1. Introduction
Hodgkin’s lymphoma (HL) is a malignant proliferation of B-lymphocytes within lymph nodes and classically has a high cure rate. HL are commonly classified as cHL or uncommonly nodular lymphocyte predominant Hodgkin’s lymphoma (NLPHL) which behaves like indolent non-Hodgkin’s lymphoma (NHL). Initiation of therapy leads to a 80% cure rate, however, 9-11% of patients will be refractory to first line therapy and up to 30% will have relapse following remission [
1]. In 8-12% of cases, extra-nodal structures such as the liver and lung are involved [
2]. Central nervous system (CNS) involvement of cHL is unusual and represents relapse, an immunocompromised state with higher rates in males, or predisposing infection with Epstein-Barr virus (EBV) [
3]. The most common abnormality found on neuroimaging when suspecting intracranial neoplasm, is ring enhancement [
4]. These lesions are hypodense on non-contrast computed tomography (NCCT) scans or hypointense on brain magnetic resonance imaging (MRI). The most common pathologies found based on neuroimaging were gliomas (40%) followed by metastatic brain (30%) lesions [
5]. Clinically, 70% of patients have focal neurological deficits, 40% with nonspecific neurocognitive behavioral changes, and 30% with intracranial hypertension [
6]. Neuroimaging can presumptively diagnose the lesion; however, brain histopathology sampling is required to confirm and/or rule out other causes such as infectious, primary cancerous, and non-neoplastic mimics. Treatment of first relapse in cHL is salvage chemotherapy followed by autologous stem cell transplantation (ASCT) for young and fit patients. In the recent years, there is a paradigm shift to use novel antibodies such as brentuximab vedotin and programmed death – 1(PD-1) inhibitors [
7,
8,
