Submitted:
16 April 2024
Posted:
16 April 2024
You are already at the latest version
Abstract
Keywords:
Introduction
Literature Review
Understanding the Effects of IMIDs on Risk of COVID-19
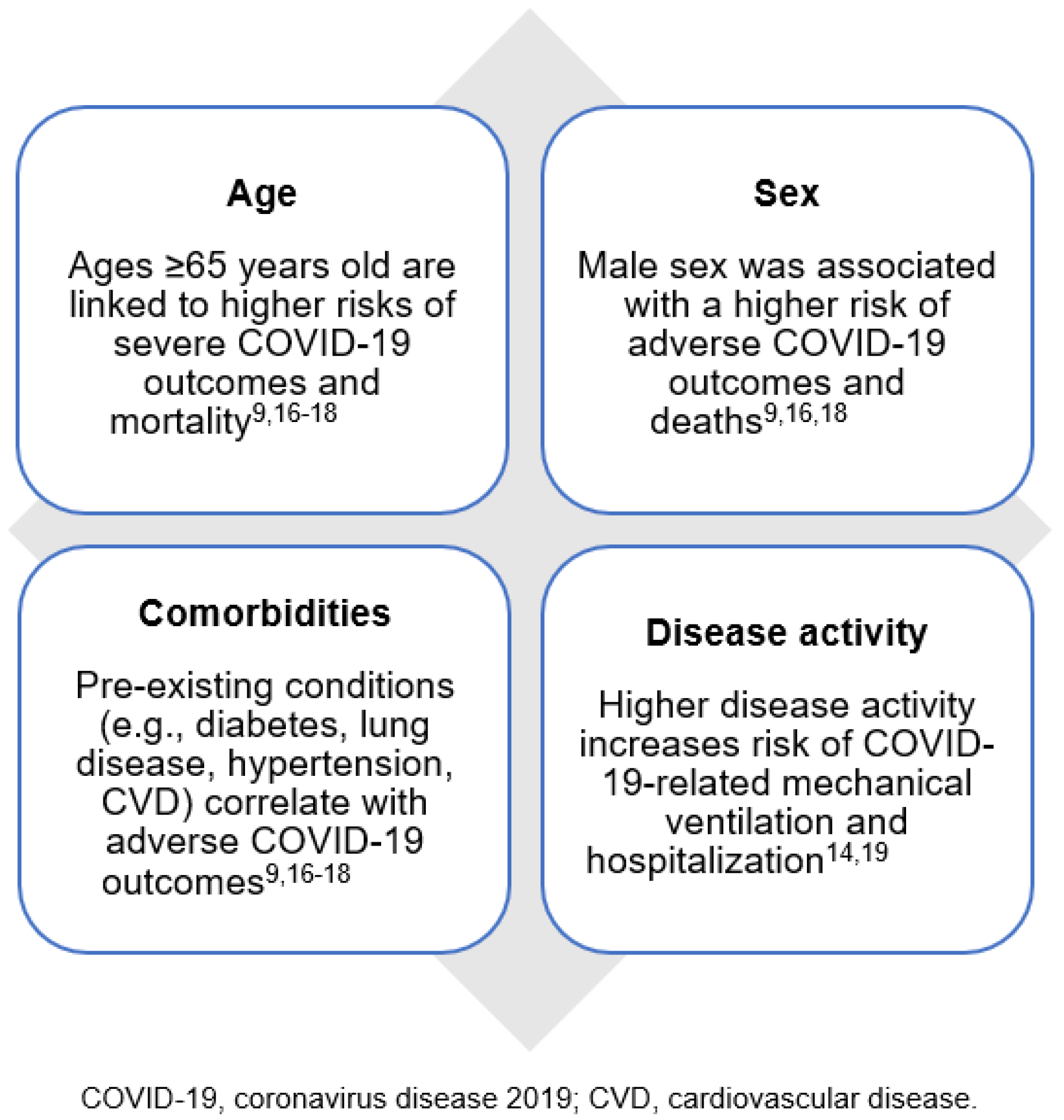
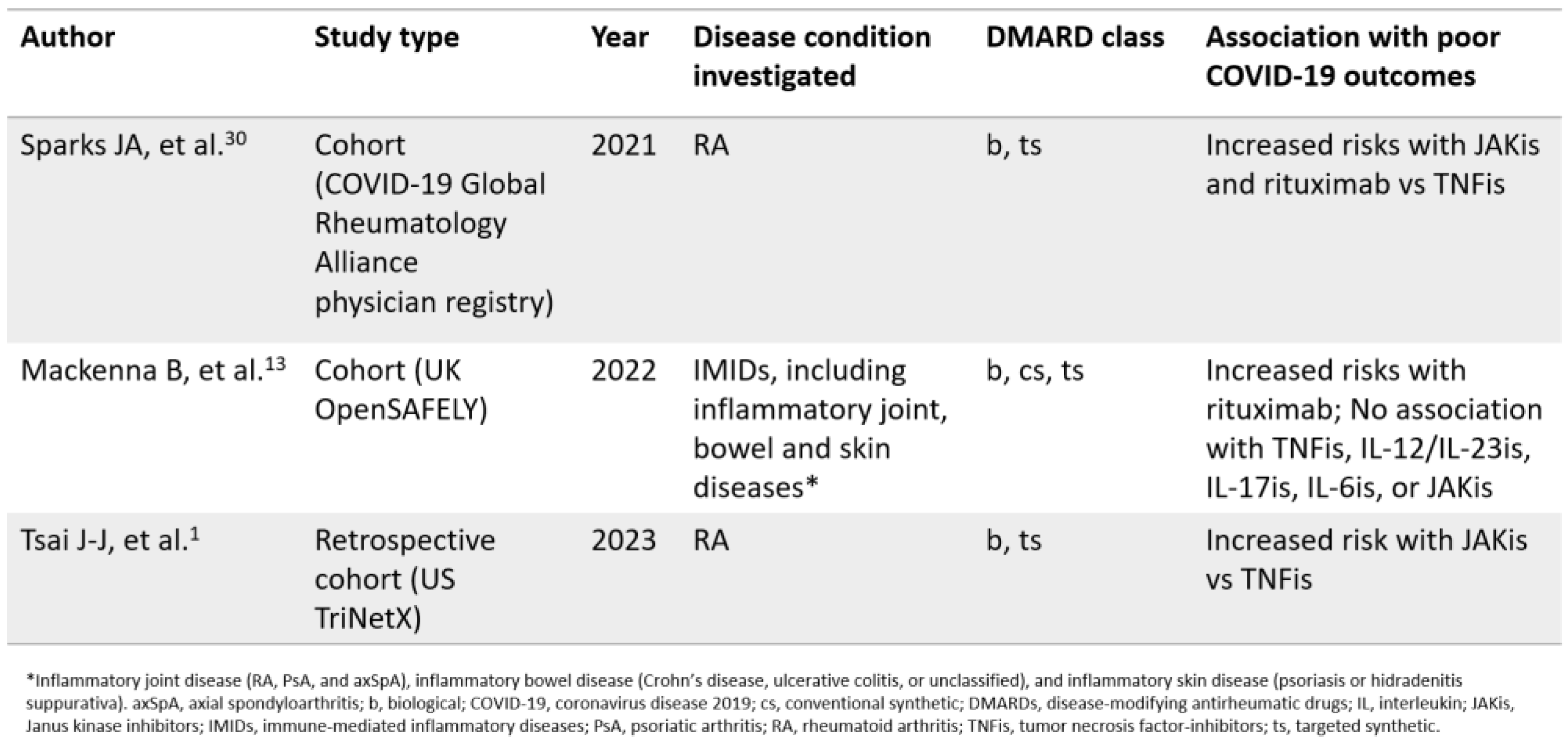
Understanding the Effects of DMARDs on COVID-19 Outcomes in Patients with IMIDs

Illustrative Scenario: A Patient with Severe Active RA on Multiple cs/bDMARDs
Discussion
Therapeutic Strategies for Treatment of COVID-19 in Patients with IMIDs
Protecting Patients with IMIDs: Vaccination Recommendations and Additional Considerations
Conclusions and Clinical Implications
Funding
Acknowledgment
Conflicts of interest/Competing interests
Ethics approval
Consent to participate
Consent for publication
Availability of data and material
Code availability
Authors’ contribution
References
- Tsai J-J, Liu L-T, Chen C-H, Chen L-J, Wang S-I, Wei JC-C. COVID-19 outcomes in patients with rheumatoid arthritis with biologic or targeted synthetic DMARDs. RMD Open. 2023;9:e003038.
