1. Introduction
Determining the quality of surface water reservoirs and their habitats is one of the most important tasks in both basic and applied science; the quality of surface water is one of the main determinants of economic and social development. Therefore, a number of tools have been developed to investigate or assess this factor. Originally, the assessment consisted of the determination of water quality classes and was based on hydrochemical studies (
REF). A major shortcoming of this method was that the results described the short-term conditions of the waters (at the time of measurement), giving either inaccurate results or requiring frequent repetition of measurements. Consequently, a number of methods based on the study of biological indicators were introduced: macrophyte method (vascular vegetation), diatom index (diatoms), macroinvertebrates (benthic macroinvertebrates). The River Habitat Survey (RHS) is a method for assessing the conditions of a river channel and valley, taking into account hydromorphological factors, and assessing the degree of naturalness or the degree of anthropogenic alteration within the channel and valley based on physical and landscape data [
1,
2,
3,
4,
5,
6,
7], thus complementing methods based on biological indicators.
The authors of this study started from the assumption that the quality of river habitats demonstrated using the RHS should translate into the composition of their fauna. Furthermore, the RHS method assesses the parameters of the river channel itself and the landscape parameters of the valley, which are often crucial for the migration of fauna along a river valley or between valleys (REF). To test this hypothesis, we used aquatic bugs (Heteroptera Aquatica), which are a group with considerable dispersal abilities and, at the same time, composed of stenotopic species associated with a particular environment, e.g. lotic waters, as well as of eurytopic elements inhabiting a wide range of environments (REF).
2. Materials and Methods
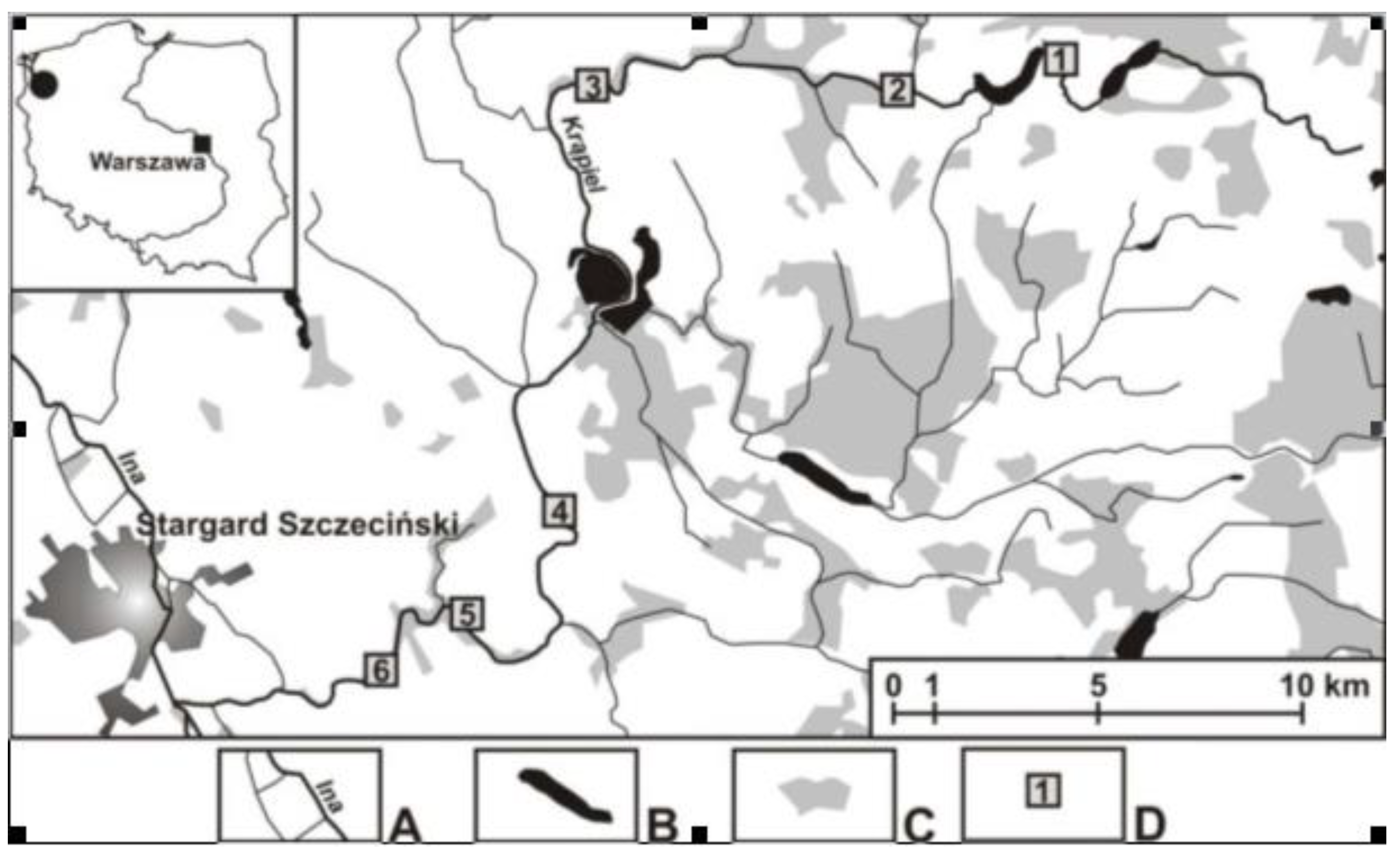
The small lowland river Krąpiel (north-western Poland), which was the areas of a number of ecological studies [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21,
31,
45], was sampled. Fieldwork was conducted from May till November, 2011. Samples were taken quarterly from April to October, at 6 sites with 43 sub-sites included (
Figure 1). At each site, surveys included a rapids-plo stream pool' system. The "rapids-stream pool" layout included all environments present: rapids - sandy, rocky, with vegetation; stream pool - sandy, silty, rocky, with vegetation. In a given layout, sites from the rapid were always located above sites from the stream pool. In the case of springs, different microhabitats (muddy, sandy, vegetated) and springs located at different distances from the river and in different vegetation communities were included.
A hydromorphological assessment of the river was also carried out for each of the study sites. The assessment was based on the River Habitat Survey (RHS) method, which is a standardised method providing results comparable to other surveys [
22]. For the purposes of this study, the RHS method was modified and the assessment was based on 100 m river sections instead of 500 m sections. It was considered that 500 m sections would cover too diverse a range of habitats, which would complicate interpretation of the results. On the basis of the field survey, the following indices were calculated [
22]:
Habitat Modification Score (HMS),
Habitat Quality Assessment (HQA),
Riverine Habitat Quality Index (RHQ),
Riverine Habitat Modification Index (RHM).
In addition, the following individual parameters were used: mineralised debris on the channel banks (BN), exposed boulders (OG), channel debris with plants (SU), complex vegetation structure on the top and slope of the bank (Z), fine gravel in the channel (Zr(S)).
The water parameters: temperature (temp.), pH, electrolytic conductivity (cond.) and dissolved oxygen (O2) content were measured with an Elmetron CX-401 multiparametric sampling probe; water flow (velocity) using a SonTek acoustic FlowTracker flowmeter; BOD5 by Winkler`s method, and the remaining parameters: hardness, NH4, NO3, PO3, Fe, turbidity (turbidite) with the help of Slandi LF205 photometer. Three measurements were performed every time and the median was used for further analyses.
The following parameters were used to describe the habitat: vegetation cover (plants), mineral substrate (mineral), organic substrate (organic), insolation (insolati), mean grain size (M), dispersion of grain parameter values (W).
