1. Introduction
Alzheimer's disease (AD) presents a formidable challenge in terms of diagnosis and treatment, spurring intensified research efforts. The apelin system has recently garnered attention for its potential involvement in AD pathogenesis, as evidenced by an expanding body of literature [
1,
2].
Apelin, a biologically active peptide hormone, originates from fat cells [
3]. It exists in three active forms, comprising 13, 17, or 36 amino acids, derived from a 77-amino acid prepropeptide precursor by angiotensin-converting enzyme [
4,
5]. Notably, apelin-13 demonstrates significantly greater biological potency than apelin-36 [
4]. Moreover, apelin exhibits neuroprotective properties by mitigating oxidative damage in neurons. In vivo experiments have shown that apelin’s ability to combat reactive oxygen species (ROS) and free radicals is closely associated with its neuroprotective effects against neurodegenerative diseases [
6].
Apelin-13 and its receptor APJ are widely distributed in the hippocampus and other brain tissues. Intracerebroventricular injection of Apelin-13 improved stress-induced memory function decline in rats [
7], indicating that apelin/APJ signaling may be involved in cognitive ability [
8,
9]. Moreover, apelin has been implicated in enhancing the function of various factors, including GLP-1, eNO, and ACE2, thereby promoting synaptic plasticity and improving learning and memory. Additionally, apelin exerts a neuroprotective effect by attenuating inflammatory responses through the BDNF signaling pathway [
10]. Furthermore, apelin can modulate amyloid-beta (Aβ) metabolism by reducing amyloid precursor protein (APP) levels and inhibiting β-secretase activity, leading to decreased Aβ production and increased Aβ clearance mediated by ABCA1 and NEP. Additionally, apelin may mitigate tau protein phosphorylation and accumulation. Studies have reported that apelin can prevent neurodegeneration by reducing levels of inflammatory mediators, particularly TNF-α and IL-1β, and inhibiting neuronal apoptosis by modulating the balance of anti-apoptotic and pro-apoptotic factors [
11]. These findings underscore the multifaceted neuroprotective effects of apelin, suggesting its potential as a therapeutic target in Alzheimer's disease intervention.
While oxidative stress is recognized as a crucial mechanism underlying Apelin-13's anti-Alzheimer's disease (AD) effects, the specific oxidative stress pathway through which apelin mitigates AD-related neurological damage requires further investigation. To address this gap, we examined whether intranasal administration of Apelin-13 could ameliorate cognitive impairment in a streptozocin (STZ)-induced animal model of AD mice.
Employing a range of technical methodologies, we investigated the anti-oxidative damage effect and the mechanism of action of Apelin-13 in this STZ-induced AD mouse model. Our study aims to elucidate the precise molecular pathways through which Apelin-13 exerts its neuroprotective effects against AD-associated oxidative stress. By unraveling these mechanisms, we aim to provide valuable insights into the therapeutic potential of Apelin-13 in AD treatment.
2. Materials and Methods
Experimental Animals
Male adult (8-12 weeks) C57BL/6J mice were housed under a 12 h light/dark cycle in a temperature- and humidity-controlled environment with free access to food and water. All experimental procedures conformed to the Fudan University and International Guidelines on the Ethical Use of Animals. All efforts were made to minimize animal suffering and to reduce the number of animals used.
Drug Administration
For nasal administration, the Apelin-13 (0.2 mg/kg, 1 mg/kg) solution (10 μl) was pipetted bilaterally on the rhinarium, i.e., the glabrous skin around the nostrils, and allowed to diffuse in the squamous epithelium. After the drip, the mouse was fixed for 5-10 seconds to ensure the liquid was fully inhaled. ML385 (Medchem Express, USA) is a specific inhibitor of Nrf2. Mice were injected intraperitoneally with 30 mg/kg ML385 [
12] (dis-solved in saline containing 50% PEG300) 30 min before each administration of the Apelin-13. Zn(II)-protoporphyrin IX (ZnPP, Medchem Express, USA) is an inhibitor of HO-1. Mice were injected intraperitoneally with 25 mg/kg ZnPP [
13] (dis-solved in saline) 30 min before each administration of the Apelin-13.
Stereotaxic Surgery
Mice were anesthetized using a mixture of ketamine and xylazine. Mice were then restrained onto a stereotaxic apparatus (Stoelting Apparatus), and STZ (3 mg/kg, ICV) was injected bilaterally into the lateral ventricles to generate an STZ-AD mouse model. Control mice (Ctl) were subjected to the same procedure but with vehicle injection (citrate buffer 0.05 mol/L, pH 4.5). STZ was diluted in citrate buffer immediately before injection. The injection of STZ was performed using a Hamilton syringe (model 705), and the coordinates used from the bregma were AP −0.5 mm, ML ±1.1 mm, and DV −2.8 mm. Each lateral ventricle received a total infusion of 1.5 μL of STZ or citrate buffer at a rate of 0.5 μL/min.
Morris Water Maze Test
The Morris water maze (MWM) test was performed as previously described [
14]. Eight male mice were used for each group. During the training period, the platform was located in the same position (one of the four pool quadrants), the mouse was placed into the pool facing the wall at one of the four starting positions, and its movement was tracked using a digital tracking system. The animal was immediately removed from the water when it reached the platform. If the mouse could not locate the platform after 60 s of swimming, it was gently guided to the platform or placed on it for an additional 15 s before being removed from the pool. The animals were tested in four daily trials with an inter-trial interval of approximately 30 min. The mice were trained for five days. A probe trial was performed on the sixth day after the final training session. During the probe trial, the platform was removed from the pool, and the mouse was placed in the pool facing the wall from the diagonally opposite side of the platform. The mouse was allowed to swim freely for 2 min with digital movement tracking recorded by computer software (EthoVision software) before being withdrawn from the pool.
Y Maze Test
The Y-maze was made of opaque and non-reflective materials. During the test, the mouse was placed at the end of one of the arms with its head facing the central area, two significant markers (cues) were set opposite to each other around the device, and the mouse was allowed to explore freely in the maze for 8 min. The Anymaze video analysis software The movement trajectory of mice was recorded by a camera located directly above the device. If all four limbs of the mouse stepped into a certain arm, the mouse was judged to have entered the arm. The three arms are denoted a, b, and c, respectively. If the mouse entered three different arms in turn (regardless of the order), it was regarded as having completed spontaneous replacement behavior (spontaneous alternation behavior). The correct rate of spontaneous replacement was calculated by subtracting 2 from the number of completed replacements and the total number of arm entries and then multiplying by 100. After each mouse finished the test, the inner surface of the maze was wiped with 75% alcohol to eliminate the smell before proceeding to the next test.
In Vitro Electrophysiology
Electrophysiology was performed as described previously [
15]. Brains were dissected quickly and placed in the artificial cerebrospinal fluid (ACSF) containing 125 mM NaCl, 2.5 mM KCl, 2 mM CaCl
2, 1 mM MgCl
2, 25 mM NaHCO
3, 1.25 mM NaH
2PO
4, 10 mM Glucose and saturated with 95% O
2 and 5% CO
2 at ~0°C. Coronal brain slices (300 μm thick) were prepared with a vibratome and transferred to a chamber at 31°C. The slices were incubated for at least 1 h before patch-clamp recording. Neurons were targeted for whole-cell patch-clamp recording with glass electrodes having a 5–8 MΩ resistance when filled with the patch pipette solution. The electrode internal solution was composed of 115 mM CsMeSO
3, 10 mM HEPES, 2.5 mM MgCl
2, 20 mM CsCl
2, 0.6 mM EGTA, 10 mM Na phosphocreatine, 0.4 mM Na-GTP and 4 mM Mg-ATP. Three male mice were used in each group for LTP recording.EPSCs were recorded in CA1 neurons, while a concentrated stimulating electrode was placed in the Schaffer collaterals. LTP was induced in the 3X theta burst stimulation protocol (TBS; four pulses at 100 Hz repeated with 200 ms inter-burst intervals). The average EPSC amplitudes 30 min after LTP induction in each group were compared to determine whether the magnitude of LTP differed significantly among the groups. Data were acquired and analyzed using pClamp10.7 and Clampfit 10.7 (Axon Axopatch 700B, Molecular Devices, US).
