Submitted:
16 April 2024
Posted:
16 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
3. Results
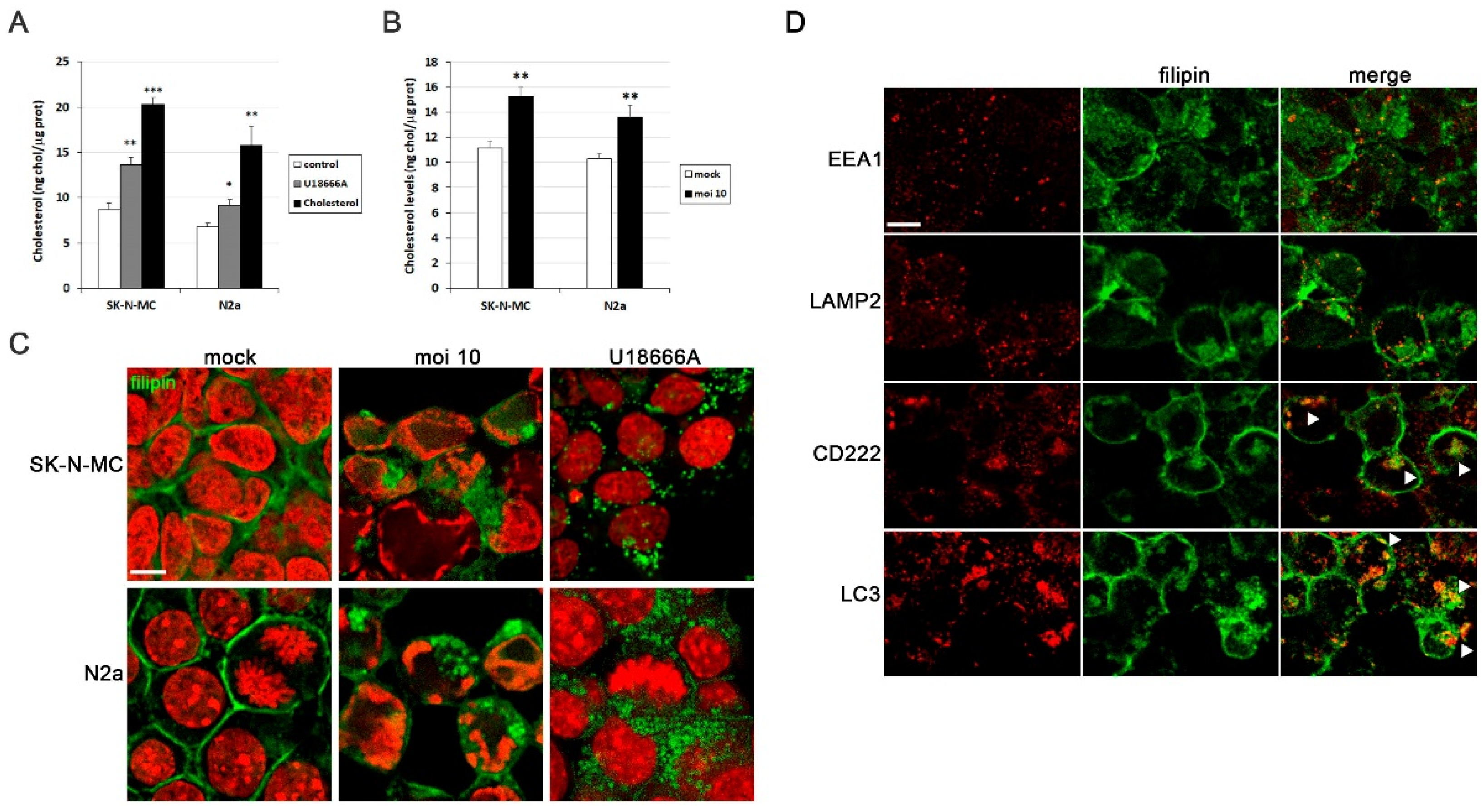
3.1.1. HSV-1 Infection Increases Intracellular Cholesterol Levels
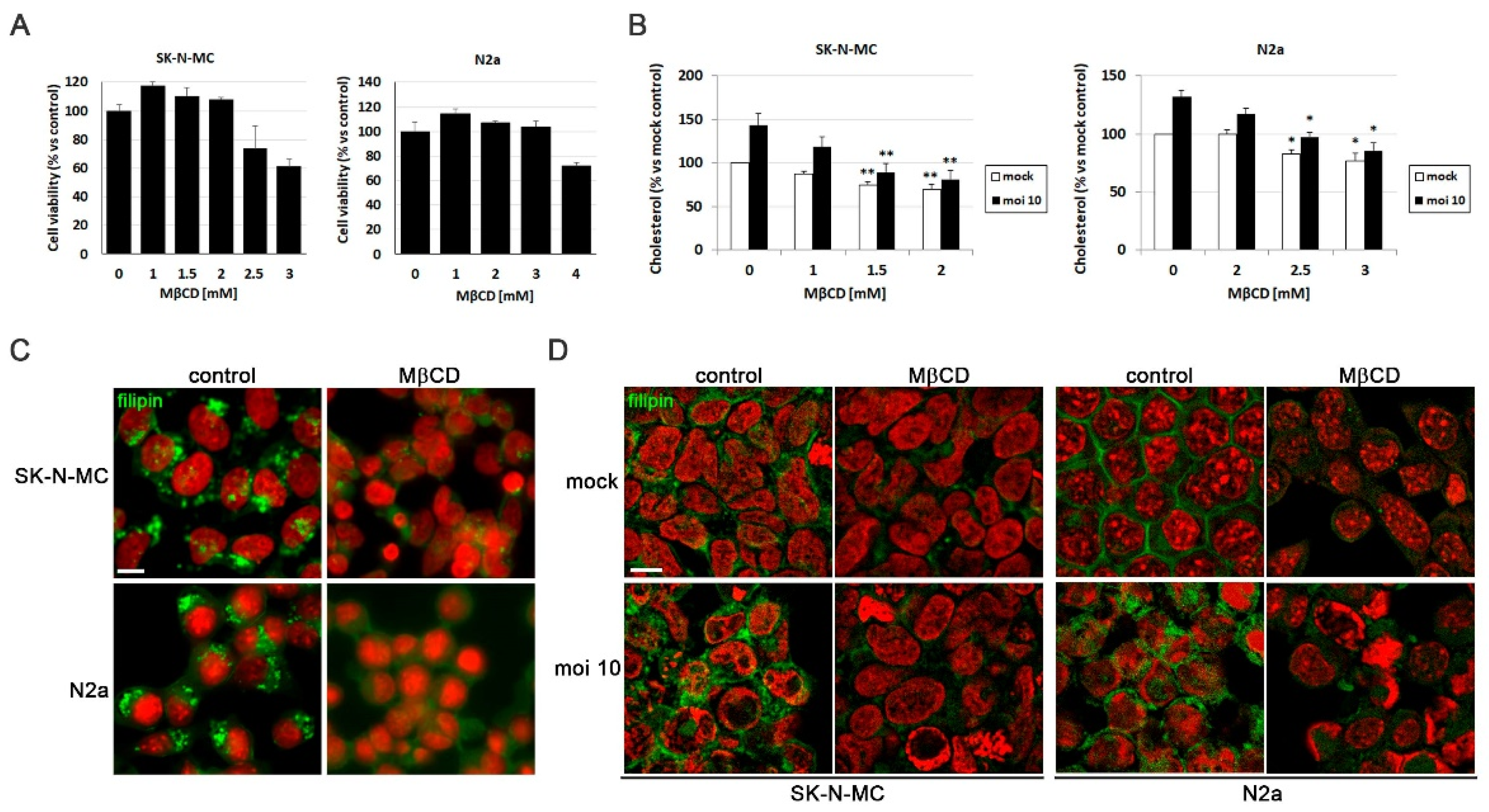
3.1.2. MβCD Reverts the Cholesterol Accumulation Induced by HSV-1
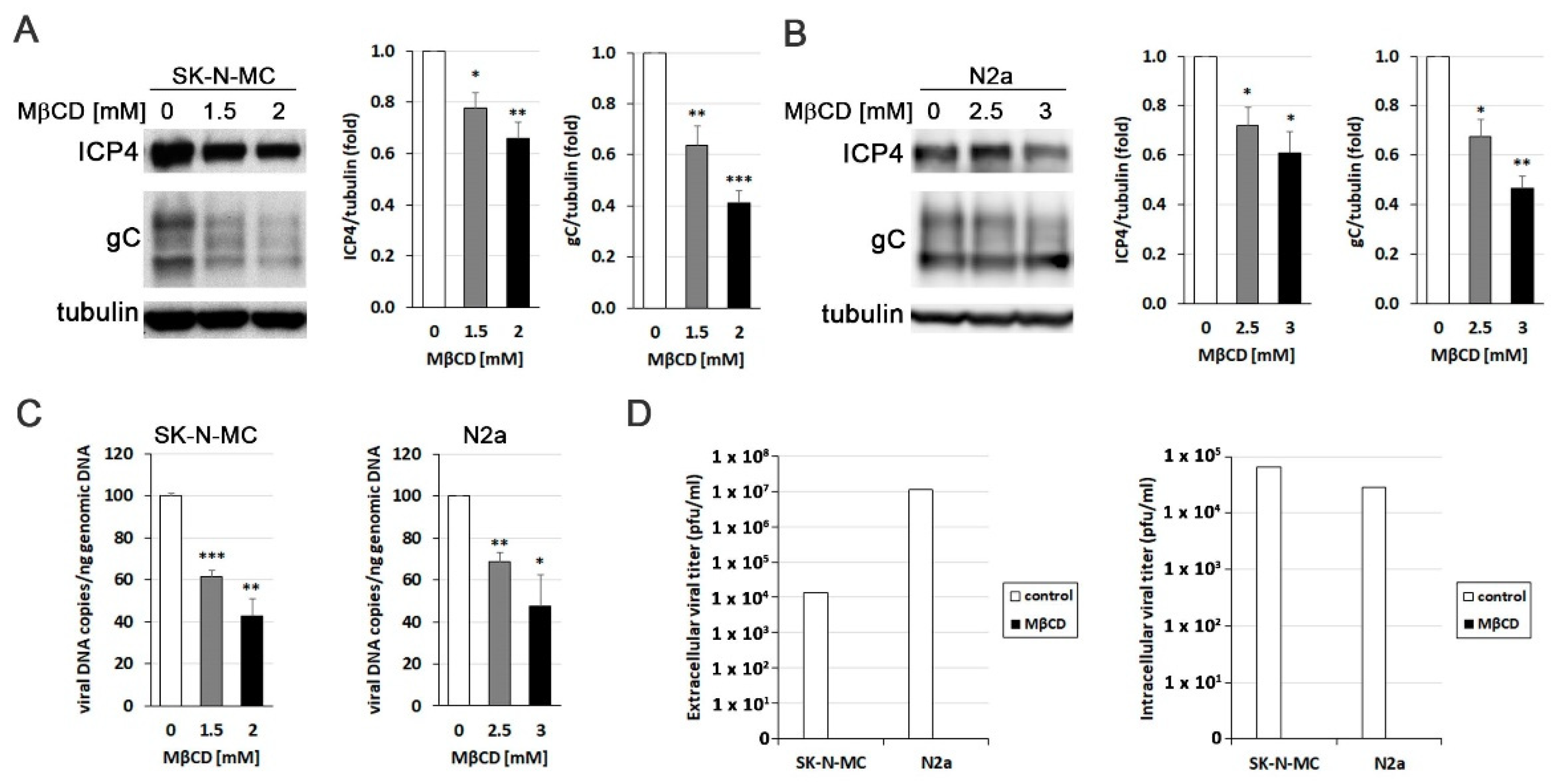
3.1.3. MβCD Inhibits the Efficiency of HSV-1 Infection
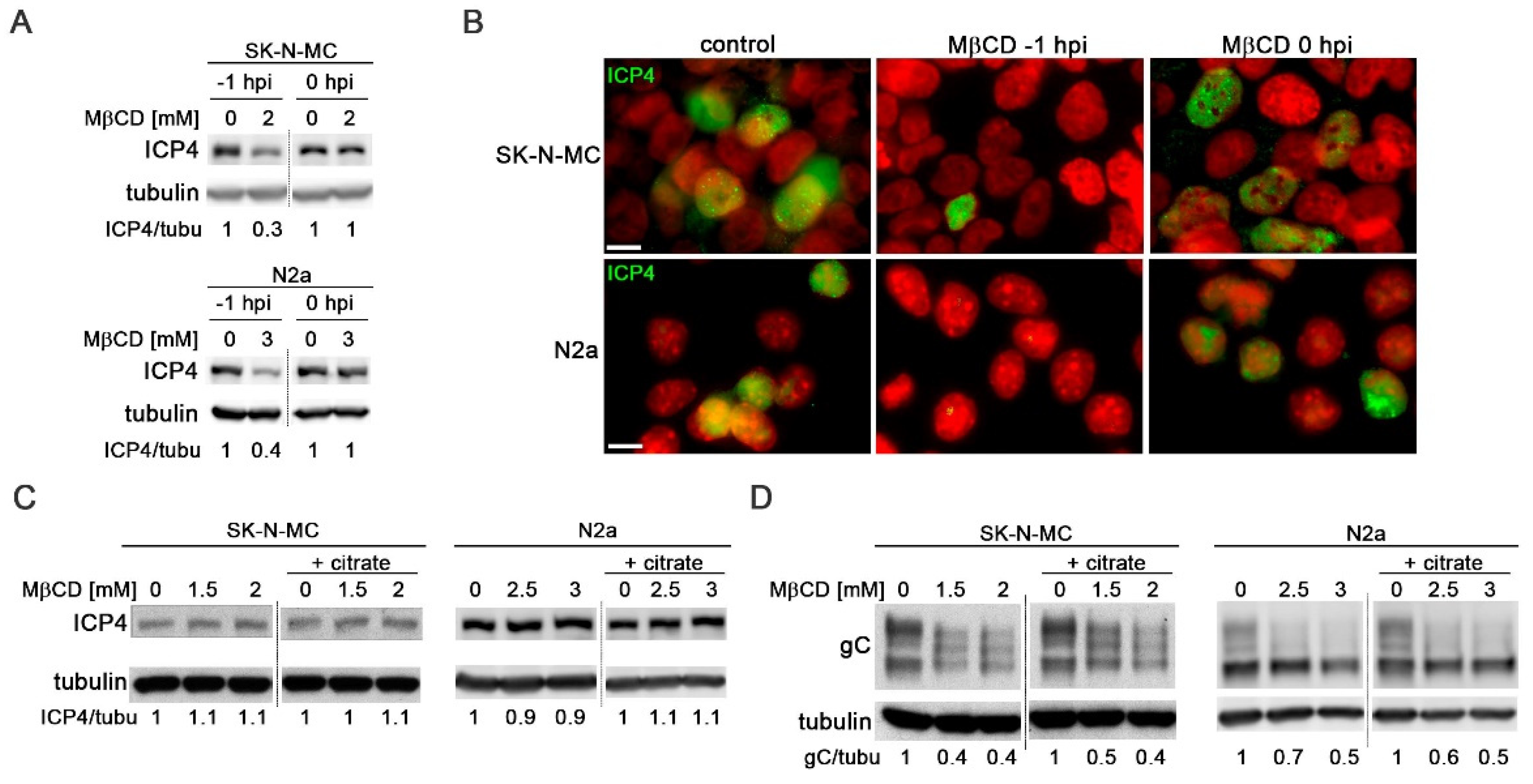
3.1.4. Cholesterol Plays a Role in Post-Entry Stages of HSV-1 Infection
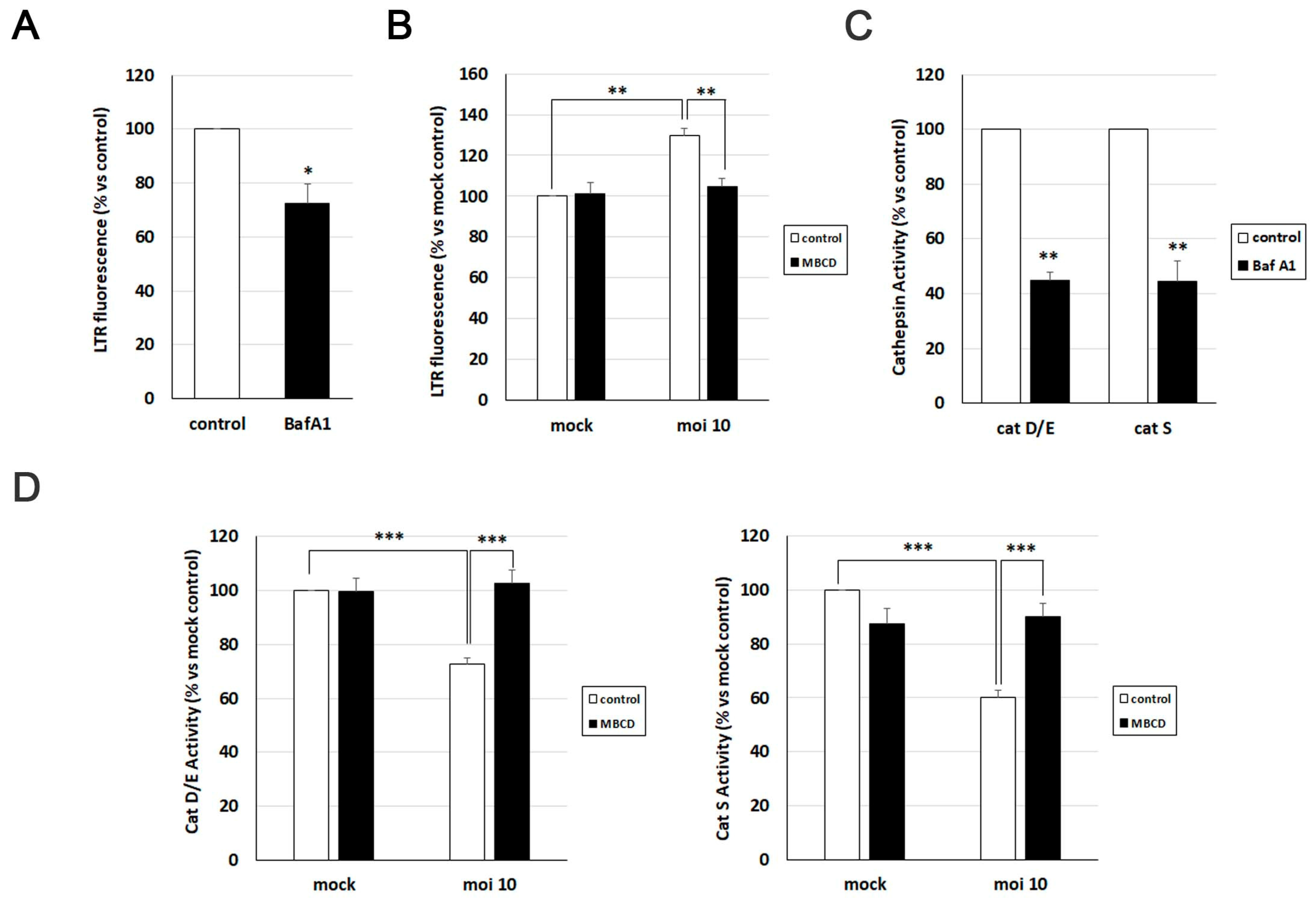
3.1.5. MβCD Reverts the Lysosomal Alterations Induced by HSV-1
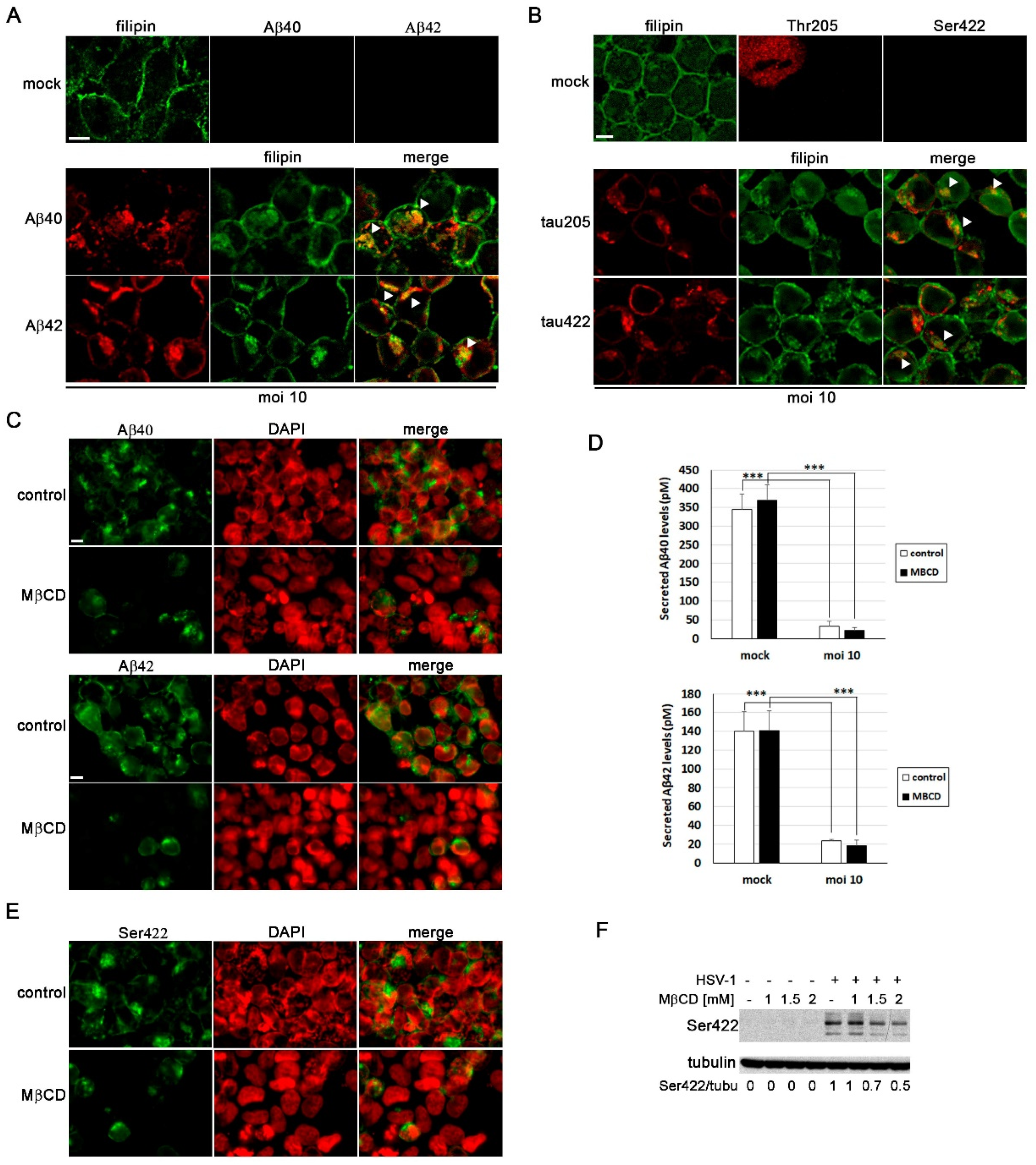
3.1.6. MβCD Attenuates the Neurodegeneration Markers Induced by HSV-1
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Luo, J.; Yang, H.; Song, B.L. Mechanisms and Regulation of Cholesterol Homeostasis. Nat. Rev. Mol. Cell Biol. 2019, 21, 225–245. [CrossRef]
- Ho, W.Y.; Hartmann, H.; Ling, S. Central Nervous System Cholesterol Metabolism in Health and Disease. IUBMB Life 2022, 74, 826–841. [CrossRef]
- Cardoso, D.; Perucha, E. Cholesterol Metabolism: A New Molecular Switch to Control Inflammation. Clin. Sci. 2021, 135, 1389–1408. [CrossRef]
- Hu, Z.L.; Yuan, Y.Q.; Tong, Z.; Liao, M.Q.; Yuan, S.L.; Jian, Y.; Yang, J.L.; Liu, W.F. Reexamining the Causes and Effects of Cholesterol Deposition in the Brains of Patients with Alzheimer’s Disease. Mol. Neurobiol. 2023, 1, 1–17. [CrossRef]
