Submitted:
16 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Field trials
2.2. Controlled-Condition Experiments
2.3. Statistical Analysis
3. Results
3.1. Field trials
3.1.1. Field Trials – Replacement System
3.1.2. Field Trials – Addition System
Controlled-Condition Experiments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Ditzler, L.; van Apeldoorn, D.F.; Pellegrini, F.; Antichi, D.; Bàrberi, P.; Rossing, W.A.H. Current research on the ecosystem service potential of legume inclusive cropping systems in Europe. A review. Agronomy for Sustainable Development 2021, 41, 26. [Google Scholar] [CrossRef]
- Rubiales, D.; Fondevilla, S.; Chen, W.D.; Gentzbittel, L.; Higgins, T.J.V.; Castillejo, M.A.; Singh, K.B.; Rispail, N. Achievements and Challenges in Legume Breeding for Pest and Disease Resistance. Crit. Rev. Plant Sci. 2015, 34, 195–236. [Google Scholar] [CrossRef]
- Plaza, M.E.H.; Bastida, F.; Gibson, D.J.; Barro, F.; Giménez, M.J.; Pallavicini, Y.; Izquierdo, J.; González-Andújar, J.L. Grain Quality as Influenced by the Structural Properties of Weed Communities in Mediterranean Wheat Crops. 2022. [Google Scholar] [CrossRef]
- Kumar, S.; Bhowmick, M.; Ray, P. Weeds as alternate and alternative hosts of crop pests. INDIAN JOURNAL OF WEED SCIENCE 2021, 53, 14–29. [Google Scholar] [CrossRef]
- Dentika, P.; Ozier-Lafontaine, H.; Penet, L. Weeds as Pathogen Hosts and Disease Risk for Crops in the Wake of a Reduced Use of Herbicides: Evidence from Yam (Dioscorea alata) Fields and Colletotrichum Pathogens in the Tropics. Journal of fungi (Basel, Switzerland) 2021, 7. [Google Scholar] [CrossRef] [PubMed]
- Carton, N.; Naudin, C.; Piva, G.; Baccar, R.; Corre-Hellou, G. Differences for traits associated with early N acquisition in a grain legume and early complementarity in grain legume-triticale mixtures. Aob Plants 2018, 10. [Google Scholar] [CrossRef]
- Duke, S.O. Why have no new herbicide modes of action appeared in recent years? Pest Management Science 2012, 68, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Avola, G.; Tuttobene, R.; Gresta, F.; Abbate, V. Weed control strategies for grain legumes. Agronomy for Sustainable Development 2008, 28, 389–395. [Google Scholar] [CrossRef]
- Pannacci, E.; Tei, F.; Guiducci, M. Evaluation of mechanical weed control in legume crops. Crop Protection 2018, 104, 52–59. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. FAOSTAT Statistical Database. 2020.
