1. Introduction
Investigation into how different factors affect dispersal probability and distance helps better understand the dispersal process [
1], knowledge of which is important for population management and invasion evaluation [
2,
3,
4,
5,
6]. Population density and individual age are two important factors that can influence dispersal [
1,
3]. However, previous studies have mainly focused on how these factors modify dispersal probability [
3,
7,
8,
9,
10,
11,
12]. To date, the effect of density and age on dispersal distance and subsequent reproduction is still poorly understood.
Many species exhibit age-specific dispersal due to the variance in reproductive costs or pressures with age [
4]. Females at the age of high fecundity may have a higher dispersal probability and a longer dispersal distance to spread their eggs [
13,
14,
15,
16]. The availability of resources is the major factor affecting immature fitness, and thus mothers are expected to disperse and select habitats with sufficient resource supply or less resource competition, aiming to increase offspring fitness [
1,
11,
17,
18,
19,
20,
21]. The intention of spreading offspring over habitats of high quality may be particularly strong for species whose immatures have limited dispersal ability and no parental care. Although many studies have investigated age-specific dispersal and reproduction, few examine the subsequent offspring competition intensity and fitness. Understanding age-specific dispersal strategies in relation to reproduction and offspring fitness is essential, because the reproductive output of dispersers at the age of dispersal can affect establishment success and population growth at new resident sites [
22,
23,
24,
25,
26].
High population density often leads to intense resource competition, reducing individual fitness [
3,
27,
28]. Increasing density would raise dispersal probability [
2,
3,
9,
29] over longer distance [
30,
31]. However, individuals living in high density may also have benefits such as reducing predation risk and increasing foraging efficiency [
32], and thus individuals may tend to live in habitats with high density. It is predicted that density-dependent dispersal in animals depends on the balance of costs and benefits of group living, which warrants species-specific investigations.
The European native spider mite
Tetranychus ludeni Zacher (Acari: Tetranychidae) has now invaded all continents except Antarctica [
33,
34]. It is a haplodiploid species where virgin females produce haploid sons and mated females produce haploid sons and diploid daughters [
35,
36].
T. ludeni attacks over 300 hosts, including many economically important crops such as beans, eggplant, hibiscus, apple, pumpkin, and other cucurbitaceous plants [
33,
37,
38,
39]. Spider mites have relatively short life cycle and high fecundity. They build up populations rapidly and exhaust resources very fast, and dispersal is common especially in overcrowding and food-depleted environments [
2,
30,
40,
41]. Previous research has demonstrated that high population density negatively affected individual reproduction and population growth of
T. ludeni [
42,
43,
44]. However, whether high density would promote the dispersal probability and distance of
T. ludeni is unknown. Adult females of spider mites usually disperse after mating [
45] through ballooning and walking (or aerial and ambulatory dispersal) [
9,
12]. Young females disperse more frequently than older females by ballooning [
9]. However, the age-specific ambulatory dispersal is still not clear [
12]. Moreover, mated females of
T. ludeni gradually increase reproductive output until they are six days old [
42]. So far, it is unknown whether age-specific ambulatory dispersal is related to reproduction.
In this study, we used T. ludeni to investigate the effect of age on dispersal probability and distance and subsequent reproduction and that of density on dispersal probability and distance. For the age experiment, we allowed females of different ages to disperse for 24 hours and then recorded their dispersal probability, dispersal distance, number of eggs laid, and the subsequent offspring survival. For the density experiment, we allowed females of different density to disperse for 24 hours and then recorded dispersal probability and distance. We hypothesized that (1) females at the age of high fecundity would have higher dispersal probability and longer dispersal distance and produce eggs over longer distances, and (2) high density would increase dispersal probability and distance.
2. Materials and Methods
2.1. Mite Colony and Experimental Conditions
We collected T. ludeni adults on Passiflora mollissima Kunth (Malpighiales: Passifloraceae) in Palmerston North, New Zealand and used 3- to 5-week-old common bean Phaseolus vulgaris L. (Fabales: Fabaceae) plants grown in pots to maintain the colony. We used leaf squares cut from the first expanded leaves of 1- to 2-week-old bean plants grown in pots for all experiments. Environmental conditions for the colony and experiments were set at 25 ± 1 ºC, 40 ± 10% RH, and 14:10 h (L:D) photoperiod.
2.2. Preparation of Mated Females for Experiments
To obtain virgin males to inseminate females, we randomly selected 50 female deutonymphs from the colony and transferred them onto a clean fresh leaf square (4 × 4 cm) on wet cotton wool in a Petri dish (9 cm in diameter × 1 cm in height). We allowed these deutonymphs to develop into virgin adult females and lay unfertilised eggs for three days. We then removed adult females and allowed the eggs to develop to virgin male adults. We prepared a total of 46 Petri dishes for this purpose. We used 1-day-old virgin adult males to mate with females prepared below.
We randomly selected 50 adult females from the colony and transferred them onto a clean fresh leaf square (4 × 4 cm) on wet cotton wool in a Petri dish for egg laying for 24 hours. We then removed the female adults and allowed eggs to develop to the quiescent deutonymphal stage. We set up approximately 230 Petri dishes for this purpose. We transferred 15 ~ 20 virgin males prepared above to female quiescent deutonymphs in each Petri dish, allowing them to stay with the newly emerged females for 5 hours to ensure that all females mated at or soon after emergence (females mate immediately after emergence [
35]). We transferred 50 newly emerged (< 24 hours old) and mated females from an above Petri dish onto a new leaf square (4 × 4 cm) on wet cotton wool in a Petri dish. We prepared 230 such dishes and replaced all leaf squares once a day to obtain mated females of 1, 3, 6, 9, and 12 days old for experiments. To keep female number on each leaf square consistent prior to experiments, we checked the leaf square every day and replaced dead females with live ones of the same age.