- Stelzmann, R.A.; Norman Schnitzlein, H.; Reed Murtagh, F. An English Translation of Alzheimer’s 1907 Paper, “Über Eine Eigenartige Erkankung Der Hirnrinde.” Clin. Anat. 1995, 8, 429–431. [CrossRef]
- Breijyeh, Z.; Karaman, R.; Muñoz-Torrero, D.; Dembinski, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [CrossRef]
- Huang, P.; Wang, X.; Lei, M.; Ma, Y.; Chen, H.; Sun, J.; Hu, Y.; Shi, J. Metabolomics Profiles Reveal New Insights of Herpes Simplex Virus Type 1 Infection. Int. J. Mol. Sci. 2023, 24, 1521–1521. [CrossRef]
- Lange, P.; Lagunoff, M.; Tarakanova, V. Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism. Viruses 2019, 11, 119. [CrossRef]
- Marcocci, M.E.; Napoletani, G.; Protto, V.; Kolesova, O.; Piacentini, R.; Li Puma, D.D.; Lomonte, P.; Grassi, C.; Palamara, A.T.; De Chiara, G. Herpes Simplex Virus-1 in the Brain: The Dark Side of a Sneaky Infection. Trends Microbiol. 2020, 28, 808–820. [CrossRef]
- Álvarez, G.; Aldudo, J.; Alonso, M.; Santana, S.; Valdivieso, F. Herpes Simplex Virus Type 1 Induces Nuclear Accumulation of Hyperphosphorylated Tau in Neuronal Cells. J. Neurosci. Res. 2012, 90, 1020–1029. [CrossRef]
- Santana, S.; Bullido, M.J.; Recuero, M.; Valdivieso, F.; Aldudo, J. Herpes Simplex Virus Type i Induces an Incomplete Autophagic Response in Human Neuroblastoma Cells. J. Alzheimers Dis. 2012, 30, 815–831. [CrossRef]
