Submitted:
15 April 2024
Posted:
16 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
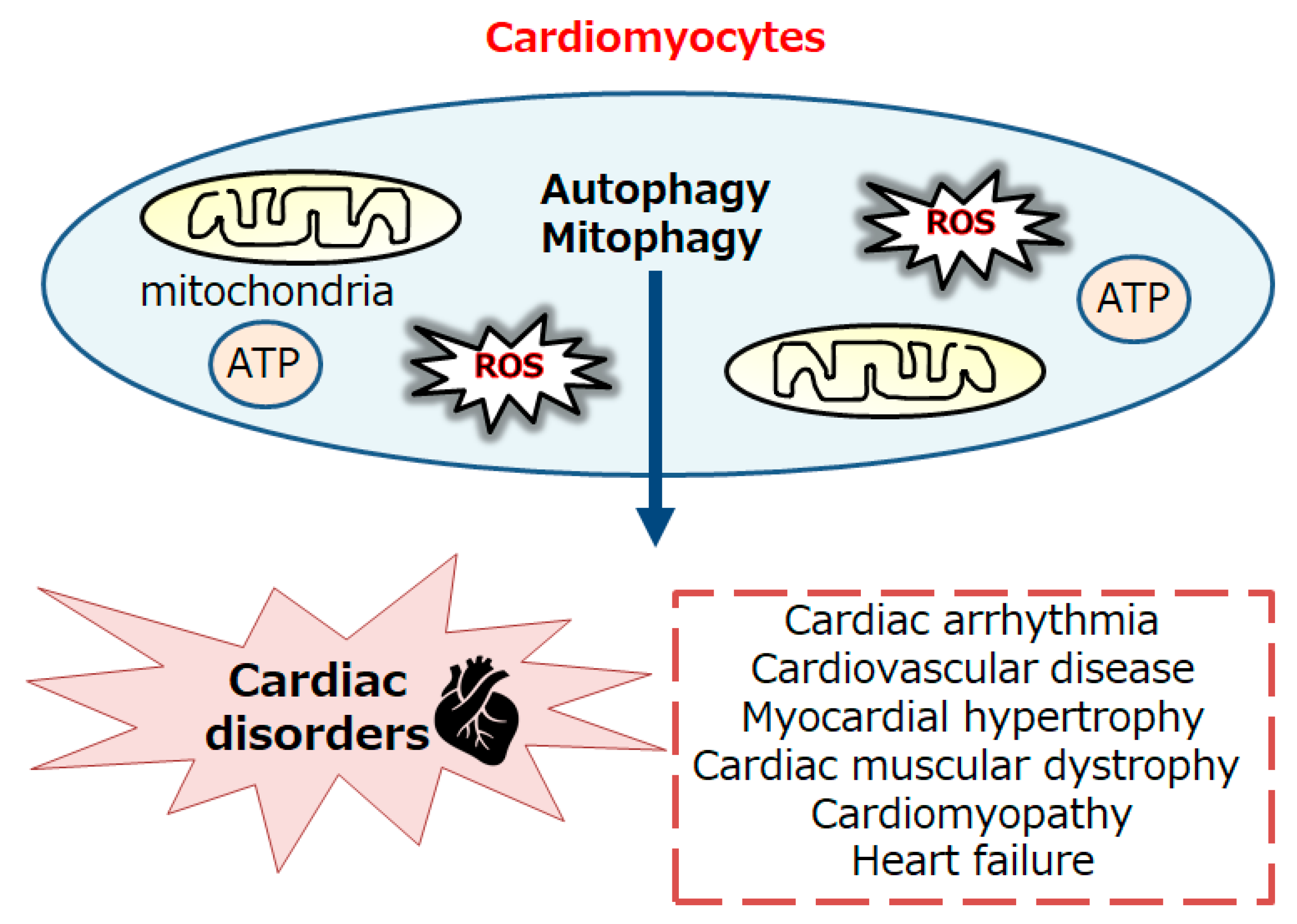
2. Relationship between Autophagy/Mitophagy and Several Diseases Including Cardiac Discorders
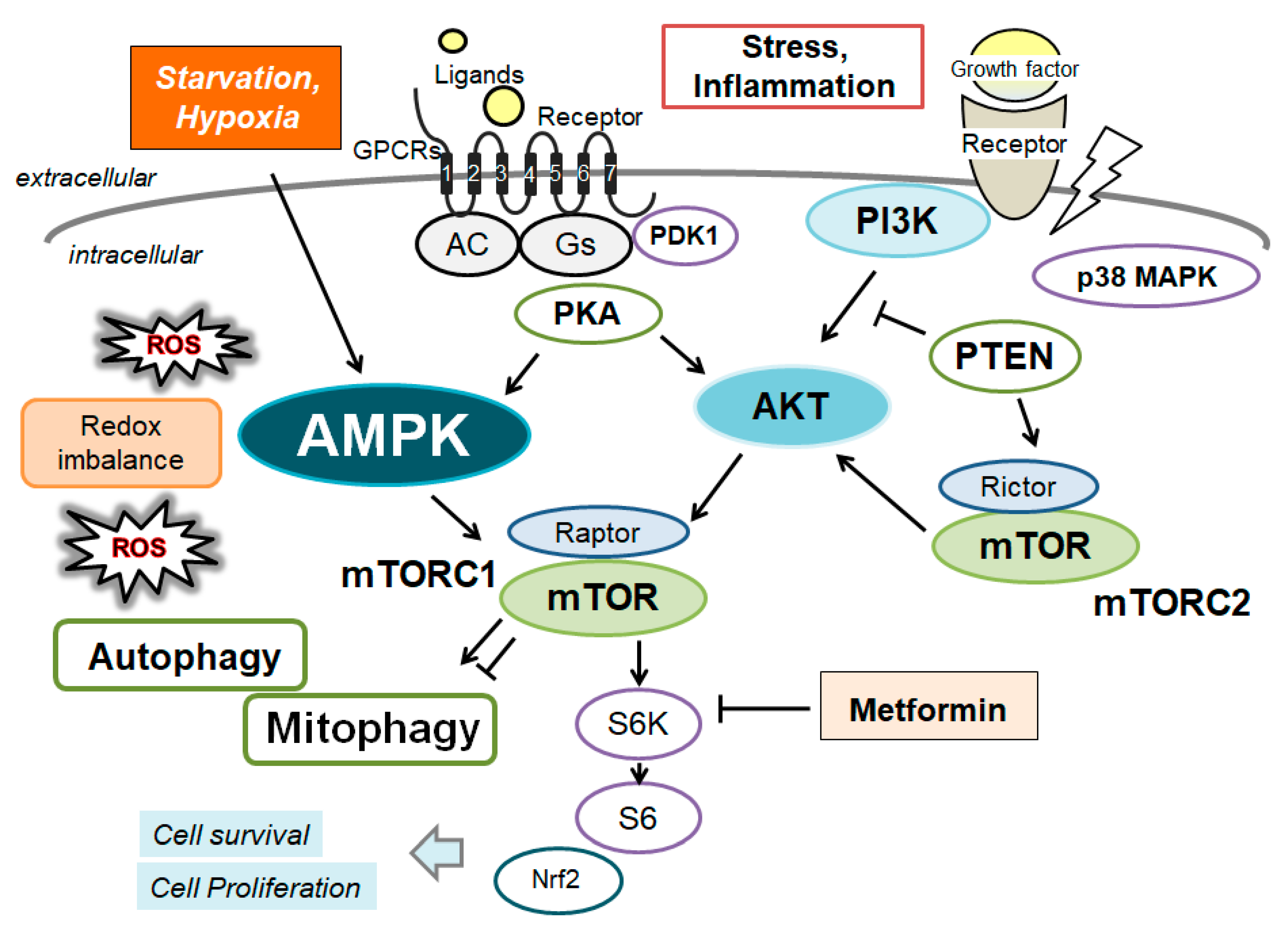
3. Characterization of AMPK and Connection between AMPK and Autophagy
4. Some Molecules Involved in the Regulation of AMPK
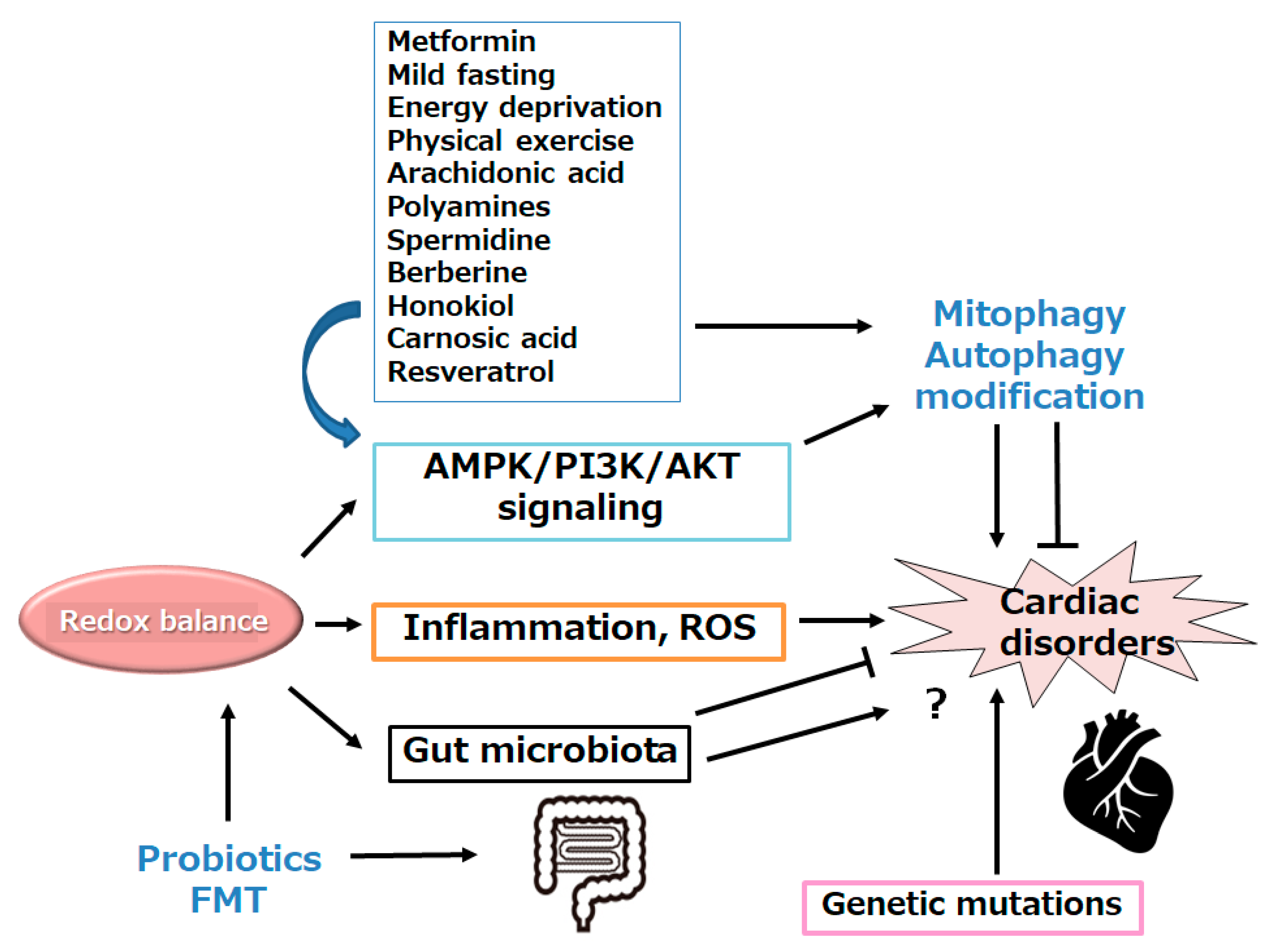
5. Possible Treatment Tactics with Certain Small Molecules against Several Heart Diseases
6. Future Perspectives
7. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMP | adenosine monophosphate |
| ATP | adenosine triphosphate |
| AMPK | adenosine monophosphate-activated protein kinase |
| FMT | fecal microbiota transplantation |
| mTOR | mechanistic/mammalian target of rapamycin |
| PDXP | pyridoxal phosphatase |
| PGC1α | peroxisome proliferator-activated receptor gamma coactivator 1-alpha |
| QOL | quality of life |
| ROS | reactive oxygen species |
| TGF-β | transforming growth factor beta |
| ULK1 | autophagy activating kinase 1 |
References
- Zhang, Z.; Yang, X.; Song, Y.Q.; Tu, J. Autophagy in Alzheimer's disease pathogenesis: therapeutic potential and future perspectives. Ageing Res. Rev. 2021, 72, 101464. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Hariharan, N.; Monden, Y.; Zablocki, D.; Sadoshima, J. Is autophagy in response to ischemia and reperfusion protective or detrimental for the heart? Pediatr. Cardiol. 2011, 32, 275–281. [Google Scholar]
- Aventaggiato, M.; Vernucci, E.; Barreca, F.; Russo, M.A.; Tafani, M. Sirtuins’ control of autophagy and mitophagy in cancer. Pharmacol. Ther. 2021, 221, 107748. [Google Scholar] [CrossRef] [PubMed]
- Hebah, H.A.; Kamel, H.M.; Bastawy, I.M.; Ahmed, F.A. Association between serum beclin 1 level and cardiac valvular calcification in hemodialysis patients. Curr Probl Cardiol. 2024, 49, 102519. [Google Scholar] [CrossRef]
- Nodaand, N.N. , Inagaki, F. Mechanisms of autophagy. Annual Review of Biophysics. 2015, 44, 101–122. [Google Scholar]
- Sharma, V.; Verma, S.; Seranova, E.; Sarkar, S.; Kumar, D. Selective Autophagy and Xenophagy in Infection and Disease. Front. Cell Dev. Biol. 2018, 6, 147. [Google Scholar] [CrossRef]
- Bravo-San, Pedro, J. M.; Kroemer, G.; Galluzzi, L. Autophagy and Mitophagy in Cardiovascular Disease. Circ. Res. 2017, 120, 1812–1824. [Google Scholar] [CrossRef]
- Kim, I.; Lemasters, J.J. Mitophagy selectively degrades individual damaged mitochondria after photoirradiation. Antioxidants & Redox Signaling. 2011, 14, 1919–1928. [Google Scholar]
- Doblado, L.; Lueck, C.; Rey, C.; Samhan-Arias, A.K.; Prieto, I.; Stacchiotti, A.; Monsalve, M. Mitophagy in human diseases. International Journal of Molecular Sciences. 2021, 22, 3903. [Google Scholar] [CrossRef] [PubMed]
- Billia, F.; Hauck, L.; Konecny, F.; Rao, V.; Shen, J.; Mak, T.W. PTEN-inducible kinase 1 (PINK1)/Park6 is indispensable for normal heart function. Proceedings of the National Academy of Sciences of the United States of America. 2011, 108, 9572–9577. [Google Scholar] [CrossRef]
- De, Gaetano, A. ; Gibellini, L.; Zanini, G.; Nasi, M.; Cossarizza, A.; Pinti, M. Mitophagy and oxidative stress: the role of aging. Antioxidants. 2021, 10, 794.
