Submitted:
15 April 2024
Posted:
16 April 2024
You are already at the latest version
Abstract
Keywords:
Significance statement
1. Introduction
2. Material and Methods
2.1. Search Strategy
2.2. In Silico Pass Prediction
- 24Z-isomasticadienolic acid (PubChem CID: 15559978),
- cardanol (PubChem CID: 11266523),
- catechin-3-o-rhamnoside (PubChem CID: 21626704),
- estragole (PubChem CID 8815:), myrcene (PubChem CID: 31253),
- oleanolic acid (PubChem CID: 10494),
- Oleanolic aldehyde (PubChem CID: 10321055),
- trans-Anethole (PubChem CID: 637563),
- β-Caryophyllene (PubChem CID: 5281515) and
- β-sitosterol-3-O-glucoside (PubChem CID: 12309057).
2.3. Pharmacokinetics and Toxicity Measurement
3. Results
3.1. The Anacardiaceae Family
3.2. Anacardiaceae Species with Anti-Candida Activity
3.3. Isolated Compounds with Anti-CANDIDA activity
3.3.1. In Silico Prediction of Anti-Candida Activity of Isolated Compounds
3.3.2. Toxicity and Oral Bioavailability of Isolated Compounds
4. Discussion
5. Conclusions
Declaration of Conflict of Interest
Abbreviations
References
- Bitar, I.; Khalaf, R.A.; Harastani, C.; Tokajian, S. Identification, typing, antifungal resistance profile, and biofilm formation of Candida albicans isolates from Lebanese hospital patients. Biomed Res Int 2014, 1, 1–10. [Google Scholar] [CrossRef]
- Kainz, K.; Bauer, M.A.; Madeo, F.; Carmona-Gutierrez, D. Fungal infections in humans: the silent crisis. Microb Cell 2020, 7, 143–145. [Google Scholar] [CrossRef]
- Pal, M. Morbidity and mortality due to fungal infections. J App Micro Biol 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Tsay, S.V.; Mu, C.; Williams, S.; Epson, E.; Nadle, J.; Bamberg, C.M.; Barter, D.M.; Johnston, C.L.; Farley, M.M.; Harb, S. Burden of candidemia in the United States, 2017. Clin Infect Dis 2020, 71, e449–e453. [Google Scholar] [CrossRef]
- Rai, L.S.; Wijlick, L.V.; Bougnoux, M.E.; Bachellier-Bassi, S.; d'Enfert, C. Regulators of commensal and pathogenic lifestyles of an opportunistic fungus-Candida albicans. Yeast 2021, 38, 243–250. [Google Scholar] [CrossRef]
- Pappas, P.G.; Lionakis, M.S.; Arendrup, M.C.; Ostrosky-Zeichner, L.; Kullberg, B.J. Invasive candidiasis. Nat Rev Dis 2018, 4, 1–20. [Google Scholar] [CrossRef]
- Banu, S.F.; Rubini, D.; Shanmugavelan, P.; Murugan, R.; Gowrishankar, S.; Pandian, S.K.; Nithyanand, P. Effects of patchouli and cinnamon essential oils on biofilm and hyphae formation by Candida species. J Mycol Med 2018, 28, 332–339. [Google Scholar] [CrossRef]
- Talapko, J.; Juzbašić, M.; Matijević, T.; Pustijanac, E.; Bekić, S.; Kotris, I.; Škrlec, I. italic>Candida albicans-The virulence factors and clinical manifestations of infection. J Fungi (Basel) 2021, 7, 79. [Google Scholar] [CrossRef]
- Lopes, J.P.; Lionakis, M.S. Pathogenesis, and virulence of Candida albicans. Virulence 2022, 13, 89–121. [Google Scholar] [CrossRef]
- Cui, X.; Wang, L.; Lü, Y.; Yue, C. Development and research progress of anti-drug resistant fungal drugs. J Infect Public Health 2022, 15, 986–1000. [Google Scholar] [CrossRef]
- Hamill, R.J. Amphotericin B formulations: a comparative review of efficacy and toxicity. Drugs 2013, 73, 919–934. [Google Scholar] [CrossRef]