- Santana, S.; Recuero, M.; Bullido, M.J.; Valdivieso, F.; Aldudo, J. Herpes Simplex Virus Type I Induces the Accumulation of Intracellular β-Amyloid in Autophagic Compartments and the Inhibition of the Non-Amyloidogenic Pathway in Human Neuroblastoma Cells. Neurobiol. Aging 2012, 33, 430.e19-430.e33. [CrossRef]
- Salgado, B.; Sastre, I.; Bullido, M.J.; Aldudo, J. Herpes Simplex Virus Type 1 Induces AD-like Neurodegeneration Markers in Human Progenitor and Differentiated ReNcell VM Cells. Microorganisms 2023, 11, 1205. [CrossRef]
- Kristen, H.; Sastre, I.; Muñoz-Galdeano, T.; Recuero, M.; Aldudo, J.; Bullido, M.J. The Lysosome System Is Severely Impaired in a Cellular Model of Neurodegeneration Induced by HSV-1 and Oxidative Stress. Neurobiol. Aging 2018, 68, 5–17. [CrossRef]
- Ball, M.J. “Limbic Predilection in Alzheimer Dementia: Is Reactivated Herpesvirus Involved?” Can. J. Neurol. Sci. 1982, 9, 303–306. [CrossRef]
- Jamieson, G.A.; Maitland, N.J.; Wilcock, G.K.; Craske, J.; Itzhaki, R.F. Latent Herpes Simplex Virus Type 1 in Normal and Alzheimer’s Disease Brains. J. Med. Virol. 1991, 33, 224–227. [CrossRef]
- Linard, M.; Letenneur, L.; Garrigue, I.; Doize, A.; Dartigues, J.F.; Helmer, C. Interaction between APOE4 and Herpes Simplex Virus Type 1 in Alzheimer’s Disease. Alzheimers Dement. 2020, 16, 200–208. [CrossRef]
- Whyte, L.S.; Lau, A.A.; Hemsley, K.M.; Hopwood, J.J.; Sargeant, T.J. Endo-Lysosomal and Autophagic Dysfunction: A Driving Factor in Alzheimer’s Disease? J. Neurochem. 2017, 140, 703–717. [CrossRef]
