1. Introduction
The retinal pigment epithelium (RPE) is a polarized monolayer of pigment cells, closely adjacent on one side to the light-sensitive photoreceptor cells of the neural retina, and on the other hand to a layer of vascular capillaries, from which it is separated by Bruch’s membrane [
1]. This arrangement of RPE cells also determines its main functions. These functions include phagocytosis of shed photoreceptor outer segments, transport and removal of metabolites from photoreceptor cells, regulation of vitamin A metabolism and control of the visual cycle, absorption of scattered light, regulation of ion currents, production of growth factors for photoreceptors, and maintenance of the blood-retinal barrier [
1,
2,
3].
There are three main types of pigment granules in RPE cells: lipofuscin granules (LG), containing the fluorescent “age pigment” lipofuscin, melanosomes, specialized organelles of RPE cells containing a protein part and a polymer of the eumelanin type, and complex granules containing simultaneously both types of pigments - melanin and lipofuscin, melanolipofuscin-like granules (MLLG). Melanosomes in RPE cells are formed during the prenatal period of development. Their functions are screening photoreceptor cells from exposure to excess light and antioxidant protection of cells from free radical oxidation [
4,
5,
6,
7]. Long-term exposure to light irradiation and intracellular oxidants are likely the factors that lead to a decrease in the number of melanosomes with age [
8,
9].
During life, lipofuscin granules accumulate in the RPE, which are a product of incomplete degradation of the outer segments of photoreceptor cells of the retina. LGs have strong fluorescence and photosensitizing properties, which are due to the bisretinoids present in their composition. The most studied bisretinoid is N-retinylidene-N-retinylethanolamine (A2E). When irradiated with visible light, LGs are capable of generating reactive oxygen species (ROS), in particular, the generation of superoxide radicals [
10,
11,
12,
13,
14]. Photodegradation of bisretinoids produces toxic water-soluble products [
15,
16,
17,
18,
19].
With ageing, melanosomes can fuse with lipofuscin granules [
20,
21] or with partially degraded phagosomes [
22,
23,
24] to form mixed melanolipofuscin-like granules. During aging, changes in the density of all pigment granules are observed. Similar processes occur when various retinal pathologies occur, such as Stargardt disease and age-related macular degeneration (AMD) [
25]. In this case, a decrease in the number of melanosomes occurs, which is accompanied by a simultaneous increase in the number of LG and MLLG [
26,
27]. The total amount of lipofuscin-containing granules can occupy up to a third of the RPE cell volume in people over 70 years of age [
26]. Melanin in MLLG can undergo photooxidative degradation caused by ROS generated by bisretinoids of the lipofuscin part of MLLG with the formation of water-soluble fluorescent products [
9,
28,
29]. Oxidative degradation of RPE melanosomes leads, on the one hand, to a decrease in their antioxidant activity, and on the other, to the appearance of products with pro-oxidant properties and increased photoreactivity [
30,
31,
32]. Moreover, as we have previously shown, the products of photooxidative degradation of melanin are themselves photoinduced superoxide generators and contain active carbonyl compounds [
29]. It can be assumed that the accumulation of pro-oxidant factors in the MLLG will contribute to their accelerated damage upon irradiation with light and the destruction of both the melanin and lipofuscin parts of the granule. In this case, the age-related decrease in the concentration of melanin in the RPE cell will be the result of a decrease in the number of melanosomes due to their fusion with LG and the subsequent degradation of melanin already inside these complex granules, i.e. in the MLLG. Degradation of melanin will lead to a decrease in the specific gravity of MLLG in the RPE cell and to the accumulation of mixed granules with small impurities of melanin that have not yet had time to be destroyed under the influence of superoxide radicals. In [
29], we showed that a decrease in the concentration of melanin in RPE cells of the human eye with age is due to its oxidative degradation by reactive oxygen species generated under the influence of light by LG in the composition of MLLG.
The purpose of this work was to study the process of melanolipofuscinogenesis during aging and under light exposure. Studying such processes in laboratory conditions seems convenient and possible when using the Japanese quail
Coturnix japonica as an experimental model. The advantages of this model are due to the short life span of birds, the structure of the retina and carotenoid metabolism similar to humans. In this model, human-like pathological and age-related changes occurring in the RPE and macular region of the retina can be observed on an accelerated time scale. In addition, the Japanese quail, as an object, has been quite well studied and is widely used in experimental studies [
33,
34]. The aging process of Japanese quail is accompanied by repeated accumulation of the aging pigment lipofuscin in RPE cells. This accumulation (by the end of a quail’s life, the amount of LG in RPE cells increases 5-8 times) occurs approximately 50 times faster than in human RPE cells.
We have previously described age-related changes in the ultrastructure of melanosomes in the pigment cells of the Japanese quail choroid, namely, a violation of the correct shape and homogeneity of the contents of the granules [
35,
36]. It has been shown that the total number of LG and MLLG in the RPE of Japanese quail increases with age [
33]. In this work, we studied the dynamics of changes in morphology, surface area and number of pigment granules in Japanese quail RPE cells depending on age and the damaging effects of blue irradiation.
