ-XRD (synchrotron) [216], XRD [215,216], ATR/FT-IR [215,216], TG-DSC [215], TG-DSC-MS [216], SEM [215] | [215,216] |
| Er(BH4)3 | 5.71 | 245-264 | ErH3, S(CH3)2*BH3 [195] ErCl3, LiBH4 [217,218] ErCl3, NaBH4 [218] |
in solution (dimethyl sulfide-toluene mixture [195]) extraction (dimethyl sulfide [217]) high energy ball milling [217,218] |
in situ-XRD (synchrotron) [195,217], XRD [217,218], FT-IR [195,218], PCI [195,217,218], TG-DSC-MS [195,217], DSC [218], TPD-MS [217], MP [195] | [195,217,218] |
| Fe(BH4)2 | 9.43 | -20 [206] -10 [164] |
FeCl2, LiBH4 [206] FeCl3, LiBH4 [164] |
in solution (diethyl ether) | VDH [164,206], EA [164] | [164,206] |
| Gd(BH4)3 | 5.99 | 250-262 | GdH3, S(CH3)2*BH3 [194,195] GdCl3, LiBH4 [200,219,220] |
in solution (toluene or tetrahydrofurane [194], S(CH3)2*BH3-toluene mixture [200], dimethyl sulfide-toluene mixture [195]) extraction (dimethyl sulfide or tetrahydrofurane [194], dimethyl sulfide [200]) high energy ball milling [200,219,220] |
in situ-XRD (synchrotron) [194,195,220], XRD [194,200,219,220], FT-IR [194,195,200,219,220], 1H-NMR [194], PCI [195,200,219,220], TG-DSC-MS [194,195,200], TG-DTA-MS [220], TPD-MS [200], DSC [220], TPPA [200], TEM [194], MP [195], conductivity measurements [220] | [194,195,200,219,220] |
| Hf(BH4)4 | 6.78 | 136.4 [221] | HfCl4, LiBH4 [221,222,223,224,225,226,227,228] NaHfF5, Al(BH4)3 [229] |
direct metathesis [221,222,223,224,225,226,229] in solution (diethyl ether [227,228]) |
VDH [222,229], EA [222,229], VPM [229], single crystal neutron diffraction [228], XRD [221], gas electron diffraction [225], DSC [221], TG-DSC-MS [221], IR [222,224,225], photoelectron spectroscopy [223], melting point [221,229], boiling point [229], CP [221], S° [221], BH [221], NMR (1H [221,222,224,227], 11B [221,222,224]), DFT [221] | [221,222,223,224,225,226,227,228,229] |
| Ho(BH4)3 | 5.77 | 252 [195] 236 [230] |
HoH3, S(CH3)2*BH3 [195] HoCl3, LiBH4 [230] |
in solution (dimethyl sulfide-toluene mixture [195]) high energy ball milling [230] |
in situ-XRD (synchrotron) [195], XRD [230], FT-IR [195,230], PCI [195], TG-DSC-MS [195], TG-DSC [230], MP [195] | [195,230] |
| La(BH4)3 | 6.59 | 242-258 | LaH3, S(CH3)2*BH3 [195] LiBH4, LaCl3 [203,205,231,232] |
in solution (dimethyl sulfide-toluene mixture [195], toluene [203,231,232]) extraction with dimethyl sulfide [203,231,232] high energy ball milling [205] |
in situ-XRD (synchrotron) [195,203], XRD [205], FT-IR [195,205], PCI [195], TG-DSC-MS [195,205], TG-DSC [203], MP [195] | [195,203,205,231,232] |
| Lu(BH4)3 | 3.94 | 220 | LuH3, S(CH3)2*BH3 | in solution (dimethyl sulfide-toluene mixture) | in situ-XRD (synchrotron), FT-IR, PCI, TG-DSC-MS, MP | [195] |
| Mn(BH4)2 | 9.53 | 125-150 | MnCl2, LiBH4 [180,181,201,233,234,235,236] MnCl2, NaBH4 [180,237] |
high energy ball milling [180,181,233] in solution (dimethyl sulfide-toluene mixture [201], diethyl ether [234,235]) extraction (dimethyl sulfide [201,234,235], diethyl ether [237]) high energy ball milling [236,237,238] |
in situ-XRD (synchrotron) [201,234,235], XRD [180,181,233,234,236,237,238], (ATR-)FT-IR [201,233,236,237,238], Raman [180,181,233,234], PCI [236,237,238], TG-MS [180,181,233], DSC [233,236], TG-DSC-MS [201,234,235,237,238], TDS-GC/MS [180,181], DFT [234], FE-SEM [237,238], ICP-OES [235] | [180,181,201,233,234,235,236,237,238] |
| Nd(BH4)3 | 6.41 | 235-245 | NdH3, S(CH3)2*BH3 | in solution (toluene or tetrahydrofurane [194], dimethyl sulfide-toluene mixture [195,239]) extraction with dimethyl sulfide or tetrahydrofurane [194] |
in situ-XRD (synchrotron) [194,195,239], XRD [194], NPD [239], FT-IR [194,195], 1H-NMR [194], PCI [195], TG-DSC-MS [194,195,239], TEM [194], MP [195], DFT [239] | [194,195,239] |
| Ni(BH4)2 | 9.12 | -20 | NiCl2, LiBH4 | in solution (diethyl ether) | / | [206] |
| Np(BH4)4 | 4.30 | < RT [240,241] | NpF4, Al(BH4)3 | direct metathesis in glass tube | VPM [240], XRD [240,242], IR [241,242,243], Raman [241,242,243], EPR [242], C°P [243], S° [243] | [240,241,242,243] |
| Pa(BH4)4 | 5.55 | > RT | PaF4, Al(BH4)3 | direct metathesis in glass tube | XRD [241,242], IR [241,242], Raman [241,242], EPR [242] | [241,242] |
| Pr(BH4)3 | 6.52 | 236-252 | PrH3, S(CH3)2*BH3 [195,239] PrCl3, LiBH4 [217] |
in solution (dimethyl sulfide-toluene mixture [195,239], diethyl ether [217]) extraction (dimethyl sulfide [217]) |
in situ-XRD (synchrotron) [195,217,239], XRD [217], NPD [239], FT-IR [195], PCI [195,217], TG-DSC-MS [195,217,239], TPD-MS [217], MP [195], DFT [239] | [195,217,239] |
| Pu(BH4)4 | 4.19 | < RT [241] | PuF4, Al(BH4)3 | direct metathesis in glass tube | XRD [241,242], IR [241,242], Raman [241,242], EPR [242] | [241,242] |
| Sc(BH4)3 | 13.52 | 207-215 | ScCl3, LiBH4 | high energy ball milling | XRD, Raman, TDS-GC/MS | [179,180,181,244] |
| Sm(BH4)2 | 4.48 | 300-318 | SmH2, S(CH3)2*BH3 [194,195] SmCl3, LiBH4 [214,215,216] |
in solution (toluene or tetrahydrofurane [194], dimethyl sulfide-toluene mixture [195], diethyl ether [214]) extraction (dimethyl sulfide or tetrahydrofurane [194], dimethyl sulfide [214]) high energy ball milling [215,216] |
in situ-XRD (synchrotron) [194,195,214,216], XRD [194,214,215,216], ATR/FT-IR [194,195,214,215,216], 1H-NMR [194], PCI [195,214], TG-DSC-MS [194,195,214,215,216], SEM [215], TEM [194], MP [195] | [194,195,214,215,216] |
| Sm(BH4)3 | 6.21 | 170 [215] 168 [216] |
SmCl3, LiBH4 | high energy ball milling | in situ-XRD (synchrotron) [216], XRD [215,216], ATR/FT-IR [215,216], TG-DSC-MS [215,216], SEM [215] | [215,216] |
| Tb(BH4)3 | 5.95 | 250[195] 243[215] |
TbH3, S(CH3)2*BH3 [195] TbCl3, LiBH4 [215] |
in solution (dimethyl sulfide-toluene mixture) [195] high energy ball milling[215] |
in situ-XRD (synchrotron) [195], XRD [215], ATR/FT-IR [195,215], PCI [195], TG-DSC-MS [195,215], SEM [215], MP [195] | [195,215] |
| Th(BH4)4 | 5.53 | > 150 – 203 [229,241,245] | ThF4, Al(BH4)3 | direct metathesis in glass tube | VDH [229], EA [229], VPM [229], XRD [241], IR [241,245], Raman [241], TG [245], melting point [229] | [229,241,245] |
| Ti(BH4)3 | 13.09 | < RT-78 [229,246,247] | TiCl4, LiBH4 [229,247] TiCl3, LiBH4 [247,248,249] TiF3, LiBH4 [246] |
direct metathesis [229] mixing in motar [247] high energy ball milling [246,247,248,249] |
VDH [229,248,249], EA [229,248,249], XRD [246], TG-DSC-MS [246], TG [247], MS [247], (FT-)IR [246,247,249] | [229,246,247,248,249] |
| Tm(BH4)3 | 5.71 | 253 | TmH3, S(CH3)2*BH3 | in solution (dimethyl sulfide-toluene mixture) | in situ-XRD (synchrotron), FT-IR, PCI, TG-DSC-MS, MP | [195] |
| U(BH4)4 | 5.42 | < RT [241,250] | UF4, Al(BH4)3 | direct metathesis in glass tube | VDH [250], EA [250,251], VPM [250,251], XRD [241,252], IR [241,245,252,253], Raman [241], thermographic investigation [252] | [241,245,250,251,252,253] |
| Y(BH4)3 | 9.06 | 191-276 | YCl3, LiBH4 [181,190,199,200,254,255,256,257,258] YH3, B2H6 [190] YH3, S(CH3)2*BH3 [194] YCl3, Li11BD4 [259] YCl3, LiBD4 [260] |
high energy ball milling[181,190,200,254,255,256,257,258,259,260] in solution (toluene or tetrahydrofurane[194], dimethyl sulfide-boran complex in toluene[200], diethyl ether[199,255]) extraction (dimethyl sulfide or tetrahydrofurane[194], dimethyl sulfide[199,200]) |
EA [258], in situ-XRD (synchrotron) [190,194,199,200,254,259], XRD [181,190,194,199,200,254,255,256,257,258,259,260], PND [259], FT-IR [194,257,258,260], Raman [181,255,258,260], NMR (1H [194,258], 11B [258], 1H-MAS [256], 11B-MAS [254], 89Y-MAS [256]), PCI [200,255,257], DSC [190,199,257], DSC-TPD [259], TG [199], TG-DSC-MS/FT-IR [256,258,260], TG-DSC [254], TG-MS [181,190], TG-DSC-MS [194,200], TDS-GC [181], TPD-MS [181,200], TG-DTA-MS [255], TPPA [199,200], SEM-EDS [258], TEM [194], BET [190] | [181,190,194,199,200,254,255,256,257,258,259,260] |
| Yb(BH4)2 | 3.98 | 329-353 | YbH3, S(CH3)2*BH3 [194] YbH2, S(CH3)2*BH3 [195] YbCl3, LiBH4 [216,261] |
in solution (toluene or tetrahydrofurane [194], dimethyl sulfide-toluene mixture [195]) extraction (dimethyl sulfide or tetrahydrofurane [194]) high energy ball milling [216,261] |
in situ-XRD (synchrotron) [194,195,216,261], XRD [194,216,261], PND [261], ATR/FT-IR [194,195,216], Raman [261], 1H-NMR [194], PCI [195], TG-DSC-MS [194,195], TG-DSC [261], TG-DSC-MS [216], TPD [261], TEM [194], MP [195] | [194,195,216,261] |
| Yb(BH4)3 | 5.56 | 122-150 | YbCl3, LiBH4 | high energy ball milling | in situ-XRD (synchrotron) [216,261], XRD [215,216,261], PND [261], Raman [261], ATR/FT-IR [215,216], TG-DSC [261], TG-DSC-MS [215,216], TPD [216,261], SEM [215] | [215,216,261] |
| Zr(BH4)4 | 10.71 | 72 [179,181,262] 82 [263] 130.4 [221] |
ZrCl4, NaBH4 [179] ZrCl4, LiBH4 [159,164,179,181,193,221,222,223,224,225,227,262,263,264,265,266,267,268,269,270] NaZrF5, Al(BH4)3 [159,229] KZrF5, Al(BH4)3 [159] Na2ZrF6, Al(BH4)3 [243] Zr(iso-propylate)4, B2H6 [159] |
direct metathesis [159,193,221,222,223,224,225,229,243,264,265,266] in solution (diethyl ether) [159,227,268,269,270] high energy ball milling [179,181,262,263,267] |
VDH [159,222,229], EA [159,222,229], VPM [229,268], in situ-XRD (synchrotron) [267], XRD [179,181,221,262,263,267], electron diffraction [225], (FT-I)IR [222,224,225,243,245,263,264,265,267,268,270], Raman [179,181,262], NMR (1H [221,222,227,269], 11B [221,222,224,269], 91Zr [221,269]), photoelectron spectroscopy [223], DSC [221,263], TPD-GC [179,181,262], TG-MS [179,181,262], TG-DSC-MS [221], melting point [159,221,263,268], boiling point [229], CP [221], S° [221], BH [221], DFT [221,262], MDS [271] | [159,179,181,193,221,222,223,224,225,227,229,243,245,262,263,264,265,266,267,268,269,270,271] |
3.2.3. Dehydrogenation and Hydrogenation Behavior
3.2.4. Stability and Diborane Formation
4. Summary and Perspective
Acknowledgements
References
- L. Schlapbach, A. Züttel, Nature (London, U. K.) 2001, 414, 353–358.