- Karkanis, A.; Ntatsi, G.; Lepse, L.; Fernandez, J.A.; Vagen, I.M.; Rewald, B.; Alsina, I.; Kronberga, A.; Balliu, A.; Olle, M.; et al. Faba Bean Cultivation - Revealing Novel Managing Practices for More Sustainable and Competitive European Cropping Systems. Frontiers in Plant Science 2018, 9. [Google Scholar] [CrossRef]
- Huss, C.P.; Holmes, K.D.; Blubaugh, C.K. Benefits and Risks of Intercropping for Crop Resilience and Pest Management. Journal of Economic Entomology 2022, 115, 1350–1362. [Google Scholar] [CrossRef]
- Weerarathne, L.V.Y.; Marambe, B.; Chauhan, B.S. Does intercropping play a role in alleviating weeds in cassava as a non-chemical tool of weed management? - A review. Crop Protection 2017, 95, 81–88. [Google Scholar] [CrossRef]
- Flores-Sanchez, D.; Pastor, A.; Lantinga, E.A.; Rossing, W.A.H.; Kropff, M.J. Exploring Maize-Legume Intercropping Systems in Southwest Mexico. Agroecology and Sustainable Food Systems 2013, 37, 739–761. [Google Scholar] [CrossRef]
- Banik, P.; Midya, A.; Sarkar, B.K.; Ghose, S.S. Wheat and chickpea intercropping systems in an additive series experiment: Advantages and weed smothering. European Journal of Agronomy 2006, 24, 325–332. [Google Scholar] [CrossRef]
- Begna, S.H.; Fielding, D.J.; Tsegaye, T.; Van Veldhuizen, R.; Angadi, S.; Smith, D.L. Intercropping of oat and field pea in Alaska: An alternative approach to quality forage production and weed control. Acta Agriculturae Scandinavica Section B-Soil and Plant Science 2011, 61, 235–244. [Google Scholar] [CrossRef]
- Corre-Hellou, G.; Dibet, A.; Hauggaard-Nielsen, H.; Crozat, Y.; Gooding, M.; Ambus, P.; Dahlmann, C.; von Fragstein, P.; Pristeri, A.; Monti, M.; et al. The competitive ability of pea-barley intercrops against weeds and the interactions with crop productivity and soil N availability. Field Crops Research 2011, 122, 264–272. [Google Scholar] [CrossRef]