- Vasquez-Trincado, C.; Garcia-Carvajal, I.; Pennanen, C.; Parra, V.; Hill, J.A.; Rothermel, B.A.; Lavandero, S. Mitochondrial dynamics, mitophagy and cardiovascular disease. The Journal of Physiology. 2016, 594, 509–525. [Google Scholar] [CrossRef]
- Jarosz, J.; Ghosh, S.; Delbridge, L.M.D.; Petzer, A.; Hickey, A.J.R.; Crampin, E.J.; Hanssen, E.; Rajagopal, V. Changes in mitochondrial morphology and organization can enhance energy supply from mitochondrial oxidative phosphorylation in diabetic cardiomyopathy. Am J Physiol-Cell Physiol. 2017, 312, C190–C197. [Google Scholar] [CrossRef] [PubMed]
- Yi, D.; Yu, H.; Lu, K.; Ruan, C.; Ding, C.; Tong, L.; Zhao, X.; Chen, D. AMPK Signaling in Energy Control, Cartilage Biology, and Osteoarthritis. Front Cell Dev Biol. 2021, 9, 696602. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhao, Y.; Guo, D.; Luo, M.; Zhang, Q.; Zhang, L.; Zhang, D. Exercise Improves Heart Function after Myocardial Infarction: The Merits of AMPK. Cardiovasc Drugs Ther. 2024. [CrossRef] [PubMed]
- Garza-González, S.; Nieblas, B.; Solbes-Gochicoa, M.M.; Altamirano, J.; García, N. Intermittent Fasting as Possible Treatment for Heart Failure. Curr Vasc Pharmacol. 2022, 20, 260–271. [Google Scholar] [CrossRef]
- Parvaresh, H.; Paczek, K.; Al-Bari, M.A.A.; Eid, N. Mechanistic insights into fasting-induced autophagy in the aging heart. World J Cardiol. 2024, 16, 109–117. [Google Scholar] [CrossRef]
- Wohlgemuth, S.E.; Julian, D.; Akin, D.E.; Fried, J.; Toscano, K.; Leeuwenburgh, C.; Dunn, W.A. Jr. Autophagy in the heart and liver during normal aging and calorie restriction. Rejuvenation Res. 2007, 10, 281–292. [Google Scholar] [CrossRef]
- Nunes, J.P.S.; Andrieux, P.; Brochet, P.; Almeida, R.R.; Kitano, E.; Honda, A.K.; Iwai, L.K.; Andrade-Silva, D.; et al. Co-exposure of cardiomyocytes to IFN-γ and TNF-α induces mitochondrial dysfunction and nitro-oxidative stress: implications for the pathogenesis of chronic chagas disease cardiomyopathy. Front Immunol. 2021, 12, 755862. [Google Scholar] [CrossRef]
- Morales, P.E.; Arias-Duran, C.; Avalos-Guajardo, Y.; Aedo, G.; Verdejo, H.E.; Parra, V.; Lavandero, S. Emerging role of mitophagy in cardiovascular physiology and pathology. Molecular Aspects of Medicine. 2020, 71, 100822. [Google Scholar] [CrossRef]
- Seidlmayer, L.K.; Kuhn, J.; Berbner, A.; Arias-Loza, P.A.; Williams, T.; Kaspar, M.; Czolbe, M.; Kwong, J.Q.; et al. Inositol 1,4,5-trisphosphate-mediated sarcoplasmic reticulum-mitochondrial crosstalk influences adenosine triphosphate production via mitochondrial Ca2+ uptake through the mitochondrial ryanodine receptor in cardiac myocytes. Cardiovasc Res. 2016, 112, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.R.; Zweier, J.L. Cardiac mitochondria and reactive oxygen species generation. Circulation Research. 2014, 114, 524–537. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, H.; Dudley, S.C. Jr. Reactive oxygen species originating from mitochondria regulate the cardiac sodium channel. Circulation Research. 2010, 107, 967–974. [Google Scholar] [CrossRef] [PubMed]
- Lobo, V.; Patil, A.; Phatak, A.; Chandra, N. Free radicals, antioxidants and functional foods: impact on human health. Pharmacognosy Reviews. 2010, 4, 118–126. [Google Scholar] [CrossRef] [PubMed]
