1. Introduction
Melanoma is one of the most common types of cancer caused by the transformation of melanocytes. In advanced stages, it is considered an aggressive type of cancer that is difficult to treat due to its risk of metastasis [
1]. To evaluate new chemotherapies against melanoma, human and mouse cell lines, such as the B16F1 mouse cell line, are often used [
2]. These cancer cell lines are commonly used in two-dimensional (2D) monolayer cell culture models to test different drugs. However, 3D cultures have become relevant in the field of oncotherapy because they provide a better model to evaluate the effect of different drugs [
3]. They confer similar properties to an in vivo tumor environment with the expression of molecules that recapitulate tumor physiology [
4,
5,
6].
Autophagy is an adaptive cellular process that maintains homeostasis under stress conditions and serves as a quality control mechanism for organelles and proteins. This mechanism can be triggered by various intra- or extracellular stimuli [
7]. The initial step of autophagy is the formation of a double-membrane phagophore in the endoplasmic reticulum. Subsequently, primarily phagophore elongation is carried out by the class III PI3K complex. During elongation, the binding of several proteins to the autophagophore membrane occurs, including the recruitment of LC3 [
8]. LC3 undergoes cleavage by ATG4 forming LC3BI and is subsequently conjugated with phosphatidylethanolamine to form LC3BII. LC3BII is capable of binding to the autophagosome membrane and functions as an anchor protein for the selection of target proteins to be introduced into the autophagosome for degradation [
9]. Selection of these proteins is carried out by the SQSTM1 protein, also known as p62. Polyubiquitinated proteins bind to p62 and deliver them to the autophagosome through its interaction with the LIR domain of LC3BII [
10,
11]. In cancer, autophagy appears to have a dual role, as it is involved in both tumor suppression and tumor development, generating resistance to anticancer treatments [
12].
PTX is a methylxanthine derivative with vasodilatory properties, which has been used to treat cardiac and cerebrovascular conditions [
13]. Recently, PTX has been tested as a potential chemotherapy for several types of cancer [
