1. Introduction
The ongoing global health crisis sparked by the COVID-19 pandemic has brought into focus various complications that arise after infection, often termed the long-term COVID-19 condition (long COVID) [
1,
2]. Long COVID manifests as a broad spectrum of symptoms persisting or emerging after the acute phase of the infection, impacting multiple organ systems, and leading to enduring health issues [
2]. The COVID-19 pandemic has underscored the critical importance of understanding the multifaceted impacts of the SARS-CoV-2 virus, particularly among populations with pre-existing health conditions [
3]. Patients undergoing hemodialysis (HD) represent a uniquely vulnerable group due to their compromised immune systems, the necessity of frequent healthcare interactions, and the high prevalence of comorbid conditions such as cardiovascular diseases and diabetes. These factors not only elevate their risk of contracting COVID-19 but also predispose them to more severe outcomes, including the development of long COVID [
4,
5]. Despite the initial steps taken to explore the underlying mechanisms and potential predictors of long COVID within the general population [
6,
7,
8], studies focusing on the HD population remain scarce.
Patients undergoing HD often experience nutritional deficiencies stemming from the nature of chronic kidney disease and dialysis treatment [
9]. Understanding the interplay between nutritional status and long COVID in this specific population is crucial for optimizing their care and improving outcomes. The Controlling Nutritional Status (CONUT) score, a tool used to assess nutritional status based on serum albumin, total cholesterol levels, and lymphocyte count, has shown promise as a predictor of hospitalization duration, severity, risk of long COVID, and mortality in the general COVID-19 patient cohort [
10,
11,
12]. However, its relevance to long COVID among HD patients remains unclear, highlighting a significant knowledge gap.
Oxidative stress, characterized by an imbalance between the production of reactive oxygen species and the body's antioxidant defenses, has been implicated in the pathogenesis of long COVID [
13,
14]. Elevated levels of oxidative stress markers have been observed in patients with long COVID, indicating that oxidative stress may play a significant role in the persistence of symptoms and the overall intensity of the condition [
14,
15,
16]. However, the precise relationship between oxidative stress and the persistence of long COVID symptoms in patients undergoing HD remains unclear. In this context, we hypothesized that malnutrition or suboptimal nutritional status may contribute to the prolonged oxidative damage observed in patients with long COVID, thereby exacerbating the severity and duration of symptoms. Therefore, the present study aimed to evaluate the association between pre-infection nutritional status, oxidative stress, and one-year long COVID persistence in patients undergoing HD.
2. Materials and Methods
2.1. Study Design and Cohort Selection
This prospective observational cohort study was part of the "Mechanisms of Development and Therapeutic Targets of Post-COVID Syndrome in Dialysis Patients" project (National Study Registration Number 0122U000144) and conducted at the State Institution "Institute of Nephrology of the National Academy of Medical Science of Ukraine" in Kyiv, between November 2021 and May 2023. The study protocol received approval from the Institute Ethics Committee (protocol number: 2-2021, dated April 6, 2021), and all participants provided written informed consent before inclusion. The research followed the principles outlined in the Declaration of Helsinki and other relevant ethical guidelines.
The study targeted patients aged 18 years or older who were undergoing HD treatments and had a confirmed history of COVID-19 infection. COVID-19 diagnosis was confirmed by either detecting SARS-CoV-2 RNA using real-time reverse transcription polymerase chain reaction (PCR) in nasopharyngeal swab specimens or observing imaging findings consistent with COVID-19. To ensure a diverse patient population, participants were recruited from three dialysis centers: the State Institution "Institute of Nephrology of the National Academy of Medical Science of Ukraine" in Kyiv, as well as two Dialysis Medical Centers operated by LLC “Nephrocenter” located in Kyiv and Zaporizhzhia, Ukraine. All enrolled patients had received at least 6 months of dialysis treatment before contracting COVID-19, maintained a stable clinical condition, and possessed a functioning arteriovenous fistula with a target Kt/V value of at least 1.2. The exclusion criteria comprised recent hospitalization, a history of cardiovascular events, undergoing immunosuppressive therapy, having systemic or malignant diseases, and experiencing acute inflammatory processes.
2.2. Sample Size
The sample size for our study was calculated using MedCalc version 19.2.6 (Ostend, Belgium) Statistical Software, based on a recent study by Zhao et al. In this study, Zhao et al. assessed the predictive value of the CONUT score in determining the risk of long COVID in a general patient cohort [
11]. They reported long COVID incidence rates of 0.65% and 0.38% in high-CONUT and mild-CONUT groups, respectively (log-rank <0.0001), with a total sample size of 151 patients. Based on these results, we determined that a minimum of 98 participants is needed to achieve a power of 0.95 and an alpha of 0.05. To accommodate potential dropouts and ensure adequate study power, we increased the sample size by 20% beyond the initial calculation, resulting in a total of 118 patients being included in the study.
2.3. Data Collection and Follow-Up
To categorize patients based on their pre-infection CONUT scores, we retrospectively recorded the latest available data before their COVID-19 contraction (on average 21±13 days prior). Baseline data collection occurred three months after the onset of COVID-19, involving the gathering of demographic information, conducting routine clinical evaluations, and assessing oxidative stress markers. Predialysis blood samples of 10 milliliters were collected after an overnight fast and immediately processed. Subsequently, patients were monitored for over one year, with long COVID symptoms checked and recorded monthly during the follow-up period. The study endpoint was the persistence of long COVID symptoms throughout the one-year follow-up duration.
Long COVID diagnosis was established based on the persistence of at least one clinical symptom following COVID-19 infection, independent of other medical conditions. Symptoms indicative of long COVID in this study encompassed fatigue, dyspnea, insomnia, concentration or memory impairment, mood changes, chest and joint discomfort, palpitations, muscle pain, alterations in smell and taste, cough, headache, skin rash or hair loss, and diarrhea.
The severity of acute COVID-19 was classified into three tiers: asymptomatic (no apparent signs of COVID-19 with a positive PCR test), mild to moderate (displaying COVID-19 symptoms or pneumonia without requiring oxygen supply), and severe (necessitating hospitalization with oxygen assistance).
