Submitted:
15 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Factors Affecting the Stability of Hypochlorous Acid
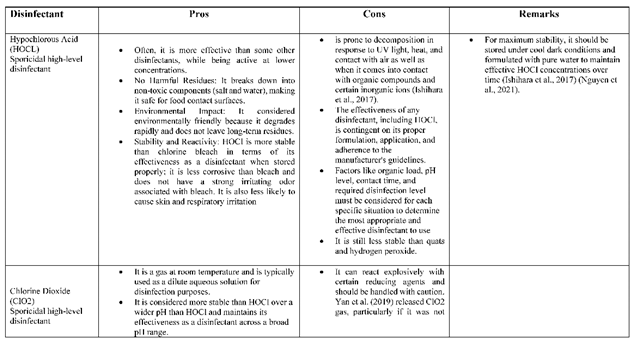
- pH Sensitivity: HOCl is the most effective disinfectant when it is in its undissociated form (HOCl). At lower pH levels (more acidic), HOCl can rapidly dissociate into hypochlorite ions (OCl-), which have reduced antimicrobial activity, whereas at lower pH, it can release chlorine gas, which is hazardous. (Ishihara et al., 2017)
- Temperature: Higher temperatures (≥ 25 °C) can accelerate the degradation of HOCl. The rate of degradation increases as the temperature increases further, causing HOCl to break down into hypochlorite ions and lose its antimicrobial effectiveness.
- Light: Exposure to sunlight or UV radiation can break down HOCl molecules, thereby reducing their disinfecting power. When HOCl absorbs light energy, it can undergo photolysis, leading to the formation of chloride and hypochlorite ions.
- Time: HOCl has a limited shelf life, and its stability decreases over time. Gradually, HOCl molecules degrade into chloride and hypochlorite ions, reducing their ability to effectively disinfect.
- Organic matter: When HOCl reacts with organic substances such as proteins and other biological materials, it can form chloramines. Chloramines have reduced antimicrobial activity compared to HOCl. (Committee, 2001)
- Metal ions: Some metal ions, such as copper, can catalyze the decomposition of HOCl into chloride and hypochlorite ions, reducing its effectiveness as a disinfectant. (Block & Rowan, 2020).(Committee, 2001)
- Generation and Delivery: The generation of hypochlorous acid as a disinfectant can be complicated and requires electrolysis of saltwater or specialized devices. Appropriate equipment and procedures must be performed to ensure the stability and effectiveness of the hypochlorous acid product.
Current Research Findings on HOCL Stability Issues
The Consequences of Instability of HOCl as a Disinfectant
- Reduced disinfectant efficacy HOCl is known for its powerful broad-spectrum antimicrobial properties. However, an unstable and less effective HOCl may require higher concentrations or longer contact times to achieve the same level of germicidal activity.(Ishihara et al., 2017).
- Shorter shelf life: Stability issues can lead to a shorter shelf life for HOCl-based products. The degradation of HOCl in solution reduces the concentration of active HOCl available for disinfection, thus shortening the shelf life of the product. This can be problematic in industries or applications in which long-term storage or infrequent use is required.
- Potential health risks: The use of degraded products containing reduced HOCl concentration or hypochlorite ions may have potential health risks.(Block & Rowan, 2020) Hypochlorite ions are less selective in their antimicrobial action compared to HOCl, and they can also interact with organic matter present on surfaces or in the environment to form potentially harmful byproducts.(Nguyen et al., 2021) These byproducts include chloramines and chlorinated organic compounds, which can be irritating to the skin, eyes, and respiratory system. (Office et al., 2004) In some cases, they can also be toxic or carcinogenic.
Challenges Faced and Current Efforts to Enhance the Stability of Hypochlorous Acid as Disinfectant
The Current Challenges
- Inconsistent disinfection efficacy: Instability of HOCl can lead to inconsistent disinfection efficacy. As HOCl degrades into less-effective hypochlorite ions (OCl-), the concentration of active disinfectants decreases. This can result in variable germicidal activity, which requires higher concentrations or longer contact times to achieve the desired level of disinfection. This inconsistent efficacy poses a significant challenge for ensuring proper sanitization and preventing the spread of infections.