14]. PTX has been found to inhibit STAT3 phosphorylation in the JAK/STAT pathway in a murine melanoma model, decreasing angiogenesis and tumor metastasis [
15,
16]. Furthermore, it decreased proliferation and induced stress-dependent endoplasmic reticulum autophagy in human melanoma cell lines A375 and MeWo [
17].
NCTD is the demethylated form of cantharidin obtained from the
Mylabris beetle and has been used in traditional Chinese medicine, showing different antitumor effects [
18]. In melanoma, NCTD induces apoptosis through the activation of caspases caused by overexpression of BAX and downregulation of Bcl-2 [
19,
20]. Furthermore, NCTD causes apoptosis in several mitophagy-mediated melanoma cell lines, by increasing LC3 expression and decreasing p62 [
21]. In this work we evaluated the effect of PTX and NCTD on autophagy in B16F1 cells using 2D and 3D cell culture models.
2. Results
2.1. 2D and 3D Cell Cultures
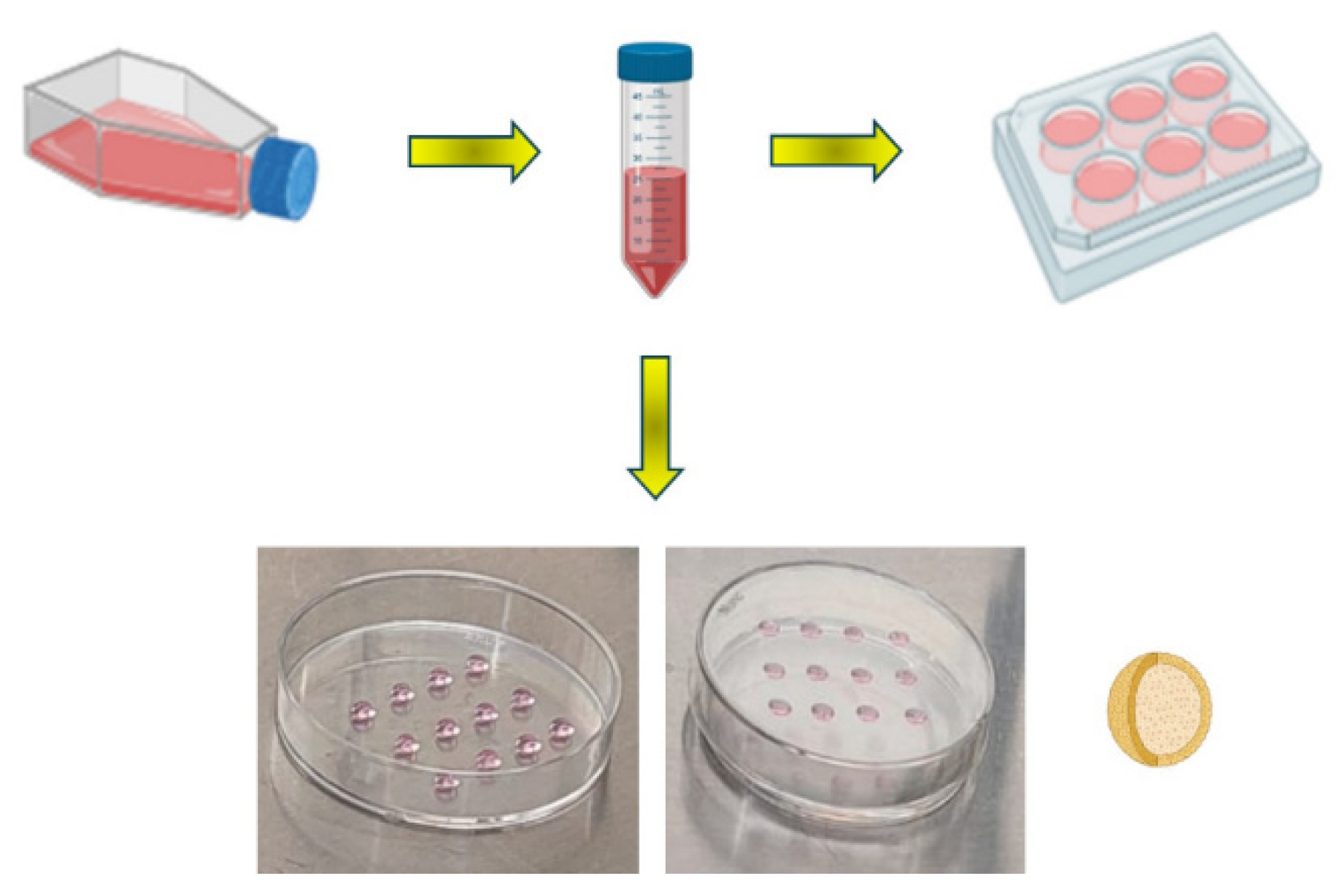
The traditional protocol was followed to obtain cultures in 2D plates. 3D cultures were obtained using the hanging drop technique in a Petri dish, where the cell suspension, due to the effect of gravity, falls to the bottom of the drop inducing the formation of spheroids because of cell aggregation [
22]. Thus, within the drop, cellular interactions and the conditions of the surrounding environment favor the formation of spheroids. This allowed us to have a cell culture that was morphologically and physiologically similar to the in vivo melanoma tumour (
Figure 1). This method proved to be very efficient and low cost, compared to other methods used for spheroid formation [
5,
23].
2.2. Treatment of 2D and 3D Cell Cultures with PXT and NCTD
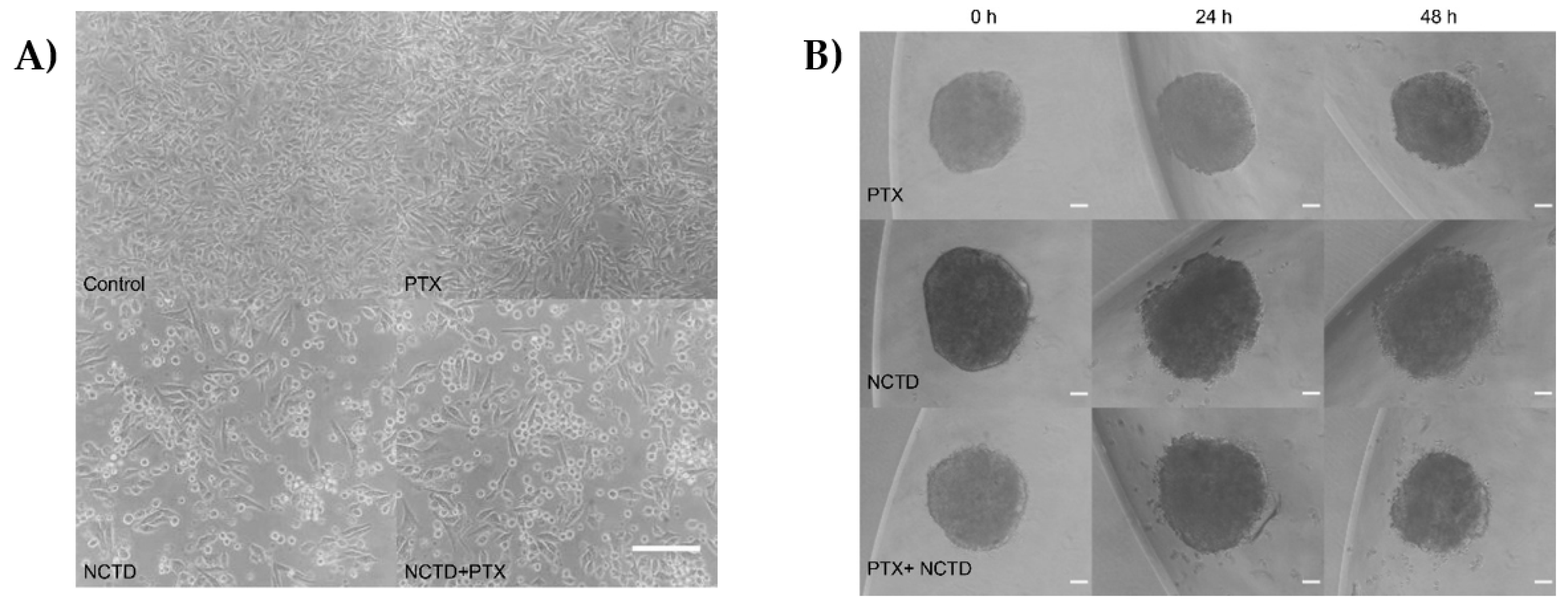
Treatments with NCTD and the combination of both PTX+NCTD induced evident morphological changes in B16F1 cells. In the 2D cultures, NCTD and PTX+NCTD treated cells present a reduction in their cytoplasm, showing a more compact and rounded shape compared to untreated controls (
Figure 2A). In 3D cultures, spheroids from the NCTD and PTX+NCTD groups showed a lower cell density in the proliferation zone. Some of the cells in this area detached in a manner of satellites, being compatible with a phenomena of spheroid lysis (
Figure 2B).
