1. Introduction
Since the late 1990´s minimally invasive surgery via right-anterolateral mini-thoracotomy (RAMT) access has been increasing and gaining popularity in cardiac surgery in order to optimize surgical procedures and results. Actually, minimally invasive surgery via RAMT has become established in many centers worldwide in the field of valve surgery [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12]. The standard surgical approach for the treatment of pathologies of the ascending aorta (AA) and the aortic root is the complete median sternotomy [
13,
14]. However, several studies report excellent results using a minimally invasive upper partial sternotomy for treating pathologies of the AA and of the aortic root [
15,
16,
17,
18,
19,
20]. In order to reduce surgical trauma and sternotomy many cardiac surgeons switch to RAMT access to treat AA and root pathologies. However, only few studies with limited selected patient present the outcomes of this approach with favorable results [
21,
22,
23,
24,
25]. At our institution, the RAMT approach has been standardized as well-established minimally invasive approach for valve surgery as well as for aortic surgical procedures including the aortic root and the aortic valve. The aim of the study is to present the steps of our surgical approach as well as the mid- and long-term outcomes after replacement of the AA (AAR) with or without involvement of the aortic root and the aortic valve using the RAMT approach.
2. Materials and Methods
2.1. Study Population
After approval by the ethics committee of the University of Bonn (ethical approval No. 464/22) and written informed consent from all participation patients, clinical data of 44 patients aged over 18 years from three participating institutions underwent elective supracoronary AAR, Wheat’s or Bentall’s procedure via RAMT between April 2017 and February 2024 were retrospectively analyzed. All enrolled patients suffered from aneurysm of the AA or the aortic root with concomitant aortic valve stenosis or regurgitation documented by computed tomography (CT) and echocardiography. Patients with acute or chronic aortic dissection, redo cardiac surgery, endocarditis patients and patients scheduled for David procedure were excluded from the study.
A comprehensive data set of pre-, intra- and postoperative parameters was analyzed by review of institutional database. Baseline characteristics, including cardiovascular risk factors, former cerebrovascular disease, comorbidities, presenting symptoms by classifying in NYHA-classification as well as echocardiographic and CT data were calculated and recorded.
2.2. Study Groups
According to the performed procedures, patients were divided into two groups:
Group A: Supracoronary AAR, n = 14
Group B: Wheat’s procedure (supracoronary AAR concomitant AVR) or Bentall’s procedure (replacement of the aortic root and AVR with a conduit) n = 30
2.3. Preoperative Diagnostic Tools
Every patient was scheduled for surgery after finishing the necessitate preoperative diagnostics. This included echocardiography to assess the pathology of the aortic valve, coronary angiography to exclude coronary artery disease, regular pulmonary function testing and CT scan to assess the anatomy of the hole aorta including the aortic root and the groin vessels as an access for cardiopulmonary bypass (CBP). In case of calcified groin vessels or abdominal aorta, we usually use the axillary artery as an access for the CPB.
2.4. Indication For Surgery
According to the EACT/STS guidelines patients with an aneurysm of the AA or the aortic root with measured diameter of >55mm or if there is a rapid progression of the aneurysm were scheduled for surgery [
26]. In patients with an indication for AVR concomitant AAR is recommended if the diameter reaches 45mm [