2.4. Routine Clinical Evaluations
The patient’s age, sex, duration of HD treatment, dialysis dose measured by single pool Kt/V level, anuria status, body mass index (BMI), comorbidities (such as diabetes mellitus, hypertension, and a history of cardiovascular disease), and COVID-19 vaccination status prior to contracting the virus were meticulously documented. BMI was calculated as weight in kilograms divided by the square of the height in meters. Anuria was defined as daily diuresis of less than 100 mL.
Biochemical and hematological parameters were measured using the automated Flexor Junior (Vital Scientific, Spankeren, the Netherlands) and the ABX Micros-60 (Horiba Medical, Montpellier, France) analyzers, respectively. Clinical laboratory parameters included blood levels of serum albumin, C-reactive protein (CRP), electrolytes, intact parathyroid hormone (PTH), total cholesterol, total lymphocyte count (TLC), and hemoglobin concentration. PTH levels were evaluated using an immunoradiometric assay, and electrolyte levels were determined through conventional autoanalyzer techniques.
2.5. CONUT Score Calculation
The CONUT score was assessed twice: once before and then 3 months after contracting COVID-19. It was determined by evaluating serum albumin level, total cholesterol concentration, and TLC. Each parameter was assigned individual scores: serum albumin (0, 2, 4, 6), total cholesterol concentration, and TLC (0, 1, 2, 3 for each). These scores were then categorized as normal, light, moderate, or severe, corresponding to ranges of 0-1, 2-4, 5-8, and 9-12, respectively, as detailed in
Table 1 [
17].
2.6. Oxidative Stress Markers Determination
The study assessed oxidative markers including malondialdehyde (MDA) in both serum (MDAs) and erythrocytes (MDAe) to gauge lipid peroxidation. Additionally, serum ceruloplasmin, transferrin, and sulfhydryl groups (SH groups) were measured to evaluate antioxidant defense [
18].
For the measurement of MDAs and MDAe, blood samples were collected and separated into serum and erythrocyte components. In the serum samples (MDAs), 0.5 mL of serum was mixed with 1.5 mL of 0.025 M Tris buffer containing potassium chloride (pH 7.4) and incubated at 37 °C for 30 minutes. Following incubation, 1 mL of 17% trichloroacetic acid solution was added, and the samples were centrifuged. Then, 1 mL of 0.8% thiobarbituric acid solution was added to the supernatant of each sample tube, followed by boiling and subsequent measurement of absorbance at 532 nm. The concentration of MDA in each sample was calculated using appropriate standard curves and expressed as µmol/L.
Serum ceruloplasmin was determined by combining 0.05 mL of serum with 4 mL of 0.4 M acetic buffer solution (pH 5.5) and 0.5 mL of a 0.5% aqueous solution of 1,2-phenylenediamine dihydrochloride. The absorbance was measured at 530 nm, and the ceruloplasmin concentration was expressed in g/L.
Serum transferrin concentration was assessed by adding 0.2 mL of serum to 2 mL of a 0.2% solution of ammonium-iron(III)-citrate (pH 5.5–5.8). The absorbance was measured at 440 nm after one minute and after 30 minutes, and transferrin concentration was calculated as the difference between the absorbance readings, expressed in g/L.
The level of SH groups in serum was determined by dissolving 0.05 mL of serum in 0.5 mL of distilled water, followed by the addition of potassium iodide solution, starch solution, and phosphate buffer. Absorbance was measured before and after the application of iodine solution, and the concentration of SH groups was expressed as mmol/L.
Reagents used in the study were sourced as follows: tris(hydroxymethyl)aminomethane, tris(hydroxymethyl)aminomethane hydrochloride, malonaldehyde bis(diethyl acetal), 1,4-phenylenediamine dihydrochloride, human ceruloplasmin, sodium fluoride, potassium chloride, and potassium iodide from Sigma-Aldrich (USA), while trichloroacetic acid, thiobarbituric acid, ferric ammonium citrate, sodium hydrogen phosphate, and sodium acetate were procured from Merck (Germany). Transferrin was obtained from BioChemica (Fluka).
2.7. Statistical Analysis
MedCalc version 19.2.6 (Ostend, Belgium) Statistical Software. Descriptive statistics were reported as means with standard deviations (M±SD) or medians with interquartile ranges [Me (Q1-Q3)] for continuous variables and as frequencies with percentages for categorical variables. The Shapiro-Wilk test was employed to assess the normality of data distribution.
Comparisons between the groups were performed using the Student's t-test for normally distributed continuous variables and the Mann-Whitney U test for non-normally distributed continuous variables. Categorical variables were compared using the Chi-square test (χ2).
Partial correlation analysis was conducted to evaluate the association between pre-infection CONUT scores and oxidative stress markers, adjusting for post-COVID CONUT scores, age, sex, and HD duration. A multivariate logistic regression analysis was performed to determine variables independently associated with MDAs concentration. Age, sex, HD duration, and all variables showing significant differences between the groups were included as potential confounders in the analysis.
The Kaplan-Meier survival analysis was employed to estimate the probability of long COVID persistence throughout the one-year follow-up period. Subsequently, to account for potential confounders, Cox proportional hazard regression models were utilized to investigate the association between pre-infection CONUT score and one-year long COVID persistence. Initially, the analysis was conducted without adjustments. Then, adjustments were made for age, sex, HD duration, and all variables demonstrating significant differences between the groups (Model 1). Model 2 included adjustments from Model 1 and additionally incorporated MDAs and ceruloplasmin concentrations.