9]. PD-1 ligand (PD-1L) receptors are highly expressed on Reed-Sternberg cells, a hallmark feature of cHL, and can be used as a target for immunotherapy. Additionally, brentuximab vedotin has been used in the first line, relapsed setting, and as a maintenance proving a substantial decrease in the risk of recurrence. We hereby discuss the management of two intracranial extra-axial metastatic cHL that have been treated with traditional chemotherapy and novel agents.
2. Materials and Methods
This is a single institution retrospective study conducted at the University of South Alabama Health System, data extraction from electronic health records was performed after obtaining IRB arrival. Here in, we are describing clinical outcomes and disease characteristics with intracranial manifestations of cHL in two cases who were treated with novel agents such as immune checkpoint inhibitors and antibody drug conjugates.
3. Case Presentation
Case 1
Thirty-one-year-old male with a history of untreated Mycobacterium nucogenicum isolated on bronchoscopy and bronchoalveolar lavage (BAL) culture 6 months prior, stage 4b mixed cellularity cHL. He was deemed asymptomatic from the mycobacterium infection, subsequent excisional lymph node biopsy confirmed the diagnosis, and the decision was made to start first line A-AVD regimen. The patient received 3 cycles with clinical improvement of his constitution symptoms. However, he subsequently presented to the hospital with 3-day onset of ataxia. He sustained a total of 3 falls with severe frontal headache that he rates as a 9/10 in severity. He denied loss of consciousness, problems with his vision, positional worsening, prior history of headaches, and neck pain. He reported worsening gait and generalized weakness over the last few days. Vital signs on admission were significant: 38.2
C, 101 beats/min, and BP 113/52 mmHg. He developed intermittent fevers as high as 39.4
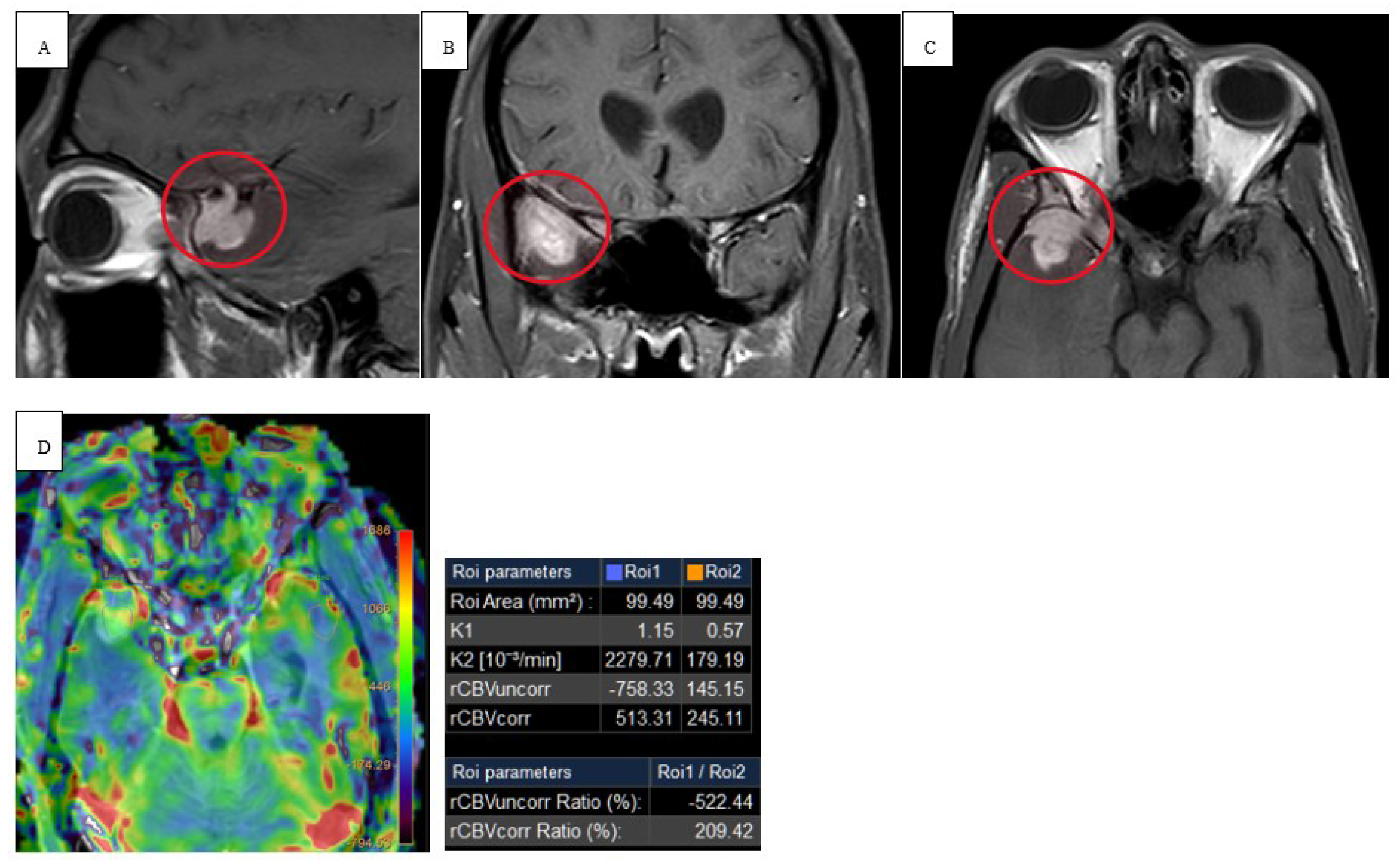
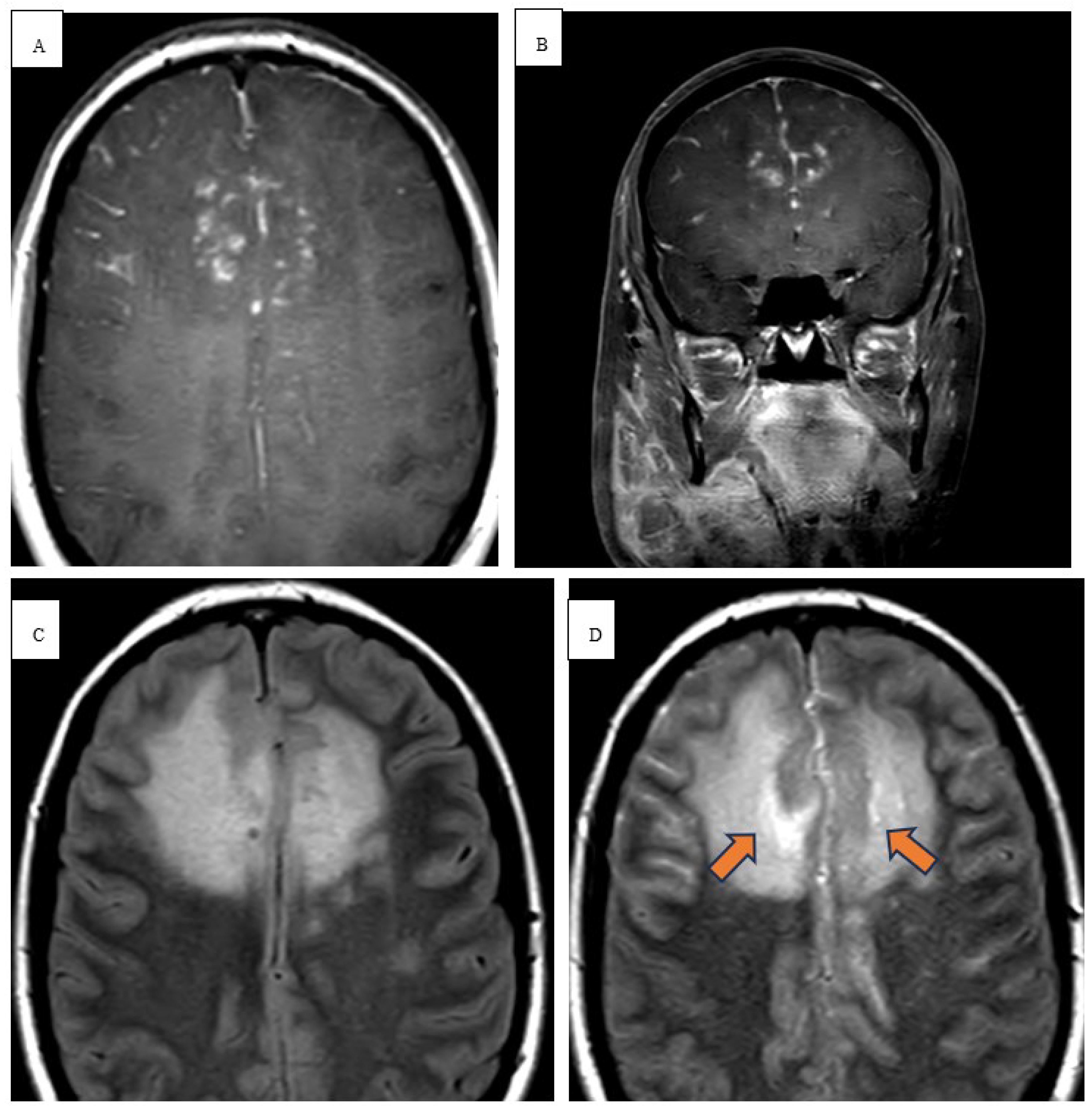