- Colombo, A.L.; Júnior, J.N.; Guinea, J. Emerging multidrug-resistant Candida species. Curr Opin Infect Dis 2017, 30, 528–538. [Google Scholar] [CrossRef]
- Gonzalez-Lara, M.F.; Ostrosky-Zeichner, L. Invasive candidiasis. Semin Respir Crit Care Med 2020, 41, 3–12. [Google Scholar] [CrossRef]
- Pedroso, R.S.; Balbino, B.L.; Andrade, G.; Dias, M.C.P.S.; Alvarenga, T.A.; Pedroso, R.C.N.; Pimenta, L.P.; Lucarini, R.; Pauletti, P.M.; Januário, A. In vitro and in vivo anti-Candida spp. activity of plant-derived products. Plants, 2019; 8, 494–521. [Google Scholar]
- Zou, X.; Zeng, M.; Huang, F.; Qin, G.; Song, Z.; Liu, F. The potential role of plant secondary metabolites on antifungal and immunomodulatory effect. Appl Miccrobio Biotechnol, 2023; 4471–4492. [Google Scholar] [CrossRef]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: a free web tool to evaluate pharmacokinetics, drug-likeness, and medicinal chemistry friendliness of small molecules. Sci Rep 2017, 7, 1–13. [Google Scholar] [CrossRef]
- Pinzi, L.; Rastelli, G. Molecular docking: shifting paradigms in drug discovery. Int J Mol Sci 2019, 20, 4331. [Google Scholar] [CrossRef]
- Mojumdar, M.; Kabir, M.S.C.; Hasan, M.S.; Ahmed, T.; Rahman, M.R.; Akter, C.; Rahman, M.M. Molecular docking and pass prediction for the analgesic activity of some isolated compounds from Acalypha idica L. and ADME/T property analysis of the compounds. World J Pharm Res 2016, 5, 1761–1770. [Google Scholar]
- Goel, R.K.; Singh, D.; Lagunin, A.; Poroikov, V. PASS-assisted exploration of new therapeutic potential of natural products. Med Chem Res 2011, 20, 1509–1514. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.C.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Pell, S.K.; Mitchell, J.D.; Miller, A.J.; Lobova, T.A. Anacardiaceae. In: KubtzkiI, K., ed. The families and genera of vascular plants. Flower. Plants, Eudicots-Sapindales, Cucurbitales, Myrtaceae 2011, 10, 7–50.
- Hall, C.F.; Bragança, A.S. Flora das cangas da Serra dos Carajás, Pará, Brasil: Anacardiaceae. Rodriguesia, 2017; 68, 911–916. [Google Scholar] [CrossRef]
- Masevhe, N.A.; McGaw, L.J.; Eloff, J.N. The traditional use of plants to manage candidiasis and related infections in Venda, South Africa. J Ethnopharmacol 2015, 168, 364–372. [Google Scholar] [CrossRef]
- Novaryatiin, S.; Indah, I. The medicinal plants used in Anjir Pulang Pisau, Central Kalimantan-Indonesia. Pharmacogn J 2019, 11, 12–32. [Google Scholar] [CrossRef]
- Baldé, M.A.; Tuenter, E.; Traoré, M.S.; Matheeussen, A.; Cos, P.; Maes, L.; Camara, A.; Haba, N.L.; Gomou, K.; Diallo, M.S.T. Antimicrobial investigation of ethnobotanically selected guinean plant species. J Ethnopharmacol 2020, 263, 113–232. [Google Scholar] [CrossRef]
- Arlandini, E.; Gelmini, F.; Testa, C.; Angioletti, S.; Beretta, G. GC-MS analysis and biological activity of hydroalcoholic extracts and essential oils of Rhus typhina L. wood (Anacardiaceae) in comparison with leaves and fruits. Nat Prod Res 2021, 35, 4764–4768. [Google Scholar] [CrossRef]
- Silva, R.A.; Liberio, S.A.; Amaral, F.M.M.; Nascimento, F.R.F.; Torres, L.M.B.; Monteiro-Neto, V.; Guerra, R.N.M. Antimicrobial and antioxidant activity of Anacardium occidentale L. flowers in comparison to bark and leaves extracts. J Biosci Med 2016, 4, 87– 99.
