Submitted:
15 April 2024
Posted:
15 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
3.1. Efficacy of Xanomeline-Trospium
| Source reviewed | RCTs | |||||
|---|---|---|---|---|---|---|
| Allocation concealment | Randomization | Blinding | Outcome data | Selective reporting | Others | |
| [68] | ||||||
| [57] | ||||||
| [77] | ||||||
| [79] | ||||||
| No risk of bias was identified | Uncertain risk of bias | The risk of bias is present | Not applicable to that research | |||
3.2. Tolerability and Safety of Xanomeline-Trospium
| Reference | Clinical trial phase and registration number | Design | Results | OQD |
|---|---|---|---|---|
| Karuna Therap. [68], Correll et al. [69], Brannan SK [70], Weiden PJ [71], Sauder C [72] | Phase 2, NCT03697252 (EMERGENT-1) |
DBRCT, N=182 adult inpatients, acute schizophrenia. The KarXT (mg xanomeline/mg trospium) dosing schedule was flexible, starting with 50 mg/20 mg b.i.d and increasing to a maximum of 125 mg/30 mg b.i.d. Main outcome- PANSS-T score at week 5; secondary outcomes- PANSS-P, PANSS-N, and PANSS-M-N scores, CGI-S, % of responders (CGI-S) at week 5 |
PANSS-T score ↓ significantly in KarXT-treated patients vs. placebo (-17.4 vs. -5.9 at week 5, p<0.001). Secondary outcomes - significant improvement in the active group vs. placebo, except for the % CGI-S responders. Response rates between 15.7-59% (defined by >20-50% ↓PANSS-T scores). The five Marden factors on PANSS showed a significant difference between the active drug and placebo from week 2 to the end of the trial. A tendency towards more significant enhancement in cognitive function with KarXT compared to placebo was reported. The most common AEs (occurring in ≥2% of patients in the KarXT group and at a more than two-fold higher incidence than in the placebo group) were nausea (16.9% vs. 4.4%), vomiting (9.0% vs. 4.4%), constipation (16.9% vs. 3.3%), and dry mouth (9.0% vs. 1.1%). |
High |
| Karuna Therap. [57], Kaul et al, 2023 [73] |
Phase 3 trial, NCT04659161 (EMERGENT-2) |
DBRCT, N=252 adult inpatients with acute schizophrenia KarXT was administered for 5 weeks: 50 mg xanomeline and 20 mg trospium b.i.d for the first 2 days + 100 mg xanomeline and 20 mg trospium b.i.d for days 3–7 + on day 8, KarXT dosing was flexible with an optional increase to 125 mg xanomeline and 30 mg trospium b.i.d and the option to return to 100 mg xanomeline and 20 mg trospium based on tolerability Main outcome- PANSS-T score at week 5; Secondary outcomes- PANSS-P, PANSS-N, PANSS-M-N, CGI-S scores at week 5, % of responders (CGI-S) |
PANSS scores decreased significantly (p < 0.0001) at endpoint vs. placebo. The most common AE with KarXT vs. placebo were: constipation (27 [21%] vs. 13 [10%]), dyspepsia (24 [19%] vs. 10 [8%]), headache (17 [14%] vs. 15 [12%]), nausea (24 [19%] vs. seven [6%]), vomiting (18 [14%] vs. one [1%]), hypertension (12 [10%] vs. one [1%]), dizziness (11 [9%] vs. four [3%]), gastro-oesophageal reflux disease (eight [6%] vs. zero [0%]), and diarrhea (seven [6%] vs. four [3%]) TEAEs rates of extrapyramidal motor symptoms (KarXT, zero [0%] vs. placebo, zero [0%]), akathisia (one [1%] vs. one [1%]), weight gain (zero [0%] vs. one [1%]), and somnolence (six [5%] vs. five [4%]) |
High |
| Karuna Therap. [74] | Phase 3, NCT04738123 (EMERGENT-3) | DBRCT, N=256 adults inpatients with schizophrenia, KarXT (125 mg xanomeline/30 mg trospium b.i.d) vs. placebo Main outcome- PANSS-T score at week 5; Secondary outcomes- PANSS-P, PANSS-N, PANSS-M-N, CGI-S scores at week 5, % of responders (PANSS-T) |
No results posted | N/A |
| Karuna Therap. [78] | Phase 3b, NCT05643170 (PENNANT) | OL, N=380 (estimated), 4 (actual enrollment) patients with schizophrenia who did not tolerate/respond to current medication, KarXT 50/20 mg b.i.d, 100/20 mg b.i.d., or 125/30 mg b.i.d, 3 years Main outcome- TEAEs leading to discontinuation, persistence and durability of KarXT effect (IAQ and CGI-S scores). Secondary outcomes- TEAEs incidence, CGI-I, MSQ scores |
Not released | N/A |
| Brannan et al, 2019 [77] | Phase I | Placebo-controlled, N=69 healthy volunteers, MAD study Drug exposure- 2-day titration period of either placebo or a KarXT dose of 50 mg xanomeline + 20 mg trospium followed by a 5-day treatment period. The doses (all b.i.d) assessed were: xanomeline 100 mg, 125 mg and 150 mg in combination with trospium 20 mg or 40 mg |
Most cholinergic AEs occurred within the first few days of starting or increasing the study drug. The majority of these AEs at 100 mg and 125 mg xanomeline-dose levels were mild and transient in nature. None of the cohorts showed meaningful changes in orthostatic HR or obvious differences in BP between placebo and KarXT compared to placebo. Increasing trospium dose ameliorated cholinergic AEs and led to the observance of some anticholinergic adverse events (AEs). Some cohorts tested on 40 mg trospium b.i.d reported signs of anticholinergic effects (i.e., dry mouth), particularly in the cohort receiving 125 mg b.i.d of xanomeline |