C throughout his hospital course. Physical exam revealed abnormal finger to nose and heel to shin testing with broad based magnetic gait and impaired tandem gait. Labs revealed white blood cells (WBCs) 1.65x10(3)/mcL with absolute neutrophil count of 1.02x10(3)/mcL, ALP 100 u/L, ALT 75 u/L, AST 118 u/L. Lumbar puncture with cerebrospinal fluid (CSF) analysis did not indicate evidence of meningitis. Serology was positive for John Cunningham (JC) virus, cytomegalovirus (CMV), Ebstein Barr virus (EBV) but the CSF was negative for JC virus, CMV and herpes simplex virus (HSV). NCCT demonstrated vasogenic edema within the right temporal lobe. MRI brain with and without contrast revealed spherical 1.0 x 1.0 x 1.1 cm lesion that increased in size to 2.4 x 2.1 x 2.2 cm in 3 weeks (
Figure 1 and
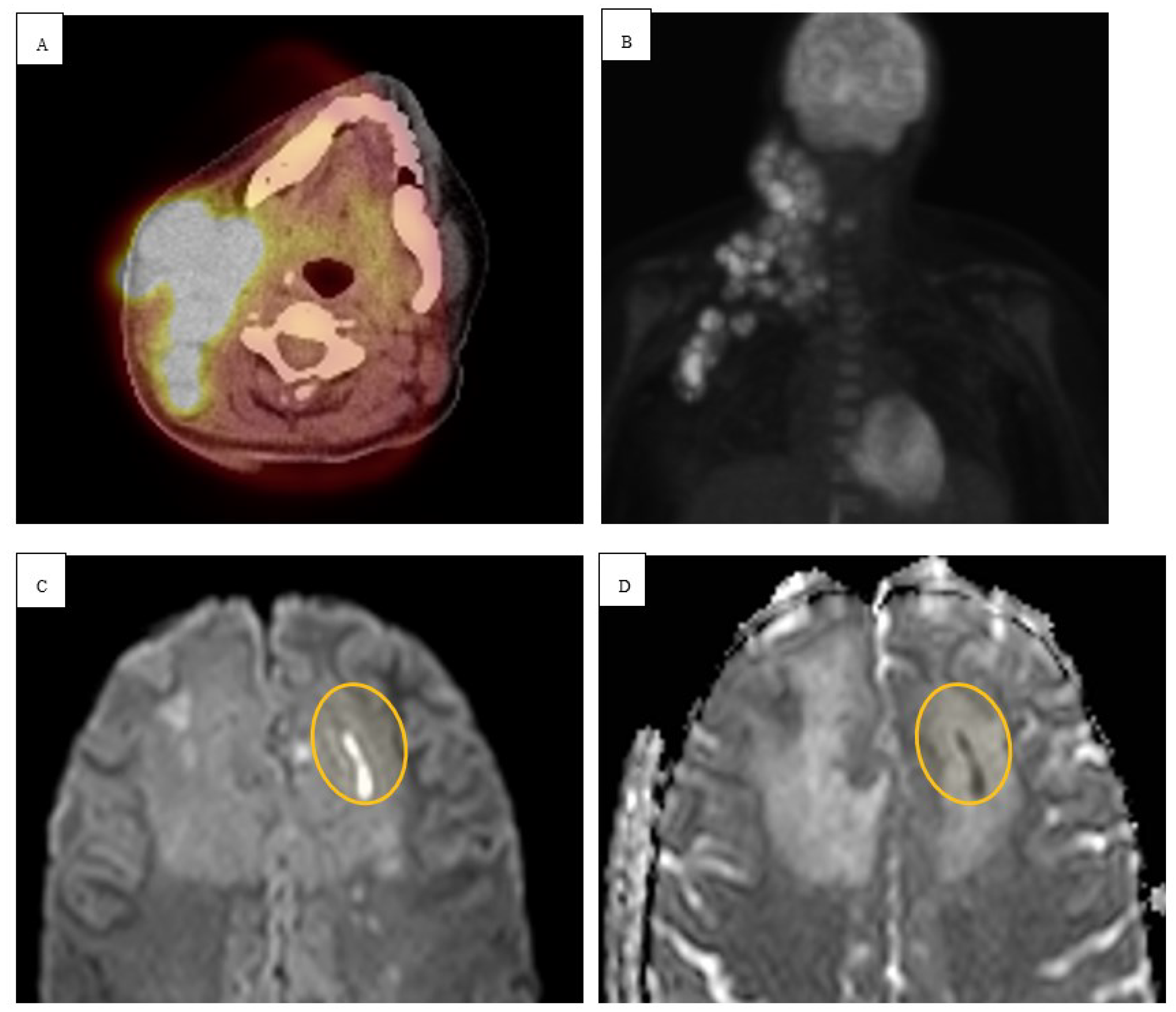
Figure 2). Patient underwent a right pteronial craniotomy with stereotactic right temporal tip dural mass biopsy by neurosurgery. Histopathology confirmed cHL of the brain with reactive gliosis of the brain parenchyma. He was initiated on a dexamethasone taper over 5 days and salvage therapy with DHAP as inpatient and eventually immunotherapy with pembrolizumab with gemcitabine, vinorelbine, and liposomal doxorubicin (pembro-GVD) as outpatient. Repeat positron emission tomography (PET) CT scan demonstrated positive response and MRI of brain negative for recurrence of disease. Subsequently, the patient had progressive disease after 3 lines of therapy and deem stem cell transplant ineligible eventually transitioned to hospice and end of life care.
Case 2
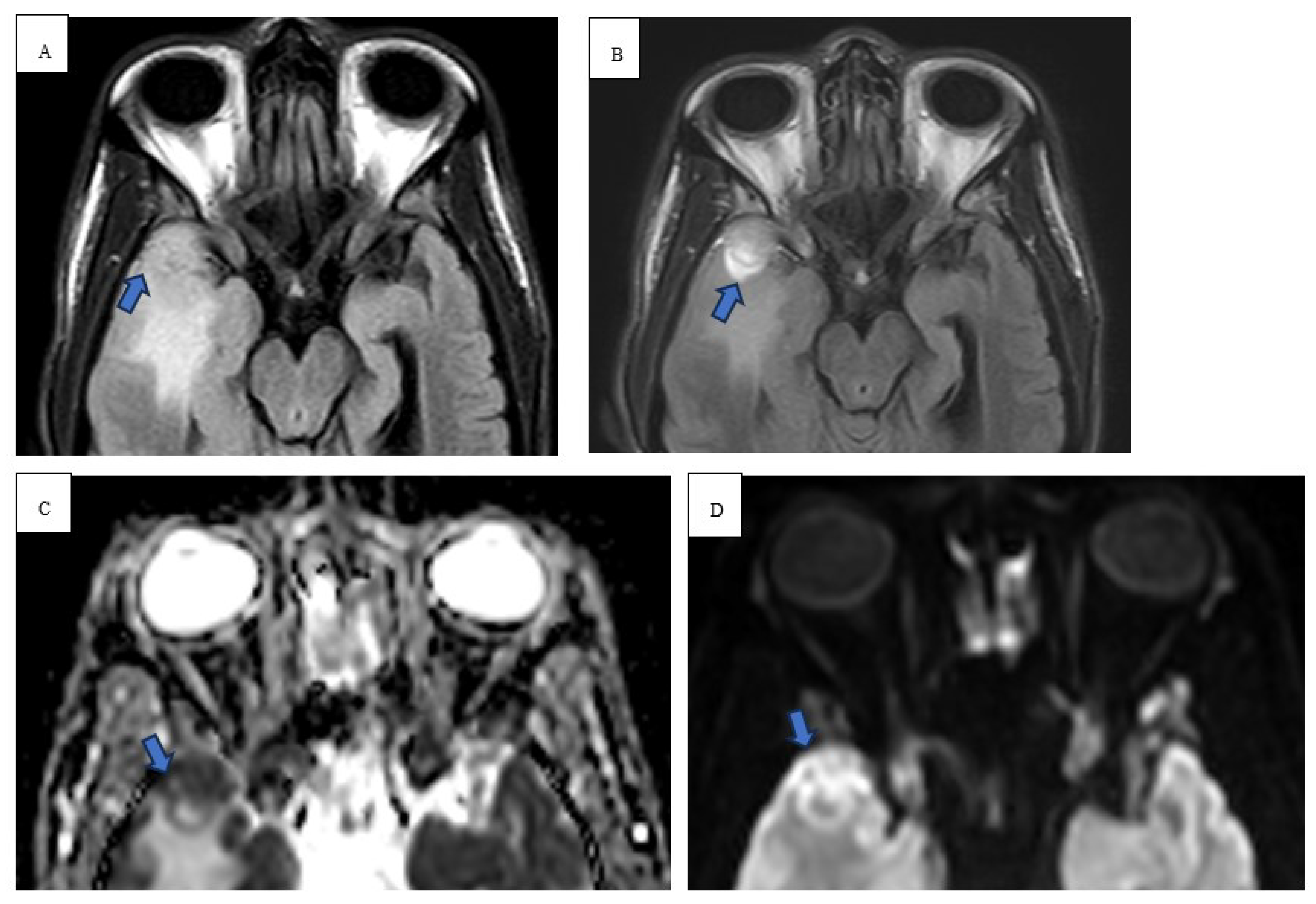
Twenty-eight-year-old female with no significant past medical history except 3 uncomplicated caesarean sections presented for neck swelling. She was found to have persistent cervical lymphadenopathy despite multiple trials of antibiotics. She underwent a right neck excisional lymph node biopsy which revealed cHL and histopathology