2.3. Dispersal and Reproduction of Females of Different Ages
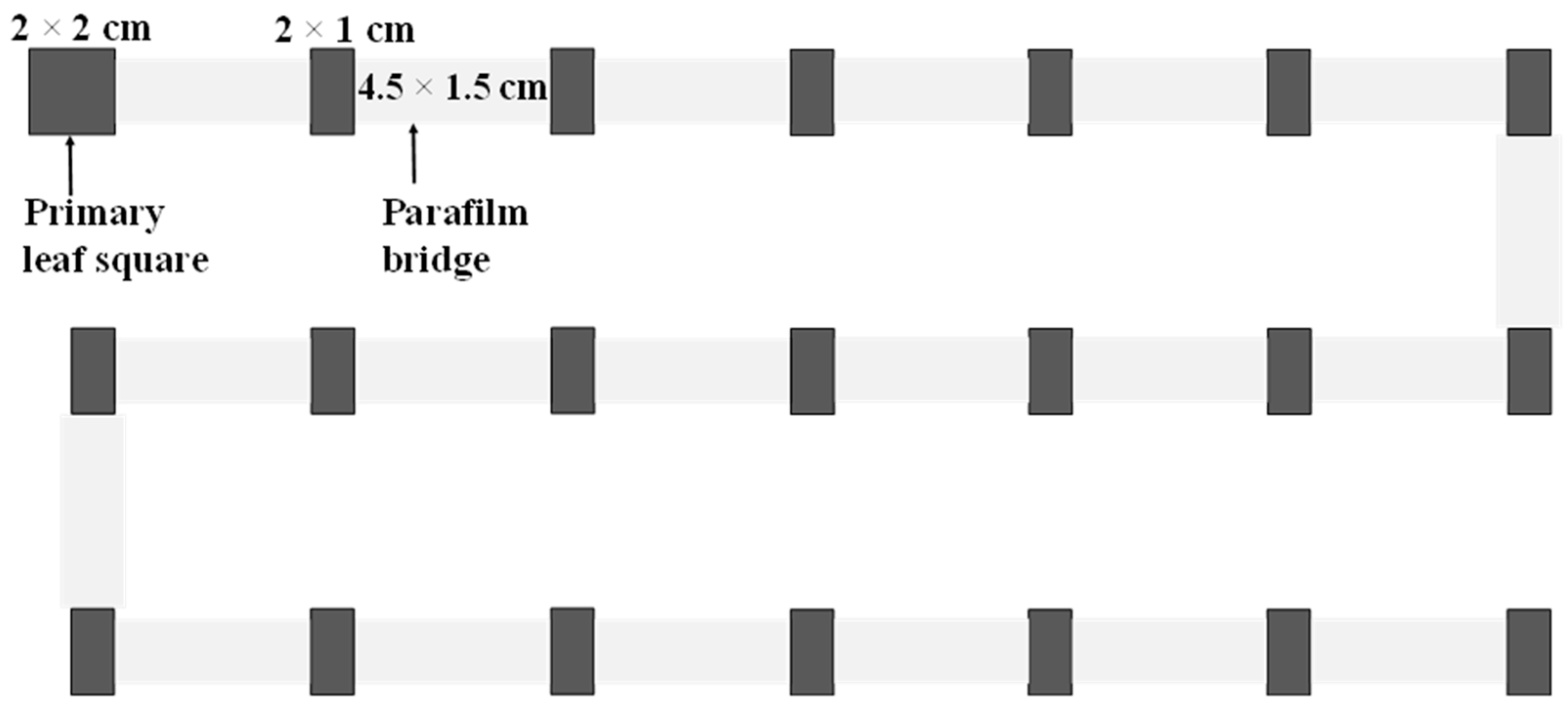
We established a dispersal arena consisting of 21 leaf squares (2 cm × 2 cm for the first leaf square and 2 cm × 1 cm for others) placed on wet cotton in a tray (45 cm length × 36 cm width × 1.5 cm heigh) (
Figure 1). We performed 17, 14, 14, 14, and 16 replicates for treatments of 1, 3, 6, 9, and 12 days old, respectively. For each replicate, we transferred 50 mated females of a desired age onto the first leaf square (primary leaf square) and allowed them to settle for 40 minutes. We then connected all leaf squares using Parafilm bridges (4.5 cm length × 1.5 cm width; Parafilm®, USA) and allowed mites to disperse freely for 24 hours, after which time, we removed all bridges, counted the number of adults on each leaf square, and removed all adults and counted the number of eggs on each leaf square. All leaf squares with eggs were individually transferred onto a wet cotton pad in a Petri dish, and the eggs were reared on their original leaf squares until adulthood.
We defined the dispersal probability as the percentage of mites that dispersed out of the primary leaf square. We used the number of leaf squares from the primary leaf square as dispersal distance and regarded the primary leaf square as zero distance. We recorded the mean dispersal distance of a replicate by averaging the dispersal distance of all individuals in the replicate and the total number of eggs laid in a replicate by summing up the number of eggs on all leaf squares. We recorded the mean distance of eggs of a replicate by averaging the distance of all eggs laid on different leaf squares.
2.4. Dispersal of Females at Different Densities
Because spider mite density in nature ranges from 0.1 to 50 individuals/cm
2 [
46,
47], we set up four densities: 2.5, 12.5, 25.0 and 37.5 females/cm
2, with 10, 50, 100 and 150 mated females on the primary leaf square, respectively. There were 15, 15, 16, and 16 replicates, respectively, for above density treatments. For each replicate, we transferred 1-d-old mated females of a desired number according to treatments on the primary leaf square and allowed them to settle for 40 minutes. We then connected all leaf squares as above and allowed mites to disperse freely for 24 hours, after which time, we removed all bridges and counted the number of adults on each leaf square. We calculated dispersal probability and distance of a replicate as described above.
2.5. Statistical Analysis
We analyzed all data using SAS software (SAS 9.4, SAS Institute Inc., Cary, NC). Data on dispersal probability in age and density experiments, dispersal distance in age experiment, total number of eggs laid, and number of eggs laid outside the primary leaf squares were normally distributed (Shapiro-Wilk test, UNIVARIATE Procedure), and thus analyzed using ANOVA with Tukey’s test for multiple comparison (GLM procedure). Data on distance of eggs, number of eggs laid on the primary leaf square, and dispersal distance in density experiment were square-root transformed to achieve normal distribution before ANOVA. The survival of offspring on and outside the primary leaf squares were not normally distributed even after transformation and analyzed using a non-parametric ANOVA (GLM procedure).