High |
| Breier et al, 2023 [76], Kavoussi et al. [77], Karuna Therap. [79] |
Phase I, NCT02831231 |
DBRCT, N=70 healthy volunteers, Xanomeline + placebo or xanomeline + trospium. The dose of xanomeline was 75 mg given three times per day and the dose of trospium was 20 mg given twice per day. Main outcome- mean weekly maximum composite VAS score (nausea, diarheea, sweating, salivation, vomiting) |
The proportion of subjects reporting any TEAEs was 81.8% on xanomeline alone and 65.7% on KarXT. There was a 46% reduction in the incidence of any cholinergic AEs reported by subjects treated with KarXT compared with xanomeline alone (34.3% vs. 63.6%, respectively). KarXT was associated with a 59% reduction in sweating. In addition, there was a reduction of ≥ 29% in the incidence of each of the four other individual cholinergic AEs by KarXT compared with xanomeline alone. ECGs, vital signs, and laboratory values were similar between the treatment arms. There were no episodes of syncope in KarXT-treated subjects (two cases occurred in the xanomeline-alone arm) and postural dizziness was noted at lower rates in the KarXT arm (11.4%) compared with xanomeline alone (27.2%). |
Moderate |
3.3. Ongoing and Future Studies
| Reference | Clinical trial phase and registration number | Design |
|---|---|---|
| Karuna Therap. [80] | Phase 3, NCT05511363 (ADEPT-1) |
DBRCT, N=380 patients with AD + psychosis. Outcomes- relapse prevention with KarXT (20/2 mg t.i.d and 66.7/6.67 t.i.d) vs. placebo during 38 weeks |
| Karuna Therap. [81] | Phase 3, NCT04820309 (EMERGENT-5) |
OL, N=568 patients with schizophrenia, KarXT (50/20 mg b.i.d up to 125/30 mg b.i.d), 56 weeks Outcomes- long-term safety and tolerability of KarXT and description of PK parameters |
| Karuna Therap. [82] | Phase 3, NCT04659174 (EMERGENT-4) |
Extension phase, OL, N=350 patients with schizophrenia, 53 weeks, fixed dose of KarXT (125/30 mg b.i.d) Outcomes- PANSS-T, PANSS subscores, CGI-S scores, and response rates |
| Karuna Therap. [83] | Phase 3, NCT05919823 (UNITE-001) |
DBRCT phase, 5 weeks + OL extension phase, 12 weeks, N=158 Chinese patients with schizophrenia Main outcome- PANSS-T |
| Karuna Therap. [84] | Phasse3, NCT05980949 (ADEPT-3) |
OL, roll-over study, 54 weeks N= 140 patients with AD + psychosis, KarXT 20/2 mg, up to 200/20 mg/day Outcome- TEAEs incidence |
| Karuna Therap. [85] | Phase 3, NCT05145413 (ARISE) |
DBRCT, N=400 patients with schizophrenia and inadequate response to their current antipsychotic, KarXT (50/20 mg b.i.d, up to 125/30 mg b.i.d) + ongoing treatment, 6 weeks Outcome- PANSS-T, PSP, CGI-S, PANSS-M-P, POM, response rate (PANSS-T) |
| Karuna Therap. [86] | Phase 3, NCT05304767 |
OL extension, 52 weeks, N=280 patients with schizophrenia and inadequate response to the ongoing antipsychotic, KarXT (50/20 mg b.i.d up to 125/30 mg b.i.d) |
| Karuna Therap. [87] | Phase 3, NCT06126224 (ADEPT-2) |
DBCRT, N=400 female patients with mild or moderate psychosis associated with AD, KarXT dose of 60/6 to 200/20 mg/day Outcome- Hallucinations and Delusions score |
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Section and Topic | Item # | Checklist item | The location where an item is reported |
|---|---|---|---|
| TITLE | |||
| Title | 1 | Identify the report as a systematic review. | Lines 2-4 |
| ABSTRACT | |||
| Abstract | 2 | See the PRISMA 2020 for the Abstracts checklist. | Lines 11-24 |
| INTRODUCTION | |||
| Rationale | 3 | Describe the rationale for the review in the context of existing knowledge. | Lines 113-133 |
| Objectives | 4 | Provide an explicit statement of the objective(s) or question(s) the review addresses. | Lines 143-149 |
| METHODS | |||
| Eligibility criteria | 5 | Specify the inclusion and exclusion criteria for the review and how studies were grouped for the syntheses. | Table 1 |
| Information sources | 6 | Specify all databases, registers, websites, organizations, reference lists, and other sources searched or consulted to identify studies. Specify the date when each source was last searched or consulted. | Lines 153-156 |
| Search strategy | 7 | Present the full search strategies for all databases, registers, and websites, including any filters and limits used. | Lines 151-173 |
| Selection process | 8 | Specify the methods used to decide whether a study met the inclusion criteria of the review, including how many reviewers screened each record and each report retrieved, whether they worked independently, and if applicable, details of automation tools used in the process. | N/A |
| Data collection process | 9 | Specify the methods used to collect data from reports, including how many reviewers collected data from each report, whether they worked independently, any processes for obtaining or confirming data from study investigators, and if applicable, details of automation tools used in the process. | N/A |