2.3. Autophagy p62 Expression in 2D and 3D Cultures
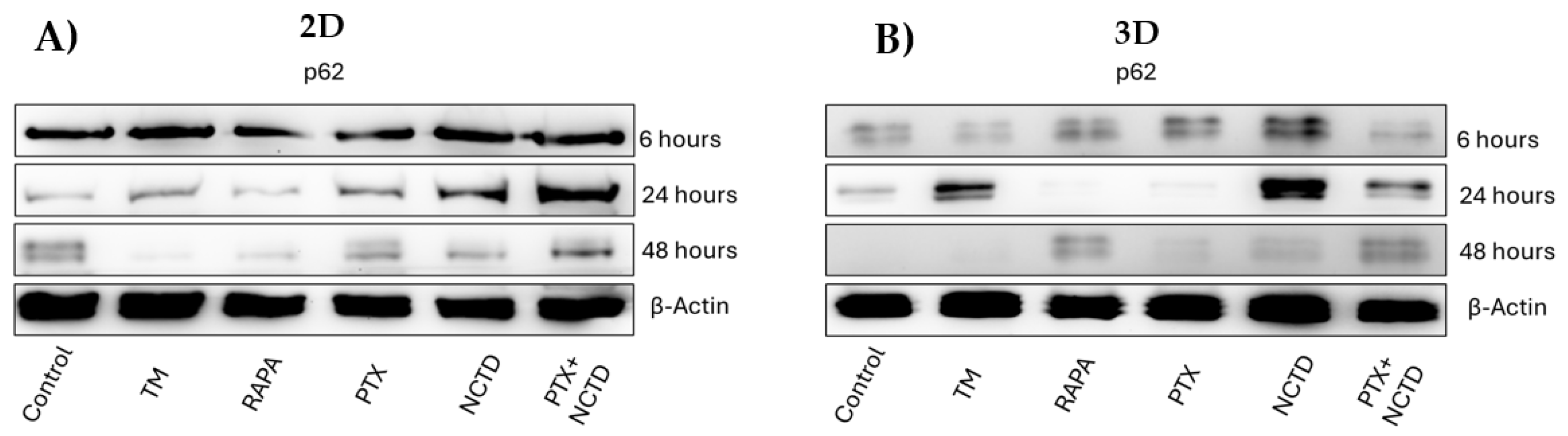
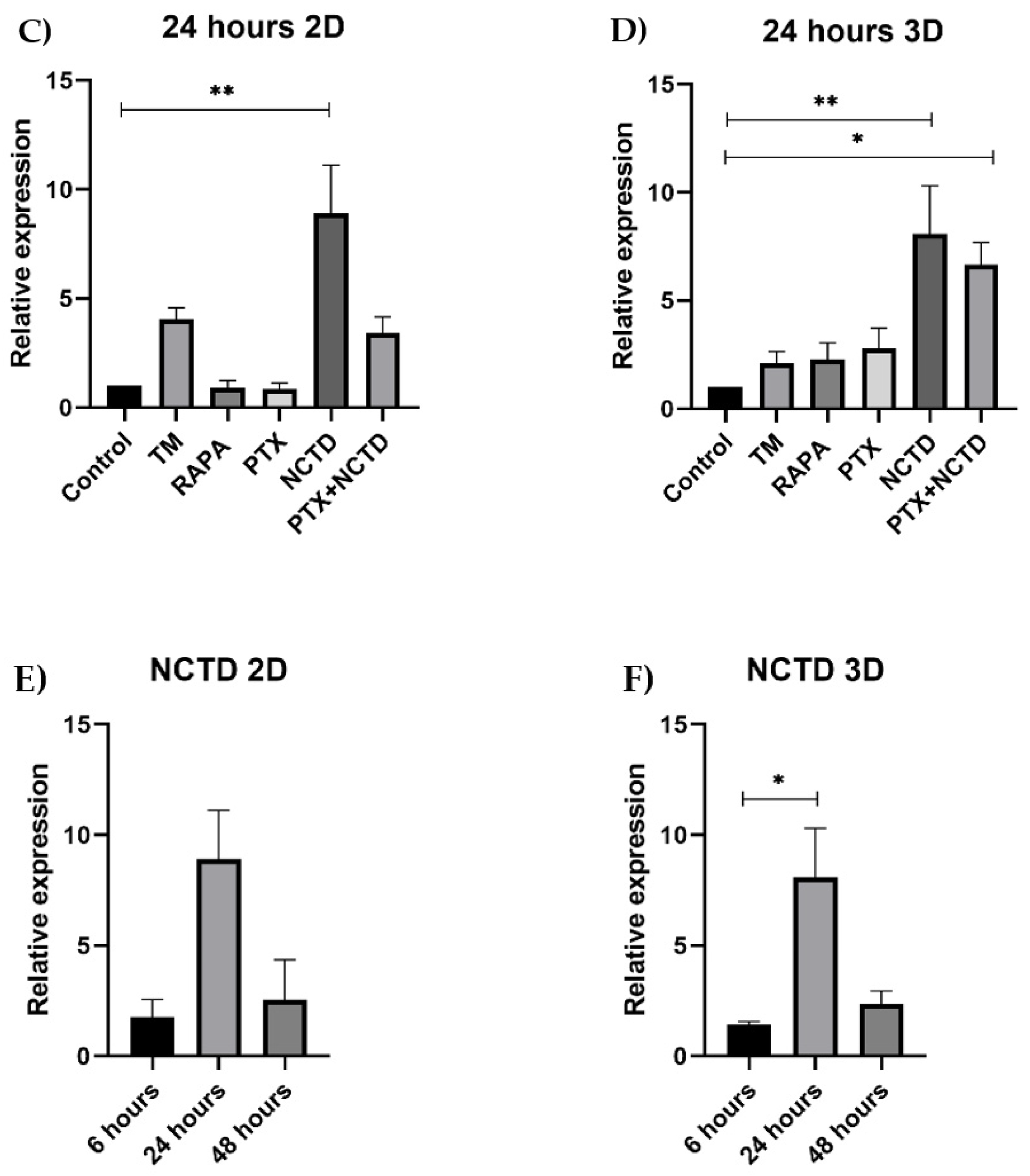
Since rapamycin induces autophagy and tunicamycin induces endoplasmic reticulum stress, we used them as positive controls to evaluate p62 expression. We observed that tunicamycin increased p62 expression at 24 h with a subsequent decrease at 48 h (
Figure 3A). In the experimental groups, we found that p62 expression decreased in 2D cell cultures treated with PTX at 24 and 48 h compared to the 6 h group (
Figure 3A). In contrast, NCTD treatment significantly increased p62 expression at 6 and 24 h compared to the untreated control (
Figure 3A). Regarding the 3D model, we found that PTX-treated spheroids decreased p62 expression at 24 h compared to 48 h (
Figure 3B). NCTD-treated spheroids exhibited high p62 expression at 24 h, but decreased at 48 h (
Figure 3B, C). For both 2D and 3D cultures, the combination of PXT+NCTD significantly increased p62 expression at 24 h of treatment (
