Submitted:
04 June 2024
Posted:
05 June 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
1.1. Inflorescence Architecture
1.2. Genotypes, Genes and Development of the Genet
1.3. Inflorescence Architecture and Environment
2. Materials and Methods
2.1. Data and Its Analysis
2.2. Sites, Cultivars and Clone-Sets
2.3. The ‘Modified’ Data Set and a Calculated Data Set for Ndihira
2.4. The Exponential Reciprocal Function and the Curvature Coefficient
2.5. The Effect of the Genet
2.6. Statistical Analyses
3. Results
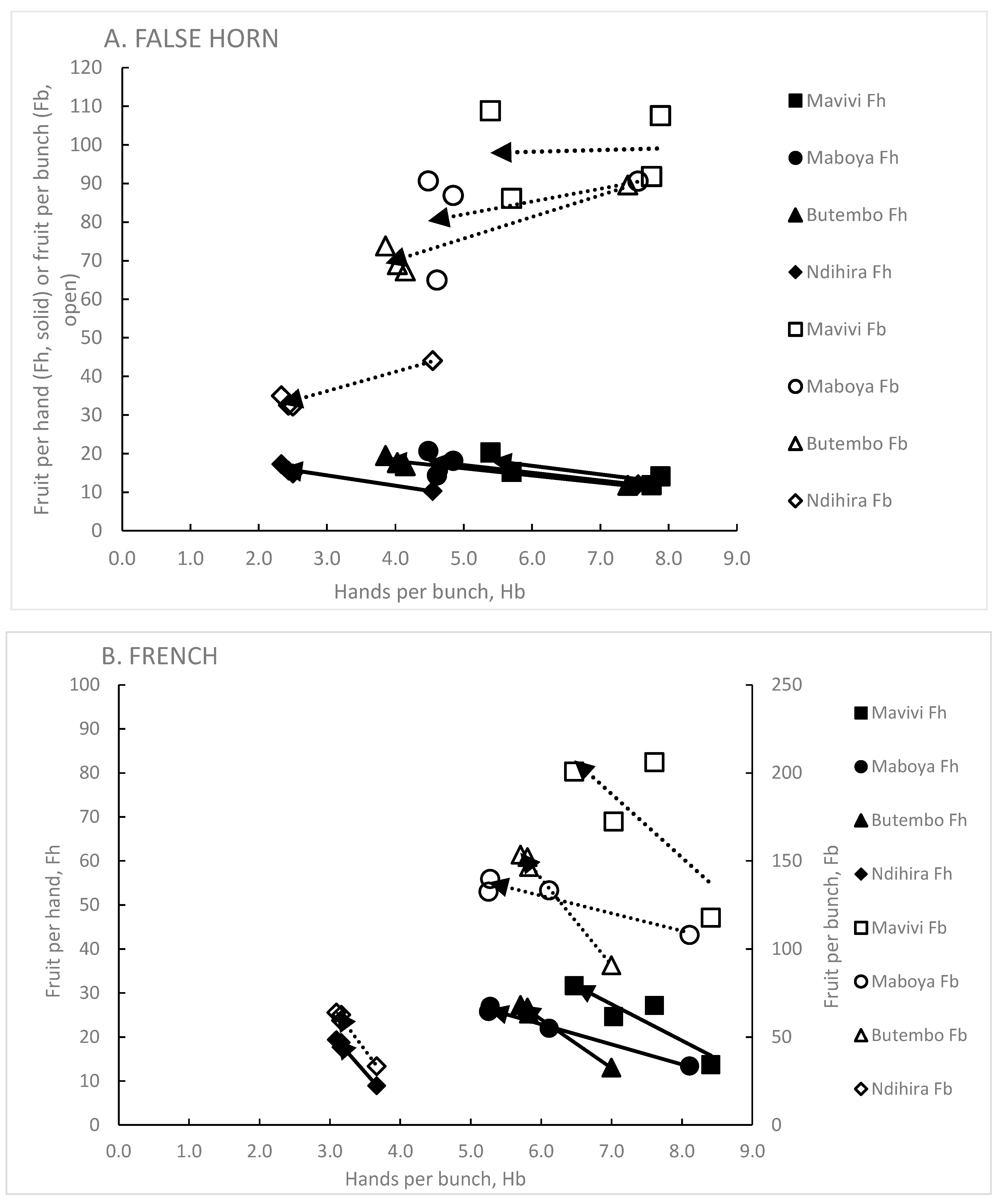
3.1. The Effect of Site, and Cultivar on Measured Data of Hb, Fh, Fb, Pr and Pf
3.2. Changes to the Data Caused by Modification
3.3. Fitting the Exponential Reciprocal Function to the Modified Data
3.4. Effect of the Genet on Hb, Fh and Fb
4. Discussion
4.1. Fruit per Bunch, Fb, per Hand, Fh, and Hands per Bunch, Hb
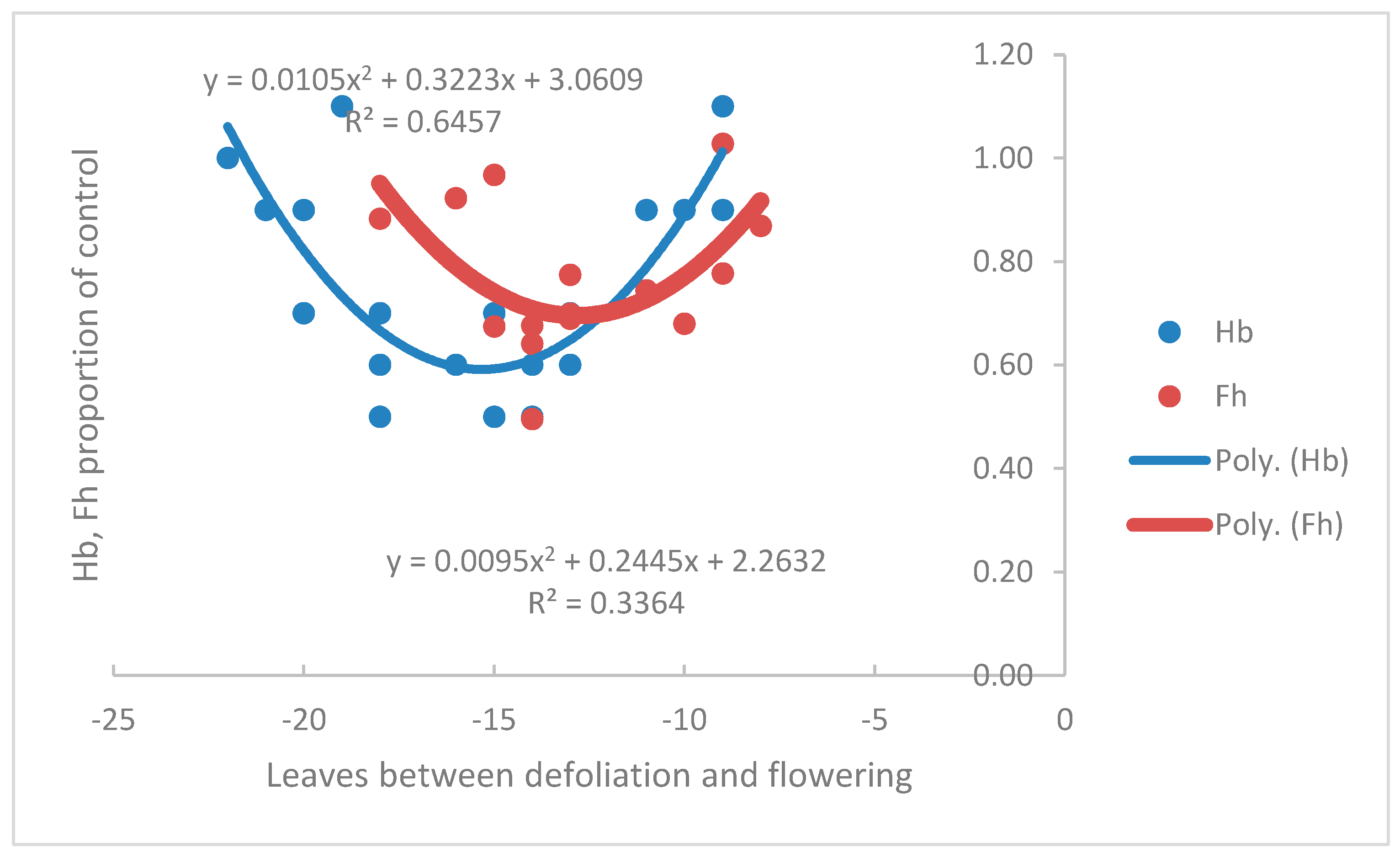
4.2. Plant Reserves and Inflorescence Architecture
4.3. Peduncle Total Length, Pr, and the Proportion that Was Female, Pf
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adheka JG, De Langhe E. (2018) Characterisation and classification of the Musa AAB Plantain subgroup in the Congo Basin. Scripta Botanica Belgica, 54, 1-104.
- Adheka JG, Dhed’a DB, Karamura D, Blomme G, Swennen R, De Langhe E. (2018) The morphological diversity of plantain in the Democratic Republic of Congo. Scientia Horticulturae, 234, 126-133. [CrossRef]
- Alexandrowicz L. (1955) ‘Etude du developpement de l’inflorescence du bananier Nain’. Annales 9, Institut des Fruits et Agrumes Coloniaux (IFAC), Paris, France.
- Argent GCG. (1976) The wild bananas of Papua New Guinea. Royal Botanical Gardens Edinburgh, Notes, 35, 77-114.
- Argent GCG. (2000) Two interesting wild Musa species (Musaceae) from Sabah, Malaysia. Gardens’ Bulletin Singapore, 52, 203-210.
- Baker JG. (1893) A synopsis of the genera and species of Musaceae. Annals of Botany, 7, 189-222.