- Jin, L.; Geng, L.; Ying, L.; Shu, L.; Ye, K.; Yang, R.; Liu, Y.; Wang, Y.; et al. FGF21-Sirtuin 3 axis confers the protective effects of exercise against diabetic cardiomyopathy by governing mitochondrial integrity. Circulation. 2022, 146, 1537–1557. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Krigman, J.; Luo, H.; Ozgen, S.; Yang, M.; Sun, N. Mitophagy in cardiovascular homeostasis. Mechanisms of Ageing and Development. 2020, 188, 111245. [Google Scholar] [CrossRef] [PubMed]
- Akar, F.G.; O'Rourke, B. Mitochondria are sources of metabolic sink and arrhythmias. Pharmacology & Therapeutics. 2011, 131, 287–294. [Google Scholar]
- Phadwal, K.; Vrahnas, C.; Ganley, I.G.; MacRae, V.E. Mitochondrial dysfunction: cause or consequence of vascular calcification? Frontiers in Cell and Developmental Biology. 2021, 9, 611922. [Google Scholar] [CrossRef]
- Brown, D.A.; O'Rourke, B. Cardiac mitochondria and arrhythmias. Cardiovascular Research. 2010, 88, 241–249. [Google Scholar] [CrossRef]
- Wong, A.K.; Howie, J.; Petrie, J.R.; Lang, C.C. AMP-activated protein kinase pathway: a potential therapeutic target in cardiometabolic disease. Clin. Sci. 2009, 116, 607–620. [Google Scholar] [CrossRef]
- Zhang, W.; Ren, H.; Xu, C.; Zhu, C.; Wu, H.; Liu, D.; Wang, J.; Liu, L.; et al. Hypoxic mitophagy regulates mitochondrial quality and platelet activation and determines severity of I/R heart injury. eLife. 2016, 5, e21407. [Google Scholar] [CrossRef]
- Jamialahmadi, T.; Hasanpour, M.; Vakilian, F.; Penson, P.E.; Iranshahy, M.; Sahebkar, A. Evaluation of Urolithin A Efficacy in Heart Failure Patients with Reduced Ejection Fraction: A Randomized, Double-blind, Crossover, Placebo-controlled Clinical Trial. Rev Recent Clin Trials. 2024. [CrossRef] [PubMed]
- Hu, X.; Xu, X.; Lu, Z.; Zhang, P.; Fassett, J.; Zhang, Y.; Xin, Y.; Hall, J.L.; et al. AMP activated protein Kinase-α2 regulates expression of estrogen-related Receptor-α, a metabolic transcription factor related to heart failure development. Hypertension. 2011, 58, 696–703. [Google Scholar] [CrossRef]
- Li, H.; Zheng, F.; Zhang, Y.; Sun, J.; Gao, F.; Shi, G. Resveratrol, novel application by preconditioning to attenuate myocardial ischemia/reperfusion injury in mice through regulate AMPK pathway and autophagy level. J. Cell. Mol. Med. 2022, 26, 4216–4229. [Google Scholar] [CrossRef]
- Shirakabe, A.; Zhai, P.; Ikeda, Y.; Saito, T.; Maejima, Y.; Hsu, C.P.; Nomura, M.; Egashira, K.; et al. Drp1-Dependent Mitochondrial Autophagy Plays a Protective Role Against Pressure Overload-Induced Mitochondrial Dysfunction and Heart Failure. Circulation. 2016, 133, 1249–1263. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.B.; Gao, W.C.; Xie, M.; Li, Z.; Ma, X.; Song, W.; Luo, D.; Huang, Y.; et al. Ang II Promotes Cardiac Autophagy and Hypertrophy via Orai1/STIM1. Front. Pharmacol. 2021, 12, 622774. [Google Scholar] [CrossRef]
- Jiang, X.; Zhang, K.; Gao, C.; Ma, W.; Liu, M.; Guo, X.; Bao, G.; Han, B.; et al. Activation of FMS-like tyrosine kinase 3 protects against isoprenaline-induced cardiac hypertrophy by improving autophagy and mitochondrial dynamics. FASEB J. 2022, 36, e22672. [Google Scholar] [CrossRef] [PubMed]
- Keerthana, C.K.; Rayginia, T.P.; Shifana, S.C.; Anto, N.P.; Kalimuthu, K.; Isakov, N, Anto, R. J. The role of AMPK in cancer metabolism and its impact on the immunomodulation of the tumor microenvironment. Front. Immunol. 2023, 14, 1114582. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, Y.; Jiang, T.; Liu, B.; Sun, H.; Zhang, Y.; Fan, B.; Li, X.; et al. Enhancing fatty acids oxidation via L-carnitine attenuates obesity-related atrial fibrillation and structural remodeling by activating AMPK signaling and alleviating cardiac lipotoxicity. Front. Pharmacol. 2021, 12, 771940. [Google Scholar] [CrossRef]
