1. Introduction
The prevalence of CKD has been increasing over the years, presenting a growing public health challenge, and it is expected to become the fifth leading cause of death by 2040 [
1].
The common therapeutic approach is hemodialysis (HD), an extracorporeal treatment, usually administered 3 times a week for 3 to 4 h each time and employing semipermeable membranes. Developments on these membranes have been long [
2].
More than 90% of the dialysis membranes are fabricated from polysulfone (PSf) or polyethersulfone (PES). Udel® PSf and Veradel® PES have emerged as the preferred polymers for crafting these membranes [
3].
The materials that make up HD membranes have changed over time, moving from synthetic alternatives to cellulose, that have better biocompatibility and control over pore size.
Nevertheless, these membranes are designed for a single use, due to their plastic composition. This imposes an environmental strain worldwide, particularly evident in the estimated 4.1 million dialysis procedures conducted annually [
4].
Progress in the advancement of bio-derived membranes, or hybrid variants with decreased plastic content, is thus imperative, aiming to attain comparable success and therapeutic effectiveness. The current investigation aims to address this objective, initiating the study of the feasibility of using chitosan derived from edible insects as a component of dialysis membranes.
Insects not only have a strong reproductive capabilities and small size, but also have a short generation time and breeding period. Research has shown that insects are a highly efficient source of protein, requiring less feed, water, and land than traditional livestock while emitting fewer greenhouse gases and contributing to a more sustainable system overall [
5].
Chitin (poly (β-(1 → 4)-N-acetyl-D-glucosamine), which serves as the exoskeleton primary building block, is produced when mealworm larvae transform into pupae [
6]. Chitin is more commonly extracted from the exoskeletons of crustaceans, such as shrimp and crab, by treating them with alkali and acid, to remove proteins, lipids, and minerals [
7]. This is the preferred source for commercial applications once they contain 15%-20% of chitin [
8]
.
Chitosan is a biopolymer composed of glucosamine and N-acetylglucosamine units [
9] typically produced by the deacetylation of chitin, which involves the removal of the acetyl groups from the N-acetylglucosamine units [
10]
. Chitosan from insects is widely available because of its high reproductive rate, ease of reproduction, and higher tolerance to changes in their environment. Furthermore, it requires more moderate conditions of extraction, compared to the ones required for crustaceans. Also, insects have, overall, a larger production of chitin material than crustaceans [
11].
Chitosan has been widely used in the medical field, with quite diverse applications, such as: as an anticoagulant and antihypertensive agent to reduce the risk of vascular disease; as an anticancer agent, as it inhibits the growth of cancer cells; as prebiotic, promoting the growth of beneficial bacteria in the intestine; an anti-cholesterol agent, as it adsorbs and promotes excretion of harmful excess cholesterol from the body [
12]; as an immune-boosting agent [