- G. Marbán, T. Valdés-Solís, Int. J. Hydrogen Energy 2007, 32, 1625–1637.
- M. D. Redwood, M. Paterson-Beedle, L. E. Macaskie, Rev. Environ. Sci. Bio/Technol. 2009, 8, 149–185.
- J. O. Bockris, Int. J. Hydrog. Energy 2013, 38, 2579–2588.
- H. T. Hwang, A. Varma, Curr. Opin. Chem. Eng. 2014, 5, 42–48.
- U. Eberle, M. Felderhoff, F. Schüth, Angew. Chem., Int. Ed. Engl. 2009, 48, 6608–6630.
- S. Satyapal, J. Petrovic, C. Read, G. Thomas, G. Ordaz, Catal. Today 2007, 120, 246–256.
- N. Heinemann, J. Alcalde, J. M. Miocic, S. J. T. Hangx, J. Kallmeyer, C. Ostertag-Henning, A. Hassanpouryouzband, E. M. Thaysen, G. J. Strobel, C. Schmidt-Hattenberger et al., Energy Environ. Sci. 2021, 14, 853–864.
- D. J. Durbin, C. Malardier-Jugroot, Int. J. Hydrogen Energy 2013, 38, 14595–14617.
- B. Zohuri, Hydrogen Energy: Challanges and Solution for a Cleaner Future, Springer International Publishing, Cham, 2019.
- T. Yoshida, K. Kojima, Electrochem. Soc. Interface 2015, 24, 45–49.
- N. Takeichi, Int. J. Hydrogen Energy 2003, 1121–1129.
- D. Mori, K. Hirose, Int. J. Hydrogen Energy 2009, 34, 4569–4574.
- D. Mori, K. D. Mori, K. Hirose, N. Haraikawa, T. Takiguchi, T. Shinozawa, T. Matsunaga, K. Toh, K. Fujita, A. Kumano, H. Kubo in SAE Technical Paper Series, SAE International400 Commonwealth Drive, Warrendale, PA, United States, 2007.
- Y. Kojima, Y. Kawai, S. Towata, T. Matsunaga, T. Shinozawa, M. Kimbara, J. Alloys Compd. 2006, 419, 256–261.
- T. MATSUNAGA, M. KON, K. WASHIO, T. SHINOZAWA, M. ISHIKIRIYAMA, Int. J. Hydrogen Energy 2009, 34, 1458–1462.
- D. Mori, N. Haraikawa, N. Kobayashi, H. Kubo, K. TOH, M. Tsuzuki, T. Shinozawa, T. Matsunaga, MRS Online Proc. Libr. 2005, 884, 72–78.
- Y. -H. Xu, C.-P. Chen, W.-X. Geng, Q.-D. Wang, Int. J. Hydrogen Energy 2001, 26, 593–596.
- K. Young, T. Ouchi, M. A. Fetcenko, J. Alloys Compd. 2009, 480, 428–433.
- M. V. Lototskyy, I. Tolj, L. Pickering, C. Sita, F. Barbir, V. Yartys, Prog. Nat. Sci.: Mater. Int. 2017, 27, 3–20.
- F. Marques, M. Balcerzak, F. Winkelmann, G. Zepon, M. Felderhoff, Energy Environ. Sci. 2021, 14, 5191–5227.
- D. P. Shoemaker, C. B. Shoemaker, J. Less-Common Met. 1979, 68, 43–58.
- D. G. Westlake, J. Less-Common Met. 1983, 90, 251–273.
- D. G. Westlake, J. Less-Common Met. 1983, 91, 1–20.
- J. Bodega, J. F. Fernández, F. Leardini, J. R. Ares, C. Sánchez, J. Phys. Chem. Solids 2011, 72, 1334–1342.
- A. R. Merlino, C. R. Luna, A. Juan, M. E. Pronsato, Int. J. Hydrogen Energy 2016, 41, 2700–2710.
- D. G. Ivey, D. O. Northwood, Z. Phys. Chem. 1986, 147, 191–209.
- J. R. Johnson, J. J. Reilly, Inorg. Chem. 1978, 17, 3103–3108.
- J. R. Johnson, J. Less-Common Met. 1980, 73, 345–354.
- S. Klyamkin, Int. J. Hydrogen Energy 1999, 24, 149–152.
- T. L. Murashkina, M. S. Syrtanov, R. S. Laptev, E. N. Stepanova, A. M. Lider, Int. J. Hydrogen Energy 2019, 44, 10732–10743.
- T. L. Murashkina, M. S. Syrtanov, R. S. Laptev, A. M. Lider, Int. J. Hydrogen Energy 2019, 44, 6709–6719.
- T. L. Murashkina, M. S. Syrtanov, A. S. Shabunin, R. S. Laptev, J. Surf. Invest.: X-Ray, Synchrotron Neutron Tech. 2019, 13, 146–153.
- Y. Moriwaki, T. Gamo, T. Iwaki, J. Less-Common Met. 1991, 172-174, 1028–1035.
- N. Nagasako, A. Fukumoto, K. Miwa, Phys. Rev. B 2002, 66.
- M. Kandavel, V. V. Bhat, A. Rougier, L. Aymard, G.-A. Nazri, J.-M. Tarascon, Int. J. Hydrogen Energy 2008, 33, 3754–3761.
- F. Li, J. Zhao, D. Tian, H. Zhang, X. Ke, B. Johansson, J. Appl. Phys. 2009, 105, 43707.
- Z. -S. Nong, J.-C. Zhu, Y. Cao, X.-W. Yang, Z.-H. Lai, Y. Liu, Phys. B 2013, 419, 11–18.
- Z. -S. Nong, J.-C. Zhu, X.-W. Yang, Y. Cao, Z.-H. Lai, Y. Liu, W. Sun, Solid State Sci. 2014, 32, 1–7.
- X. Guo, E. Wu, J. Alloys Compd. 2008, 455, 191–196.
- X. GUO, E. WU, S. WANG, Rare Met. 2006, 25, 218–223.
- W. Li, E. Wu, P. Ma, K. Sun, D. Chen, Int. J. Energy Res. 2013, 37, 686–697.
- J. J. Reilly, Z. Phys. Chem. 1979, 117, 155–184.
- J. R. Johnson, J. J. J. R. Johnson, J. J. Reilly, THE METAL HYDRIDE RESEARCH AND DEVELOPMENT PROGRAM AT BROOKHAVEN NATIONAL LABORATORY, 1978.
- J. R. Johnson, J. J. Reilly, F. Reidinger, L. M. Corliss, J. M. Hastings, J. Less-Common Met. 1982, 88, 107–114.
- O. Beeri, D. Cohen, Z. Gavra, J. R. Johnson, M. H. Mintz, J. Alloys Compd. 2000, 299, 217–226.
- O. Beeri, D. Cohen, Z. Gavra, M. H. Mintz, J. Alloys Compd. 2003, 352, 111–122.
- D. S. dos Santos, M. Bououdina, D. Fruchart, J. Alloys Compd. 2002, 340, 101–107.
- U. Ulmer, M. Dieterich, A. Pohl, R. Dittmeyer, M. Linder, M. Fichtner, Int. J. Hydrogen Energy 2017, 42, 20103–20110.
- K. Young, T. Ouchi, J. Yang, M. A. Fetcenko, Int. J. Hydrogen Energy 2011, 36, 11137–11145.
- K. Young, J. Nei, B. Huang, M. A. Fetcenko, Int. J. Hydrogen Energy 2011, 36, 11146–11154.
- S. -M. Lee, H. Lee, J.-H. Kim, P. S. Lee, J.-Y. Lee, J. Alloys Compd. 2000, 308, 259–268.
- S. -. Lee, S.-H. Kim, S.-W. Jeon, J.-Y. Lee, J. Electrochem. Soc. 2000, 4464–4469.
- Y. Xu, G. Wang, C. Chen, Q. Wang, X. Wang, Int. J. Hydrogen Energy 2007, 32, 1050–1058.
- V. S. Marinin, K. R. Umerenkova, O. V. Volovchuk, Int. J. Hydrogen Energy 2011, 36, 1359–1363.
- J. -G. Park, H.-Y. Jang, S.-C. Han, P. S. Lee, J.-Y. Lee, J. Alloys Compd. 2001, 325, 293–298.
- T. L. Pourpoint, V. Velagapudi, I. Mudawar, Y. Zheng, T. S. Fisher, Int. J. Heat Mass Transfer 2010, 53, 1326–1332.
- Z. Cao, L. Ouyang, H. Wang, J. Liu, D. Sun, Q. Zhang, M. Zhu, Int. J. Hydrogen Energy 2015, 40, 2717–2728.
- Z. Cao, L. Ouyang, H. Wang, J. Liu, L. Sun, M. Zhu, J. Alloys Compd. 2015, 639, 452–457.
- Z. Chen, X. Xiao, L. Chen, X. Fan, L. Liu, S. Li, H. Ge, Q. Wang, Int. J. Hydrogen Energy 2013, 38, 12803–12810.
- Z. Chen, X. Xiao, L. Chen, X. Fan, L. Liu, S. Li, H. Ge, Q. Wang, J. Alloys Compd. 2014, 585, 307–311.
- L. Liu, L. Chen, X. Xiao, C. Xu, J. Sun, S. Li, H. Ge, L. Jiang, J. Alloys Compd. 2015, 636, 117–123.