showcasing interfollicular proliferation of large and multilobulated and single lobe cells with prominent nucleoli consistent with Reed-Sternberg cells. Immunohistochemical staining showed diffuse positivity for CD15/30 with a Ki67 index of 80%. PET-CT neck demonstrated enlarged right neck cervical lymph nodes and MR brain demonstrating characteristic diffusion weighted images (DWI) with apparent diffusion coefficient (ADC) restrictions and T2 FLAIR enhancement within the frontal lobes (
Figure 3 and
Figure 4). MRI of the chest, abdomen, and pelvis demonstrated right supraclavicular, retroclavicular, infraclavicular, right axillary, and right subpectoral lymph nodes. Initiation of chemotherapy was delayed due to pregnancy; the patient was in her third trimester and opted to start chemotherapy after delivery. During that period, she was monitored clinically. Subsequently, the patient was started on chemotherapy with ABVD (doxorubicin, bleomycin, vinblastine, and dacarbazine). Later, developed neurological symptoms for which brain imaging, CSF evaluation, extensive infectious, and paraneoplastic testing were all negative. The patient had a negative positron emission tomography (PET) CT scan after 2 cycles of chemotherapy. Chemotherapy was continued with omitting bleomycin per The United Kingdom Risk-Adapted Therapy in Hodgkin Lymphoma (RATHL) trial. The patient’s neurological symptoms continued to improve with chemotherapy, and she was in complete remission at the end of chemotherapy. Follow up brain imaging indicated improvement in the leptomeningeal enhancement.
4. Discussion
In patients with newly diagnosed or relapse refractory cHL exhibiting neurological symptoms, an evaluation for potential direct and or indirect involvement of the nervous system is necessary. CNS involvement occurs in only 0.01-0.5% of cases and any intracranial space-occupying lesion in a patient with a history of cHL demands consideration of secondary etiologies [