The Detection of Oxidative Stress Levels
Each group collected Hippocampal tissues for cell disruption using a cell sonicator (Huxi, China). Then, centrifuge at 3500 r/min for 10 min. Supernatants from groups C, L, M, and H were analyzed to measure SOD, MDA, GSH-Px, and CAT levels. The SOD (A001-1-2), MDA (A00-1-2), CAT (A007-1-1), and GSH-Px (A005-1-2) test kits were purchased from the Nanjing Jiancheng Biological Research Institute, China. The SOD, CAT, MDA, and GSH-Px kits were detected using Coomassie brilliant blue, hydroxylamine, ammonium molybdate, thiobarbituric acid, and colorimetric methods, respectively.
Western Blot Analysis
Following treatment, the tissues were washed and collected in PBS. After centrifugation, cell lysis was carried out at 4°C by vigorous shaking for 15 min in RIPA buffer (150 mM NaCl, 1% NP-40, 0.5% sodiumdeoxycholate, 0.1%SDS, 50 mMTris–HCl (pH7.4), 50 mM β-glycerol phosphate, 20 mM NaF, 20 mM EGTA, 1 mM DTT, 1 mM Na3VO4, and protease inhibitors). After centrifugation at 15,000 rpm for 15 min, the supernatant was separated and stored at −70 °C until use. Protein concentration was determined using the Bradford method. The lysates were boiled for 5 min. Denatured proteins were separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 8% or 10% polyacrylamide gels and then transferred onto PVDF membranes (Millipore, Billerica, MA, USA). After blocking overnight at 4°C in 5% BSA in Tris-buffered saline/Tween [with 0.05% (v/v) Tween 20], the membranes were first incubated with each antibody at dilutions of 1:2000 [phospho-ERK1/2 (Thr202/Tyr204), ERK, anti-Nrf2 and anti-HO-1]. A second incubation was performed using a horseradish peroxidase-conjugated secondary anti-rabbit IgG antibody. The blots were developed using the ECL western blotting detection reagent (Santa Cruz Biotechnology).
Statistical Analysis
Numerical data were expressed as mean ± SEM. Offline data analysis was performed using Clampfit software (Axon Instruments, USA) and GraphPad Prism 6 (GraphPad Software, USA). Statistical significance was determined by ANOVA followed by Bonferroni post-tests for multiple comparisons among more than two groups. In the electrophysiological studies, n refers to the number of cells. Each cell group in each experiment was obtained from at least four animals. Statistical significance was set at p < 0.05. significant.
3. Results
Intranasal Administration of Apelin-13 Improves Cognitive Impairment in STZ-Induced Animal Model of AD Mice
Morris water maze and Y maze tests were conducted after 30 days of intranasal apelin-13 treatment in an STZ-induced animal model of AD to determine whether intranasal administration of Apelin-13 improves spatial learning and memory. We designed parallel positive drug control experiments using donepezil as an effective positive control. First, we examined the open-field behavior of the different treatment groups, and the experimental results showed that there was no difference in the total movement distance of the different drug treatment groups within the 15-minute test time (
Figure 1B, n =7-10, one-way ANOVNs, P > 0.05). There was no difference between the different treatment groups in movement time or proportion of edge movement (
Figure 1C, D, n = 7-10, one-way ANOVN, P > 0.05). Trace records (
Figure 1E) showed that the swimming trajectory of the STZ-induced animal model of AD mice in the target quadrant was shorter than that of control mice and Apelin-13 treated mice. There was no significant difference in swimming speed between the groups (
Figure 1F). The STZ-induced animal model of AD required more time to find the target quadrant and had fewer platform crossings. Furthermore, both parameters significantly improved after Apelin-13 treatment in the STZ-induced animal model of AD (
Figure 1G, H, n = 7-10, one-way ANOVNs, P < 0.05).
In the Y-maze test (
Figure 1I), there was no significant difference in the total number of entries between the different groups (
Figure 1J, n = 7-10, one-way ANOVA, P > 0.05). No significant difference in the alternation ratio between the control and high Apelin-13 treatment group existed. However, the alternation rate of the high Apelin-13 treatment group was higher than that of the STZ-induced AD mouse model (
Figure 1K, n = 7-10, one-way ANOVA, P < 0.05). In summary, our data indicates that the intranasal administration of Apelin-13 effectively improves cognitive impairment in an STZ-induced animal model of AD.
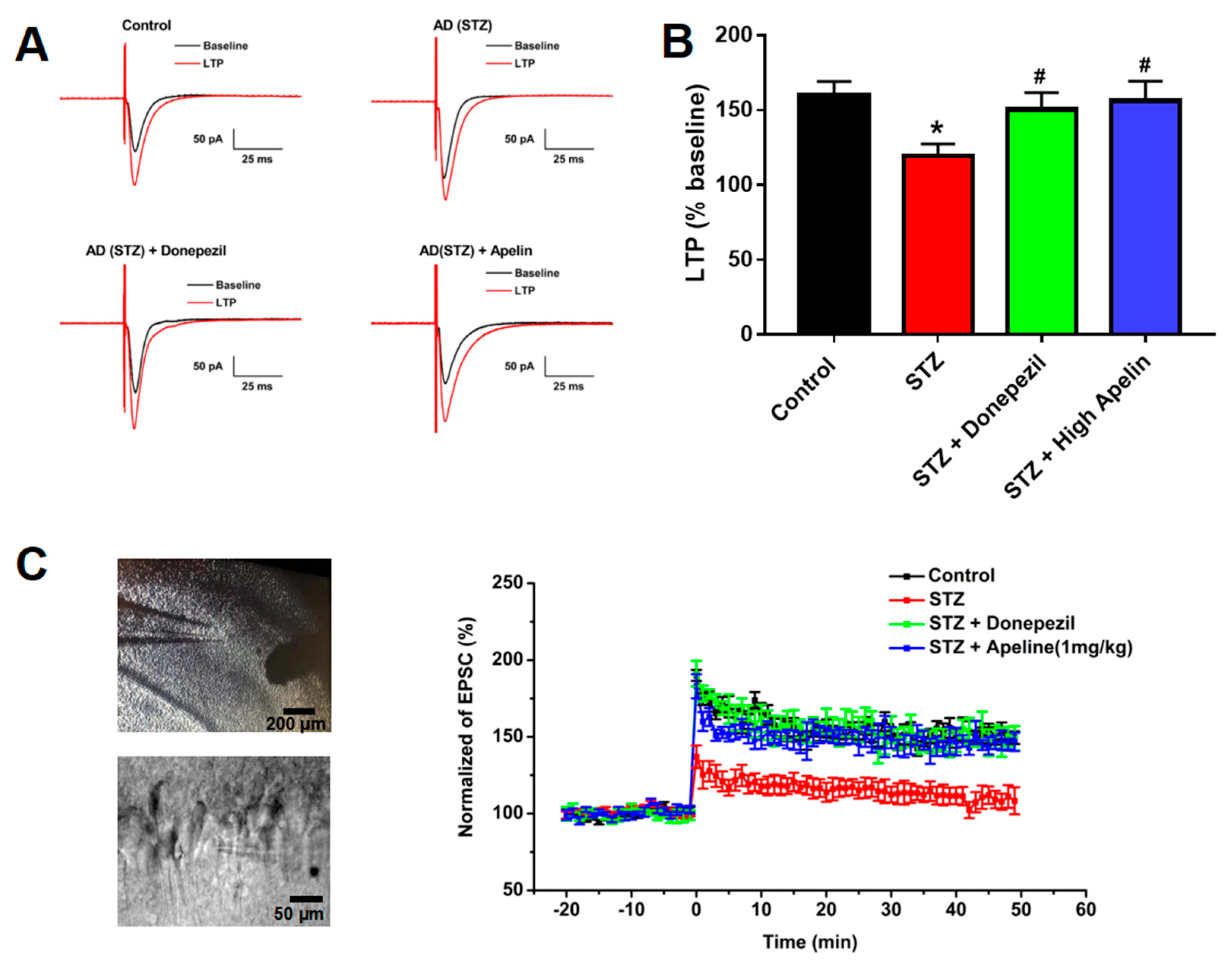
Intranasal Administration of Apelin-13 Restores LTP in CA1 Neurons of STZ-Induced Animal Model of AD Mice
We measured evoked EPSC and LTP in hippocampal slices to investigate the effect of intranasal Apelin-13 administration on synaptic plasticity in the CA1 region of the hippocampus.