- Malik, B.R.; Maddison, D.C.; Smith, G.A.; Peters, O.M. Autophagic and Endo-Lysosomal Dysfunction in Neurodegenerative Disease. Mol. Brain 2019, 12. [CrossRef]
- Kristen, H.; Sastre, I.; Aljama, S.; Fuentes, M.; Recuero, M.; Frank-García, A.; Martin, A.; Sanchez-Juan, P.; Lage, C.; Bullido, M.J.; et al. LAMP2 Deficiency Attenuates the Neurodegeneration Markers Induced by HSV-1 Infection. Neurochem. Int. 2021, 146, 105032. [CrossRef]
- Wudiri, G.A.; Nicola, A.V. Cellular Cholesterol Facilitates the Postentry Replication Cycle of Herpes Simplex Virus 1. J. Virol. 2017, 91, e00445-17. [CrossRef]
- Staege, M.S.; Hutter, C.; Neumann, I.; Foja, S.; Hattenhorst, U.E.; Hansen, G.; Afar, D.; Burdach, S.E.G. DNA Microarrays Reveal Relationship of Ewing Family Tumors to Both Endothelial and Fetal Neural Crest-Derived Cells and Define Novel Targets. Cancer Res. 2004, 64, 8213–8221. [CrossRef]
- Rothaug, M.; Stroobants, S.; Schweizer, M.; Peters, J.; Zunke, F.; Allerding, M.; D’Hooge, R.; Saftig, P.; Blanz, J. LAMP-2 Deficiency Leads to Hippocampal Dysfunction but Normal Clearance of Neuronal Substrates of Chaperone-Mediated Autophagy in a Mouse Model for Danon Disease. Acta Neuropathol. Commun. 2015, 3, 6. [CrossRef]
- Burgos, J.S.; Ramirez, C.; Sastre, I.; Bullido, M.J.; Valdivieso, F. Involvement of Apolipoprotein E in the Hematogenous Route of Herpes Simplex Virus Type 1 to the Central Nervous System. J. Virol. 2002, 76, 12394–12398. [CrossRef]