- Bartlett ME, Specht CD. (2010) Evidence for the involvement of GLOBOSA-like gene duplications and expression divergence in the evolution of floral morphology in the Zingiberales. New Phytologist, 187, 521-541. [CrossRef]
- Blomme G, Ocimati W, Sivirihauma C, Vutseme L, Turner DW. (2020) The performance of a wide range of plantain cultivars at three contrasting altitude sites in North Kivu, Eastern Democratic Republic of Congo. Fruits, 75, 21-35.
- Bowman JL, Moyroud E (2024) Reflections on the ABC model of flower development. The Plant Cell, 36, 1334-1357.
- Daniells, JW, O’Farrell PJ. (1987) Effect of cutting height of the parent pseudostem on yield and time of production of the following sucker in banana. Scientia Horticulturae, 31, 89-94. [CrossRef]
- De Langhe E. (1961) La taxonomie du bananier plantain en Afrique Equatoriale. Journal d’agriculture tropicale et de botanique appliquee, 8 (10-11), 417-449.
- Dodsworth S. (2017) Petal, sepal, or tepal? B-genes and monocot flowers. Trends in Plant Science, 22 (1), 8-10. [CrossRef]
- Eckstein K, Robinson JC. (1999) The influence of the mother plant on sucker growth, development, and photosynthesis in banana (Musa AAA: Dwarf Cavendish). Journal of Horticultural Science and Biotechnology, 74, 347-350.
- Fahn A. (1953) The origin of the banana inflorescence. Kew Bulletin 1953, (3), 299-306. [CrossRef]
- Ganry J. (1977) Determination ‘in situ’ du stade de transition entre la phase vegetative et la phase florale chez le bananier, utilisant le ‘coefficient de Vitesse de croissance des feuilles’. Essai d’interpretation de quelques processus de developpement durant la periode florale. Fruits, 32, 373-386.
- Irish V. (2017) The ABC model of flower development. Current Biology, 27, R853-R909.
- Kirchoff BK. (2017) Inflorescence and flower development in Musa velutina H. Wendl. & Drude (Musaceae), with a consideration of developmental variability, restricted phyllotactic direction, and hand initiation. International Journal of Plant Science, 178, 259-272.
- Lahav E, Turner DW. (1989) ‘Fertilising for high yield – Banana’ 2nd edn. IPI Bulletin 7, International Potash Institute, Berne, Switzerland.
- McIntyre BD, Speijer P, Riha SJ, Kizito F. (2000) Effects of mulching on biomass, nutrients, and soil water in banana inoculated with nematodes. Agronomy Journal, 92, 1081-1085. [CrossRef]
- Mead R, Curnow RN, Hasted AM.(1993) ‘Statistical Methods in Agriculture and Experimental Biology’. 2nd Edn. Chapman & Hall, London, UK.
- Moncur MW. (1988) ‘Floral development of tropical and subtropical fruit and nut species. An atlas of scanning electron micrographs. Division of Water and Land Resources, Natural Resources Series No 8, CSIRO, Australia.
- Nyine M, Uwimana B, Akech V, Brown A, Ortiz R, Dolezel J, Lorenzen J, Swennen R. (2019) Association genetics of bunch weight and its component traits in East African highland banana (Musa spp. AAA group). Theoretical and Applied Genetics, 132, 3295-3308. [CrossRef]
- Nyine M, Uwimana B, Swennen R, Batte M, Brown A, Christelova P, Hribova E, Lorenzen J, Dolezel J. (2017) Trait variation and genetic diversity in a banana genomic selection training population. PLoS ONE, 12(6), e0178734. [CrossRef]
- R Core Team. (2019) ‘R: A language and environment for statistical computing’. R Foundation for Statistical Computing, Vienna, Austria.
- Ram HYM, Ram M, Steward FC. (1962) Growth and development of the banana plant, 3a. The origin of the inflorescence and the development of flowers. 3b. The structure and development of the fruit. Annals of Botany, 26, 657-673.
- Robinson JC, Nel DJ. (1985) Comparative morphology, phenology, and production potential of banana cultivars ‘Dwarf Cavendish’ and ‘Williams’ in the Eastern Transvaal Lowveld. Scientia Horticulturae, 25, 149-161.
- Robinson JC, Nel DJ. (1990) Competitive inhibition of yield potential in a ‘Williams’ banana plantation due to excessive sucker growth. Scientia Horticulturae, 43, 225-236.
- Savvides A, Dieleman JA, van Ieperen W, Marcelis LFM. (2016) A unique approach to demonstrating that apical bud temperature specifically determines leaf initiation rate in the dicot Cucumis sativus. Planta, 243, 1071-1079. [CrossRef]
- Sikyolo I, Sivirihauma C, Ndungo V, De Langhe E, Ocimati W, Blomme G. (2013) ‘Growth and yield of plantain cultivars at four sites of differing altitude in North Kivu, Eastern Democratic Republic of Congo’. In ‘Banana Systems in the Humid Highlands of Sub-Saharan Africa – Enhancing Resilience and Productivity’. Eds. G Blomme, P van Asten, B Vanlauwe. CAB International, Wallingford, UK, pp. 48-57.
- Simmonds NW. (1954) Varietal identification in the Cavendish group of bananas. Journal of Horticultural Science, 29, 81-88. [CrossRef]
- Simmonds NW. (1966) ‘Bananas’. 2nd edn. Longman, London, UK.