3. Results
3.1. Baseline Patient Characteristics
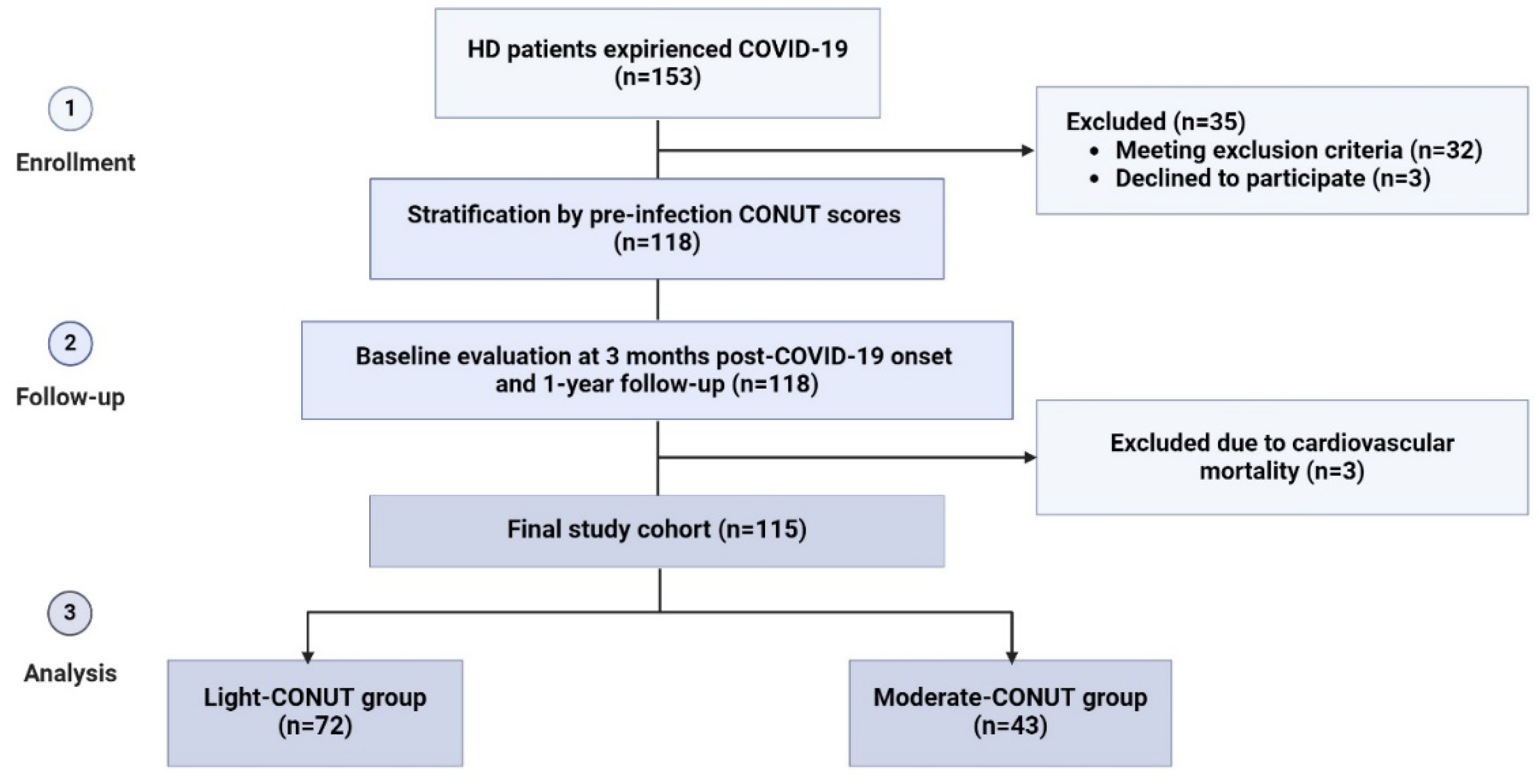
During the enrollment period, 153 patients undergoing HD and experienced COVID-19 were initially considered for inclusion in the study. Among them, 35 patients were excluded for various reasons, including a history of cardiovascular events (n=9), short duration of dialysis before COVID-19 (n=7), temporary vascular access (n=7), recent hospitalization (n=6), malignant or systemic disease (n=5), and age less than 18 years (n=1). Additionally, 3 out of the remaining 118 patients died due to heart failure during the follow-up period and were subsequently excluded from the study, resulting in a final study cohort of 115 patients (
Figure 1).
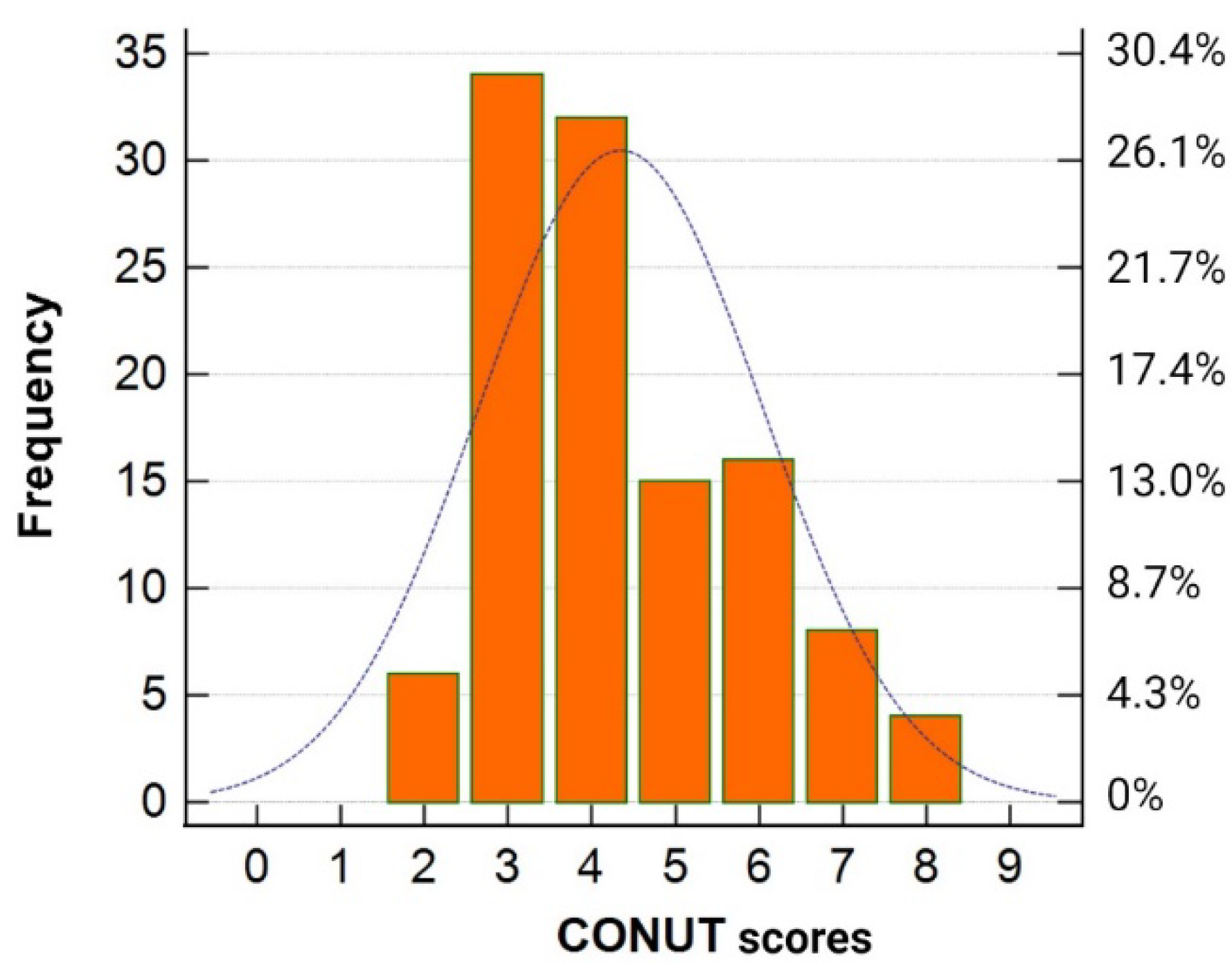
The pre-infection CONUT scores ranged from 2 to 8 points (
Figure 2), indicating that none of the patients in the study cohort were classified as having low or severe degrees of undernutrition before the onset of COVID-19.