- Lee, C.C.; Chen, W.T.; Chen, S.; Lee, T.M. Dapagliflozin attenuates arrhythmic vulnerabilities by regulating connexin43 expression via the AMPK pathway in post-infarcted rat hearts. Biochem. Pharmacol. 2021, 192, 114674. [Google Scholar] [CrossRef]
- Zhang, M.; Alemasi, A.; Zhao, M.; Xu, W.; Zhang, Y.; Gao, W.; Yu, H.; Xiao, H. Exercise Training Attenuates Acute beta-Adrenergic Receptor Activation-Induced Cardiac Inflammation via the Activation of AMP-Activated Protein Kinase. Int J Mol Sci. 2023, 24, 9263. [Google Scholar] [CrossRef] [PubMed]
- Yao, Y.; Wang, H.; Yang, Y.; Jiang, Z.; Ma, H. Dehydroepiandrosterone activates the GPER-mediated AMPK signaling pathway to alleviate the oxidative stress and inflammatory response in laying hens fed with high-energy and low-protein diets. Life Sci. 2022, 308, 120926. [Google Scholar] [CrossRef] [PubMed]
- Garcia, D.; Shaw, R.J. AMPK: mechanisms of cellular energy sensing and restoration of metabolic balance. Mol. Cell. 2017, 66, 789–800. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Yang, G.; Kim, Y.; Kim, J.; Ha, J. AMPK activators: mechanisms of action and physiological activities. Exp. Mol. Med. 2016, 48, e224. [Google Scholar] [CrossRef] [PubMed]
- Mihaylova, M.M.; Shaw, R.J. The AMPK signalling pathway coordinates cell growth, autophagy and metabolism. Nat. Cell Biol. 2011, 13, 1016–1023. [Google Scholar] [CrossRef]
- Muraleedharan, R.; Dasgupta, B. AMPK in the brain: its roles in glucose and neural metabolism. Febs J. 2022, 289, 2247–2262. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Chen, C.; Dong, B.; Xing, F.; Huang, H.; Yao, F.; Ma, Y.; He, J.; et al. AMPK attenuates ventricular remodeling and dysfunction following aortic banding in mice via the Sirt3/Oxidative stress pathway. Eur. J. Pharmacol. 2017, 814, 335–342. [Google Scholar] [CrossRef] [PubMed]
- Cantó, C.; Gerhart-Hines, Z.; Feige, J.N.; Lagouge, M.; Noriega, L.; Milne, J.C.; Elliott, P.J.; Puigserver, P.; et al. AMPK regulates energy expenditure by modulating NAD+ metabolism and SIRT1 activity. Nature. 2009, 458, 1056–1060. [Google Scholar] [CrossRef]
- Pagan, L.U.; Gomes, M.J.; Gatto, M.; Mota, G.A.; Okoshi, K.; Okoshi, M.P. The role of oxidative stress in the aging heart. Antioxidants. 2022, 11, 336. [Google Scholar] [CrossRef]
- Trefts, E; Shaw, R. J. AMPK: restoring metabolic homeostasis over space and time. Mol Cell. 2021, 81, 3677–3690. [Google Scholar] [CrossRef]
- Qi, X.; Wang, J. Melatonin improves mitochondrial biogenesis through the AMPK/PGC1α pathway to attenuate ischemia/reperfusion-induced myocardial damage. Aging. 2020, 12, 7299–7312. [Google Scholar] [CrossRef]
- Li, S.X.; Li, C.; Pang, X.R.; Zhang, J.; Yu, G.C.; Yeo, A.J.; Lavin, M.F.; Shao, H.; et al. Metformin attenuates silica-induced pulmonary fibrosis by activating autophagy via the AMPK-mTOR signaling pathway. Front Pharmacol. 2021, 12, 719589. [Google Scholar] [CrossRef]
- Timm, K.N.; Tyler, D.J. The role of AMPK activation for cardioprotection in doxorubicin-induced cardiotoxicity. Cardiovasc Drugs Ther. 2020, 34, 255–269. [Google Scholar] [CrossRef]