26]. If the root is involved and David procedure was not possible, patients received Bentall procedure. Otherwise, a Wheat procedure was performed in case of normal diameter of the aortic root.
2.5. Surgical Procedure
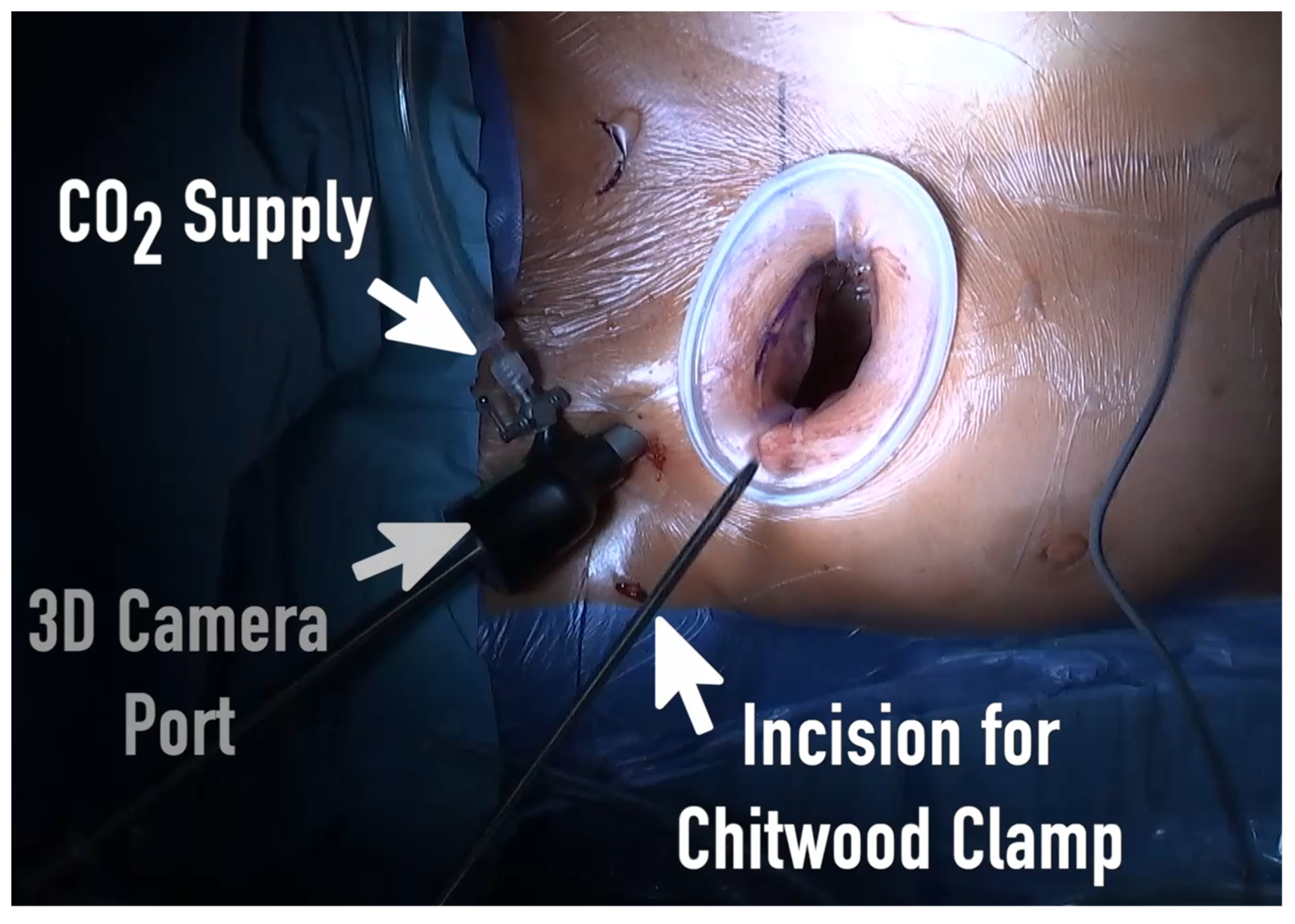
A standard anesthetic protocol was performed using sufentanil, propofol, and rocuronium for induction of general anesthesia followed by single-lumen endotracheal intubation. Anesthesia was maintained using sufentanil and sevoflurane and was continued during CPB. Routinely, standard Monitoring including electrocardiogram, invasive blood pressure, near infrared spectroscopy (NIRS) and transesophageal echocardiography (TEE) were applied. Every patient got external defibrillation electrode pads placed behind the right scapula and on the lateral side of the left anterior axillary line. Anticoagulation with 400-500 U/kg sodium heparin was initiated to achieve an activating clotting time of >450 seconds. After sterile washing and draping of the patient, femoral venous and arterial cannulation was performed percutaneously in Seldinger technique and under TEE control to conduct the CPB (Terumo Cardiovascular Systems, Ann Arbor, MI, USA). In case of calcified groin vessels or abdominal aorta, the arterial canulation was performed via axillary artery. Standard size of arterial canula was 16-18Fr (Bio-Medicus arterial cannulas; Medtronic, Minneapolis, MN, USA) the femoral vein was canulated with 25-27Fr multistage canula (Bio-Medicus arterial cannulas; Medtronic, Minneapolis, MN, USA) with placement in the superior vena cava. In patients with a body weight more than 80kg an additional canula was placed via the right jugular vein in the superior vena cava. After establishment of the CBP, RAMT approach was performed via a small skin incision of 3-5cm in the second or third intercostal space (ICS). Afterward, the pleural space was entered without transection of the mammary vessels or resection of the rips. Extension of the intercostal space was performed by insertion of the soft tissue retractor (Valve GateTM Soft Tissue Protector, Geister, Germany). The use of a rip spreader for further visualization was avoided. The whole surgical procedure was performed as totally endoscopic approach in 3D visualization. Two further small incisions of 5mm were made in the second ICS for the 3D camera port (Aesculap EinsteinVision, Tuttlingen, Germany) and for aortic cross-clamping (
Figure 1).
The pericardium was opened while sparing the phrenic nerve and sutures for pericardial stay were placed to provide sufficient exposure. In case of sufficient aortic valve, a needle vent catheter was placed in the AA. Left ventricular vent catheter was placed in the right superior pulmonary vein in all cases. Transincisional direct aortic cross-clamping was performed, using a Chitwood cross-clamp (Scanlan International, Inc. St. Paul, MN, USA). The carbon dioxide (CO2) diffuser was connected to the camera port. Myocardial protection was achieved using Brettschneider cardioplegia (Custadiol, Dr. Franz Kohler Chemie GmbH, Bensheim, Germany) via the inserted needle vent catheter. In case of aortic valve regurgitation cardioplegic solution was performed directly in both coronary ostia after the aortotomy. A non-pulsatile pump flow of 2.2-2.6 L/min/m2 was conducted to maintain a mean arterial pressure (MAP) of 50-60 mm Hg during CPB. Based on aortic pathology the proximal AA was resected. If the aneurysm was localized only to the AA, a supracoronary AAR was performed. In case of additional aortic valve pathology (aortic valve stenosis or regurgitation) with or without involvement of the aortic root, either a Wheat procedure or the Bentall procedure was performed.