- Porter, K.; Nallathambi, J.; Lin, Y.; Liton, P.B. Lysosomal Basification and Decreased Autophagic Flux in Oxidatively Stressed Trabecular Meshwork Cells. Autophagy 2013, 9, 581–594. [CrossRef]
- Bender, F.C.; Whitbeck, J.C.; Ponce De Leon, M.; Lou, H.; Eisenberg, R.J.; Cohen, G.H. Specific Association of Glycoprotein B with Lipid Rafts during Herpes Simplex Virus Entry. J. Virol. 2003, 77, 9542–9552. [CrossRef]
- Kristen, H.; Santana, S.; Sastre, I.; Recuero, M.; Bullido, M.J.; Aldudo, J. Herpes Simplex Virus Type 2 Infection Induces AD-like Neurodegeneration Markers in Human Neuroblastoma Cells. Neurobiol. Aging 2015, 36, 2737–2747. [CrossRef]
- Gao, Y.; Ye, S.; Tang, Y.; Tong, W.; Sun, S. Brain Cholesterol Homeostasis and Its Association with Neurodegenerative Diseases. Neurochem. Int. 2023, 171, 105635. [CrossRef]
- Wang, Y.; Gao, L. Cholesterol: A Friend to Viruses. Int. Rev. Immunol. 2024, 1–15. [CrossRef]
- Llorente, P.; Mejías, V.; Sastre, I.; Recuero, M.; Aldudo, J.; Bullido, M.J. Matrix Metalloproteinase 14 Regulates HSV-1 Infection in Neuroblastoma Cells. Antiviral Res. 2021, 192, 105116. [CrossRef]
- Recuero, M.; Vicente, M.C.; Martínez-García, A.; Ramos, M.C.; Carmona-Saez, P.; Sastre, I.; Aldudo, J.; Vilella, E.; Frank, A.; Bullido, M.J.; et al. A Free Radical-generating System Induces the Cholesterol Biosynthesis Pathway: A Role in Alzheimer’s Disease. Aging Cell 2009, 8, 128–139. [CrossRef]