- Z. Yao, L. Liu, X. Xiao, C. Wang, L. Jiang, L. Chen, J. Alloys Compd. 2018, 731, 524–530.
- J. Li, L. Xu, X. Jiang, X. Li, Prog. Nat. Sci.: Mater. Int. 2018, 28, 470–477.
- J. Li, X. Jiang, G. Li, X. Li, Int. J. Hydrogen Energy 2019, 44, 15087–15099.
- J. Li, X. Jiang, Z. Li, L. Jiang, X. Li, Int. J. Energy Res. 2019, 43, 5759–5774.
- J. Puszkiel, J. M. Bellosta von Colbe, J. Jepsen, S. V. Mitrokhin, E. Movlaev, V. Verbetsky, T. Klassen, Energies 2020, 13, 2751.
- M. S. Granovsky, D. Arias, J. Nucl. Mater. 1996, 229, 29–35.
- T. Zotov, E. Movlaev, S. Mitrokhin, V. Verbetsky, J. Alloys Compd. 2008, 459, 220–224.
- S. M. Filipek, I. Jacob, V. Paul-Boncour, A. Percheron-Guegan, I. Marchuk, D. Mogilyanski, J. Pielaszek, ChemInform 2002, 33, no.
- V. Paul-Boncour, F. Bourée-Vigneron, S. M. Filipek, I. Marchuk, I. Jacob, A. Percheron-Guégan, J. Alloys Compd. 2003, 356-357, 69–72.
- E. D. Koultoukis, S. S. Makridis, E. Pavlidou, P. de Rango, A. K. Stubos, Int. J. Hydrogen Energy 2014, 39, 21380–21385.
- S. Banerjee, A. Kumar, C. Pillai, Intermetallics 2014, 51, 30–36.
- A. Jain, R. K. Jain, S. Agarwal, R. K. Sharma, S. K. Kulshrestha, I. P. Jain, J. Alloys Compd. 2008, 454, 31–37.
- R. B. Sivov, T. A. Zotov, V. N. Verbetsky, D. S. Filimonov, K. V. Pokholok, J. Alloys Compd. 2011, 509, S763–S769.
- R. B. Sivov, V. A. Somenkov, V. P. Glazkov, V. N. Verbetsky, Inorg. Mater. 2012, 48, 792–795.
- V. A. Yartys, R. V. Denys, C. J. Webb, J. P. Mæhlen, E. M. Gray, T. Blach, O. Isnard, L. C. Barnsley, J. Alloys Compd. 2011, 509, S817–S822.
- R. B. Sivov, T. A. Zotov, V. N. Verbetsky, Inorg. Mater. 2010, 46, 372–376.
- M. H. Mendelsohn, D. M. Gruen, J. Less-Common Met. 1980, 74, 449–453.
- T. B. Zhang, X. F. Wang, R. Hu, J. S. Li, X. W. Yang, X. Y. Xue, H. Z. Fu, Int. J. Hydrogen Energy 2012, 37, 2328–2335.
- T. P. Yadav, R. R. Shahi, O. N. Srivastava, Int. J. Hydrogen Energy 2012, 37, 3689–3696.
- K. Kanematsu, J. Phys. Soc. Jpn. 1971, 31, 1355–1360.
- H. Fujii, V. K. Sinha, F. Pourarian, W. E. Wallace, J. Less-Common Met. 1982, 85, 43–54.
- A. Jain, Jain, R. Agarwal, S., Ganesan, V. Lalla, N. P., D. M. Phase, I. P. Jain, Int. J. Hydrogen Energy 2007, 32, 3965–3971.
- S. Hirosawa, F. Pourarian, V. K. Sinha, W. E. Wallace, J. Magn. Magn. Mater. 1983, 38, 159–164.
- M. Bereznitsky, I. Jacob, J. Bloch, M. H. Mintz, J. Alloys Compd. 2003, 351, 180–183.
- J. Coaquira, H. R. Rechenberg, J. Mestnik Filho, J. Magn. Magn. Mater. 1999, 196-197, 677–679.
- J. Coaquira, H. R. Rechenberg, J. Mestnik Filho, J. Alloys Compd. 1999, 288, 42–49.
- G. Y. Yu, W. E. Wallace, J. Solid State Chem. 1986, 65, 356–362.
- V. Sinha, F. Pourarian, W. Wallace, J. Less-Common Met. 1982, 87, 283–296.
- R. S. T. Zotov, E. R. S. T. Zotov, E. Mitrohkin, E. A. Movlaev, V. Verbetsky, Carbon Nanomaterials in Clean Energy Hydrogen Systems 2008, 699–704.
- T. A. Zotov, R. B. Sivov, E. A. Movlaev, S. V. Mitrokhin, V. N. Verbetsky, J. Alloys Compd. 2011, 509, S839–S843.
- D. J. Davidson, O. N. Srivastava, Int. J. Hydrog. Energy 2001, 26, 219–223.
- D. Davidson, Int. J. Hydrog. Energy 2003, 28, 1425–1431.
- R. B. Sivov, T. A. Zotov, V. N. Verbetsky, Int. J. Hydrogen Energy 2011, 36, 1355–1358.
- L. Jiang, Y. Tu, H. Tu, L. Chen, J. Alloys Compd. 2015, 627, 161–165.
- Z. Cao, L. Ouyang, H. Wang, J. Liu, L. Sun, M. Felderhoff, M. Zhu, Int. J. Hydrogen Energy 2016, 41, 11242–11253.
- C. Zhou, H. Wang, L. Z. Ouyang, J. W. Liu, M. Zhu, J. Alloys Compd. 2019, 806, 1436–1444.
- C. Qin, C. Zhou, L. Ouyang, J. Liu, M. Zhu, T. Sun, H. Wang, Int. J. Hydrogen Energy 2020, 45, 9836–9844.
- M. Kandavel, S. Ramaprabhu, J. Phys.: Condens. Matter 2003, 15, 7501–7517.
- S. L. Li, H. H. Cheng, X. X. Deng, W. Chen, D. M. Chen, K. Yang, J. Alloys Compd. 2008, 460, 186–190.
- M. Kandavel, S. Ramaprabhu, J. Alloys Compd. 2004, 381, 140–150.
- E. Akiba, H. Iba, Intermetallics 1998, 6, 461–470.
- X. Yu, Z. Wu, B. Xia, T. Huang, J. Chen, Z. Wang, N. Xu, J. Mater. Res. 2003, 18, 2533–2536.
- K. Nomura, E. Akiba, J. Alloys Compd. 1995, 231, 513–517.
- H. Yukawa, A. Teshima, D. Yamashita, S. Ito, S. Yamaguchi, M. Morinaga, J. Alloys Compd. 2002, 337, 264–268.
- J. -H. Yoo, G. Shim, C.-N. Park, W.-B. Kim, S.-W. Cho, Int. J. Hydrogen Energy 2009, 34, 9116–9121.
- H. Arashima, F. Takahashi, T. Ebisawa, H. Itoh, T. Kabutomori, J. Alloys Compd. 2003, 356-357, 405–408.
- C. Wu, Y. Yan, Y. Chen, M. Tao, X. Zheng, Int. J. Hydrogen Energy 2008, 33, 93–97.
- S. Ono, K. Nomura, Y. Ikeda, J. Less-Common Met. 1980, 72, 159–165.
- B. Nowak, S. Hayashi, K. Hayamizu, O. Yamamoto, J. Less-Common Met. 1986, 123, 75–84.
- J. Matsuda, E. Akiba, J. Alloys Compd. 2013, 581, 369–372.
- J. F. Lynch, J. J. Reilly, F. Millot, Solid State Commun. 1978, 26, iv.
- V. N. Verbetsky, T. A. Zotov, A. V. Tatarintsev, E. A. Movlaev, Inorg. Mater. 2013, 49, 149–152.
- K. Asano, S. Hayashi, Y. Nakamura, E. Akiba, J. Alloys Compd. 2012, 524, 63–68.
- K. Asano, S. Hayashi, Y. Nakamura, E. Akiba, J. Alloys Compd. 2010, 507, 399–404.
- V. N. Verbetsky, T. A. Zotov, E. A. Movlaev, Inorg. Mater.: Appl. Res. 2014, 5, 70–74.
- T. V. B. Phung, H. Ogawa, an van Dinh, O. H. Nguyen, Y. Shibutani, K. Asano, Y. Nakamura, E. Akiba, Theor. Chem. Acc. 2019, 138.
- S. Miraglia, P. de Rango, S. Rivoirard, D. Fruchart, J. Charbonnier, N. Skryabina, J. Alloys Compd. 2012, 536, 1–6.
- T. Tamura, T. Kazumi, A. Kamegawa, H. Takamura, M. Okada, J. Alloys Compd. 2003, 356-357, 505–509.
- H. Itoh, H. Arashima, K. Kubo, T. Kabutomori, K. Ohnishi, J. Alloys Compd. 2005, 404-406, 417–420.
- J. F. Lynch, A. J. Maeland, G. G. Libowitz, Z. Phys. Chem. 1985, 145, 51–59.
- G. Fu, F. Wang, J. Wang, M. H. Rong, Z. M. Wang, G. H. Rao, H. Y. Zhou, Mater. Sci. Forum 2016, 847, 3–7.
- A. Guéguen, J.-M. Joubert, M. Latroche, J. Alloys Compd. 2011, 509, 3013–3018.
- S. K. Callear, A. J. Ramirez-Cuesta, K. Kamazawa, S. Towata, T. Noritake, S. F. Parker, M. O. Jones, J. Sugiyama, M. Ishikiriyama, W. I. F. David, Phys. Chem. Chem. Phys. 2014, 16, 16563–16572.
- S. Basak, K. Shashikala, P. Sengupta, S. K. Kulshrestha, Int. J. Hydrogen Energy 2007, 32, 4973–4977.
- Y. Yan, Y. Chen, H. Liang, C. Wu, M. Tao, T. Mingjing, J. Alloys Compd. 2006, 426, 253–255.
- T. Kuriiwa, T. Maruyama, A. Kamegawa, M. Okada, Int. J. Hydrogen Energy 2010, 35, 9082–9087.
- K. Goshome, N. Endo, M. Tetsuhiko, Int. J. Hydrogen Energy 2019, 44, 10800–10807.
- S. Selvaraj, A. Jain, S. Kumar, T. Zhang, S. Isobe, H. Miyaoka, Y. Kojima, T. Ichikawa, Int. J. Hydrogen Energy 2018, 43, 2881–2889.
- Y. Nonobe, IEEJ Trans. Electr. Electron. Eng. 2017, 12, 5–9.
- S. Kumar, A. Jain, T. Ichikawa, Y. Kojima, G. K. Dey, Renewable Sustainable Energy Rev. 2017, 72, 791–800.
- A. Lys, J. O. Fadonougbo, M. Faisal, J.-Y. Suh, Y.-S. Lee, J.-H. Shim, J. Park, Y. W. Cho, Hydrogen 2020, 1, 38–63.
- K. Young, D. F. Wong, S. Yasuoka, J. Ishida, J. Nei, J. Koch, J. Power Sources 2014, 251, 170–177.
- T. Bibienne, C. Gosselin, J.-L. Bobet, J. Huot, Appl. Sci. 2018, 8, 1151.
- Y. Yan, Y. Chen, C. Wu, M. Tao, H. Liang, J. Power Sources 2007, 164, 799–802.
- C. Wu, X. Zheng, Y. Chen, M. Tao, G. Tong, J. Zhou, Int. J. Hydrogen Energy 2010, 35, 8130–8135.