13]. Given these beneficial bioactivities of chitosan, this study is set on encouraging the production and valorization of chitosan from edible insects, for HD-membrane design.
Due to its anti-coagulant, anti-inflammatory, and antibacterial properties, chitosan may lower the chance of coagulation, inflammation, and/or infection in subjects undergoing dialysis. This upvalues properties make it a viable opportunity for future study and advancement in dialyzer technology.
We report the extraction of chitin, followed by deacetylation, to obtain chitosan from T. molitor. We compare its structure and biological properties with commercial chitosan. Also, this study aimed to optimize a process for the extraction of chitin from insects, since all the processes known so far are very time-consuming, and, also, the application of the chitosan into a hemodialysis membrane, studying their permeation characteristics.
2. Materials and Methods
2.1. Materials
All standards and reagents, including commercial chitosan of medium molecular weight (approximate degree of deacetylation of 75%, viscosity-based molecular weight of 190,000-310,000 Da) were obtained from Sigma-Aldrich (Sintra, Portugal), unless otherwise noted.
2.1.1. Microbial Strains Used for Antimicrobial Activity Tests
For the antimicrobial assays, Staphylococcus aureus (MRSA, DSM 11729), Candida albicans (DSM 3454), and Staphylococcus epidermidis (DSM 20044) were provided by DSM Pharmaceuticals Inc. (Durham, North Carolina, USA) and Escherichia coli (ATCC 25922), Staphylococcus aureus (MSSA, ATCC 29213) and Pseudomonas aeruginosa (ATCC 10145) were provided from American Type Culture Collection (Manassas, Virginia, USA).
2.2. Chitin and Chitosan Extraction
The samples of
T. molitor larvae (Gotanbug, Portugal) were ground to obtain a powder. To extract the chitin and chitosan from the
T. molitor powder, the method of Chae-Shim Shin
et al. [
15] was used with modifications.
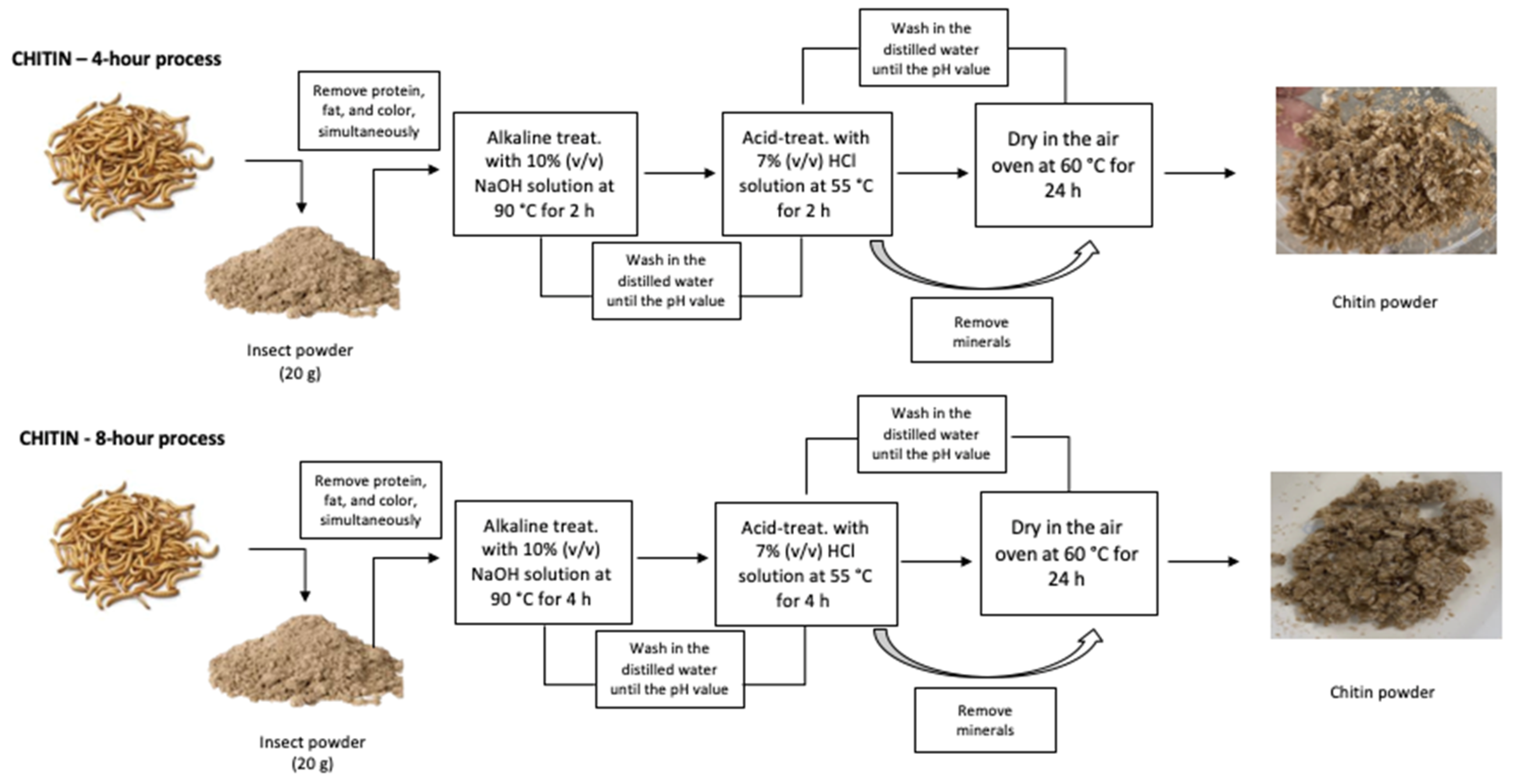
2.2.1. Chitin Extraction
Two processes were tested varying the extraction time. In each, the dried insect powder (20 g) was subjected to an alkaline treatment, with 10% (w/v) NaOH solution (Merck, Darmstadt, Germany), at 90 °C, for 2 h (E4), or 4 h (E8), to remove protein, fat, and colour. The samples obtained were washed in distilled water until the pH value became neutral. Subsequently, samples were acid-treated with 7% (v/v) HCl solution (Merck, Darmstadt, Germany), at 55 °C, for more than 2 h (E4) or 4 h (E8), respectively, to remove minerals, and then they were washed with distilled water until the pH value became neutral. After drying in an air oven at 60 °C for 24 h, chitin was obtained, with a colour corresponding to a light brown in both samples (E4 and E8). The overall procedure is schematically represented in
Figure 1.
After weighing the dried chitin, the yields were calculated according to the following equation;
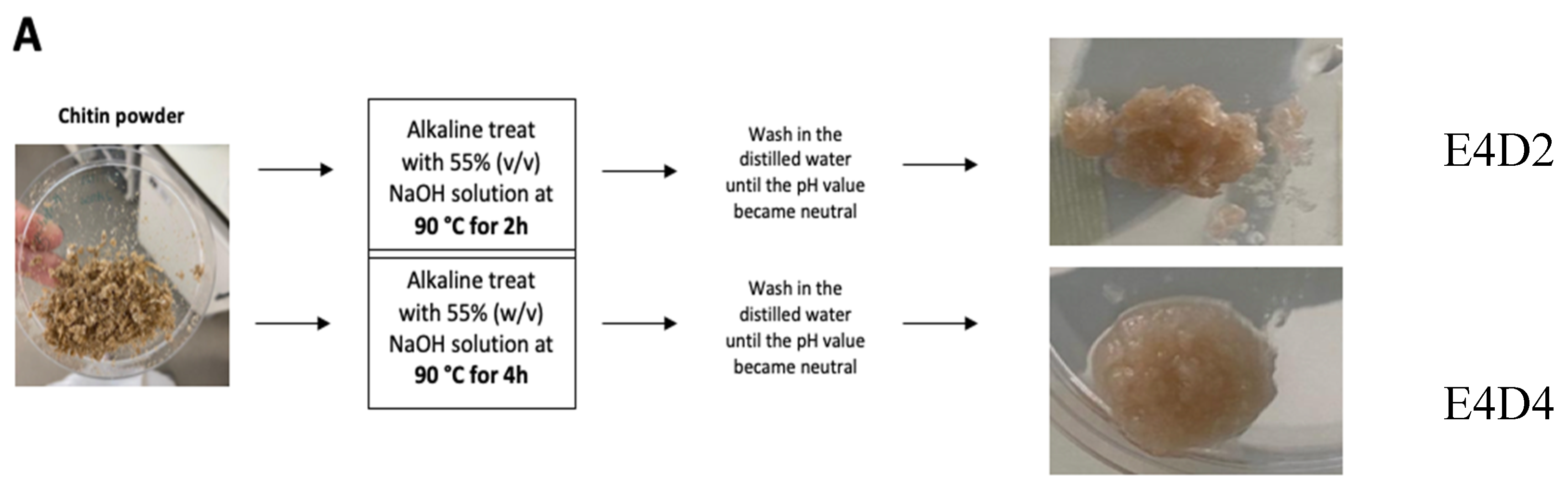
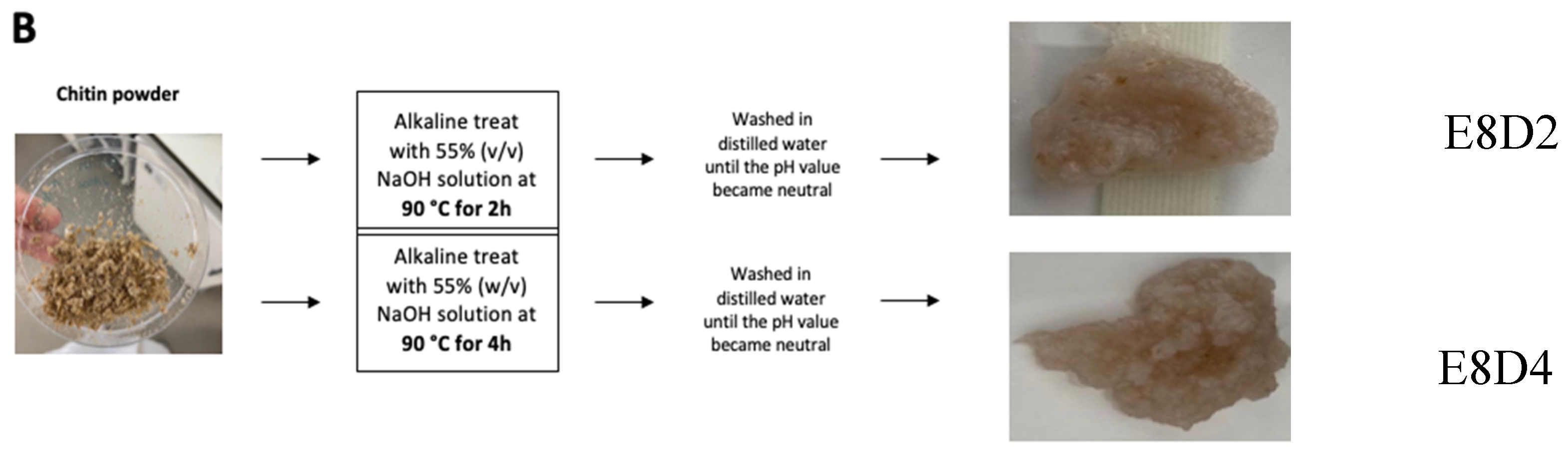
2.2.2. Chitin Deacetylation into Chitosan
Two processes were performed, varying the deacetylation time. To remove the acetyl groups, the powder of each chitin sample (E4 and E8) from
T. molitor was treated with a 55% (w/v) NaOH solution, at 90 °C, for 2 h (E4D2 and E8D2), or 4 h (E4D4 and E8D4), and it was washed with distilled water until the pH value became neutral (
Figure 2).