Consequently, for further analysis, the patients were stratified into two groups: light-CONUT and moderate-CONUT.
As presented in
Table 2, the patient characteristics, including age, sex, dialysis dose and vintage, prevalence of diabetes, arterial pressure, BMI, hemoglobin, electrolytes, and PTH levels, as well as medication used were comparable between the light-CONUT and moderate-CONUT groups at three months post-COVID-19.
However, the moderate-CONUT group demonstrated a higher proportion of anuric patients and lower levels of serum albumin, TLC, CRP, and cholesterol compared to the light-CONUT group. Additionally, the moderate-CONUT group had a lower prevalence of vaccinated patients and fewer cases of asymptomatic acute COVID-19. Although there was a tendency towards more hospitalizations with oxygen supply during the acute phase of COVID-19 in the moderate-CONUT group, this difference did not achieve statistical significance. Moreover, the moderate-CONUT group showed a significantly higher post-COVID CONUT score compared to the light-CONUT group. Additionally, at three months post-COVID-19, severe undernutrition was present in 9 (7.8%) patients across the cohort, with 2 (2.8%) in the light-CONUT group and 7 (16.3%) in the moderate-CONUT group (χ2 = 74.9, p < 0.0001).
3.2. Pre-Infection CONUT Scores and Post-COVID Oxidative Damage
Analysis of oxidative stress markers indicated a significantly elevated concentration of MDAs and reduced ceruloplasmin level in the moderate-CONUT group compared to the light-CONUT group (
Table 3).
In the partial correlation analysis, with post-COVID CONUT scores, age, sex, and HD duration as covariates, the pre-infection CONUT score showed a significant association with MDAs (r = 0.65, p < 0.0001) and ceruloplasmin levels (r = -0.27, p = 0.004).
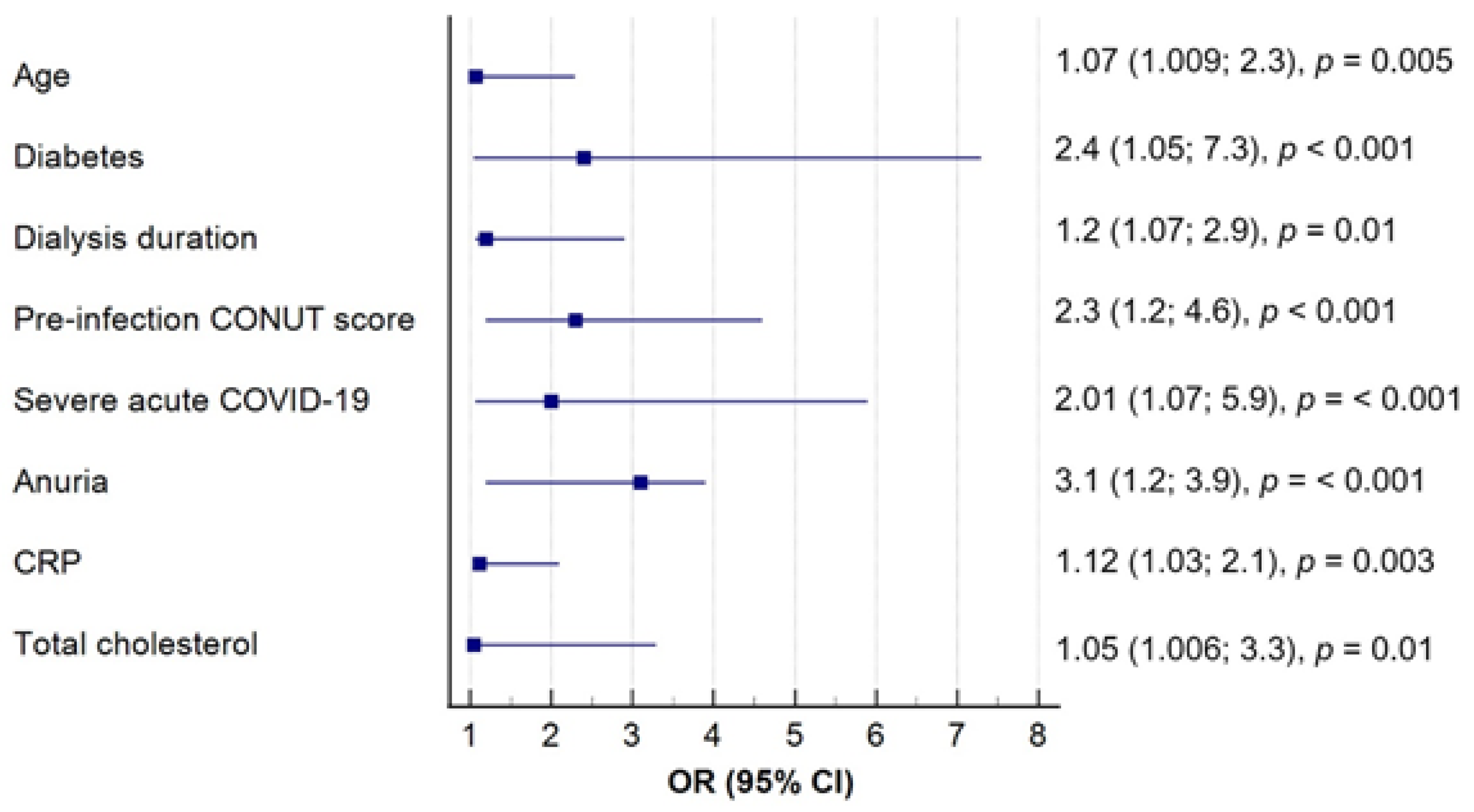
To further address potential confounding factors that could affect the intensity of oxidative stress, the patients were categorized based on median MDAs levels (<269 and ≥ 269 μmol/L). Subsequently, a multivariate logistic regression analysis was conducted to determine variables significantly affecting MDAs concentration. Notably, the pre-infection CONUT score emerged as an independent predictor of post-COVID oxidative damage, independently of other factors (
Figure 3).