2.5.1. Supracoronary Replacement of the Ascending Aorta
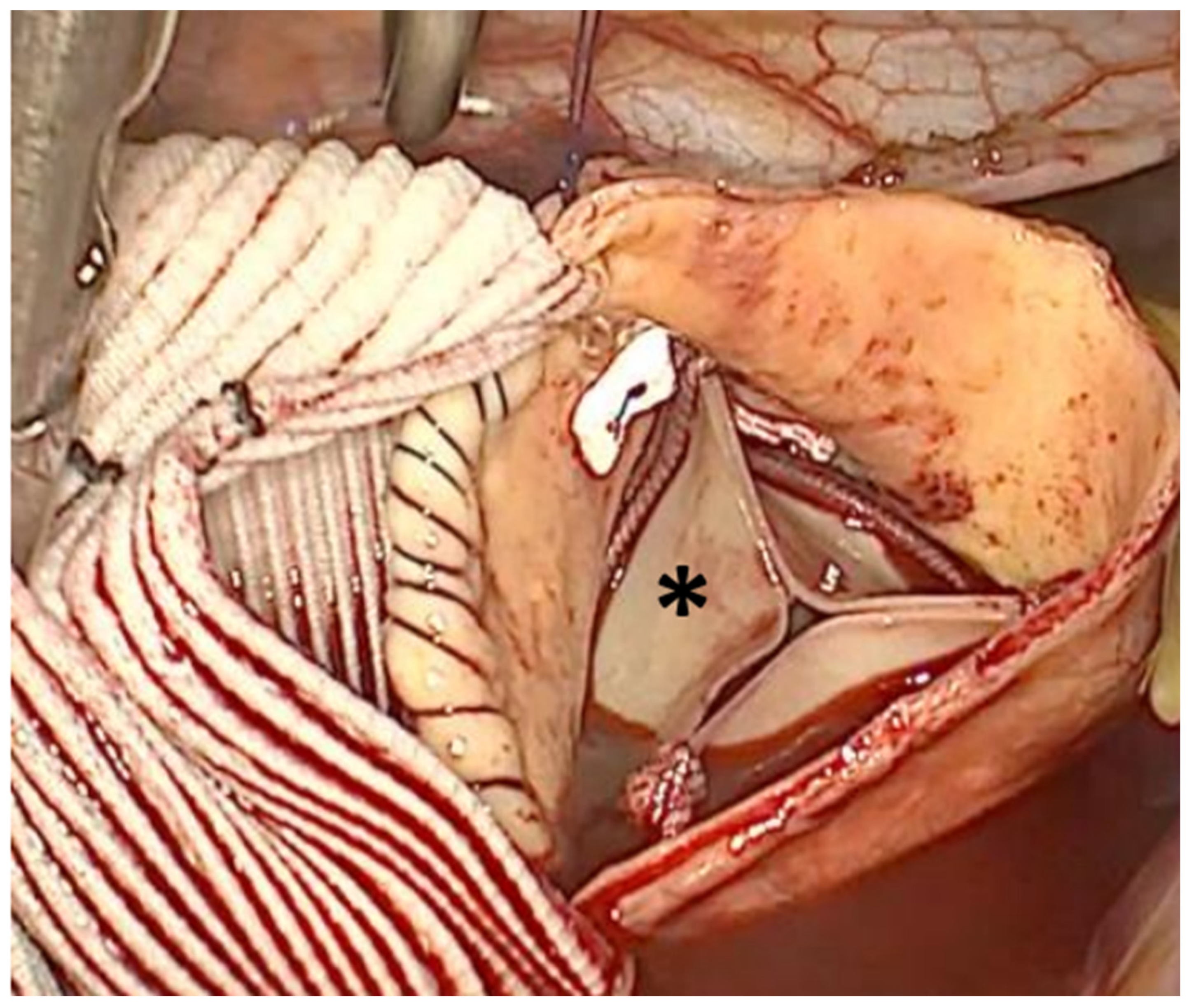
After removing the AA, first the distal than the proximal anastomosis between the distal and proximal part of the native aorta and the suitable vascular graft with usual size between 28mm and 34mm (Vascutek Gelweave grafts, Vascutek Terumo Inc, Scotland, UK) was performed in a running fashion using 4–0 polypropylene suture (
Figure 2).
2.5.2. Wheat-Procedure (Video Included as Supplementary Material)
After removing the AA, a prolene stay sutures were placed to exposure the aortic valve. The leaflets were excised and in case of calcified valves a debridement of the annulus was performed. According to actual ESC/EACTs-guidelines and patients wish, biological aortic valves were implanted in all patients [
27]. The size of the prosthesis was based on intraoperative measurement using the according valve sizer. For AVR, pledgeted mattress annular sutures were placed using the automatically RAM suture device (RAM COR-SUTURE QUICK LOAD, LSI Solutions, USA) starting with the base of the non-coronary cusp, followed by the left coronary cusp and finally the right coronary cusp, respectively. Before the introduction of the RAM suture device (RAM COR-SUTURE QUICK LOAD, LSI Solutions, USA) into our routine practice, annular sutures were traditionally placed manually using interrupted non-everted pledgeted mattress sutures, starting at the base of the left coronary cusp and proceeding in a clockwise fashion. An automatically knot tie device (Cor-Knot, LSI Solutions, USA) was used to tie the knots of the aortic valve prosthesis. After finishing the AVR the supracoronary AA was replaced in the same technique described above (
Figure 3).
2.5.3. Bentall-Procedure
After performing the aortotomy, the complete AA including the aortic root was resected. The coronary buttons were cut out and retracted using stay sutures, and the remaining portion of aortic sinuses was removed. Afterwards, the leafleats of the aortic valve were removed. After sizing the aortic anulus a suitable biological conduit (size of vascular graft= 5mm > of size of aortic valve prosthesis) was constructed, whereas a vascular graft (Vascutek Gelweave grafts, Vascutek Terumo Inc, Scotland, UK) and a bioprosthetic valve was sewn into the graft using a running 3–0 polypropylene. Then, pledgeted mattress annular sutures were either placed manually using interrupted non-everted pledgeted mattress sutures, starting at the base of the left coronary cusp and proceeding in a clockwise fashion or using the automatically RAM suture device (RAM COR-SUTURE QUICK LOAD, LSI Solutions, USA) starting with the base of the non-coronary cusp, followed by the left coronary cusp and finally the right coronary cusp, respectively. The automatically knot tie device (Cor-Knot, LSI Solutions, USA) was used to tie the sutures with the constructed conduit. Further sutures were placed from aortic side and are also tied with the conduit to stabilize the conduit with the aortic root. Thereafter, both coronary buttons (starting with the left main artery) were anastomosed on the vascular graft using 5-0 polypropylene sutures. After determination of the length of the vascular graft the distal anastomosis between the graft and the remaining aorta was completed using 4–0 polypropylene suture.