Figure 3 B–D). A relative expression analysis of p62 was performed in 2D and 3D cultures at the time points tested, finding a similar pattern in which p62 was highly expressed at 24 h but decreased at 48 h (
Figure 3D, F).
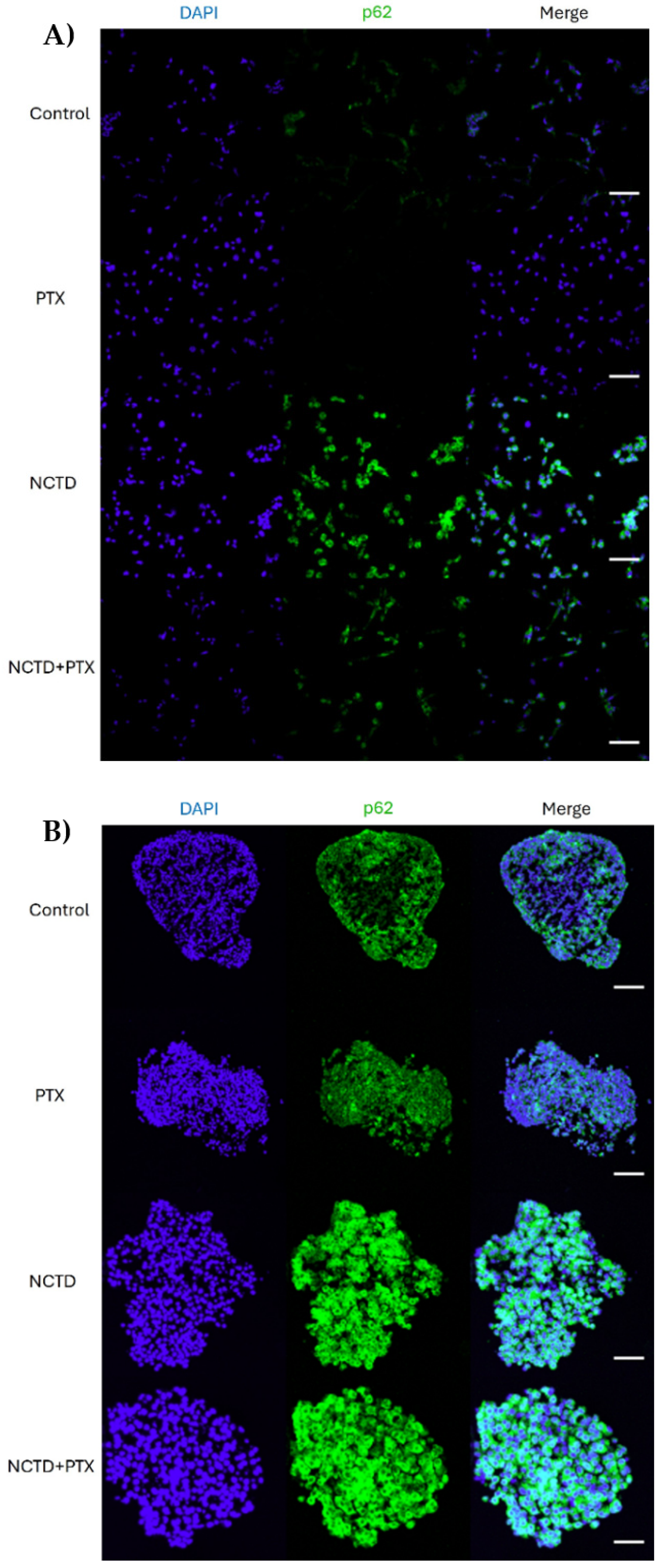
2.4. Expression of p62 in 2D and 3D Cultures by Immunofluorescence
The effect on p62 expression of PXT, NCTD and the combination of both drugs was analyzed by immunofluorescence in 2D and 3D cultures at 24h. In 2D cultures, untreated controls showed basal expression of p62, which decreased when cells were treated with PXT. NCTD induced high expression of p62. Interestingly, although cells treated with PXT+NCTD also showed high expression of p62, but it was not higher than cells treated with NCTD alone (
Figure 4A). For 3D cultures, basal expression of p62 was observed in some areas of control and PTX spheroids at 24 h. However, spheroids treated with NCTD and NCTD + PTX showed strong expression of p62 in all cells of the spheroid (
Figure 4B).