| Data items | 10a | List and define all outcomes for which data were sought. Specify whether all results that were compatible with each outcome domain in each study were sought (e.g. for all measures, time points, analyses), and if not, the methods used to decide which results to collect. | Table 1 |
| 10b | List and define all other variables for which data were sought (e.g. participant and intervention characteristics, funding sources). Describe any assumptions made about any missing or unclear information. | Table 1 | |
| Study risk of bias assessment | 11 | Specify the methods used to assess the risk of bias in the included studies, including details of the tool(s) used, how many reviewers assessed each study and whether they worked independently, and if applicable, details of automation tools used in the process. | Lines 170-171 |
| Effect measures | 12 | Specify for each outcome the effect measure(s) (e.g. risk ratio, mean difference) used in the synthesis or presentation of results. | N/A |
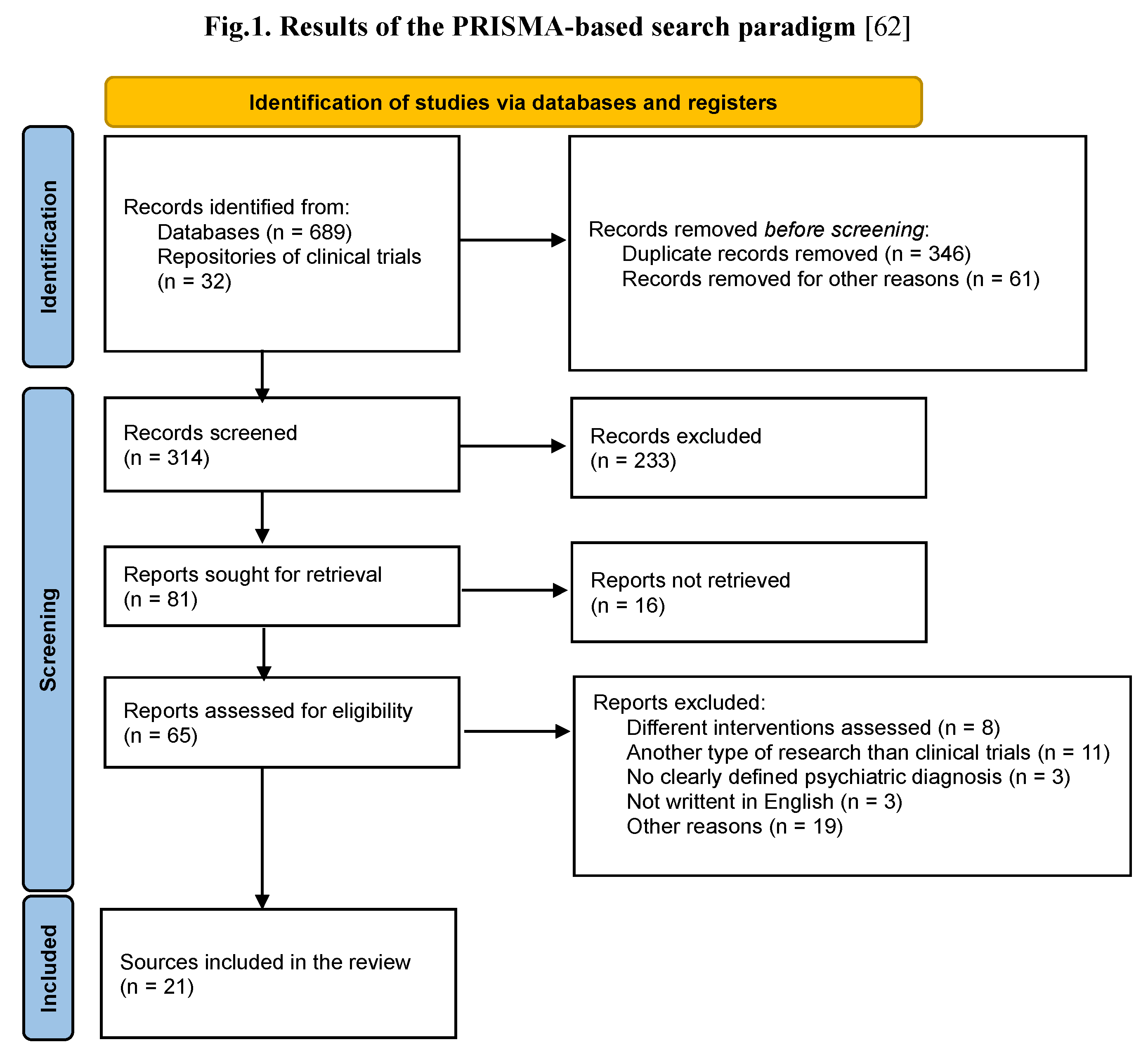
| Synthesis methods | 13a | Describe the processes used to decide which studies were eligible for each synthesis (e.g. tabulating the study intervention characteristics and comparing against the planned groups for each synthesis (item #5)). | Figure 1 |
| 13b | Describe any methods required to prepare the data for presentation or synthesis, such as handling of missing summary statistics, or data conversions. | N/A | |
| 13c | Describe any methods used to tabulate or visually display the results of individual studies and syntheses. | Table 3, Table 4 | |
| 13d | Describe any methods used to synthesize results and provide a rationale for the choice(s). If meta-analysis was performed, describe the model(s), method(s) to identify the presence and extent of statistical heterogeneity, and software package(s) used. | Lines 178-185 | |
| 13e | Describe any methods used to explore possible causes of heterogeneity among study results (e.g. subgroup analysis, meta-regression). | N/A | |
| 13f | Describe any sensitivity analyses conducted to assess the robustness of the synthesized results. | N/A | |
| Reporting bias assessment | 14 | Describe any methods used to assess the risk of bias due to missing results in a synthesis (arising from reporting biases). | Lines 170-171 |
| Certainty assessment | 15 | Describe any methods used to assess certainty (or confidence) in the body of evidence for an outcome. | Lines 171-173 |
| RESULTS | |||
| Study selection | 16a | Describe the results of the search and selection process, from the number of records identified in the search to the number of studies included in the review, ideally using a flow diagram. | Figure 1 |
| 16b | Cite studies that might appear to meet the inclusion criteria, but which were excluded, and explain why they were excluded. | N/A | |
| Study characteristics | 17 | Cite each included study and present its characteristics. | Table 2 |
| Risk of bias in studies | 18 | Present assessments of risk of bias for each included study. | Table 3 and Table 4 |
| Results of individual studies | 19 | For all outcomes, present, for each study: (a) summary statistics for each group (where appropriate) and (b) an effect estimate and its precision (e.g. confidence/credible interval), ideally using structured tables or plots. | Table 3 and Table 4 |
| Results of syntheses | 20a | For each synthesis, briefly summarise the characteristics and risk of bias among contributing studies. | Table 2 |
| 20b | Present results of all statistical syntheses conducted. If meta-analysis was done, present for each the summary estimate and its precision (e.g. confidence/credible interval) and measures of statistical heterogeneity. If comparing groups, describe the direction of the effect. | N/A | |
| 20c | Present results of all investigations of possible causes of heterogeneity among study results. | N/A | |
| 20d | Present results of all sensitivity analyses conducted to assess the robustness of the synthesized results. | N/A | |
| Reporting biases | 21 | Present assessments of risk of bias due to missing results (arising from reporting biases) for each synthesis assessed. | N/A |
| Certainty of evidence | 22 | Present assessments of certainty (or confidence) in the body of evidence for each outcome assessed. | Table 3 |
| DISCUSSION | |||
| Discussion | 23a | Provide a general interpretation of the results in the context of other evidence. | Lines 363-453 |
| 23b | Discuss any limitations of the evidence included in the review. | Lines 438-444 | |
| 23c | Discuss any limitations of the review processes used. | Lines 438-444 | |
| 23d | Discuss the implications of the results for practice, policy, and future research. | Lines 445-452 | |
| OTHER INFORMATION | |||