2. Results
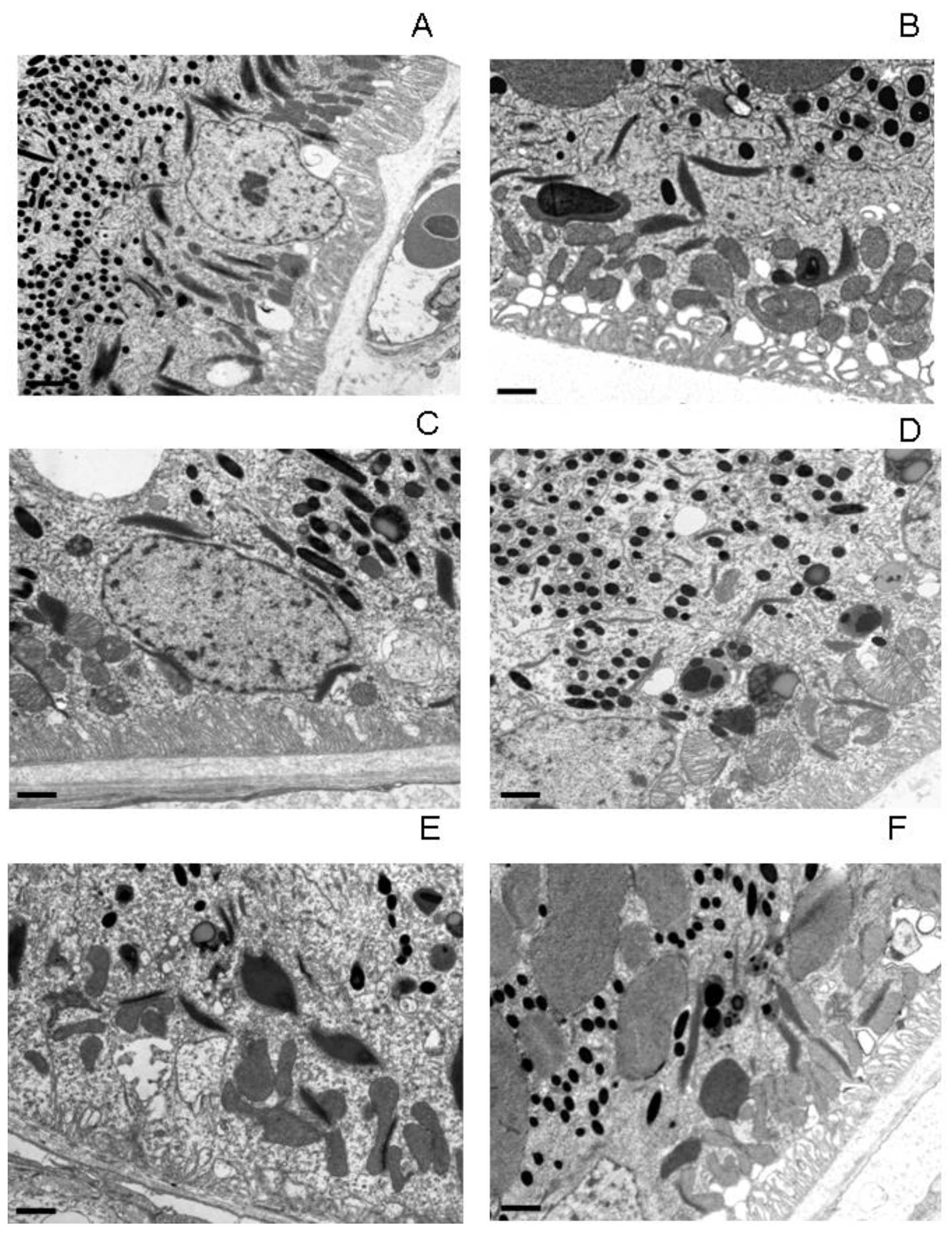
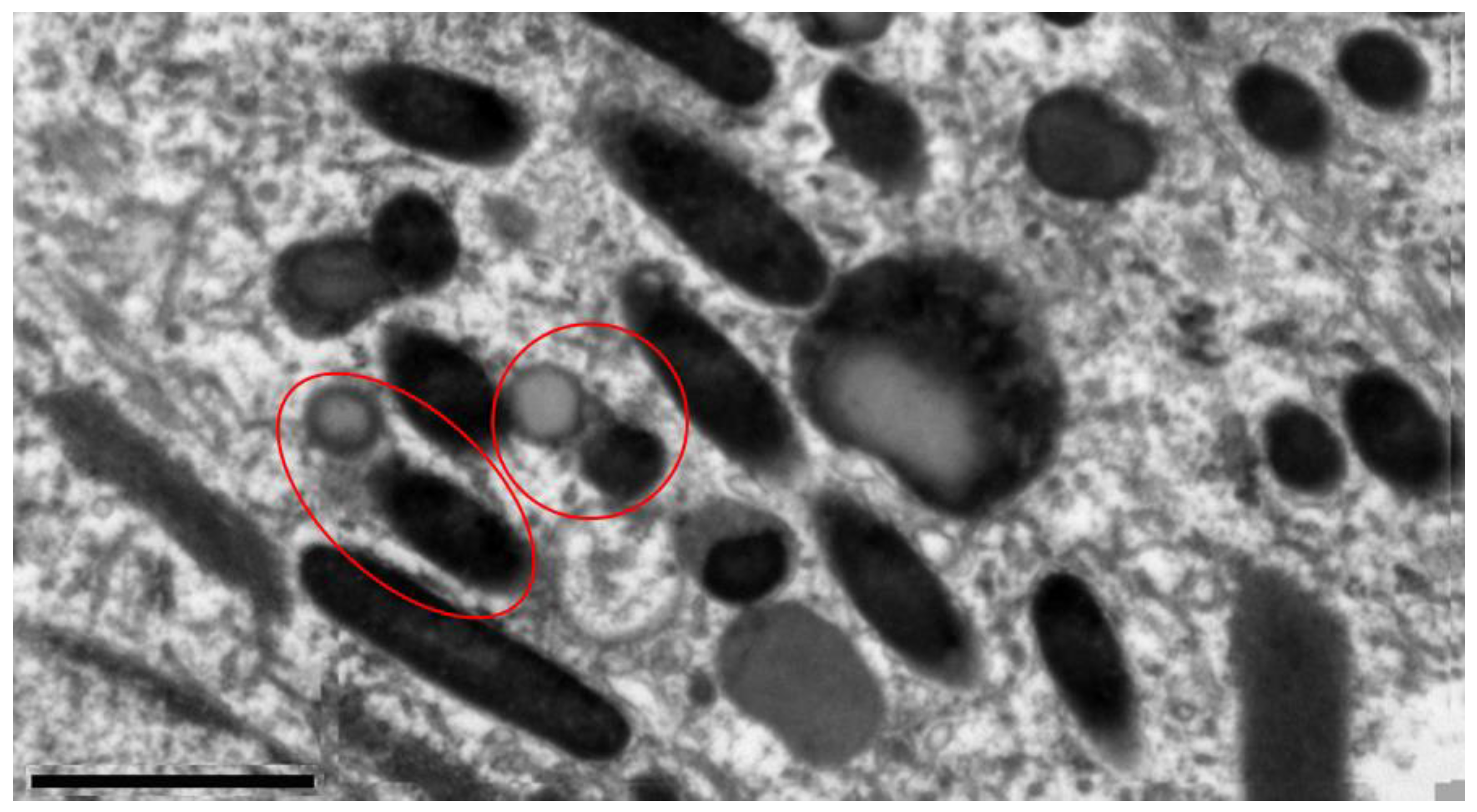
Electron microscopic analysis of RPE cells showed (
Figure 1) that with aging and irradiation with blue light, pronounced changes occur the subcellular level: the structure of the main cellular components - nuclei and mitochondria - was disrupted, LG and MLLG were formed and accumulated, and the basal infoldings were deformed.