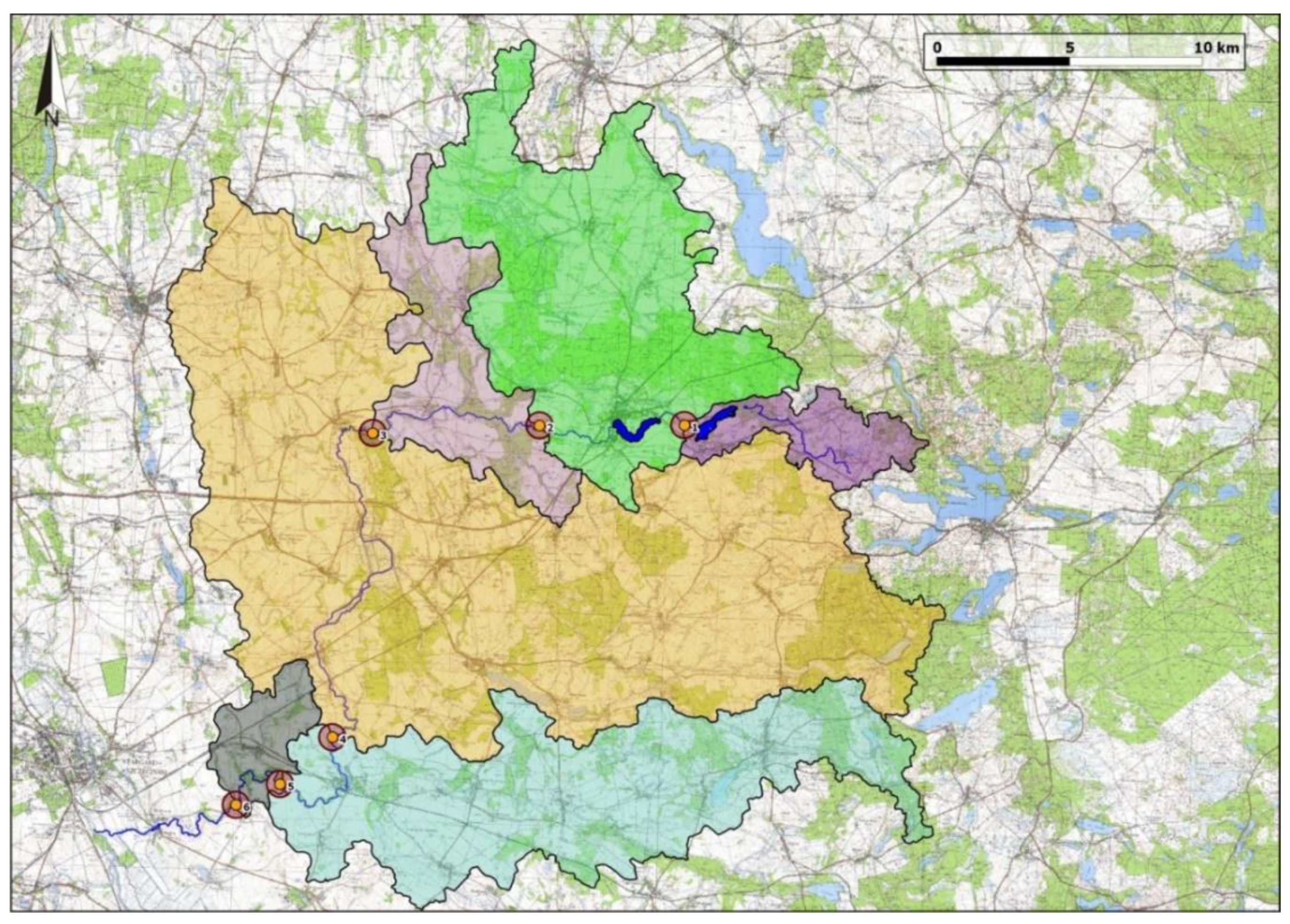
The landscape analysis was based on the buffer zones and catchment areas defined for each site (macrohabitat) (
Figure 2). The buffer zone was defined as a circle around the site with a radius of 500 m. The catchment of a site is defined as the catchment bounded by two consecutive sites (
Figure 2).
The analysis of the spatial structure of the buffer zones and catchments was based on a set of landscape metrics calculated in TNTmips software (MicroImagesTM). Classification was based on Landsat TM7 data 28-05-2003, boundaries and linear elements were based on map 1:10 000, classes according to the Corine classification - simplified in the case of meadows (meadows and pastures were combined due to the minimal share of grazing in the surveyed areas), after the initial classification by the isoclass method the extents and correctness of the separation of classes were verified in the field, the isoclass classification was repeated with the combination of classes according to the dendrogram, then the class boundaries were manually drawn in vector form; rivers, roads and drainage ditches appear in the form of polygons, minimum areas of separated patches were equal to ca. 200m. GPS was used to determine the coordinates of the sampling point - the area was delimited with a 500 m radius.
The following measures and indicators were used to analyse landscape structure: 1. measures of patch areas - area (AREA); 2. measures of patch density and size - number of patches (NUMP), mean patch size (MPS), median patch size (MEDPS), standard deviation of mean (PSSD), patch density (PD); 3. edge measures - total edge length (TE), edge density (ED), mean edge length (MTE); 4. Shape measures-mean shape index (MSI), mean patch fractal size (MPFD), sum of lobe shape indices (SUM); 5. Diversity and distribution indices -measure of mean distance to nearest neighbour (MNN), measure of boundary diversity (IJI), Shannon's index of patch diversity (SDI), index of spatial distribution and number of patches (SEI), catchment area measured from sources, catchment area, length of catchment boundaries, roughness (Ra), compactness (C), river gradient, distance from sources, distance of individual patches from the river (forests, fields, swamps, buildings, meadows, shrubland, wasteland and water).
After analysing the data, we selected only those landscape parameters, whose effects on faunal distribution were statistically significant.
3. Results
A total of 555 individuals of aquatic bugs belonging to 14 species were caught in the River Krąpiel (
Table 1).
The bugs collected in the Krąpiel River were classified into two synecological groups: rheophiles and stagnophiles. The rheophiles were by far the more abundant despite belonging to only three species (Aphelocheirus aestivalis, Aquarius najas, Velia caprai) and constituted 78.6% of the total collected material.
The DCA analysis for the distribution of sites and bug species showed that the length of the gradient represented by the first ordination axis ranks below 3.0, which mandates direct ordination analyses of the RDA type, in order to determine the relationship between species occurrence and the included environmental parameters [
23,
24].
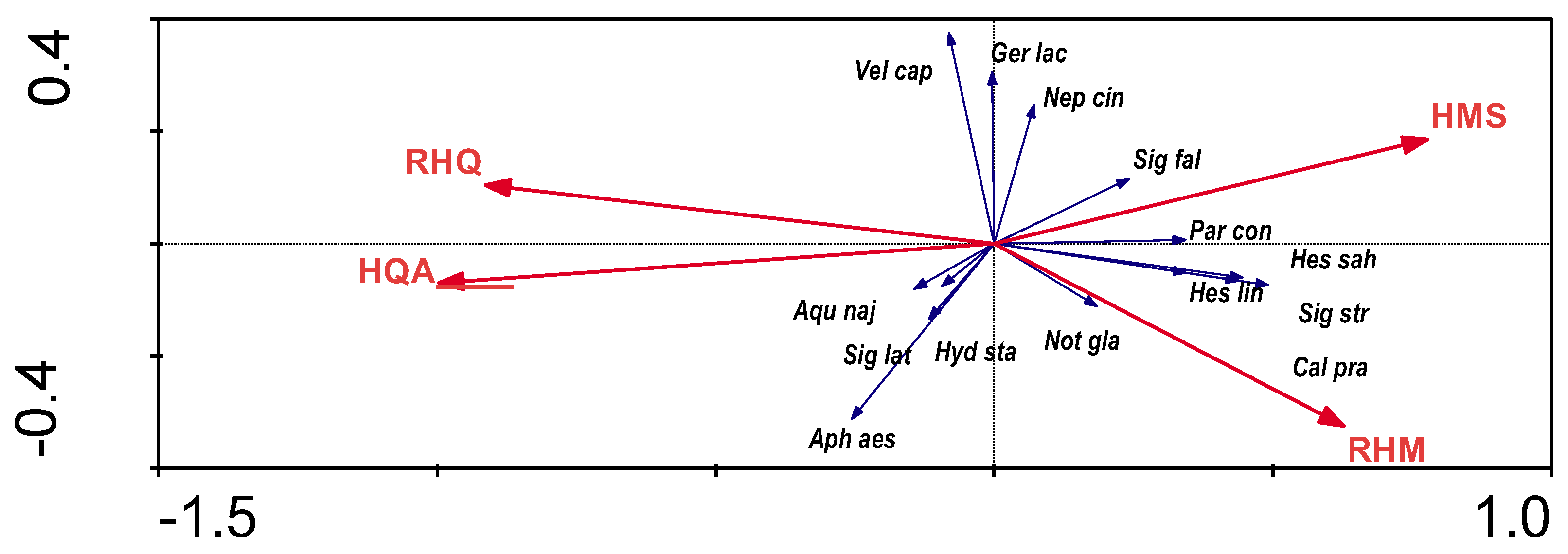
When analysing the RDA for the dependence of distribution of bugs on the characteristics of the hydromorphological indicators of the riverbed, only one parameter (HQA) was statistically significant (
Figure 3). The results of the direct RDA analysis indicate that the variables used in the ordination explain 15.1% of the total variation of species. The habitat quality index was positively correlated with presence of some rheophilic bug species and negatively correlated with presence of eurytopic species.