After drying in the air oven at 60 °C for 24 h, the weight of the dried chitosan was evaluated, and the yields of the procedure were calculated according to the following equation:
2.3. Chitosan Physicochemical Characterization
2.3.1. Fourier Transform Infrared Spectroscopy (FTIR)
The extracted chitin and chitosan specimens underwent analysis through Fourier-transform infrared spectroscopy (FTIR-ATR analysis), utilizing a Perkin Elmer spectrometer (Waltham, MA, USA) equipped with a diamond/ZnSe crystal and an attenuated total reflectance (ATR) sampling accessory from PIKE Technologies (Beaconsfield, UK). Each sample underwent 32 scans, covering the wavenumber range of 600-4000 cm-1, with a spectral resolution of 4 cm-1. Additionally, baseline point correction and spectra normalization procedures were implemented.
The degree of deacetylation (DD) in the chitosan samples was determined via the absorbance ratio (A1655/A3450), as the two parameters exhibit a linear correlation. The following equation was employed for DD calculation:
2.4.2. Dynamic Light Scattering and Zeta Potential
10 mg of extracted and commercial chitosan were dissolved in 1% (v/v) acetic acid solution. The samples zeta potential (ZP) was evaluated with a NanoZSP device (Worcestershire, UK). All tests were performed using a disposable foldable capillary cell with a 90° laser angle (Malvern, Worcestershire, UK) at room temperature (25 °C).
2.4. In Vitro Bioactivities of the Extracted Chitosan
2.4.1. Antioxidant Activity
The antioxidant activity was assessed using the 2,2-azinobis-3-ethylbenzothiazoline-6-sulfonic acid (ABTS) radical as a photometric technique. The fundamental principle underlying ABTS involves the attenuation of a well-established metastable radical (ABTS+) by antioxidant compounds. The experimental procedure, as detailed in Coscueta et al. 2020 [
16], was executed in a 96-well microplate format. The assay was conducted using a multidetector plate reader (Synergy H1, Vermont, USA), operating under the guidance of Gen5 Biotek software version 3.04. To generate the ABTS radical cation (ABTS•+), a reaction mixture of 2.45 mM potassium persulfate and 7 mM 2,20-azinobis (3-ethylbenzothiazoline-6-sulfonic acid) diammonium salt was prepared. An absorbance of 0.70 ± 0.02 at 734 nm was maintained, achieved by combining 180 µL of the ABTS•+ working solution with 20 µL of either the sample or Trolox for the standard calibration curve (ranging from 25 to 175 M).
The scavenging activity for the control was expressed as a percentage reduction in absorbance. Trolox concentration was determined through regression equations, and the results were presented in units of µmol TE (Trolox equivalent) per gram.
2.4.2. Antimicrobial Activity
Each microbial culture was grown aerobically in Muller Hinton agar (Biokar, France), for 24 h, at a temperature of 37 ºC. The bacteria were then moved to a sterile saline solution, and their turbidity was adjusted to 0.5 MacFarland scale, which is equivalent to an optical density of 0.08-0.1 at a wavelength of 600 nm. Dilutions within a concentration range of 1–10 mg/mL of commercial and extracted chitosan were prepared in Muller-Hinton broth. The chitosan solutions were then mixed with 2% (v/v) of each microbial inoculum, and the resultant mixtures were incubated at 37 ºC for 24 h. These inoculum-containing chitosan solutions were then placed onto Muller Hinton agar plates and kept incubating for an additional 24 h at 37 ºC.
The lowest chitosan concentration at which microbial growth was suppressed, and the initial viability of the microbial population was reduced by at least 99.9%, was identified as the Minimum Lethal Concentrations (MLCs).
2.5. Design and Development of a Bio-Based HD Membrane
The method of chitosan extraction E4D2 was chosen, since it was the shortest, and with the best antimicrobial and antioxidant activities.
For membrane production, a 2% (w/v) solution of polyvinyl chloride (PVC) in tetrahydrofuran was prepared, and then E4D2 chitosan powder was added to 1% (w/v) concentration. This solution was mixed until becoming homogeneous.
The PVC/chitosan solution was transferred to a petri dish and was left to dry at room conditions.
After the membrane was dried, it was cut into approximately 6 x 1 cm rectangles.