Figure 2A shows representative EPSC traces before and after TBS stimulation in the four groups of mice used in this study. The ratio of LTP was significantly reduced in the STZ-induced animal model of AD compared to that in control mice. The depression of LTP 30 min after TBS in the STZ-induced animal model of AD treated with Apelin-13 confirmed that Apelin-13 significantly enhances the magnitude of LTP (
Figure 2B,C, n =8, one-way ANOVA, P < 0.05). These data confirm that intranasal administration of Apelin-13 can rescue impaired LTP in APP/PS1 mice.
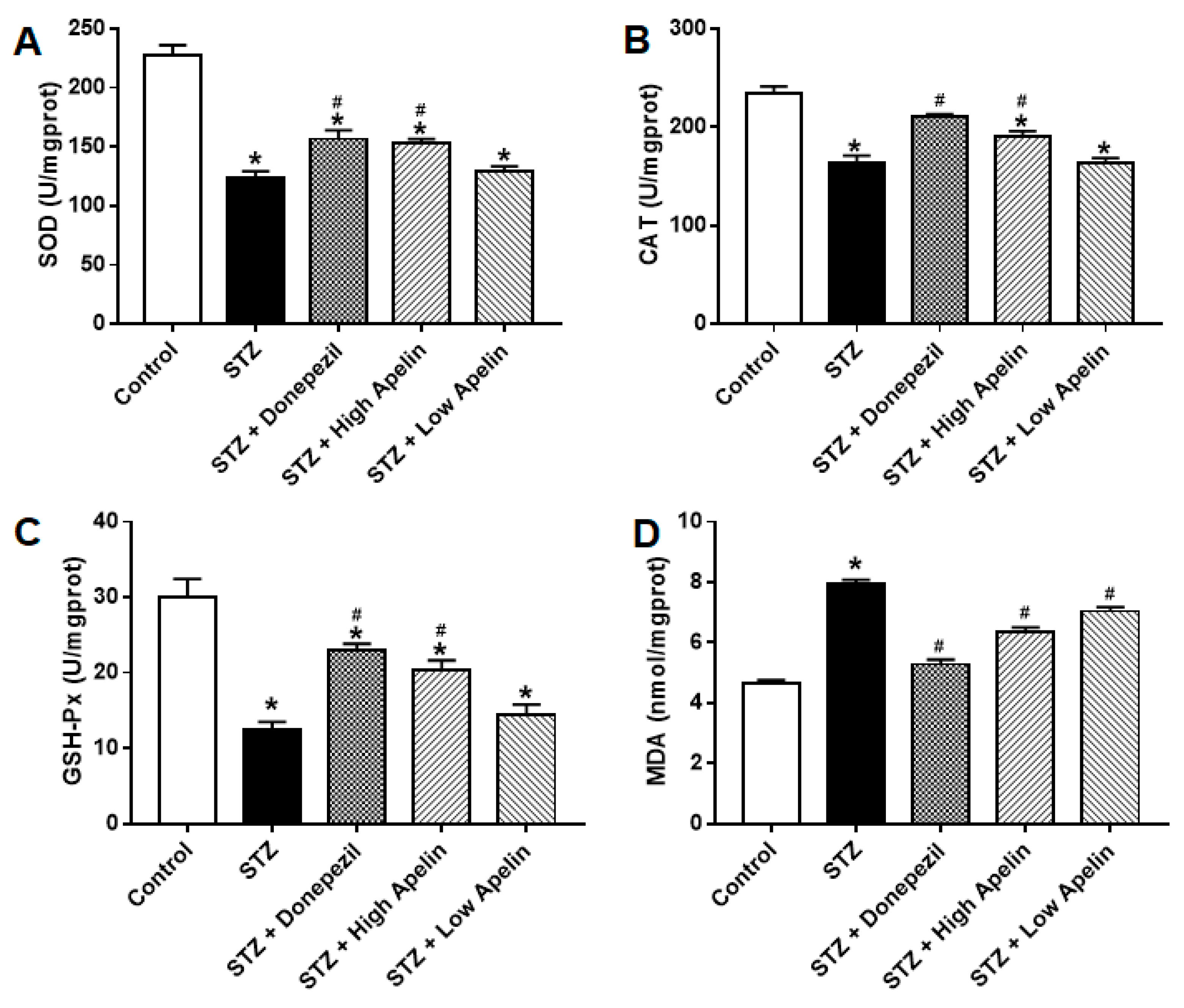
Intranasal Administration of Apelin-13 Reduces the Oxidative Stress of the Hippocampal in STZ-Induced Animal Models of AD Mice
Many in vivo experiments have shown that Apelin-13 can improve oxidative stress-related indicators in animal models of AD [
10]. Therefore, we used biochemical methods to observe the performance of a series of oxidative stress-related systems in the different treatment groups. After the behavioral tests in each group, we measured the activity of superoxide dismutase (SOD), glutathione peroxidase (GSH), catalase (CAT), and malondialdehyde (MDA) in the hippocampal tissue. The results showed that the activities of SOD, GSH, and CAT in the STZ-induced AD mouse model were significantly lower than those in the control group, and the activity of MDA was increased, which was consistent with previous reports. Donepezil treatment improved these oxidative stress indicators in an animal model of STZ-induced AD. High and low doses of Apelin-13 also improved these oxidative stress indicators in the STZ-induced animal models of AD (
Figure 3, n = 3, one-way ANOVA, P < 0.05). These results indicate that intranasal administration of Apelin-13 can reduce oxidative stress by improving the free radical scavenging capacity in vivo.