| Registration and protocol | 24a | Provide registration information for the review, including register name and registration number, or state that the review was not registered. | N/A |
| 24b | Indicate where the review protocol can be accessed, or state that a protocol was not prepared. | N/A | |
| 24c | Describe and explain any amendments to information provided at registration or in the protocol. | N/A | |
| Support | 25 | Describe sources of financial or non-financial support for the review, and the role of the funders or sponsors in the review. | Line 468 |
| Competing interests | 26 | Declare any competing interests of review authors. | Lines 475-476 |
| Availability of data, code and other materials | 27 | Report which of the following are publicly available and where they can be found: template data collection forms; data extracted from included studies; data used for all analyses; analytic code; any other materials used in the review. | Line N/A |
References
- Solmi, M.; Seitidis, G.; Mavridis, D.; Correll, C.U.; Dragioti, E.; Guimond, S.; et al. Incidence, prevalence, and global burden of schizophrenia- data, with critical appraisal, from the Global Burden of Disease (GBD) 2019. Mol. Psychiatry 2023. [Google Scholar] [CrossRef] [PubMed]
- Potkin, S.G.; Kane, J.M.; Correll, C.U.; Lindenmayer, J.P.; Agid, O.; Marder, S.R.; et al. The neurobiology of treatment-resistant schizophrenia: paths to antipsychotic resistance and a roadmap for future research. npj Schizophrenia 2020, 6, 1. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O.; Vasile, D.; Voicu, V. Efficacy and tolerability of antibiotic augmentation in schizophrenia spectrum disorders- A systematic literature review. RJMM 2020, CXXIII(1), 3–20. [Google Scholar] [CrossRef]
- Lally, J.; Ajnakina, O.; Di Forti, M.; Trotta, A.; Demjaha, A.; Kolliakou, A.; et al. Two distinct patterns of treatment resistance: clinical predictors of treatment resistance in first-episode schizophrenia spectrum psychoses. Psychol. Med. 2016, 46, 3231–3240. [Google Scholar] [CrossRef] [PubMed]
- Li, C.T. Overview of treatment-resistant depression. Prog. Brain Res. 2023, 278, 1–23. [Google Scholar] [CrossRef]
- Diaz, A.P.; Fernandes, B.S.; Quevedo, J.; Sanches, M.; Soares, J.C. Treatment-resistant bipolar depression: concepts and challenges for novel interventions. Braz. J. Psychiatry 2022, 44, 178–186. [Google Scholar] [CrossRef] [PubMed]
- Pigott, H.E. The STAR*D Trial: It is time to reexamine the clinical beliefs that guide the treatment of major depression. Can. J. Psychiatry 2015, 60, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Rush, A.J.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; et al. Evaluation of outcomes with citalopram for depression using measurement-based care in STAR*D: implications for clinical practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef]
- Vasiliu, O. Investigational drugs for the treatment of depression (Part 2): Glutamatergic, cholinergic, sestrin modulators, and other agents. Front. Pharmacol. 2022, 13, 884155. [Google Scholar] [CrossRef]
- Tandon, R. Antipsychotics in the treatment of schizophrenia: an overiew. J. Clin. Psychiatry 2011, 72 (Suppl.1), 4–8. [Google Scholar] [CrossRef]
- Faden, J. How do we select an antipsychotic for those with schizophrenia? Expert. Opin. Pharmacother. 2019, 20, 2195–2199. [Google Scholar] [CrossRef] [PubMed]
- Vasiliu, O.; Vasile, D.; Făinărea, A.F.; Pătrașcu, M.C.; Morariu, E.A.; Manolache, R.; Alexandru, I.; Androne, F.T. Analysis of risk factors for antipsychotic-resistant schizophrenia in young patients- a retrospective analysis. RJMM 2018, CXXI(1), 25–29. [Google Scholar] [CrossRef]
- Kishimoto, T.; Hagi, K.; Kurokawa, S.; Kane, J.M.; Correll, C.U. Efficacy and safety/tolerability of antipsychotics in the treatment of adult patients with major depressive disorder: a systematic review and meta-analysis. Psychol. Med. 2023, 53, 4064–4082. [Google Scholar] [CrossRef] [PubMed]
- Mühlbauer, V.; Mȍhler, R.; Dichter, M.N.; Zuidema, S.U.; Kȍpke, S.; Luijendijk, H.J. Antipsychotics for agitation and psychosis in people with Alzheimer’s disease and vascular dementia. Cochrane Database Syst. Rev. 2021, 12, CD013304. [Google Scholar] [CrossRef]
- Sultzer, D.L. Psychosis and antipsychotic medications in Alzheimer’s disease: clinical management and research perspectives. Dement. Geriatr. Cogn. Disord. [CrossRef]
- Vinkers, C.H. Antipsychotics with no dopamine receptor blockade; promise or hype? Ned. Tijdschr. Geneeskd. 2020, 164, D5325. [Google Scholar] [PubMed]
- Seeman, P.; Tallerico, T. Antipsychotic drugs elicit little or no parkinsonism bind more loosely than dopamine to brain D2 receptors, yet occupy high levels of these receptors. Mol. Psychiatry 1998, 3, 123–34. [Google Scholar] [CrossRef] [PubMed]
- Cusik, E.; Gupta, V. Pimavanserin. StatPearls (Internet), Treasure Island (FL), StatPearls Publishing, 2024. Retrieved online at https://pubmed.ncbi.nlm.nih.gov/32491644/ (accessed 31 Mar 2024).