2.5.1. Diffusion and Retention Parameters for Permeation Studies to Simulate HD
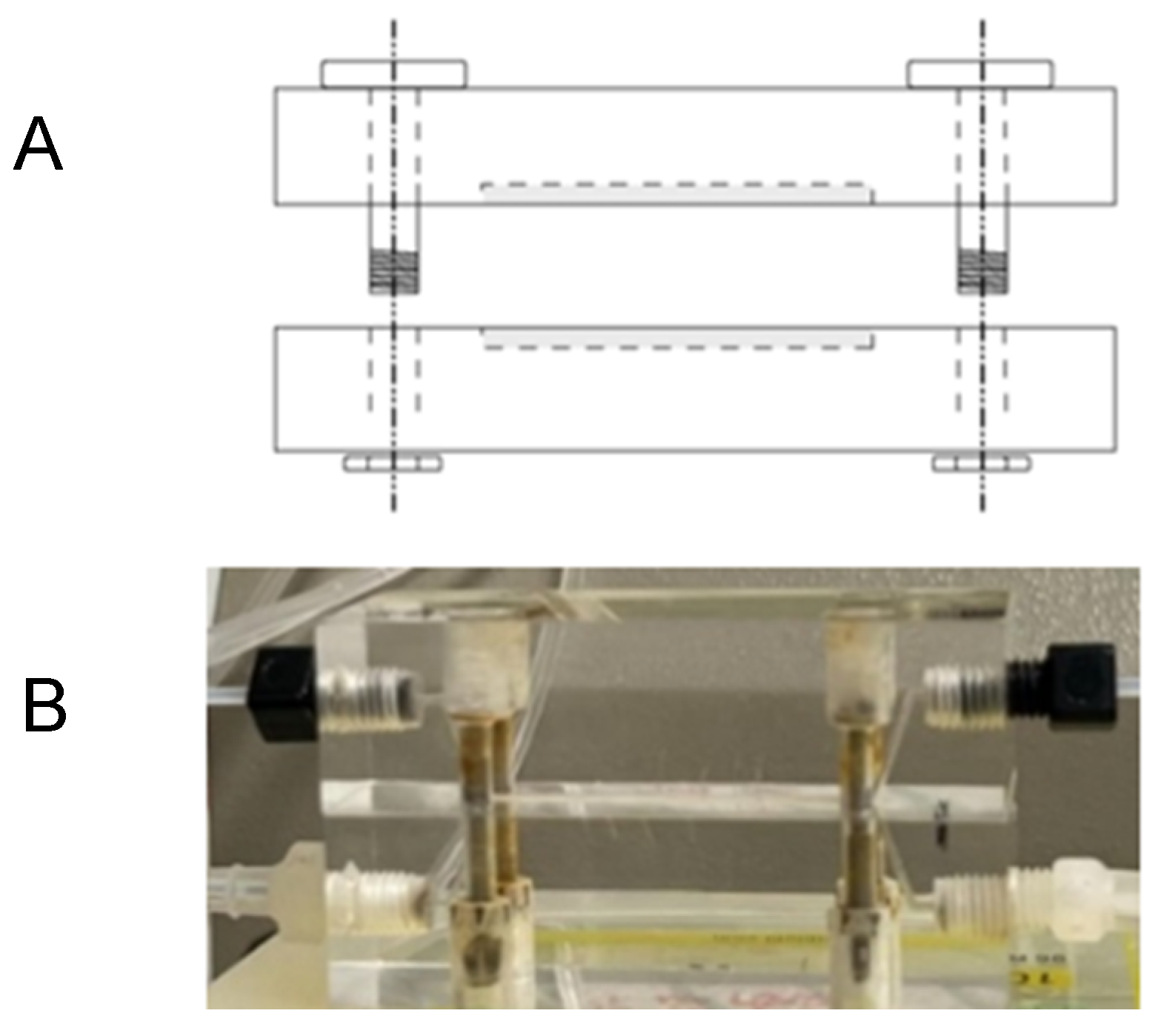
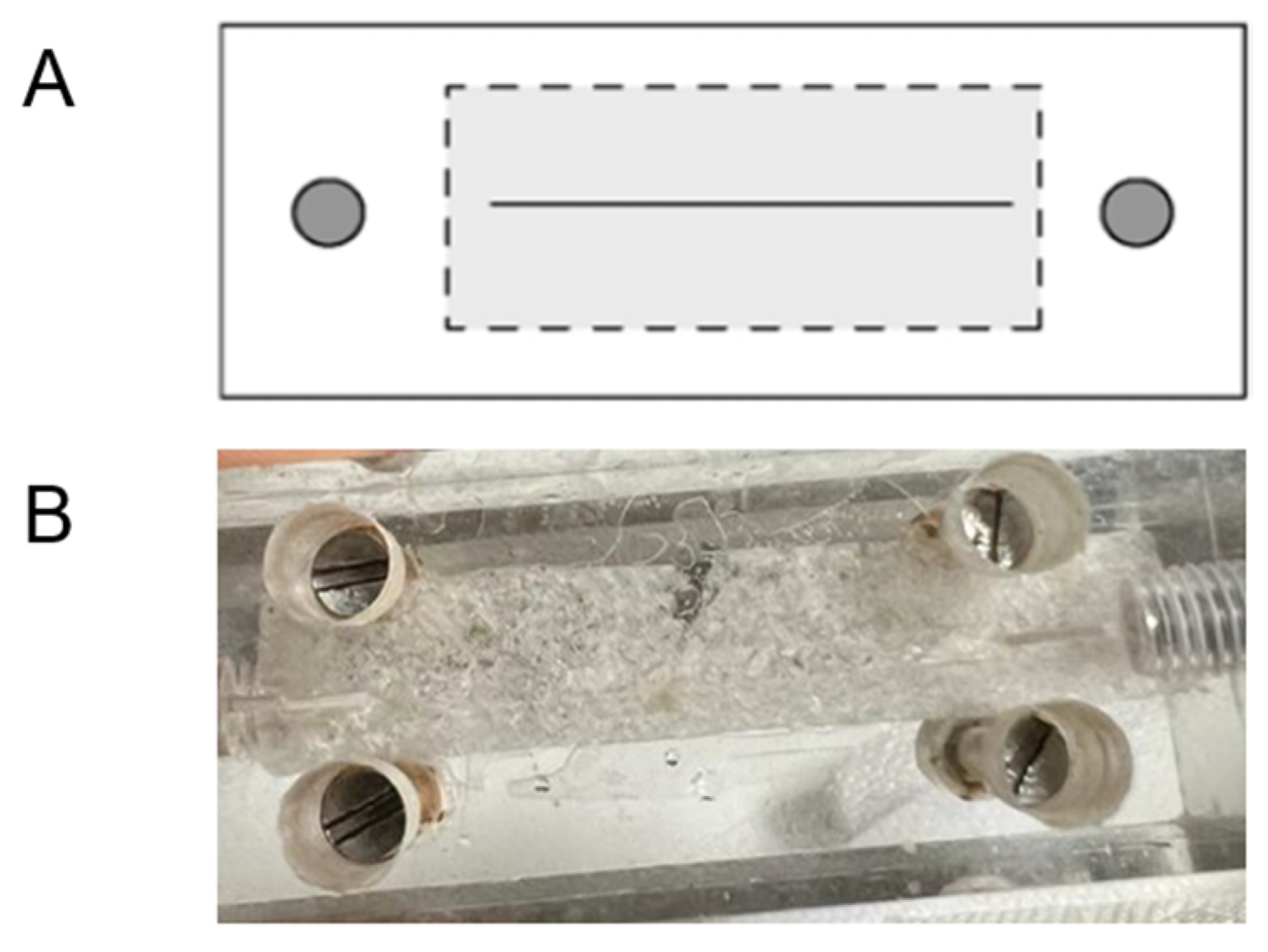
The semi-permeability of the chitosan-based membrane (CH – M) was evaluated with a permeation study of urea and albumin. The goal was to understand the ability of the membrane to selectively allow the passage of certain molecules (urea) while restricting others (albumin), which is fundamental to its HD application. Solutions were propelled by a Gilson Minipuls 3 peristaltic pump with PhthalateFREE® PVC pump tubes (
Figure 3) in order to simulate the HD process.