3. Discussion
Recently, 3D cell cultures have become important for anticancer drug screening, offering great similarity to solid tumours in vitro [
3,
5]. Spheroids provide a niche with several layers of cells that allows a better evaluation of different markers under different treatment models, reducing the use of animals for the screening of new therapies [
24]. Here, we constructed 3D spheroids of mouse B16F1 melanoma cells using the hanging drop method to test the effect of PTX and NCTD. We obtained spheroids with well-defined cell proliferation and a senescent zone. This 3D model allowed us to demonstrate part of the effect of NCTD and PTX. In this work we demonstrate that NCTD promotes high expression of p62 which in turn is related to important morphological changes in melanocytes. The p62 protein is a scaffold molecule with multiple domains. This molecule is also known as sequestosome 1 (p62//SQSTM 1) and can interact with phagosomes through interaction with LC3. Because p62 possesses multifunctional and signaling properties involved in the regulation of autophagy and apoptosis, it is believed to play an important role in cancer [
25,
26,
27,
28]. Its regulation has been the subject of several studies and for that, several modulators of autophagy have gained importance in melanoma [
29]. In the early stages of the disease, autophagy promotes the removal of misfolded or mutated proteins [
30]. This alteration in autophagy together with the accumulation of p62 contains tumour progression [
31]. Once metastasis occurs, tumour cells can activate autophagy and p62 levels decrease. This reactivation of autophagy, under treatments such as chemotherapy, makes tumour cells resistant, preventing their death [
32]. Interestingly, we found that NCTD induces high expression of p62 in both 2D and 3D models at 24 hours. Indeed, in our 3D model NCTD promoted spheroid disaggregation. This is relevant since higher autophagic activity has been reported in 3D cultures, which usually leads to lower effectiveness of anticancer drugs [
33,
34]. Despite the antitumoral properties of NCTD, some studies have shown that p62 accumulation is a marker of poor prognosis in different types of cancer [
35,
36,
37]. However, in melanoma it has been shown that the decrease in p62 in late stages of the disease is related to metastasis [
38]. Therefore, since autophagy in melanoma improves tumor survival, the use of an autophagy inhibitor such as NCTD could be useful in advanced stages of the disease to contain metastasis.
On the other hand, PTX also possess anti-cancer effects [
14,
39]. Previous studies have shown that it has antimetastatic properties in human and mouse melanoma cell lines [
40,
41]. In this work, we demonstrate that PTX can increase autophagy in mouse B16F1 melanoma cells through the degradation of p62 in 2D and 3D cultures, promoting the preservation of cell morphology and avoiding spheroids lysis (
Figure 3A, 3B). This effect could be a result of increased endoplasmic reticulum stress triggered by PXT, which is a mechanism that degrades misfolded proteins and can activate autophagy in cells [
17].
In conclusion, NCTD is a potent inducer of p62 that induces a deregulation of autophagy, causing important morphological changes in B16F1 melanoma cells and spheroid lysis in vitro. Future in vivo studies are necessary to determine the potential role of NCTD to control metastasis in advanced stages of the disease.
4. Materials and Methods
4.1. Two-Dimensional (2D) and Three-Dimensional (3D) Cell Culture Models
For cell culture, murine melanoma cell line B16F1 obtained from the American Type Culture Collection (ATCC, #CRL, 1872, MD, U.S.A.) was used. It was cultured using DMEM/F12 medium + GlutaMAXTM (cat# 10565-018, Gibco) supplemented with 10% fetal bovine serum at 37°C in 5% CO2. Cells were detached with Trypsin-EDTA (cat# 25200-056, Gibco), when they reached >80% confluent. Then, 2 washes were performed with PBS 1x and resuspended in 1 mL of medium. For 2D assays, cells were grown in 6-well plates at a concentration of 1x106 and maintained for 24 hours for attaching to the plate. For 3D assays, the hanging drop technique was employed. On the inside of a Petri dish lid, cells were placed in droplets at a concentration of 2x103 cells/20 μL. The lid was gently turned upside down to prevent the droplets from falling. Cells were cultured for 2 weeks until spheroid formation. The change of culture medium was performed every 3 days. Cell cultures were treated with PTX (100 μM), NCTD (80 μM) and the combination of both drugs, for 6, 24 and 48 hours. In addition, Rapamycin (RAPA, 10 μM) and Tunicamycin (TM, 7 μM) were used as controls for autophagy and endoplasmic reticulum stress, respectively.