- Sharma, G.; Shrestha, S.; Kunwar, S.; Tseng, T. Crop Diversification for Improved Weed Management: A Review. Agriculture-Basel 2021, 11. [Google Scholar] [CrossRef]
- Kemper, R.; Rinke, N.; Gerhards, R.; Böhm, H. Weed suppression and crop yield performance in sole and intercrops of common vetch and spring wheat depending on seed density ratio in organic farming. Journal fur Kulturpflanzen 2020, 72, 12–24. [Google Scholar] [CrossRef]
- Lithourgidis, A.S.; Dordas, C.A.; Damalas, C.A.; Vlachostergios, D.N. Annual intercrops: an alternative pathway for sustainable agriculture. Australian Journal of Crop Science 2011, 5, 396–410. [Google Scholar]
- Boudreau, M.A. Diseases in Intercropping Systems. In Annual Review of Phytopathology, Vol 51, VanAlfen, N.K., Ed.; Annual Review of Phytopathology; 2013; Volume 51, pp. 499–519.
- Khamare, Y.; Chen, J.J.; Marble, S.C. Allelopathy and its application as a weed management tool: A review. Frontiers in Plant Science 2022, 13. [Google Scholar] [CrossRef]
- Maver, M.; Miras-Moreno, B.; Lucini, L.; Trevisan, M.; Pii, Y.; Cesco, S.; Mimmo, T. New insights in the allelopathic traits of different barley genotypes: Middle Eastern and Tibetan wild-relative accessions vs. cultivated modern barley. PloS one 2020, 15, e0231976. [Google Scholar] [CrossRef] [PubMed]
- Belz, R.G. Allelopathy in crop/weed interactions - an update. Pest Management Science 2007, 63, 308–326. [Google Scholar] [CrossRef]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates, Inc.: New Jersey, 1988. [Google Scholar]