- González, A.; Hall, M.N.; Lin, S.C.; Hardie, D.G. AMPK and TOR: the Yin and Yang of cellular nutrient sensing and growth control. Cell Metab. 2020, 31, 472–492. [Google Scholar] [CrossRef]
- Steinberg, G.R.; Hardie, D.G. New insights into activation and function of the AMPK. Nat Rev Mol Cell Biol. 2023, 24, 255–272. [Google Scholar] [CrossRef]
- Maharajan, N.; Ganesan, C.D.; Moon, C.; Jang, C.H.; Oh, W.K.; Cho, G.W. Licochalcone D ameliorates oxidative stress-induced senescence via AMPK activation. Int J Mol Sci. 2021, 22, 7324. [Google Scholar] [CrossRef]
- Sharma, A.; Anand, S.K.; Singh, N.; Dwivedi, U.N.; Kakkar, P. AMP-activated protein kinase: an energy sensor and survival mechanism in the reinstatement of metabolic homeostasis. Exp Cell Res. 2023, 428, 113614. [Google Scholar] [CrossRef]
- Hu, Y.; Dong, Y.D.; Wu, Y.C.; Wang, Q.X.; Nan, X.; Wang, D.L. AMPK inhibitor BML-275 induces neuroprotection through decreasing cyt c and AIF expression after transient brain ischemia. Bioorg Med Chem. 2021, 52, 116522. [Google Scholar] [CrossRef]
- Spaulding, HR.; Yan, Z. AMPK and the adaptation to exercise. Annu Rev Physiol. 2022, 84, 209–227. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Z.; Li, Y.; Fu, Y.; Yang, Y. Research progress of AMP-activated protein kinase and cardiac aging. Open Life Sci. 2023, 18, 20220710. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.; Hao, R.; Wang, W.; Gao, H.; Wang, C. SIRT1/Atg5/autophagy are involved in the antiatherosclerosis effects of ursolic acid. Mol. Cell. Biochem. 2016, 420, 171–184. [Google Scholar] [CrossRef] [PubMed]
- LaMoia, TE.; Shulman, GI. Cellular and molecular mechanisms of metformin action. Endocr. Rev. 2021, 42, 77–96. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Li, T.; Li, Z.; Liu, N.; Yan, Y.; Liu, B. Role of Mitophagy in cardiovascular disease. Aging and Disease. 2020, 11, 419–437. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, P.; Guo, J.; Ma, T.; Hu, Y.; Huang, L.; Xing, B.; He, Y.; et al. Zinc Overload Induces Damage to H9c2 Cardiomyocyte Through Mitochondrial Dysfunction and ROS-Mediated Mitophagy. Cardiovasc Toxicol. 2023, 23, 388–405. [Google Scholar] [CrossRef]
- Sun, W.; Lee, T.S.; Zhu, M.; Gu, C.; Wang, Y.; Zhu, Y.; Shyy, J.Y. Statins activate AMP-activated protein kinase in vitro and in vivo. Circulation. 2006, 114, 2655–2662. [Google Scholar] [CrossRef] [PubMed]
- Shah, S.A.; Yoon, G.H.; Chung, S.S.; Abid, M.N.; Kim, T.H.; Lee, H.Y.; Kim, M.O. Novel osmotin inhibits SREBP2 via the AdipoR1/AMPK/SIRT1 pathway to improve Alzheimer's disease neuropathological deficits. Mol. Psychiatry. 2017, 22, 407–416. [Google Scholar] [CrossRef] [PubMed]
- Mao, Y.; Cheng, J.; Yu, F.; Li, H.; Guo, C.; Fan, X. Ghrelin attenuated lipotoxicity via autophagy induction and nuclear factor-κB inhibition. Cell Physiol. Biochem 2015, 37, 563–576. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Moon, N.R.; Kim, D.S.; Kim, S.H.; Park, S. Central acylated ghrelin improves memory function and hippocampal AMPK activation and partly reverses the impairment of energy and glucose metabolism in rats infused with β-amyloid. Peptides. 2015, 71, 84–93. [Google Scholar] [CrossRef]