- Ketkar A, Willey V, Pollack M, Glasser L, Dobie C, Wenziger C, et al. Assessing the risk and costs of COVID-19 in immunocompromised populations in a large United States commercial insurance health plan: the EPOCH-US Study. Current Medical Research and Opinion. 2023;0:1–16.
- Bieber A, Brikman S, Novack L, Ayalon S, Abu-Shakra M, Zeller L, et al. SARS-CoV-2 infection among patients with autoimmune rheumatic diseases; comparison between the Delta and Omicron waves in Israel. Semin Arthritis Rheum. 2023;58:152129.
- Barnes E, Goodyear CS, Willicombe M, Gaskell C, Siebert S, I de Silva T, et al. SARS-CoV-2-specific immune responses and clinical outcomes after COVID-19 vaccination in patients with immune-suppressive disease. Nat Med. 2023;29:1760–74.
- Tang CY, Boftsi M, Staudt L, McElroy JA, Li T, Duong S, et al. SARS-CoV-2 and influenza co-infection: A cross-sectional study in central Missouri during the 2021–2022 influenza season. Virology. 2022;576:105–10.
- Chu RBH, Zhao S, Zhang JZ, Chan KCK, Ng PY, Chan C, et al. Comparison of COVID-19 with influenza A in the ICU: a territory-wide, retrospective, propensity matched cohort on mortality and length of stay. BMJ Open. 2023;13:e067101.
- Alhowaish TS, Alhamadh MS, Alhabeeb AY, Aldosari SF, Masuadi E, Alrashid A. Outcomes of COVID-19 in Inflammatory Rheumatic Diseases: A Retrospective Cohort Study. Cureus. 2022;14:e26343.
- Figueroa-Parra G, Gilbert EL, Valenzuela-Almada MO, Vallejo S, Neville MR, Patel NJ, et al. Risk of severe COVID-19 outcomes associated with rheumatoid arthritis and phenotypic subgroups: a retrospective, comparative, multicentre cohort study. Lancet Rheumatol. 2022;4:e765–74.
- Wang F, Ma Y, Xu S, Liu H, Chen Y, Yang H, et al. Prevalence and risk of COVID-19 in patients with rheumatic diseases: a systematic review and meta-analysis. Clin Rheumatol. 2022;41:2213–23.
- Grainger R, Kim AHJ, Conway R, Yazdany J, Robinson PC. COVID-19 in people with rheumatic diseases: risks, outcomes, treatment considerations. Nat Rev Rheumatol. 2022;18:191–204.
- Simon D, Tascilar K, Fagni F, Krönke G, Kleyer A, Meder C, et al. SARS-CoV-2 vaccination responses in untreated, conventionally treated and anticytokine-treated patients with immune-mediated inflammatory diseases. Ann Rheum Dis. 2021;80:1312–6.
- Ruscitti P, Conforti A, Cipriani P, Giacomelli R, Tasso M, Costa L, et al. Pathogenic implications, incidence, and outcomes of COVID-19 in autoimmune inflammatory joint diseases and autoinflammatory disorders. Adv Rheumatol. 2021;61:45.
- MacKenna B, Kennedy NA, Mehrkar A, Rowan A, Galloway J, Matthewman J, et al. Risk of severe COVID-19 outcomes associated with immune-mediated inflammatory diseases and immune-modifying therapies: a nationwide cohort study in the OpenSAFELY platform. The Lancet Rheumatology. 2022;4:e490–506.
- Bruera S, Lei X, Zhao H, Yazdany J, Chavez-MacGregor M, Giordano SH, et al. Risks of mortality and severe coronavirus disease 19 (COVID-19) outcomes in patients with or without systemic lupus erythematosus. Lupus Sci Med. 2023;10:e000750.
- Chang R, Yen-Ting Chen T, Wang S-I, Hung Y-M, Chen H-Y, Wei C-CJ. Risk of autoimmune diseases in patients with COVID-19: A retrospective cohort study. eClinicalMedicine. 2023;56:101783.
- Williamson EJ, Walker AJ, Bhaskaran K, Bacon S, Bates C, Morton CE, et al. Factors associated with COVID-19-related death using OpenSAFELY. Nature. 2020;584:430–6.