- Increased costs: Reduced disinfectant efficacy due to stability issues can increase costs for organizations. Higher concentrations of HOCl may be required to compensate for degradation and achieve the desired level of disinfection. This can lead to increased consumption of HOCl and additional expenses associated with the procurement, storage, and application of disinfectants. The need for larger quantities and increased frequency of applications can affect budgets and potentially strain resources.
- Shortened shelf life: Stability issues can shorten the shelf life of HOCl-based disinfectants, resulting in a higher risk of expired or ineffective disinfectants within the organization. This challenge can disrupt supply chains, require more frequent reordering, and increase inventory management complexity.
- Regulatory compliance: Industries and organizations that utilize HOCl as a disinfectant must comply with the regulatory guidelines and standards for efficacy and safety. Stability issues that compromise the efficiency of disinfectants or generate potentially harmful degradation byproducts can pose challenges in meeting regulatory and accreditation requirements.
- Reputation and customer trust: Stability issues with HOCl-based disinfectants can affect an organization's reputation and customer trust. Businesses in industries such as healthcare, hospitality, and food services rely heavily on effective disinfection to maintain a safe environment for both staff and visitors. Any resultant instability can result in inconsistent disinfection or inferior quality of products, which can erode customer confidence and lead to reputational damage.
Ongoing Research and Developments on Stability of HOCl
- Formulation optimization, including pH adjustment and the addition of buffering agents, can improve stability and shelf life. Researchers are currently investigating different formulations and techniques that can enhance the stability of HOCl. This includes the study of the effects of pH, temperature, and other factors on HOCl stability. By perfecting the formulation, researchers have aimed to minimize the degradation of HOCl into less effective hypochlorite ions and extend its shelf life. (Khalid et al., 2021) (McBain AJ, et al. 2003)
- Stabilizing agents: Adding stabilizers such as inorganic salts or phosphates can help minimize the decomposition of HOCl(Nguyen et al., 2021). Research is ongoing to identify suitable stabilizing agents to enhance the stability of HOCl. Some studies have explored the use of additives such as buffering agents, chelating agents, and surfactants to stabilize HOCl solutions.(Nguyen et al., 2021), (Fox LJ, et al, 1992)
- Alternative delivery systems: Researchers are exploring alternative delivery systems, including the use of encapsulation techniques that involve enclosing HOCl in micro- or nanosized particles to protect them from degradation and interactions with the environment. These encapsulated systems can provide controlled release of HOCl, maximizing its stability and efficacy. See, for example, Doganay and Doganay H. (2019)
- Storage and handling conditions: Researchers are currently studying the impact of storage and handling conditions on the stability of HOCl. Factors such as light exposure, humidity, and packaging materials can affect the stability of the HOCl solutions. By this, researchers aim to develop recommended storage and handling guidelines to maintain the stability of HOCl.(Ishihara et al., 2017) (Wang et al., 2007).