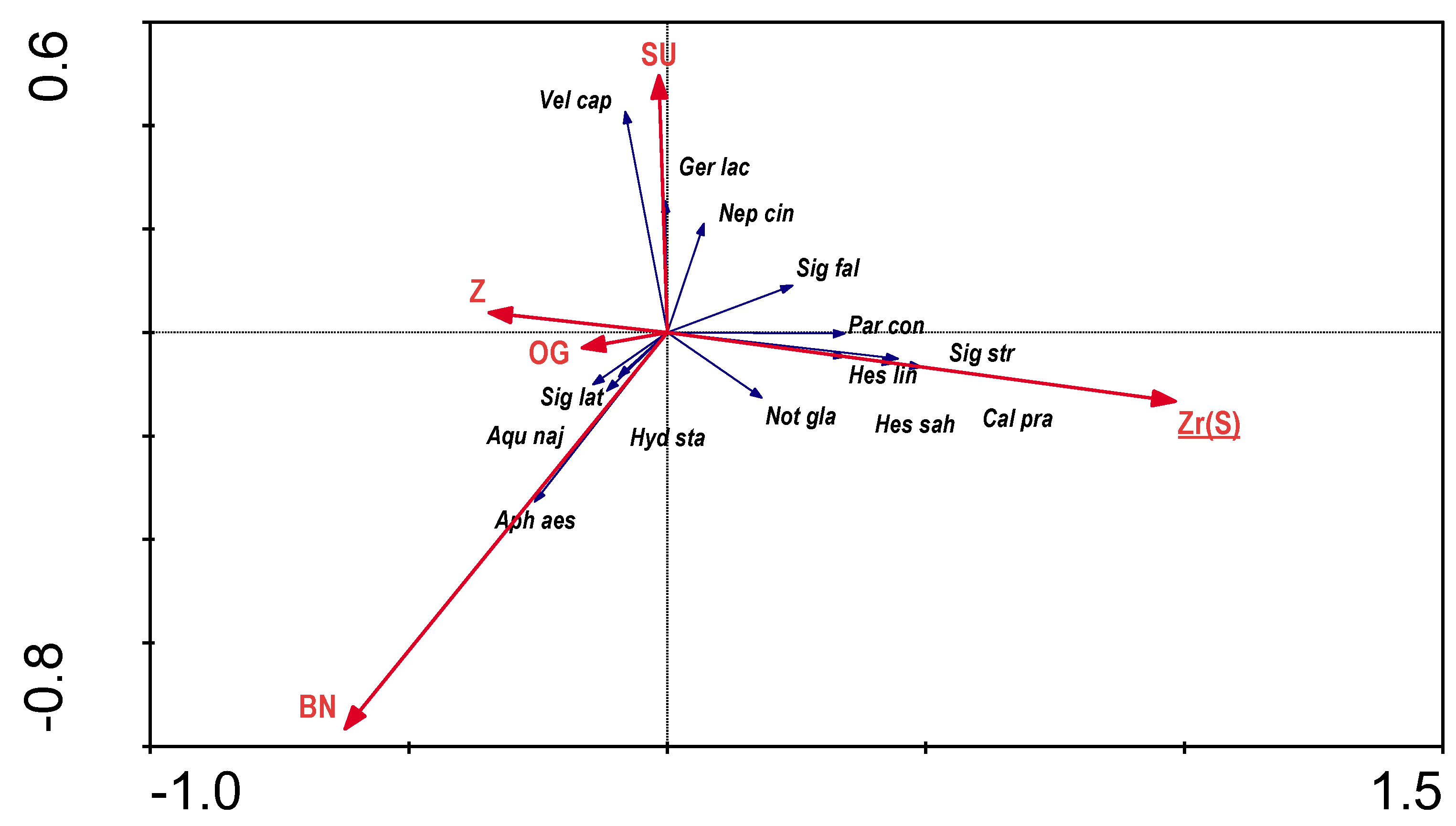
When performing the RDA for the dependence of presence of the aquatic bugs on hydromorphological characteristics of the river channel, colinearity of many variables was detected, so only five that were not colinear were used for further analyses: morphological elements of the channel: exposed boulders (OG), channel debris with plants (SU); bed material: gravel (Zr); morphological elements of the banks: bank debris without plants (BN); vegetation structure: complex (Z). Only one parameter (Zr(S)) was statistically significant (
Figure 4). The results of the direct RDA analysis indicate that the variables used in the ordination explain 16.8% of the total variation of species distribution.