- Dhima, K.; Vasilakoglou, I.; Gatsis, T.; Gougoulias, N. Faba bean-barley intercrops for high productivity and corn poppy suppression. Experimental Agriculture 2016, 54, 163–180. [Google Scholar] [CrossRef]
- Eskandari, H. Intercropping of wheat (Triticum aestivum) and bean (Vicia faba): Effects of complementarity and competition of intercrop components in resource consumption on dry matter production and weed growth. African Journal of Biotechnology 2011, 10, 17755–17762. [Google Scholar] [CrossRef]
- Boutagayout, A.; Belmalha, S.; Nassiri, L.; el Alami, N.; Jiang, Y.; Lahlali, R.; Bouiamrine, E.H. Weed competition, land equivalent ratio and yield potential of faba bean (Vicia faba L.)-cereals (Triticum aestivum L. and/or Avena sativa L.) intercropping under low-input conditions in Meknes region, Morocco. Vegetos. [CrossRef]
- Fernandez-Aparicio, M.; Sillero, J.C.; Rubiales, D. Intercropping with cereals reduces infection by Orobanche crenata in legumes. Crop Protection 2007, 26, 1166–1172. [Google Scholar] [CrossRef]
- Villegas-Fernandez, A.M.; Amarna, A.A.; Moral, J.; Rubiales, D. Crop Diversification to Control Rust in Faba Bean Caused by Uromyces viciae-fabae. Journal of Fungi 2023, 9. [Google Scholar] [CrossRef] [PubMed]
- Gu, C.; Bastiaans, L.; Anten, N.P.R.; Makowski, D.; van der Werf, W. Annual intercropping suppresses weeds: A meta-analysis. Agriculture Ecosystems & Environment 2021, 322. [Google Scholar] [CrossRef]
- Poggio, S.L.; Satorre, E.H.; de la Fuente, E.B. Structure of weed communities occurring in pea and wheat crops in the Rolling Pampa (Argentina). Agriculture, Ecosystems & Environment 2004, 103, 225–235. [Google Scholar] [CrossRef]
- Stefan, L.; Engbersen, N.; Schöb, C. Crop–weed relationships are context-dependent and cannot fully explain the positive effects of intercropping on yield. Ecological Applications 2021, 31. [Google Scholar] [CrossRef]