- Vingtdeux, V.; Giliberto, L.; Zhao, H.; Chandakkar, P.; Wu, Q.; Simon, J.E.; Janle, E.M.; Lobo, J.; et al. AMP-activated protein kinase signaling activation by resveratrol modulates amyloid-beta peptide metabolism. J. Biol. Chem. 2010, 285, 9100–9113. [Google Scholar] [CrossRef]
- Zhao, H.; Wang, Z.C.; Wang, K.F.; Chen, X.Y. Aβ peptide secretion is reduced by Radix Polygalae-induced autophagy via activation of the AMPK/mTOR pathway. Mol. Med. Rep. 2015, 12, 2771–2776. [Google Scholar] [CrossRef]
- Feng, J.; Chen, X.; Guan, B.; Li, C.; Qiu, J.; Shen, J. Inhibition of peroxynitrite-induced mitophagy activation attenuates cerebral ischemia-reperfusion injury. Molecular Neurobiology. 2018, 55, 6369–6386. [Google Scholar] [CrossRef]
- Fu, Q.; Li, T.; Zhang, C.; Ma, X.; Meng, L.; Liu, L.; Shao, K.; Wu, G.; et al. Butyrate mitigates metabolic dysfunctions via the ERalpha-AMPK pathway in muscle in OVX mice with diet-induced obesity. Cell Commun Signal. 2023, 21, 95. [Google Scholar] [CrossRef] [PubMed]
- Hawley, S.A.; Fullerton, M.D.; Ross, F.A.; Schertzer, J.D.; Chevtzoff, C.; Walker, K.J.; Peggie, M.W.; Zibrova, D.; et al. The ancient drug salicylate directly activates AMP-activated protein kinase. Science. 2012, 336, 918–922. [Google Scholar] [CrossRef]
- O'Neill, E.J.; Moore, J.; Song, J.; Tsiani, E.L. Inhibition of Non-Small Cell Lung Cancer Proliferation and Survival by Rosemary Extract Is Associated with Activation of ERK and AMPK. Life (Basel). 2021, 12, 52. [Google Scholar] [CrossRef]
- Mao, R.W.; He, S.P.; Lan, J.G.; Zhu, W.Z. Honokiol ameliorates cisplatin-induced acute kidney injury via inhibition of mitochondrial fission. Br J Pharmacol. 2022, 179, 3886–3904. [Google Scholar] [CrossRef]
- Rajabian, N.; Choudhury, D.; Ikhapoh, I.; Saha, S.; Kalyankar, A.S.; Mehrotra, P.; Shahini, A.; Breed, K.; et al. Reversine ameliorates hallmarks of cellular senescence in human skeletal myoblasts via reactivation of autophagy. Aging Cell. 2023, 22, 13764. [Google Scholar] [CrossRef] [PubMed]
- Kandadi, M.R.; Roe, N.D.; Ren, J. Autophagy inhibition rescues against leptin-induced cardiac contractile dysfunction. Curr. Pharm. Des. 2014, 20, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Xu, X.; Wang, Q.; Ren, S.Y.; Dong, M.; Zhang, Y. Permissive role of AMPK and autophagy in adiponectin deficiency-accentuated myocardial injury and inflammation in endotoxemia. J. Mol. Cell Cardiol. 2016, 93, 18–31. [Google Scholar] [CrossRef]
- Fu, Z.; Liegl, R.; Wang, Z.; Gong, Y.; Liu, C.H.; Sun, Y.; Cakir, B.; Burnim, S.B.; et al. Adiponectin mediates dietary omega-3 long-chain polyunsaturated fatty acid protection against choroidal neovascularization in mice. Invest Ophthalmol. Vis. Sci. 2017, 58, 3862–3870. [Google Scholar]
- Al-Kuraishy, H.M.; Al-Gareeb, A.I.; Bungau, S.G.; Radu, A.F.; Batiha, G.E. The potential molecular implications of adiponectin in the evolution of SARS-CoV-2: inbuilt tendency. J. King Saud. Univ. Sci. 2022, 34, 102347. [Google Scholar] [CrossRef]
- Guo, Z.; Yan, X.; Wang, L.; Wu, J.; Jing, X.; Liu, J. Effect of Telmisartan or Insulin on the Expression of Adiponectin and its Receptors in the Testis of Streptozotocin-Induced Diabetic Rats. Horm. Metab. Res. 2016, 48, 404–412. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Tan, J.X.; He, Y.; Bai, F.; Li, S.W.; Hou, Y.W.; Ji, L.S.; Gao, Y.T.; et al. Atractylenolide III ameliorates Non-Alcoholic Fatty Liver Disease by activating Hepatic Adiponectin Receptor 1-Mediated AMPK Pathway. Int J. Biol. Sci. 2022, 18, 1594–1611. [Google Scholar]