10,
11,
12,
13]. Intracranial cHL typically results from direct invasion from surrounding bony structures or perineural spread from cranial nerves [
14]. Several case repots and series have been published in the literature and are summarized in
Table 1. The current first line chemotherapy regimens have minimal CNS penetration. The 5 years follow up on the ECHELON trial showed a survival advantage with A-AVD compared to ABVD. Further, the SWOG S1826 trial inducting progression free survival advantage with nivolumab as compared to brentuximab with AVD. Probably, rapid control of the systemic disease aid to decrease risk of lymphomatous CNS involvement. However, this type of lymphoma is not well known to have CNS involvement. In the relapse or refractory setting if the malignancy is driven by an infectious oncogene such as EBV, this very low risk CNS disease might increase.
4.1. Radiological Findings
Radiologically, the first-line imaging modality should be a NCCT scan [
14,
15]. Characteristic findings include a hyperattenuating lesion associated with edema, while immunocompromised patients may have a more heterogenous or even hypoattenuating appearance [
14]. MRI reveals iso- to hypointense lesion(s) on T1 weighted scans and iso- to hyperintensity on T2 weighted images [
16]. DWI in immunocompetent patients show strong restricted diffusion with low ADC, however, lymphomatous spread in immunocompromised patients is more heterogenous with scattered lesions [
17]. In high grade tumors and other metastatic tumors, there is high ADC due to significant vasogenic edema [
15]. Our immunocompromised patient in case 1 revealed peripheral restricted diffusion on DWI with T1 isotensity, T2/FLAIR central hyperintensity, and peripheral T2/FLAIR hypotensity (
Figure 1 and
Figure 2). In a similar sense, case 2 demonstrated restricted diffusion on DWI and low ADC (
Figure 3).