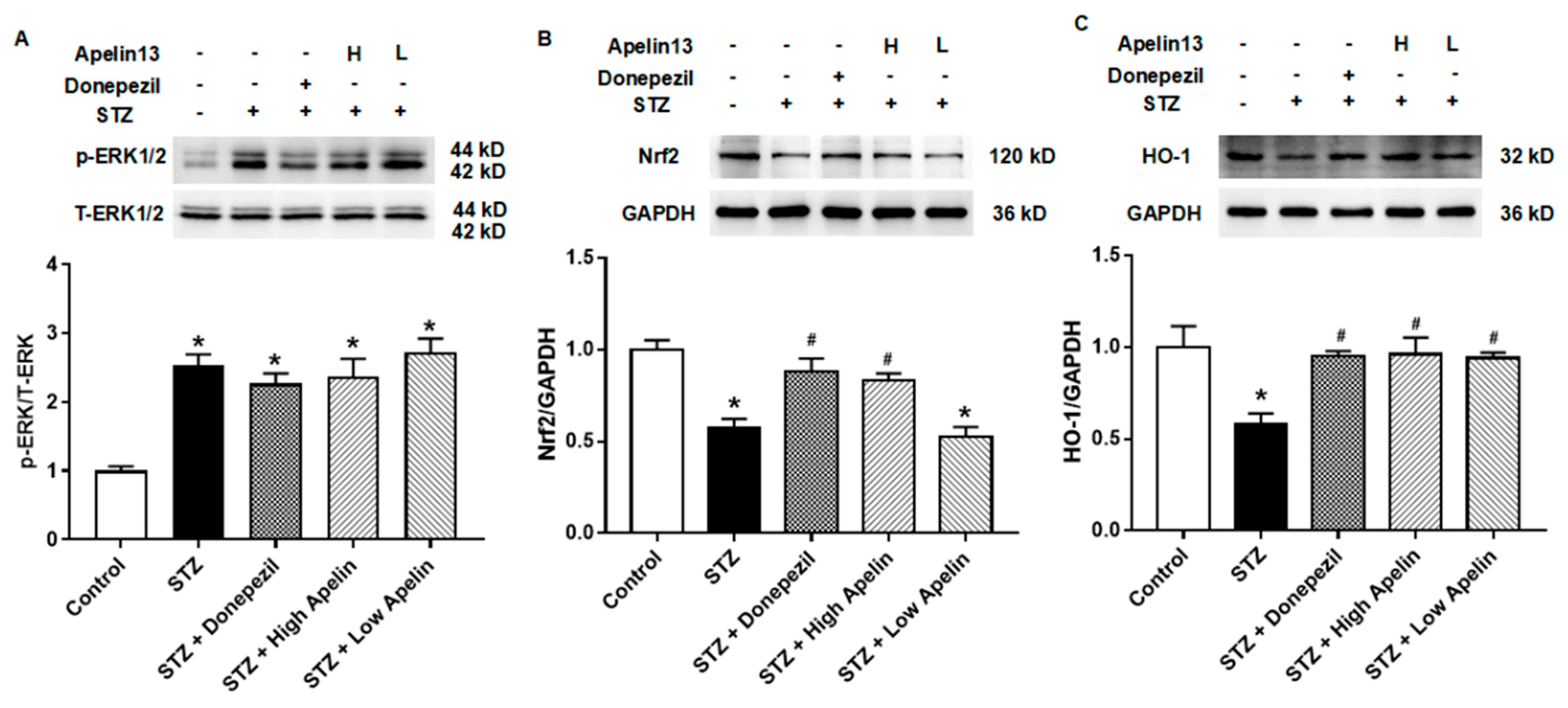
Effect of Intranasal Administration of Apelin-13 on the Expression of ERK-Nrf2-HO-1 in STZ-Induced Animal Model of AD Mice
After confirming that intranasal administration of apelin can improve oxidative stress factors in the hippocampus, we further verified the signaling pathway through which apelin exerts its anti-oxidative stress effect. Previous studies have shown that Aβ25-35 treatment can inhibit the expression of Nrf2 and HO-1 in SH-SY5Y cells [
15]. These findings indicate that Apelin-13 may exert anti-neural injury effects in AD cell models by activating the Nrf2-HO-1 pathway.
First, we examined whether the classic upstream molecule, ERK, of the Nrf2-HO-1 signaling pathway changes in hippocampal tissue. The results showed that STZ caused an increase in the expression of phosphorylated ERK, and this increase was not altered by the positive control drugs donepezil and Apelin-13 (
Figure 4A, n = 3, one-way ANOVA, P < 0.05). These results indicate that phosphorylated ERK protein may be involved in the anti-AD damage mechanism of intranasal administration of Apelin-13.
We then observed the expression of Nrf2 and HO-1 proteins in the different treatment groups. The results showed that both Nrf2 and HO-1 proteins decreased in the STZ-induced animal model of AD. This effect can be altered by donepezil and high-dose Apelin-13. There was no difference in Nrf2 and HO-1 proteins between the donepezil and high-dose Apelin-13 treatment groups and the control group (
Figure 4B, C, n = 3, one-way ANOVA, P < 0.05). These results indicate that the ERK-Nrf2-HO-1 signaling pathway may be one of the mechanisms of action of Apelin-13 against STZ-induced nerve injury.
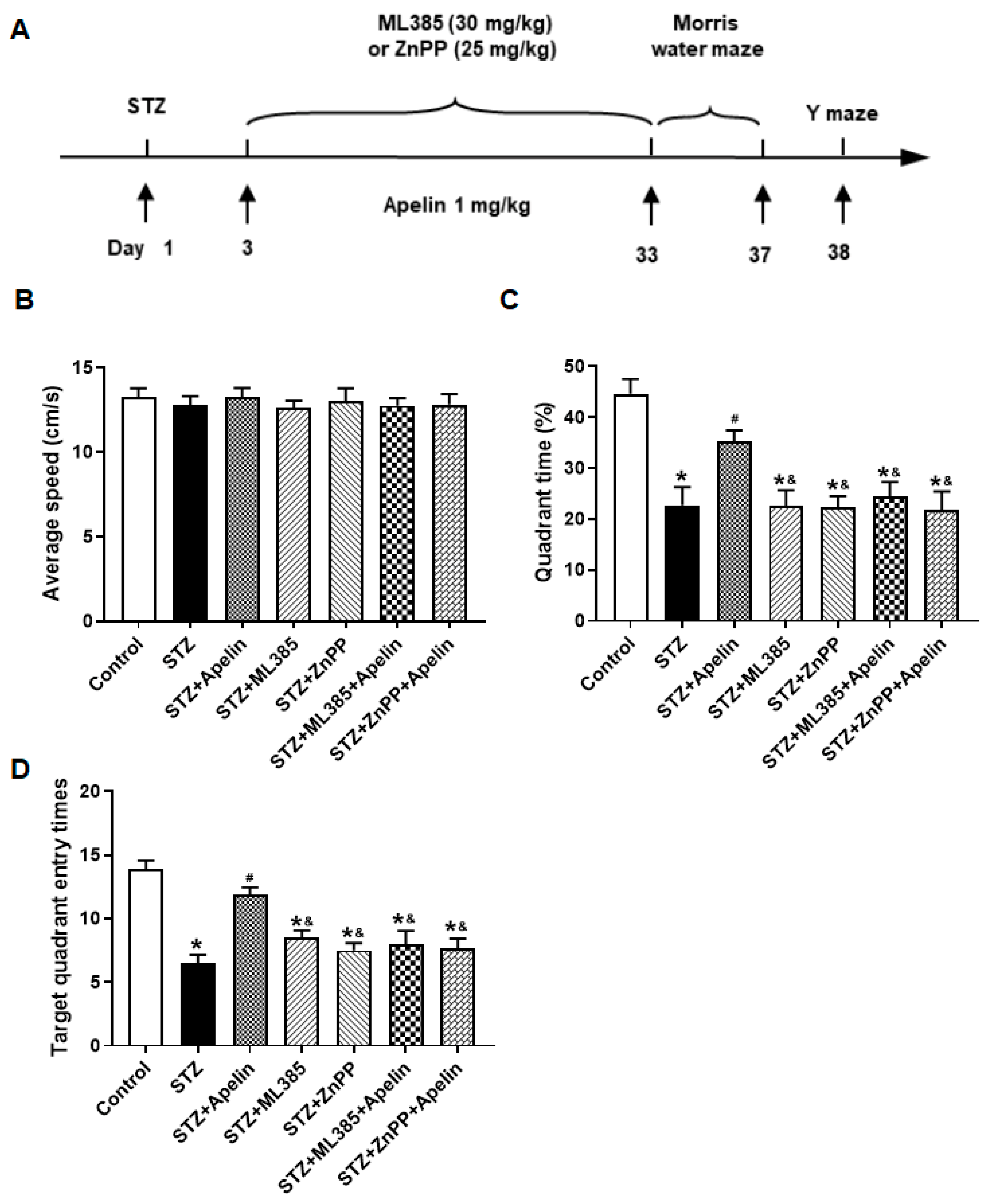
Inhibition of Nrf2 and HO-1 Pathways Attenuates the Cognitive Benefits of Intranasal Apelin-13 Administration in STZ-Induced AD Mice
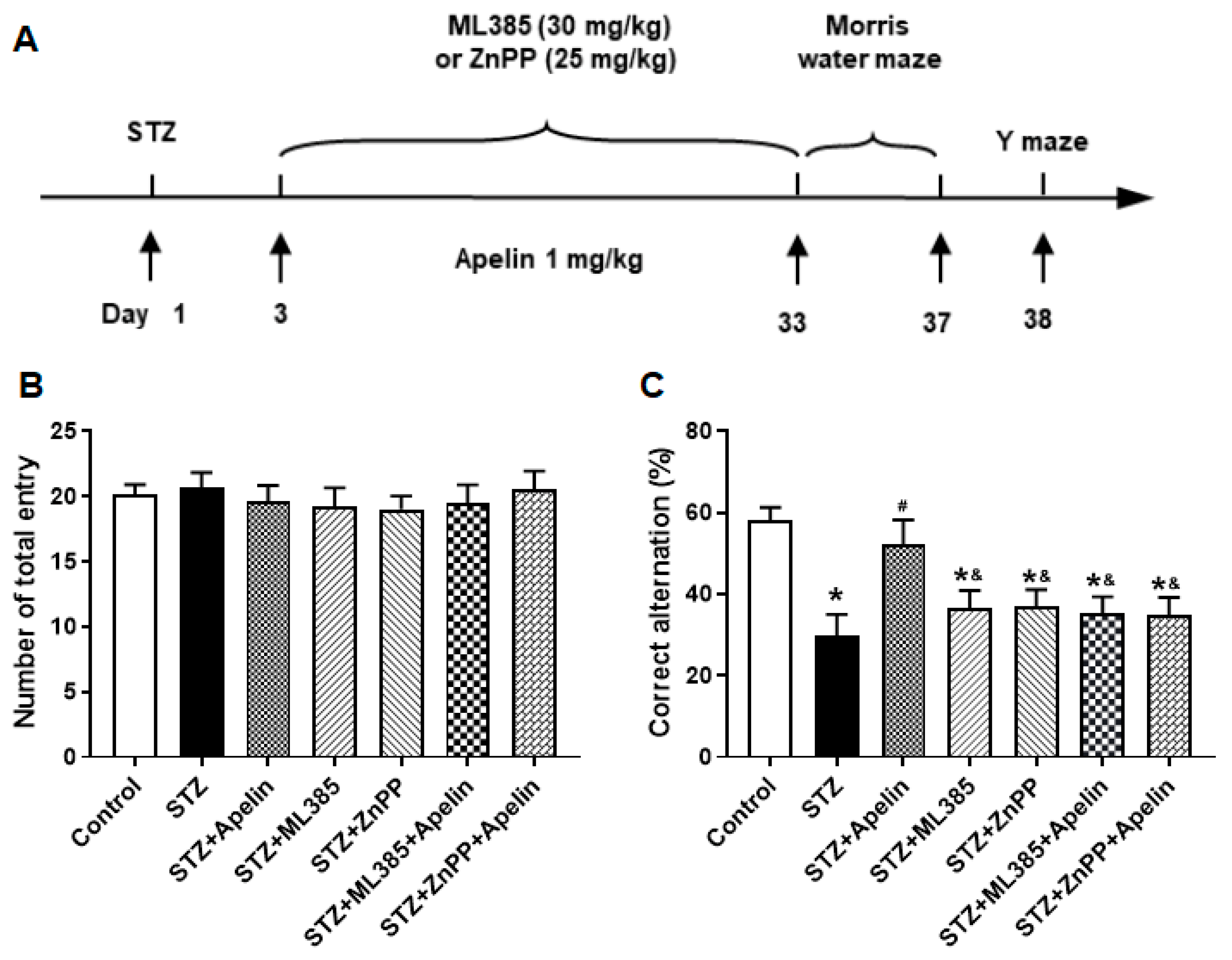
Furthermore, to elucidate the involvement of the Nrf2-HO-1 pathway in the behavioral effects of Apelin-13, we investigated the involvement of the Nrf2-HO-1 pathway in mediating the cognitive effects of Apelin-13. Mice were treated with the Nrf2 inhibitor (ML385, 30 mg/kg) or the HO-1 inhibitor (ZnPP, 25 mg/kg) before receiving high-dose Apelin-13, followed by assessment of spatial learning and memory in the Morris water maze and Y-maze tests (
Figure 5A).
The Morris water maze test revealed that mice treated with high-dose Apelin-13 exhibited significantly improved spatial learning compared to the control group. However, pretreatment with the Nrf2 inhibitor or the HO-1 inhibitor attenuated these improvements, as evidenced by shorter time in the target quadrant and fewer platform crossings compared to the Apelin-13-treated group (