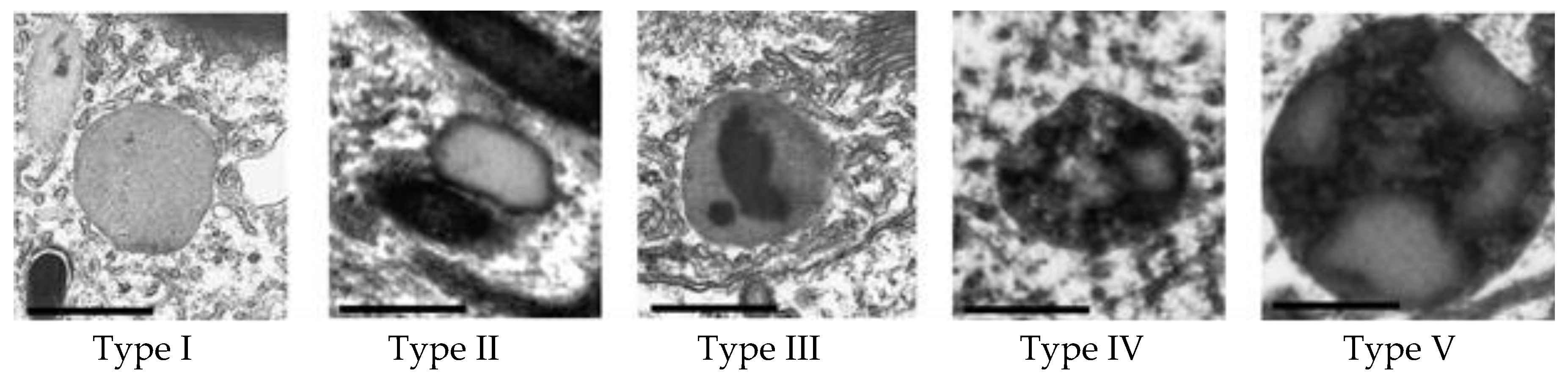
To study the dynamics of melanolipofuscinogenesis in RPE cells of different age groups of quails depending on age and light exposure, we identified and characterized 5 types of granules containing lipofuscin and melanin (
Figure 2).
Type I granule is a typical lipofuscin granule with homogeneous light content, surrounded by a membrane and containing no melanin. Granules of types II−V contain both lipofuscin and melanin (MLLG - melanolipofuscin-like granules). Type II granule is a melanolipofuscin granule, which is surrounded by a dense rim of melanin nature and has an internal content similar to lipofuscin. Melanolipofuscin granule type III is a lipofuscin granule containing melanin inside, which may be partially degraded. Type IV granule is a type III cohesive melanolipofuscin granule and is characterized by optically highly heterogeneous content with a large number of inclusions of different shapes and sizes. Type V granule is a megamelanolipofuscin granule (˃0.5 µm2), apparently formed as a result of the fusion of smaller type IV granules.
Visual electron microscopic analysis revealed, after light exposure, increased formation of MLLG due to the fusion of melanosomes and LG in RPE cells of quails of different ages (
Figure 1). It can be assumed that there are two mechanisms for this process: in the first case, the melanosome absorbs LG, enveloping it (type II,
Figure 2), and in the second case, on the contrary, the lipofuscin granule absorbs the melanosome (type III,
Figure 2). Similar results were obtained in [38], in which the authors discovered MLLGs containing lipofuscin both in the center and at the periphery of the granule. These two mechanisms appear to occur with equal probability throughout the life of the organism. When irradiated with intense blue light, LGs begin to actively generate ROS, which, in turn, are deactivated by melanosomes. As a result, during the interaction of melanin with ROS, melanin pigment is degraded, and the damaged melanosome can be absorbed by LG.
The most pronounced effect of the formation and accumulation of MLLG as a result of light exposure is observed in middle-aged quails (
Figure 1C,D). This is probably due to the fact that the RPE of young birds still contains too little LGs necessary for the formation of MLLG (
Figure 1A,B), while in old birds the number of melanosomes becomes significantly smaller and, as a consequence, their protective function, such as the formation of MLLGis weakened (
Figure 1E,F). Therefore, middle-aged quails, which, on the one hand, still have many intact melanosomes, and, on the other hand, have accumulated quite a lot of lipofuscin granules, were chosen for a detailed study of the effect of light irradiation on morphological changes in RPE cells.
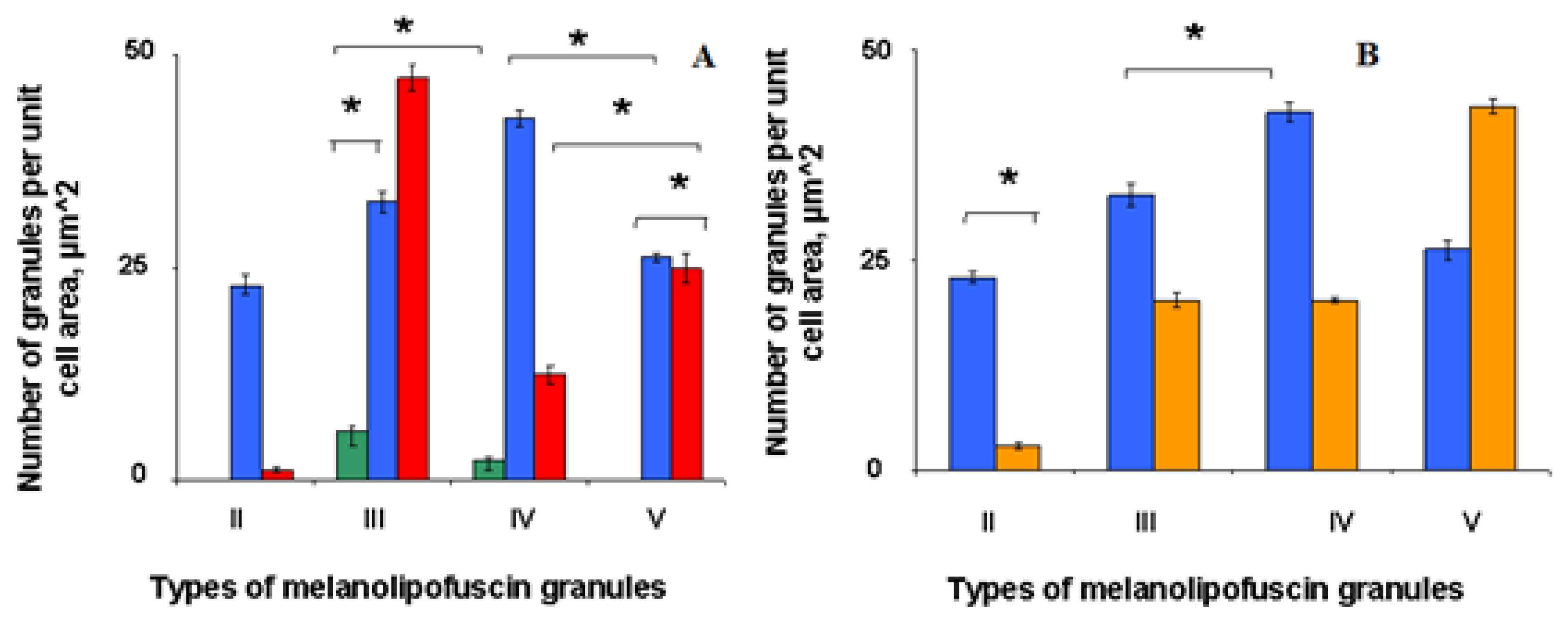
Morphometric analysis showed (
Figure 3) that in middle-aged birds the content of MLLG granules of types II, III, IV per cell decreased after light irradiation, and for type V granules it reliably increased almost twice (
Figure 3). This is probably due to the fact that these granules further fuse with each other to form giant MLPGs.