- Mathis, M.V.; Muoio, B.M.; Andreason, P.; Avila, A.M.; Farchione, T.; Atrakchi, A.; Temple, R.J. The US Food and Drug Administration’s perspective on the new antipsychotic pimavanserin. J. Clin. Psychiatry 2017, 78, e668–e673. [Google Scholar] [CrossRef] [PubMed]
- Seeman, M.V. History of the dopamine hypothesis of antipsychotic action. World J. Psychiatry 2021, 11, 355–364. [Google Scholar] [CrossRef]
- Heinz, A.; Schlagenhauf, F. Dopaminergic dysfunction in schizophrenia: salience attribution revisited. Schizophr. Bull. 2010, 36, 472–85. [Google Scholar] [CrossRef]
- Matsuzaki, H.; Hatano, M.; Iwata, M.; Saito, T.; Yamada, S. Effectiveness of clozapine on employment outcomes in treatment-resistant schizophrenia: A retrospective bidirectional mirror-image study. Neuropsychiatr. Dis. Treat. 2023, 19, 615–622. [Google Scholar] [CrossRef]
- Fenton, C.; Kang, C. Clozapine is the approved option in treatment-resistant schizophrenia and requires careful management. Drugs Ther. Perspect. 2023, 39, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Stephens, P. A review of clozapine: an antipsychotic for treatment-resistant schizophrenia. Compr. Psychiatry 1990, 31, 315–26. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Ciccarelli, M.; Vellucci, L.; Fornaro, M.; Iasevoli, F.; Barone, A. Update on novel antipsychotics and pharmacological strategies for treatment-resistant schizophrenia, Expert. Opinion on Pharmacotherapy 2022, 23, 2035–2052. [Google Scholar] [CrossRef] [PubMed]
- Howes, O.D.; Kapur, S. The dopamine hypothesis of schizophrenia: version III- the final common pathway. Schizophr. Bull. 2009, 35, 549–62. [Google Scholar] [CrossRef] [PubMed]
- Weinberger, D.R. Implications of normal brain development for the pathogenesis of schizophrenia. Arch. Gen. Psychiatry 1987, 44, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Toda, M.; Abi-Dargham, A. Dopamine hypothesis of schizophrenia: making sense of it all. Curr. Psychiatry Rep. 2007, 9, 329–336. [Google Scholar] [CrossRef]
- Peng, A.; Chai, J.; Wu, H.; Bai, B.; Yang, H.; He, W.; Zhao, Y. New therapeutic targets and drugs for schizophrenia beyond dopamine D2 receptor antagonists. Neuropsychiatric Dis. Treat. 2024, 20, 607–620. [Google Scholar] [CrossRef]
- Manseau, M.W.; Goff, D.C. Cannabinoids and schizophrenia: Risks and therapeutic potential. Neurotherapeutics 2015, 12, 816–824. [Google Scholar] [CrossRef] [PubMed]
- de Bartolomeis, A.; Barone, A.; Begni, V.; Riva, M.A. Present and future antipsychotic drugs: A systematic review of the putative mechanisms of action for efficacy and a critical appraisal under a translational perspective. Pharmacol. Res. 2022, 176, 106078. [Google Scholar] [CrossRef]
- Luykx, J.J. The future of antipsychotics studies: How innovative designs may benefit patients with psychotic disorders. Eur. Neuropsychopharmacol. 2022, 62, 46–48. [Google Scholar] [CrossRef]
- Citrome, L.; Meyer, J.M. Reviewing non-dopaminergic mechanisms for positive and negative schizophrenia symptom management. J. Clin. Psychiatry 2023, 84, 4. [Google Scholar] [CrossRef]
- Archtyes, E.D.; Hopkins, S.C.; Dedic, N.; Dworak, H.; Zeni, C.; Koblan, K. Ulotaront: review of preliminary evidence for the efficacy and safety of a TAAR1 agonist in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2023, 273, 1543–1556. [Google Scholar] [CrossRef] [PubMed]
- Scarr, E.; Gibbons, A.S.; Neo, J.; Udawela, M.; Dean, B. Cholinergic connectivity: it’s implications for psychiatric disorders. Front. Cell Neurosci. 2013, 7, 55. [Google Scholar] [CrossRef]
- Higley, M.J.; Picciotto, M.R. Neuromodulation by acetylcholine: examples from schizophrenia and depression. Curr. Opin. Neurobiol. 2014, 0, 88–95. [Google Scholar] [CrossRef]
- Vasiliu, O. Third-generation antipsychotics in patients with schizophrenia and non-responsivity or intolerance to clozapine regimen: What is the evidence? Front. Psychiatry 2022, 13, 1069432. [Google Scholar] [CrossRef]
- Caton, M.; Ochoa, E.L.M.; Barrantes, F.J. The role of nicotinic cholinergic neurotransmission in delusional thinking. npj Schizophrenia 2020, 6, 16. [Google Scholar] [CrossRef]
- Radcliffe, K.A.; Fisher, J.L.; Gray, R.; Dani, J.A. Nicotinic modulation of glutamate and GABA synaptic transmission of hypocampal neurons. Ann. N. Y. Acad. Sci. 1999, 868, 591–610. [Google Scholar] [CrossRef] [PubMed]
- Richardson, B.D.; Sottile, S.Y.; Caspary, D.M. Mechanisms of GABAergic and cholinergic neurotransmission in auditory thalamus: impact of aging. Hear. Res. 2021, 402, 108003. [Google Scholar] [CrossRef]
- Langmead, C.J.; Watson, J.; Reavill, C. Muscarinic acetylcholine receptors as CNS drug targets. Pharmacol. Ther. 2008, 117, 232–243. [Google Scholar] [CrossRef]
- Conn, P.J.; Christopoulos, A.; Lindsley, C.W. Allosteric modulators of GPCRs: a novel approach for the treatment of CNS disorders. Nat. Rev. Drug Discov. 2009, 8, 41–54. [Google Scholar] [CrossRef]
- Burger, W.A.C.; Pham, V.; Vuckovic, Z.; Powers, A.S.; Mobbs, J.I.; Laloudakis, Y.; et al. Xanomeline displays concomitant orthosteric and allosteric binding modes at the M4 mAChR. Nature Communications 2023, 14, 5440. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.H.; Adrover, M.F.; Wess, J.; Alvarez, V.A. Muscarinic regulation of dopamine and glutamate transmission in the nucleus accumbens. Proc. Natl. Acad. Sci. U. S. A. 2015, 112, 8124–8129. [Google Scholar] [CrossRef]