Figure 5B, C and D, n = 8, one-way ANOVA, P < 0.05).
In the Y-maze test, mice administered high-dose Apelin-13 demonstrated enhanced alternation behavior, indicative of improved spatial working memory, compared to the control group. Conversely, pretreatment with the Nrf2 inhibitor or the HO-1 inhibitor reversed this effect, resulting in a significant decrease in alternation behavior compared to the Apelin-13-treated group (
Figure 6B and C, n = 8, one-way ANOVA, P < 0.05). The results revealed that pretreatment with Nrf2 inhibitor or the HO-1 inhibitor abolished the cognitive improvement induced by Apelin-13, suggesting that the Nrf2-HO-1 pathway may play a crucial role in mediating the behavioral effects of Apelin-13 in AD mice.
4. Discussion
The main findings of the present study were that (1) intranasal administration of Apelin-13 improves cognitive impairment in an STZ-induced animal model of AD, and (2) the anti-STZ-induced nerve injury effect of intranasal administration of Apelin-13 may be achieved by improving synaptic plasticity and anti-oxidative stress signaling pathways.
The innovation of this study is the new exploration of Apelin-13 drug delivery. Previous studies have shown that Apelin-13, as a short peptide, cannot be administered intraperitoneally or subcutaneously, similar to conventional drugs, and most can only be administered intraventricularly. Many studies have reported that insulin administered through the nasal cavity can be directly absorbed by the nasal mucosa without passing through the peripheral blood circulation and can enter the brain through the blood-brain barrier to treat neurodegenerative diseases such as AD and cognitive impairment [16–18]. Therefore, we used the intranasal administration of Apelin-13 in mice to explore the feasibility of its drug effects.
After a series of behavioral tests in the open field, Y-maze, and water maze, we found that intranasal administration of Apelin-13 can improve cognitive dysfunction in mice with STZ-induced nerve injury. Our behavioral results are consistent with the latest research reports [
10,
19,
20], the way of Apelin-13 administration in these studies is still using the previous ventricular cannula administration. Our experimental results confirmed that nasal administration of Apelin-13 treatment can improve the behavioral performance of animal models of nerve injury, indicating that intranasal administration of Apelin-13 is effective and feasible. In future studies, we will explore the drug effects at multiple concentrations and time points.