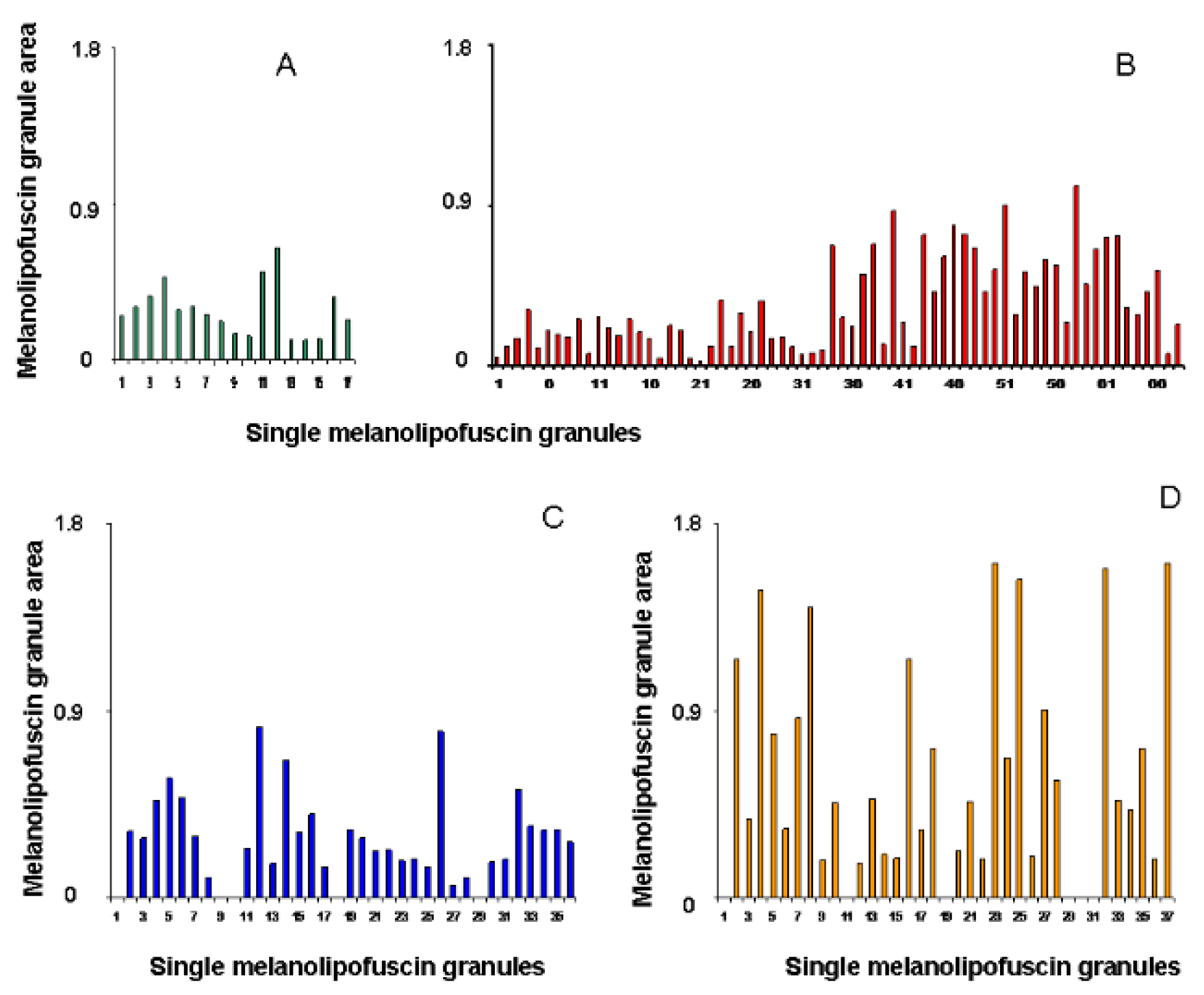
The histogram of the distribution of areas of individual granules showed an enlargement of MLLGs of different types with age and, mainly, after light exposure, as a result of which the area of MLLGs increased by 2 or more times (
Figure 4).
The number of different types of MLLGs formed may reflect the priority mechanism of their formation. Based on our data, we can assume that the process of formation of melanolipofuscin granules during the aging process in the initial stage occurs with the formation, mainly, of type II MLPG, which then transforms into granules of types III, IV and V. In old birds, MLLG type III predominates in RPE cells. The damaging effect of light in the RPE of middle-aged birds (
Figure 3B) leads to a decrease in the number of granules of types II, III and IV, while the number of granules of type V increases. The development of photooxidative stress, accompanied by the generation of ROS by lipofuscin granules, probably causes accelerated formation of MLLG, destruction of melanin in the composition of these complex granules and their further fusion with the formation of megagranules (
Figure 5).
3. Discussion
The main result of the work comes down to to the following: a) when blue light acts on the quail’s eye, active fusion of melanosomes and lipofuscin granules occurs with the formation of various types of mixed MLLGs; b) irradiation leads to twofold decrease in the amount of LG(dark - 19.6±0.8 and light - 9.8±0.5) and a synchronous increase in the amount of MLLG in the quail RPE cell; c) increased accumulation of MLLG was also observed in non-irradiated quails with age - in old birds.
The results of our morphometric analysis of granules in the quail RPE are somewhat different from the results obtained previously in works [
31,
32]. Thus, in [
31], when assessing morphological changes in the RPE, not only canonical LGs, but also all types of melanolipofuscin-like granules were taken into account as lipofuscin granules. For this reason, the diameters of the granules were averaged in such a way that, as a result of irradiation, the total area of the LG per unit cell area decreased.We took into account all subtypes of LG and MLLG, including those whose area increased after exposure to light. It turned out that it was with these granules that the greatest changes, both qualitative and quantitative, occurred (
Figure 3 and
Figure 4). At the same time, the ultrastructure of RPE cells, both in our work and in the works [
33,
34], was visually identical.
In [38], the authors assessed the number of LG and MLLG granules in the RPE of the human eye using the SIM method based on the intensity of the fluorescence signal. In this case, it is possible that some of the melanosomes containing partially oxidized, fluorescent products could be classified as MLLG, since there was no confirmation of the type of granules by electron microscopy. It is possible that the visualization parameters were not set quite correctly during the calculation, as a result of which a significant number of granules could be taken into account repeatedly and, as a result, distort the final value of the number of granules per cell. According to the same authors [38], more MLLG is present in the foveal region, while more LG is present in the periphery of the retina, which is consistent with the results presented in [39] about more intense fluorescence of retinal areas located closer to the center of the fundus. This fluorescence is most likely due to the fluorescence of oxidized bisretinoids [40], while more unoxidized bisretinoids are located in the periphery.
Thus, under photooxidative stress, active fusion of LG and melanosomes occurs with the formation of MLLG. In this case, an inverse correlation is observed between the content of LG and MLLG: the amount of LG decreases, and MLLG increases. It is logical to assume that MLLG contains more oxidized and degraded products than the original LG, therefore the maximum of their fluorescence should be shifted to a shorter wavelength region. This assumption is confirmed by the data presented in [41], where it was shown that larger granules are located closer to the center of the RPE. These were probably melanolipofuscin granules [38], whose fluorescence maximum is in a shorter wavelength region than that of smaller granules (most likely the original LGs) located on the periphery of the fundus [38,41].