The membrane was placed between the two chambers of equal volume (
Figure 4).
One of the chambers, the donor chamber, was filled with solution of either urea at 37 mg/dL, or albumin AT 8 g/dL). the other chamber was filled with deionised water [
17] (
Figure 5).
At appropriate time intervals (2, 4 and 6 min), 1.5 mL sample from the chamber was collected, and the quantification of the solute diffused through the membrane was determined. For urea, a direct UV spectrophotometric assay, at 200 nm, was used. The linearity of the method was verified by constructing a calibration curve with a serial dilution of a standard urea solution at 37 mg/dl. For the quantification of albumin, the Pierce BCA Protein Assay Kit (Thermo Fisher, USA) was used.
2.6. Statistical Analysis
Statistical analysis was conducted using the IBM® SPSS® Statistics 26 software. For comparing the means across multiple groups, the analysis involved a one-way ANOVA for datasets with a normal distribution, complemented by Tukey's HSD for post hoc analysis. When comparing just two groups, the data underwent analysis with a Student's t-test, if normally distributed. The threshold for statistical significance was established at p < 0.05.
3. Results and Discussion
The chitosan extracted from insects using four different processes (varying, in total, extraction times between 6 h and 12 h) was characterized in terms of physical properties, including analysis by FTIR, Zeta potential, and degree of deacetylation.
In addition, for each sample, some bioactivities were also characterised, namely antioxidant capacity and antimicrobial activity, in a range of selected concentrations (1, 2, 4, 6, 8, 10 mg/mL), against MRSA, MSSA, S. epidermis, E. coli, P. aeruginosa and C. albicans.
The chitosan extracted by the E4D2 process was chosen for the development of a semi-permeable membrane (CH-M), since it was the one that showed the best performance in antimicrobial activity. To simulate the HD process and to test the semi-permeability of the CH-M, albumin retention and urea permeation studies were carried out.
3.1. Extraction of Chitin
The procedure was developed into two approaches, one lasting 4 h (E4) and the other 8 h (E8).
The data presented in
Table 1 compares the chitin yield from
T. molitor at two different extraction times and reveals E4 and E8 yields of 5.2 ± 0.8% and 5.0 ± 0.1% respectively. The difference in yield is not statistically significant (
p > 0.05), suggesting that the additional time spent on extraction in E8 does not yield a proportionally higher amount of chitin.
A shorter time represents an increase in efficiency, particularly relevant when considering the scale-up of production for commercial purposes, where cost is a crucial factor.
In essence, the findings from
Table 2 reinforce the hypothesis that not only it is possible to derive chitosan from insects like
T. molitor efficiently, but also that it can be done in a manner that is cognizant of economic and environmental sustainability.
Also, these yield results are in line with previous studies using
Beetle holotrichia and
Melolontha melolontha. These reports have indicated a range of values for chitin content from 5.3% to 16%. It is important to keep in mind that the chitin extracted from different insects might vary, depending on the insect species, stage of development, and growth conditions [
18].
In order to obtain chitosan from E4 and E8, a deacetylation procedure was used, varying also the deacetylation time.
The first deacetylation lasted 2 h (E4D2, E8D2), giving a total of 6 h and 10 h process, and the second deacetylation took 4 h (E4D4, E8D4), giving a 8 h or 12 h process, respectively.
From the data in
Table 2, one concludes that extending the deacetylation time from 2 h to 4 h did lead to a significant increase in chitosan yield (
p > 0.05), indicating that the duration of this procedure is a more critical factor than the duration of the chitin extraction.
The most notable increase in yield was observed when comparing the E4D4 and E8D4 samples to their E4D2 and E8D2 counterparts, highlighting the importance of the deacetylation phase. A longer period for the deacetylation phase allows for a more complete conversion of chitin to chitosan, perhaps due to a more thorough deacetylation process.
Despite the highest yield obtained with the E8D4 sample, further work will clarify whether the longer process is justified.
In conclusion, the findings suggest that optimizing the deacetylation time, rather than the chitin extraction time, may be a more viable strategy for improving yield.
Also, in other studies, with differing time conditions, chitosan yields from the insects
Beetle holotrichia and
Melolontha melolontha ranged from 4% to 74% [
19,
20], which highlights the value of the extraction processes presented.
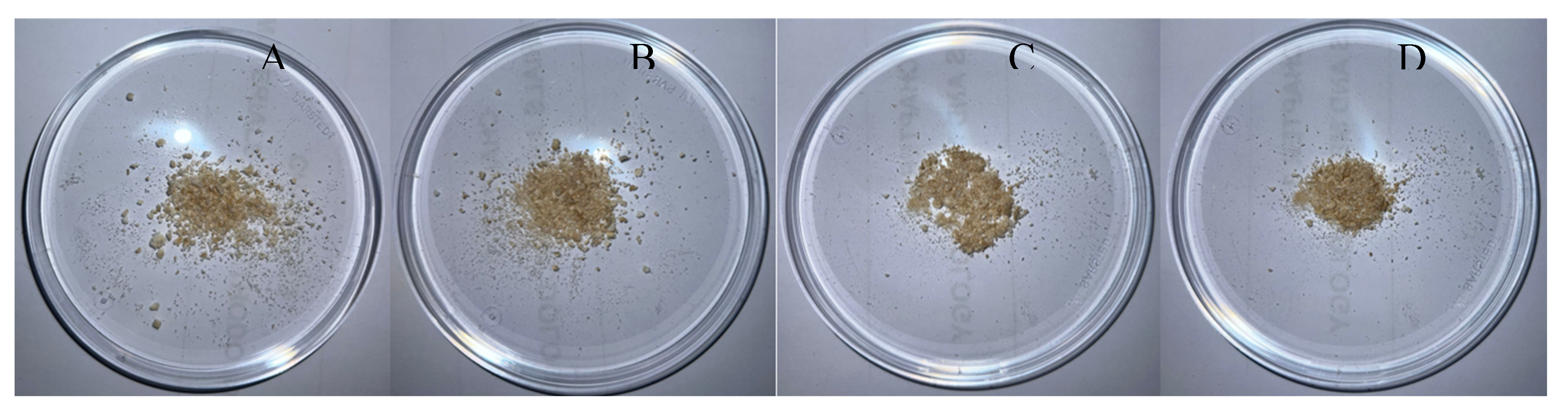
Additionally, the four chitosan samples obtained showed, empirically, very similar macroscopic features to commercial chitosan (
Figure 6).