- Leblanc, P.; Vorberg, I.M. Viruses in Neurodegenerative Diseases: More than Just Suspects in Crimes. PLOS Pathog. 2022, 18, e1010670. [CrossRef]
- Omasta, B.; Tomaskova, J. Cellular Lipids—Hijacked Victims of Viruses. Viruses 2022, 14, 1896. [CrossRef]
- Hajjar, D.P.; Pomerantz, K.B.; Falcone, D.J.; Weksler, B.B.; Grant, A.J. Herpes Simplex Virus Infection in Human Arterial Cells. Implications in Arteriosclerosis. J. Clin. Invest. 1987, 80, 1317–1321. [CrossRef]
- Hsu, H.Y.; Nicholson, A.C.; Pomerantz, K.B.; Kaner, R.J.; Hajjar, D.P. Altered Cholesterol Trafficking in Herpesvirus-Infected Arterial Cells. Evidence for Viral Protein Kinase-Mediated Cholesterol Accumulation. J Biol Chem 1995, 270, 19630–19637. [CrossRef]
- Braga, S.S. Molecular Mind Games: The Medicinal Action of Cyclodextrins in Neurodegenerative Diseases. Biomolecules 2023, 13, 666. [CrossRef]
- Dai, S.; Dulcey, A.E.; Hu, X.; Wassif, C.A.; Porter, F.D.; Austin, C.P.; Ory, D.S.; Marugan, J.; Zheng, W. Methyl-β-Cyclodextrin Restores Impaired Autophagy Flux in Niemann-Pick C1-Deficient Cells through Activation of AMPK. Autophagy 2017, 13, 1435–1451. [CrossRef]
- Zidovetzki, R.; Levitan, I. Use of Cyclodextrins to Manipulate Plasma Membrane Cholesterol Content: Evidence, Misconceptions and Control Strategies. Biochim Biophys Acta 2007, 1768, 1311–1324. [CrossRef]