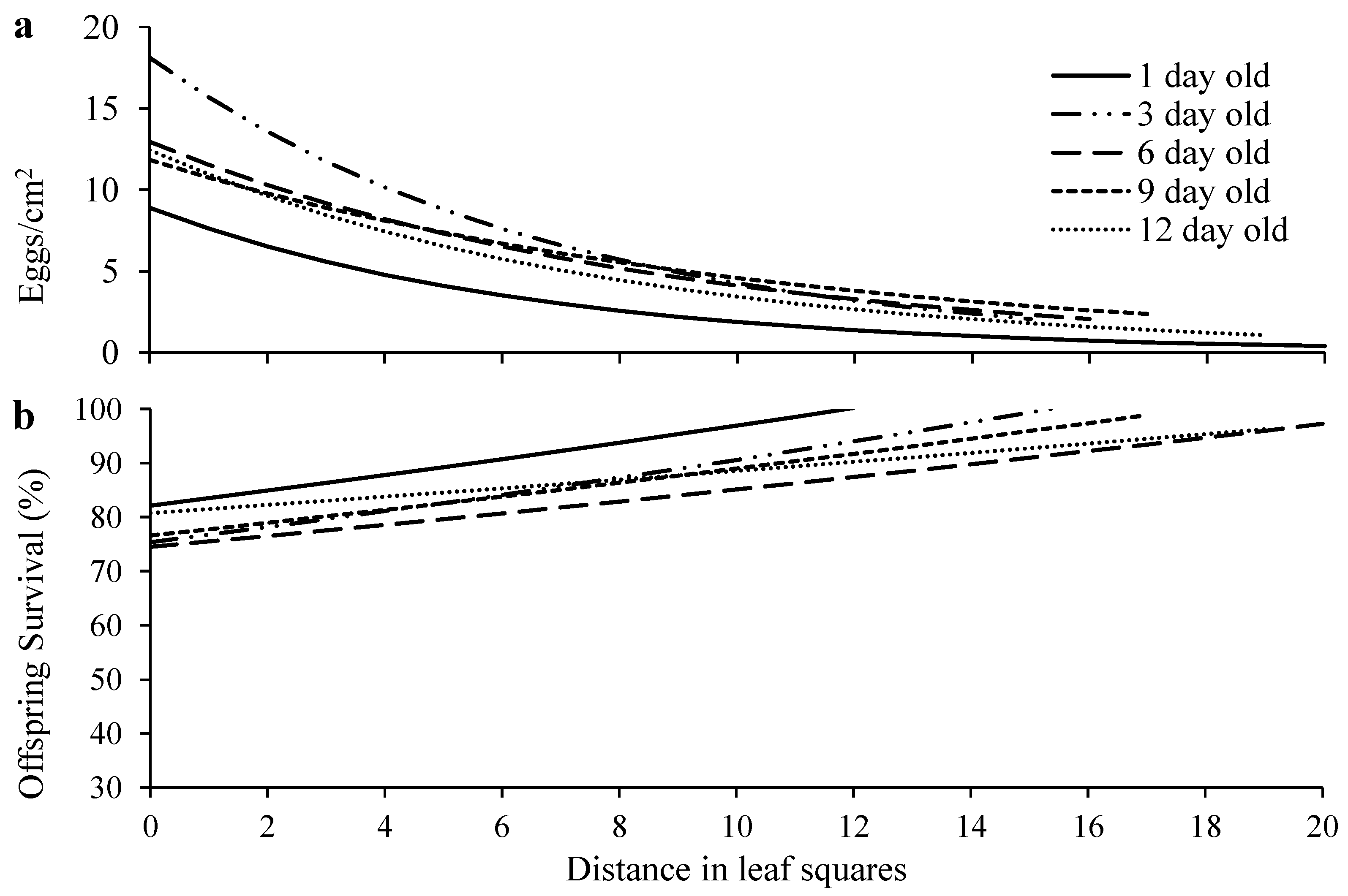
We used a logistic linear model, y = exp(a + bx), to determine the distribution of egg density (number of eggs/leaf square area) and changes of offspring survival rate over distance with a Gamma distribution and a log link function in all age treatments, where y is the response variable, x is distance, and a and b are constant parameters of the model (GLIMMIX Procedure).
3. Results
3.1. Dispersal and Reproduction of Females of Different Ages
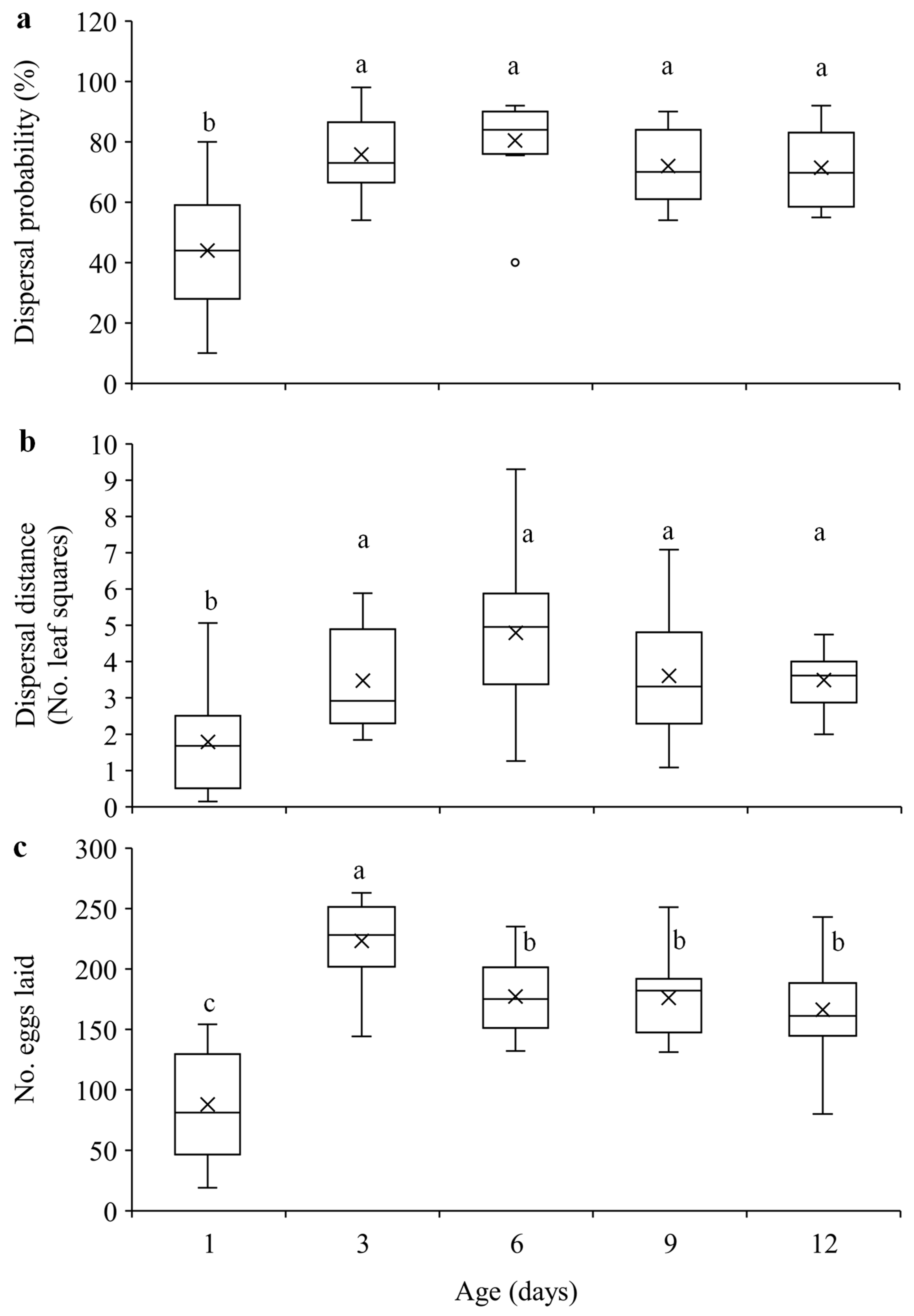
Our results show that within 24-h experimental period, 1-d-old females were significantly less likely to disperse compared to the older females (F
4, 70 = 15.51,
p < 0.0001) (
Figure 2a), and they dispersed significantly shorter distance than older females (F
4, 70 = 7.55,
p < 0.0001) (
Figure 2b). Furthermore, 1-d-old females laid significantly fewer eggs than older females and 3-d-old females laid significantly more eggs than females of other ages (F