4.2. Infectious Considerations
Brain abscess occur from either a hematogenous spread, neighboring infective infiltration, or immunocompromised state. Mechanistically, brain abscesses begin as a focal area of cerebritis and form a fibrovascular capsule within 1-2 weeks [
18]. Multiple studies reported specific features favoring brain abscesses including peripheral edema, central necrosis, extra parenchymal spread, and capsular enhancement. Peripheral edema, central necrosis, and extraparenchymal spread commonly produces short TE/short TR hypointensity with long TE/long TR hyperintensity with respect to CSF. Capsular features included short TE/short TR iso-to hyperintensity with long TE/long TR iso-to hypointensity secondary to paramagnetic hydroxyl free radicals produced by glial macrophages [
19]. In addition to the above intra-axial abscesses commonly demonstrate high signal on DWI and low ADC values. However, in instances of untreated abscesses, higher ADC signaling can be seen and therefore a premature closure of intracerebral malignancy may masquerade as an underlying abscess. A study using a high-resolution 3D gradient echo (susceptibility weighted imaging [SWI]) sequence determined that 9/12 pyogenic abscess had features of the dual rim sign. This was defined as one ring most adjacent to a lesion being hyperintense and outer most rim being hypointense [
18,
20].
4.3. Treatment
Lesions outside the blood-brain barrier (extra-axial, leptomeningeal and dural based lesions), are permeable to immunotherapy antibodies and chemotherapy drugs [
16]. Due to a lack of clinical trials, there are no definitive treatment guidelines. Voorheesof and Beaven reviewed 16 patients undergoing varied therapies reported radiological evidence of treatment response in 11 patients and complete response in 9 patients [
9]. It is essential to obtain tissue sampling for confirmatory diagnosis and to avoid administering corticosteroids preemptively, as retrospective studies have shown an increased rate of inconclusive biopsies following steroid use. In Case 1, the patient underwent a stereotactic brain biopsy confirming cHL. Subsequently, he was initiated on a dexamethasone taper followed by DHAP (dexamethasone, high dose cytarabine, and cisplatin). In patients with refractory or relapsed cHL, novel therapies include pembrolizumab in combination with traditional chemotherapy with liposomal doxorubicin, gemcitabine and vinorelbine (Pembro + GVD) which showed a curative rate prior to autologous hematopoietic stem cell transplant (Auto-SCT) of 93% [
9]. Our patients received a combination of multiple regimens encompassing both traditional and novel therapies during and before the diagnosis of intracranial disease.
Conclusion
The key learning points from these cases are (1) to investigate all space-occupying lesions with confirmatory biopsy (2) to request comprehensive workup including neuroimaging with cHL exhibiting CNS symptoms to exclude secondary diseases processes such as infectious etiology and non-neoplastic mimics. Case reports with CNS involvement in various locations document complete remission with appropriate therapy, see
Table 1. However additional large-scale studies should be conducted for additional exploration of presentation, underlying pathophysiology and tailored treatment approaches.
Table 1.
Summary of Intracranial Hodgkin’s Lymphoma Cases Reported in Literature from 1986 to 2024.
Table 1.
Summary of Intracranial Hodgkin’s Lymphoma Cases Reported in Literature from 1986 to 2024.
| Ref |
Study/year |
Age(years)/Gender(M=male, F=female) |
Location |
Type |
Outcome |
| [21] |
Bender and Mayernik/1986 |
34M |
Parietofrontal, Frontal lobe and dura |
Nodular sclerosis |
Excellent 12 months remission with XRT and CT |
| [22] |
Deckert-Schluter/1998 |
62F |
Fronto-parietal lobe |
Lymphocytic rich |
Excellent 13 months remission with XRT and CT |
| [23] |
Figueroa et al/2004 |
23F |
Posterior Fossa |
Nodular sclerosis |
Excellent 3 months remission with XRT and CT |
| [24] |
Hirmiz et al/2004 |
38M |
Parietal lobe |
Nodular sclerosis |
Excellent 12 months remission with XRT and CT |
| [25] |
Subklewe/2007 |
65F |
Temporal lobe |
Mixed cellularity |
Excellent 18 months remission with reduction of immunotherapy medications and XRT |
| [26] |
Morawa et al/2007 |
51M |
Frontal lobe |
Relapse of mixed-cellularity |
Excellent, unknown follow up, remission with XRT and CT |
| [27] |
Apollonsky et al/2008 |
22F |
Frontal lobe |
Nodular sclerosis |
Excellent, 48 months remission with XRT and CT |
| [28] |
Gerstner et al/2008 |
46M, 72M, 37F, 19M, 44M, 23M |
Unknown |
Unknown |
5/8 patients are alive without disease progression at 10 months, only 2/8 alive at 35 months |
| [29] |
Almhanna et al/2009 |
65M |
Temporal lobe |
Nodular sclerosis |
Poor response, resection followed by XRT and CT |
| [30] |
Torgerson et al/2011 |
79F |
Temporal lobe |
Nodular sclerosis |
Poor response, received only XRT |
| [31] |
Gressi et al/2013 |
77M, 59M, 65F |
Cerebellum, Brain stem, Temporal lobe |
Unknown |
77M – Excellent with XRT, NED 59 M – N/A65 F – Excellent with CT, NED |
| [32] |
Van Blydenstein et al/2014 |
41M |
Parietal lobe |
Nodular sclerosis |
Poor response, no treatment given, DOD |
| [33] |
Szczepanek et al/2020 |
33F |
Temporal lobe and cerebellum |
mixed cellularity |
Excellent, complete regression with XRT and CT |
| [34] |
Ahmed et al/2021 |
35M |
Parietal lobe |
Lymphocyte rich |
Disease progression after multiple rounds of CT |
| [35] |
Lee et al/2024 |
27M |
Dura |
Lymphocyte rich |
Excellent response with CT and SCT |
Author Contributions
K.S., K.M., and D.P. wrote the initial manuscript; O.A., E.M., B.M., and M.P. provided critical feedback and supervised the project. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of University of South Alabama (protocol code 24-015 and approved on January 24, 2024).