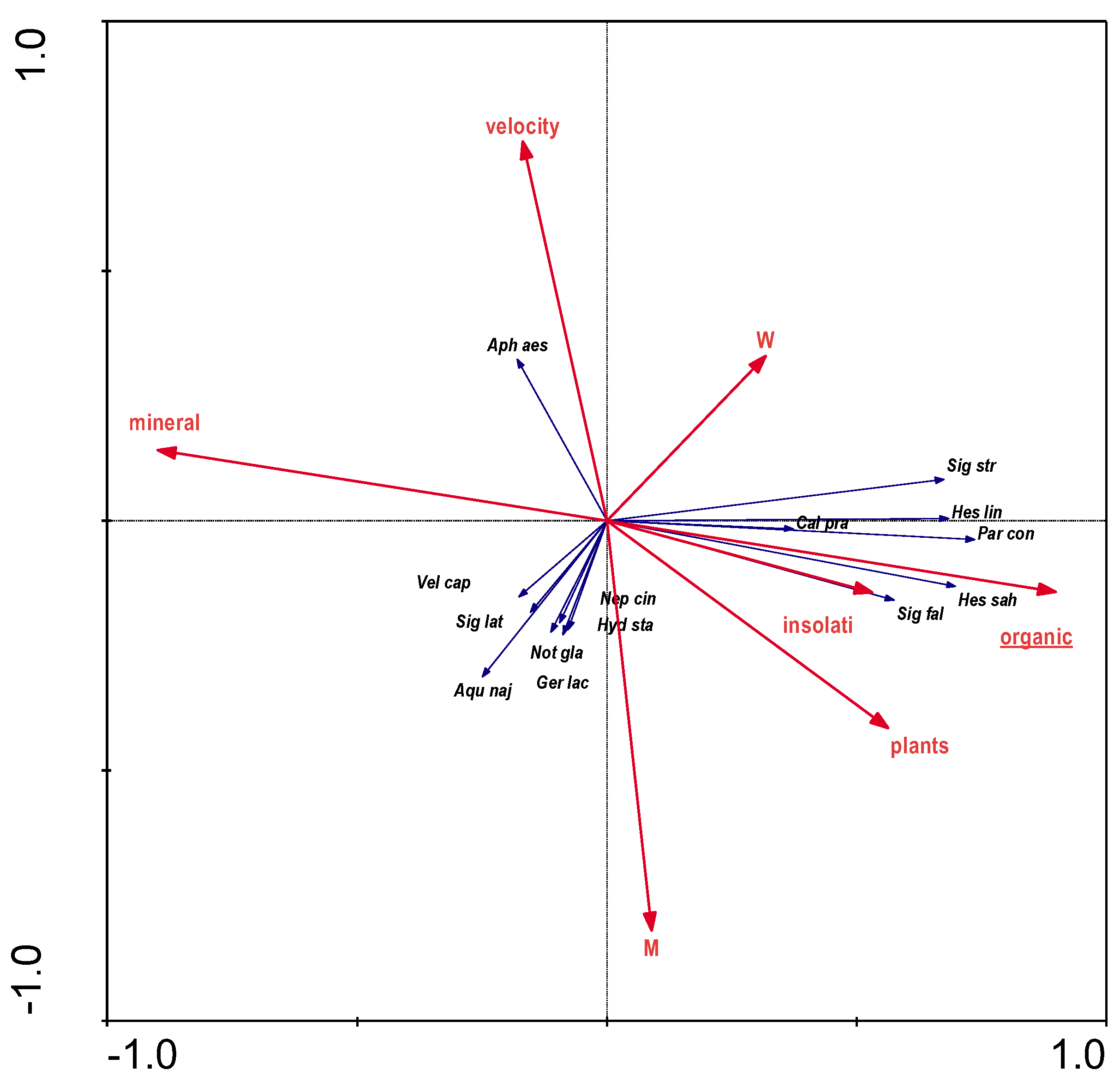
The results of direct RDA analysis for the distribution of bugs as a function of substrate structure, water current velocity, degree of overgrowth by aquatic plants and degree of sunshine indicate that the variables used in the ordination explain 26.2% of the total variation of species distribution. The results of the stepwise selection of environmental variables showed that one variable (organic) is statistically significant (
Figure 5) and is connected with Corixidae, which is related to their diet.
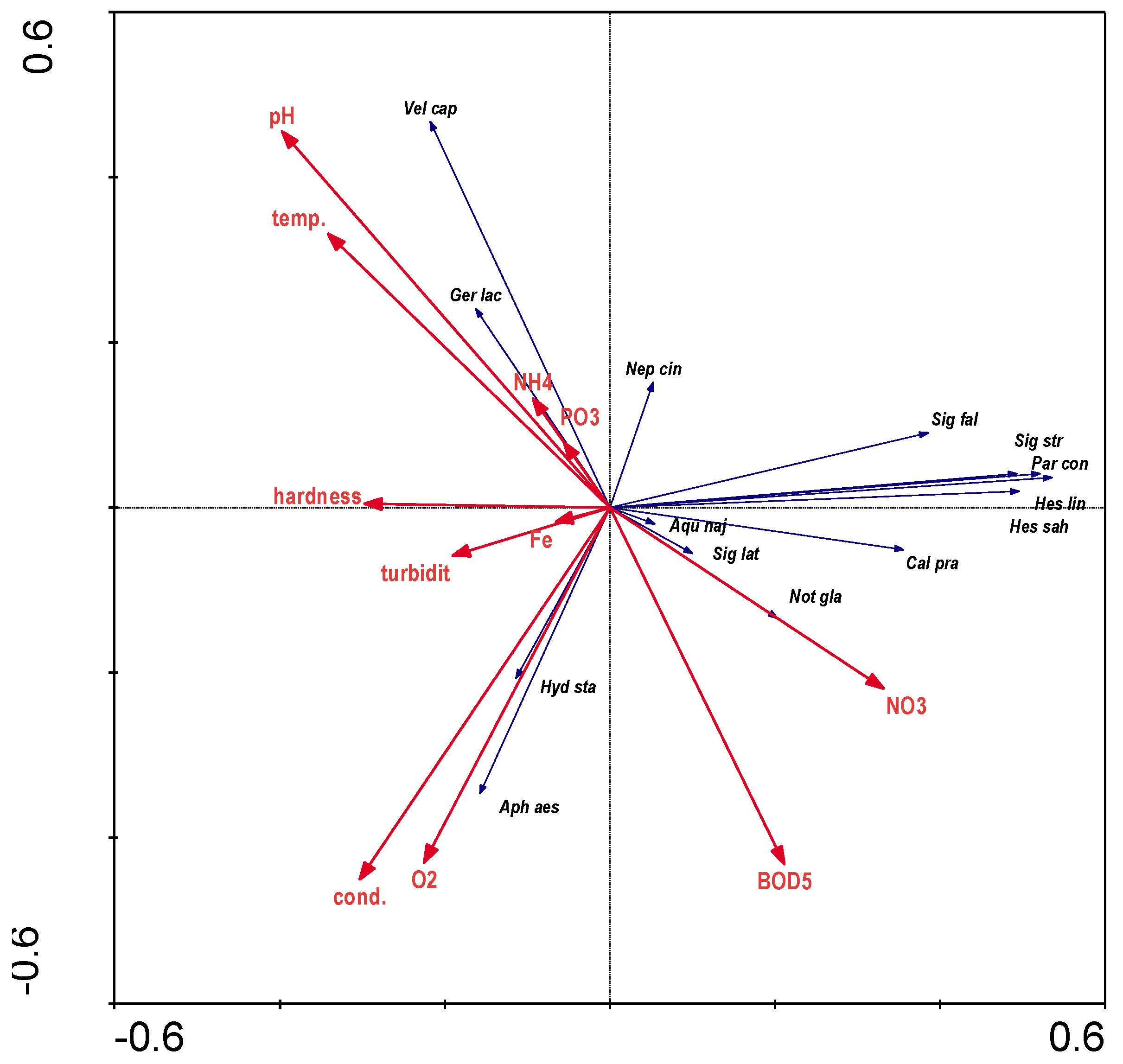
The results of direct RDA analysis for the distribution of bugs in relation to hydrochemical parameters indicate that the variables used in the ordination explained 26.5% of the total species variability. The results of stepwise selection of environmental variables showed that none of the variables explained the range of total species variation in a statistically significant way (p≤0.05) (
Figure 6). In contrast, a correlation can be observed between dissolved oxygen content and
Aphelocheirus aestivalis abundance.