- Sivirihauma C, Blomme G, Ocimati W, Vutseme L, Sikyolo I, Valimuzigha K, De Langhe E, Turner DW. (2016) Altitude effect on plantain growth and yield during four production cycles in North Kivu, Eastern Democratic Republic of Congo. Acta Horticulturae, 1114, 139-148.
- Summerville WAT. (1944) Studies on nutrition as qualified by development in Musa cavendishii Lamb. The Queensland Journal of Agricultural Science, 1, 1-127.
- Swennen R, Vuylsteke D. (1987) ‘Morphological taxonomy of plantain (Musa cultivars AAB) in West Africa’. In ‘Banana and Plantain Breeding Strategies’. Eds. GJ Persley, EA De Langhe pp. 165-171. ACIAR Proceedings No 21, ACIAR Canberra, pp 165-171.
- Swennen R, Vuylsteke D, Ortiz R. (1995) Phenotypic diversity, and patterns of variation in West and Central African plantains (Musa spp., AAB group, Musaceae). Economic Botany, 49, 329-327. [CrossRef]
- Tezenas du Montcel H, De Langhe E, Swennen R. (1983) Essai de classification des bananiers plantains (AAB). Fruits, 38, 461-474.
- Turner DW. (1971) Effects of climate on rate of banana leaf production. Tropical Agriculture (Trinidad), 48, 283-287.
- Turner DW.(1981) Crop physiology of bananas – quo vadis? Madras Agricultural Journal, 68, 78-84.