After completing the surgical procedure and before de-clamping the aorta, temporary epicardial pacing wire was positioned on the right ventricle. Before releasing the aortic cross-clamp, the left ventricle and the vascular graft were de-aired under TEE guidance using an aortic de-airing cannula and the left ventricular vent. TEE was also used to evaluate aortic prosthesis function, global ventricle functions and blood flow of the coronary arteries. CPB was ended and vein canula was removed. During protamine administration and obtaining further hemostasis, the arterial canula was removed and the femoral artery was closed using the MANTATM closure system (Essential Medical Inc., Malvern, PA, USA). A pleural and a pericardial tube were placed, respectively. All incisions were closed in usual fashion.
2.6. Postoperative Care
After the surgical procedure was finished, all patients were transferred to the intensive care unit (ICU), where early extubation and transfer to the intermediate care (IMC) ward were attempted. In some cases, the patients were extubated in the operating room (fast track). After arrival on the ICU, blood loss from chest tube drainages was monitored after 1, 2, 3, 6 and 24 h postoperatively. In addition, transfusion of red blood cells (RBC), fresh frozen plasma (FFP) and platelets concentrates (PC) during surgery and ICU stay were evaluated, as well as any additional surgery or adverse event during the hospital stay. According to institutional policy, bleeding was defined as a mean blood loss exceeding 400ml/h measured between arrival on ICU and the earliest of the following events: the elapse of 3 hours or re-thoracotomy due to major blood loss, as generally accepted.
Intra- and postoperative data including operative time, based on aortic cross-clamping time and duration of the CPB, as well as postoperative outcome including length of ventilation, length of ICU and postoperative hospital stay, re-thoracotomy due to bleeding, requirement of blood transfusion and in-hospital and 30-day mortality were also analyzed.
2.7. Follow-up Data
During follow-up all patients were regularly contacted at 1-, 3- and 6-month and at 1-, 3- and 5-years postoperatively. Follow-up data were obtained through telephone contact and included questions on survival and aortic reintervention.
2.8. Statistical Analysis
Statistical analysis was performed using the IBM SPSS statistics version 25 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 8.4.3 (LaJolla). Continuous variables were expressed as mean ± standard deviation (SD) and categorical variables were given as absolute values and percentages. Data were tested for normal distribution by using Kolmogorov-Smirnov and Shapiro-Wilk tests. Normally distributed demographic and clinical data were analyzed using the students t-test. Not normally distributed data were compared using the Mann–Whitney U-test. Categorial variables were evaluated with the Pearson chi-square-test or Fisher’s exact test, as indicated. A p-value of <0.05 was considered statistically significant.
3. Results
Baseline characteristics, aortic valve pathologies, comorbidities, symptoms at admission and preoperative CT and echocardiographic parameters are summarized in
Table 1. In total 44 patients could be included in the actual study (14 patients with supracoronary AAR and 30 patients with Wheat- or Bentall-procedure). There were 28 (63.6%) male and 16 (36.4%) female patients with a mean age of 61.4 ± 10.7 years.
Of total, 41 (93.2%) patients suffered from hypertension. A medical history of cardiac artery disease (CAD) was present in 18.2% of the study population. Previous stroke without neurological deficit was recorded in 9.1% of the patients. Nearly half of the patients had bicuspid aortic valves and 6.8% had a bovine arch configuration. In patients needed AVR, Aortic regurgitation and aortic stenosis were present in 14 (31.8%) and 16 (36.4%) patients, respectively.
Most of the patients presented with dyspnea according to NYHA class II and III. The pre-operative echocardiographic data revealed normal LVEF in both groups. The mean aortic diameter, measured in the CT scans, was 51.2 ± 4.6 mm.
Regarding the previous medical history, no differences were documented between both groups.
In
Table 2, the intraoperative data and the postoperative outcome were presented. Adequate surgical exposure without the need of conversion to sternotomy was obtained in all patients. Mean procedure time was 140.6 ± 33.2 minutes; mean aortic cross-clamp time was 63.8 ± 25.9 minutes, whereas the mean CBP time was 94.9 ± 32.5 minutes. None of the patients needed circulatory arrest for performing the distal anastomosis. Intraoperative transfusion rate of RBC and FFP was in total very low.