3.3. Pre-Infection CONUT Scores and One-Year Long COVID Persistence
Throughout the follow-up period, 83 (72.2%) patients receiving HD were diagnosed with long COVID. Among them, 41 (95.3%) belonged to the moderate CONUT group, while 42 (58.3%) were in the light-CONUT group (χ
2 = 22.1, p < 0.0001). At the end of the follow-up period, 48 (41.7%) patients still presented with long COVID, with 32 (74.4%) in the moderate-CONUT group and 16 (22.2%) in the light-CONUT group (χ
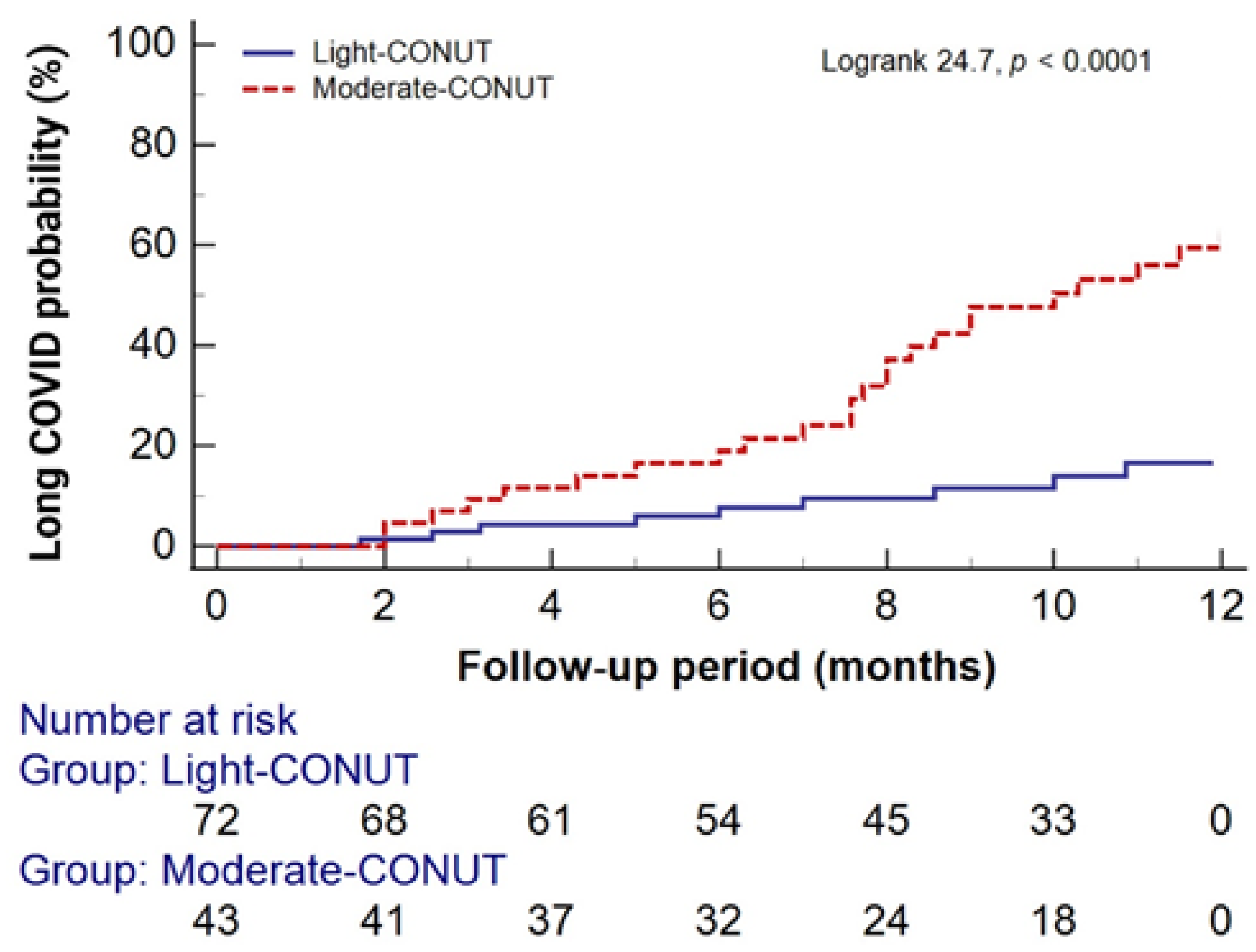
2 = 29.9, p < 0.0001). Kaplan-Meier analysis revealed a significantly higher probability of one-year long COVID persistence in the moderate-CONUT group compared to the light-CONUT group (
Figure 4).
Cox proportional hazard models were utilized to assess the risk of one-year long COVID persistence based on CONUT scores. As shown in
Table 4, both unadjusted and adjusted models demonstrated a significantly higher risk for the one-year persistence of long COVID in the moderate-CONUT group compared to the light-CONUT group.