After confirming the validity of the behavioral results, we focused on specific brain regions. It has been reported that Apelin and its receptors are expressed in the whole brain, but the hippocampus is one of the regions with relatively high expression [21–23], and this region is also strongly associated with AD neurodegenerative disease. Therefore, we focused on changes that occur in the hippocampus.
We first observed the long-term potentiation of hippocampal synaptic plasticity, which is closely related to learning and memory functions, in different treatment groups. The results showed that LTP was significantly impaired in the STZ-induced nerve injury group, and high-dose Apelin-13 treatment could improve the LTP impairment caused by STZ-induced nerve injury.
Many in vivo experiments have shown that Apelin-13 can improve oxidative stress-related indicators in STZ-induced nerve injury models [
10]. Our experiments showed that the activities of SOD, GSH, and CAT in STZ-induced nerve injury mice were significantly lower than those in the control group, and the activity of MDA was increased, which is consistent with previous reports. These results indicate that nerve injury mice’s free radical scavenging ability is decreased, leading to oxidative damage. High and low doses of Apelin-13 also improved oxidative stress indicators in STZ-induced nerve injury mice to a certain extent.
Next, we explored the mechanism by which Apelin-13 improves STZ-induced nerve injury. Studies have reported that Apelin-13 exerts neuroprotective effects through anti-inflammatory factors, BDNF/TrkB, and other signaling pathways [
10,
24,
25], GLP-1 can improve learning and memory function of STZ-induced injured rats and inhibit Tau Protein hyperphosphorylation [26–28]. In this study, we investigated whether the ERK-Nrf2-HO-1 pathway, which is related to oxidative stress, plays a role in the Apelin-13 anti-AD cell model of nerve injury.
A recent report confirmed that Aβ25-35 treatment can inhibit the expression of Nrf2 and HO-1 in SH-SY5Y cells [
29]. Our study demonstrates that STZ treatment increases phosphorylated ERK levels and decreases Nrf2 and HO-1 expression. However, the Nrf2 and HO-1 proteins were increased compared with the STZ group after treatment with Apelin-13. The additional behavioral results further corroborate the notion that Apelin-13 exerts an ameliorative effect on cognitive impairment through the Nrf2-HO1 pathway. These findings provide additional support for the involvement of the Nrf2-HO1 pathway in mediating the cognitive-enhancing effects of Apelin-13.
Initially, we explored the protective mechanism of intranasal administration of Apelin-13 against STZ-induced nerve injury. Importantly, it was confirmed for the first time that the administration of Apelin-13 through the nose can produce effective drug effects, similar to the effects of previous intraventricular administration. Overall, these behavioral findings provide important insights into the mechanisms underlying the cognitive-enhancing effects of Apelin-13 and underscore the therapeutic potential of targeting the Nrf2-HO1 pathway for the treatment of Alzheimer's disease. Further research aimed at elucidating the precise molecular mechanisms involved and evaluating the efficacy of Nrf2-HO1 pathway modulation in preclinical and clinical settings is warranted.
Author Contributions
Conceptualization, H.L., M.C. and C.Z.; methodology, H.L. and M.C.; formal analysis, H.L. and M.C.; resources, C.Z.; writing—original draft preparation, H.L., M.C. and C.Z.; supervision, C.Z.; project administration, C.Z.. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal study protocol was approved by the Animal Care and Use Committee of the Shanghai Medical College of Fudan University (No. 20170223-098).
Data Availability Statement
The datasets used and analyzed in this study are available from the corresponding author upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, H.; Han, L.; Xu, J. Apelin/APJ system: a novel promising target for neurodegenerative diseases. Journal of cellular physiology 2020, 235, 638–657. [Google Scholar] [CrossRef] [PubMed]
- Wan, T.; Fu, M.; Jiang, Y.; Jiang, W.; Li, P.; Zhou, S. Research progress on mechanism of neuroprotective roles of Apelin-13 in prevention and treatment of Alzheimer’s disease. Neurochemical research 2022, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.-X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochemical and biophysical research communications 1998, 251, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Simpkin, J.C.; Yellon, D.M.; Davidson, S.M.; Lim, S.Y.; Wynne, A.M.; Smith, C.C. Apelin-13 and apelin-36 exhibit direct cardioprotective activity against ischemiareperfusion injury. Basic research in cardiology 2007, 102, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Hosoya, M.; Kawamata, Y.; Fukusumi, S.; Fujii, R.; Habata, Y.; Hinuma, S.; Kitada, C.; Honda, S.; Kurokawa, T.; Onda, H. Molecular and functional characteristics of APJ: tissue distribution of mRNA and interaction with the endogenous ligand apelin. Journal of Biological Chemistry 2000, 275, 21061–21067. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Jiang, W.; Sun, W.; Guo, W.; Xia, B.; Shen, X.; Fu, M.; Wan, T.; Yuan, M. Neuroprotective roles of Apelin-13 in neurological diseases. Neurochemical Research 2023, 48, 1648–1662. [Google Scholar] [CrossRef] [PubMed]
- Dai, T.-T.; Wang, B.; Xiao, Z.-Y.; You, Y.; Tian, S.-W. Apelin-13 upregulates BDNF against chronic stress-induced depression-like phenotypes by ameliorating HPA axis and hippocampal glucocorticoid receptor dysfunctions. Neuroscience 2018, 390, 151–159. [Google Scholar] [CrossRef] [PubMed]
- Han, R.-w.; Xu, H.-j.; Wang, R. The role of apelin-13 in novel object recognition memory. Peptides 2014, 62, 155–158. [Google Scholar] [CrossRef] [PubMed]
- Haghparast, E.; Esmaeili-Mahani, S.; Abbasnejad, M.; Sheibani, V. Apelin-13 ameliorates cognitive impairments in 6-hydroxydopamine-induced substantia nigra lesion in rats. Neuropeptides 2018, 68, 28–35. [Google Scholar] [CrossRef]
- Luo, H.; Xiang, Y.; Qu, X.; Liu, H.; Liu, C.; Li, G.; Han, L.; Qin, X. Apelin-13 suppresses neuroinflammation against cognitive deficit in a streptozotocin-induced rat model of Alzheimer’s disease through activation of BDNF-TrkB signaling pathway. Frontiers in pharmacology 2019, 10, 395. [Google Scholar] [CrossRef]