It is known that in the pathogenesis of AMD there is an increased accumulation of MLLG, and not LG [
26,42,43]. This is in good agreement with our assumption that under oxidative stress, melanosomes more actively fuse with lipofuscin granules, which helps prevent the release of ROS generated by lipofuscin bisretinoids into the cell cytoplasm.
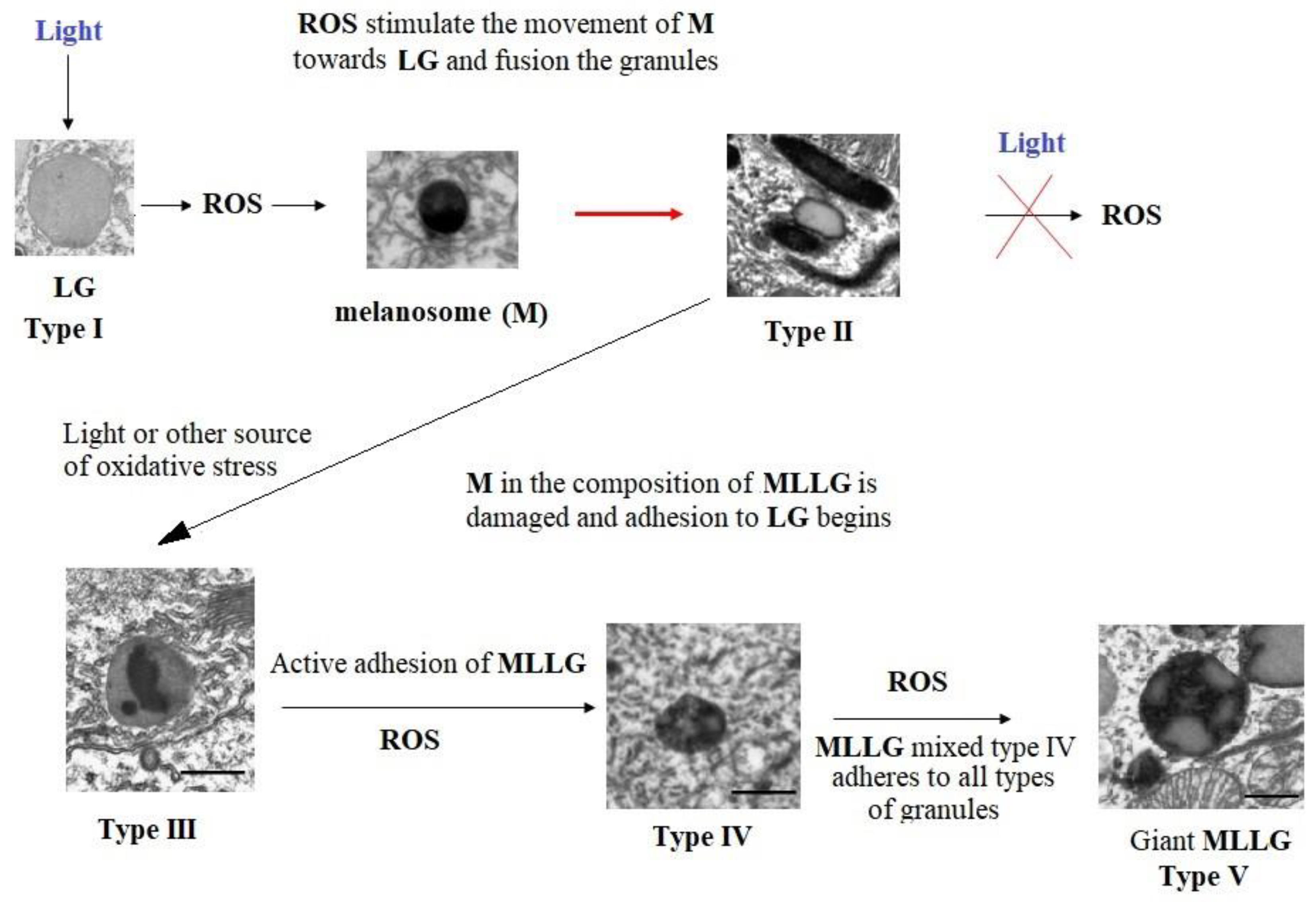
To summarize, we can propose a hypothetical scheme for the development of melanolipofuscinogenesis in RPE cells (
Figure 6).
According to the above scheme, the action of light causes the generation of ROS toxic to the RPE cell by lipofuscin granules, triggering cascades of intracellular signaling mechanisms that promote the movement of melanosomes toward to lipofuscin granules and their fusion with the formation of type II granules and preventing the release of ROS into the cell cytoplasm. If oxidative stress does not stop, then melanin gradually begins to degrade under the influence of superoxide radicals and its water-soluble products contribute to its further destruction, which leads to the formation of type III granules. With further mutual degradation of LG and melanin in the composition of MLLG, additional fusion of granules of types I, II and III occurs with the formation of complex mixed granules of type IV. With the damaging effects of light and with age, all this leads to the formation of giant melanolipofuscin granules of type V. And, ultimately, to the gradual disappearance of melanin in the composition of RPE cells.
The mechanism of accelerated formation of complex melanolipofuscin granules under photooxidative stress may be due to the activation of melanosome transport towards the source of generation of superoxide radicals, i.e. towards lipofuscin granules. It is known that intracellular organelles do not move by free diffusion [44]. For this purpose, the cell uses motor proteins (kinesins, dyneins and myosins), which move either along microtubules or along actin filaments [45,46]. The interaction of organelles with motor proteins is mediated by small GTPases, which are involved in the selection of translocated organelles, as well as in their intracellular transport, docking and fusion [46–48]. In RPE cells, such functions are performed by proteins of the Rab family [46,49,50]. It is known that superoxide anion radicals can enhance the dissociation of GDP from the Rab protein molecule with the subsequent addition of a GTP molecule to it, which leads to the activation of Rab GTPase [51,52]. It can be assumed that activation of the Rab-melanosome complex in RPE cells causes the movement of the melanosome and its fusion with the lipofuscin granule, which leads to increased accumulation of type II melanolipofuscin granules. At the same time, the release of ROS into the cell cytoplasm is blocked, since the resulting radicals are utilized on the melanin matrix. Thus, this process is a protective reaction that reduces the toxic effect of lipofuscin granules. However, with prolonged exposure to photo-oxidative stress, gradual degradation of melanin occurs in MLLG granules under the action of superoxide radicals generated by lipofuscin, with the formation of water-soluble destruction products that can also induce light-dependent degradation of melanin [29]. This leads to the formation of various types of melanolipofuscin granules, including giant MLLGs. As part of MLLG, melanin degrades under natural conditions of the retina both as a result of interaction with ROS generated by lipofuscin bisretinoids, and as a result of reaction with ROS generated by melanin destruction products.