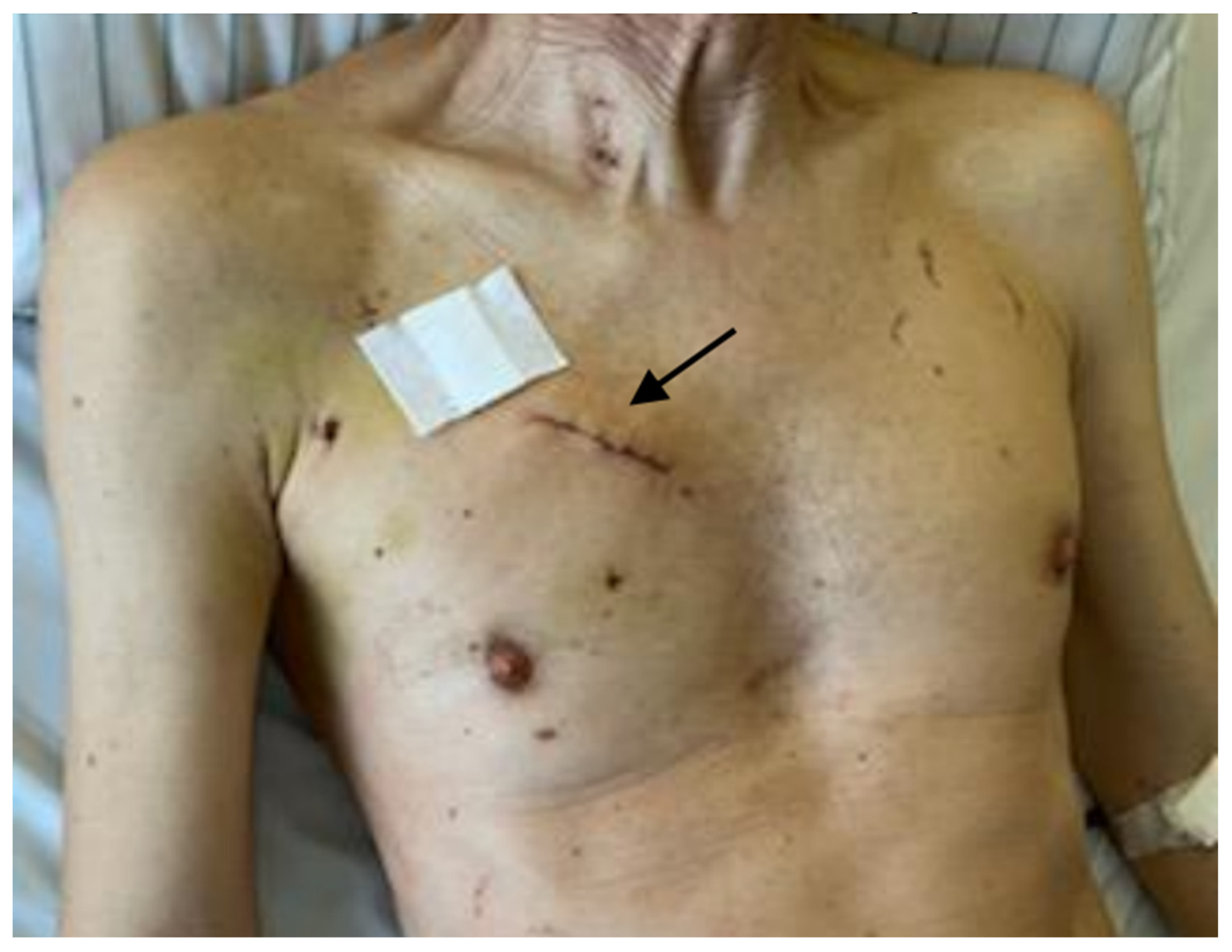
There was no significant postoperative complication noted including bleeding, pericardial effusion needing re-thoracotomy, myocardial infarction, conversion to sternotomy, renal failure, postoperative stroke or in hospital deaths. Postoperative delirium was present in 9.1% of the entire cohort. The rate of postoperative atrial fibrillation was 20.5%. Requirement of postoperative transfusion was also very low. None of the patients needed pacemaker implantation. The rate of wound infection was zero. The cosmetic result was excellent (
Figure 4).
Mean mechanical ventilation time was 5.8 hours. Patients stayed nearly 36 hours at the ICU and were discharged after a mean stay of 7.8 ± 3.0 days. In-hospital, 30-day and one-year mortality was zero, respectively. Throughout midterm follow-up period of 18.2 months (range: 1-62 months), no occurrences of valve deterioration, cardiovascular events necessitating reintervention, or mortality were observed.
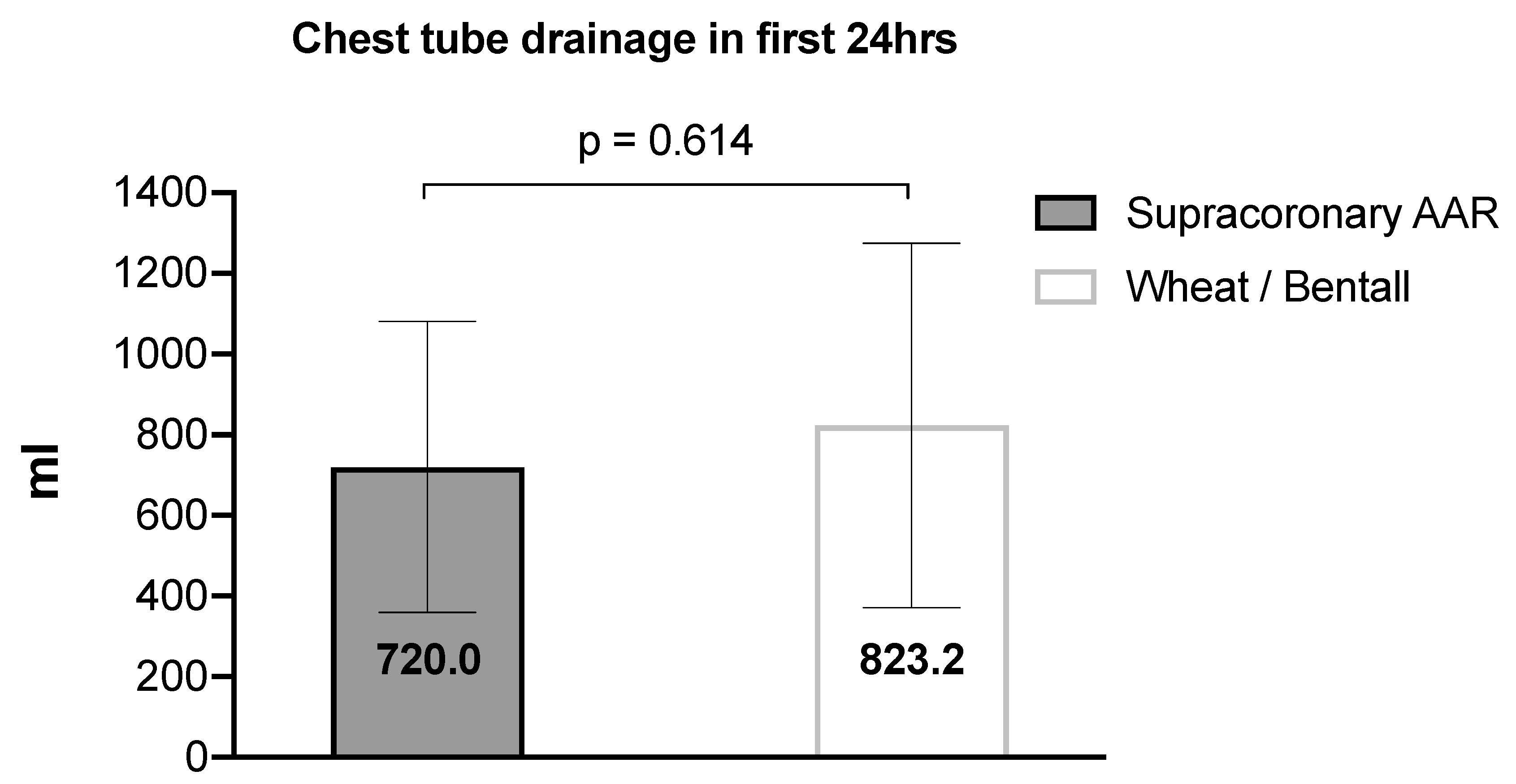
Figure 5 shows the recorded amount of postoperative bleeding from the chest tube drainage with a mean loss of 790.3 ± 423.6 ml during the first 24hours.