- Masoumi, J.; Abbasloui, M.; Parvan, R.; Mohammadnejad, D.; Pavon-Djavid, G.; Barzegari, A.; Abdolalizadeh, J. Apelin, a promising target for Alzheimer disease prevention and treatment. Neuropeptides 2018, 70, 76–86. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Gong, Z.; Zhai, D.; Yang, C.; Lu, G.; Wang, S.; Xiao, S.; Li, C.; Chen, L.; Lin, X. Unveiling the therapeutic potential of Dl-3-n-butylphthalide in NTG-induced migraine mouse: activating the Nrf2 pathway to alleviate oxidative stress and neuroinflammation. The Journal of Headache and Pain 2024, 25, 50. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.-S.; Chau, L.-Y. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nature medicine 2002, 8, 240–246. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Wang, L.; Xiao, W.; Su, Z.; Zheng, C.; Zhang, Z.; Wang, Y.; Xu, B.; Yang, X.; Hoi, M.P.M. Memantine improves cognitive function and alters hippocampal and cortical proteome in triple transgenic mouse model of Alzheimer's disease. Experimental neurobiology 2019, 28, 390. [Google Scholar] [CrossRef]
- Wei, H.; Dobkin, C.; Sheikh, A.M.; Malik, M.; Brown, W.T.; Li, X. The therapeutic effect of memantine through the stimulation of synapse formation and dendritic spine maturation in autism and fragile X syndrome. PloS one 2012, 7, e36981. [Google Scholar] [CrossRef]
- Benedict, C.; Frey II, W.H.; Schiöth, H.B.; Schultes, B.; Born, J.; Hallschmid, M. Intranasal insulin as a therapeutic option in the treatment of cognitive impairments. Experimental gerontology 2011, 46, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Freiherr, J.; Hallschmid, M.; Frey, W.H.; Brünner, Y.F.; Chapman, C.D.; Hölscher, C.; Craft, S.; De Felice, F.G.; Benedict, C. Intranasal insulin as a treatment for Alzheimer’s disease: a review of basic research and clinical evidence. CNS drugs 2013, 27, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Reger, M.A.; Watson, G.S.; Green, P.S.; Wilkinson, C.W.; Baker, L.D.; Cholerton, B.; Fishel, M.A.; Plymate, S.; Breitner, J.; DeGroodt, W. Intranasal insulin improves cognition and modulates β-amyloid in early AD. Neurology 2008, 70, 440–448. [Google Scholar] [CrossRef] [PubMed]
- Nasseri, B.; Zareian, P.; Alizade, H. Apelin attenuates streptozotocin-induced learning and memory impairment by modulating necroptosis signaling pathway. International Immunopharmacology 2020, 84, 106546. [Google Scholar] [CrossRef]
- Aminyavari, S.; Zahmatkesh, M.; Farahmandfar, M.; Khodagholi, F.; Dargahi, L.; Zarrindast, M.-R. Protective role of Apelin-13 on amyloid β25–35-induced memory deficit; Involvement of autophagy and apoptosis process. Progress in Neuro-Psychopharmacology and Biological Psychiatry 2019, 89, 322–334. [Google Scholar] [CrossRef]
- Reaux, A.; De Mota, N.; Skultetyova, I.; Lenkei, Z.; El Messari, S.; Gallatz, K.; Corvol, P.; Palkovits, M.; Llorens-Cortès, C. Physiological role of a novel neuropeptide, apelin, and its receptor in the rat brain. Journal of neurochemistry 2001, 77, 1085–1096. [Google Scholar] [CrossRef] [PubMed]
- Reaux, A.; Gallatz, K.; Palkovits, M.; Llorens-Cortes, C. Distribution of apelin-synthesizing neurons in the adult rat brain. Neuroscience 2002, 113, 653–662. [Google Scholar] [CrossRef] [PubMed]
- O’Carroll, A.-M.; Selby, T.L.; Palkovits, M.; Lolait, S.J. Distribution of mRNA encoding B78/apj, the rat homologue of the human APJ receptor, and its endogenous ligand apelin in brain and peripheral tissues. Biochimica et Biophysica Acta (BBA)-Gene Structure and Expression 2000, 1492, 72–80. [Google Scholar] [CrossRef]
- Shen, P.; Yue, Q.; Fu, W.; Tian, S.-W.; You, Y. Apelin-13 ameliorates chronic water-immersion restraint stress-induced memory performance deficit through upregulation of BDNF in rats. Neuroscience Letters 2019, 696, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Chen, J.; Bai, B.; Xin, Q. Neuroprotection of apelin and its signaling pathway. Peptides 2012, 37, 171–173. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Duffy, K.B.; Ottinger, M.A.; Ray, B.; Bailey, J.A.; Holloway, H.W.; Tweedie, D.; Perry, T.; Mattson, M.P.; Kapogiannis, D. GLP-1 receptor stimulation reduces amyloid-β peptide accumulation and cytotoxicity in cellular and animal models of Alzheimer's disease. Journal of Alzheimer's disease 2010, 19, 1205–1219. [Google Scholar] [CrossRef] [PubMed]
- Hölscher, C. Potential role of glucagon-like peptide-1 (GLP-1) in neuroprotection. CNS drugs 2012, 26, 871–882. [Google Scholar] [CrossRef]
- Perry, T.A.; Greig, N.H. A new Alzheimer's disease interventive strategy: GLP-1. Current drug targets 2004, 5, 565–571. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, Y.; Wang, H.; Zhao, L.; Ma, Z.; Li, T.; Liu, J.; Sun, M.; Jian, Y.; Yao, L. Edaravone reduces Aβ-induced oxidative damage in SH-SY5Y cells by activating the Nrf2/ARE signaling pathway. Life sciences 2019, 221, 259–266. [Google Scholar] [CrossRef]
Figure 1.
Intranasal administration of Apelin-13 improves cognitive impairment in STZ-induced animal model of AD mice. (A) Experimental timeline. (B) Distance of mouse movement during the 15-minute test period in different groups (one-way ANOVA,P > 0.05). (C) Move mouse time during the 15-minute test period in different groups (one-way ANOVA,P > 0.05). (D) Percentage of time spent in the margin area in different groups (one-way ANOVA,P > 0.05). (E) The swimming trajectory of mice during the probe test. (F) Average speed in different groups (one-way ANOVA,P > 0.05). (G) Quadrant time in different groups (one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group). (H) Target quadrant entry times in different groups (one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group). (I) Y maze test. (J) Number of arm entrances in different groups (one-way ANOVA,P > 0.05). (K) Alternation ratio in different groups (one-way ANOVA, *P < 0.05 compared to control group, #P < 0.05 compared to STZ treatment group). Data are shown as the mean ± s.e.m.
Figure 1.
Intranasal administration of Apelin-13 improves cognitive impairment in STZ-induced animal model of AD mice. (A) Experimental timeline. (B) Distance of mouse movement during the 15-minute test period in different groups (one-way ANOVA,P > 0.05). (C) Move mouse time during the 15-minute test period in different groups (one-way ANOVA,P > 0.05). (D) Percentage of time spent in the margin area in different groups (one-way ANOVA,P > 0.05). (E) The swimming trajectory of mice during the probe test. (F) Average speed in different groups (one-way ANOVA,P > 0.05). (G) Quadrant time in different groups (one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group). (H) Target quadrant entry times in different groups (one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group). (I) Y maze test. (J) Number of arm entrances in different groups (one-way ANOVA,P > 0.05). (K) Alternation ratio in different groups (one-way ANOVA, *P < 0.05 compared to control group, #P < 0.05 compared to STZ treatment group). Data are shown as the mean ± s.e.m.