- Rahn, E.; Petermann, P.; Hsu, M.-J.; Rixon, F.J.; Knebel-Mörsdorf, D. Entry Pathways of Herpes Simplex Virus Type 1 into Human Keratinocytes Are Dynamin- and Cholesterol-Dependent. PLoS ONE 2011, 6, e25464. [CrossRef]
- Davidson, C.D.; Ali, N.F.; Micsenyi, M.C.; Stephney, G.; Renault, S.; Dobrenis, K.; Ory, D.S.; Vanier, M.T.; Walkley, S.U. Chronic Cyclodextrin Treatment of Murine Niemann-Pick C Disease Ameliorates Neuronal Cholesterol and Glycosphingolipid Storage and Disease Progression. PloS One 2009, 4, e6951. [CrossRef]
- Cermak, S.; Kosicek, M.; Mladenovic-Djordjevic, A.; Smiljanic, K.; Kanazir, S.; Hecimovic, S. Loss of Cathepsin B and L Leads to Lysosomal Dysfunction, NPC-Like Cholesterol Sequestration and Accumulation of the Key Alzheimer’s Proteins. PLOS ONE 2016, 11, e0167428. [CrossRef]
- Barbero-Camps, E.; Roca-Agujetas, V.; Bartolessis, I.; De Dios, C.; Fernández-Checa, J.C.; Marí, M.; Morales, A.; Hartmann, T.; Colell, A. Cholesterol Impairs Autophagy-Mediated Clearance of Amyloid Beta While Promoting Its Secretion. Autophagy 2018, 14, 1129–1154. [CrossRef]
- Van Der Kant, R.; Langness, V.F.; Herrera, C.M.; Williams, D.A.; Fong, L.K.; Leestemaker, Y.; Steenvoorden, E.; Rynearson, K.D.; Brouwers, J.F.; Helms, J.B.; et al. Cholesterol Metabolism Is a Druggable Axis That Independently Regulates Tau and Amyloid-β in iPSC-Derived Alzheimer’s Disease Neurons. Cell Stem Cell 2019, 24, 363-375.e9. [CrossRef]