- Kihara M, Sugihara T, Asano J, Sato M, Kaneko H, Muraoka S, et al. Clinical characteristics of COVID-19 patients with underlying rheumatic diseases in Japan: data from a multicenter observational study using the COVID-19 Global Rheumatology Alliance physician-reported registry. Clin Rheumatol. 2022;41:3661–73.
- Ye Y, Yue X, Krueger WS, Wegrzyn LR, Maniccia AW, Winthrop KL, et al. Factors Associated with Severe COVID-19 Among Patients with Rheumatoid Arthritis: A Large, Nationwide Electronic Health Record Cohort Study in the United States. Adv Ther. 2023;40:3723–38.
- Strangfeld A, Schäfer M, Gianfrancesco MA, Lawson-Tovey S, Liew JW, Ljung L, et al. Factors associated with COVID-19-related death in people with rheumatic diseases: results from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the Rheumatic Diseases. 2021;80:930–42.
- Geng Y, Fan Y, Deng X, Wang Y, Zhao J, Ji L, et al. The Recent Outbreak of COVID-19 in China During the Omicron Variant Predominance: Clinical Features and Outcomes in Patients with Autoimmune Inflammatory Rheumatic Diseases. Rheumatol Ther. 2023;10:1039–53.
- Kashiwado Y, Kimoto Y, Oku K, Yamamoto M, Ohshima S, Ito S, et al. Prognostic improvement and treatment of COVID-19 in patients with rheumatic diseases until December 2022: Analysis of the JCR COVID-19 registry in Japan. Modern Rheumatology. 2023;road057.
- Montero F, Martínez-Barrio J, Serrano-Benavente B, González T, Rivera J, Molina Collada J, et al. Coronavirus disease 2019 (COVID-19) in autoimmune and inflammatory conditions: clinical characteristics of poor outcomes. Rheumatol Int. 2020;40:1593–8.
- Hsu C-Y, Ko C-H, Wang J-L, Hsu T-C, Lin C-Y. Comparing the burdens of opportunistic infections among patients with systemic rheumatic diseases: a nationally representative cohort study. Arthritis Res Ther. 2019;21:211.
- Fagni F, Simon D, Tascilar K, Schoenau V, Sticherling M, Neurath MF, et al. COVID-19 and immune-mediated inflammatory diseases: effect of disease and treatment on COVID-19 outcomes and vaccine responses. Lancet Rheumatol. 2021;3:e724–36.
- Petri M, Joyce D, Haag K, Fava A, Goldman DW, Zhong D, et al. Effect of Systemic Lupus Erythematosus and Immunosuppressive Agents on COVID-19 Vaccination Antibody Response. Arthritis Care & Research. 2023;75:1878–85.
- Meunier L, Sanavio M, Dumortier J, Meszaros M, Faure S, Ursic Bedoya J, et al. Mycophenolate mofetil decreases humoral responses to three doses of SARS-CoV-2 vaccine in liver transplant recipients. Liver Int. 2022;42:1872–8.
- Fraenkel L, Bathon JM, England BR, St Clair EW, Arayssi T, Carandang K, et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2021;73:1108–23.
- Di Iorio M, Cook CE, Vanni KMM, Patel NJ, D’Silva KM, Fu X, et al. DMARD disruption, rheumatic disease flare, and prolonged COVID-19 symptom duration after acute COVID-19 among patients with rheumatic disease: A prospective study. Semin Arthritis Rheum. 2022;55:152025.
- Sieiro Santos C, Calleja Antolin S, Moriano Morales C, Garcia Herrero J, Diez Alvarez E, Ramos Ortega F, et al. Immune responses to mRNA vaccines against SARS-CoV-2 in patients with immune-mediated inflammatory rheumatic diseases. RMD Open. 2022;8:e001898.
- Sparks JA, Wallace ZS, Seet AM, Gianfrancesco MA, Izadi Z, Hyrich KL, et al. Associations of baseline use of biologic or targeted synthetic DMARDs with COVID-19 severity in rheumatoid arthritis: Results from the COVID-19 Global Rheumatology Alliance physician registry. Ann Rheum Dis. 2021;annrheumdis-2021-220418.