- Foster, D.J.; Bryant, Z.K.; Conn, P.J. Targeting muscarinic receptors to treat schizophrenia. Behav. Brain Res. 2021, 405, 113201. [Google Scholar] [CrossRef] [PubMed]
- Dean, B.; Bakker, G.; Ueda, H.R.; Tobin, A.B.; Brown, A.; Kanaan, R.A.A. A growing understanding of the role of muscarinic receptors in the molecular pathology and treatment of schizophrenia. Front. Cell Neurosci. 2023, 17, 1124333. [Google Scholar] [CrossRef]
- Raedler, T.J.; Bymaster, F.P.; Tandon, R.; Copolov, D.; Dean, B. Towards a muscarinic hypothesis of schizophrenia. Mol. Psychiatry 2007, 12, 232–246. [Google Scholar] [CrossRef]
- Bakker, G.; Vingerhoets, C.; Boucherie, D.; Caan, M.; Bloemen, O.; Eersels, J.; et al. Relationship between muscarinic M1 receptor binding and cognition in medication-free subjects with psychosis. Neuroimage Clin. 2018, 18, 713–719. [Google Scholar] [CrossRef]
- Singh, A. Xanomeline and trospium: A potential fixed drug combination (FDC) for schizophrenia- A brief review of current data. Innov. Clin. Neurosci. 2022, 19, 43–47. [Google Scholar]
- Kidambi, N.; Elsayed, O.H.; El-Mallakh, R.S. Xanomeline-trospium and muscarinic involvement in schizophrenia. Neuropsychiatr. Dis. Treat. 2023, 2023, 1145–1151. [Google Scholar] [CrossRef]
- Sramek, J.J.; Hurley, D.J.; Wardle, T.S.; Satterwhite, J.H.; Hourani, J.; Dies, F.; Cutler, N.R. The safety and tolerance of xanomeline tartrate in patients with Alzheimer’s disease. J. Clin. Pharmacol. 1995, 35, 800–806. [Google Scholar] [CrossRef]
- Paul, S.M.; Yohn, S.E.; Popiolek, M.; Miller, A.C.; Felder, C.C. Muscarinic acetylcholine receptor agonists as novel treatments for schizophrenia. Am. J. Psychiatry 2022, 179, 611–627. [Google Scholar] [CrossRef]
- Lieberman, J.A.; Javitch, J.A.; Moore, H. Cholinergic agonists as novel treatments for schizophrenia: The promise of rational drug development for psychiatry. Am. J. Psychiatry 2008, 165, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Thorn, C.A.; Moon, J.; Bourbonnais, C.A.; Harms, J.; Edgerton, J.R.; Stark, E.; et al. Striatal, Hippocampal, and Cortical Networks Are Differentially Responsive to the M4- and M1-Muscarinic Acetylcholine Receptor-Mediated Effects of Xanomeline. ACS Chem. Neurosci. 2019, 10, 3910. [Google Scholar] [CrossRef] [PubMed]
- Woolley, M.L.; Carter, H.J.; Gartlon, J.E.; Watson, J.M.; Dawson, L.A. Attenuation of amphetamine-induced activity by the non-selective muscarinic receptor agonist, xanomeline, is absent in muscarinic M4 receptor knockout mice and attenuated in muscarinic M1 receptor knockout mice. Eur. J. Pharmacol. 2009, 603(1-3), 147-9. [CrossRef]
- Burger, W.A.C.; Pham, V.; Vuckovic, Z.; Powers, A.S.; Mobbs, J.I.; Laloudakis, Y.; et al. Xanomeline displays concomitant orthosteric and allosteric binding modes at the M4 mAChR. Nat. Commun. 2023, 14, 5440. [Google Scholar] [CrossRef] [PubMed]
- Karuna Therapeutics. A study to assess efficacy and safety of KarXT in acutely psychotic hospitalized adult patients with schizophrenia (EMERGENT-2). Retrieved online: https://clinicaltrials.gov/study/NCT04659161 (accessed 26 October 2023).
- Flotros (trospium chloride). Summary of product characteristics. Retrieved online from https://www.hpra.ie/img/uploaded/swedocuments/Licence_PA22747-001-001_28052019110340.pdf (accessed Apr 13, 2024).
- Karuna Therapeuticals. Karuna Therapeutics Announces U.S. Food and Drug Administration Accepts New Drug Application for KarXT for the Treatment of Schizophrenia. Retrieved online from https://investors.karunatx.com/news-releases/news-release-details/karuna-therapeutics-announces-us-food-and-drug-administration (accessed Apr 13, 2024).
- O’Brien, E. FDA accepts NDA, grants PDUFA date for investigational schizophrenia treatment. Psychiatric Times., Nov 30, 2023. Retrieved online from https://www.psychiatrictimes.com/view/fda-accepts-nda-grants-pdufa-date-for-investigational-schizophrenia-treatment (accessed Apr 13, 2024).
- Mullard, A. Novel schizophrenia therapy filed for FDA approval. Nature Reviews Drug Discov. 2023, 22, 862. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed]
- Brown, D. A review of the PubMed PICO tool: using evidence-based practice in health education. Health Promot. Pract. 2020, 21, 496–498. [Google Scholar] [CrossRef] [PubMed]
- Schünemann, H.; Brożek, J.; Guyatt, G.; Oxman, A. (Eds.) GRADE Handbook for grading the quality of evidence and strength of recommendations. Updated 13. The GRADE Working Group, 2013. Retrieved from guidelinedevelopment.org/handbook (accessed Apr 02, 2024). 20 October.
- Kay, S.R.; Opler, L.A.; Lindemayer, J.P. Reliability and validity of the positive and negative syndrome scale for schizophrenics. Psychiatry Res. 1988, 23, 99–110. [Google Scholar] [CrossRef] [PubMed]
- Overall, J. E. , & Gorham, D. R. The Brief Psychiatric Rating Scale. Psychological Reports 1962, 10, 799–812. [Google Scholar] [CrossRef]
- Bell, M.; Milstein, R.; Beam-Goulet, J.; Lysaker, P.; Cicchetti, D. The Positive and Negative Syndrome Scale and the Brief Psychiatric Rating Scale. Reliability, comparability, and predictive validity. J. Nerv. Ment. Dis. 1992, 180, 723–8. [Google Scholar] [CrossRef]
- Karuna Therapeutics. A study to assess safety and efficacy of KarXT in adult patients with schizophrenia (EMERGENT-1). Retrieved online from https://clinicaltrials.gov/study/NCT03697252 (accessed 26 October 2023).