Comparing with reported studies, the method presented has a significantly reduced processing time, from 1 ½ days to a timeframe of 6-12 h. Also, by employing a hot alkali treatment for decolorization, the method demonstrates remarkable efficiency in achieving similar macroscopic features to commercial chitosan.
3.2. Molecular Characteristics of Chitosan
3.2.1. Fourier Transform Infrared Spectroscopy (FTIR)
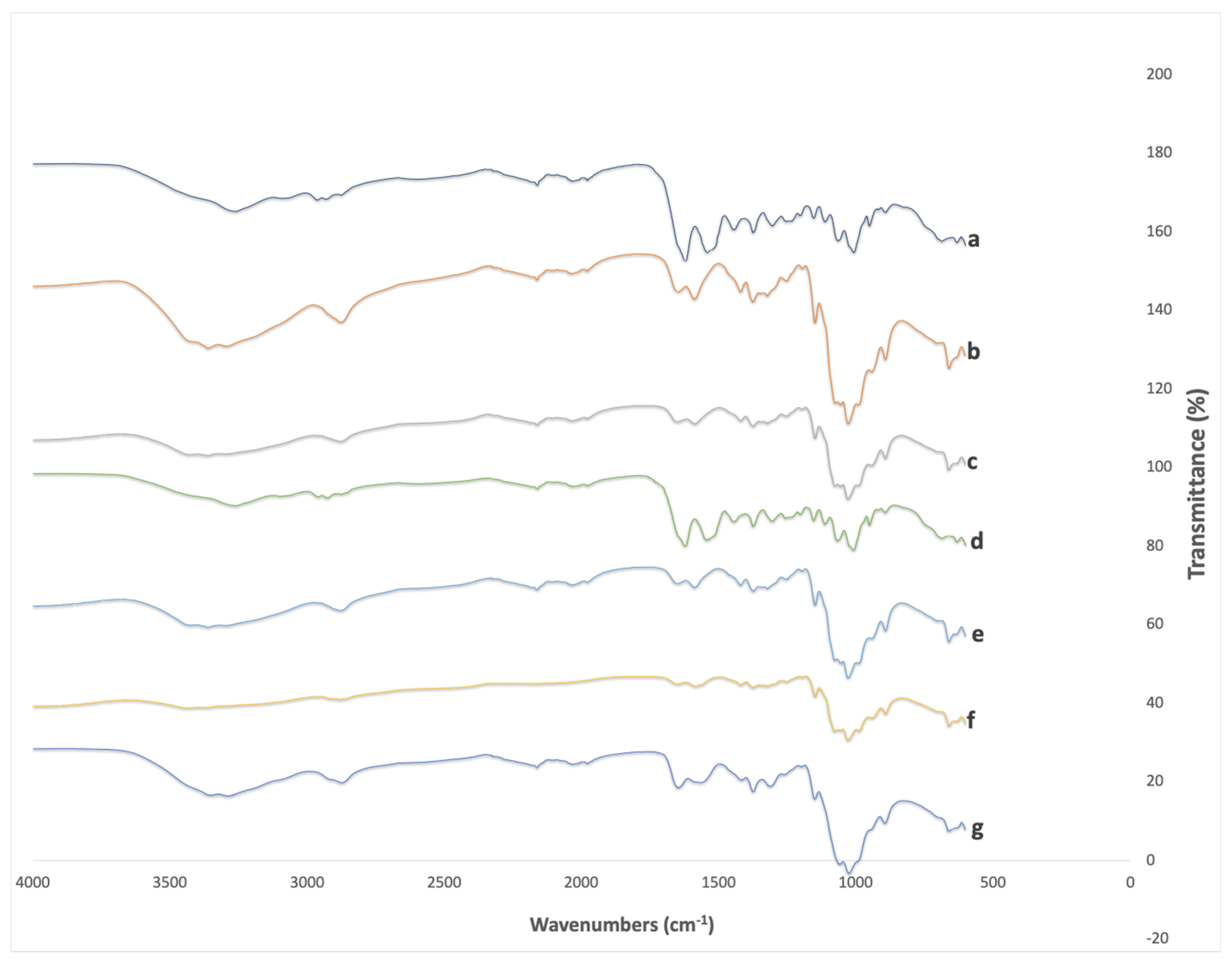
The FTIR spectra of chitosan and chitin obtained from
T. molitor, and of commercial chitosan, are compared in
Figure 7. The four samples of chitosan from this work showed FTIR spectra extremely comparable to that of the brand chitosan.
A broad absorption peak centered around 3300 cm-1 is observed in the IR spectrum of chitin (E4 and E8), indicating the presence of hydrogen bonding associated with O-H or N-H groups, which are functional groups found in chitin. Additionally, a sharp peak at approximately 1650 cm-1 is discerned, signifying the presence of carbonyl (C=O), or potentially conjugated double bond (C=C) groups. Two additional peaks at 1450 cm-1 and 1250 cm-1 correspond to C-H bending in alkanes and C-O stretching in esters or ethers, respectively, suggesting the existence of these functional groups within the chitin structure. Chitosan E8D4 exhibits absorption peaks in the range of 3000-2850 cm-1, indicative of C-H stretching vibrations in aliphatic (alkane) groups. Furthermore, a distinct peak near 1650 cm-1 is observed, implying the presence of carbonyl (C=O) groups or potential double bonds (C=C) within the chitosan molecular framework. Chitosan E8D2 shares similarities with chitin in displaying a broad absorption peak around 3300 cm-1, indicating the presence of O-H or N-H groups and the associated hydrogen bonding. Peaks near 1650 cm-1, akin to chitin, signify the existence of carbonyl (C=O) groups. The appearance of peaks at 1450 cm-1 and 1250 cm-1 suggests the presence of C-H bending in alkanes and C-O stretching in esters or ethers, corroborating the molecular composition.
Chitosan E4D2 showcases a broad absorption peak around 3300 cm
-1, akin to other chitosan samples, suggesting the presence of O-H or N-H groups and associated hydrogen bonding. Chitosan E4D4 manifests sharp peaks within the 3000-2850 cm
-1 range, signifying C-H stretching vibrations in aliphatic (alkane) groups. The sharpness of these peaks may suggest a more ordered or crystalline structural arrangement in comparison to other samples, possibly due to differences in processing [
21,
22,
23].