- Gianfrancesco M, Hyrich KL, Al-Adely S, Carmona L, Danila MI, Gossec L, et al. Characteristics associated with hospitalisation for COVID-19 in people with rheumatic disease: data from the COVID-19 Global Rheumatology Alliance physician-reported registry. Annals of the Rheumatic Diseases. 2020;79:859–66.
- Price E, MacPhie E, Kay L, Lanyon P, Griffiths B, Holroyd C, et al. Identifying rheumatic disease patients at high risk and requiring shielding during the COVID-19 pandemic. Clin Med (Lond). 2020;20:256–61.
- Hasseli R, Mueller-Ladner U, Hoyer BF, Krause A, Lorenz H-M, Pfeil A, et al. Older age, comorbidity, glucocorticoid use and disease activity are risk factors for COVID-19 hospitalisation in patients with inflammatory rheumatic and musculoskeletal diseases. RMD Open. 2021;7:e001464.
- Md Yusof MY, Arnold J, Saleem B, Vandevelde C, Dass S, Savic S, et al. Breakthrough SARS-CoV-2 infections and prediction of moderate-to-severe outcomes during rituximab therapy in patients with rheumatic and musculoskeletal diseases in the UK: a single-centre cohort study. Lancet Rheumatol. 2023;5:e88–98.
- Simon D, Tascilar K, Kleyer A, Fagni F, Krönke G, Meder C, et al. Impact of Cytokine Inhibitor Therapy on the Prevalence, Seroconversion Rate, and Longevity of the Humoral Immune Response Against SARS-CoV-2 in an Unvaccinated Cohort. Arthritis Rheumatol. 2022;74:783–90.
- Widdifield J, Kwong JC, Chen S, Eder L, Benchimol EI, Kaplan GG, et al. Vaccine effectiveness against SARS-CoV-2 infection and severe outcomes among individuals with immune-mediated inflammatory diseases tested between March 1 and Nov 22, 2021, in Ontario, Canada: a population-based analysis. Lancet Rheumatol. 2022;4:e430–40.
- Frey S, Chiang TP-Y, Connolly CM, Teles M, Alejo JL, Boyarsky BJ, et al. Antibody durability 6 months after two doses of SARS-CoV-2 mRNA vaccines in patients with rheumatic and musculoskeletal disease. Lancet Rheumatol. 2022;4:e241–3.
- Finckh A, Ciurea A, Raptis CE, Rubbert-Roth A. Susceptibility to COVID-19 and Immunologic Response to Vaccination in Patients With Immune-Mediated Inflammatory Diseases. J Infect Dis. 2023;228:S13–23.
- Schreiber K, Graversgaard C, Petersen R, Jakobsen H, Bojesen AB, Krogh NS, et al. Reduced Humoral Response of SARS-CoV-2 Antibodies following Vaccination in Patients with Inflammatory Rheumatic Diseases-An Interim Report from a Danish Prospective Cohort Study. Vaccines (Basel). 2021;10:35.
- Saleem B, Ross RL, Bissell L-A, Aslam A, Mankia K, Duquenne L, et al. Effectiveness of SARS-CoV-2 vaccination in patients with rheumatoid arthritis (RA) on DMARDs: as determined by antibody and T cell responses. RMD Open. 2022;8:e002050.
- Tobudic S, Simader E, Deimel T, Straub J, Kartnig F, Heinz LX, et al. The accelerated waning of immunity and reduced effect of booster in patients treated with bDMARD and tsDMARD after SARS-CoV-2 mRNA vaccination. Frontiers in Medicine [Internet]. 2023 [cited 2023 Jun 7];10. Available from: https://www.frontiersin.org/articles/10.3389/fmed.2023.1049157.
- Zhao T, Wang B, Shen J, Wei Y, Zhu Y, Tian X, et al. Third dose of anti-SARS-CoV-2 inactivated vaccine for patients with RA: Focusing on immunogenicity and effects of RA drugs. Front Med (Lausanne). 2022;9:978272.
- Dayam RM, Law JC, Goetgebuer RL, Chao GYC, Abe KT, Sutton M, et al. Accelerated waning of immunity to SARS-CoV-2 mRNA vaccines in patients with immune-mediated inflammatory diseases. JCI Insight [Internet]. 2022 [cited 2023 Aug 9];7. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC9220925/.