- Y. Mao, S. Yang, C. Wu, L. Luo, Y. Chen, J. Alloys Compd. 2017, 705, 533–538.
- S. Yang, F. Yang, C. Wu, Y. Chen, Y. Mao, L. Luo, J. Alloys Compd. 2016, 663, 460–465.
- M. B. Ley, L. H. Jepsen, Y.-S. Lee, Y. W. Cho, J. M. Bellosta von Colbe, M. Dornheim, M. Rokni, J. O. Jensen, M. Sloth, Y. Filinchuk et al., Mater. Today 2014, 17, 122–128.
- B. Bogdanović, M. Schwickardi, J. Alloys Compd. 1997, 253-254, 1–9.
- K. Suárez-Alcántara, J. R. Tena-Garcia, R. Guerrero-Ortiz, Materials 2019, 12.
- G. Monnier, Bull. Soc. Chim. Fr. 1955, 1138.
- M. E. Kost, A. I. Golovanova, Izv. Akad. Nauk SSSR, Neorg. Mater. 1978, 14, 1732–1734.
- H. Neumaier, D. Büchel, G. Ziegelmaier, Z. Anorg. Allg. Chem. 1966, 345, 46–52.
- M. E. Kost, A. I. Golovanova, Izv. Akad. Nauk SSSR, Ser. Khim. 1975, 5, 991–994.
- N. T. Kuznetsov, N. N. Mal'tseva, A. I. Golovanova, Russ. J. Inorg. Chem. 1985, 30, 1604–1606.
- G. Monnier, Ann. chim. 1957, 13, 33.
- C. Weidenthaler, A. Pommerin, M. Felderhoff, W. Sun, C. Wolverton, B. Bogdanović, F. Schüth, J. Am. Chem. Soc. 2009, 131, 16735–16743.
- E. Wiberg, W. Henle, Z. Naturforsch. B 1951, 6, 461–462.
- E. Wiberg, H. Neumaier, Inorg. Nucl. Chem. Lett. 1965, 1, 35–37.
- E. Wiberg, W. Henle, Z. Naturforsch. B 1951, 6, 461.
- H. Neumaier, Dissertation, Ludwig-Maximilians-Universität, München, 1961.
- F. Habermann, K. Burkmann, B. Hansel, B. Störr, C. Schimpf, J. Seidel, M. Bertau, F. Mertens, Dalton Trans. 2023, 52, 4880–4890.
- E. Wiberg, R. Usón, Z. Naturforsch. B 1951, 6, 393.
- A. I. Golovanova, M. E. Kost, V. I. Mikheeva, Bull. Acad. Sci. USSR, Div. Chem. Sci. (Engl. Transl.) 1973, 22, 1410–1413.
- G. Jander, K. Kraffczyk, Z. Anorg. Allg. Chem. 1956, 283, 217–229.
- E. Wiberg, W. Henle, Z. Naturforsch. B 1952, 7, 250–251.
- W. E. Reid, J. M. Bish, A. Brenner, J. Electrochem. Soc. 1957, 104, 21–29.
- G. L. Soloveichik, B. M. Bulychev, Russ. Chem. Rev. 1983, 52, 43–60.
- E. C. Ashby, R. A. Kovar, Inorg. Chem. 1977, 16, 1437–1440.
- E. C. Ashby, H. S. Prasad, Inorg. Chem. 1975, 14, 1608–1614.
- A. Pommerin, A. Wosylus, M. Felderhoff, F. Schüth, C. Weidenthaler, Inorg. Chem. 2012, 51, 4143–4150.
- G. W. Schaeffer, J. S. Roscoe, A. C. Stewart, J. Am. Chem. Soc. 1956, 78, 729–733.
- M. E. Kost, A. I. Golovanova, Zh. Neorg. Khim. 1977, 22, 977–979.
- E. Wiberg, H. Neumaier, Z. Anorg. Allg. Chem. 1965, 340, 189–200.
- E. Wiberg, R. Usón, Z. Naturforsch. B 1951, 6, 392–393.
- M. E. Kost, A. I. Golovanova, Zh. Neorg. Khim. 1977, 22, 832–833.
- Z. Cao, L. Ouyang, H. Wang, J. Liu, M. Felderhoff, M. Zhu, J. Mater. Chem. A 2017, 5, 6042–6046.
- Z. Cao, M. Felderhoff, Int. J. Hydrogen Energy 2021, 46, 26437–26444.
- H. -W. Li, Y. Yan, S.-I. Orimo, A. Züttel, C. M. Jensen, Energies 2011, 4, 185–214.
- J. J. Vajo, F. Mertens, C. C. Ahn, R. C. Bowman, B. Fultz, J. Phys. Chem. B 2004, 108, 13977–13983.
- J. J. Vajo, S. L. Skeith, F. Mertens, J. Phys. Chem. B 2005, 109, 3719–3722.
- K. Suárez-Alcántara, J. R. Tena García, Materials 2021, 14, 2561–2616.
- H. Nöth, M. H. Nöth, M. Seitz, J. Chem. Soc., Chem. Commun. 1976, 1004a-1004a.
- B. Lobkovskii, S. E. Kravchenko, K. N. Semenenko, J. Struct. Chem. 1977, 18, 312–314.
- J. H. Morris, W. E. Smith, J. Chem. Soc. D 1970, 245a.
- H. Nöth, L. P. Winter, Z. Anorg. Allg. Chem. 1972, 389, 225–234.
- Y. Nakamori, H. Li, K. Miwa, S. Towata, S.-I. Orimo, Mater. Trans. 2006, 47, 1898–1901.
- Y. Nakamori, H.-W. Li, K. Kikuchi, M. Aoki, K. Miwa, S. Towata, S. Orimo, J. Alloys Compd. 2007, 446-447, 296–300.
- Y. Nakamori, H.-W. Li, M. Matsuo, K. Miwa, S. Towata, S. Orimo, J. Phys. Chem. Solids 2008, 69, 2292–2296.
- E. Jeon, Y. Cho, J. Alloys Compd. 2006, 422, 273–275.
- M. Y. Song, Y. J. Kwak, S. N. Kwon, S. H. Lee, I. W. Park, Korean J. Met. Mater. 2015, 53, 500–505.
- S. Srinivasan, D. Escobar, M. Jurczyk, Y. Goswami, E. Stefanakos, J. Alloys Compd. 2008, 462, 294–302.
- D. Ravnsbaek, Y. Filinchuk, Y. Cerenius, H. J. Jakobsen, F. Besenbacher, J. Skibsted, T. R. Jensen, Angew. Chem. Int. Ed. 2009, 48, 6659–6663.
- Q. Gu, L. Gao, Y. Guo, Y. Tan, Z. Tang, K. S. Wallwork, F. Zhang, X. Yu, Energy Environ. Sci. 2012, 5, 7590.
- V. I. Mikheeva, N. N. Mal'tseva, L. S. Alekseeva, Russ. J. Inorg. Chem. 1968, 13, 682–685.
- C. -H. Yang, W.-T. Tsai, J.-K. Chang, Int. J. Hydrogen Energy 2011, 36, 4993–4999.
- D. Korablov, D. B. Ravnsbæk, V. Ban, Y. Filinchuk, F. Besenbacher, T. R. Jensen, Int. J. Hydrogen Energy 2013, 38, 8376–8383.
- K. Park, H.-S. Lee, A. Remhof, Y.-S. Lee, Y. Yan, M.-Y. Kim, S. J. Kim, A. Züttel, Y. W. Cho, Int. J. Hydrogen Energy 2013, 38, 9263–9270.
- H. Nöth, Angew. Chem. 1961, 73, 371–383.
- E. Wiberg, W. Henle, Z. Naturforsch. B 1952, 7, 575–576.
- G. W. Rice, R. L. Woodin, J. Am. Chem. Soc. 1988, 71, C–181.
- B. Richter, J. B. Grinderslev, K. T. Møller, M. Paskevicius, T. R. Jensen, Inorg. Chem. 2018, 57, 10768–10780.
- J. B. Grinderslev, K. T. Møller, M. Bremholm, T. R. Jensen, Inorg. Chem. 2019, 58, 5503–5517.
- L. J. Bannenberg, M. Heere, H. Benzidi, J. Montero, E. M. Dematteis, S. Suwarno, T. Jaroń, M. Winny, P. A. Orłowski, W. Wegner et al., Int. J. Hydrogen Energy 2020, 45, 33687–33730.
- M. Sharma, E. Didelot, A. Spyratou, L. M. Lawson Daku, R. Černý, H. Hagemann, Inorg. Chem. 2016, 55, 7090–7097.
- A. Remhof, A. Borgschulte, O. Friedrichs, P. Mauron, Y. Yan, A. Züttel, Scr. Mater. 2012, 66, 280–283.
- E. Roedern, Y.-S. Lee, M. B. Ley, K. Park, Y. W. Cho, J. Skibsted, T. R. Jensen, J. Mater. Chem. A 2016, 4, 8793–8802.
- M. B. Ley, M. Paskevicius, P. Schouwink, B. Richter, D. A. Sheppard, C. E. Buckley, T. R. Jensen, Dalton Trans. 2014, 43, 13333–13342.
- B. Richter, D. B. Ravnsbæk, N. Tumanov, Y. Filinchuk, T. R. Jensen, Dalton Trans. 2015, 44, 3988–3996.
- Deutsches Institut für Normung e., V. , Kunststoffe - Dynamische Differenz-Thermoanalyse (DSC) - Teil 1: Allgemeine Grundlagen (ISO 11357-1:2016), 83.080.01, 2017-02-00, Beuth Verlag GmbH, Berlin.
- M. B. Ley, M. Jørgensen, R. Černý, Y. Filinchuk, T. R. Jensen, Inorg. Chem. 2016, 55, 9748–9756.
- F. C. Gennari, M. R. Esquivel, J. Alloys Compd. 2009, 485, L47–L51.
- B. J. Zhang, B. H. Liu, Z. P. Li, J. Alloys Compd. 2011, 509, 751–757.
- J. Aubry, G. Monnier, Bull. Soc. Chim. Fr. 1955, 438.
- A. C. Stewart, G. W. Schaeffer, J. Inorg. Nucl. Chem. 1956, 3, 194–197.
- E. Wiberg, Angew. Chem. 1953, 65, 16–33.
- T. J. Klingen, Inorg. Chem. 1964, 3, 1058–1059.
- E. Wiberg, W. Henle, Z. Naturforsch. B 1952, 7, 582.
- N. N. Mal'tseva, Russ. J. Inorg. Chem. 1697, 12, 1338–1341.
- D. Blanchard, M. Zatti, T. Vegge, J. Alloys Compd. 2013, 547, 76–80.
- D. B. Ravnsbæk, L. H. Sørensen, Y. Filinchuk, F. Besenbacher, T. R. Jensen, Angew. Chem. Int. Ed. 2012, 51, 3582–3586.
- T. D. Humphries, M. B. Ley, C. Frommen, K. T. Munroe, T. R. Jensen, B. C. Hauback, J. Mater. Chem. A 2015, 3, 691–698.
- W. Wegner, T. Jaroń, W. Grochala, J. Alloys Compd. 2018, 744, 57–63.
- J. E. Olsen, C. Frommen, T. R. Jensen, M. D. Riktor, M. H. Sørby, B. C. Hauback, RSC Adv. 2014, 4, 1570–1582.
- M. Heere, S. H. Payandeh GharibDoust, C. Frommen, T. D. Humphries, M. B. Ley, M. H. Sørby, T. R. Jensen, B. C. Hauback, Phys. Chem. Chem. Phys. 2016, 18, 24387–24395.