- Correll, C.U.; Angelov, A.S.; Miller, A.C.; et al. Safety and tolerability of KarXT (xanomeline–trospium) in a phase 2, randomized, double-blind, placebo-controlled study in patients with schizophrenia. Schizophrenia 2022, 8, 109. [Google Scholar] [CrossRef]
- Brannan, S.K.; Sawchak, S.; Miller, A.C.; Lieberman, J.A.; Paul, S.M.; Breier, A. Muscarinic Cholinergic Receptor Agonist and Peripheral Antagonist for Schizophrenia. N. Engl. J. Med. 2021, 384, 717–726. [Google Scholar] [CrossRef] [PubMed]
- Weiden, P.J.; Breier, A.; Kavanagh, S.; Miller, A.C.; Brannan, S.K.; Paul, S.M. Antipsychotic efficacy of KarXT (xanomeline-trospium): Post hoc analysis of positive and negative syndrome scale categorical response rates, time course of response, and symptom domains of response in a phase 2 study. J. Clin. Psychiatry 2022, 83, 21m14316. [Google Scholar] [CrossRef] [PubMed]
- Sauder, C.; Allen, L.A.; Baker, E.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Effectiveness of KarXT (xanomeline-trospium) for cognitive impairment in schizophrenia: post hoc analyses from a randomised, double-blind, placebo-controlled phase 2 study. Transl. Psychiatry 2022, 12, 491. [Google Scholar] [CrossRef] [PubMed]
- Kaul, I.; Sawchak, S.; Correll, C.U.; Kakar, R.; Breier, A.; Zhu, H.; Miller, A.C.; Paul, S.M.; Brannan, S.K. Efficacy and safety of the muscarinic receptor agonist KarXT (xanomeline-trospium) in schizophrenia (EMERGENT-2) in the USA: results from a randomised, double-blind, placebo-controlled, flexible-dose phase 3 trial. Lancet 2024, 403, 160–170. [Google Scholar] [CrossRef] [PubMed]
- Karuna Therapeutics. A study to assess efficacy and safety of KarXT in acutely psychotic hospitalized adult patients with schizophrenia (EMERGENT-3). Retrieved online: https://clinicaltrials.gov/study/NCT04738123 (accessed 26 October 2023).
- Kavoussi, R.; Miller, A.; Brannan, S.K.; Breier, A. Results of a double-blind, placebo-controlled, tolerability study of KarXT, a novel combination targeting muscarinic acetylcholine receptors using xanomeline with trospium chloride to mitigate cholinergic side effects. Poster presented at American Society of Clinical Psychopharmacology Annual Meeting, Miami Beach, FL. 2017.
- Breier, A.; Brannan, S.K.; Paul, S.M.; Miller, A.C. Evidence of trospium's ability to mitigate cholinergic adverse events related to xanomeline: phase 1 study results. Psychopharmacology (Berl). 2023, 240, 1191–1198. [Google Scholar] [CrossRef] [PubMed]
- Brannan, S.; Miller, A.; Felder, C.; Paul, S.; Breier, A. T106. KarXt: a m1/m4 preferring muscarinic agonist for the treatment of schizophrenia. Schizophr. Bull. 2019, 45 (Suppl 2), S244–5. [Google Scholar] [CrossRef]
- Karuna Therapeutics. An open-label study to assess the long-term safety, tolerability, effectiveness, and durability of effect of KarXT in patients with DSM-5 diagnosis of schizophrenia (PENNANT). Retrieved online: https://clinicaltrials.gov/study/NCT05643170 (accessed 9 March 2024).
- Karuna Therapeutics. Pilot study comparing effects of xanomeline alone to xanomeline plus trospium. Retrieved online from https://clinicaltrials.gov/study/NCT02831231 (accessed 01 Mar 2024).
- Karuna Therapeutics. A study to assess efficacy and safety of KarXT for the treatment of psychosis associated with Alzheimer’s disease (ADEPT-1). Retrieved online: https://clinicaltrials.gov/study/NCT05511363 (accessed 9 March 2024).
- Karuna Therapeutics. An open-label study to assess the long-term safety, tolerability, and efficacy of KarXT in adult patients with schiziphrenia (EMERGENT-5). Retrieved online: https://clinicaltrials.gov/study/NCT04820309 (accessed 26 October 2023).
- Karuna Therapeutics. An extension study to assess long-term safety, tolerability, and efficacy of KarXT in adult patients with schizophrenia (EMERGENT-4). Retrieved online: https://clinicaltrials.gov/study/NCT04659174 (accessed 26 October 2023).
- Karuna Therapeutics. A study to assess the efficacy and safety of KarXT in acutely psychotic hospitalized Chinese adult subjects with DSM-5 schizophrenia (UNITE-001). Retrieved online: https://clinicaltrials.gov/study/NCT05919823 (accessed 9 March 2024).
- Karuna Therapeutics. Open-label extension study to assess the long-term safety and tolerability of KarXT in subjects with psychosis associated with Alzheimer’s disease (ADEPT-3). Retrieved online: https://clinicaltrials.gov/study/NCT05980949 (accessed 9 March 2024).
- Karuna Therapeutics. A study to assess efficacy and safety of adjunctive KarXT in subjects with inadequately controlled symptoms of schizophrenia (ARISE). Retrieved online: https://clinicaltrials.gov/study/NCT05145413 (accessed 9 March 2024).
- Karuna Therapeutics. An extension study to assess long-term safety and tolerability of adjunctive KarXT in subjects with inadequately controlled symptoms of schizophrenia. Retrieved online: https://clinicaltrials.gov/study/NCT05304767 (accessed 9 March 2024).
- Karuna Therapeutics. A Study to Assess Efficacy and Safety of KarXT for the Treatment of Psychosis Associated With Alzheimer's Disease (ADEPT-2). Retrieved online: https://clinicaltrials.gov/study/NCT06126224?intr=xanomeline-trospium&aggFilters=results:without&rank=10 (accessed 9 March 2024).