4. Materials and Methods
4.1. Animals
The work was carried out on sexually mature individuals of Japanese quail aged from two to seventeen months. Experiments were conducted on females, which age faster than males and exhibit higher sensitivity to photodamage [
33,
34]. Based on data on the egg production of birds and the lipofuscin content in RPE cells depending on age, the following age groups were identified: young (9-25 weeks), middle (35-40 weeks), and old (52-78 weeks). In each age group, 3-6 birds were studied. Quails were kept under standard daily illumination with an incandescent lamp (15 hours of light / 9 hours of darkness) at the experimental base of the State Research Center of the Russian Federation Institute of Biomedical Problems of the Russian Academy of Sciences within the framework of the Agreement on scientific and technical cooperation No. 59-23 dated 04/03/2023. The birds received balanced feed for adult quail brand PK1P-18154-776 (JSC Istra-Hleboproduct, veterinary certificate No. 10015 dated September 19, 2017, GOST R 51851-2001). Monitoring the life support of birds and their removal from the experiment by decapitation was carried out in accordance with the “Rules of Laboratory Practice” approved by Order of the Ministry of Health of the Russian Federation dated April 1, 2016 No. 199n.
4.2. Blue Light Irradiation
For light irradiation, a blue LED source (λ
max = 450 nm) (LED450) was used in a photodamaging (sublethal) dose for the retina (4.0 J/cm
2 of the corneal surface when irradiated for 40 min) [
37]. Eye irradiation was performed on intact animals. The right eye of the birds was exposed to irradiation with “manual” fixation of the bird’s head in relation to the light flux and dilated eyelids, the left eye remained without exposure (control). The effects of photodamaging light were assessed 24 hours after irradiation. The birds were removed from the experiment by decapitation, the eyes were enucleated, the posterior hemisphere was isolated, cut into fragments with an area of approximately 1.0 cm
2, containing the central part of the retina with RPE and then placed in fixative solutions.
4.3. Microscopic Studies
Tissue samples were fixed in a mixture of 2.5% glutaraldehyde (Pan Real, Spain) with 2% formaldehyde (MP Biomedicals, France) in 0.1 M PBS (Amresco, USA; pH 7.2-7.4) for 6-8 hours at +40C. Then the material was postfixed with 1% OsO4 (Electron Microscopy Sciences, USA), contrasted with 70% alcohol with 2% uranyl acetate (Electron Microscopy Sciences, USA), dehydrated in alcohols of increasing concentration and in acetone (ChimMed, Russia) and filled with a mixture of epoxy resins (Epon 812, Fluka, Germany). To orient the material, semi-thin sections (1-2 µm thick) were prepared on a pyromicrotome (LKB, Sweden), stained with a 1% aqueous solution of methylene blue (Isolab, Germany. Ultrathin transverse sections of the retina were additionally contrasted with 2% uranyl acetate (Electron Microscopy Sciences, USA) and lead citrate (Electron Microscopy Sciences, USA) according to Reynolds, and then viewed in a JEM-1011 transmission electron microscope (JEOL, Japan) at magnifications of 8000x−25000x.
4.4. Morphometric Analysis
The number and area of different types of lipofuscins and melanolipofuscin granules were measured using electron microscopy photographs in RPE cells using ImageJ software (Wayne Rasband, USA). At least 10 RPE cells were analyzed for each animal.
4.5. Statistical Analysis
Statistical processing was performed using GraphPad Prism 8.00 software (GraphPad Software, USA). Normality of distribution was determined using the Shapiro-Wilk test (ɑ˃0.05). Between-group differences were assessed using one-way ANOVA with Tukey's multiple comparison test. P-values ≤ 0.05 were considered statistically significant.
Author Contributions
Conceptualization, N.S., N.T., M.Y., A.D., and M.O.; methodology, N.S., N.T., P.Z. and M.O.; investigation, N.S., N.T., M.Y., A.D. and P.Z.; data curation, N.T., M.Y., A.D., P.Z. and M.O.; writing—review and editing, N.S.; N.T., M.Y., A.D., and M.O.; writing—original draft preparation, N.S, N.T. and M.Y.; funding acquisition, M.O. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Ministry of Science and Higher Education of the Russian Federation, Project no. 122041400102-9.
Institutional Review Board Statement
Breeding chickens and monitoring their life support was carried out at the Institute of Biomedical Problems of the Russian Academy of Sciences within the framework of the Agreement on scientific and technical cooperation No. 59-23 dated 04/03/2023. The quails were kept under standard lighting with an incandescent lamp with a daily cycle of 15 hours of light and 9 hours of darkness at the experimental base of the State Research Center of the Russian Federation, Institute of Biomedical Problems, Russian Academy of Sciences. The birds were fed balanced feed for adult quails PK1P-18154-776 (JSC Istra-Khleboprodukt, veterinary certificate No. 10015 dated September 19, 2017, GOST R 51851-2001). The keeping of birds and their removal from the experiment by decapitation was carried out in accordance with the “Rules of Laboratory Practice” approved by Order of the Ministry of Health of the Russian Federation dated April 1, 2016 N 199n.The breeding of chickens and control of their life support were carried out at the Institute of Biomedical Problems of the Russian Academy of Sciences within the framework of the Agreement on Scientific and Technical Cooperation.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the article and on request from the corresponding author.