- Synergistic combinations: Several studies have explored the use of synergistic combinations of different stabilizing agents or techniques to enhance the stability of HOCl. For example, combining pH stabilization with encapsulation, or using a combination of ROS scavengers and cross-linking agents. For example, Kim, J. H., Moon, J. H., Cheong, K. C. (2016), Shi, B., Zhang, D., Tsui, T.H., & Chen, R. (2018), Yang, C., Li, H., Wei, H., & Zhang, Y. (2014), Oh, J.C., Kim, Y., & Oh, S.G. (2018), Lee, J.H., Kim, H.K., Park, S.H., & Kang, M.H. (2014)
- Analytical methods: Regular monitoring of the pH, concentration, and other relevant parameters of the HOCl solution can help identify any changes in stability and take necessary actions to maintain its effectiveness.(Ishihara et al., 2017). The development of robust analytical methods for assessing the stability of HOCl is an ongoing research effort. Researchers are developing rapid and accurate analytical techniques to monitor the degradation of HOCl over time. These methods can help understand the kinetics of HOCl degradation and evaluate the efficacy of stabilizing agents and formulation optimization strategies. See, for example, Lee, J., Lee, Y., and Kim, H. (2017), Abu-Omar, M.M., Abu-Rumeileh, R.G., & Kodeh, F.S. (2019), Martins, R.C., Miquelini, M., Neves, A.A.R., Moreira, N.C., 2019. (2017), Buettner, K., & Lackmann, J.-W. (2012), and
- Anode materials and electrode modifications: Researchers are currently investigating various anode materials and modifications to improve the stability of HOCl during electrolysis. For example, some studies have explored the use of electrodes coated with metal oxides or alloys to enhance stability and minimize the degradation of HOCl. For instance, (Chen et al. (2013) (Zhang et al. 2015)
Current Manufacturing Techniques and Formulations to Enhance its Stability
- Acidic electrolyzed water (AEW) generators: AEW generators produce HOCl via a process called electrolysis. The generators created a stable HOCl solution using salt, water, and electricity. By generating HOCl on demand, these generators can provide a fresh and stable solution with an extended shelf life.
- Optimizing electrolysis conditions, such as current density and pH control, can affect the stability of HOCl and extend its shelf life.
- Controlled-release systems: Researchers have developed controlled-release systems that can release HOCl slowly over a specified period. These systems typically involve encapsulation of HOCl in polymers or nanostructures. Controlled release of HOCl can prevent its degradation and maintain its stability for an extended period.
- Reactive oxygen species (ROS) scavengers: Reactive oxygen species, such as superoxide anions, can degrade HOCl. The addition of scavengers that neutralize ROS can help stabilize HOCl and prevent its degradation. Examples of ROS scavengers include antioxidants such as ascorbic acid, tocopherols, and thioctic acid. See for example, (Eryilmaz & Palabiyik, 2013; Geir Hermod Almås, 2018; Issa-Zacharia et al., 2011; Li et al., 2023; Zhang et al., 2021)
- Cross-linking agents: Cross-linking agents enhance the stability of HOCl by forming chemical bonds that protect it from degradation. One example is the use of polyvinylpyrrolidone (PVP) as a crosslinking agent to stabilize HOCl solutions.
Summary, Conclusion and Recommendations
Summary
Conclusion
Recommendations to End-Users on Maintaining the Stability of HOCl
- pH Control: Maintain the pH of the HOCl solution around neutral (pH 7), where HOCl is most stable, to ensure maximum efficacy. (Ishihara et al., 2017)
- Proper Storage: Store HOCl in dark, cool environments, away from sunlight, and heat to prevent decomposition. Use containers that do not promote breakdown and ensure that they are labeled and sealed correctly.(Block & Rowan, 2020)
- Minimizing Exposure to Contaminants: Prevent the solution from meeting metals or organic materials that could accelerate degradation or reduce disinfecting power. (Mayer & Ryan, 2017)
- Monitor concentration: The concentration of HOCL is regularly checked and adjusted to ensure that it maintains the appropriate available chlorine levels and disinfecting properties, avoiding concentrations that are too low to be effective or so high that they can damage surfaces or equipment. (Nguyen et al., 2021)
- Water Quality: Use water that is as free from organic contaminants and impurities as possible to reduce the formation of harmful disinfection by-products and maintain available chlorine levels for disinfection.(Nguyen et al., 2021)
- Safe Handling Procedures: Follow safe handling procedures, including wearing appropriate personal protective equipment, to protect against potential hazards.
- Adhere to industry-specific guidelines and manufacturer recommendations for the use of HOCL, considering the type of application and required disinfection strength.
- Use As Intended: Apply HOCL only as directed for the specific disinfection tasks intended to maximize its benefits and reduce wastage or potential hazards. (Nguyen et al., 2021)
- End users should carefully assess their usage and consider alternative disinfection methods that may offer similar or better efficacy without the associated risks.
- Monitoring disinfection by-products and vigilance in managing microbial resistance are essential to ensure the safe and effective use of HOCL as a disinfectant.