- Shekhar, A.; Potter, W.Z.; Lightfoot, J.; Lienemann, J.; Dube, S.; Mallinckrodt, C.; et al. Selective muscarinic receptor agonist xanomeline as a novel treatment approach for schizophrenia. Am. J. Psychiatry 2008, 165, 1033–9. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, R.; Cookson, J.; Taylor, M. Numbers-needed-to-treat analysis: an explanation using antipsychotic trials in schizophrenia. Advances in Psychiatric Treatment 2011, 17, 63–71. [Google Scholar] [CrossRef]
- Stauffer, V.; Karagianis, J.; Sutton, V.; Ascher-Svanum, H.; Treuer, T.; de Lima, M.S.; et al. Number needed to treat (NNT) and number needed to harm (NNH) in randomized, blinded trials comparing olanzapine to other atypical antipsychotics for treatment of schizophrenia. Clinical Schizophrenia and Related Psychoses 2008, 2, 136–146. [Google Scholar] [CrossRef]
- Popovic, D.; Reinares, M.; Amann, B.; Salamero, M.; Vieta, E. Number needed to treat analyses of drugs used for maintenance treatment of bipolar disorder. Psychopharmacology (Berl). 2011, 213, 657–67. [Google Scholar] [CrossRef]
- Schneider, L.S. Risperidone for treating patients with dementia: systematic review of randomized, placebo-controlled clinical trials. Aging Health 2005, 1, 39–48. [Google Scholar] [CrossRef]
- Tandon, R.; Nasrallah, H.; Akbarian, S.; Carpenter, W.T. Jr.; DeLisi, L.E.; Gaebel, W.; et al. The schizophrenia syndrome, circa 2024: What we know and how that informs its nature. Schizophr. Res. 2024, 264, 1–28. [Google Scholar] [CrossRef] [PubMed]
- Davidson, M.; Carpenter, W.T. Jr. Targeted treatment of schizophrenia symptoms as they manifest, r continuous treatment to reduce the risk of psychosis recurrence. Schizophr. Bull. 2024, 50, 14–21. [Google Scholar] [CrossRef] [PubMed]
- Luvsannyam, E.; Jain, M.S.; Pormento, M.K.L.; Siddiqui, H.; Balagtas, A.R.A.; Emuze, B.O.; Poprawski, T. Neurobiology of schizophrenia: A comprehensive review. Cureus 2022, 14, e23959. [Google Scholar] [CrossRef] [PubMed]
- Foley, C.; Corvin, A.; Nakagome, S. Genetics of schizophrenia: ready to translate? Curr. Psychiatry Rep. 2017, 19, 61. [Google Scholar] [CrossRef] [PubMed]
- Leber, A.; Ramachandra, R.; Ceban, F.; Kwan, A.T.H.; Rhee, T.G.; Wu, J.; et al. Efficacy, safety, and tolerability of xanomeline for schizophrenia spectrum disorders: a systematic review. Expert Opin. Pharmacother. 2024, 1–10. [Google Scholar] [CrossRef]
- Spoelstra, S.K.; Visser, L.; Knegtering, H. Muscarinic M1 and/or M4 receptor agonists as potential novel treatments for psychoses. Tijdschr. Psychiatr. 2023, 65, 555–562. [Google Scholar] [PubMed]
- Pahwa, M.; Sleem, A.; Elsayed, O.H.; Good, M.E.; El-Mallakh, R.S. New antipsychotic medications in the last decade. Curr. Psychiatry Rep. 2021, 23, 87. [Google Scholar] [CrossRef] [PubMed]
- Tsapakis, E.M.; Diadaki, K.; Miliaras, A.; Fountoulakis, K.N. Novel compounds in teh treatment of schizophrenia- A selective review. Brain Sci. 2023, 13, 1193. [Google Scholar] [CrossRef]
- Bender, A.M.; Jones, C.K.; Lindsley, C.W. Classics in Chemical Neuroscience: Xanomeline. ACS Chem. Neurosci. 2017, 8, 435–443. [Google Scholar] [CrossRef]
- Vaidya, S.; Guerin, A.A.; Walker, L.C.; Lawrence, A.J. Clinical effectiveness of muscarinic receptor-targeted interventions in neuropsychiatric disorders: A systematic review. CNS Drugs 2022, 36, 1171–1206. [Google Scholar] [CrossRef] [PubMed]
| Operational criteria | Inclusion criteria | Exclusion criteria |
|---|---|---|
| Population | All populations were allowed, regardless of the participants' age (no inferior or superior age limit was pre-defined). The primary diagnoses allowed were schizophrenia and schizoaffective disorder, but patients with all types of severe mental disorders were included in the review. Chronic organic or psychiatric co-morbidities were allowed if screened for and managed adequately, as specified by the trial protocol. Diagnoses should be based on clearly defined criteria, according to ICD10, ICD-11, DSM IV-TR, DSM 5, or DSM 5-TR. No limitation on the initial severity of the disorder (as assessed by a validated scale) was imposed. |
Trials that did not specify the demographic and clinical characteristics of the participants. The presence of psychiatric co-morbidities with a significant impact on cognition, mood, and behavior if they were not managed during the trial, based on the specific protocols. |
| Intervention | Pharmacological intervention with xanomeline-trospium, either as monotherapy or as an add-on. No limitations regarding the dose, way of administration, or duration of the intervention were applied. |
Concomitant medication that was not monitored according to the study protocol. |
| Environment | Both in-patient and out-patient regimen. |
Unspecified environment. |
| Primary and secondary variables |
Evaluation of the efficacy, safety, and/or tolerability of xanomeline-trospium. |
All research that was using unclear outcomes. |
| Study design | Any phase of clinical investigation, from to III, that was focused on evaluating the effects of xanomeline-trospium was admitted. |
Studies with unspecified or poorly defined design (e.g., insufficiently validated instruments for monitoring symptom severity, unclear reporting procedures for adverse events, and unspecified study duration). Studies focused on the evaluation of other pharmacological agents as a primary intervention. Case reports, case series, reviews, meta-analyses. Preclinical studies. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