- F. C. Gennari, J. Alloys Compd. 2013, 581, 192–195.
- J. Andrade-Gamboa, J. A. Puszkiel, L. Fernández-Albanesi, F. C. Gennari, Int. J. Hydrog. Energy 2010, 35, 10324–10328.
- M. B. Ley, S. Boulineau, R. Janot, Y. Filinchuk, T. R. Jensen, J. Phys. Chem. C 2012, 116, 21267–21276.
- K. Burkmann, F. Habermann, E. Schumann, J. Kraus, B. Störr, H. Schmidt, E. Brendler, J. Seidel, K. Bohmhammel, J. Kortus et al., New J. Chem. 2024, 48, 2743–2754.
- B. D. James, R. K. Nanda, M. G. H. Wallbridge, J. Chem. Soc. A 1966, 1, 182–184.
- J. C. Green, M. de Simone, M. Coreno, A. Jones, H. M. I. Pritchard, G. S. McGrady, Inorg. Chem. 2005, 44, 7781–7793.
- T. J. Marks, L. A. Shimp, J. Am. Chem. Soc. 1972, 94, 1542–1550.
- A. Haaland, D. J. Shorokhov, A. V. Tutukin, H. V. Volden, O. Swang, G. S. McGrady, N. Kaltsoyannis, A. J. Downs, C. Y. Tang, J. F. C. Turner, Inorg. Chem. 2002, 41, 6646–6655.
- E. R. Bernstein, T. A. Keiderling, J. Chem. Phys. 1973, 59, 2105–2122.
- A. L. Wayda, L. F. Schneemeyer, R. L. Opila, Appl. Phys. Lett. 1988, 53, 361–363.
- R. W. Broach, I. S. Chuang, T. J. Marks, J. M. Williams, Inorg. Chem. 1983, 22, 1081–1084.
- H. R. Hoekstra, J. J. Katz, J. Am. Chem. Soc. 1949, 71, 2488–2492.
- W. Wegner, T. Jaroń, W. Grochala, Int. J. Hydrogen Energy 2014, 39, 20024–20030.
- S. Payandeh GharibDoust, M. Heere, M. H. Sørby, M. B. Ley, D. B. Ravnsbæk, B. C. Hauback, R. Černý, T. R. Jensen, Dalton Trans. 2016, 45, 19002–19011.
- S. Payandeh GharibDoust, M. Brighi, Y. Sadikin, D. B. Ravnsbæk, R. Černý, J. Skibsted, T. R. Jensen, J. Phys. Chem. C 2017, 121, 19010–19021.
- R. Liu, D. Reed, D. Book, J. Alloys Compd. 2012, 515, 32–38.
- N. A. Tumanov, E. Roedern, Z. Łodziana, D. B. Nielsen, T. R. Jensen, A. V. Talyzin, R. Černý, D. Chernyshov, V. Dmitriev, T. Palasyuk et al., Chem. Mater. 2016, 28, 274–283.
- E. Roedern, T. R. Jensen, J. Phys. Chem. C 2014, 118, 23567–23574.
- R. A. Varin, A. S. Bidabadi, Int. J. Hydrogen Energy 2014, 39, 11620–11632.
- R. A. Varin, D. K. Mattar, A. S. Bidabadi, M. Polanski, J. Energy Chem. 2017, 26, 24–34.
- R. A. Varin, A. S. Bidabadi, M. Polanski, M. Biglari, L. Stobinski, Mater. Res. Bull. 2018, 100, 394–406.
- S. Payandeh GharibDoust, M. Heere, C. Nervi, M. H. Sørby, B. C. Hauback, T. R. Jensen, Dalton Trans. 2018, 47, 8307–8319.
- R. H. Banks, N. M. Edelstein, B. Spencer, D. H. Templeton, A. Zalkin, J. Am. Chem. Soc. 1980, 102, 620–623.
- R. H. Banks, N. M. Edelstein, R. R. Rietz, D. H. Templeton, A. Zalkin, J. Am. Chem. Soc. 1978, 100, 1957–1958.
- R. H. Banks, N. M. R. H. Banks, N. M. Edelstein in ACS Symposium Series, Vol. 131 (Hrsg.: N. M. Edelstein), American Chemical Society, Washington, D.C., 1980, S. 331–348.
- R. H. Banks, N. Edelstein, J. Chem. Phys. 1980, 73, 3589–3599.
- Y. Nakamori, S. Y. Nakamori, S. Orimo in Woodhead Publishing Series in Electronic and Optical Materials (Hrsg.: G. Walker), Elsevier Reference Monographs, Cambridge, 2008, S. 420–449.
- V. V. Volkov, K. G. Myakishev, Z. A. Grankika, Russ. J. Inorg. Chem. 1970, 15, 1490–1491.
- Z. Z. Fang, L. P. Ma, X. D. Kang, P. J. Wang, P. Wang, H. M. Cheng, Appl. Phys. Lett. 2009, 94, 44104.
- E. Callini, A. Borgschulte, C. L. Hugelshofer, A. J. Ramirez-Cuesta, A. Züttel, J. Phys. Chem. C 2014, 118, 77–84.
- V. V. Volkov, K. G. Myakishev, Sib. Khim. Zh. 1992, 5, 105–108.
- V. V. Volkov, K. G. Myakishev, Izv. Akad. Nauk SSSR, Ser. Khim. 1987, 6, 1429.
- H. I. Schlesinger, H. C. Brown, J. Am. Chem. Soc. 1953, 75, 219–221.
- J. J. Katz, E. 251. J. J. Katz, E. Rabinowitch, The Chemistry of Uranium. The Element, Its Binary and Related Compounds, 1951. [Google Scholar]
- V. V. Volkov, Z. A. Grankika, K. G. Myakishev, Radiochemistry 1971, 13, 416–419.
- N. Ghiassee, P. G. Clay, G. N. Walton, J. Inorg. Nucl. Chem. 1981, 43, 2909–2913.
- D. B. Ravnsbaek, Y. Filinchuk, R. Cerný, M. B. Ley, D. Haase, H. J. Jakobsen, J. Skibsted, T. R. Jensen, Inorg. Chem. 2010, 49, 3801–3809.
- Y. Yan, H.-W. Li, T. Sato, N. Umeda, K. Miwa, S. Towata, S.-I. Orimo, Int. J. Hydrogen Energy 2009, 34, 5732–5736.
- T. Jaroń, W. Koźmiński, W. Grochala, Phys. Chem. Chem. Phys. 2011, 13, 8847–8851.
- F. C. Gennari, Int. J. Hydrogen Energy 2012, 37, 18895–18903.
- T. Jaroń, W. Grochala, Dalton Trans. 2010, 39, 160–166.
- C. Frommen, N. Aliouane, S. Deledda, J. E. Fonneløp, H. Grove, K. Lieutenant, I. Llamas-Jansa, S. Sartori, M. H. Sørby, B. C. Hauback, J. Alloys Compd. 2010, 496, 710–716.
- T. Jaroń, W. Grochala, J. Nucl. Mater. 2012, 420, 307–313.
- J. E. Olsen, C. Frommen, M. H. Sørby, B. C. Hauback, RSC Adv. 2013, 3, 10764.
- Y. Nakamori, K. Miwa, A. Ninomiya, H. Li, N. Ohba, S. Towata, A. Züttel, S.-I. Orimo, Phys. Rev. B 2006, 74, 1–9.
- F. C. Gennari, L. Fernández Albanesi, I. J. Rios, Inorg. Chim. Acta 2009, 362, 3731–3737.
- N. Davies, D. Saunders, M. G. H. Wallbridge, J. Chem. Soc., A 1970, 15, 2915–2917.
- T. J. Marks, W. J. Kennelly, J. R. Kolb, L. A. Shimp, Inorg. Chem. 1972, 11, 2540–2546.
- J. Sung, D. M. Goedde, G. S. Girolami, J. R. Abelson, J. Appl. Phys. 2002, 91, 3904–3911.
- L. H. Rude, M. Corno, P. Ugliengo, M. Baricco, Y.-S. Lee, Y. W. Cho, F. Besenbacher, J. Overgaard, T. R. Jensen, J. Phys. Chem. C 2012, 116, 20239–20245.
- B. D. James, B. E. Smith, Synth. React. Inorg. Met.-Org. Chem. 1974, 4, 461–465.
- B. G. Sayer, J. I. A. Thompson, N. Hao, T. Birchall, D. R. Eaton, M. J. McGlinchey, Inorg. Chem. 1981, 20, 3748–3750.
- B. E. Smith, H. F. B. E. Smith, H. F. Shurvell, B. D. James, Phys. Rev. B 1978, 710.
- A. M. Igoshkin, I. F. Golovnev, V. V. Krisyuk, I. K. Igumenov, J Struct Chem 2016, 57, 1068–1073.
- Y. Yan, A. Remhof, D. Rentsch, Y.-S. Lee, Y. Whan Cho, A. Züttel, Chem. Commun. 2013, 49, 5234–5236.
- A. A. Guda, I. A. Pankin, A. L. Bugaev, K. A. Lomachenko, S. A. Guda, V. P. Dmitriev, A. V. Soldatov, Bull. Russ. Acad. Sci. Phys. 2015, 79, 139–143.
- I. A. Pankin, A. A. Guda, N. A. Tumanov, Y. Filinchuk, K. A. Lomachenko, A. L. Bugaev, S. A. Guda, V. V. Shapovalov, C. Lamberti, A. V. Soldatov, J. Alloys Compd. 2018, 735, 277–284.
- Z. Łodziana, Phys. Rev. B 2010, 81, 1–12.
- M. Paskevicius, L. H. Jepsen, P. Schouwink, R. Černý, D. B. Ravnsbæk, Y. Filinchuk, M. Dornheim, F. Besenbacher, T. R. Jensen, Chem. Soc. Rev. 2017, 46, 1565–1634.
- R. A. Varin, L. Zbroniec, M. Polanski, Y. Filinchuk, R. Černý, Int. J. Hydrogen Energy 2012, 37, 16056–16069.
- D. Harrison, T. Thonhauser, Int. J. Hydrogen Energy 2016, 41, 3571–3578.
- Borgschulte, E. Callini, B. Probst, A. Jain, S. Kato, O. Friedrichs, A. Remhof, M. Bielmann, A. J. Ramirez-Cuesta, A. Züttel, J. Phys. Chem. C 2011, 115, 17220–17226. [Google Scholar]
- H. -W. Li, S. Orimo, Y. Nakamori, K. Miwa, N. Ohba, S. Towata, A. Züttel, J. Alloys Compd. 2007, 446-447, 315–318.
- J. R. Rumble, T. J. 281. J. R. Rumble, T. J. Bruno (Hrsg.) CRC handbook of chemistry and physics. A ready-reference book of chemical and physical data, 2020. [Google Scholar]
- E. Riedel, C. E. Riedel, C. Janiak, Anorganische Chemie, 9. Aufl., De Gruyter, Berlin, Boston, 2015.
- E. Callini, A. Borgschulte, A. J. Ramirez-Cuesta, A. Züttel, Dalton Trans. 2013, 42, 719–725.
- A. J. Du, S. C. Smith, G. Q. Lu, Phys. Rev. B 2006, 74, 1–4.
- P. F. McMillan, Nat. Mater. 2002, 1, 19–25.
- T. Takeshita, K. A. Gschneidner, J. F. Lakner, J. Less-Common Met. 1981, 78, 43–47.