Regarding the procedure time, aortic cross-clamp time and CBP time, these were significantly lower in patients of group A (A: 121.0 ± 27.1, 42.9 ± 19.4 and 68.8 ± 19.7 minutes vs. B: 149.8 ± 32.2, 73.5 ± 22.7 and 107.2 ± 30.0 minutes; p<0.05, respectively.
4. Discussion
Minimally invasive cardiac surgery has been widely adopted worldwide and is showing a growing trend. Complex cardiac surgical procedures using the RAMT approach are increasingly performed in many cardiac surgery centers worldwide [
7,
8,
9,
10,
11,
12]. The aim of minimally invasive surgery is to avoid a complete sternotomy and their associated complications by reducing the morbidity and mortality [
7,
28,
29]. Recently, minimally invasive surgeons are increasingly using the RAMT approach to perform aortic valve surgery [
7,
8,
9,
10,
11]. RAMT has shown better results in terms of blood loss and length of hospital stay than the sternotomy approach [
11]. As surgeons, employing minimally invasive approaches, increase their experience, their interest in treating further complex pathology, particularly in the AA or the aortic root via smaller surgical approaches is also increasing. However, there is limited literature available specifically on treating aortic pathologies via the RAMT approach. Due to the technical challenges involved in aortic surgery, surgeons tend to approach these procedures with caution and prefer incisions that are closer to the aorta. In the past, various minimal-invasive approaches, including upper partial sternotomy (T- or J-incision) as well as S-, L-, Z- and C-shaped mini-sternotomy are described in the literature, which have been used by aortic surgeons performing surgeries of the AA or the aortic root with good feasibility [
16,
17,
18,
19,
20]. These techniques have already been shown to be as safe and effective as the conventional median sternotomy. All these minimally invasive techniques showed favorable postoperative outcome with reduced postoperative bleeding, reduced re-thoracotomy rate, faster extubation, reduced stay on ICU, lower risk of mediastinitis, faster discharge from hospital and recovery and better esthetic results [
3,
7,
28,
29].
To the best of our knowledge, the actual study is the largest experience in the field of aortic surgery using the full endoscopic RAMT approach with 3D visualization published to date. Conventional instruments and innovative suturing techniques were used without total circulatory arrest for distal anastomosis. Neither rib resection was done, nor the right internal mammary artery was injured during the procedures.
LaPeitra et al firstly described the surgical AVR with concomitant AAR via a right anterior thoracotomy approach with dislocation of the third or fourth costochondral cartilage. In their study, they included 20 patients with a mean age of 61 years and 80% of male gender with excellent postoperative outcome and short-term results. Distal anastomosis was performed under circulatory arrest in most of these patients (95%) and it is not known, if video guidance was used [
21].
In another retrospective study, Johnson et al. presented their surgical technique in seven selected patients received elective Bentall procedure using the RAMT approach. It was the first study, describing the Bentall procedure through the RAMT approach. Also, they received satisfied short-term results with favorable outcome. Here, circulatory arrest was also used for suturing the distal anastomosis. Video guidance was used in all patients and for performing the anastomosis an automated suturing technology was applied in some cases [
22].
Recently, Ji et al. presented their experience using the RAMT approach for performing the Bentall procedure in 15 selected patients. So far, it was the largest study presenting results about the Bentall procedure using the RAMT approach. In this small series conventional instruments and suturing techniques were used without total circulatory arrest for distal anastomosis. The authors also did not disarticulate the ribs or injured the right internal mammary artery. They could show a shortened length of intensive care unit as well as hospital stay coincided with neither in-hospital death nor neurological complication nor postoperative bleeding or re-thoracotomy. In this study, the follow-up period was at least 6 months. During this time all 15 patients were alive without reoperation [
25].
So far, the longer procedure, as well as the CBP- and aortic clamp time have been discussed as a major disadvantage of the RAMT approach in the field of aortic surgery in comparison to sternotomy approaches. In our study, we have a relatively short CBP and aortic clamping time. Compared to the previously described studies, our times were significantly lower. The mean CPB time in this study was 94.9 minutes, which is much shorter than the time of 138.5 minutes in the case series by Ji et al. and 202.9 minutes in the series by Johnson et al [
22,
30]. Similarly, the mean aortic cross-clamp time in our study was 63.8 minutes, which is also meaningfully shorter than the mean cross-clamp time of 95.0 minutes and 161.9 minutes reported in their study.