- Turner DW, Fortescue JA, Ocimati W, Blomme G. (2016) Plantain cultivars (Musa spp. AAB) grown at different altitudes demonstrate cool temperature and photoperiod responses relevant to genetic improvement. Field Crops Research, 194, 103-111. [CrossRef]
- Turner DW, Gibbs DJ. (2018) ‘A functional approach to bunch formation in banana’. In ‘Achieving sustainable cultivation of bananas, Vol 1. Cultivation techniques. Eds. GHJ Kema, A Drenth. Burleigh Dodds Scientific Publishing: Cambridge UK, pp. 93-116.
- Turner DW, Gibbs DJ, Ocimati W, Blomme G. (2020) The suckering behaviour of plantain (Musa, AAB) can be viewed as part of an evolved reproductive strategy. Scientia Horticulturae, 261, 108975. [CrossRef]
- Turner DW, Hunt N. (1984) ‘Growth, yield, and nutrient composition of 30 banana varieties in subtropical New South Wales’. Technical Bulletin 31, Department of Agriculture NSW, Sydney.
- Turner DW, Hunt N. (1987) Planting date and defoliation influence the time of harvest of bananas. Scientia Horticulturae, 32, 233-248. [CrossRef]
- Twyford IT. (1967) Banana nutrition: a review of principles and practice. Journal of the Science of Food and Agriculture, 18, 177-183. [CrossRef]
- Walmsley D, Twyford IT 1968, The translocation of phosphorus within a stool of ‘Robusta’ bananas. Tropical Agriculture (Trinidad) 45, 229-233.
- White PR. (1928) Studies on the banana. An investigation of the floral morphology and cytology of certain types of the genus Musa L. Zeitschrift. Fuer. Zellforschung und Mikroskopische Anatomie Bd., 7, 673-733. [CrossRef]