Informed Consent Statement
Patient consent was waived due to there being no more than minimal risk to the privacy of the individual subjects based on the presence of the required elements. In addition, it is not practicable to conduct the research without access to the use of the PHI.
Conflicts of Interest
The authors declare no conflict of interest.
References
- H. Kaseb and H. M. Babiker, “Hodgkin Lymphoma.,” in StatPearls, Treasure Island (FL): StatPearls Publishing, 2023.
- Guermazi et al., “Extranodal Hodgkin Disease: Spectrum of Disease,” RadioGraphics, vol. 21, no. 1, pp. 161–179, Jan. 2001. [CrossRef]
- S. A. Blydenstein et al., “Classical Hodgkin Lymphoma involving the central nervous system (brain) – an unusual presentation,” Clin. Case Rep., vol. 2, no. 3, pp. 88–92, Jun. 2014. [CrossRef]
- R. Archana, P. S. Kumar, and A. Kishore, “Role of MRI in Evaluation of Ring Enhancing Lesions of Brain in Correlation with Mr Spectroscopy,” Int. J. Contemp. Med. Surg. Radiol., vol. 3, no. 4, Dec. 2018. [CrossRef]
- K. M. Schwartz, B. J. Erickson, and C. Lucchinetti, “Pattern of T2 hypointensity associated with ring-enhancing brain lesions can help to differentiate pathology.,” Neuroradiology, vol. 48, no. 3, pp. 143–149, Mar. 2006. [CrossRef]
- Grommes, J. L. Rubenstein, L. M. DeAngelis, A. J. M. Ferreri, and T. T. Batchelor, “Comprehensive approach to diagnosis and treatment of newly diagnosed primary CNS lymphoma.,” Neuro-Oncol., vol. 21, no. 3, pp. 296–305, Feb. 2019. [CrossRef]
- von Tresckow and C. H. Moskowitz, “Treatment of relapsed and refractory Hodgkin Lymphoma.,” Semin. Hematol., vol. 53, no. 3, pp. 180–185, Jul. 2016. [CrossRef]
- T. P. Vassilakopoulos, J. V. Asimakopoulos, K. Konstantopoulos, and M. K. Angelopoulou, “Optimizing outcomes in relapsed/refractory Hodgkin lymphoma: a review of current and forthcoming therapeutic strategies.,” Ther. Adv. Hematol., vol. 11, p. 2040620720902911, 2020. [CrossRef]
- T. J. Voorhees and A. W. Beaven, “Therapeutic Updates for Relapsed and Refractory Classical Hodgkin Lymphoma.,” Cancers, vol. 12, no. 10, Oct. 2020. [CrossRef]
- F. Bruno et al., “P14.85 Brain metastasis from Hodgkin’s Lymphoma: case report and literature review,” Neuro-Oncol., vol. 21, no. Supplement_3, pp. iii87–iii88, Sep. 2019. [CrossRef]
- N. L. Wood and C. A. Coltman, “Localized primary extranodal Hodgkin’s disease.,” Ann. Intern. Med., vol. 78, no. 1, pp. 113–118, Jan. 1973. [CrossRef]
- M. D. Sapozink and H. S. Kaplan, “Intracranial Hodgkin’s disease. A report of 12 cases and review of the literature.,” Cancer, vol. 52, no. 7, pp. 1301–1307, Oct. 1983. [CrossRef]
- S. Grimm and M. Chamberlain, “Hodgkin’s Lymphoma: A Review of Neurologic Complications.,” Adv. Hematol., vol. 2011, p. 624578, 2011. [CrossRef]
- Chourmouzi, “Dural lesions mimicking meningiomas: A pictorial essay,” World J. Radiol., vol. 4, no. 3, p. 75, 2012. [CrossRef]
- K. Fink and J. Fink, “Imaging of brain metastases,” Surg. Neurol. Int., vol. 4, no. 5, p. 209, 2013. [CrossRef]
- Lyndon, J. A. Lansley, J. Evanson, and A. S. Krishnan, “Dural masses: meningiomas and their mimics,” Insights Imaging, vol. 10, no. 1, p. 11, Dec. 2019. [CrossRef]
- Scheichel, D. Pinggera, B. Popadic, C. Sherif, F. Marhold, and C. F. Freyschlag, “An Update on Neurosurgical Management of Primary CNS Lymphoma in Immunocompetent Patients.,” Front. Oncol., vol. 12, p. 884724, 2022. [CrossRef]
- Carloni et al., “Can MRI differentiate between ring-enhancing gliomas and intra-axial abscesses?,” Vet. Radiol. Ultrasound, vol. 63, no. 5, pp. 563–572, Sep. 2022. [CrossRef]
- Haimes et al., “MR imaging of brain abscesses,” Am. J. Roentgenol., vol. 152, no. 5, pp. 1073–1085, May 1989. [CrossRef]
- H. Toh et al., “Differentiation of Pyogenic Brain Abscesses from Necrotic Glioblastomas with Use of Susceptibility-Weighted Imaging,” Am. J. Neuroradiol., vol. 33, no. 8, pp. 1534–1538, Sep. 2012. [CrossRef]
- Bender BL, Mayernik DG. Hodgkin’s disease presenting with isolated craniospinal involvement. Cancer. 1986;58:1745–1748.