- I. Vedel, A.-M. Redon, J.-M. Mignot, J.-M. Leger, J. Phys. F 1987, 849.
- J. F. Cannon, H. Hall, J. Less-Common Met. 1975, 40, 313–328.
- M. Sekar, N. Chandra Shekar, P. Sahu, N. Sanjay Kumar, K. Rajan, Phys. B 2002, 324, 240–244.
- R. Griessen, A. Driessen, Phys. Rev. B 1984, 30, 4372–4381.
- R. Griessen, A. Driessen, D. G. de Groot, J. Less-Common Met. 1984, 103, 235–244.
- B. A. Pudjanto (Hrsg.) Proceedings of Asian Physics Symposium, 2005.
- P. Vajeeston, P. Ravindran, A. Kjekshus, H. Fjellvåg, Appl. Phys. Lett. 2006, 89, 71906.
- S. V. Alapati, J. K. Johnson, D. S. Sholl, J. Phys. Chem. B 2006, 110, 8769–8776.
- J. Lu, Z. Z. Fang, Y. J. Choi, H. Y. Sohn, MRS Online Proc. Libr. 2007, 1042.
- S. V. Alapati, J. Karl Johnson, D. S. Sholl, Phys. Chem. Chem. Phys. 2007, 9, 1438–1452.
- A. R. Akbarzadeh, V. Ozoliņš, C. Wolverton, Adv. Mater. 2007, 19, 3233–3239.
- C. Wolverton, D. J. Siegel, A. R. Akbarzadeh, V. Ozoliņš, J. Phys.: Condens. Matter 2008, 20, 64228.
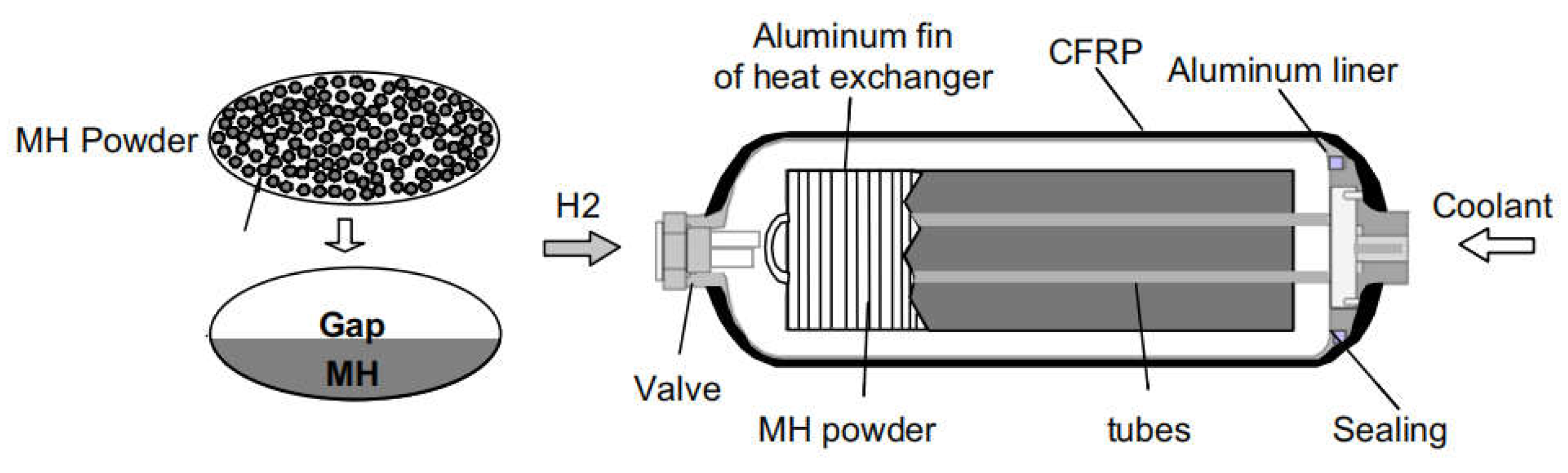

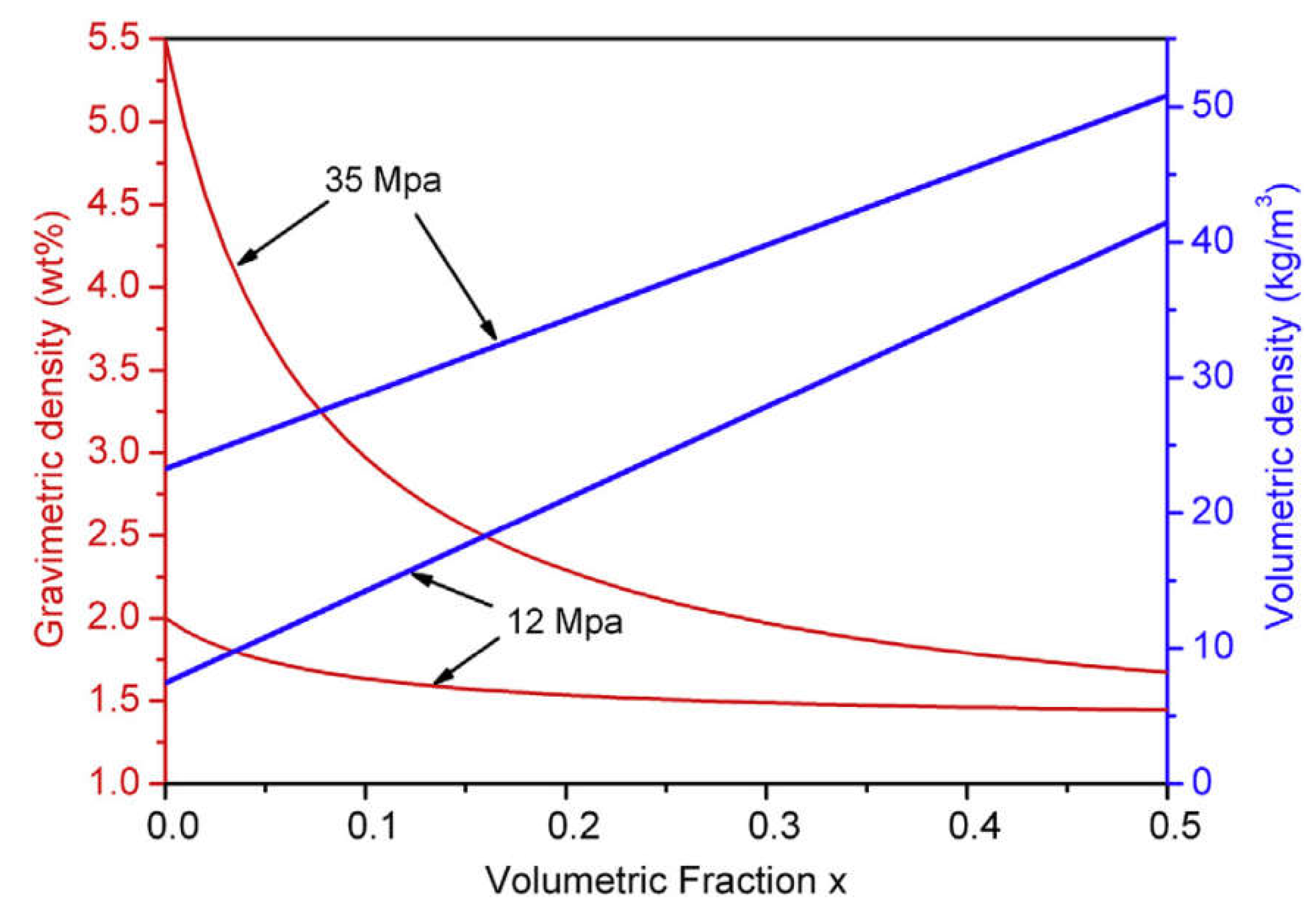
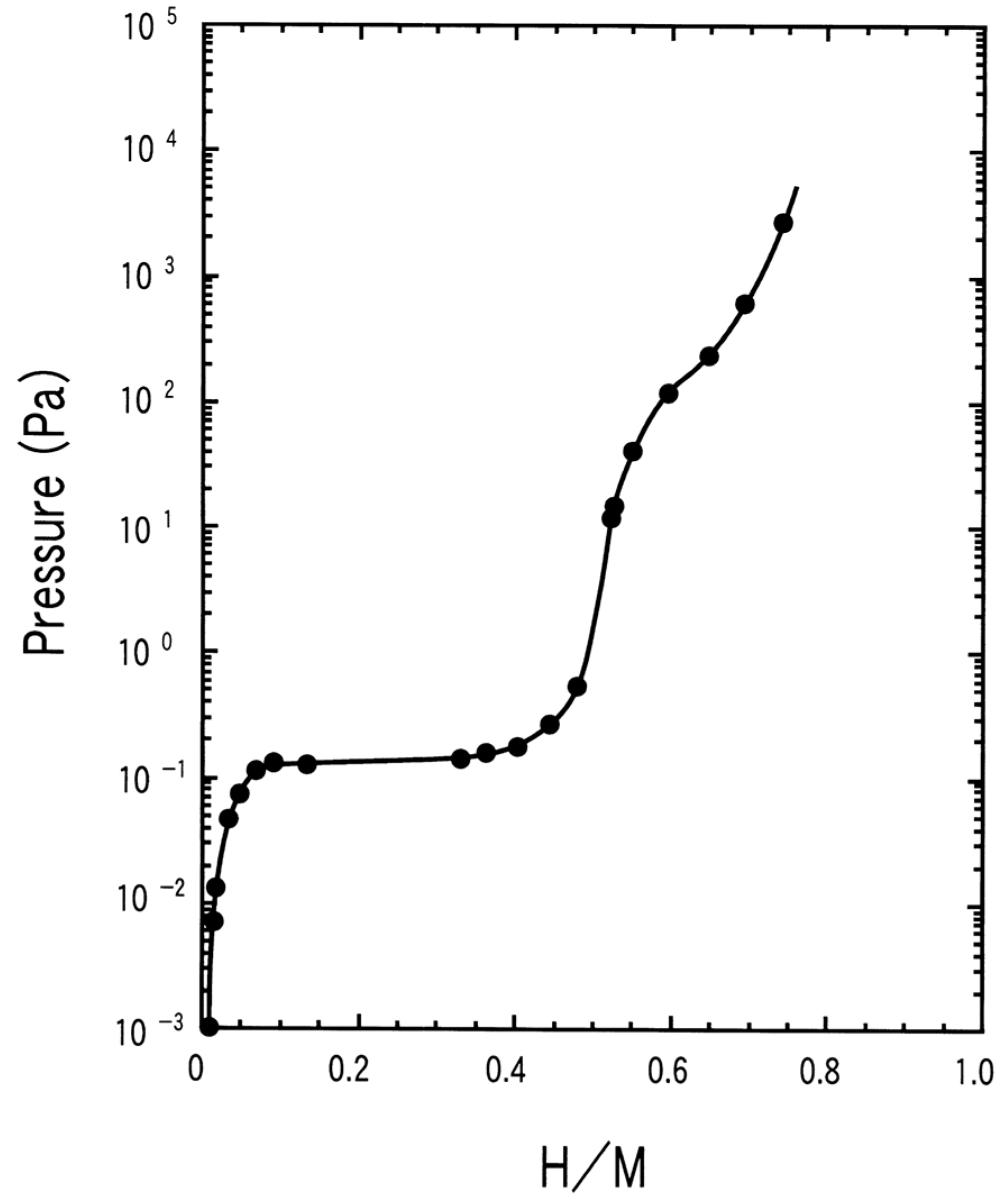
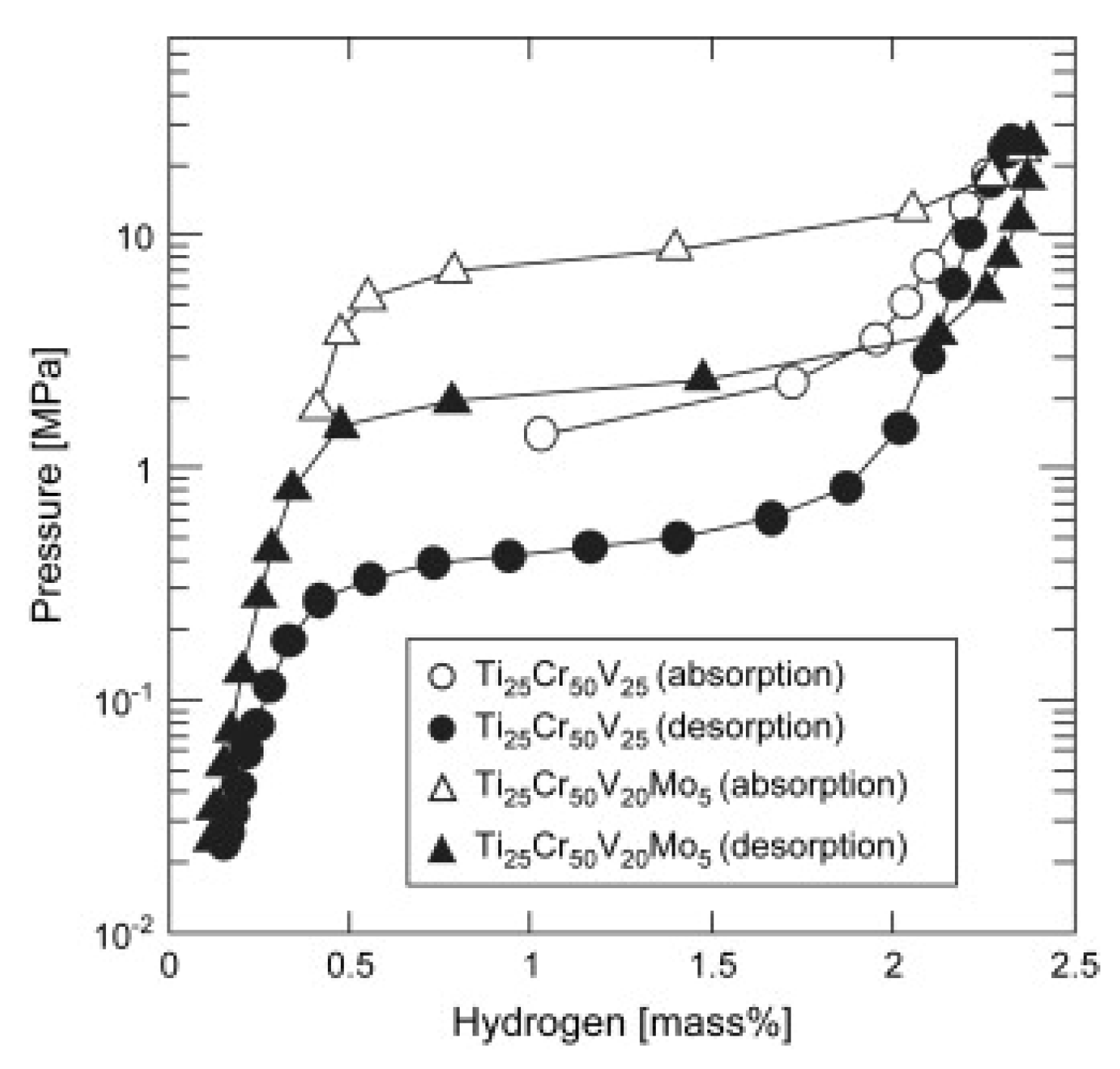
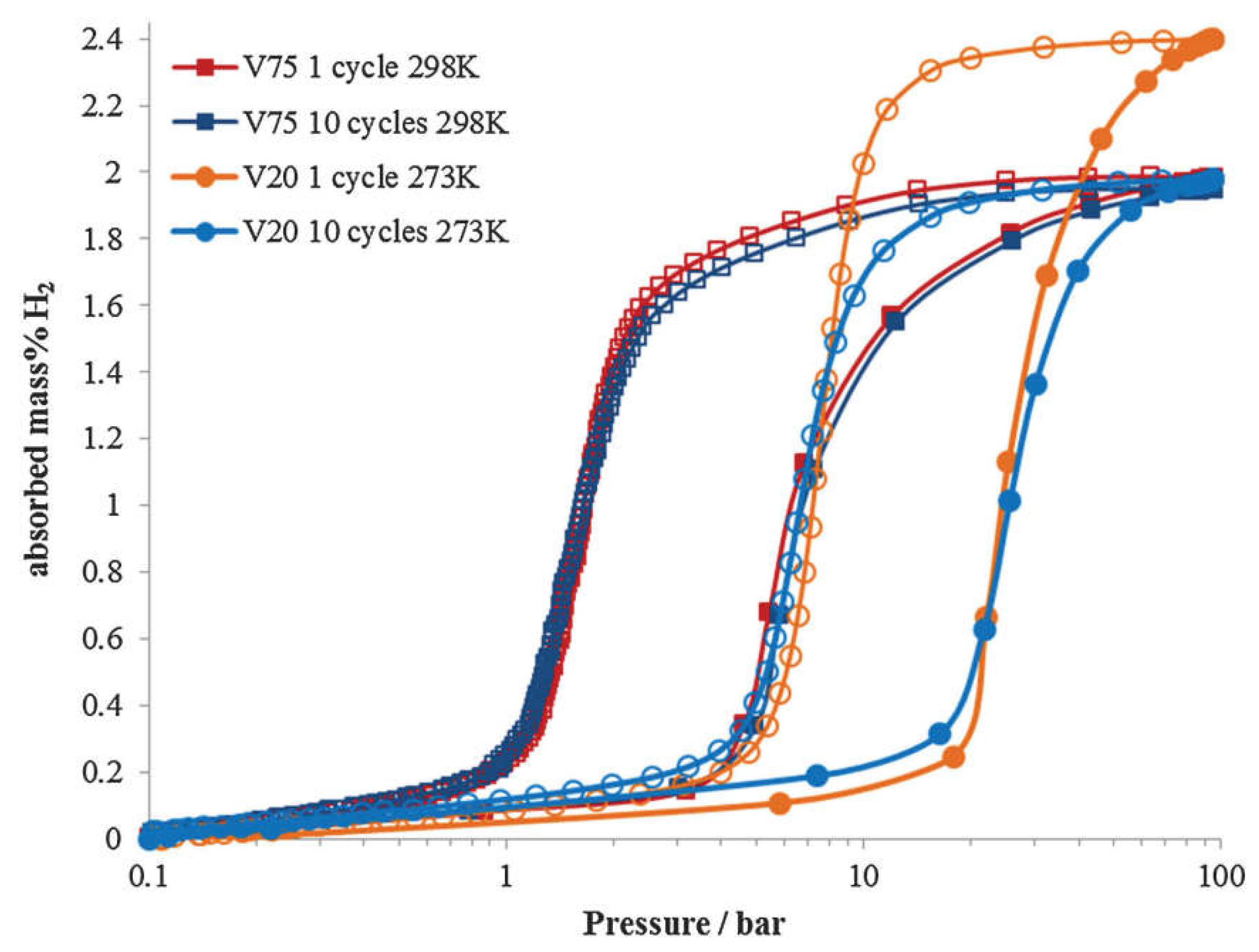
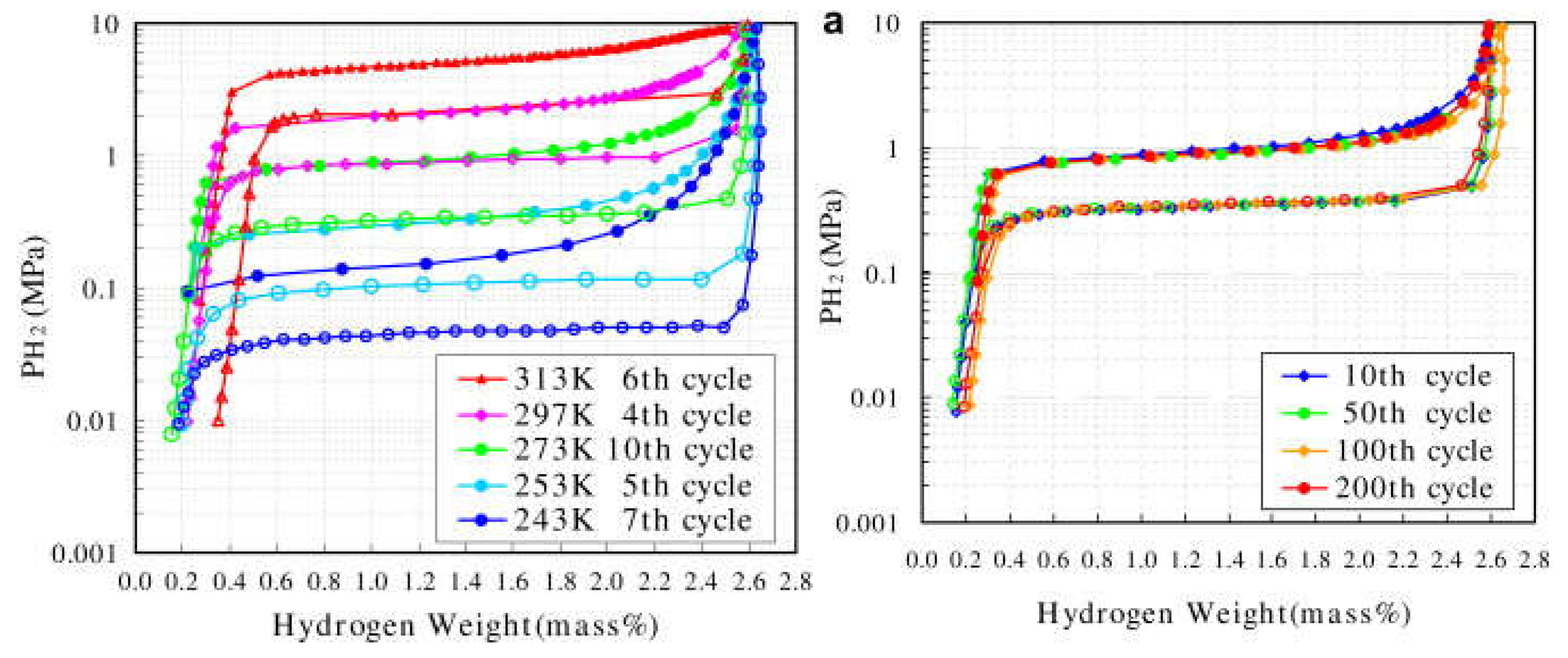