- Yao, J.; Ho, D.; Calingasan, N.Y.; Pipalia, N.H.; Lin, M.T.; Beal, M.F. Neuroprotection by Cyclodextrin in Cell and Mouse Models of Alzheimer Disease. J. Exp. Med. 2012, 209, 2501–2513. [CrossRef]
| Primary Antibodies | Species | Dilution | Reference | |
| HSV-1 infection | ICP4 | Mouse | 1/1000 (WB) 1/100 (IF) |
Abcam ab6514 |
| gC | Mouse | 1/3000 (WB) 1/300 (IF) |
Abcam ab6509 | |
| Neurodegeneration markers | Aβ40 | Rabbit | 1/100 (IF) | Invitrogen 44348A |
| Aβ42 | Rabbit | 1/100 (IF) | Invitrogen 44-344 | |
| p-Tau Thr205 | Rabbit | 1/250 (WB) 1/50 (IF) |
Invitrogen 44-738G | |
| p-Tau Ser422 | Rabbit | 1/250 (WB) 1/50 (IF) |
Invitrogen 44-764G | |
| Autophagy-lysosomal pathway | EEA1 | Mouse | 1/1000 (WB) 1/100 (IF) |
BD Biosciences 610457 |
| Human LAMP2 | Mouse | 1/1000 (WB) 1/50 (IF) |
DSHB H4B4 | |
| CD222 | Mouse | 1/100 (IF) | BioLegend 315902 | |
| LC3B | Rabbit | 1/500 (WB) 1/100 (IF) |
Sigma L7543 | |
|
Housekeeping protein |
α-Tubulin | Mouse | 1/10000 (WB) | Sigma T5168 |
| Secondary Antibodies | Species | Dilution | Reference | |
| Mouse-POD | Horse | 1/25000 (WB) | Vector PI-2000 | |
| Rabbit-POD | Goat | 1/25000 (WB) | Nordic GAR/IgG (H+L)/PO | |
| Alexa-555 anti-mouse | Goat | 1/1000 (IF) | Thermo Fisher A-21137 |
|
| Alexa-488 anti-rabbit | Donkey | 1/1000 (IF) | Thermo Fisher A-21206 |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