3.2.2. Degree of Deacetylation
In general, the amount of alkali solution, reaction temperature, reaction time, and solid chitin structure [
24] affect the degree of deacetylation. There are two different ways to modify chitin to obtain chitosan: chemically (using intense alkali solutions, alkali catalysis, alkali fusion, and hydrazine hydrate techniques) [
25], and biologically (using enzymes). Since the concentrated alkali solution technique is the most well-known and regularly used due to reduced cost and efficiency, it was selected in this study. To produce chitosan, raw materials were heated in a NaOH solution for 2 h or 4 h. The degree of deacetylation (DD) of the four samples of chitosan obtained from
T. molitor was determined using FTIR and is presented in
Table 3.
The E4D2 sample exhibits the highest degree of deacetylation at 75.06%. Increasing the deacetylation time from 2 h to 4 h had a negligible effect on deacetylation, given that the extraction conditions remain the same.
The E8D2 sample shows a further reduced degree of deacetylation at 73.14%, indicating that prolonging the chitin extraction time to 8 h may not be optimal for maximizing deacetylation. Also, the E8D4 sample reflects a deacetylation degree of 73.36%, which is slightly higher than that of E8D2 but still lower than the E4 series.
This could suggest that while extending chitosan extraction time does increase deacetylation, the initial 8 h chitin extraction may limit the potential for higher deacetylation levels.
The commercial sample presents the lowest degree of deacetylation at 70.22%, which could be due to various factors, including the source of chitin, and industrial processing techniques.
The results obtained are consistent with data previously reported in the literature, which indicates that the DD of medium-molecular-weight commercial chitosan (from crustaceans) ranges from 70 to 85% and from other insects, such as
Blaps luthier, Pimelia fernandezlopezi, Musca domestica rages from 86.9 to 88% [
26].
3.2.3. Zeta Potential
Zeta potential is a crucial factor that controls the physicochemical characteristics of polymers [
27].
It stands for the electric surface charge that, as a result of the electrostatic attraction, has a substantial impact on the stability of particles in suspension. A high mutual repulsion between the particles in the medium will prevent particle aggregation when they all have either a noticeably negative or positive zeta potential. Typically, entities with zeta potential greater than +30 mV or lower than -30 mV are regarded as being stable [
28].
The zeta potential value for the commercial chitosan sample was found to be 65.5 mV and the values of the four chitosan produced presented an average value of 58.1 mV, which may guarantee the stability of the developed chitosan formulations and opening the range of its possible future applications.
3.3. Biological Activities of Chitosan from Tenebrio Molitor
3.3.1. Antimicrobial Activity
The extracted chitosan was evaluated for antimicrobial activity against a wide range of microorganisms associated with infections.
It was proved that all four chitosan samples are active against
E. coli,
S. aureus (MSSA and MRSA),
C. albicans, S. epidermidis, and
P. aeruginosa.
Table 4 summarizes the minimum lethal concentration (MLC) values found for each chitosan, including the commercial one.
The MLC is the lowest broth dilution of an antimicrobial compound that prevents the growth on an agar plate. The failure to develop on the plate means that only nonviable microorganisms are available [
29].
The antimicrobial agent exhibiting the highest potency is characterized by possessing the lowest MLC value. While all extracted chitosan showed antimicrobial properties similar to the commercial sample, the analysis highlighted E4D2 as having the most significant inhibitory effects, requiring the lowest minimum lethal concentrations against a wide array of microorganisms. We omit the connection between E4D4 antimicrobial activity and its higher degree of deacetylation. It is noted that E4D2 also exhibited a higher degree of deacetylation, aligning with literature that suggests a higher degree of deacetylation correlates with increased antimicrobial activity.
In contrast, chitosan E4D4 showed the highest MLC for the majority of bacteria tested. Until now, the antimicrobial activity of insect chitosans has been explored by a limited number of studies, however, there is already some evidence for insects such as
T. molitor [
15].
The notable antimicrobial properties underscore the potential utilization of chitosan in crafting biodegradable membranes for hemodialysis (HD).
3.3.2. Antioxidant Activity
Using the ABTS method, the antioxidant activity was assessed, with results shown in
Table 5.
The chitosan E8D4, E4D2 and the commercial chitosan showed that their antioxidant activities are not significantly different (p > 0.05).
The results suggest that the extraction and deacetylation times have no impact on the antioxidant activity of the chitosan.
In conclusion, variations in processing times did not affect the antioxidant properties of chitosan. The antioxidant activity of the commercial chitosan falls within the range of the extracted samples, suggesting that the extraction methodology used for the T. molitor samples was not more deleterious.
Also, the observed values are within the range described in the literature, which range from 48.5 to 80.9 µmol Trolox equivalent/g for crustacean chitosan [
30].
3.4. CH-M Development and HD Simulation
For an HD purpose, it is important to analyze whether the CH-M effectively retains albumin, a critical protein in blood plasma, while allowing smaller molecular constituents (in particular urea) to pass through [
31].
It is crucial to note, also, that this setup did not entirely replicate the typical HD process. The membrane used is not of the hollow fiber type, and the experiment did not involve blood contact, nor were hemocompatibility tests conducted. These are important distinctions from the clinical setting and should be taken into consideration when interpreting the results.
As far as our understanding extends, this is the sole flow model (dynamic and counter-current) validated for albumin and urea for simulating HD.
3.4.1. Analysis of the Urea Permeation
For the urea permeation, over a time range of 2 to 6 min, the analysis demonstrated a consistent permeation of urea across the membrane, with concentrations ranging from 0.27 to 0.31 mg/mL (
Table 6), once the initial solution presented a concentration of 0.37 mg/mL.
After 2 min, the urea permeated was measured at 0.29 ± 0.019 mg/mL, which equates to 77.6 ± 5.2%. This high permeation in such a short time underscores the membrane rapid response to urea diffusion, a critical aspect on HD.
Subsequently, at 4 min, there was a slight increase in both the concentration and percentage of urea permeated, reaching 0.31 ± 0.013 mg/mL and 82.6 ± 3.7%, respectively. This can suggest that the membrane maintains its permeability over time, allowing for continued diffusion of urea without saturation or loss of function.
After this, at the 6-min interval, there was a slight decrease in urea permeation to 0.27 ± 0.003 mg/mL, which corresponds to 72.8 ± 0.9% permeation. This reduction could be attributed to the approach towards an equilibrium state, where the concentration gradient between the two sides of the membrane diminishes over time [
32].
During a real HD process, approximately 60 to 70% of urea is typically removed from the blood. While it is challenging to specify a definitive percentage for adequate dialysis, it is observed that patients tend to have a longer lifespan and fewer hospital admissions when the urea reduction ratio (URR) - a critical measure of the dialysis treatment ability to remove urea from the blood - is 60% or higher [
32].