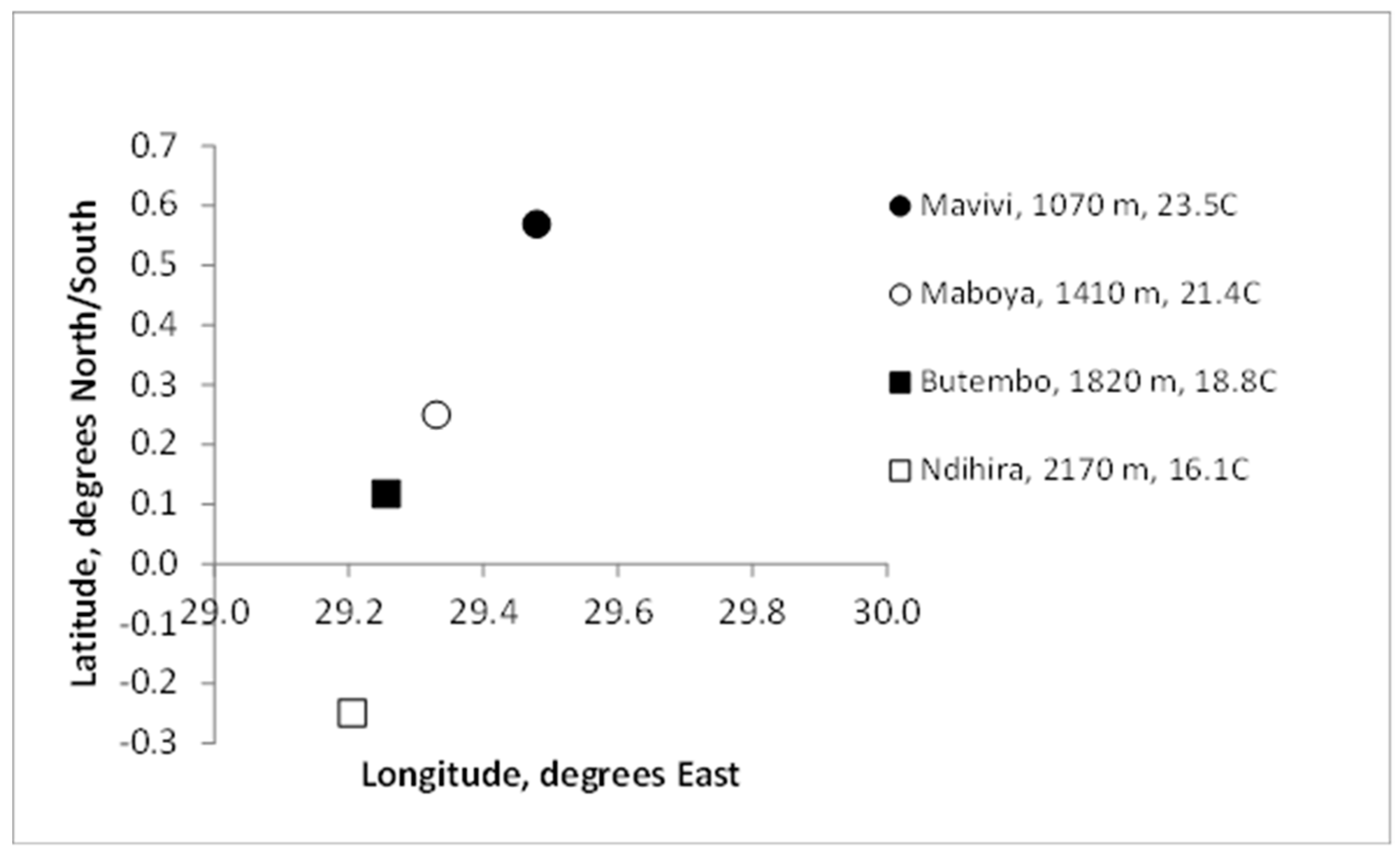
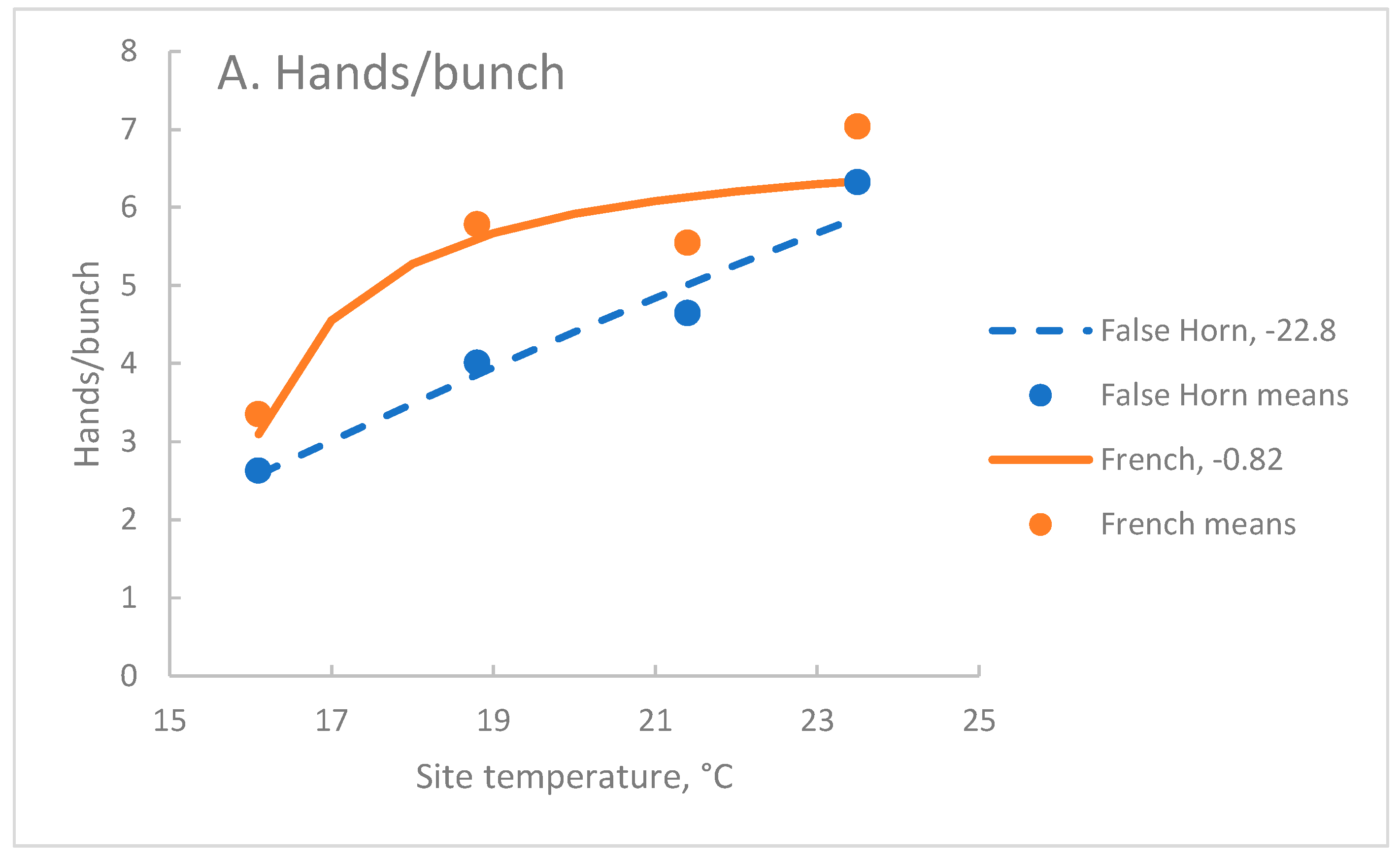
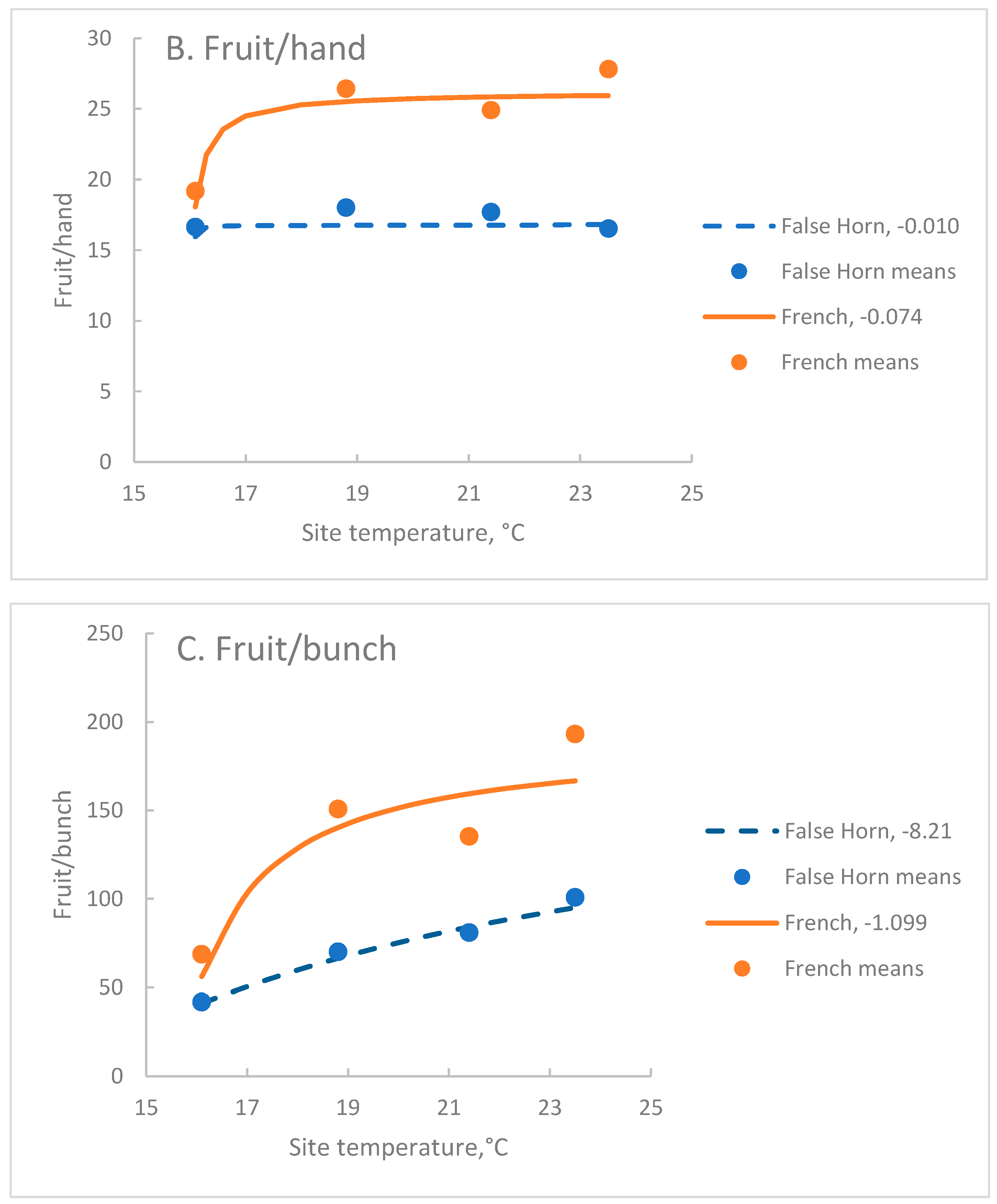
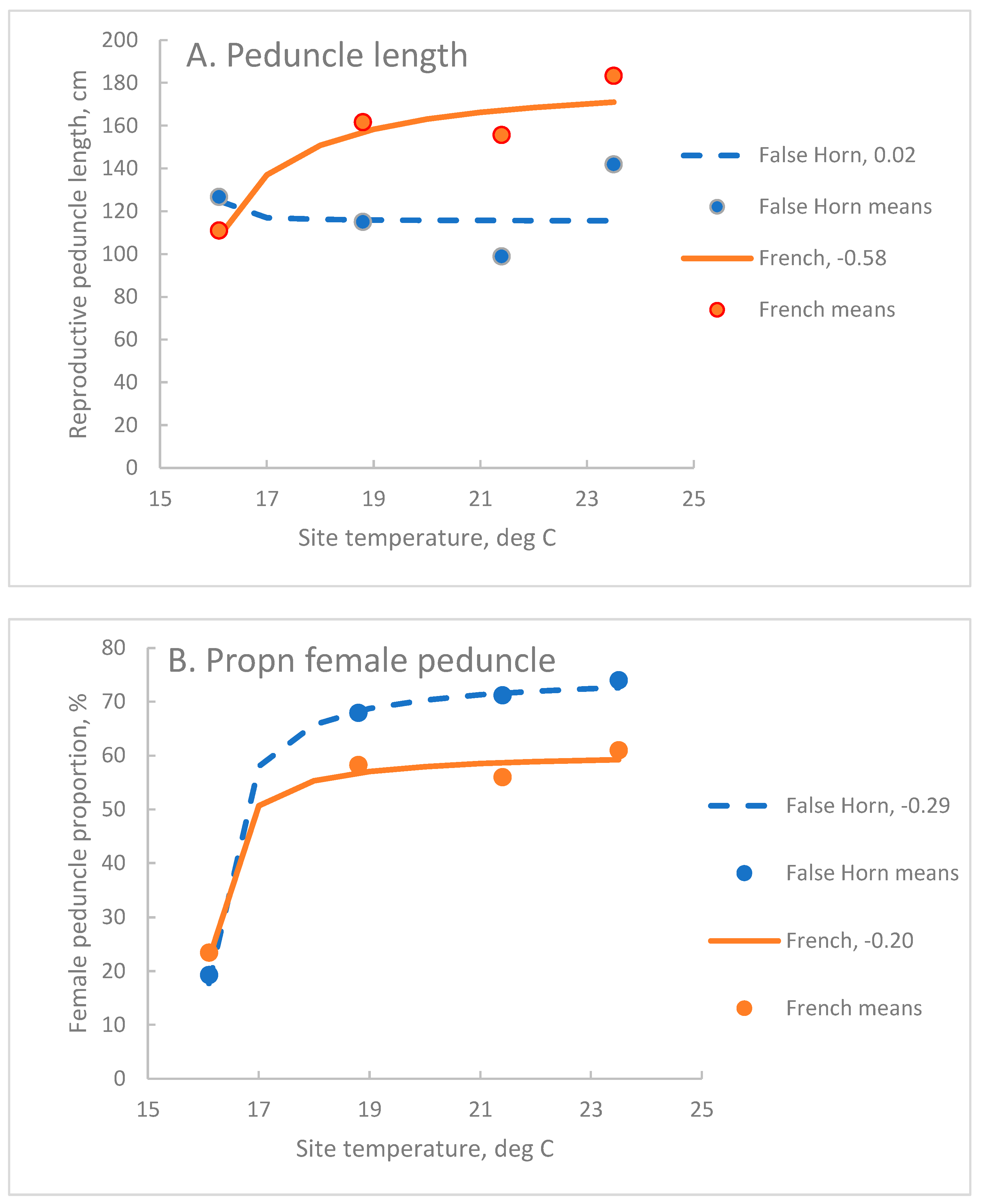
| Cultivar | Ndhi ratio | ||
|---|---|---|---|
| Fh | Hb | Fb | |
| ‘Kotina’ | 0.97 | 0.60 | 0.58 |
| ‘Vuhembe’ | 0.87 | 0.71 | 0.62 |
| ‘Musilongo’ | 0.49 | 0.35 | 0.17 |
| ‘Nguma’ | 0.85 | 0.70 | 0.60 |
| ‘Vuhindi’ | 0.81 | 0.66 | 0.53 |
| A | Parameters of the female peduncle | ||||
|---|---|---|---|---|---|
| Parameter | Hands/bunch, Hb | ||||
| Clone set | False Horn | French | |||
| Site/Cultivar | ‘Kotina’ | ‘Vuhembe’ | ‘Musilongo’ | ‘Nguma’ | ‘Vuhindi’ |
| Mavivi, 1066 m | 5.29 | 4.67 | 5.57 | 5.42 | 5.63 |
| Maboya, 1412 m | 3.69 | 3.73 | 3.91 | 4.73 | 4.67 |
| Butembo, 1815 m | 3.60 | 3.49 | 4.73 | 5.86 | 4.73 |
| Ndihira, 2172 m | 2.12 | 2.04 | 2.36 | 2.07 | 1.94 |
| LSD, P=0.05 | 0.23 | ||||
| Parameter | Fruit per hand, Fh | ||||
| Mavivi | 11.8 | 14.4 | 20.2 | 24.1 | 21.8 |
| Maboya | 14.6 | 13.9 | 21.9 | 20.9 | 17.2 |
| Butembo | 17.4 | 14.6 | 21.6 | 24.2 | 24.4 |
| Ndihira | 2.6 | 3.3 | 2.4 | 2.9 | 3.5 |
| LSD, P=0.05 | 0.2 | ||||
| Parameter | Fruit per bunch, Fb | ||||
| Mavivi | 58.8 | 65.7 | 110.8 | 127.4 | 120.2 |
| Maboya | 52.9 | 50.4 | 83.1 | 97.1 | 79.0 |