- Paoli, A.; Tinsley, G.M.; Mattson, M.P.; De, Vivo, I. ; Dhawan, R.; Moro, T. Common and divergent molecular mechanisms of fasting and ketogenic diets. Trends Endocrinol Metab. 2024, 35, 125–141. [Google Scholar] [CrossRef] [PubMed]
- Cavezzi, A.; Colucci, R.; d'Errico, G. Mitoresilience: Hormesis, Psycho-physical Resilience, Mitochondria and Heart Rate Variability as Relevant Interplaying Elements in Longevity Medicine. Curr Aging Sci. 2023, 16, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Lau, K.; Eby, B.; Lozano, P.; He, C.; Pennington, B.; Li, H.; Rathi, S.; et al. Improvement of cardiac functions by chronic metformin treatment is associated with enhanced cardiac autophagy in diabetic OVE26 mice. Diabetes. 2011, 60, 1770–1778. [Google Scholar] [CrossRef] [PubMed]
- Marek-Iannucci, S.; Ozdemir, A.B.; Moreira, D.; Gomez, A.C.; Lane, M.; Porritt, R.A.; Lee, Y.; Shimada, K.; et al. Autophagy-mitophagy induction attenuates cardiovascular inflammation in a murine model of Kawasaki disease vasculitis. JCI Insight. 2021, 6, e151981. [Google Scholar] [CrossRef] [PubMed]
- Bland, A.R.; Payne, F.M.; Ashton, J.C.; Jamialahmadi, T.; Sahebkar, A. The cardioprotective actions of statins in targeting mitochondrial dysfunction associated with myocardial ischaemia-reperfusion injury. Pharmacol Res. 2022, 175, 105986. [Google Scholar] [CrossRef] [PubMed]
- Lv, Y.; Li, K.; Wang, S.; Wang, X.; Yue, G.; Zhang, Y.; Lv, X.; Zhao, P.; et al. Protective role of arachidonic acid against diabetic myocardial ischemic injury: a translational study of pigs, rats, and humans. Cardiovasc Diabetol. 2024, 23, 58. [Google Scholar] [CrossRef] [PubMed]
- Messerer, J.; Wrede, C.; Schipke, J.; Brandenberger, C.; Abdellatif, M.; Eisenberg, T.; Madeo, F.; Sedej, S.; et al. Spermidine supplementation influences mitochondrial number and morphology in the heart of aged mice. Mühlfeld C.J Anat. 2023, 242, 91–101. [Google Scholar] [CrossRef]
- Galasso, L.; Cappella, A.; Mulè, A.; Castelli, L.; Ciorciari, A.; Stacchiotti, A.; Montaruli, A. Polyamines and Physical Activity in Musculoskeletal Diseases: A Potential Therapeutic Challenge. Int J Mol Sci. 2023, 24, 9798. [Google Scholar] [CrossRef]
- Chai, N.; Zheng, H.; Zhang, H.; Li, L.; Yu, X.; Wang, L.; Bi, X.; Yang, L.; et al. Spermidine Alleviates Intrauterine Hypoxia-Induced Offspring Newborn Myocardial Mitochondrial Damage in Rats by Inhibiting Oxidative Stress and Regulating Mitochondrial Quality Control. Iran J Pharm Res. 2023, 21, e133776. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Yang, T.; Mei, L.; Zhang, Y.; Liang, C.; Bai, X.; Zhang, Z.; Shi, Y.; et al. The Potential of Berberine to Target Telocytes in Rabbit Heart. Planta Med. 2024, 90, 84–95. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Li, J.; Li, Y.; Zhang, Y.; Du, Q.; Hao, P.; Li, J.; Cao, X.; et al. Berberine Protects Against Simulated Ischemia/Reperfusion Injury-Induced H9C2 Cardiomyocytes Apoptosis In Vitro and Myocardial Ischemia/Reperfusion-Induced Apoptosis In Vivo by Regulating the Mitophagy-Mediated HIF-1alpha/BNIP3 Pathway. Front Pharmacol. 2020, 11, 367. [Google Scholar] [CrossRef] [PubMed]