- Costa, A.R.; Almeida-Bezerra, J.C.; Silva, T.G.; Pereira, P.S.; Oliveira Borba, E.F.; Braga, A.L.; Fonseca, V.J.A.; Menezes, S.A.; Silva, F.S.C.; Sousa Fernandes, P.A. Phytochemical profile and anti-Candida and cytotoxic potential of Anacardium occidentale L. (cashew tree). Biocatalisis Agric Biotechnol 2021, 37, 102–192. [Google Scholar] [CrossRef]
- Mahata, D.; Mandal, S.M.; Bharti, R.; Gupta, V.K.; Mandal, M.; Nag, A.; Nando, G.B. Self-assembled cardanol azo derivatives as antifungal agent with chitin-binding ability. Int J Biol Macromol 2014, 69, 5–11. [Google Scholar] [CrossRef]
- Ulukanli, Z.; Karabörklü, S.; Bozok, F.; Çenet, M.; Oztürk, B.; Balcilar, M. Antimicrobial, insecticidal and phytotoxic activities of Cotinus coggyria Scop. essential oil (Anacardiaceae). Nat Prod Res. 2014, 28, 2150–2157. [Google Scholar] [CrossRef]
- Sukhikh, S.; Noskova, S.; Pungin, A.; Ivanova, S.; Skrypnik, L.; Chupakhin, E.; Babich, O. Study of the Biologically Active Properties of Medicinal Plant Cotinus coggygria. Plants (Basel) 2021, 10, 1224. [Google Scholar] [CrossRef]
- Njinga, N.S.; Sule, M.I.; Pateh, U.U.; Hassan, C.S.; Abdullahi, S.T.; Ache, R.N. Isolation and antimicrobial activity of β-sitosterol-3-O-glucoside from Lannea kerstingii Engl. & K. Krause (Anacardiacea). J Heal Allied Sci 2016, 6, 4–8. [Google Scholar]
- Stanislaus, N.N.; Ibrahim, S.M.; Usman, P.U.; Sa'adiya, C.C.; Garba, M.M.; Toyin, A.S.; Moji, B.-O.T.; Ndifor, A.R.; Osas, E.G.; Oyetunji, S.A. Antimicrobial and antioxidant activity of catechin-3-o-rhamnoside isolated from the stem bark of Lannea kerstingii Engl. and K. Krause (Anacardiaceae). Pakistan J Pharm Sci 2021, 34, 1–10. [Google Scholar]
- Dorta, E.; González, M.; Lobo, M.G.; Laich, F. Antifungal activity of mango peel and seed extracts against clinically pathogenic and food spoilage yeasts. Nat Prod Res 2016, 30, 2598–2604. [Google Scholar] [CrossRef]
- Benabdallah, F.Z.; Kouamé, R.O.; El Bentchikou, M.; Zellagui, A.; Gherraf, N. Études ethnobotanique, phytochimique et valorisation de l'activité antimicrobienne des feuilles et de l'oléorésine du pistachier de l'atlas (Pistacia atlantica Desf.). Phytothérapie 2017, 15, 222–229. [Google Scholar] [CrossRef]
- Othman, S.; El-Hashash, M.; Hussein, S.; El-Mesallamy, A.; Rizk, S.; Elabbar, F.A. Phenolic content as antioxidant and antimicrobial activities of Pistacia atlantica Desf. (Anacardiaceae) extract from Libya. Egypt. J Chem 2019, 62, 21–28. [Google Scholar] [CrossRef]
- Hasheminya, S.-M.; Dehghannya, J. Composition, phenolic content, antioxidant, and antimicrobial activity of Pistacia atlantica subsp. Kurdica Hulls' essential oil. Food Biosci 2020, 34, 100–510. [Google Scholar] [CrossRef]
- Karygianni, L.; Cecere, M.; Argyropoulou, A.; Hellwig, E.; Skaltsounis, A.L.; Wittmer, A.; Tchorz, J.P.; Al-Ahmad, A. Compounds from Olea europaea and Pistacia lentiscus inhibit oral microbial growth. BMC Complement Altern Med 2019, 19, 1–10. [Google Scholar] [CrossRef]
- Brahmi F, Haddad S, Bouamara K, Yalaoui-Guellal D, Prost-Camus E, Barros J-PP, Prost M, Atanasov AG, Madani K, Boulekbache-Makhlouf L (2020) Comparison of chemical composition and biological activities of Algerian seed oils of Pistacia lentiscus L., Opuntia ficus indica (L.) Mill. and Argania spinosa L. Skeels. Ind Crops Prod. 151, 112-456.
- Milia E, Usai M, Szotáková B, Elstnerová M, Králová V, D'hallewin G, Spissu C, Barberis A, Marchetti M, Bortone A, Campanella V, Mastandrea G, Langhansová L, Eick S (2020) The pharmaceutical ability of Pistacia lentiscus L. leaves essential oil against periodontal bacteria and Candida sp. and its anti-inflammatory potential. Antibiotics 9, 1-10.
- Piras A, Marzouki C, Falconieri D, Porcedda S, Gonçalves MJ, Cavaleiro C, Salgueiro L (2017) Chemical composition and biological activity of volatile extracts from leaves and fruits of Schinus terebinthifolius Raddi from Tunisia. Rec Nat Prod 11.