4. Discussion
The emergence of long COVID as a significant health concern post-acute SARS-CoV-2 infection has prompted heightened attention towards its predisposing factors [
1,
8,
19]. Nutritional deficiency has been identified as a significant risk factor for severe acute COVID-19 and long COVID in the general patient population [
10,
11,
20]. However, there is a lack of data on this topic in patients undergoing HD, who often have malnutrition at onset. To our knowledge, our study is the first to investigate the association between pre-infection nutritional status, measured by the CONUT score, post-COVID oxidative stress, and one-year long COVID persistence. The findings revealed that patients with moderate pre-infection CONUT scores faced a heightened risk of severe undernutrition and increased oxidative stress three months post-acute COVID-19 compared to those with light CONUT scores. Moreover, moderate pre-infection undernutrition significantly contributed to oxidative damage and the persistence of long COVID symptoms at one year, independent of other known factors. It's worth noting that none of the patients in our cohort were classified as severely undernourished before COVID-19 infection, highlighting the importance of even mild to moderate nutritional deficiencies in affecting long-term COVID-19 outcomes.
Although data on the HD population are limited, our findings align with broader research suggesting that malnutrition can worsen the severity of COVID-19 and contribute to prolonged recovery times in the general patient population [
12,
21,
22,
23,
24]. Malnutrition, characterized by deficiencies in essential nutrients, compromises immune function and increases vulnerability to infections [
22,
23,
25]. This is particularly relevant for patients undergoing HD, who are already at an increased risk for malnutrition due to factors such as reduced dietary intake, nutrient losses during dialysis, and chronic inflammation [
9,
26,
27]. Consistent with our results, Lin et al. have emphasized the notable impairment in humoral response to COVID-19 vaccination among HD patients identified as malnourished based on the CONUT score [
27]. This corresponds with our observation that moderate pre-infection CONUT score status predisposes HD patients to prolonged long COVID symptoms, even among those who have been vaccinated.
Furthermore, our study highlights the role of oxidative stress in the development and persistence of long COVID symptoms. Oxidative stress has been implicated in the pathogenesis of long COVID, as evidenced by previous research [
14,
16,
28]. Elevated levels of oxidative damage markers, such as MDAs, and reduced levels of ceruloplasmin were observed in our cohort of HD patients with moderate pre-infection nutritional deficiencies, suggesting that oxidative stress may play a significant role in the chronicity of COVID-19 sequelae. Our previous report also demonstrated significantly increased oxidative stress and decreased antioxidant markers in patients undergoing HD with long COVID compared to fully recovered patients [
29]. Additionally, in line with our findings, Stufano et al. proposed MDAs as an independent predictor of long COVID in Italian workers [
30], while Restea et al. demonstrated decreased ceruloplasmin levels in patients with type 2 diabetes and severe COVID-19 [
31].
The relationship between nutritional status and oxidative stress is complex and bidirectional, especially in HD patients who already contend with heightened oxidative stress [
26,
32]. Nutritional deficiencies can lead to diminished antioxidant defenses, thereby increasing vulnerability to oxidative damage [
23,
33,
34,
35]. Conversely, oxidative stress can further deplete nutrients, creating a vicious cycle that can be particularly detrimental in the context of long COVID [
28,
36]. For instance, the depletion of antioxidants such as glutathione, superoxide dismutase, and catalase has been associated with severe COVID-19 outcomes and may also play a role in the persistence of long COVID symptoms [
37,
38].
Our study supports the hypothesis that optimizing nutritional status could mitigate oxidative stress levels, potentially reducing the severity and duration of long COVID symptoms in vulnerable populations like those undergoing HD. Strategies aimed at optimizing nutritional status, such as dietary interventions and supplementation, may offer potential avenues for mitigating the severity and duration of long COVID symptoms in HD patients.
Despite the novel insights provided by our study, several limitations warrant consideration. First, the observational nature of our study precludes causal inference, and unidentified confounding factors may influence the observed associations. Second, the relatively small sample size limits the generalizability of our findings. Third, the exclusion criteria, such as excluding patients with recent hospitalization or cardiovascular events, may introduce selection bias and limit the representativeness of the study cohort. Finally, variability in the measurement of oxidative stress markers and other variables could affect the accuracy and reliability of the results. Future prospective studies with larger, multicenter cohorts are warranted to validate our findings and elucidate the underlying mechanisms linking nutritional status, oxidative stress, and long COVID persistence in HD patients.
5. Conclusions
Together, our study highlights the association between pre-infection nutritional status, oxidative stress, and the one-year persistence of long COVID in patients undergoing HD. Patients with moderate pre-infection nutritional deficiencies, as assessed by the CONUT score, faced a heightened risk of severe undernutrition following acute COVID-19, increased oxidative stress, and prolonged long COVID symptoms compared to those with light nutritional deficiencies. The findings underscore the importance of addressing even mild to moderate nutritional deficiencies in improving long COVID outcomes among HD patients. Strategies aimed at optimizing nutritional status may help mitigate oxidative stress levels and reduce the severity and duration of long COVID symptoms in this vulnerable population.
Further research, including larger prospective studies with diverse cohorts, is needed to validate these findings and elucidate the underlying mechanisms linking nutritional status, oxidative stress, and long COVID persistence in HD patients. Additionally, interventional studies evaluating the efficacy of nutritional interventions in mitigating long COVID symptoms in this population are warranted.
Author Contributions
NS: Conceptualization, Methodology, Formal analysis, Visualization, Writing - Original Draft. LK: Investigation, Writing - Review & Editing. T.O., V.M., O.B. and L.S: Resources. I.S.: Writing - Review & Editing. M.O.: Supervision. All the authors reviewed the manuscript and approved it for publication.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of the State Institution "Institute of Nephrology of the National Academy of Medical Sciences", Kyiv, Ukraine (protocol number: 2-2021, dated April 6, 2021).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
The data used in the study are available upon reasonable request to the corresponding author.