- Deckert-Schlüter M, Marek J, Setlík M, Marková J, Pakos E, Fischer R, et al. Primary manifestation of Hodgkin’s disease in the central nervous system. Virchows Arch. 1998;432:477–481.
- Figueroa BE, Brown JR, Nascimento A, Fisher DC, Tuli S. Unusual sites of Hodgkin’s lymphoma: case 2. Hodgkin’s lymphoma of the CNS masquerading as meningioma. J Clin Oncol. 2004;22:4228–4230.
- Hirmiz K, Foyle A, Wilke D, Burrell S, Brownstone R, Ago C, et al. Intracranial presentation of systemic Hodgkin’s disease. Leuk Lymphoma. 2004;45:1667–1671.
- Subklewe M, Anagnostopoulos I. Radiologic and pathologic features of a posttransplantation primary central nervous system lymphoma demonstrating Epstein-Barr virus-positive Hodgkin lymphoma. Clin Lymphoma Myeloma. 2007;7:535–537.
- Morawa E, Ragam A, Sirota R, Nabhan C. Hodgkin’s lymphoma involving the CNS. J Clin Oncol. 2007;25:1437–1438.
- Apollonsky N, Edelman M, Johnson A, Bhuiya T, Karayalcin G. Intracerebral presentation of Hodgkin disease mimicking meningioma in a young woman: case presentation with literature review. J Pediatr Hematol Oncol. 2008;30:369–372.
- Gerstner ER, Abrey LE, Schiff D, Ferreri AJ, Lister A, Montoto S, Tsang R, Thiel E, Graus F, Behringer D, Illerhaus G, Weaver S, Wen P, Voloschin A, Harris NL, Batchelor TT. CNS Hodgkin lymphoma. Blood. 2008 Sep 1;112(5):1658-61. Epub 2008 Jun 30. PMID: 18591379; PMCID: PMC3710443. [CrossRef]
- Almhanna K, Wongchaowart N, Sweetenham J. Intracerebral Hodgkin's lymphoma in a patient with chronic lymphocytic leukemia/small lymphocytic lymphoma: a case report and literature review. Cancer Invest. 2009 Feb;27(2):215-20.
- Torgerson, S.,Olteanu, H., Tinguely, M. et al. Central nervous system Hodgkin lymphoma: case report and review of the literature. J Neurooncol 102, 329–334 (2011). [CrossRef]
- Gessi M, Kuchelmeister K, Kellner U, Ritter M, Morgner A, Urbach H, et al. Unusual clinico-pathological features in primary Hodgkin’s lymphomas of the central nervous system. Acta Neurochir (Wien) 2013;155:19–24.
- Van Blydenstein SA, Patel M, Philip V, Lakha A, Pather S, Westgarth-Taylor T, et al. Classical Hodgkin lymphoma involving the central nervous system (brain) - an unusual presentation. Clin Case Rep. 2014;2:88–92.
- Szczepanek D, Szumiło J, Stoma F, Szymczyk A, Jarosz B, Szczepanek A, et al. A case report of a female patient with Hodgkin lymphoma localized in the central nervous system and with concomitant pulmonary lymphomatoid granulomatosis. Front Neurol. 2020;11:963.
- Ahmed S, Irfan B, Raza M, Haider G. Atypical involvement of central nervous system in classic Hodgkin lymphoma: a case report. J Med Case Rep. 2021;15:532.
- Lee H, Ahn S, Cha SH, Cho WH. Intracranial Involvement of Systemic Hodgkin Lymphoma: A Case Report and Literature Review. Brain Tumor Res Treat. 2024 Jan;12(1):63-69. PMID: 38317490; PMCID: PMC10864131. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).