Compared to LaPietra et al. and Johnson et al. none of our patients needed circulatory arrest for distal anastomosis. Our significant shorter times could be justified to the high standardization of the operative steps, the high experience of the surgeon, the use of 3D camera for better visualization and the use of new innovations like the RAM and Cor-Knot devices. All cases presented here were performed by a single surgeon with excellent surgical skills using minimally invasive surgery. In the study by Ji et al. it is not described if they used 3D visualization.
Our postoperative outcome showed comparable results to the results of, LaPeitra et al., Johnson et al. and Ji et al [
21,
22,
25]. We could show a lower ventilation time (5.8hrs) compared to LaPietra et al., Johnson et al. and Ji et al. (11hrs, 10.6hrs and 12.5hrs, respectively). The ICU stay of our patients was 35.9hrs, which was comparable to the ICU times of Johnson et al. (31.8hrs) and Ji et al. (36hrs) [
21,
22,
25]. Our patients were discharged from the hospital after 7.8 day and was slightly higher than the discharge time of Ji et al. (5.8 days) [
25]. The re-thoracotomy rate in our study due to bleeding was zero and similar to LaPietra et al. results [
21]. Cerebrovascular complication and 30-day mortality were also zero, which is in line with the aforementioned studies [
21,
22,
25]. Summarized the excellent results of the previous studies could be confirmed through our results.
During a median follow-up time of 18.2 months, which is much longer than the follow-up time of Ji et al. with 8.0 months, all our patients were alive and did not need re-interventions [
25]. No follow-up data were collected in the studies of LaPietra et al. and Johnson et al. [
21,
22].
We could show that the endoscopic RAMT approach can be safely transferred to the field of aortic surgery in selected patients. The high standardization of the operative steps by an expert team and the use of new surgical tools could make RAMT as fast and effective as sternotomy procedures, but with the advantages of minimally invasive surgery as less surgical trauma, faster extubation, shorter ICU and in hospital stay, lower pain, faster recovery and better cosmetic results. Reducing the length of hospital stay is an important aspect of resource use, since ICU and hospital stays are the main determinants of cost after cardiac surgery [
31].
In our center, the RAMT approach has become a common practice over the years with an annual volume of over 50% of all surgical cases, and aortic surgical procedures through the RAMT approach is a common option in selected patients. Patients benefit enormously from the RAMT approach. Together with the use of a totally endoscopic approach smaller incisions with preserving the integrity and stability of the chest wall are achieved, while an excellent visualization of the surgical field and access to the aorta, aortic valve and aortic root could be ensured. Therefore, the RAMT approach can be used for both isolated and complex AAR involving the aortic root and the aortic valve with a high degree of safety. They have a very short ventilation time and ICU stay and can be discharged very quickly, which leads to faster recovery. Moreover, the minimally invasive procedure offers a better cosmetic result of a smaller scar with a very low risk of wound infection, and mediastinitis. The perioperative safety and feasibility as well as the mid- and long-term results should be the goal of a new surgical technique, which should improve the existence evidence-based results of a standard procedure.
5. Conclusions
The full endoscopic RAMT approach with 3D visualization is a safe and feasible technique which can be transferred in the field of aortic surgery without compromising surgical quality, postoperative outcomes, or patient safety when performed the procedure by an experienced team in minimal invasive surgery in a large-volume center.
6. Limitation
The main limitation of our study is its retrospective and non-randomized design, which may introduce selection bias. Further limitation point is that all procedures have been performed by a single surgeon with excellent expertise in minimally invasive surgery. The lack of comparison with other approaches, like the upper sternotomy, should also be considered as a limitation point. The patient cohort is small but present the largest case number worldwide. Further lager studies, especially in multicenter and randomized design should be initiated to confirm the results of this study.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Video S1: Minimally invasive Aortic valve and Ascending Aorta Replacement.