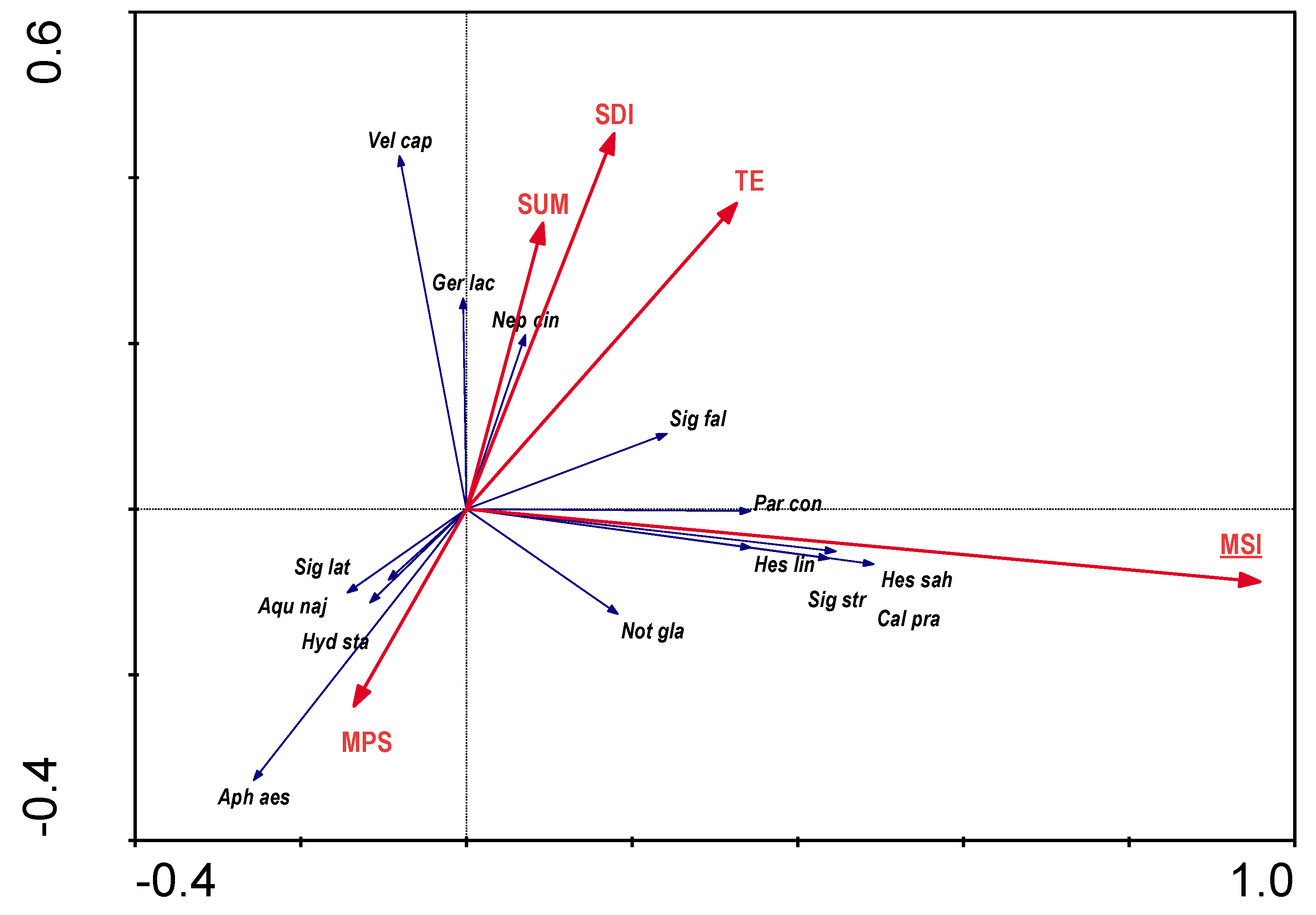
When analysing the RDA for the dependence of distribution of bugs on buffer zone characteristics, colinearity of many variables was detected, so only five variables that were not colinear were used for further analyses (
Figure 7). Only one parameter (MSI) was statistically significant. The variables used in the ordination explain 16.8% of the total variability. Mean shape index was clearly related to the stagnophilous Corixidae and
Notonecta glauca.
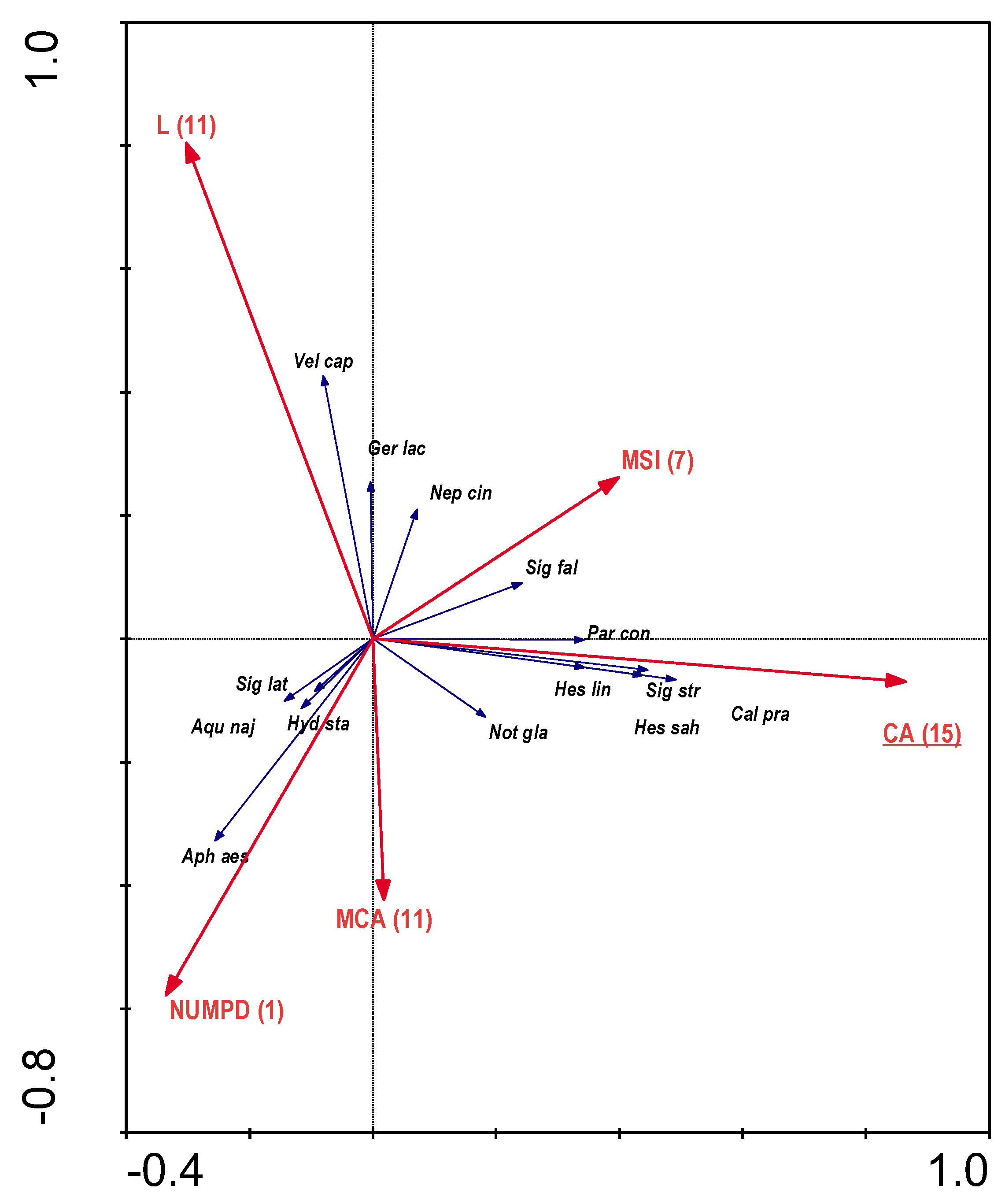
When analysing RDA for the dependence of the distribution bugs on the characteristics of individual patches in the buffer zones, colinearity of many variables was also detected, so that only five variables that were not colinear were used for further analyses. These were: total area of patches of a given class: willow thickets - CA (15); mean shape index: arable land - MSI (7); distance from survey point: deciduous forests - L (11); number of patches: compact development - NUMPD (1); mean patch size: deciduous forests - MCA (11) (statistically significant parameters (p≤0.05) were
bolded and
underlined in the figure) (
Figure 8). The variables used in the ordination explain 13.7% of the total variation. The abundance of Corixidae was associated to presence of willow thickets.