- D'Arrigo M, Bisignano C, Irrera P, Smeriglio A, Zagami R, Trombetta D, Romeo O, Mandalari G (2019). In vitro evaluation of the activity of essential oil from Pistacia vera L. variety Bronte hull against Candida sp. BMC Complement Altern Med. 2019, 6. [CrossRef]
- Gharibi, S.; Matkowski, A.; Sarfaraz, D.; Mirhendi, H.; Fakhim, H.; Szumny, A.; Rahimmalek, M. Identification of polyphenolic compounds responsible for antioxidant, anti-Candida activities and nutritional properties in different pistachio (Pistacia vera L.) hull cultivars. Molecules 2020, 28, 4772. [Google Scholar] [CrossRef]
- Yilmaz, G.; Ekşi, G.; Demirci, B.; Demirci, F. Characterization of the fatty acid compositions and antimicrobial activity of sumac (Rhus coriaria L.) fruits, growing naturally in Turkey and sold in herbalist markets. J Fac Pharm Ankara 2020, 44, 61–69. [Google Scholar]
- Vandal, J.; Abou-Zaid, M.M.; Ferroni, G.; Leduc, L. Antimicrobial activity of natural products from the flora of Northern Ontario, Canada. Pharm Biol 2015, 53, 800–806. [Google Scholar] [CrossRef]
- Donati, M.; Mondin, A.; Chen, Z.; Miranda, F.M.; Nascimento, B.B., Jr.; Schirato, G.; Pastore, P.; Froldi, G. Radical scavenging and antimicrobial activities of Croton zehntneri, Pterodon emarginatus, and Schinopsis brasiliensis essential oils and their major constituents: estragole, trans-anethole, β-caryophyllene, and myrcene. Nat Prod Res 2015, 29, 939–946. [Google Scholar] [CrossRef]
- Gehrke IT, Neto AT, Pedroso M, Mostardeiro CP, Da Cruz IB, Silva UF, Ilha V, Dalcol II, Morel AF (2013). Antimicrobial activity of Schinus lentiscifolius (Anacardiaceae). J Ethnopharmacol 2013, 148, 486–491. [CrossRef]
- Turchetti, G.; Garzoli, S.; Laghezza Masci, V.; Sabia, C.; Iseppi, R.; Giacomello, P.; Tiezzi, A.; Ovidi, E. Antimicrobial Testing of Schinus molle (L.) Leaf Extracts and Fractions Followed by GC-MS Investigation of Biological Active Fractions. Molecules 2020, 25, 1977. [Google Scholar] [CrossRef]
- El-Nashar HAS, Mostafa NM, El-Badry MA, Eldahshan OA, Singab ANB. Chemical composition, antimicrobial and cytotoxic activities of essential oils from Schinus polygamus (Cav.) cabrera leaf and bark grown in Egypt. Nat Prod Res 2021, 35, 5369–5372. [CrossRef]
- Hernandes, C.; Taleb-Contini, S.C.; Bartolomeu, A.C.D.; Bertoni, B.C.; Franҫa, S.C.; Pereira, A.M.S. Chemical composition and antifungal activity of the essential oils of Schinus weinmannifolius collected in the spring and winter. Nat Prod Commun 2014, 9, 1383–1386. [Google Scholar] [CrossRef]
- Freitas MA, Cruz RP, Santos ATL, Almeida-Bezerra JC, Machado AJT, Santos JFS, Rocha JE, Boligon AA, Bezerra CF, Freitas TS. HPLC–DAD analysis and antimicrobial activities of Spondias mombin (Anacardiaceae). Biotechnology 2022, 12, 1–15.
- Costa-Cordeiro, B.M.P.; Lima Santos, N.D.; Ferreira, M.R.A.; Araújo, L.C.C.; Junior, A.R.C. Conceição Santos AD, Oliveira AP, Silva AG, Silva Falcão EP, Santos Correia MT. Silva Almeida JRG, Silva LCN, Soares LAL, Napoleão TC, Silva MV, Paiva PMG. Hexane extract from Spondias tuberosa (Anacardiaceae) leaves has antioxidant activity and is an anti-Candida agent by causing mitochondrial and lysosomal damage. BMC Complement Altern Med 2018, 18, 1–10.
- Santos A, Carneiro JNP, Cruz RP, Sales DL, Andrade JC, Almeida CO, Costa JGM, Ribeiro RV, Brito ES, Batista FLA). UPLC-MS-ESI-QTOF analysis and antifungal activity of the Spondias tuberosa - arruda leaf and root hydroalcoholic extracts. Antibiotics 2019, 8, 240.
- WFO – World Flora online, https://www.worldfloraonline.org/ captured in December 2023.