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Prashar, J. Long Covid: Conceptualizing the Challenges for Public Health. J. Public Health (Bangkok). 2023, 45, 771–779. [CrossRef]
- Davis, H.E.; McCorkell, L.; Vogel, J.M.; Topol, E.J. Long COVID: Major Findings, Mechanisms and Recommendations. Nat. Rev. Microbiol. 2023, 21, 408. [CrossRef]
- Jacobs, E.T.; Catalfamo, C.J.; Colombo, P.M.; Khan, S.M.; Austhof, E.; Cordova-Marks, F.; Ernst, K.C.; Farland, L. V.; Pogreba-Brown, K. Pre-Existing Conditions Associated with Post-Acute Sequelae of COVID-19. J. Autoimmun. 2023, 135, 102991. [CrossRef]
- Salerno, S.; Messana, J.M.; Gremel, G.W.; Dahlerus, C.; Hirth, R.A.; Han, P.; Segal, J.H.; Xu, T.; Shaffer, D.; Jiao, A.; et al. COVID-19 Risk Factors and Mortality Outcomes Among Medicare Patients Receiving Long-Term Dialysis. JAMA Netw. Open. 2021, 4, e2135379. [CrossRef]
- Demiray, A.; Kanbay, A.; Kanbay, M. Long-Term Effect of COVID-19 Infection on Hemodialysis Patients: Should We Follow Hemodialysis Patients More Closely? Clin. Kidney J. 2022, 15, 369-371. [CrossRef]
- Subramanian, A.; Nirantharakumar, K.; Hughes, S.; Myles, P.; Williams, T.; Gokhale, K.M.; Taverner, T.; Chandan, J.S.; Brown, K.; Simms-Williams, N.; et al. Symptoms and Risk Factors for Long COVID in Non-Hospitalized Adults. Nat. Med. 2022, 28, 1706-1714. [CrossRef]
- Asadi-Pooya, A.A.; Akbari, A.; Emami, A.; Lotfi, M.; Rostamihosseinkhani, M.; Nemati, H.; Barzegar, Z.; Kabiri, M.; Zeraatpisheh, Z.; Farjoud-Kouhanjani, M.; et al. Risk Factors Associated with Long COVID Syndrome: A Retrospective Study. Iran. J. Med. Sci. 2021, 46, 428-436. [CrossRef]
- Tsampasian, V.; Elghazaly, H.; Chattopadhyay, R.; Debski, M.; Naing, T.K.P.; Garg, P.; Clark, A.; Ntatsaki, E.; Vassiliou, V.S. Risk Factors Associated With Post−COVID-19 Condition: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2023, 183, 566–580. [CrossRef]
- Sahathevan, S.; Khor, B.H.; Ng, H.M.; Gafor, A.H.A.; Daud, Z.A.M.; Mafra, D.; Karupaiah, T. Understanding Development of Malnutrition in Hemodialysis Patients: A Narrative Review. Nutrients. 2020, 12, 3147. [CrossRef]
- Bengelloun, A.K.; Ortega, G.J.; Ancochea, J.; Sanz-Garcia, A.; Rodríguez-Serrano, D.A.; Fernández-Jiménez, G.; Girón, R.; Ávalos, E.; Soriano, J.B.; de Ulíbarri, J.I. Usefulness of the CONUT Index upon Hospital Admission as a Potential Prognostic Indicator of COVID-19 Health Outcomes. Chin. Med. J. (Engl). 2022, 135, 187-193. [CrossRef]
- Zhao, Z.W.; Chen, Q.; Zhang, X.T.; Luo, Y.K. The CONUT Score Predicts the Length of Hospital Stay and the Risk of Long COVID. Nutr. Hosp. 2024, 41, 138–144. [CrossRef]
- Wang, X.; Deng, W.; Zhao, J.; Guo, Y.; Lai, H.; G Hu, Y.; Kang, W.; Li, Y.; Zuo, J. Improving Nutritional Status Was Associated with Decreasing Disease Severity and Shortening of Negative Conversion Time of PCR Test in Non-ICU Patients with COVID-19. Infect. Drug. Resist. 2023, 16, 4443–4452. [CrossRef]
- Golabi, S.; Ghasemi, S.; Adelipour, M.; Bagheri, R.; Suzuki, K.; Wong, A.; Seyedtabib, M.; Naghashpour, M. Oxidative Stress and Inflammatory Status in COVID-19 Outpatients: A Health Center-Based Analytical Cross-Sectional Study. Antioxidants. 2022, 11, 606. [CrossRef]
- Vollbracht, C.; Kraft, K. Oxidative Stress and Hyper-Inflammation as Major Drivers of Severe COVID-19 and Long COVID: Implications for the Benefit of High-Dose Intravenous Vitamin C. Front. Pharmacol. 2022, 13, 899198. [CrossRef]
- Al-Hakeim, H.K.; Al-Rubaye, H.T.; Al-Hadrawi, D.S.; Almulla, A.F.; Maes, M. Long-COVID Post-Viral Chronic Fatigue and Affective Symptoms are Associated with Oxidative Damage, Lowered Antioxidant Defenses and Inflammation: A Proof of Concept and Mechanism Study. Mol. Psychiatry. 2022, 28, 564–578. [CrossRef]
- Georgieva, E.; Ananiev, J.; Yovchev, Y.; Arabadzhiev, G.; Abrashev, H.; Abrasheva, D.; Atanasov, V.; Kostandieva, R.; Mitev, M.; Petkova-Parlapanska, K.; et al. COVID-19 Complications: Oxidative Stress, Inflammation, and Mitochondrial and Endothelial Dysfunction. Int. J. Mol. Sci. 2023, 24, 14876. [CrossRef]
- Médica, G.A.; Ulibarri, D.; González-Madroño, J.I.; Villar, A. De; González, N.G.; González, P.; Mancha, B.; Rodríguez, A.; Fernández, F.; Hospitalaria, N.; et al. CONUT: A Tool for Controlling Nutritional Status. First Validation in a Hospital Population. Nutr. Hosp. 2005, 20, 38-45.