4.2. Western Blot Analysis
Cells from 2D and 3D cultures were lysed in RIPA buffer (cat# 89900, Thermo scientificTM) with protease inhibitor (cat# P8340, Sigma Aldrich). Protein concentration was quantified with the BCA kit (cat# 23225, Thermo scientificTM). Samples were adjusted to 30 μg/well and separated on a 12% SDS-PAGE gel. Electrophoresis was performed at 100 V for 1 hour followed by a transference to a nitrocellulose membrane (BioRAD, Berkeley, California). The nitrocellulose membrane was blocked with 5% BSA (bovine serum albumin, cat# 30063-721, Gibco) in 0.5% TBS-Tween 0.5% (Tris-HCl 20 mM, pH 7.6, NaCl 150 mM, Tween 20 0.5%). Subsequently, anti-p62 (1:1000, cat# 39749, Cell Signaling Technology) and anti-LC3B (1:1000, cat# 2775, Cell Signaling Technology) primary antibodies were added and incubated overnight under constant agitation. Subsequently, 5 washes were performed with 0.5% TBS-Tween and incubated for 1 hour with anti-rabbit antibody, HRP-linked (1:1000, cat# 7074, Cell Signaling Technology) and anti-β-actin HRP conjugate (1:3000, cat# 5125, Cell Signaling Technology). Finally, 5 washes were performed with TBS-Tween and SignalFire™ ECL Reagent (cat# 6883s, Cell Signaling Technology) was added for development on ChemiDocTM Touch (BioRad, Berkeley, California). The images obtained were analyzed with Image Lab software (BioRad).
4.2. Slices of the 3D Cultures of the Spheroids
3D cultures in spheroids were collected in microtubes with the aid of a pipette. They were washed 2 times with PBS and fixed in 100 μL of 4% paraformaldehyde for 24 hours. Then, they were washed 2 times with PBS and placed in cryomolds. Tissue Tek (The brand) was added until completely covered. Subsequently, they were frozen at -20°C and cut at 5 microns in the cryostat (HM525 NX, Epredia™ Company City). The slices were placed on 1% gelatin-coated slides. The slides were stored at -20°C until use.
4.3. Immunofluorescence of 2D and 3D Cultures
For 2D staining, 5x105 cells were cultured on sterile coverslips in a 6-well plate. Cells were cultured for 24 hours at 37°C in a humidified atmosphere with 5% CO2 to allow adherence and drug treatments and controls were added as above. Slides with spheroid sections were thawed at room temperature. The same staining procedure was followed for both conditions. They were fixed with 4% paraformaldehyde for 5 minutes. Then, 2 washes were performed with PBS and 0.25% NH4 Cl was added for 5 min to quench endogenous fluorescence. Two washes were performed with 0.2% PBS-Triton for 5 minutes. Subsequently, blocking solution (PBS-Triton 0.2%, albumin 1%) was added for 1 hour. The first anti-p62 antibody (1:200, cat# 39749, Cell Signaling Technology) diluted in the blocking solution was incubated for 2 hours. Three washes were performed with 0.2% PBS-Triton. Mouse anti-rabbit FITC secondary antibody (1:200, sc-2359, Santa Cruz) diluted in blocking solution was kept for 1 hour in the dark. Two washes were performed with 0.2% PBS-Triton. Slides were mounted in Fluoromount-GTM, with DAPI (cat# 4959-52, eBioscience) and stored at 4°C in the dark. Slides were observed under the LMS 5 Exciter confocal microscope (Zeiss).
4.3. Statistical Analysis
Western blot assays were performed in triplicate. Shapiro-Wilk test was performed to test the normality of the data. One-way ANOVA test (one-way ANOVA) was performed for comparison analysis between treatments, post hoc Dunnet.
Author Contributions
J.L.G-Q performed all experiments and writing manuscript. J.M.O-G design experiments and analysis the results. V.N.H-G design experiments. E.R-M, R.A.L., A.V-L reviewed manuscript. M.L.D-L was responsive for fundings, review and editing manuscript. All authors have agreed to the published final version of manuscript.
Funding
This work was supported in part by grants from Secretaría de Investigación y Posgrado-Instituto Politécnico Nacional 20210213, 20220577 y 20231529.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this work are available on request from the corresponding author.