Author Contributions
Conceptualization, M.H. A.E.-S.A. and F.B. Methodology, M.H. Validation, M.H. Formal analysis, M.H. Investigation, S.S.(Sami Sirat). and M.A. Resources, A.S., S.S. (Saad Salamate) Data curation, M.A.N. Writing—original draft preparation, M.H. Writing—review and editing, M.H., S.S., M.S. and A.E.-S.A. Visualization, M.H. Supervision, F.B., A.E.-S.A. and M.D., Project administration, M.H. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Ethics Committee of University of Bonn, Germany (protocol code No. 464/22) on 23 January 2022.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Written informed consent has been obtained from the patients to publish this paper. Dat Availability Statement: The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Seeburger, J.; et al., Minimal invasive mitral valve repair for mitral regurgitation: Results of 1339 consecutive patients. Eur J Cardiothorac Surg, 2008. 34(4): p. 760-5. [CrossRef]
- Svensson, L.G.; et al., Minimally invasive versus conventional mitral valve surgery: A propensity-matched comparison. J Thorac Cardiovasc Surg, 2010. 139(4): p. 926-32 e1-2. [CrossRef]
- Akowuah, E.F.; et al., Minithoracotomy vs Conventional Sternotomy for Mitral Valve Repair: A Randomized Clinical Trial. JAMA, 2023. 329(22): p. 1957-1966. [CrossRef]
- Moscarelli, M.; et al., Minimal Access Versus Sternotomy for Complex Mitral Valve Repair: A Meta-Analysis. Ann Thorac Surg, 2020. 109(3): p. 737-744. [CrossRef]
- Sakaguchi, T.; et al., Minimally Invasive Mitral Valve Repair Through Right Minithoracotomy - 11-Year Single Institute Experience. Circ J, 2018. 82(6): p. 1705-1711. [CrossRef]
- Berretta, P.; et al., Minimally invasive approach: Is this the future of aortic surgery? Indian J Thorac Cardiovasc Surg, 2022. 38(Suppl 1): p. 171-182. [CrossRef]
- Bakhtiary, F.; et al., Comparison of Right Anterior Mini-Thoracotomy Versus Partial Upper Sternotomy in Aortic Valve Replacement. Adv Ther, 2022. 39(9): p. 4266-4284. [CrossRef]
- El-Sayed Ahmad, A.; et al., The First 100 Cases of Two Innovations Combined: Video-Assisted Minimally Invasive Aortic Valve Replacement Through Right Anterior Mini-Thoracotomy Using a Novel Aortic Prosthesis. Adv Ther, 2021. 38(5): p. 2435-2446. [CrossRef]
- Seitz, M.; et al., Minimally Invasive Aortic Valve Replacement Via Right Anterior Mini-Thoracotomy: Propensity Matched Initial Experience. Heart Lung Circ, 2019. 28(2): p. 320-326. [CrossRef]
- Malaisrie, S.C.; et al., Current era minimally invasive aortic valve replacement: Techniques and practice. J Thorac Cardiovasc Surg, 2014. 147(1): p. 6-14. [CrossRef]
- Bowdish, M.E.; et al., A comparison of aortic valve replacement via an anterior right minithoracotomy with standard sternotomy: A propensity score analysis of 492 patients. Eur J Cardiothorac Surg, 2016. 49(2): p. 456-63. [CrossRef]
- Fatehi Hassanabad, A.; et al., Right anterior mini thoracotomy approach for isolated aortic valve replacement: Early outcomes at a Canadian center. J Card Surg, 2021. 36(7): p. 2365-2372. [CrossRef]
- Sioris, T.; et al., Clinical outcomes after separate and composite replacement of the aortic valve and ascending aorta. J Thorac Cardiovasc Surg, 2004. 128(2): p. 260-5. [CrossRef]
- Mok, S.C.; et al., Twenty-five year outcomes following composite graft aortic root replacement. J Card Surg, 2017. 32(2): p. 99-109. [CrossRef]
- Haunschild, J.; et al., Supracommissural replacement of the ascending aorta and the aortic valve via partial versus full sternotomy-a propensity-matched comparison in a high-volume centre. Eur J Cardiothorac Surg, 2022. 61(2): p. 479-487. [CrossRef]
- Mikus, E.; et al., Mini-Bentall: An Interesting Approach for Selected Patients. Innovations (Phila), 2017. 12(1): p. 41-45. [CrossRef]
- Shah, V.N.; et al., Upper Hemisternotomy Versus Full Sternotomy for Replacement of the Supracoronary Ascending Aorta and Aortic Valve. Innovations (Phila), 2024. 19(1): p. 39-45. [CrossRef]
- Helms, F.; et al., Expanding the Minimally Invasive Approach towards the Ascending Aorta-A Practical Overview of the Currently Available Techniques. Medicina (Kaunas), 2023. 59(9). [CrossRef]
- Shrestha, M.L.; et al., David procedure through an upper partial sternotomy. Ann Cardiothorac Surg, 2015. 4(2): p. 212-3. [CrossRef]
- Perrotta, S. and S. Lentini, Ministernotomy approach for surgery of the aortic root and ascending aorta. Interact Cardiovasc Thorac Surg, 2009. 9(5): p. 849-58. [CrossRef]
- LaPietra, A.; et al., Outcomes of aortic valve and concomitant ascending aorta replacement performed via a minimally invasive right thoracotomy approach. Innovations (Phila), 2014. 9(5): p. 339-42; discussion 342. [CrossRef]
- Johnson, C.A., Jr.; et al., Right Mini-thoracotomy Bentall Procedure. Innovations (Phila), 2018. 13(5): p. 328-331. [CrossRef]
- Johnson, C.A., Jr.; et al., Right mini-thoracotomy Bentall with traditional and automated suturing devices. Multimed Man Cardiothorac Surg, 2018. 2018. [CrossRef]
- Lamelas, J. and A. LaPietra, Right Minithoracotomy Approach for Replacement of the Ascending Aorta, Hemiarch, and Aortic Valve. Innovations (Phila), 2016. 11(4): p. 301-4. [CrossRef]
- Ji, Q.; et al., Mini-Invasive Bentall Procedure Performed via a Right Anterior Thoracotomy Approach With a Costochondral Cartilage Sparing. Front Cardiovasc Med, 2022. 9:p. 841472. [CrossRef]
- Authors/Task Force, M.; et al., EACTS/STS Guidelines for Diagnosing and Treating Acute and Chronic Syndromes of the Aortic Organ. Ann Thorac Surg, 2024. [CrossRef]
- Vahanian, A.; et al., 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J, 2022. 43(7): p. 561-632. [CrossRef]
- Chang, C.; et al., Minimally Invasive Approaches to Surgical Aortic Valve Replacement: A Meta-Analysis. Ann Thorac Surg, 2018. 106(6): p. 1881-1889. [CrossRef]
- Phan, K.; et al., A meta-analysis of minimally invasive versus conventional sternotomy for aortic valve replacement. Ann Thorac Surg, 2014. 98(4): p. 1499-511. [CrossRef]
- Omura, A.; et al., Early and late results of graft replacement for dissecting aneurysm of thoracoabdominal aorta in patients with Marfan syndrome. Ann Thorac Surg, 2012. 94(3): p. 759-65. [CrossRef]
- Hamilton, A.; et al., Cost reduction in cardiac surgery. Can J Cardiol, 1994. 10(7): p. 721-7.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).