- Korol, L. V.; Mygal, L.Y.; Stepanova, N.M. Intensity of Oxidative Stress and Activity of Angiotensin Converting Enzyme in Blood of Patients with Uncomplicated Pyelonephritis. Ukr. Biochem. J. 2017, 89, 99–105. [CrossRef]
- Stepanova, N.; Rysyev, A.; Rusyn, O.; Ostapenko, T.; Snisar, L.; Kompaniets, O.; Kolesnyk, M. High-Density Lipoproteins and Clinical Outcomes of COVID-19 in Hemodialysis Patients: A Multicenter, Propensity-Score Matched Case-Control Study. Ukr. J. Nephrol. Dial. 2022, 1, 22–30. [CrossRef]
- Tosato, M.; Calvani, R.; Ciciarello, F.; Galluzzo, V.; Martone, A.M.; Zazzara, M.B.; Pais, C.; Savera, G.; Robles, M.C.; Ramirez, M.; et al. Malnutrition in COVID-19 Survivors: Prevalence and Risk Factors. Aging Clin. Exp. Res. 2023, 35, 2257–2265. [CrossRef]
- Deer, R.R.; Hosein, E.; Harvey, M.; Nguyen, T.; Givan, A.; Hamilton, M.; Turner, K.; Kretzmer, R.; Rock, M.; Swartz, M.C.; et al. Impact of COVID-19 Infection and Persistent Lingering Symptoms on Patient Reported Indicators of Nutritional Risk and Malnutrition. Nutrients. 2022, 14, 642. [CrossRef]
- Mortaz, E.; Bezemer, G.; Alipoor, S.D.; Varahram, M.; Mumby, S.; Folkerts, G.; Garssen, J.; Adcock, I.M. Nutritional Impact and Its Potential Consequences on COVID-19 Severity. Front. Nutr. 2021, 8, 698617. [CrossRef]
- Schloss, J. V. Nutritional Deficiencies That May Predispose to Long COVID. Inflammopharmacology. 2023, 31, 573–583. [CrossRef]
- De Araújo Morais, A.H.; Aquino, J.D.S.; Da Silva-Maia, J.K.; Vale, S.H.D.L.; MacIel, B.L.L.; Passos, T.S. Nutritional Status, Diet and Viral Respiratory Infections: Perspectives for Severe Acute Respiratory Syndrome Coronavirus 2. Br J Nutr. 2021, 125, 851-862. [CrossRef]
- Rodriguez-leyva, D.; Pierce, G.N. The Impact of Nutrition on the COVID-19 Pandemic and the Impact of the COVID-19 Pandemic on Nutrition. Nutrients. 2021, 13, 1752. [CrossRef]
- Shifris, I.; Korol, L.; Krasiuk, E.; Dudar, S. Activation of Oxidative Stress, Comorbidity and Survival of End-Stage Renal Disease Patients Treated with Hemodialysis. Ukr. J. Nephrol. Dial. 2021, 4, 67–77. [CrossRef]
- Lin, T.Y.; Hung, N.K.; Hung, S.C. Association of Malnutrition with SARS-CoV-2 Vaccine Response in Patients Undergoing Hemodialysis. Clin. Nutr. 2022, 41, 2683-2690. [CrossRef]
- Hofmann, H.; Önder, A.; Becker, J.; Gröger, M.; Müller, M.M.; Zink, F.; Stein, B.; Radermacher, P.; Waller, C. Markers of Oxidative Stress during Post-COVID-19 Fatigue: A Hypothesis-Generating, Exploratory Pilot Study on Hospital Employees. Front. Med. (Lausanne). 2023, 10, 1305009. [CrossRef]
- Stepanova, N.; Korol, L.; Snisar, L.; Rysyev, A.; Ostapenko, T.; Marchenko, V.; Belousova, O.; Popova, O.; Malashevska, N.; Kolesnyk, M. Long-COVID Sequelae Are Associated with Oxidative Stress in Hemodialysis Patients. Ukr. J. Nephrol. Dial. 2023, 1, 31–39. [CrossRef]
- Stufano, A.; Isgrò, C.; Palese, L.L.; Caretta, P.; De Maria, L.; Lovreglio, P.; Sardanelli, A.M. Oxidative Damage and Post-COVID Syndrome: A Cross-Sectional Study in a Cohort of Italian Workers. Int. J. Mol. Sci. 2023, 24, 7445. [CrossRef]
- Reștea, P.A.; Țigan, Ștefan; Vicaș, L.G.; Fritea, L.; Marian, E.; Jurca, T.; Pallag, A.; Mureșan, I.L.; Moisa, C.; Micle, O.; et al. Serum Level of Ceruloplasmin, Angiotensin-Converting Enzyme and Transferrin as Markers of Severity in SARS-CoV-2 Infection in Patients with Type 2 Diabetes. Microbiology Research. 2023, 14, 1670–1686. [CrossRef]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Hemodialysis: Causative Mechanisms, Clinical Implications, and Possible Therapeutic Interventions. Semin. Dial. 2019, 32, 58–71. [CrossRef]
- Trujillo-Mayol, I.; Guerra-Valle, M.; Casas-Forero, N.; Sobral, M.M.C.; Viegas, O.; Alarcón-Enos, J.; Ferreira, I.M.; Pinho, O. Western Dietary Pattern Antioxidant Intakes and Oxidative Stress: Importance During the SARS-CoV-2/COVID-19 Pandemic. Adv. Nutr. 2021, 12, 670–681. [CrossRef]
- Chaudhary, P.; Janmeda, P.; Docea, A.O.; Yeskaliyeva, B.; Abdull Razis, A.F.; Modu, B.; Calina, D.; Sharifi-Rad, J. Oxidative Stress, Free Radicals and Antioxidants: Potential Crosstalk in the Pathophysiology of Human Diseases. Front. Chem. 2023, 11, 1158198. [CrossRef]
- Voelkle, M.; Gregoriano, C.; Neyer, P.; Koch, D.; Kutz, A.; Bernasconi, L.; Conen, A.; Mueller, B.; Schuetz, P. Prevalence of Micronutrient Deficiencies in Patients Hospitalized with COVID-19: An Observational Cohort Study. Nutrients. 2022, 14, 1862. [CrossRef]
- Bakadia, B.M.; Boni, B.O.O.; Ahmed, A.A.Q.; Yang, G. The Impact of Oxidative Stress Damage Induced by the Environmental Stressors on COVID-19. Life Sci. 2021, 264, 118653. [CrossRef]
- Muhammad, Y.; Kani, Y.A.; Iliya, S.; Muhammad, J.B.; Binji, A.; El-Fulaty Ahmad, A.; Kabir, M.B.; Umar Bindawa, K.; Ahmed, A. Deficiency of Antioxidants and Increased Oxidative Stress in COVID-19: A Cross-Sectional Comparative Study in Jigawa, Northwestern. SAGE Open Med. 2021, 9, 2050312121991246. [CrossRef]
- Naidu, A.S.; Wang, C.K.; Rao, P.; Mancini, F.; Clemens, R.A.; Wirakartakusumah, A.; Chiu, H.F.; Yen, C.H.; Porretta, S.; Mathai, I.; et al. Precision Nutrition to Reset Virus-Induced Human Metabolic Reprogramming and Dysregulation (HMRD) in Long-COVID. NPJ Sci. Food. 2024, 8, 19. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).