Recommendations for Further Research and Development
- Understanding the degradation pathways of HOCl,
- Optimizing its formulation and pH levels,
- Studying its compatibility with materials,
- Assessment of the potential for microbial resistance to HOCl and development of strategies to mitigate this risk.
- Customizing application research focused on developing specialized formulations tailored to specific applications, such as wound care, food sanitation, or water treatment, that can maintain stability under conditions unique to those environments, and
- Update and refine regulatory guidelines and standards for the production, storage, and application of HOCl to ensure stable and effective use across industries.
The Significance of This Study, Its Novelty and Limitations
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflict of interest
Author Contributions
References
- Asghar, A., Raman, A. A., Daud, W M A. W. (2015, January 5, 2015). Advanced oxidation processes for in-situ production of hydrogen peroxide/hydroxyl radical for textile wastewater treatment: A review. https://www.sciencedirect.com/science/article/pii/S0959652614009408.
- Block, M S., & Rowan, B G. (2020, September 1). Hypochlorous Acid: A Review. [CrossRef]
- Boecker, D., Zhang, Z., Breves, R., Herth, F J., Kramer, A., & Bulitta, C. (2023, January 1, 2023). Antimicrobial efficacy, mode of action and in vivo use of hypochlorous acid (HOCl) for prevention or therapeutic support of infections. https://pubmed.ncbi.nlm.nih.gov/37034111.
- Committee, N R C S D W. (2001, January 1, 2001). The Chemistry of Disinfectants in Water: Reactions and Products. https://www.ncbi.nlm.nih.gov/books/NBK234591/#ddd00180.
- Eryilmaz, M. and Palabiyik, I. M. (2013). Hypochlorous acid: analytical methods and antimicrobial activity. In Tropical Journal of Pharmaceutical Research (Vol. 12, Issue 1). [CrossRef]
- Geir Hermod Almås. (2018). Preparation for controlled release of hypochlorous acid (Patent US20180117081A1). US Patent.
- Gustin, J. (2005, January 1, 2005). Safety of chlorine production and chlorination processes.
- Issa-Zacharia, A., Kamitani, Y., Miwa, N., Muhimbula, H., & Iwasaki, K. (2011). Application of slightly acidic electrolyzed water as a potential non-thermal food sanitizer for the decontamination of fresh ready-to-eat vegetables and sprouts. Food Control, 22(3–4), 601–607. [CrossRef]
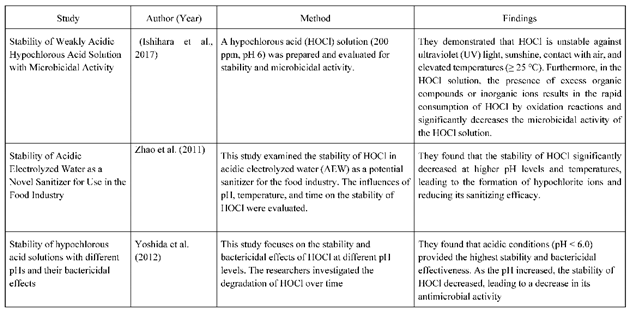
- Ishihara, M., Murakami, K., Fukuda, K., Nakamura, S., Kuwabara, M., Hattori, H., Fujita, M., Kiyosawa, T. and Yokoe, H. (2017, January 1, 2017). Stability of Weakly Acidic Hypochlorous Acid Solution with Microbicidal Activity. [CrossRef]
- Jeffcoat, S B. (2011, February 1). On-site Sodium Hypochlorite Generation Reduces Storage and Risks. [CrossRef]
- Khalid, N., Sulaiman, N., Aziz, N A., Taip, F S., Sobri, S., M.A.R., N. (2021, January 3, 2021). Stability of electrolyzed water: from the perspective of food industry. [CrossRef]
- Kuwertz, R., Martinez, I G., Vidaković-Koch, T., Sundmacher, K., Turek, T., & Kunz, U. (2013, September 1). Energy-efficient chlorine production by gas-phase HCl electrolysis using an oxygen-depolarized cathode. Electrochemistry communications, 34, 320-322. [CrossRef]