Acknowledgments
We thank Gurieva T.S., Mednikova E.I. for technical support for experimental and model animals from Institute of medical-biological problems RAS.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Strauss, O. The retinal pigment epithelium in visual function. Physiological Reviews. 2005, 85, 845–881. [Google Scholar] [CrossRef] [PubMed]
- Bonilha, V.L. Age and disease-related structural changes in the retinal pigment epithelium. Clinical Ophthalmology. 2008, 2, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Kaarniranta, K.; Hyttinen, J.; Ryhanen, T.; Viiri, J.; Paimela, T.; Toropainen, E.; Sorri, I.; Salminen, A. Mechanisms of protein aggregation in the retinal pigment epithelial cells. Frontiers in Bioscience-Landmark (Elite Ed). 2010, 2, 1374–1384. [Google Scholar] [CrossRef] [PubMed]
- Ostrovsky, M.A.; Sakina, N.L.; Dontsov, A.E. Antioxidative role of eye screening pigments. Vision Res. 1987, 27, 893–899. [Google Scholar] [CrossRef]
- Wang, Z.; Dillon, J.; Gaillard, E.R. Antioxidant Properties of Melanin in Retinal Pigment Epithelial Cells. Photochem. Photobiol. 2006, 82, 474–479. [Google Scholar] [CrossRef]
- Ostrovsky, M.A.; Zak, P.P.; Dontsov, A.E. Vertebrate eye melanosomes and invertebrate eye ommochromes as screening cell organelles. Biology Bulletin. 2018, 45, 570–579. [Google Scholar] [CrossRef]
- Ostrovsky, M.A.; Dontsov, A.E. Vertebrate Eye Melanosomes and Invertebrate Eye Ommochromes as Antioxidant Cell Organelles: Part 2. Biol. Bulletin. 2019, 46, 105–116. [Google Scholar] [CrossRef]
- Sarna, T.; Burke, J.M.; Korytowski, W.; Rózanowska, M.; Skumatz, C.M.B.; Zareba, A.; Zareba, M. Loss of melanin from human RPE with aging: possible role of melanin photooxidation. Exp Eye Res. 2003, 76, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Dontsov, A.E.; Sakina, N.L.; Ostrovsky, M.A. Loss of melanin by eye retinal pigment epithelium cells is associated with its oxidative destruction in melanolipofuscin granules. Biochemistry (Moscow). 2017, 82, 916–924. [Google Scholar] [CrossRef]
- Boulton, M.; Dontsov, A.; Jarvis-Evans, J.; Ostrovsky, M.; Svistunenko, D. Lipofuscin is a photoinducible free radical generator. J. Photochem. Photobiol. B. Biol. 1993, 19, 201–204. [Google Scholar] [CrossRef]
- Rozanowska, M.; Jarvis-Evans, J.; Korytowski, W.; Boulton, M.E.; Burke, J.M.; Sarna, T. Blue light-induced reactivity of retinal age pigment: In vitro generation of oxygen-reactive species. Journal of Biological Chemistry. 1995, 270, 18825–18930. [Google Scholar] [CrossRef] [PubMed]
- Rozanowska, M.; Wessels, J.; Boulton, M.; Burke, J.M.; Rodgers, MA.J.; Truscott, T.G.; Sarna, T. . Blue light-induced singlet oxygen generation by retinal lipofuscin in non-polar media. Free Radical in Biology and Medicine. 1998, 24, 1107–1112. [Google Scholar] [CrossRef] [PubMed]
- Boulton, M.; Rozanowska, M.; Rozanowski, B.; Wess, T. The photoreactivity of ocular lipofuscin. Photochemical & Photobiological Sciences. 2004, 3, 759–764. [Google Scholar]
- Dontsov, A.E.; Sakina, N.L.; Ostrovsky, M.A. . Comparative study of the dark and light induced toxicity of lipofuscin granules from human retinal pigment epithelium and their chromophore A2E on the cardiolipin liposome model. Russian Chemical Bulletin. 2012, 61, 442–448. [Google Scholar] [CrossRef]
- Sparrow, J.R.; Vollmer-Snarr, H.R.; Zhou, J.; Jang, Y.P.; Jockusch, S.; Itagaki, Y.; Nakanishi, K. A2E-epoxides damage DNA in retinal pigment epithelial cells. Vitamin E and other antioxidants inhibit A2E-epoxide formation. J. Biol. Chem. 2003, 278, 18207–18213. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Keller, LM.M.; Dillon, J.; Gaillard, E.R. Oxidation of A2E results in the formation of highly reactive aldehydes and ketones. Photochemistry and Photobiology. 2006, 82, 1251–1257. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.A.; Sakina, N.L.; Kononikhin, A.S.; Feldman, T.B.; Nikolaev, E.N.; Dontsov, A.E.; Ostrovsky, M.A. . Detection and study of the products of photooxidation of N-Retinylidene-N-retinylethanolamine (A2E), the fluorophore of lipofuscin granules from retinal pigment epithelium of human donor eyes. Dokl. Biochemistry and Biophysics. 2006, 409, 223–225. [Google Scholar] [CrossRef] [PubMed]
- Yoon, K.D.; Yamamoto, K.; Ueda, K.; Zhou, J.; Sparrow, J.R. A novel source of methylglyoxal and glyoxal in retina: implications for age-related macular degeneration. PLoS ONE. 2012, 7, e41309. [Google Scholar] [CrossRef] [PubMed]
- Yakovleva, M.; Dontsov, A.; Trofimova, N.; Sakina, N.; Kononikhin, A.; Aybush, A.; Gulin, A.; Feldman, T.; Ostrovsky, M. Lipofuscin granule bisretinoid oxidation in the human retinal pigment epithelium forms cytotoxic carbonyls. Int. J. Mol. Sci. 2022, 23, 222. [Google Scholar] [CrossRef]
- Feeney, L. Lipofuscin and melanin of human retinal pigment epithelium: fluorescence, enzyme cytochemical, and ultrastructural studies. Invest. Ophthalmol. Vis. Sci. 1978, 17, 583–600. [Google Scholar] [PubMed]
- Taubitz, T.; Tschulakow, A.V.; Tikhonovich, M.; Illing, B.; Fang, Y.; Biesemeier, A.; Julien-Schraermeyer, S.; Schraermeyer, U. Ultrastructural alterations in the retinal pigment epithelium and photoreceptors of a Stargardt patient and three Stargardt mouse models: indication for the central role of RPE melanin in oxidative stress. Peer J. 2018, 6, e5215. [Google Scholar] [CrossRef] [PubMed]
- Schraermeyer, U.; Stieve, H. A newly discovered pathway of melanin formation in cultured retinal pigment epithelium of cattle. Cell Tissue Res. 1994, 276, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Wavre-Shapton, S.T.; Meschede, I.P.; Seabra, M.C.; Futter, C.E. Phagosome maturation during endosome interaction revealed by partial rhodopsin processing in retinal pigment epithelium. J Cell Sci. 2014, 127, 3852–3861. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Y.; Tschulakow, A.V.; Schraermeyer, U. Melanosomes degrade lipofuscin and precursors that are derived from photoreceptor membrane turnover in the retinal pigment epithelium—an explanation for the origin of the melanolipofuscin granule. BioRxiv. 2022. [Google Scholar] [CrossRef]
- Meleppat, R.K.; Ronning, K.E.; Karlen, S.J.; Burns, M.E.; Pugh, E.N. Jr; Zawadzki, R.J. In vivo multimodal retinal imaging of disease-related pigmentary changes in retinal pigment epithelium. Nature portfolio. 2021, 11, 16252. [Google Scholar] [CrossRef] [PubMed]
- Feeney-Burns, L.; Hilderbrand, E.S.; Eldridge, S. Aging human RPE: morphometric analysis of macular, equatorial, and peripheral cells. Invest. Ophthalmol. Vis. Sci. 1984, 25, 195–200. [Google Scholar] [PubMed]