| Butembo | 59.8 | 49.5 | 100.3 | 139.2 | 113.3 |
| Ndihira | 5.0 | 6.0 | 5.0 | 5.5 | 5.9 |
| LSD, P=0.05 | 1.3 | ||||
| B | Dimensions of the reproductive peduncle | ||||
| Parameter | Length of reproductive peduncle, Pr, cm | ||||
| Cultivar | ‘Kotina’ | ‘Vuhembe’ | ‘Musilongo’ | ‘Nguma’ | ‘Vuhindi’ |
| Mavivi | 128 | 95 | 143 | 148 | 142 |
| Maboya | 78 | 80 | 121 | 141 | 111 |
| Butembo | 109 | 95 | 137 | 159 | 132 |
| Ndihira | 107 | 130 | 105 | 100 | 107 |
| LSD, P=0.05 | 3 | ||||
| Parameter | Female proportion of reproductive peduncle, Pf, % | ||||
| Mavivi | 74 | 74 | 57 | 66 | 60 |
| Maboya | 73 | 69 | 52 | 61 | 56 |
| Butembo | 70 | 66 | 58 | 59 | 57 |
| Ndihira | 22 | 17 | 26 | 26 | 18 |
| LSD, P=0.05 | 1 | ||||
| Site | Mavivi | Maboya | Butembo | Ndihira | LSD |
|---|---|---|---|---|---|
| Site Temperature, °C | 23.5 | 21.4 | 18.8 | 16.1 | P=0.05 |
| Data/parameter | Hands/bunch, Hb | ||||
| Measured | 5.31 | 4.15 | 4.48 | 2.11 | 0.10 |
| SQRT(PK) | 6.75 | 5.19 | 5.07 | 2.25 | 0.12 |
| SQRT(PK), Ndhi calc | 6.75 | 5.19 | 5.07 | 2.86 | 0.12 |
|
Parameter |
Fruit/hand, Fh Fruit/hand, Fh |
||||
| Measured | 18.3 | 17.6 | 18.9 | 2.8 | 0.6 |
| SQRT(PK) | 23.3 | 22.0 | 23.1 | 3.0 | 0.7 |
| SQRT(PK), Ndhi calc | 23.3 | 22.0 | 23.1 | 17.6 | 0.8 |
|
Parameter |
Fruit/bunch, Fb Fruit/bunch, Fb |
||||