- Hashemi, S.E.; Shokohi, T.; Abastabar, M.; Aslani, N.; Ghadamzadeh, M.; Haghani, I. Species distribution and susceptibility profiles of Candida species isolated from vulvovaginal candidiasis, emergence of C. lusitaniae. Curr Med Mycol 2019, 5, 26–36. [Google Scholar] [CrossRef]
- Gangneux, J.P.; Dannaoui, E.; Fekkar, A.; Luyt, C.E.; Botterel, F.; De Prost, N.; Bougnoux, M.E. Fungal infections in mechanically ventilated patients with COVID-19 during the first wave: the French Multicenter MYCOVID study. Lancet Respirat Med 2022, 10, 180–190. [Google Scholar] [CrossRef]
- Costa-de-Oliveira, S.; Rodrigues, A.G. Candida albicans Antifungal resistance and tolerance in bloodstream infections: the triad yeast-host-antifungal. Microrganisms 2020, 8, 154. [Google Scholar] [CrossRef]
- Teodoro, G.R.; Ellepola, K.; Seneviratne, C.J.; Koga-Ito, C.Y. Potential use of phenolic acids as anti-Candida agents: A Review. Front Microbiol 2015, 6, 1420. [Google Scholar] [CrossRef]
- Ahmed, Z.B.; Yousfi, M.; Viaene, J.; Dejaegher, B.; Demeyer, K.; Vander Heyden, C. Four Pistacia atlantica subspecies (atlantica, cabulica, kurdica, and mutica): A review of their botany, ethnobotany, phytochemistry, and pharmacology. J Ethnopharmacol 2021, 265, 113–329. [Google Scholar]
- Iranshahy M, Javadi B, Sahebkar A. Protective effects of functional foods against Parkinson's disease: A narrative review on pharmacology, phytochemistry, and molecular mechanisms. Phytother Res 2022, 36, 1952– 1989. [CrossRef]
- CLSI - Clinical and Laboratory Standards Institute. Method for broth dilution antifungal susceptibility testing of yeasts: approved M27-A3. CLSI 2012, Wayne, PA, USA.
- Abubakar, A.R.; Haque, M. Preparation of medicinal plants: Basic extraction and fractionation procedures for experimental purposes. J Pharm Bioallied Sci 2020, 12, 1–10. [Google Scholar] [CrossRef]
- Donadu, M.G.; Peralta-Ruiz, C.; Usai, D.; Maggio, F.; Molina-Hernandez, J.B.; Rizzo, D.; Bussu, F.; Rubino, S.; Zanetti, S.; Paparella, A.; Chaves-Lopez, C. Colombian essential oil of Ruta graveolens against nosocomial antifungal resistant Candida strains. J Fungi 2021, 7, 1–17. [Google Scholar] [CrossRef]
- Giordani B, Basnet P, Mishchenko E, Luppi B, Škalko-Basnet N (2019) Utilizing liposomal quercetin and gallic acid in localized treatment of vaginal Candida infections. Pharmaceutics 2019, 12, 9–15.
- Rhimi, C.; Aneke, C.I.; Annoscia, G.; Otranto, D.; Boekhout, T.; Cafarchia, C. Effect of chlorogenic and gallic acids combined with azoles on antifungal susceptibility and virulence of multidrug-resistant Candida spp. and Malassezia furfur isolates. Med Mycol 2020, 58, 1091–1101. [Google Scholar] [CrossRef]
- Maiyoa, F.; Moodley, R.; Singh, M. Phytochemistry, cytotoxicity and apoptosis studies of β-sitosterol-3-glucoside and β-amyrin from Prunus africana. African J Tradit Complement Altern Med 2016, 13, 105–112. [Google Scholar] [CrossRef]
- Kim, J.E.; Kim, S.S.; Hyun, C.-G.; Lee, N.C. Antioxidative chemical constituents from the stems of Cleyera japonica Thunberg. Int J Pharmacol 2012, 8, 410–415. [Google Scholar] [CrossRef]
- El-Alfy, T.S.; Ezzat, S.M.; Hegazy, A.K.; Amer, A.M.; Kamel, G.M. Isolation of biologically active constituents from Moringa peregrina (Forssk.) family: Moringaceae growing in Egypt. Pharmacogn Mag 2011, 7, 109–115. [Google Scholar]
- Shin, S.; Pyun, M. Anti-Candida effects of estragole in combination with ketoconazole or amphotericin B. Phyther Res 2004, 18, 827–830. [Google Scholar] [CrossRef]
- Dąbrowska M, Zielińska-Bliźniewska C, Kwiatkowski P, Łopusiewicz Ł, Pruss A, Kostek M, Kochan E, Sienkiewicz M. Inhibitory effect of eugenol and trans-anethole alone and in combination with antifungal medicines on Candida albicans clinical isolates. Chem Biodivers 2021, 18, 200–223.