4, 70 = 25.73,
p < 0.0001) (
Figure 2c).
Egg density significantly decreased over distance in all age treatments (
Figure 3a;
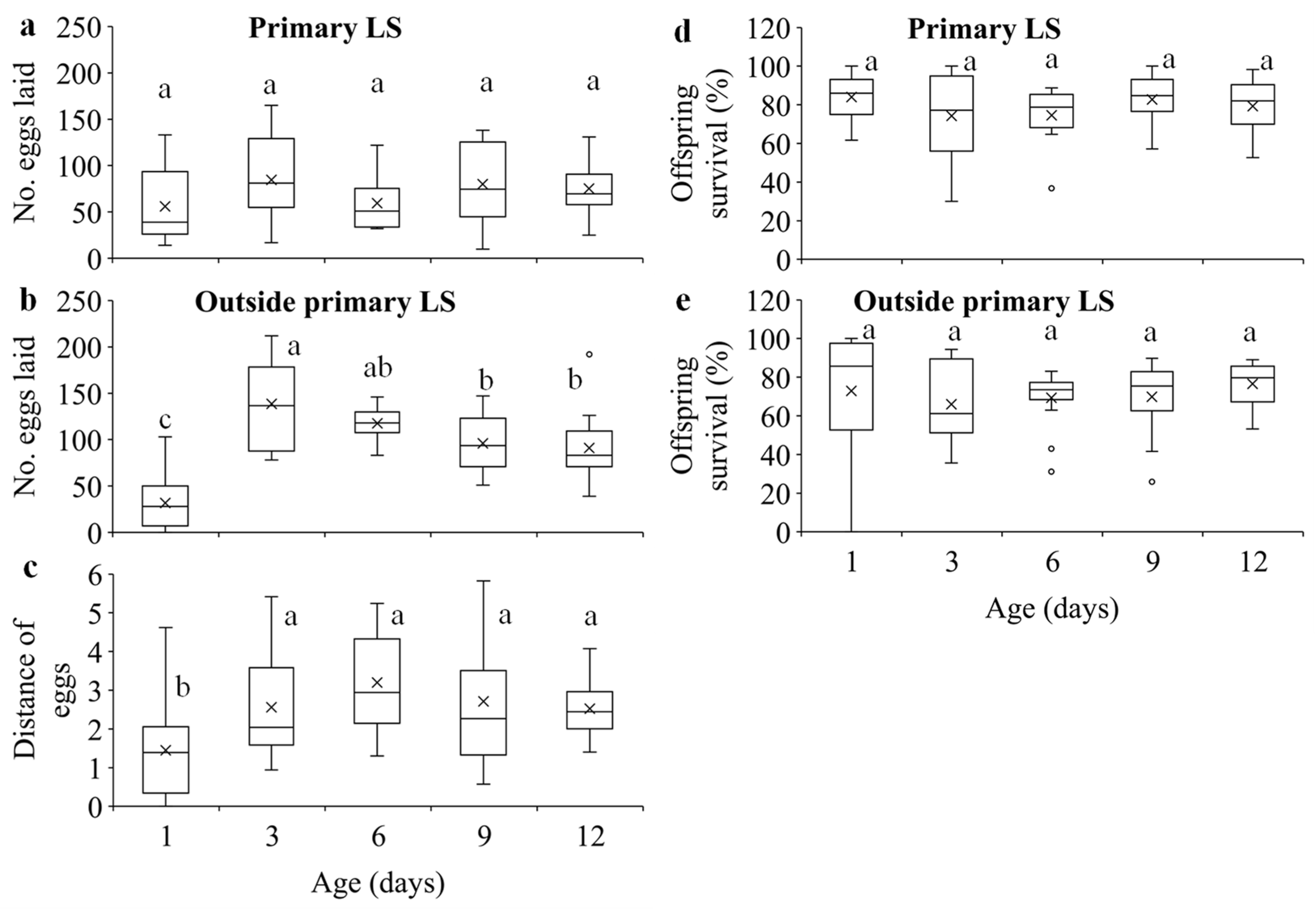
Table S1). Females of different ages laid similar number of eggs on the primary leaf squares (F
4, 70 = 1.62,
p = 0.1797) (
Figure 4a), but older females laid significantly more eggs outside the primary leaf squares than 1-d-old females (F
4, 70 = 22.59,
p < 0.0001) (
Figure 4b). In addition, older females produced eggs over significantly longer distance than 1-d-old females (F
4, 70 = 5.35,
p = 0.0008) (
Figure 4c). Immature survival rate significantly increased over distance in all treatments (
Figure 3b;
Table S1). Offspring survival on or outside the primary leaf square were similar between females of different ages (F
4, 70 = 1.16,
p = 0.3339 for primary leaf square; F
4, 69 = 1.80,
p = 0.1379 for outside the primary leaf square) (
Figure 4d,e).