- Zhu, N.; Cao, X.; Hao, P.; Zhang, Y.; Chen, Y.; Zhang, J.; Li, J.; Gao, C.; et al. Berberine attenuates mitochondrial dysfunction by inducing autophagic flux in myocardial hypoxia/reoxygenation injury. Cell Stress Chaperones. 2020, 25, 417–426. [Google Scholar] [CrossRef]
- Tan, Z.; Liu, H.; Song, X.; Ling, Y.; He, S.; Yan, Y.; Yan, J.; Wang, S.; et al. Honokiol post-treatment ameliorates myocardial ischemia/reperfusion injury by enhancing autophagic flux and reducing intracellular ROS production. Chem Biol Interact. 2019, 307, 82–90. [Google Scholar] [CrossRef]
- Zhang, Q.L.; Yang, J.J.; Zhang, H.S. Carvedilol (CAR) combined with carnosic acid (CAA) attenuates doxorubicin-induced cardiotoxicity by suppressing excessive oxidative stress, inflammation, apoptosis and autophagy. Biomed Pharmacother. 2019, 109, 71–83. [Google Scholar] [CrossRef]
- Thai, P.N.; Miller, C.V.; King, M.T.; Schaefer, S.; Veech, R.L.; Chiamvimonvat, N.; Bers, D.M.; Dedkova, E.N. Ketone Ester D-beta-Hydroxybutyrate-(R)-1,3 Butanediol Prevents Decline in Cardiac Function in Type 2 Diabetic Mice. J Am Heart Assoc. 2021, 10, e020729. [Google Scholar] [CrossRef]
- Bal, N.B.; Bostanci, A.; Sadi, G.; Dönmez, M.O.; Uludag, M.O.; Demirel-Yilmaz, E. Resveratrol and regular exercise may attenuate hypertension-induced cardiac dysfunction through modulation of cellular stress responses. Life Sci. 2022, 296, 120424. [Google Scholar] [CrossRef]
- Kuno, A.; Hosoda, R.; Sebori, R.; Hayashi, T.; Sakuragi, H.; Tanabe, M.; Horio, Y. Resveratrol Ameliorates Mitophagy Disturbance and Improves Cardiac Pathophysiology of Dystrophin-deficient mdx Mice. Sci Rep. 2018, 8, 15555. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Guo, J.; Ma, T.; Hu, Y.; Huang, L.; He, Y.; Xi, J. Resveratrol Inhibits Zinc Deficiency-Induced Mitophagy and Exerts Cardiac Cytoprotective Effects. Biol Trace Elem Res. 2024, 202, 1669–1682. [Google Scholar] [CrossRef]
- Ye, H.; Zhang, Y.; Yun, Q.; DU, R.; Li, L.; Li, Y.; Gao, Q. Resveratrol alleviates hyperglycemia-induced cardiomyocyte hypertrophy by maintaining mitochondrial homeostasis via enhancing SIRT1 expression]. Nan Fang Yi Ke Da Xue Xue Bao. 2024, 44, 45–51. [Google Scholar]
- Mori, A.; Ezawa, Y.; Asano, D.; Kanamori, T.; Morita, A.; Kashihara, T.; Sakamoto, K.; Nakahara, T. Resveratrol dilates arterioles and protects against N-methyl-d-aspartic acid-induced excitotoxicity in the rat retina. Neurosci Lett. 2023, 793, 136999. [Google Scholar] [CrossRef] [PubMed]
- Inoue, T.; Miura, K.; Han, R.; Seto-Tetsuo, F.; Arioka, M.; Igawa, K.; Tomooka, K.; Sasaguri, T. Differentiation-inducing factor 1 activates cofilin through pyridoxal phosphatase and AMP-activated protein kinase, resulting in mitochondrial fission. J Pharmacol Sci. 2023, 152, 39–49. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.M.; Choi, S.C.; Song, M.H.; Kim, K.S.; Jun, S.; Park, J.H.; Kim, J.H.; Kim, K.; et al. The Activation of the LIMK/Cofilin Signaling Pathway via Extracellular Matrix-Integrin Interactions Is Critical for the Generation of Mature and Vascularized Cardiac Organoids. Cells. 2023, 12, 2029. [Google Scholar] [CrossRef] [PubMed]
- Huan,g F. ; Li, M.L.; Fang, Z.F.; Hu, X.Q.; Liu, Q.M.; Liu, Z.J.; Tang, L.; Zhao, Y.S.; Zhou, S.H. Overexpression of MicroRNA-1 improves the efficacy of mesenchymal stem cell transplantation after myocardial infarction. Cardiology. 2013, 125, 18–30. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).