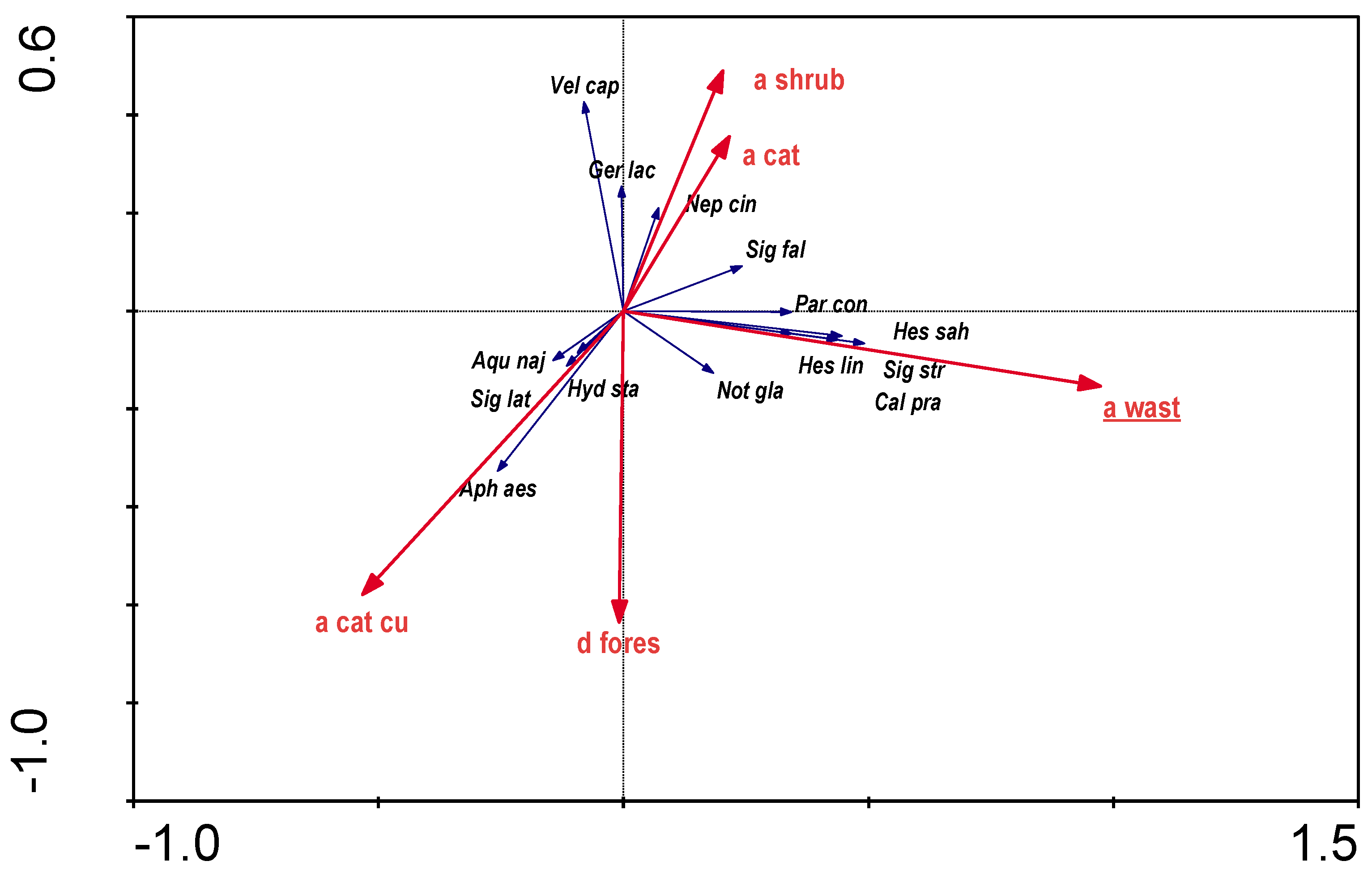
In the RDA analysis of the influence of catchment elements on the distribution of aquatic bugs, all parameters that did not show colinearity were considered. Parameters that were statistically significant (p≤0.05) are highlighted (
Figure 9). The variables used in the ordination explain 16.8% of the total variation. Presence of the stagnophilous Corixidae and
Notonecta glauca was associated with area of wastelands.
4. Discussion
Aquatic bugs are insects commonly found in both flowing and standing waters. Most species are eurytopic, so they inhabit a wide variety of habitats and their possible preferences are difficult to describe. In addition, these species are highly migratory, making them macroinvertebrates that colonise new habitats very quickly. However, they are often associated with a zone along river banks covered by natural vegetation and may therefore be indicative of the naturalness of the river channel, especially in its lower section. Unfavourable habitat conditions including, but not limited to, certain abiotic factors (e.g. high salinity, overfertilisation, low oxygen and others) and biotic factors (e.g. poor development of littoral vegetation, high predation pressure) may significantly reduce both abundance and species richness of aquatic bugs [
24,
25,
26,
27,
28,
29,
30,
31,
32,
33,
34,
35,
36]. On the other hand, there are also several stenotopic species of aquatic bugs, associated with relatively fast water current and having high oxygen-level demands, making them good indicators of clean running waters [
27,
38].
Three stenotopic species associated with running water were recorded in our study:
Aphelocheirus aestivalis,
Aquarius najas and
Velia caprai. In the Krąpiel River, presence of the first two species, together with
Hydrometra stagnorum and
Sigara lateralis, were associated with the middle and lower sections of the river (a cat cu -
Figure 8) in open areas (
Figure 8) and mineral debris on the banks of the riverbed (BN -
Figure 3; mineral -
Figure 4) and partly (
Aphelocheirus aestivalis,
Hydrometra stagnorum) with highly oxygenated water and increased mineralisation (O2 and cond.-
Figure 5). High values of electrolytic conductivity can significantly limit or even prevent the occurrence of many aquatic bug species, consequently significantly reducing species diversity. Such results were obtained e.g. in the fauna of oligohaline waters discharged from the Bogdanka coal mine [
25,
26]. Regarding landscape indicators, the species are associated with patches of natural, low vegetation with average patch sizes and undifferentiated boundaries (Figs. 6 and 7). Explaining their association with riverbed habitat and water physicochemical parameters is relatively easy, as the autecological characteristics of these species clearly indicate an association with relatively clean rivers of medium size, not very fast current and coarse mineral substrate [
40,
41,
42,
43].
The relationship with landscape parameters, on the other hand, is more enigmatic. It seems that in the case of Aquarius najas and Velia caprai, the open habitats directly next to the river facilitate the dispersal of these species. The presence of these species (with the exception of Velia caprai, whose abundance is too low to infer any conclusion) is related to the habitat quality index (HQA). This indicator in the case of the Krąpiel River, which is a small lowland river but having a mountainous character over much of its course, is associated with meandering sections, with a relatively fast main river current, numerous channel debris and a gravel bed. In the water current, vegetation is present in insolated places, and the stagnant areas are characterised mostly by mineral (sand and gravel) bottoms, vegetated occasionally.
The second abundant synecological group of aquatic bugs represented in the Krąpiel River is the group of eurytopic species (
Callicorixa praeusta,
Hesperocorixa linnaei,
H. sahlbergi,
Paracorixa concinna,
Sigara falleni,
S. striata), which in the study area was associated with organic substrate, covered with vegetation and insolated (
Figure 4), water of low turbidity and hardness (
Figure 5), and, among landscape parameters, a valley area covered with patches of medium shape variation (
Figure 6), with a predominance of willow thickets immediately adjacent to the river (
Figure 7) and a predominance of wastelands located in the catchment (
Figure 8). Many authors emphasise the positive influence of temperature and insolation on the activity of aquatic bugs, with a particularly strong influence of water thermals, particularly in the shallow littoral, which can locally heat up strongly, creating favourable conditions for the development of thermophilic species [
24,
25,
26,
27,
44]. In the Krąpiel River, such habitat and landscape characteristics correspond to transformed sections with a low habitat quality index (HQA) (
Figure 2) and with a gravelly bottom outside the main river current (Zr(S) -
Figure 3).