3.2. Dispersal of Females at Different Densities
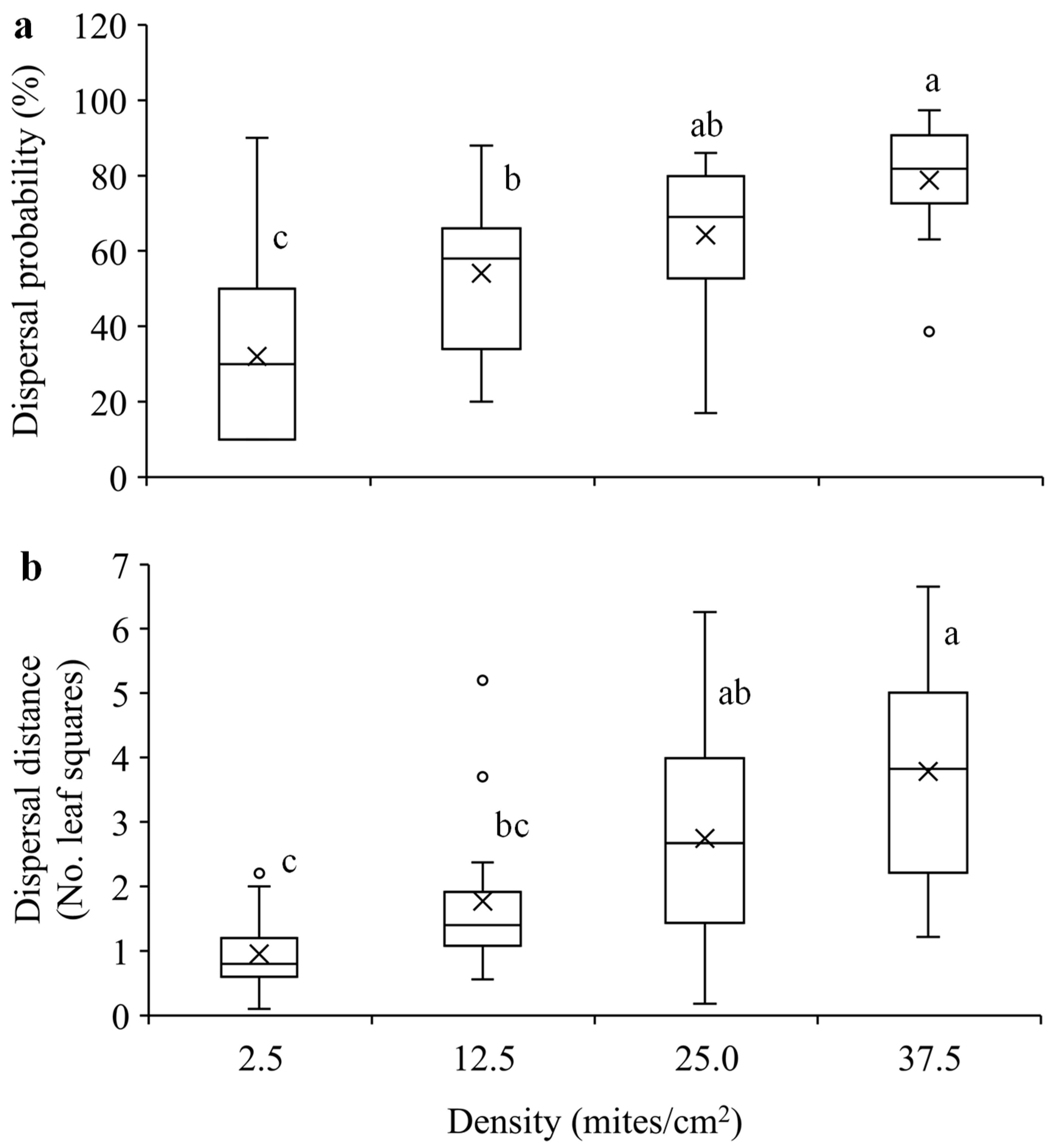
Mites were significantly more likely to disperse with the increase of population density (F
3, 58 = 15.33,
p < 0.0001) (
Figure 5a). Similarly, they dispersed significantly longer distance within 24 hours when population density was higher (F
3, 58 = 14.06,
p < 0.0001) (
Figure 5b).
4. Discussion
Our results show that compared to 1-d-old females, older ones had significantly higher dispersal probability and longer dispersal distance and laid more eggs in
T. ludeni (
Figure 2). These findings strongly suggest that the age-dependent dispersal is caused by reproductive readiness when older females carry more mature eggs [
42,
48]. In general, females with more eggs tend to disperse in wider areas [
1]. We also show that 3-d-old females laid the highest number of eggs under our experimental setting (
Figure 2c) where the population density is relatively high [
46,
47]. This may be attributed to a resource allocation strategy of
T. ludeni to maximizing reproduction during the early lifespan at high population densities [
42]. The synchronization of dispersal and reproduction has also been reported in many insects such as the light brown apple moth,
Epiphyas postvittana (Walker) (Lepidoptera: Tortricidae) [
14], the tarnished plant bug,
Lygus lineolaris (Palisot de Beauvois) (Hemiptera: Miridae) [
25,
49], the rice bug,
Leptocorisa chinensis Dallas (Hemiptera: Alydidae) [
15], and the butterfly,
Maculinea (
Phengaris)
teleius Bergsträsser (Lepidoptera: Lycaenidae) [
11].