- Swanton, C.; Ondoua, R.; Blackshaw, R.E. Experimental methods for crop-weed competition studies. Weed Science 2015, 63, 2–11. [Google Scholar] [CrossRef]
- Singh, M.; Kukal, M.S.; Irmak, S.; Jhala, A.J. Water Use Characteristics of Weeds: A Global Review, Best Practices, and Future Directions. Frontiers in Plant Science 2022, 12. [Google Scholar] [CrossRef]
- Lemerle, D.; Cousens, R.; Gill, G.; Peltzer, S.; Moerkerk, M.; Murphy, C.; Collins, D.; Cullis, B. Reliability of higher seeding rates of wheat for increased competitiveness with weeds in low rainfall environments. The Journal of Agricultural Science 2004, 142. [Google Scholar] [CrossRef]
- Korav, S.; Dhaka, A.; Singh, R.; Reddy, G. A study on crop weed competition in field crops. 2018, 7, 3235–3240.
- Jha, P.; Kumar, V.; Godara, R.; Chauhan, B. Weed management using crop competition in the United States: A review. Crop Protection 2017, 95, 31–37. [Google Scholar] [CrossRef]
- Bertholdsson, N.O. Early vigour and allelopathy - Two useful traits for enhanced barley and wheat competitiveness against weeds. Weed Research 2005, 45, 94–102. [Google Scholar] [CrossRef]
- Dhima, K.; Vasilakoglou, I.; Gatsis, T.; Eleftherohorinos, I. Competitive interactions of fifty barley cultivars with Avena sterilis and Asperugo procumbens. Field Crops Research 2010, 117, 90–100. [Google Scholar] [CrossRef]
- Jabran, K.; Mahajan, G.; Sardana, V.; Chauhan, B.S. Allelopathy for weed control in agricultural systems. Crop Protection 2015, 72, 57–65. [Google Scholar] [CrossRef]
- Kremer, R.; Ben-Hammouda, M. Allelopathic Plants. 19. Barley (Hordeum vulgare L). Allelopathy Journal 2009, 24, 225–242. [Google Scholar]