- Benny L, Mehta P, Ahmed S, Paul A, Sukumaran A, Mohanan M, et al. Correlates of breakthrough Omicron (B.1.1.529) infections in a prospective cohort of vaccinated patients with rheumatic diseases. Rheumatol Int. 2023;43:1033–9.
- Drosos AA, Pelechas E, Voulgari PV. Treatment strategies of COVID-19: A rheumatology perspective. Eur J Intern Med. 2022;102:17–23.
- Clinical Spectrum [Internet]. COVID-19 Treatment Guidelines. [cited 2023 Aug 24]. Available from: https://www.covid19treatmentguidelines.nih.gov/overview/clinical-spectrum/.
- Nonhospitalized Adults: Therapeutic Management [Internet]. COVID-19 Treatment Guidelines. [cited 2023 Aug 24]. Available from: https://www.covid19treatmentguidelines.nih.gov/management/clinical-management-of-adults/nonhospitalized-adults--therapeutic-management/.
- Mikuls TR, Johnson SR, Fraenkel L, Arasaratnam RJ, Baden LR, Bermas BL, et al. American College of Rheumatology Guidance for the Management of Rheumatic Disease in Adult Patients During the COVID-19 Pandemic: Version 3. Arthritis Rheumatol. 2021;73:e1–12.
- Landewé RB, Machado PM, Kroon F, Bijlsma HW, Burmester GR, Carmona L, et al. EULAR provisional recommendations for the management of rheumatic and musculoskeletal diseases in the context of SARS-CoV-2. Ann Rheum Dis. 2020;79:851–8.
- Zhang X, Zhang Y, Qiao W, Zhang J, Qi Z. Baricitinib, a drug with potential effect to prevent SARS-COV-2 from entering target cells and control cytokine storm induced by COVID-19. Int Immunopharmacol. 2020;86:106749.
- Gómez RI, Méndez R, Palanques-Pastor T, Ballesta-López O, Almenar CB, Vericat JEM, et al. Baricitinib against severe COVID-19: effectiveness and safety in hospitalised pretreated patients. Eur J Hosp Pharm. 2022;29:e41–5.
- Costanzo G, Cordeddu W, Chessa L, Del Giacco S, Firinu D. COVID-19: Considerations about immune suppression and biologicals at the time of SARS-CoV-2 pandemic. World J Clin Cases. 2021;9:5352–7.
- Alunno A, Najm A, Machado PM, Bertheussen H, Burmester G-RR, Carubbi F, et al. 2021 update of the EULAR points to consider on the use of immunomodulatory therapies in COVID-19. Ann Rheum Dis. 2022;81:34–40.
- Information on COVID-19 Treatment, Prevention and Research [Internet]. COVID-19 Treatment Guidelines. [cited 2023 Aug 25]. Available from: https://www.covid19treatmentguidelines.nih.gov/.
- Curtis JR, Johnson SR, Anthony DD, Arasaratnam RJ, Baden LR, Bass AR, et al. American College of Rheumatology Guidance for COVID-19 Vaccination in Patients With Rheumatic and Musculoskeletal Diseases: Version 5. Arthritis Rheumatol. 2023;75:E1–16.
- Guideline Update: ACR Advises on Vaccinations for Patients with Rheumatic Diseases [Internet]. 2023 [cited 2023 Aug 25]. Available from: https://www.practicalpainmanagement.com/resources/clinical-practice-guidelines/acr-advises-vaccinations-patients-with-rheumatic-disease.
- Bellino S. COVID-19 treatments approved in the European Union and clinical recommendations for the management of non-hospitalized and hospitalized patients. Ann Med. 54:2856–60.
- AstraZeneca. A Phase I/III Randomized, Double Blind Study to Evaluate the Safety, Efficacy and Neutralizing Activity of AZD5156/AZD3152 for Pre Exposure Prophylaxis of COVID 19 in Participants With Conditions Causing Immune Impairment. Sub-study: Phase II Open Label Sub-study to Evaluate the Safety, PK, and Neutralizing Activity of AZD3152 for Pre-exposure Prophylaxis of COVID-19 [Internet]. clinicaltrials.gov; 2023 Jun. Report No.: NCT05648110. Available from: https://clinicaltrials.gov/study/NCT05648110.
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