Higher dispersal probability and longer dispersal distance of females ladened with more eggs may contribute to fast population growth at new sites, promoting pest outbreak and range expansion [
22,
24,
49]. Furthermore, the similarly high dispersal probability and long dispersal distance among older females (3–12 days old) (
Figure 2) suggest that
T. ludeni females can quickly disperse and infest new host plants in most of their adult lives. In their study on dispersal behaviour of
T. urticae, Li and Margolies reveal that the aerial dispersal is much more frequent in younger females because they are smaller in body size and may be more easily carried aloft by air currents [
9]. We provide the first evidence that females of ≥ 3 days old carrying more eggs are more likely to disperse through walking.
Dispersal serves multiple functions such as avoiding harsh conditions and reducing competition for resources between their own [
50,
51] and their offspring [
1,
11,
17,
18,
19,
20,
52]. We show that although older females had higher fecundity (
Figure 2c), they laid similar number of eggs on primary leaf squares (
Figure 3a) but produced significantly more eggs outside the primary leaf squares over longer distances compared to the 1-d-old ones (
Figure 3b,
c). The results suggest that older females of
T. ludeni can reduce offspring competition for food resources by dispersing from their natal habitats to the new habitats (
Figure 2a), and the spread of local population and increase of population size of spider mites within and between the adjacent habitats are through the females with high reproductive output.
We show that with the decrease of egg density over dispersal distance, offspring survival significantly increased (
Figure 4), implying that crowding and competition negatively affect offspring fitness and rich resources available at their birthplace increases their fitness. This strategy is critical to offspring survival in spider mites because immatures have limited dispersal ability [
9,
45,
53] and no parental care. Similar strategy has also been reported in the predatory mites,
Phytoseiulus persimilis Athias-Henriot and
Neoseiulus californicus McGregor (Acari: Phytoseiidae). Mothers of these two species produce eggs on a patch according to the number of prey eggs on the patch that are sufficient for their offspring to survival/develop before dispersing away [
54,
55].
The present study indicates that
T. ludeni females significantly increased dispersal probability and distance with the increase of population density (
Figure 5). This could be attributed to the fact that high population density reduces individual and population fitness [
3,
42,
43,
44,
56,
57]. In
T. ludeni, high population density negatively affected individual reproduction and population growth [
42,
43,
44]. Our results suggest that females would avoid overcrowding condition to reduce competition by dispersal with a longer distance. The density-dependent dispersal strategy has been reported in many animal species such as the butterflies
Maculinea nausithous and
M. teleius (Lepidoptera: Lycaenidae) [
19],
T. urticae [
9,
30], and the brown garden snail
Cornu aspersum (Müller) (Gastropoda: Helicidae) [
58]. This strategy allows individuals to leave high density habitats and emigrate into low density habitats and/or colonize new habitats [
3,
6,
8,
59], promoting the invasion speed of
T. ludeni.
In conclusion, increasing age and density promote dispersal probability and distance in T. ludeni. Females with more mature eggs are more likely to disperse and move longer distances. These dispersal strategies spread eggs out of their natal habitats and over longer distances, which reduces competition and increases their own and offspring fitness. These strategies may facilitate pest outbreak and invasion success of T. ludeni.
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org, Table S1: Statistical results of models of the egg density and offspring survival over distance in
T. ludeni females of different ages in
Figure 3.
Author Contributions
Conceptualization, P.Z., Q.W. and X.Z.H.; experimental design, P.Z., Q.W. and X.Z.H.; data collection, P.Z. and C.C.; data analysis, P.Z. and X.Z.H.; manuscript preparation and revision, P.Z., Q.W. and X.Z.H. All authors have read and agreed to the published version of the manuscript.
Funding
The work reported here was funded by the New Zealand-China Doctoral Research Scholarships Programme, and an open fund project of Anhui Key Laboratory for Biodiversity Research and Ecological Protection in Southwest Anhui (Wxn202307) to P.Z., the China Scholarship Council-Massey University Joint Scholarship Program to C.C., and a Massey University Research Fund to Q.W.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors on request.
Acknowledgments
We thank Professor Z.-Q. Zhang for identification of this spider mite to species, and K. Sinclair for technical assistance.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Matthysen, E. Multicausality of dispersal: a review. In Dispersal Ecology and Evolution; Clobert, J., Baguette, M., Benton, T.G., Bullock, J.M., Eds.; Oxford University Press: London, UK, 2012; Volume 27, pp. 3–18. [Google Scholar]
- Azandémè-Hounmalon, G.Y.; Fellous, S.; Kreiter, S.; Fiaboe, K.K.; Subramanian, S.; Kungu, M.; Martin, T. Dispersal behavior of Tetranychus evansi and T. urticae on tomato at several spatial scales and densities: implications for integrated pest management. PLoS ONE 2014, 9, e95071. [Google Scholar] [CrossRef] [PubMed]
- Bowler, D.E.; Benton, T.G. Causes and consequences of animal dispersal strategies: relating individual behaviour to spatial dynamics. Biol. Rev. Camb. Philos. Soc. 2005, 80, 205–225. [Google Scholar] [CrossRef] [PubMed]
- Clobert, J.; Le Galliard, J.F.; Cote, J.; Meylan, S.; Massot, M. Informed dispersal, heterogeneity in animal dispersal syndromes and the dynamics of spatially structured populations. Ecol. Lett. 2009, 12, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Regniere, J.; Nealis, V.G. Density dependence of egg recruitment and moth dispersal in spruce budworms. Forests 2019, 10, 706. [Google Scholar] [CrossRef]