- Asghari, J.; Tewari, J.P. Allelopathic Potentials of Eight Barley Cultivars on Brassica jucea (L) Czern. and Setaria viridis (L) p. Beauv. Journal of Agricultural Science and Technology 2007, 9, 165–176. [Google Scholar]
- Copaja, S.; López, M.; Bravo, H. Dynamic of indole alkaloids in a soil and its relationships with allelopathic properties. Journal of the Chilean Chemical Society 2023, 68, 5787–5795. [Google Scholar] [CrossRef]
- Wu, H.; Pratley, J.; Lemerle, D.; Haig, T.; An, M. Screening methods for the evaluation of crop allelopathic potential. Botanical Review 2001, 67, 403–415. [Google Scholar] [CrossRef]
- Vasilakoglou, I.; Dhima, K.; Lithourgidis, A.; Eleftherohorinos, I. Allelopathic potential of 50 barley cultivars and the herbicidal effects of barley extract. Allelopathy Journal 2009, 24, 309–319. [Google Scholar]
- Modhej, A.; Rafatjoo, A.; Behdarvandi, B. Allelopathic inhibitory potential of some crop species (wheat, barley, canola, and safflower) and wild mustard (Sinapis arvensis). International Journal of Biosciences (IJB) 2013, 3, 212–220. [Google Scholar]
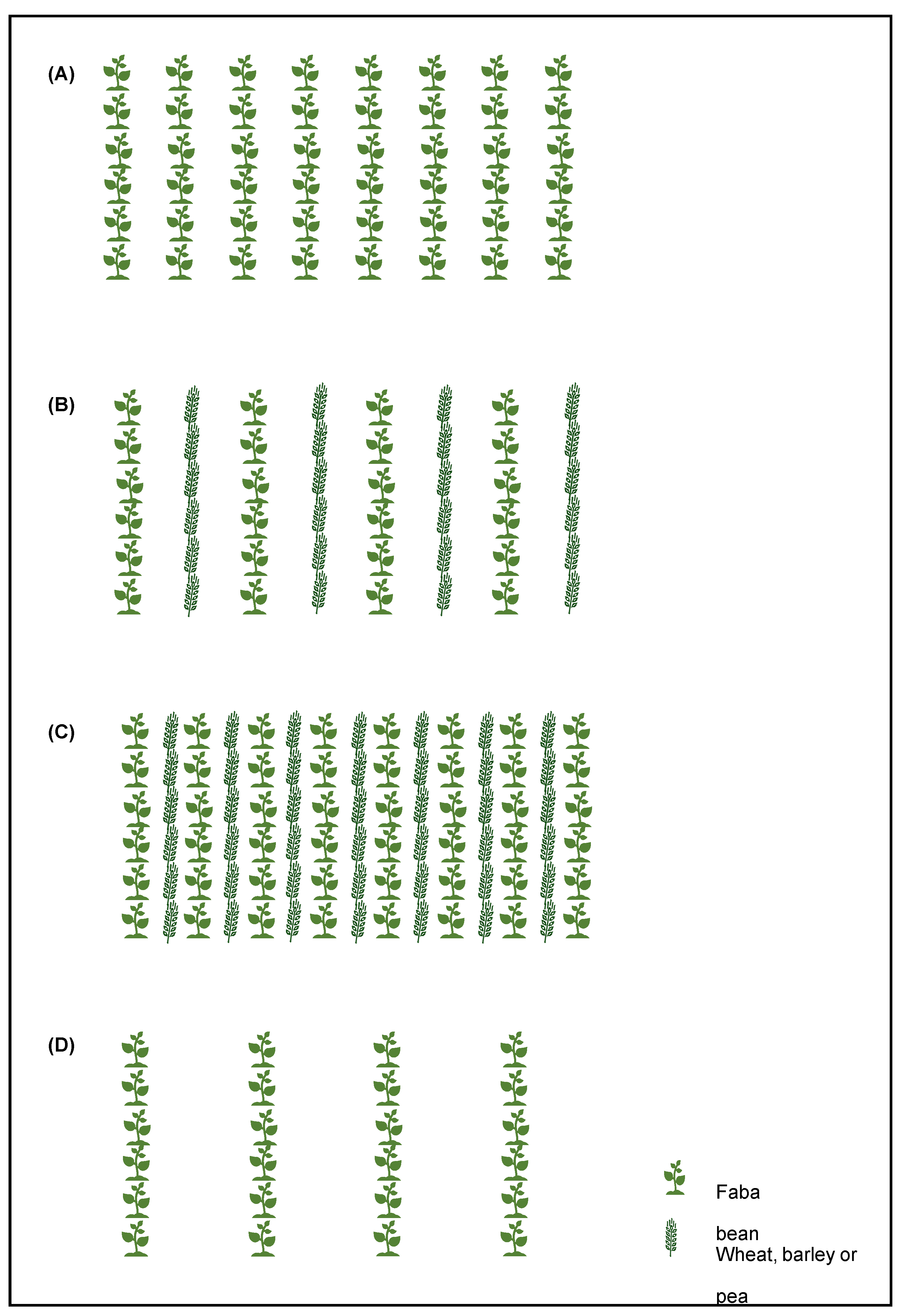
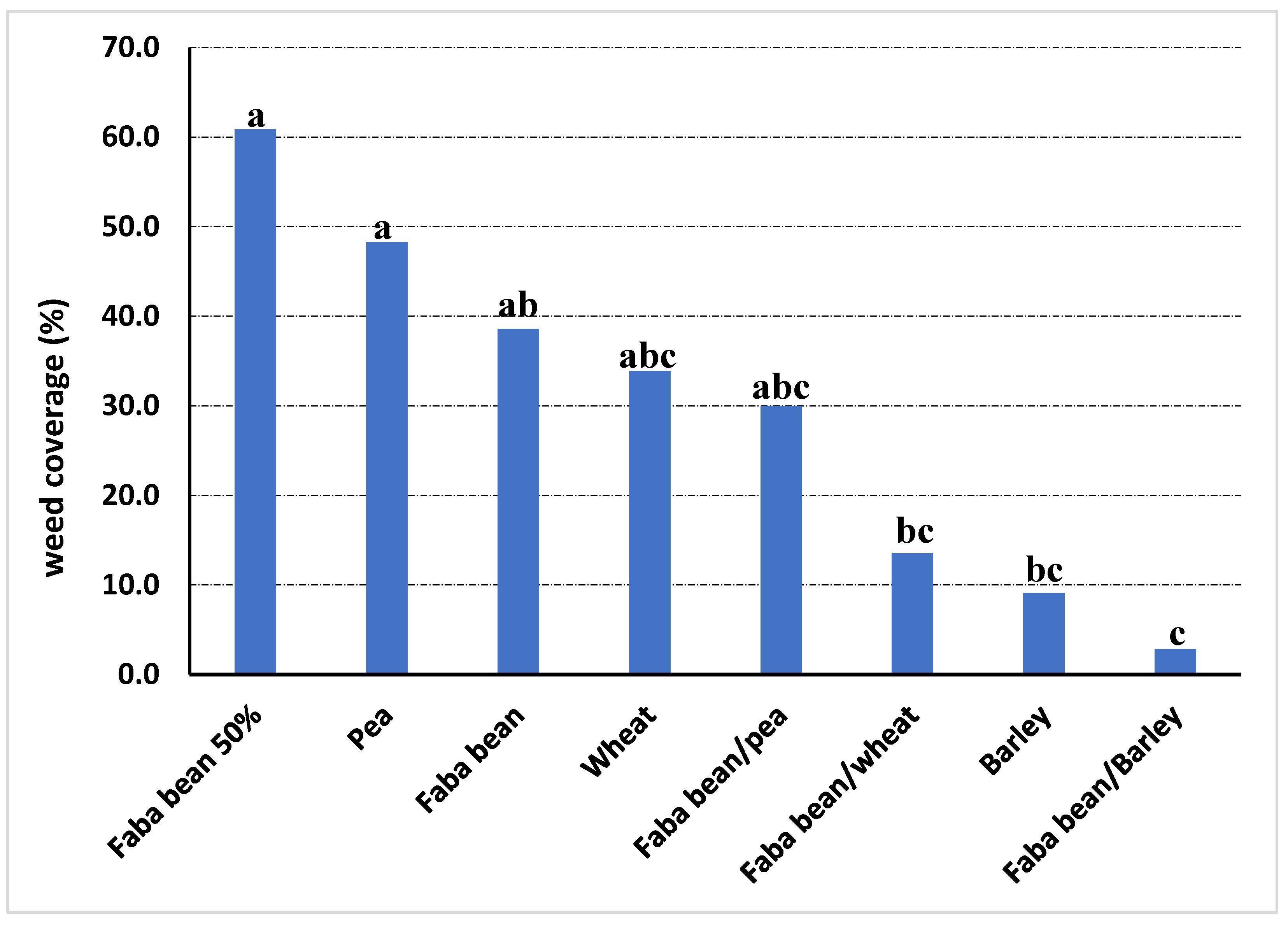
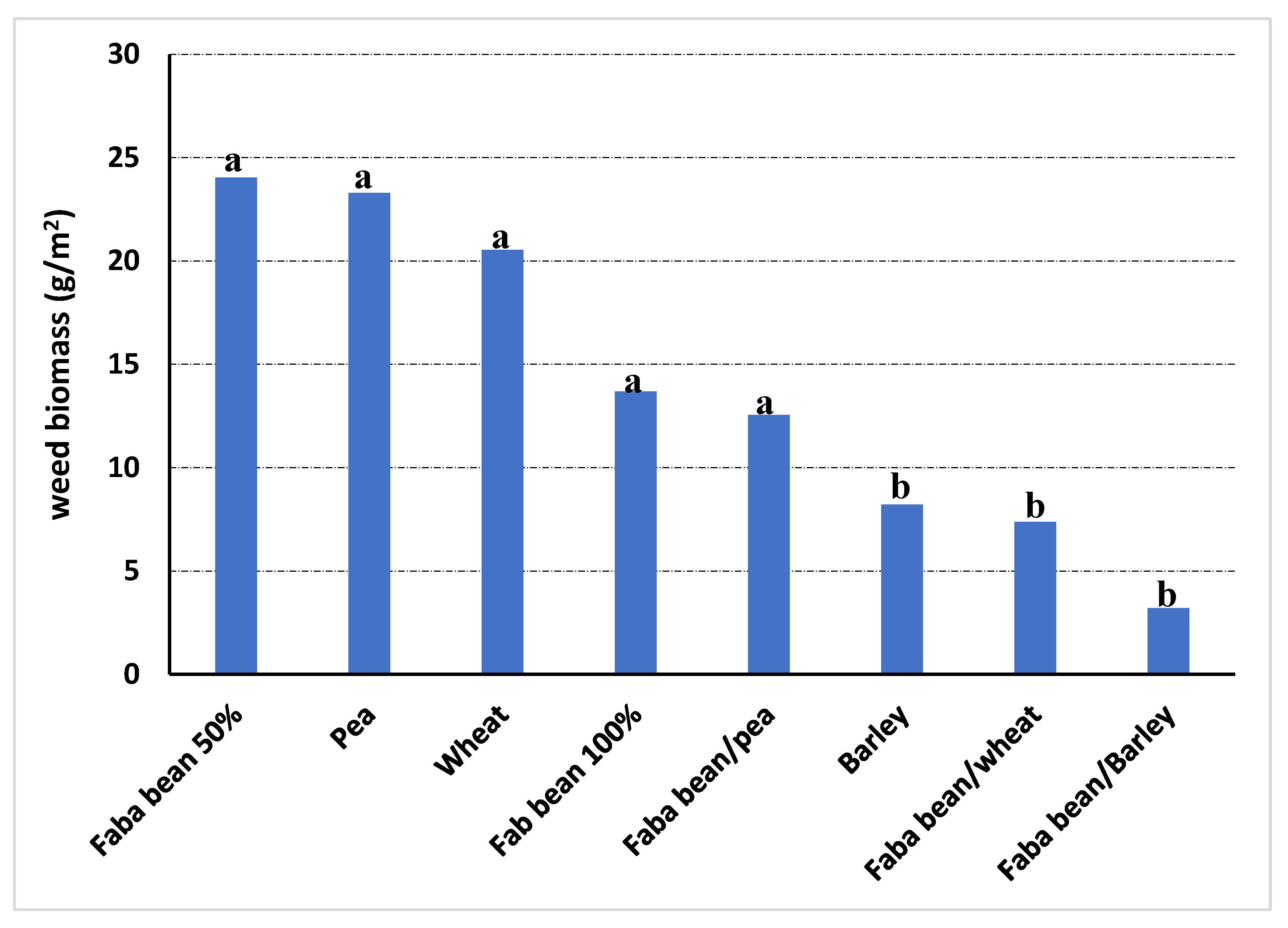
| Trial | Córdoba-15-r | Córdoba-16-r | Córdoba-16-a | Córdoba-17-a | Córdoba-18-a |
|---|---|---|---|---|---|
| Season | 2014/15 | 2015/16 | 2015/16 | 2016/17 | 2017/18 |
| Intercropping system | Replacement | Replacement | Addition | Addition | Addition |
| Maximum T (ºC) | 35.3 | 31.7 | 31.7 | 32.2 | 29.7 |
| Minimum T (ºC) | −3.3 | -2.3 | -2.3 | −3.4 | -3.4 |
| Mean T (ºC) | 12.3 | 12.6 | 12.6 | 14.3 | 12.0 |
| Precipitation (ml) | 150 | 336 | 336 | 143.8 | 444.6 |
| Córdoba-16 | Córdoba-17 | Córdoba-18 | |
|---|---|---|---|
| Amarantaceae | x | x | |
| Asteraceae | x | ||
| Convolvulaceae | x | x | x |