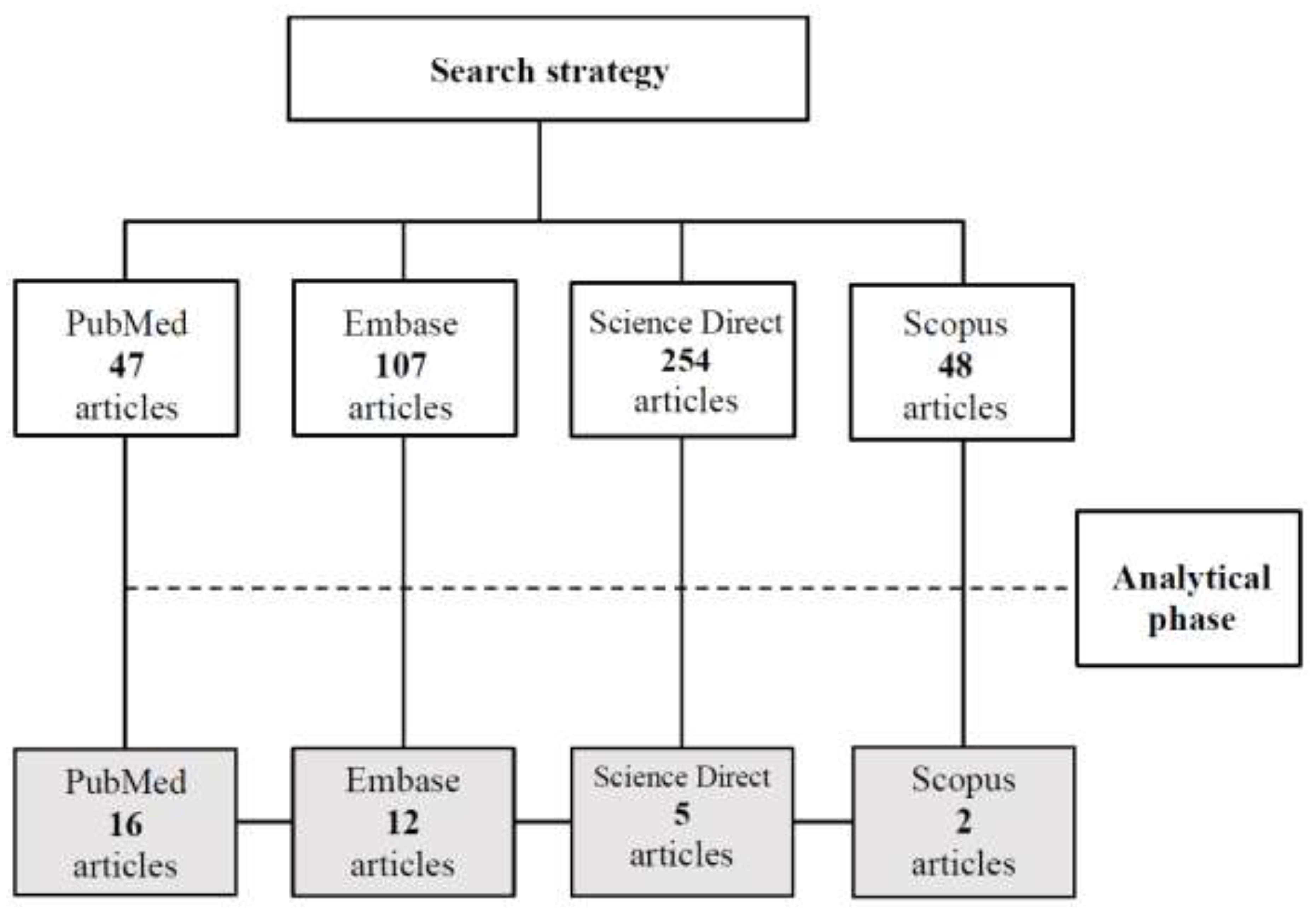
| Plant Species | Type of extract or fraction (Plant part) | Compounds identified and/or isolated | Candida species tested | Type of assay (methods)* |
Reference |
|---|---|---|---|---|---|
| Anacardium occidentale L. | Ethanolic (Flowers, leaves, stem bark) |
Phosphoric acid, dodecanoic acid, ethylgallic acid, sorbitol, glucose, gallic acid, hexadecanoic acid, octadecanoic acid and 1, 2-benzenedicarboxylic acid |
C. albicans C. tropicalis |
In vitro (Halo diffusion, MIC, MFC) |
[27] |
| Ethanolic (bark) |
Gallic acid, luteolin, epicatechin gallate and flavone |
C. albicans, C. krusei C. tropicalis |
In vitro (MIC) |
[28] | |
| (NI)* Cashew nutshell |
Cardanol (Isolated compound) |
C. albicans |
In vitro (MIC) |
[29] | |
| Cotinus coggyria Scop | Essential oil (leaves) |
α-pinene β-pinene limonene α-terpinolene β-terpinene β-myrcene β-caryophyllene |
C. albicans C. parapsilosis |
In vitro (Halo diffusion) |
[30] |
| Ethyl alcohol (Leaves and flowers) |
Rutin ferulic acid quercetin gallic acid kaempferol sulphurein, 3, 3’4’, 5, 6, 7 -hexahydroxyflavone, 7-O-β-D glucopyranoside |
C. albicans |
In vitro (Halo diffusion) |
[31] | |
| Lannea kerstingii Engl. and K. Krause. | Ethyl acetate (Stem bark) |
β-sitosterol-3-O-glucoside (Isolated compound) |
C. albicans , C. tropicalis C. krusei |
In vitro (Halo diffusion, MIC, MFC) |
[32] |
| Ethyl acetate (Stem bark) |
catechin-3-o-rhamnoside (Isolated compound) |
C. albicans C. tropicalis |
In vitro (MIC, MFC) |
[33] | |
|
Mangifera indica L. |
NI (peel and seed) |
Proanthocyanidins gallates gallotannins |
C. parapsilosis C. glabrata |
In vitro (Halo diffusion MIC) |
[34] |
| Pistacia atlantica Desf. | Essential oil (Leaves, fruits) |
α-pyrene, terpinen-4-ol acid | C. albicans | In vitro (MIC) | [35] |
| Methanolic (leaves) |
gallic acid, ellagic acid, 3, 3’-dimethoxyellagic acid, gallotannins, 2, 3-di-O-galloyl-(α/β)-4 C1 - glucopyranose, nilocitin, 1, 3-di-O-galloyl-β-D-4, C1-glucopyranose and 1, 2, 3, 4, 6- penta-O-galloyl-β-D-4 |
C. albicans |
In vitro (Halo diffusion) |
[36] | |
|
Pistacia atlantica subsp. |
Essential oil (hulls) |
α-Pinene, β-citral, carvone hydrate, myristic acid, p-acetyltoluene, pinocarveol and palustrol |
C. albicans |
In vitro (Halo diffusion, MIC) |
[37] |
| Mastic gum |
24Z-isomasticadienolic acid, oleanolic acid, oleanonic aldehyde (Isolated compounds) |
C. albicans |
In vitro (MIC) |
[38] | |
| Oils (seeds) |