| Measured | 97 | 73 | 92 | 6 | 2 |
| PK | 156 | 113 | 118 | 6 | 3 |
| PK, Ndhi calc | 156 | 113 | 118 | 52 | 3 |
| Parameter | Total peduncle length, Pr, cm | ||||
| Measured French | 144 | 125 | 143 | 104 | 4 |
| Measured False Horn | 112 | 79 | 102 | 118 | 4 |
| SQRT(PK) French | 183 | 156 | 162 | 111 | 3 |
| SQRT(PK) False Horn | 142 | 99 | 115 | 127 | 3 |
| Clone set | A | Tb, °C | Curvature coeff, C | r | P |
|---|---|---|---|---|---|
| Hands per bunch, Hb | |||||
| French | 6.98 A | 15.1 | -0.81 A | 0.75 | *** |
| False Horn | 20.1 B | 5.0 | -22.78 B | 0.87 | *** |
| Fruit per hand, Fh | |||||
| French | 26.2 A | 15.9 | -0.074 B | 0.51 | *** |
| False Horn | 16.8 B | 15.9 | -0.010 C | 0.08 | 0.097 |
| Fruit per bunch, Fb | |||||
| French | 190 A | 15.2 | -1.09 B | 0.74 | *** |
| False Horn | 179 B | 10.5 | -8.21 C | 0.83 | *** |
| Length of reproductive peduncle, Pr, cm | |||||
| French | 183 B | 15.0 | -0.581 B | 0.73 | *** |
| False Horn | 115 C | 15.9 | -0.016 C | 0.16 | 0.001 |
| Proportion of peduncle with female flowers, Pf | |||||
| French | 60.8 A | 15.9 | -0.201 B | 0.92 | *** |
| False Horn | 75.5 B | 15.9 | -0.290 C | 0.94 | *** |
| Genome (n) | Increase in fruit/hand, Fh, % | Increase in hands/bunch, Hb, % |
|---|---|---|
| AAA (17) | 60 | 15 |
| AAB + ABB (7) | 23 | 16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