| Cruciferae | x | x | |
| Cyperaceae | x | ||
| Fabaceae | x | x | x |
| Papaveraceae | x | x | |
| Poaceae | x | x | |
| Polygonaceae | x | x | x |
| Quenopodiaceae | x |
| Richness (R) | |||
|---|---|---|---|
| Córdoba-16 | Córdoba-17 | Córdoba-18 | |
| Faba bean 100% | 35.7 a | 78.6 ab | 76.2 ab |
| Faba bean / barley | 25.0 a | 50.0 ab | 42.3 a |
| Faba bean /wheat | 25.0 a | 46.4 a | 61.9 ab |
| Faba bean / pea | 50.0 a | 64.3 ab | 85.7 b |
| Faba bean 50% | 46.4 a | 75.0 ab | 85.7 b |
| Barley 100% | 83.3 b | 76.2 ab | |
| Wheat 100% | 75.0 ab | 95.0 b | |
| Pea 100% | 83.3 b | 80.9 ab | |
| Barley removed | Barley not removed | |||
|---|---|---|---|---|
| Control | Barley sown | Control | Barley sown | |
| Polypogon monspeliensis | 19.9 a | 5.0 b | 19.7 a | 4.6 b |
| Matricaria chamomilla | 18.9 a | 5.9 b | 18.6 a | 5.1 b |
| Sinapis arvensis | 11.1 a | 3.8 b | 11.9 a | 2.7 b |
| Medicago truncatula | 5.2 a | 1.4 b | 7.8 a | 1.4 b |
| Barley removed | Barley not removed | |||
|---|---|---|---|---|
| Control | Barley sown | Control | Barley sown | |
| Polypogon monspeliensis | 39.9 a | 20.2 b | 55.5 a | 24.9 b |
| Matricaria chamomilla | 115.4 a | 49.0 b | 131.6 a | 25.6 b |
| Sinapis arvensis | 70.6 a | 41.7 b | 104.6 a | 10.1 b |
| Medicago truncatula | 16.3 a | 10.6 b | 35.0 a | 4.8 b |
| No of weeds (%) | Biomass of weeds (%) | |||
|---|---|---|---|---|
| Barley removed | Barley not removed | Barley removed | Barley not removed | |
| Polypogon monspeliensis | 25.2 a | 23.6 a | 70.1 a | 61.9 a |
| Matricaria chamomilla | 31.1 a | 27.3 a | 65.5 a | 43.7 b |
| Sinapis arvensis | 32.1 a | 23.0 a | 77.0 a | 29.3 b |
| Medicago truncatula | 24.9 a | 18.4 a | 58.9 a | 31.9 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).