Linoleic acid, oleic acid, fatty acid, β-sitosterol, protocatechuic acid, p-coumaric, t-cinnamic |
C. albicans |
In vitro (halo diffusion) |
[39] | |
| Pistacia lentiscus L. | Essential oil (leaves) |
α-pinene, terpinen-4-ol and other 62 compounds |
C. albicans C. glabrata |
In vitro (MIC) |
[40] |
| Pistacia terebinthus L. | Essential oils (leaves) |
Monoterpene hydrocarbons, α-pinene camphene, β-pinene terpinolene, β-phellandrene |
C. albicans |
In vitro (MIC) |
[41] |
|
Pistacia vera L. |
Essential oil (hulls) |
α-Pinene α-terpineol, camphene D-limonene and 3-carene |
C. albicans, C. parapsilosis C. glabrata |
In vitro (MIC, MFC, growth curve) |
[42] |
| Cyanidin-3- O-galactoside, gallic acid, catechin, eriodictyol-7- O-glucoside |
C. albicans C. glabrata C. parapsilosis C. auris |
In vitro (MIC) |
[43] | ||
| Rhus coriaria L. | Essential oil (seeds) |
Linoleic acid, oleic acid palmitic acid |
C. albicans |
In vitro (Halo diffusion, MIC) |
[44] |
| Rhus typhina L | Hydroalcoholic extract, essential oil (Branches, leaves, and fruits) |
Gallic acid, 1-cyclohexane-3, 4, 5-hydroxy-carboxylic acid, malic acid, d-cadinene, β-pinene, phenylacetaldehyde |
C. albicans |
In vitro (Halo diffusion, MIC) |
[26] |
| Ethanolic (leaves and berries) |
Gallic acid, chlorogenic acid, gentisic acid, sinapic acid, caffeic acid, ethyl gallate |
C. albicans |
In vitro (MIC) |
[45] | |
|
Schinopsis brasiliensis Engl. |
Essential oil (leaves) |
Estragole trans-anethole, β-caryophyllene myrcene (Isolated compounds) |
C. parapsilosis |
In vitro (MIC) |
[46] |
|
Schinus lentiscifolius Marchand. |
Aqueous, n-hexane, ethyl acetate and n-butanol fractions (leaves) |
Nonadecanol moronic acid gallic acid methyl ester, gallic acid quercetin quercitrin |
C. albicans, C. tropicalis |
In vitro (MIC) |
[47] |
| Schinus molle L. | Petroleum ether, diethylether, acetone, aqueous (leaves) |
Sesquiterpenes, sesquiterpenoids and other terpenes | C. albicans |
In vitro (Halo diffusion; MIC) |
[48] |
|
Schinus polygamus Cav. |
Essential oil (Bark and leaves) |
dl -limonene myrtenal caryophyllene oxide (bark). E-caryophyllene dl-limonene β-pinene (leaves) |
C. albicans |
In vitro (MIC) |
[49] |
| Essential oil (Leaves and fruits) |
A-phellandrene, β-phellandrene, α-pinene, and germacrene D |
C. albicans, C. tropicalis, C. krusei, C. guillermondii C. parapsilosis |
In vitro (MIC) |
[41] | |
| Schinus weinmannifolius Engl | Essential oil (leaves) | Bicyclogermacrene, limonene | C. albicans |