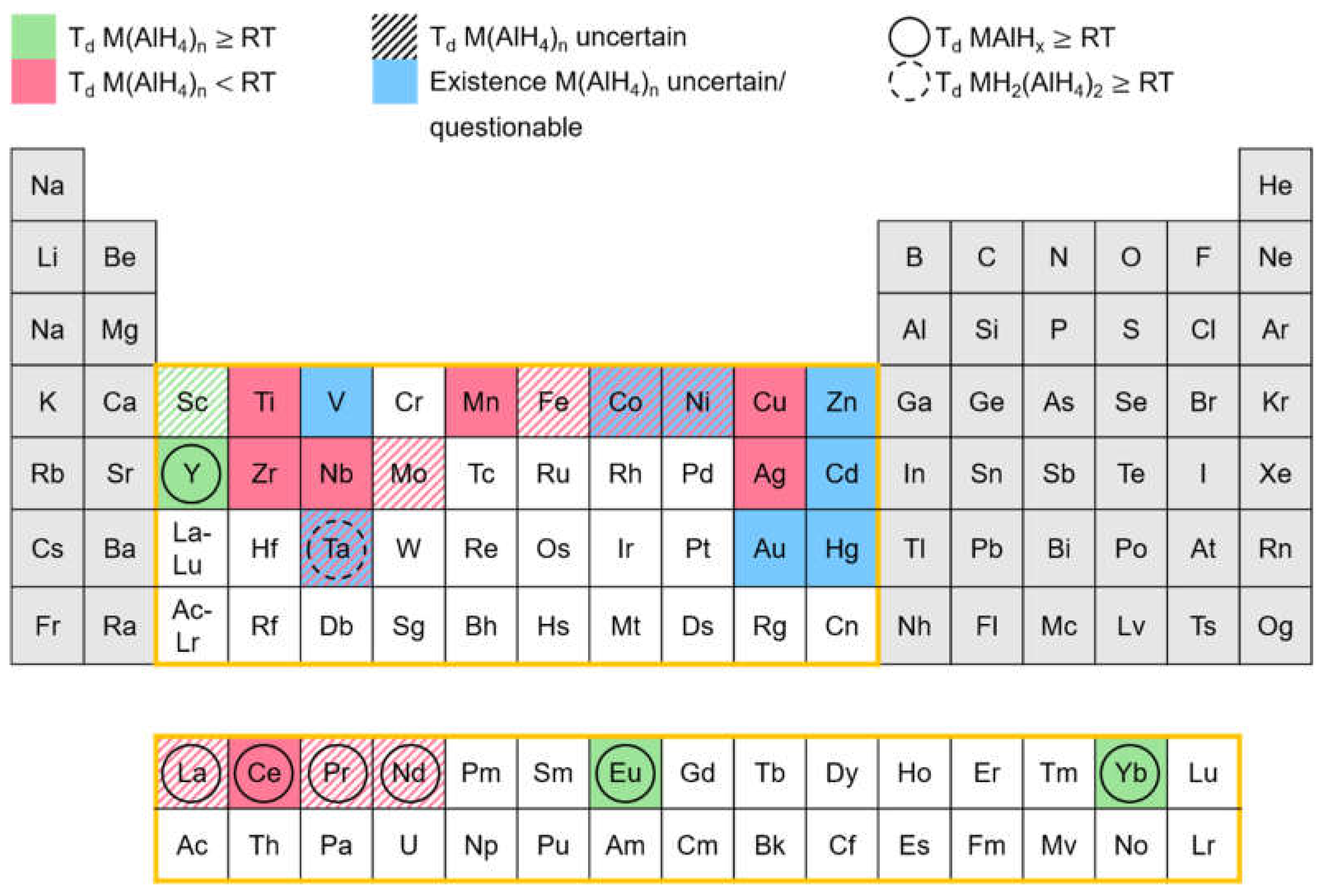
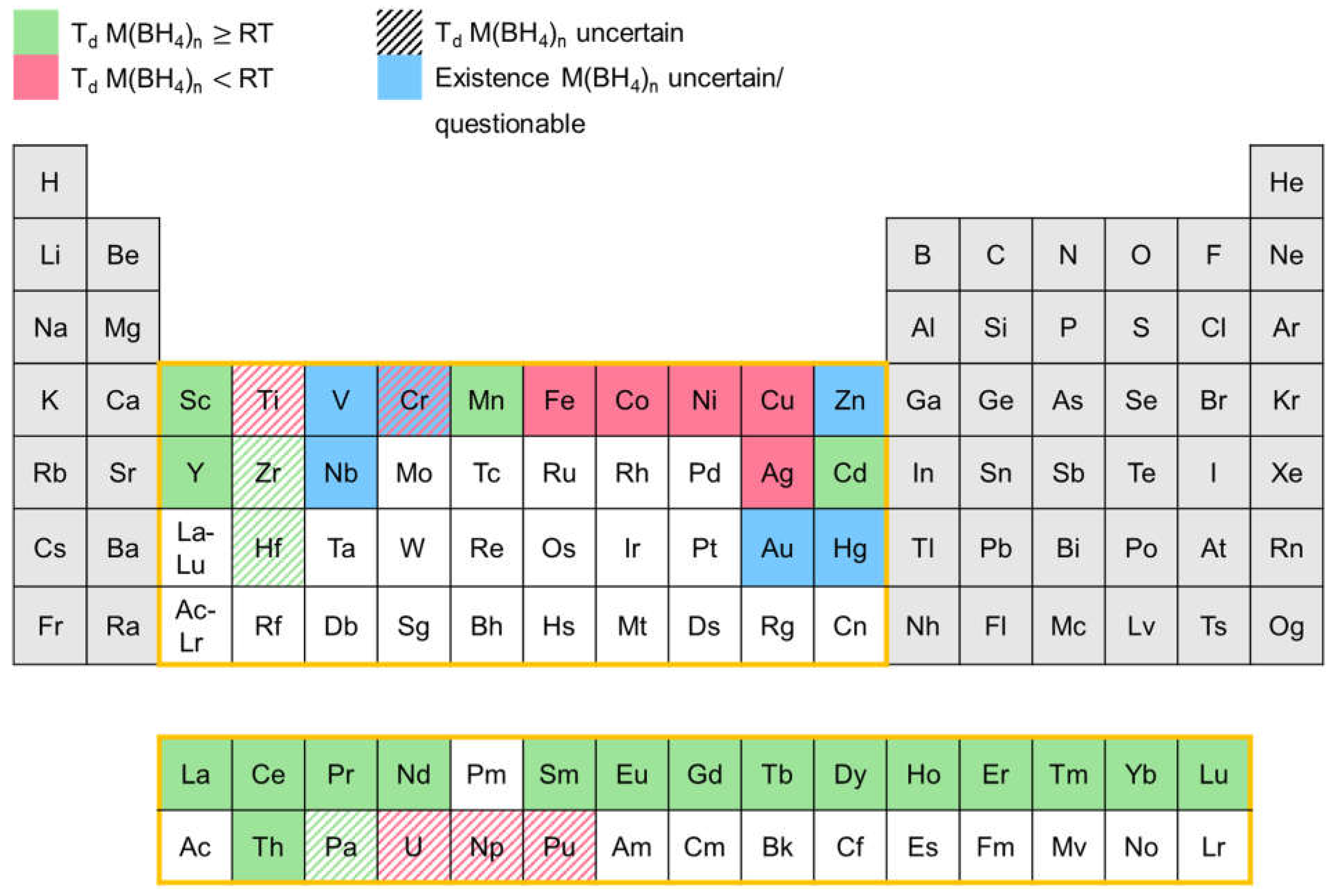
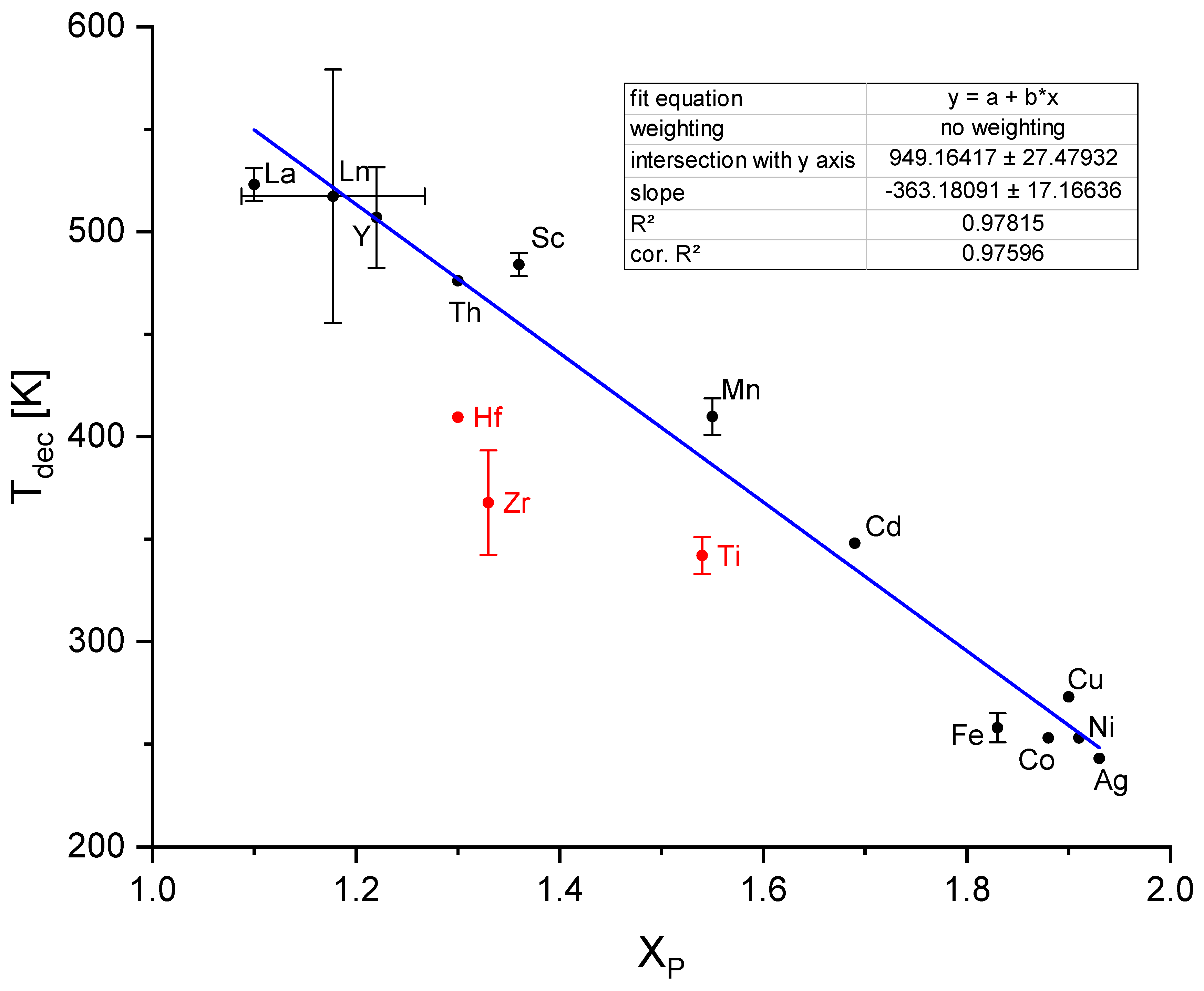
| Priority | Specification | Note |
|---|---|---|
|
Hydrogen storage density |
Weight > 3-4 wt% Volume (V/V0) > 1,800-2,400 |
V = stored hydrogen gas volume (273 K, 0.1 MPa) V0 = volume of MH |
| Enthalpy | |∆RH| < 20 kJ/mol H2 | |
| Equilibrium pressure | > 1.0 MPa at 243 K (desorbing) < 35 MPa at 393 K (absorbing) |
|
|
Cyclic durability |
Decrease of storage capacity < 10% at 1,000 cycles < 5% at 100 cycles |
H2 purity > 99.99% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