Our analyses suggest the River Habitat Survey (RHS) method can predict the composition of the aquatic bug fauna inhabiting a given river. The use of this method for such an estimation can be very convenient as, in addition to the habitat parameters of the river itself, it takes into account the landscape character of its immediate surroundings, which has a major influence on the migration of aquatic bugs along the river valley. However, this estimate is relative to the type and nature of the river itself. It will depend on the geographical location of the river (the different nature of mountain/submountain rivers and lowland rivers), its size and order. Indicators of river habitat quality (HQA and RHQ) will be associated with different habitat types depending on the geographic location and type of river, once indicating habitats associated with the mainstream river and mineral substrate (mountain and small lowland rivers) and at other times habitats covered by vegetation with relatively slow water flow (large and medium lowland rivers). As such, they will indicate the presence of rheophilic or vegetation-associated fauna. In summary, RHS may be a good method for estimating the water bug fauna, linking it to the size and character of the river. But such estimating is useful of general synecological groups (the number of individuals and species), whereas at the species level, distribution of particular species is better explained by structure of habitatas in the river. However, these two components complement one another, and ultimately both of them together explain the nature of the water bugs fauna in the river. Similar relationships were observed for the water mite fauna [
45].
Acknowledgments
The project was supported by the Minister of Science under the "Regional Excellence Initiative" Program for 2024-2027.
References
- Stryjecki, R.; Zawal, A.; Stępień, E.; Buczyńska, E.; Buczyński, P.; Czachorowski, S.; Szenejko, M.; Śmietana, P. Water mites (Acari, Hydrachnidia) of water bodies of the Krąpiel River valley: interactions in the spatial arrangement of a river valley. Limnology 2016, 17, 247–261. [Google Scholar] [CrossRef]
- Pakulnicka, J.; Buczyński, P.; Dąbkowski, P.; Buczyńska, E.; Stępień, E.; Stryjecki, R.; Szlauer-Łukaszewska, A.; Zawal, A. (a). Aquatic beetles (Coleoptera) in springs of a small lowland river: habitat factors vs landscape factors. Knowledge and Management of Aquatic Ecosystems 2016, 417, 1–13. [Google Scholar] [CrossRef]
- Pakulnicka, J.; Buczyński, P.; Dąbkowski, P.; Buczyńska, E.; Stępień, E.; Stryjecki, R.; Szlauer-Łukaszewska, A.; Zawal, A. (b). Development of fauna of water beetles (Coleoptera) in waters bodies of a river valley: habitat factors, landscape and geomorphology. Knowledge and Management of Aquatic Ecosystems 2016, 417, 1–20. [Google Scholar] [CrossRef]
- Pakulnicka, J.; Buczyński, P.; Buczyńska, E.; Stępień, E.; Szlauer-Łukaszewska, A.; Stryjecki, R.; Bańkowska, A.; Pešić, V.; Filip, E.; Zawal, A. Sequentiality of beetle communities in the longitudinal gradient of a lowland river in the context of the river continuum concept. PeerJ 2022, 10, e13232. [Google Scholar] [CrossRef] [PubMed]
- Buczyńska, E.; Czachorowski, S.; Buczyński, P.; Pakulnicka, J.; Stępień, E.; Szlauer-Łukaszewska, A.; Stryjecki, R.; Zawal, A. Environmental heterogeneity at different scales: key factors affecting caddisfly larvae assemblages in standing waters within a lowland river catchment. Journal of Limnology 2017, 76, 305–325. [Google Scholar] [CrossRef]
- Zawal, A.; Stryjecki, R.; Stępień, E.; Buczyńska, E.; Buczyński, P.; Czachorowski, S.; Pakulnicka, J.; Śmietana, P. The influence of environmental factors on water mite assemblages (Acari, Hydrachnidia) in a small lowland river: an analysis at different levels of organization of the environment. Limnology 2017, 18, 333–343. [Google Scholar] [CrossRef]
- Zawal, A.; Stryjecki, R.; Buczyńska, E.; Buczyński, P.; Pakulnicka, J.; Bańkowska, A.; Czernicki, T.; Janusz, K.; Szlauer-Łukaszewska, A.; Pešić, V. Water mites (Acari, Hydrachnidia) of riparian springs in a small lowland river valley: what are the key factors for species distribution? PeerJ 2018, 6, e4797. [Google Scholar] [CrossRef] [PubMed]
- Szlauer-Łukaszewska, A.; Zawal, A. The impact of river dredging on ostracod assemblages in the Krąpiel River (NW Poland). Fundam. Appl. Limnol. 2014, 185 (3–4), 295–305. [CrossRef]
- Zawal, A.; Stępień, E.; Szlauer-Łukaszewska, A.; Michoński, G.; Kłosowska, M.; Bańkowska, A.; Myśliwy, M.; Stryjecki, R.; Buczyńska, E.; Buczyński, P. The influence of a lowland river dredging (the Krąpiel in NW Poland) on water mite fauna (Acari: Hydrachnidia). Fundam. Appl. Limnol. 2015, 186, 217–232. [Google Scholar] [CrossRef]
- Zawal, A.; Śmietana, P.; Stępień, E.; Pešić, V.; Kłosowska, M.; Michoński, G.; Bańkowska, A.; Dąbkowski, P.; Stryjecki, R. Habitat comparison of Mideopsis orbicularis (O. F. Müller, 1776) and M. crassipes Soar, 1904 (Acari: Hydrachnidia) in the Krąpiel River. Belgian Journal of Zoology 2015, 145, 94–101. [Google Scholar]
- Zawal, A.; Czachorowski, S.; Stępień, E.; Buczyńska, E.; Szlauer-Łukaszewska, A.; Buczyński, P.; Stryjecki, R.; Dąbkowski, P. Early post-dredging recolonization of caddisflies (Insecta: Trichoptera) in a small lowland river (NW Poland). Limnology 2016, 17, 71. [Google Scholar] [CrossRef]
- Zawal, A.; Sulikowska-Drozd, A.; Stępień, E.; Jankowiak, Ł.; Szlauer-Łukaszewska, A. Regeneration of the molluscan fauna of a small lowland river after dredging. Fundam. Appl. Limnol. 2016, 187, 281–293. [Google Scholar] [CrossRef]
- Dąbkowski, P.; Buczyński, P.; Zawal, A.; Stępień, E.; Buczyńska, E.; Stryjecki, R.; Czachorowski, S.; Śmietana, P.; Szenejko, M. The impact of dredging of a small lowland river on water beetle fauna (Coleoptera). Journal of Limnology 2016, 75(3), 472–487. [Google Scholar] [CrossRef]
- Buczyńska, E.; Buczyński, P.; Zawal, A.; Stępień, E. Environmental factors affecting micro-distribution of larval caddisflies (Trichoptera) in a small lowland reservoir under different types of watershed usage. Fundam. Appl. Limnol. 2016, 188, 157–170. [Google Scholar] [CrossRef]
- Zawal, A.; Lewin, I.; Stępień, E.; Szlauer-Łukaszewska, A.; Buczyńska, E.; Buczyński, P.; Stryjecki, R. The influence of the landscape structure within buffer zones, catchment land use and instream environmental variables on mollusc communities in a medium-sized lowland river. Ecol Res., 2016, 31, 853–867. [Google Scholar] [CrossRef]