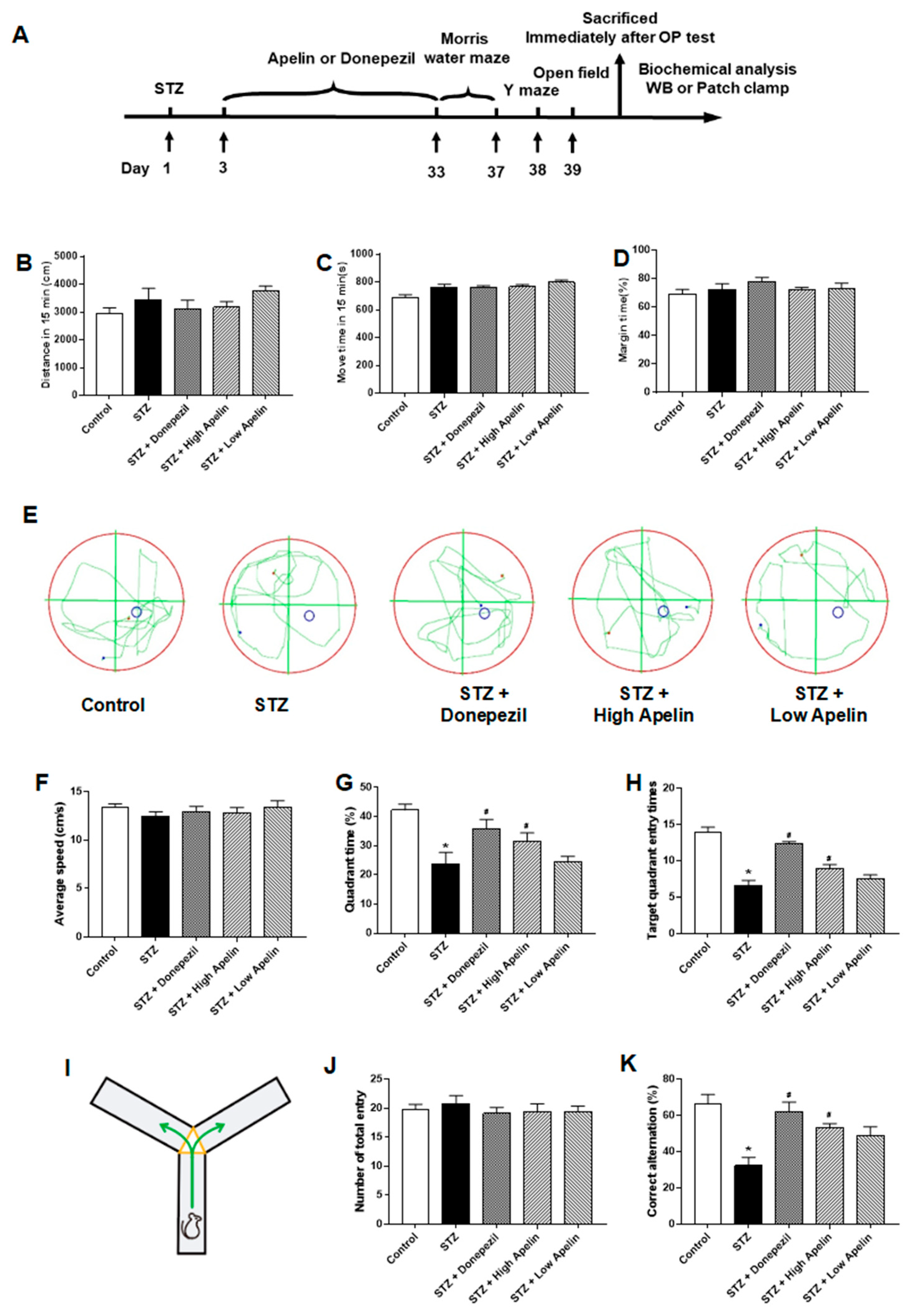
Figure 2.
Intranasal administration of Apelin-13 restores LTP in CA1 neurons of STZ-induced animal model of AD mice. (A) Typical traces in different treatment groups. (B) Bar graphs showing changes of LTP in different treatment groups (n = 6, one-way ANOVA, *P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (C) Left: Typical recording cell image (Up panel: Bar = 200 μm, Down panel: Bar = 50 μm). Right: Time course of the LTP in different treatment groups (n = 6). Data are shown as the mean ± s.e.m.
Figure 2.
Intranasal administration of Apelin-13 restores LTP in CA1 neurons of STZ-induced animal model of AD mice. (A) Typical traces in different treatment groups. (B) Bar graphs showing changes of LTP in different treatment groups (n = 6, one-way ANOVA, *P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (C) Left: Typical recording cell image (Up panel: Bar = 200 μm, Down panel: Bar = 50 μm). Right: Time course of the LTP in different treatment groups (n = 6). Data are shown as the mean ± s.e.m.
Figure 3.
Effect of Intranasal administration of Apelin-13 on STZ induced oxidative stress in the hippocampus. (A) Quantitative mean U/mg of SOD in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (B) Quantitative mean U/mg of CAT in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (C) Quantitative mean U/mg of GSH in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (D) Quantitative mean nmol/mg of MDA in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). Data are shown as the mean ± s.e.m.
Figure 3.
Effect of Intranasal administration of Apelin-13 on STZ induced oxidative stress in the hippocampus. (A) Quantitative mean U/mg of SOD in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (B) Quantitative mean U/mg of CAT in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (C) Quantitative mean U/mg of GSH in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (D) Quantitative mean nmol/mg of MDA in each treatment group (n = 3, one-way ANOVA,*P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). Data are shown as the mean ± s.e.m.
Figure 4.
Effect of intranasal administration of Apelin-13 on the expression of ERK-Nrf2-HO-1 in STZ-induced animal model of AD mice. (A) Representative image of immunoblots and densitometric analysis of changes in levels for ERK family proteins in different treatment groups (n = 3, one-way ANOVA, *P < 0.05 compared to control). (B) Representative image of immunoblots and densitometric analysis of changes in levels for Nrf2 proteins in different treatment groups (n = 3, one-way ANOVA, *P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (C) Representative image of immunoblots and densitometric analysis of changes in levels for HO-1 proteins in different treatment groups (n = 3, one-way ANOVA, *P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). Data are shown as the mean ± s.e.m.
Figure 4.
Effect of intranasal administration of Apelin-13 on the expression of ERK-Nrf2-HO-1 in STZ-induced animal model of AD mice. (A) Representative image of immunoblots and densitometric analysis of changes in levels for ERK family proteins in different treatment groups (n = 3, one-way ANOVA, *P < 0.05 compared to control). (B) Representative image of immunoblots and densitometric analysis of changes in levels for Nrf2 proteins in different treatment groups (n = 3, one-way ANOVA, *P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). (C) Representative image of immunoblots and densitometric analysis of changes in levels for HO-1 proteins in different treatment groups (n = 3, one-way ANOVA, *P < 0.05 compared to control, #P < 0.05 compared to STZ treatment group). Data are shown as the mean ± s.e.m.
Figure 5.
Effect of Nrf2-HO-1 pathway blockade on Apelin-13-mediated improvement of Morris maze performance in STZ-Induced animal model of AD mice. (A) Experimental timeline. (B) Average speed in different groups (one-way ANOVA,P > 0.05). (C) Quadrant time in different groups (n = 8, one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group, &P < 0.05 compared to STZ+Apelin-13 treatment group). (D) Target quadrant entry times in different groups (n = 8, one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group, &P < 0.05 compared to STZ+Apelin-13 treatment group). Data are shown as the mean ± s.e.m.
Figure 5.
Effect of Nrf2-HO-1 pathway blockade on Apelin-13-mediated improvement of Morris maze performance in STZ-Induced animal model of AD mice. (A) Experimental timeline. (B) Average speed in different groups (one-way ANOVA,P > 0.05). (C) Quadrant time in different groups (n = 8, one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group, &P < 0.05 compared to STZ+Apelin-13 treatment group). (D) Target quadrant entry times in different groups (n = 8, one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group, &P < 0.05 compared to STZ+Apelin-13 treatment group). Data are shown as the mean ± s.e.m.
Figure 6.
Effect of Nrf2-HO-1 pathway blockade on Apelin-13-mediated improvement of Y maze performance in STZ-Induced animal model of AD mice. (A) Experimental timeline. (B) Number of arm entrances in different groups (one-way ANOVA,P > 0.05). (C) Alternation ratio in different groups (n = 8, one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group, &P < 0.05 compared to STZ+Apelin-13 treatment group). Data are shown as the mean ± s.e.m.
Figure 6.
Effect of Nrf2-HO-1 pathway blockade on Apelin-13-mediated improvement of Y maze performance in STZ-Induced animal model of AD mice. (A) Experimental timeline. (B) Number of arm entrances in different groups (one-way ANOVA,P > 0.05). (C) Alternation ratio in different groups (n = 8, one-way ANOVA, *P < 0.05 compared to the control group, #P < 0.05 compared to STZ treatment group, &P < 0.05 compared to STZ+Apelin-13 treatment group). Data are shown as the mean ± s.e.m.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).