Acknowledgments
J.L.G-Q: J.M.O-G and V.N.H-G were beneficiaries of scholarship of Consejo Nacional de Humanidades, Ciencias y Tecnologías (CONHACYT). E.R-M, R.A.L., A.V-L and M.L.D-L.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dhanyamraju P.K.; Patel T.N. Melanoma therapeutics: a literature review. J Biomed Res. 2022, 36, 77–97. [CrossRef]
- Patton E.E.; Mueller K.L.; Adams D.J.; Anandasabapathy N.; Aplin A.E. Melanoma models for the next generation of therapies. Cancer Cell. 2021, 39, 610–631. [CrossRef]
- Brancato V.; Oliveira J.M.; Correlo V.M.; Reis R.L.; Kundu S.C. Could 3D models of cancer enhance drug screening? Biomaterials, 232, 119744. [CrossRef]
- Sherman H.; Gitschier H.J.; Rossi A.E. A Novel Three-Dimensional Immune Oncology Model for High-Throughput Testing of Tumoricidal Activity. Front. Immunol. 2018, 9, 857. [CrossRef]
- Kapałczyńska M.; Kolenda T.; Przybyła W.; Zajączkowska M.; Teresiak A.; Filas V.; Ibbs M.; Bliźniak R.; Łuczewski L.; Lamperska K. 2D and 3D cell cultures – a comparison of different types of cancer cell cultures. Arch Med Sci. 2018, 14, 910–919. [CrossRef]
- Cave D.D; Rizzo R.; Sainz B., Jr.; Gigli G.; del Mercato L.L.; Lonardo E. The Revolutionary Roads to Study Cell–Cell Interactions in 3D In Vitro Pancreatic Cancer Models. Cancers, 2021, 13, 930. [CrossRef]
- Khandia R.; Dadar M.; Munjal A.; Dhama K.; Karthik K.; Tiwari R.; Yatoo M.I.; Iqbal H.M.N; Singh K.P.; Joshi S.K; Chaicumpa W. A Comprehensive Review of Autophagy and Its Various Roles in Infectious, Non-Infectious, and Lifestyle Diseases: Current Knowledge and Prospects for Disease Prevention, Novel Drug Design, and Therapy. Cells. 2019, 8, 674. [CrossRef]
- Brier L.W.; Ge L.; Stjepanovic G.; Thelen A.M.; Hurley J.H.; Schekman R. Regulation of LC3 Lipidation by the Autophagy-Specific Class III phosphatidylinositol-3 Kinase Complex. Mol Biol Cell., 2019, 30, 1098-1107. [CrossRef]
- Nath S.; Dancourt J.; Shteyn V.; Puente G.; Fong W.M.; Nag S.; Bewersdorf J.; Yamamoto A.; Antonny B.; Melia T.J. Lipidation of the LC3/GABARAP family of autophagy proteins relies upon a membrane curvature-sensing domain in Atg3. Nat Cell Biol., 2014, 16, 415–424. [CrossRef]
- Lin X.; Li S.; Zhao Y.; Ma X.; Zhang K.; He X.; Wang Z. Interaction domains of p62: a bridge between p62 and selective autophagy. DNA Cell Biol. 2013, 32, 220-227. [CrossRef]
- Pankiv S.; Clausen T.H.; Lamark T.; Brech A.; Bruun J.A.; Outzen H.; Øvervatn A.; Bjørkøy G.; Johansen T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J Biol Chem. 2007, 282, 24131-24145. [CrossRef]
- Yun C.W.; Lee S.H. The Roles of Autophagy in Cancer. Int J Mol Sci., 2018, 19(11), 3466. [CrossRef]
- Frampton J.E.; Brogden R.N. Pentoxifylline (Oxpentifylline) A Review of its Therapeutic Efficacy in the Management of Peripheral Vascular and Cerebrovascular Disorders. Drugs & Aging, 1995, 7, 480-503. [CrossRef]
- Golunski G.; Woziwodzka A.; Piosik J. Potential Use of Pentoxifylline in Cancer Therapy. Curr Pharm Biotechnol., 2018, 19, 206-216. [CrossRef]
- Pratibha D.; Yuvraj N.; Rajiv P.G. Pentoxifylline: A Potent Inhibitor of Angiogenesis via Blocking STAT3 Signaling in B16F10 Melanoma. Int. J. Tumor Ther., 2013, 2, 1-9. [CrossRef]
- Mohammad Z.K.; Rajiv P.G. Pentoxifylline Inhibits Melanoma Tumor Growth and Angiogenesis by Targeting STAT3 Signaling Pathway. Biomed Pharmacother, 2013, 67, 399-405. [CrossRef]
- Sharma K.; Ishaq M.; Sharma G.; Khan M.A.; Dutta R.K.; Majumdar S. Pentoxifylline triggers autophagy via ER stress response that interferes with Pentoxifylline induced apoptosis in human melanoma cells. Biochem Pharmacol., 2016, 103, 17-28. [CrossRef]
- Wang G.S. Medical uses of Mylabris in ancient China and recent studies. J Ethnopharmacol, 1989, 26, 147-162. [CrossRef]
- An W.W.; Wang M.W.; Tashiro S.; Onodera S.; Ikejima T. Norcantharidin induces human melanoma A375-S2 cell apoptosis through mitochondrial and caspase pathways. J Korean Med Sci., 2004, 19, 560–566. [CrossRef]
- Liu S.; Yu H.; Kumar S.M.; Martin J.S.; Bing Z.; Sheng W.; Bosenberg M.; Xu X. Norcantharidin induces melanoma cell apoptosis through activation of TR3 dependent pathway. Cancer Biol Ther., 2011, 12, 1005-1014. [CrossRef]