- Buczyński, P.; Zawal, A.; Buczyńska, E.; Stępień, E.; Dąbkowski, P.; Michoński, G.; Szlauer-Łukaszewska, A.; Pakulnicka, J.; Stryjecki, R.; Czachorowski, S. Early recolonization of a dredged lowland river by dragonflies (Insecta: Odonata). Knowl. Manag. Aquat. Ecosyst. 2016, 417, 43. [Google Scholar] [CrossRef]
- Stępień, E.; Zawal, A.; Buczyński, P.; Buczyńska, E.; Szenejko, M. Effects of dredging on the vegetation in a small lowland river. PeerJ 7:e6282. 2019. [Google Scholar] [CrossRef]
- Szlauer-Łukaszewska, A.; Pešić, V.; Zawal, A. Environmental factors shaping assemblages of ostracods (Crustacea: Ostracoda) in springs situated in the River Krapiel valley (NW Poland). Knowl. Manag. Aquat. Ecosyst. 2021, 422, 14. [Google Scholar] [CrossRef]
- Stryjecki, R.; Zawal, A.; Krepski, T.; Stępień, E.; Buczyńska, E.; Buczyński, P.; Czachorowski, S.; Jankowiak, Ł.; Pakulnicka, J.; Sulikowska-Drozd, A.; Pešić, V.; Michoński, G.; Grabowski, M.; Jabłońska, A.; Achrem, M.; Olechwir, T.; Pietrzak, L.; Szlauer-Łukaszewska, A. Anthropogenic transformations of river ecosystems are not always bad for the environment: Multi-taxa analyses of changes in aquatic and terrestrial environments after dredging of a small lowland river. PeerJ 2021, 9, e12224. [CrossRef]
- Savić, A.; Zawal, A.; Stępień, E.; Pešić, V.; Stryjecki, R.; Pietrzak, L.; Filip, E.; Skorupski, J.; Szlauer-Łukaszewska, A. Main macroinvertebrate community drivers and niche properties for characteristic species in urban/rural and lotic/lentic systems. Aquatic Sciences 2021, 84, 1. [Google Scholar] [CrossRef]
- Lewin, I.; Stępień, E.; Szlauer-Łukaszewska, A.; Pakulnicka, J.; Stryjecki, R.; Pešić, V.; Bańkowska, A.; Szućko-Kociuba, I.; Michoński, G.; Krzynówek, Z.; Krakowiak, M.; Chatterjee, T.; Zawal, A. Drivers of the Structure of Mollusc Communities in the Natural Aquatic Habitats along the Valley of a Lowland River: Implications for Their Conservation through the Buffer Zones. Water 2023, 15, 2059. [Google Scholar] [CrossRef]
- Szoszkiewicz, K. , Gebler, D. Polska wersja systemu oceny stanu hydromorfologicznego rzek River Habitat Survey – nowe zastosowania w praktyce. Gospodarka Wodna 2012, 4, 141–146. [Google Scholar]
- ter Braak, C.J.F. Canonical correspondence analysis: a new eigenvector technique for ultivariate direct gradient analysis. Ecology 1986, 67, 1167–1179. [Google Scholar] [CrossRef]
- ter Braak, C.J.F. , Verdonschot, P. F.M. Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquatic Sci. 1995, 57, 255–289. [Google Scholar]
- Jaczewski, T.; Wróblewski, A. Klucze do oznaczania owadów Polski. Część XVIII. Pluskwiaki różnoskrzydłe – Heteroptera, zeszyt 2. Wyd. PWN: Warszawa – Wrocław, Poland, 1977; pp. 68.
- Jaczewski, T.; Wróblewski, A. Klucze do oznaczania owadów Polski. Część XVIII. Pluskwiaki różnoskrzydłe – Heteroptera, zeszyt 4. Wyd. PWN: Warszawa–Wrocław, Poland, 1978; pp. 32.
- Wróblewski, A. Fauna słodkowodna Polski. Pluskwiaki (Heteroptera). PWN: Warszawa-Poznań, Poland, 1980; 8, pp. 157.
- Klausnitzer, B. Käfer im und am Wasser. Die Neue Brehm-Bücherei. Bd.567 Westarp Wissenschafter: Magdeburg-Heidelberg-Berlin-Oxford, 1996; pp. 237.
- Chenrikson, L.; Oscarson, H.G. Water bugs (Corixidae, Hemiptera-Heteroptera) in acidified lakes: Habitat selection and adaptions. Ecol. Bull. 1985, 37, 232–238. [Google Scholar]
- Bell, H.L. Effects of low pH on the emergence of aquatic insects. Water Res. 1971, 5, 313–319. [Google Scholar] [CrossRef]
- Płaska, W.; Kurzątkowska, A.; Stępień, A.; Buczyńska, E.; Pakulnicka, J.; Szlauer-Łukaszewska, A.; Zawal, A. The effect of dredging of a small lowaland river on aquatic Heteroptera. Ann. Zool. Fennici 2016, 53, 139–153. [Google Scholar] [CrossRef]
- Jansson, A. Distribucion of Micronectinae (Heteroptera, Corixidae) in Lake Päijänne, central Finland: Correlation with eutrophication and pollution. Ann. Zool. Fennici 1977, 14, 105–117. [Google Scholar]
- Savage, A.A. Use of water boatmen (Corixidae) in the classification of lakes. Biological Conservation 1982, 23, 55–70. [Google Scholar] [CrossRef]
- Savage, A.A. The distribution of Corixidae in lakes at the ecological status of the North West. Midlands Meres Field Studies 1990, 7, 516–530. [Google Scholar]
- Nam, V.S.; Yen, N.T.; Holynska, M.; Reid, J.W.; Kay, B.H. National progress dengue vector in Vietnam: survey for Mesocyclops (Copepoda), Micronecta (Corixidae) and fish, as a biological control agents. Am. J. [of] Trop. Med. Hyg. 2000, 6, 5–10. [Google Scholar] [CrossRef]
- Płaska, W.; Tarkowska-Kukuryk, M. Influence of abiotic factors on species spectrum of zoopleuston in different types of peatlands. Pol. J. Environ. Stud. 2014, 23, 441–447. [Google Scholar]
- Mieczan, T.; Tarkowska-Kukuryk, M.; Płaska, W.; Rechulicz, J. Abiotic predictors of faunal communities in an ombrotrophic peatland lagg and an open peat bog. Isr. J. Ecol. Evol. 2014, 60, 62–74. [Google Scholar] [CrossRef]
- Kurzątkowska, A. Micronectinae (Heteroptera: Corixidae) jezior Polski północnowschodniej. In: Szacunek dla wody. Materiały zjazdowe XVIII Zjazdu Hydrobiologów Polskich w Białymstoku, Poland, 2000; pp. 147–148.
- Kowalik, W.; Buczyński, P. Beetles (Coleoptera) of saline waters from „Bogdanka” Stone Coal Mine (south-eastern Poland). Acta Agrophisica 2003, 1, 115–121. [Google Scholar]
- Płaska, W. . Pluskwiaki wodne (Heteroptera aquatica) jako wskaźniki stanu ekologicznego wód płynących (badania wstępne). Acta Agrophisica 2003, 88, 493–499. [Google Scholar]
- Czachorowski, S.; Lewandowski, K.; Wasilewska, A. The importance of 414 aquatic insects for landscape integration in the catchment area of the River Gizela (Masurian 415 Lake District, north eastern Poland). Acta Hydrobiologica 1993, 35, 49–64. [Google Scholar]
- Ilie, D.M.; Olosutean, H. Aquatic and semiaquatic Heteroptera from areas 428 rivier basin: methods in estimating biodiversity. Transylvanian Review of Systematical and 429 Ecological Researches 2009, 7, 1–9. [Google Scholar]
- Lechowski, L. , Buczyński, P. Aquatic and semiaquatic bugs (Heteroptera: Nepomorphaet Gerromorpha) of water bodies in the middle reach of the River Bug and its valley. ActaBiol. Univ. Daugavp., 2006, 6, 109–116. [Google Scholar]
- Macan, T.T. A revised key to the British water bugs (Hemiptera - Heteroptera) with notes on their Ecology. Freshwater Biological Association 1976; 16, pp. 120.
- Stryjecki, R.; Pešić, V.; Szlauer-Łukaszewska, A.; Michoński, G.; Bańkowska, A.; Pakulnicka, J.; Filip, E.; Lewin, I.; Chatterjee, T.; Zawal, A. River Habitat Survey: Does This Help to Explain the Nature of Water Mite (Acari and Hydrachnidia) Assemblages? Water 2023, 15, 3751. [Google Scholar] [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).