- Travis, J.M.J.; Mustin, K.; Benton, T.G.; Dytham, C. Accelerating invasion rates result from the evolution of density-dependent dispersal. J. Theor. Biol. 2009, 259, 151–158. [Google Scholar] [CrossRef] [PubMed]
- Hovestadt, T.; Mitesser, O.; Poethke, H.-J. Gender-specific emigration decisions sensitive to local male and female density. Am. Nat. 2014, 184, 38–51. [Google Scholar] [CrossRef]
- Hovestadt, T.; Poethke, H.J. The control of emigration and its consequences for the survival of populations. Ecol. Modell. 2006, 190, 443–453. [Google Scholar] [CrossRef]
- Li, J.; Margolies, D.C. Effects of mite age, mite density, and host quality on aerial dispersal behavior in the twospotted spider mite. Entomol. Exp. Appl. 1993, 68, 79–86. [Google Scholar] [CrossRef]
- Mishra, A.; Tung, S.; Sruti, V.R.S.; Sadiq, M.A.; Srivathsa, S.; Dey, S. Pre-dispersal context and presence of opposite sex modulate density dependence and sex bias of dispersal. Oikos 2018, 127, 1596–1604. [Google Scholar] [CrossRef]
- Plazio, E.; Margol, T.; Nowicki, P. Intersexual differences in density-dependent dispersal and their evolutionary drivers. J. Evol. Biol. 2020, 33, 1495–1506. [Google Scholar] [CrossRef]
- Suiter, K.A.; Gould, F. Effects of mating status and age on dispersal behavior in the twospotted spider mite, Tetranychus urticae in response to fenvalerate-treated leaf surfaces. Entomol. Exp. Appl. 1992, 62, 1–8. [Google Scholar] [CrossRef]
- Fadamiro, H.Y. Free flight capacity determination in a sustained flight tunnel: effects of age and sexual state on the flight duration of Prostephanus truncatus. Physiol. Entomol. 1997, 22, 29–36. [Google Scholar] [CrossRef]
- Gu, H.; Danthanarayana, W. Age-related flight and reproductive performance of the light brown apple moth, Epiphyas postvittana. Entomol. Exp. Appl. 1990, 54, 109–115. [Google Scholar] [CrossRef]
- Ishizaki, M.; Watanabe, T.; Moriya, S.; Tabuchi, K. Diurnal locomotion activity of adult rice bug, Leptocorisa chinensis (Hemiptera: Alydidae), at different ages, measured by actograph and video camera. Appl. Entomol. Zool. 2011, 46, 135–142. [Google Scholar] [CrossRef]
- Perez-Mendoza, J.; Campbell, J.F.; Throne, J.E. Influence of age, mating status, sex, quantity of food, and long-term food deprivation on red flour beetle (Coleoptera: Tenebrionidae) flight initiation. J. Econ. Entomol. 2011, 104, 2078–2086. [Google Scholar] [CrossRef] [PubMed]
- Benton, T.G.; Bowler, D.E. Dispersal in invertebrates: influences on individual decisions. In Dispersal Ecology and Evolution; Clobert, J., Baguette, M., Benton, T.G., Bullock, J.M., Eds.; Oxford University Press: London, UK, 2012; pp. 41–49. [Google Scholar]
- Lakovic, M.; Poethke, H.-J.; Hovestadt, T. Dispersal timing: emigration of insects living in patchy environments. PLoS ONE 2015, 10, e0128672. [Google Scholar] [CrossRef] [PubMed]
- Nowicki, P.; Vrabec, V. Evidence for positive density-dependent emigration in butterfly metapopulations. Oecologia 2011, 167, 657–665. [Google Scholar] [CrossRef] [PubMed]
- Reiskind, M.H.; Wilson, M.L. Culex restuans (Diptera: Culicidae) oviposition behavior determined by larval habitat quality and quantity in southeastern Michigan. J. Med. Entomol. 2004, 41, 179–186. [Google Scholar] [CrossRef]
- Salomão, R.P.; Arellano, L.; Huerta, C.; León-Cortés, J.L. Do sexual gonadic maturity and age determine habitat occupancy of Canthon cyanellus LeConte, 1859 (Coleoptera: Scarabaeidae)? Can. Entomol. 2021, 153, 412–427. [Google Scholar] [CrossRef]
- Dingle, H. Relation between age and flight activity in milkweed bug, Oncopeltus. J. Exp. Biol. 1965, 42, 269–283. [Google Scholar] [CrossRef]
- Järemo, J.; Bengtsson, G. On the importance of life history and age structure in biological invasions. Ecol. Modell. 2011, 222, 485–492. [Google Scholar] [CrossRef]
- Schumacher, P.; Weyeneth, A.; Weber, D.C.; Dorn, S. Long flights in Cydia pomonella L. (Lepidoptera: Tortricidae) measured by a flight mill: influence of sex, mated status and age. Physiol. Entomol. 1997, 22, 149–160. [Google Scholar] [CrossRef]
- Stewart, S.D.; Gaylor, M.J. Age, sex, and reproductive status of the tarnished plant bug (Heteroptera: Miridae) colonizing mustard. Environ. Entomol. 1991, 20, 1387–1392. [Google Scholar] [CrossRef]
- Williamson, J.A.; Charlesworth, B. The effect of age of founder on the probability of survival of a colony. J. Theor. Biol. 1976, 56, 175–190. [Google Scholar] [CrossRef] [PubMed]
- Bonte, D.; De Roissart, A.; Wybouw, N.; Van Leeuwen, T. Fitness maximization by dispersal: evidence from an invasion experiment. Ecology 2014, 95, 3104–3111. [Google Scholar] [CrossRef]
- Burgess, S.C.; Powell, J.; Bueno, M. Dispersal, kin aggregation, and the fitness consequences of not spreading sibling larvae. Ecology 2022, 104, e3858. [Google Scholar] [CrossRef] [PubMed]
- De Meester, N.; Bonte, D. Information use and density-dependent emigration in an agrobiont spider. Behav. Ecol. 2010, 21, 992–998. [Google Scholar] [CrossRef]
- Bitume, E.V.; Bonte, D.; Ronce, O.; Bach, F.; Flaven, E.; Olivieri, I.; Nieberding, C.M. Density and genetic relatedness increase dispersal distance in a subsocial organism. Ecol. Lett. 2013, 16, 430–437. [Google Scholar] [CrossRef] [PubMed]
- Poethke, H.J.; Gros, A.; Hovestadt, T. The ability of individuals to assess population density influences the evolution of emigration propensity and dispersal distance. J. Theor. Biol. 2011, 282, 93–99. [Google Scholar] [CrossRef]
- Ventura, L.; Smith, D.R.; Lubin, Y. Crowding leads to fitness benefits and reduced dispersal in a colonial spider. Behav. Ecol. 2017, 28, 1384–1392. [Google Scholar] [CrossRef]
- Bolland, H.R.; Gutierrez, J.; Flechtmann, C.H. World Catalogue of the Spider Mite Family (Acari: Tetranychidae); Leiden, Brill: the Netherlands, 1998; p. 392. [Google Scholar]
- CABI. Tetranychus ludeni (red spider mite). Available online: https://www.cabi.org/isc/datasheet/53351 (accessed on 6 April 2024).