In vitro (MIC) |
[50] |
|
Spondias mombin L. |
Aqueous (leaves) hydroethanolic (bark) |
Quercetin caffeic acid catechin kaempferol phenols flavonoids |
C. albicans C. tropicalis |
In vitro (MIC; MFC) |
[51] |
|
Spondias tuberosa Arruda. |
Hexane (leaves) |
Flavonoids, hydrolysable tannins, saponins, terpenes; gallic acid, saturated and unsaturated fatty acids |
C. albicans, C. parapsilosis, C. glabrata, C. krusei |
In vitro (MIC; MFC) |
[52] |
| Hydroalcoholic (Leaves and roots) |
Alkaloids, steroids, phenols, flavonoids, triterpenoids, xanthones; dehydroascorbicacid, quinic acid, and others |
C. albicans, C. tropicalis |
In vitro (MIC, morphological transition) |
[53] |
| Isolated compounds | Potential activity (Pa) | Potential inactivity (Pi) |
|---|---|---|
| 24Z-isomasticadienolic acid | 0.687 | 0, 010 |
| Cardanol | 0.543 | 0.024 |
| Catechin-3-o-rhamnoside | 0.740 | 0, 008 |
| Estragole | 0.425 | 0.045 |
| Myrcene | 0.584 | 0.020 |
| Oleanolic acid | 0.575 | 0.021 |
| Oleanolic aldehyde | 0.590 | 0.019 |
| Trans-Anethole | 0.444 | 0.040 |
| β-Caryophyllene | 0.582 | 0.020 |
| β-sitosterol-3-O-glucoside | 0.722 | 0.009 |
| Compounds | MWa(g/mol) | HBDb | HBAc | LogPd(o/w) | MRe | GAf |
|---|---|---|---|---|---|---|
| 24Z-isomasticadienolic acid | 454.6 | 1 | 3 | 4.09 | 137.8 | Low |
| Cardanol | 298.4 | 1 | 1 | 4.61 | 99.3 | Low |
| Catechin-3-o-rhamnoside | 436.5 | 7 | 10 | 1.58 | 105.5 | Low |
| Estragole | 148.2 | 0 | 1 | 2.47 | 47.0 | High |
| Myrcene | 136.2 | 0 | 0 | 2.89 | 48.7 | Low |
| Oleanolic acid | 456.7 | 2 | 3 | 3.89 | 136.6 | Low |
| Oleanolic aldehyde | 440.7 | 1 | 2 | 4.33 | 135.0 | Low |
| Trans-Anethole | 145.2 | 0 | 1 | 2.55 | 47.8 | High |
| β-Caryophyllene | 204.3 | 0 | 0 | 3.29 | 68.7 | Low |
| β-sitosterol-3-O-glucoside | 576.8 | 4 | 6 | 4.98 | 165.6 | Low |
| Compounds | MPa | TPb | IRc | REd | Oral toxicity(LD50e mg/Kg) |
|---|---|---|---|---|---|
| 24Z-isomasticadienolic acid | No | No | No | No | 1688 |
| Cardanol | No | No | No | No | 3737 |
| Catechin-3-o-rhamnoside | No | No | No | No | 2452 |
| Estragole | No | No | No | No | 1290 |
| Myrcene | No | Yes | Yes | Yes | 2561 |
| Oleanolic acid | No | No | No | Yes | 369.6 |
| Oleanolic aldehyde | No | No | No | No | 260.2 |
| Trans-Anethole | Yes | Yes | Yes | No | 3243 |
| β-Caryophyllene | No | No | No | No | 2331 |
| β-sitosterol-3-O-glucoside | No | No | No | No | 1279 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