- Feeney-Burns, L.; Burns, R.P.; Gao, C.L. Age-related Macular Changes in Humans Over 90 Years old. American J. Ophthalmol. 1990, 109, 265–278. [Google Scholar] [CrossRef] [PubMed]
- Gulin, A.A.; Dontsov, A.E.; Yakovleva, M.A.; Trofimova, N.N.; Aybush, A.V.; Vasin, A.A.; Ostrovsky, M.A. Oxidative destruction of human RPE melanosomes induced by superoxide radicals leads to the formation of reactive aldehydes and ketones. St. Petersburg State Polytechnical University Journal. Physics and Mathematics. 2022, 15, 311–316. [Google Scholar]
- Dontsov, A.E.; Yakovleva, M.A.; Vasin, A.A.; Gulin, A.A.; Aybush, A.V.; Nadtochenko, V.A.; Ostrovsky, M.A. . Understanding the Mechanism of Light-Induced Age-Related Decrease in Melanin Concentration in Retinal Pigment Epithelium Cells. International Journal of Molecular Sciences. 2023, 24, 13099. [Google Scholar] [CrossRef]
- Zadlo, A.; Rozanowska, M.B.; Burke, J.M.; Sarna, T.J. Photobleaching of retinal pigment epithelium melanosomes reduces their ability to inhibit iron induced peroxidation of lipids. Pigment Cell Res. 2007, 20, 52–60. [Google Scholar] [CrossRef]
- Rózanowski, B.; Burke, J.M.; Boulton, M.E.; Sarna, T.; Rózanowska, M. Human RPE melanosomes protect from photosensitized and iron-mediated oxidation but become pro-oxidant in the presence of iron upon photodegradation. Invest.Ophthalmol. Vis. Sci. 2008, 49, 2838–2847. [Google Scholar] [CrossRef]
- Olchawa, M.M.; Szewczyk, G.M.; Zadlo, A.C.; Krzysztynska-Kuleta, O.I.; Sarna, T.J. The effect of aging and antioxidants on photoreactivity and phototoxicity of human melanosomes: in vitro study. Pigment Cell Melanoma Res. 2020, 34, 670–682. [Google Scholar] [CrossRef] [PubMed]
- Fite, K.V.; Bengston, L. Aging and sex-related changes in the outer retina of Japanese quail. Current Eye Research. 1989, 8, 1039–1048. [Google Scholar] [CrossRef] [PubMed]
- Fite, K.V.; Bengston, L.; Donaghey, B. Experimental Light Damage Increases Lipofuscin in the Retinal Pigment Epithelium of Japanese Quail (Coturnix coturnix Japonica). Exp. Eye Res. 1993, 57, 449–460. [Google Scholar] [CrossRef] [PubMed]
- Sereznikova, N.N.; Pogodina, L.S.; Lipina, T.V.; Trofimova, N.N.; Gurieva, T.S.; Zak, P.P. Age-related adaptive responses of mitochondria of the retinal pigment epithelium to the everyday blue LED lighting. Doklady Biological Sciences. 2017, 475, 141–143. [Google Scholar] [CrossRef]
- Serejnikova, N.B.; Pogodina, L.S.; Trofimova, N.N.; Zak, P.P. Study of short-wave visible light on retinal pigment epithelial cells of the Japanese quail Coturnix japonica (electron microscopic study). IX International theoretical and practical conference "Present scientific achievement-2013"Praha, Publishing House «Education and Science» s.r.o. 2013, 59, 78–81. [Google Scholar]
- Van Norren, D.; Gorgels, T.G. The action spectrum of photochemical to the retina: a review of monochromatical threshold data. Photochem. Photobiol. 2011, 87(4), 747–753. [Google Scholar] [CrossRef] [PubMed]
- Bermond, K.; von der Emde, L.; Tarau, I.-S.; Bourauel, L.; Heintzmann, R.; Holz, F.G.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent Organelles Within the Retinal Pigment Epithelium in Human Donor Eyes with and without Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2022, 63, 23. [Google Scholar] [CrossRef]
- Ablonczy, Z.; Higbee, D.; Grey, A.C.; Koutalos, Y.; Schey, K.L.; Crouch, R.K. Similar molecules spatially correlate with lipofuscin and N-retinylidene-N-retinylethanolamine in the mouse but not in the human retinal pigment epithelium. Archives of Biochemistry and Biophysics. 2013, 539, 196–202. [Google Scholar] [CrossRef]
- Feldman, T.B.; Yakovleva, M.A.; Arbukhanova, P.M.; Borzenok, S.A.; Kononikhin, A.S.; Popov, I.A.; Nikolaev, E.N.; Ostrovsky, M.A. Changes in spectral properties and composition of lipofuscin fluorophores from human retinal pigment epithelium with age and pathology. Analytical and Bioanalytical Chemistry. 2015, 407, 4, 1075 1088. [Google Scholar] [CrossRef]
- Bindewald-Wittich, A.; Han, M.; Schmitz-Valckenberg, S.; Snyder, S.R.; Giese, G.; Bille, J.F.; Holz, F.G. Two-Photon–Excited Fluorescence Imaging of Human RPE Cells with a Femtosecond Ti: Sapphire. Laser Investigative Ophthalmology & Visual Science. 2006, 47, 10, 4553-4557. [Google Scholar]
- Gliem, M.; Muller, P.L.; Finger, R.P.; McGuinness, M.B.; Holz, F.G.; Issa, P.C. Quantitative Fundus Autofluorescence in Early and Intermediate Age-Related Macular Degeneration. JAMA Ophthalmol. 2016, 134, 817–824. [Google Scholar] [CrossRef] [PubMed]
- Bermond, K.; von der Emde, L.; Tarau, I.S.; Bourauel, L.; Heintzmann, R.; Holz, F.G.; Curcio, C.A.; Sloan, K.R.; Ach, T. Autofluorescent Organelles Within the Retinal Pigment Epithelium in Human Donor Eyes with and without Age Related Macular Degeneration. Investig. Ophthalmol. Vis. Sci. 2022, 63, 23. [Google Scholar] [CrossRef] [PubMed]
- Reits, E.A.; Neefjes, J.J. From fixed to FRAP: measuring protein mobility and activity in living cells. Nat. Cell Biol. 2001, 3, E145–E147. [Google Scholar] [CrossRef]
- Cabukusta, B.; Neefjes, J. Mechanisms of lysosomal positioning and movement. Traffic. 2018, 19, 761–769. [Google Scholar] [CrossRef] [PubMed]
- Hazim, R.A.; Williams, D.S. Microtubule Motor Transport of Organelles in a Specialized Epithelium: The RPE. Frontiers in Cell and Developmental Biology. 2022, 10, 852468. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Hiragi, S.; Fukuda, M. Rab family of small GTPases: an updated view on their regulation and functions. The FEBS Journal. 2020, 288, 36–55. [Google Scholar] [CrossRef] [PubMed]
- Sastre, A.A.; Montoro, M.L.; Lacerda, H.M.; Llavero, F.; Zugaza, J.L. Small GTPases of the Rab and Arf Families: Key Regulators of Intracellular Trafficking in Neurodegeneration. Int. J. Mol. Sci. 2021, 22, 4425. [Google Scholar] [CrossRef] [PubMed]
- Futter, C.E. The molecular regulation of organelle transport in mammalian retinal pigment epithelial cells. Pigment Cell Res. 2006, 19, 104–111. [Google Scholar] [CrossRef]
- Fukuda, M. Rab GTPases: key players in melanosome biogenesis, transport, and transfer. Pigment Cell and Melanoma Research. 2020, 34, 222–235. [Google Scholar] [CrossRef]
- Heo, J.; Campbell, S.L. Superoxide anion radical modulates the activity of Ras and Ras-related GTPases by a radical-based mechanism similar to that of nitric oxide. J. Biol. Chem. 2005, 280, 12438–12445. [Google Scholar] [CrossRef] [PubMed]
- Heo, J.; Campbell, S.L. Ras Regulation by Reactive Oxygen and Nitrogen Species. Biochemistry. 2006, 45, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).