- Lee, S L., O’Connor, T., Yang, X., Cruz, C N., Chatterjee, S., Madurawe, R D., Moore, C., Yu, L X., & Woodcock, J. (2015, March 19). Modernizing Pharmaceutical Manufacturing: from Batch to Continuous Production. Journal of pharmaceutical innovation, 10(3), 191-199. [CrossRef]
- 14. Li, Z., Wang, X., Li, X., Guo, S., Li, S., Chen, B., Cheng, Y., Xu, H., & Yan, W. (2023). Optimization of electrolysis process, storage conditions, and sterilization effect of slightly acidic electrolytic water prepared using a titanium suboxide electrode. Journal of Environmental Chemical Engineering, 11(3), 109679. [CrossRef]
- Mayer, B K., Ryan, D R. (2017, January 1, 2017).017). Impact of advanced oxidation processes on disinfection byproducts for drinking water treatment. The handbook of environmental chemistry, 345-386. [CrossRef]
- McDonnell, G., & Russell, A D. (1999, January 1). Antiseptics and Disinfectants: Activity, Action, and Resistance. [CrossRef]
- Nguyen, K., Bui, D., Hashemi, M., Hocking, D M., Mendis, P., Strugnell, R A., Dharmage, S C. (2021, January 1, 2021). The Potential Use of Hypochlorous Acid and a Smart Prefabricated Sanitizing Chamber to Reduce Occupation-Related COVID-19 Exposure. [CrossRef]
- Nguyen, K., Bui, D., Hashemi, M., Hocking, D M., Mendis, P., Strugnell, R A., Dharmage, S C. (2021, January 1, 2021). The Potential Use of Hypochlorous Acid and a Smart Prefabricated Sanitizing Chamber to Reduce Occupation-Related COVID-19 Exposure. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7837568/.
- Office, N., Restoration., & GOV, U. (2004, January 1). Reactivity Documentation. https://cameochemicals.noaa.gov/reactivity/documentation/RG44-RG1.
- Omidbakhsh, N., & Sattar, S A. (2006, June 1). Broad-spectrum microbicidal activity, toxicological assessment, and material compatibility of a new generation of accelerated hydrogen peroxide-based environmental surface disinfectants. American journal of infection control, 34(5), 251-257. [CrossRef]
- Overholt, B., Reynolds, K., & Wheeler, D. (2018, November 25). A Safer, More Effective Method for Cleaning and Disinfecting GI Endoscopic Procedure Rooms. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6255518/.
- Park, G W., Boston, D M., Kase, J A., Sampson, M N., & Sobsey, M D. (2007, July 15). Evaluation of Liquid- and Fog-Based Applications of Sterilox Hypochlorous Acid Solution for Surface Inactivation of Human Norovirus. Applied and environmental microbiology, 73(14), 4463-4468. [CrossRef]
- Sudrajat, A., Handayani, E M., Tamaldin, N., & Yamin, A K M. (2018, January 1). Principle of generator HHO hybrid multistack-type production technologies to increase HHO gas volume. SHS web of conferences, 49, 02016-02016. [CrossRef]
- Wang, L., Bassiri, M., Najafi, R., Najafi, K H B R M A N R., Yang, J., Khosrovi, B., Hwong, W., Barati, E., Belisle, B., Celeri, C., & Mc, R. (2007, April 11). Hypochlorous acid as a potential wound care agent: part I. Stabilized hypochlorous acid: a component of the inorganic armamentarium of innate immunity.
- Why does Hype affect chlorine gas safety? (2023, January 1, 2023). https://www.wateronline.com/doc/why-all-the-hype-about-chlorine-gas-safety-0001.
- Zhang, C., Wang, X., Du, J., Gu, Z., & Zhao, Y. (2021). Reactive Oxygen Species-Regulating Strategies Based on Nanomaterials for Disease Treatment. Advanced Science, 8(3). [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).