- Zhou, P.; He, X.Z.; Wang, Q. Sons from virgin mothers produce more daughters in a haplodiploid mite. Syst. Appl. Acarol. 2018, 23, 1869–1878. [Google Scholar] [CrossRef]
- Zhou, P.; He, X.Z.; Chen, C.; Wang, Q. Resource relocations in relation to dispersal in Tetranychus ludeni Zacher. Syst. Appl. Acarol. 2021, 26, 2018–2026. [Google Scholar] [CrossRef]
- Migeon, A.; Nouguier, E.; Dorkeld, F. Spider mites web: a comprehensive database for the Tetranychidae. Proceedings of the 12th International Congress, Dordrecht; Sabelis, M.W., Bruin, J. Eds.; 2010, pp. 557–560. [CrossRef]
- Ragusa, E.; Sinacori, M.; Tsolakis, H. First record of Tetranychus ludeni Zacher (Acariformes: Tetranychidae) in Italy. Int. J. Acarol. 2019, 45, 26–28. [Google Scholar] [CrossRef]
- Zhang, Z.-Q. Mites of Greenhouses: Identification, Biology and Control; CABI Publishing: Wallingford, UK, 2003; p. 244. [Google Scholar]
- Mcenroe, W.D. Spreading and inbreeding in the spider mite. J. Hered. 1969, 60, 343–345. [Google Scholar] [CrossRef] [PubMed]
- Smitley, D.R.; Kennedy, G.G. Aerial dispersal of the two-spotted spider mite (Tetranychus urticae) from field corn. Exp. Appl. Acarol. 1988, 5, 33–46. [Google Scholar] [CrossRef]
- Weerawansha, N.; Wang, Q.; He, X.Z. Effect of foundress population density and size on reproduction and population growth of a haplodiploid mite. Syst. Appl. Acarol. 2020, 25, 2063–2076. [Google Scholar]
- Weerawansha, N.; Wang, Q.; He, X.Z. A haplodiploid mite adjusts fecundity and sex ratio in response to density changes during the reproductive period. Exp. Appl. Acarol. 2022, 88, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Weerawansha, N.; Wang, Q.; He, X.Z. Adjustment of fecundity and sex ratio in response to social environments in a haplodiploid mite. Syst. Appl. Acarol. 2022, 27, 61–70. [Google Scholar] [CrossRef]
- Mitchell, R. Growth and population dynamics of a spider mite (Tetranychus urticae K., Acarina: Tetranychidae). Ecology 1973, 54, 1349–1355. [Google Scholar] [CrossRef]
- Geroh, M.; Gulati, R.; Sharma, S.; Kaushik, H.; Aneja, D. Effect of abiotic stresses on population build up of Tetranychus urticae Koch and its predator, Stethorus punctillum Weise on okra. Ann. Plant Prot. Sci. 2010, 18, 108–113. [Google Scholar]
- Helle, W.; Sabelis, M.W. Spider Mites: Their Biology, Natural Enemies and Control; Elsevier: Amsterdam, Netherlands, 1985; p. 458. [Google Scholar]
- Adango, E.; Onzo, A.; Hanna, R.; Atachi, P.; James, B. Comparative demography of the spider mite, Tetranychus ludeni, on two host plants in West Africa. J. Insect Sci. 2006, 6, 1–9. [Google Scholar] [CrossRef]
- Stewart, S.D.; Gaylor, M.J. Effects of age, sex, and reproductive status on flight by the tarnished plant bug (Heteroptera: Miridae). Environ. Entomol. 1994, 23, 80–84. [Google Scholar] [CrossRef]
- Bonte, D.; Dahirel, M. Dispersal: a central and independent trait in life history. Oikos 2017, 126, 472–479. [Google Scholar] [CrossRef]
- Buoro, M.; Carlson, S.M. Life-history syndromes: integrating dispersal through space and time. Ecol. Lett. 2014, 17, 756–767. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-Y.; Kokko, H. Intersexual resource competition and the evolution of sex-biased dispersal. Front. Ecol. Evol. 2019, 7, 111. [Google Scholar] [CrossRef]
- Brandenburg, R.L.; Kennedy, G.G. Intercrop relationships and spider mite dispersal in a corn/peanut agro-ecosystem. Entomol. Exp. Appl. 1982, 32, 269–276. [Google Scholar] [CrossRef]
- Nadeali, T.; Golpayegani, A.Z.; Saboori, A. When do the predators leave their patch? Leaving tendency in Phytoseiulus persimilis and Neoseiulus californicus (Phytoseiidae). Syst. Appl. Acarol. 2014, 19, 263–274. [Google Scholar]
- Vanas, V.; Enigl, M.; Walzer, A.; Schausberger, P. The predatory mite Phytoseiulus persimilis adjusts patch-leaving to own and progeny prey needs. Exp. Appl. Acarol. 2006, 39, 1–11. [Google Scholar] [CrossRef]
- Denno, R.F.; Roderick, G.K. Density-related dispersal in planthoppers-effects of interspecific crowding. Ecology 1992, 73, 1323–1334. [Google Scholar] [CrossRef]
- Li, G.-Y.; Zhang, Z.-Q. The costs of social interaction on survival and reproduction of arrhenotokous spider mite Tetranychus urticae. Entomol. Gen. 2021, 41, 49–57. [Google Scholar] [CrossRef]
- Dahirel, M.; Vardakis, M.; Ansart, A.; Madec, L. Density-dependence across dispersal stages in a hermaphrodite land snail: insights from discrete choice models. Oecologia 2016, 181, 1117–1128. [Google Scholar] [CrossRef] [PubMed]
- Sæther, B.-E.; Engen, S.; Lande, R. Finite metapopulation models with density-dependent migration and stochastic local dynamics. Proc. R. Soc. London, B 1999, 266, 113–118. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).