- Liu Z.; Li B.; Cao M.; Jiang J. Norcantharidin triggers apoptotic cell death in non-small cell lung cancer via a mitophagy-mediated autophagy pathway. Ann Transl Med., 2021, 9, 971. [CrossRef]
- Müller I.; Kulms D. A 3D Organotypic Melanoma Spheroid Skin Model. J Vis Exp. 2018, 135, 57500. [CrossRef]
- Ocampo-Godinez J.M.; Gonzalez-Quiroz J.L.; Cote-Palafox H.; George E.; Vergara-Lope Nuñez J.A.; Villagomez-Olea G.; Vazquez-Vazquez F.C.; Lopez-Villegas E.O.; Leon-Avila G.; Dominguez-Lopez M.L.; Alvarez-Perez M.A. Primary explants of the postnatal thymus allow the expansion of clonogenic thymic epithelial cells that constitute thymospheres. Stem Cell Res Ther., 2023 ,14, 312. [CrossRef]
- Nayak P.; Bentivoglio V.; Varani M.; Signore A. Three-Dimensional In Vitro Tumor Spheroid Models for Evaluation of Anticancer Therapy: Recent Updates. Cancers., 2023, 15, 4846. [CrossRef]
- Zhou J.; Ren Y.; Tan L.; Song X.; Wang M.; Li Y.; Cao Z.; Guo C. Norcantharidin: research advances in pharmaceutical activities and derivatives in recent years. Biomed Pharmacother. 2020, 131, 110755. [CrossRef]
- Xiao W.; Dai B.; Zhu Y.; Ye D. Norcantharidin induces autophagy-related prostate cancer cell death through Beclin-1 upregulation by miR-129-5p suppression. Tumour Biol., 2015. [CrossRef]
- Han Z.; Li B.; Wang J.; Zhang X.; Li Z.; Dai L.; Cao M.; Jiang J. Norcantharidin Inhibits SK-N-SH Neuroblastoma Cell Growth by Induction of Autophagy and Apoptosis. Technol Cancer Res Treat., 2017, 16, 33-44. [CrossRef]
- Xu L.; Su B.; Mo L.; Zhao C.; Zhao Z.; Li H.; Hu Z.; Li J. Norcantharidin Induces Immunogenic Cell Death of Bladder Cancer Cells through Promoting Autophagy in Acidic Culture. Int J Mol Sci., 2022, 23, 3944. [CrossRef]
- Pangilinan C.; Xu X.; Herlyn M.; Liang C. Autophagy Paradox: Strategizing Treatment Modality in Melanoma. Curr Treat Options Oncol., 2023, 2, 130-145. [CrossRef]
- Kumar A.V.; Mills J.; Lapierre L.R. Selective Autophagy Receptor p62/SQSTM1, a Pivotal Player in Stress and Aging. Front Cell Dev Biol., 2022, 10, 793328. [CrossRef]
- Li H.C.; Xia Z.H.; Chen Y.F.; Yang F.; Feng W.; Cai H.; Mei Y.; Jiang Y.M.; Xu K.; Feng D.X. Cantharidin Inhibits the Growth of Triple-Negative Breast Cancer Cells by Suppressing Autophagy and Inducing Apoptosis in Vitro and in Vivo. Cell Physiol Biochem., 2017, 43, 1829-1840. [CrossRef]
- Yun C.W.; Jeon J.; Go G.; Lee J.H.; Lee S.H. The Dual Role of Autophagy in Cancer Development and a Therapeutic Strategy for Cancer by Targeting Autophagy. Int. J. Mol. Sci., 2021, 22, 179. [CrossRef]
- Bingel C.; Koeneke E.; Ridinger. Three-dimensional tumor cell growth stimulates autophagic flux and recapitulates chemotherapy resistance. Cell Death Dis., 2017, 8, e3013. [CrossRef]
- Imamura Y.; Mukohara T.; Shimono Y.; Funakoshi Y.; Chayahara N.; Toyoda M.; Kiyota N.; Takao S.; Kono S.; Nakatsura T.; Minami H. Comparison of 2D- and 3D-culture models as drug-testing platforms in breast cancer. Oncol Rep., 2015, 33, 1837-1843. [CrossRef]
- Islam M.A.; Sooro M.A.; Zhang P. Autophagic Regulation of p62 is Critical for Cancer Therapy. Int J Mol Sci., 2018, 19, 1405. [CrossRef]
- Philipson E.; Engström C.; Naredi P.; Bourghardt Fagman J. High expression of p62/SQSTM1 predicts shorter survival for patients with pancreatic cancer. BMC Cancer. 2022, 22, 347. [CrossRef]
- Moscat J.; Karin M.; Diaz-Meco M.T. p62 in Cancer: Signaling Adaptor Beyond Autophagy. Cell., 2016, 167, 606-609. [CrossRef]
- Tang D.Y.; Ellis R.A.; Lovat P.E. Prognostic Impact of Autophagy Biomarkers for Cutaneous Melanoma. Front Oncol., 2016, 6, 236. [CrossRef]
- Ward A.; Clissold S.P. Pentoxifylline. A review of its pharmacodynamic and pharmacokinetic properties, and its therapeutic efficacy. Drugs. 1987, 34, 50-97. [CrossRef]
- Dua P.; Gude R.P. Antiproliferative and antiproteolytic activity of pentoxifylline in cultures of B16F10 melanoma cells. Cancer Chemother Pharmacol., 2006, 58, 195-202. [CrossRef]
- Kamran M.Z.; Gude R.P. Preclinical evaluation of the antimetastatic efficacy of Pentoxifylline on A375 human melanoma cell line. Biomed Pharmacother. 2012, 66, 617-26. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).