The choice of these specific time intervals was strategic, aiming to capture the initial efficiency and progression towards equilibrium of the membrane filtration capacity. Nonetheless, the permeability rate is directly proportional to the filtration surface (i.e. filter and/or membrane). In this case, the surface with size 6 x 1 cm allows a test permeability of low volumes and flow rates (i.e. 1.5 mL/min), and consequently a few minutes (i.e. less than 10 min), until establishing its equilibrium. Therefore, this was a pilot permeability test that is still far from real dialysis conditions.
In this study, when analyzing the samples collected at 2, 4, and 6-minute intervals, it was observed a complete absence of albumin in the filtrate. This was conclusively verified through BCA assay, which displayed no deviation from the baseline, clearly indicating the CH - M effectiveness in retaining albumin molecules.
The ability of the membrane to selectively filter solutes while preventing the passage of larger molecules like albumin is of paramount importance in its application in HD.
In typical HD processes, not all albumin is retained and, according to the literature, dialysis-related albumin loss is up to 26.4 g/4 h [
33].
The ability of a dialyzer to sieve or reject solutes during dialysis has been known as a marker for the clinician to determine the suitable dialyzer for the patient. The sieving coefficient (that indicates the potential of different solutes to pass across a particular dialyzer membrane) is < 0.001 for albumin for the most common commercial polysulfone dialyzers [
34].
The study revealed total retention of albumin by the CH-M, suggesting a very high level of selectivity.
Despite these promising results, several limitations must be recognized. Firstly, the time frame of the analysis was relatively short, restricted to intervals of 2, 4, and 6 minutes. This duration may not fully capture the membrane performance over the extended periods typical of HD treatments, as mentioned previously.
Secondly, while the focus on albumin retention was critical, it does not cover the entire spectrum of molecules that are involved in HD, thereby necessitating a more comprehensive range of testing for a thorough evaluation.
Furthermore, the study was conducted under laboratory conditions, which may not completely reflect the complexities encountered in a clinical HD setup.
Another significant limitation was the absence of actual blood contact in the performance tests, a factor that is essential in real-world HD scenarios and can significantly impact the performance of the membrane. Lastly, the study did not include hemocompatibility tests, which are crucial for assessing how the membrane interacts with blood components in clinical applications [
35].
Understanding these limitations is vital for interpreting the results within the appropriate context and for guiding future research aimed at optimizing the membrane design and functionality for practical HD applications.
Nonetheless, this study provides us with a conceptual examination validating the semi-permeable capacity of the membrane for molecules with both higher and lower molecular weights.
Conclusions
In this work, chitin extraction from T. molitor and conversion to chitosan were carried out with significantly reduced processing times. Comparing with previously published research about chitosan produced from various insect species, the yields here reported are comparable. The analyses of the extraction times for obtaining both chitin and chitosan have that longer time does not necessarily result in a higher yield This finding is particularly relevant for the industrial scalability of chitosan production, where time efficiency directly correlates with economic viability.
Moreover, the investigation into the deacetylation phase has underscored its critical role in the chitosan yield. It was observed that extending the deacetylation time significantly improves the yield, as seen in the difference between yields from E4D2 to E4D4 samples, as well as from the E8D2 to E8D4 samples. This enhancement in yield with prolonged deacetylation time could lead to a more effective use of resources, but it implies the environmental footprint of the production process.
The physicochemical characterization of chitosan, employing FTIR, along with the bioactive assessments through antimicrobial and antioxidant activities, has provided a diverse overview of the material properties. The degree of deacetylation, a key parameter in determining the application potential of chitosan, was quantified, offering insights into the structural integrity and functionality of the biopolymer.
In the context of bioactive potential, chitosan derived from T. molitor demonstrated noteworthy activity against a variety of microorganisms, including bacteria Gram-positive, bacteria Gram-negative, and a yeast. Interestingly, earlier research has demonstrated that chitosan generated from insects is non-toxic and safe for the environment. This antimicrobial activity alongside its antioxidant capacity evaluated through ABTS radical scavenging, shows chitosan potential in biomedical applications, particularly in the realm of sustainable HD membranes.
This study introduced a a novelty, the semipermeable membrane with chitosan (CH – M). The choice to use chitosan extracted from insects breaks away from traditional sources, aligning with sustainable practices while showing potential for medical applications. Throughout the research process, from extraction to application, insect-derived chitosan has demonstrated adequate permeability to urea and albumin, a requirement for dialysis membranes.
Acknowledgements
This work was supported by National Funds from project BUGS@PETS (POCI-01-0247-FEDER-047042) funded by Fundo Europeu de Desenvolvimento Regional (FEDER), under Programa Operacional Competitividade e Internacionalização (POCI). We would also like to thank the scientific collaboration from Fundação para a Ciência e a Tecnologia (FCT) through project UIDB/50016/2020.
References
- Foreman, K. J. et al. Forecasting life expectancy, years of life lost, and all-cause and cause-specific mortality for 250 causes of death: reference and alternative scenarios for 2016–40 for 195 countries and territories. The Lancet 392, 2052–2090 (2018). [CrossRef]
- Fox, A. & Franklin, G. Clinical Practice Guideline Haemodialysis. www.nice.org.uk/accreditation.
- Hestekin, C. N. et al. High flux novel polymeric membrane for renal applications. Sci Rep 13, 11703 (2023).
- Fresenius Medical Care. Facts & Figures. (2023).
- Huis, A. et al. EDIBLE INSECTS Future Prospects Fo Food and Feed Security. vol. 171 (2013).
- Song, Y.-S. et al. Extraction of chitin and chitosan from larval exuvium and whole body of edible mealworm, Tenebrio molitor. Entomol Res 48, 227–233 (2018). [CrossRef]
- No, H. K., Young Park, N., Ho Lee, S. & Meyers, S. P. Antibacterial activity of chitosans and chitosan oligomers with different molecular weights. Int J Food Microbiol 74, 65–72 (2002). [CrossRef]
- Mathur, N. K. & Narang, C. K. Chitin and chitosan, versatile polysaccharides from marine animals. J Chem Educ 67, 938 (1990). [CrossRef]
- Ravi Kumar, M. N. V. A review of chitin and chitosan applications. React Funct Polym 46, 1–27 (2000). [CrossRef]
- Kumirska, J., Weinhold, M. X., Thöming, J. & Stepnowski, P. Biomedical Activity of Chitin/Chitosan Based Materials—Influence of Physicochemical Properties Apart from Molecular Weight and Degree of N-Acetylation. Polymers vol. 3 1875–1901 (2011). [CrossRef]
- Zainol Abidin, N. A., Kormin, F., Zainol Abidin, N. A., Mohamed Anuar, N. A. F. & Abu Bakar, M. F. The Potential of Insects as Alternative Sources of Chitin: An Overview on the Chemical Method of Extraction from Various Sources. Int J Mol Sci 21, (2020). [CrossRef]
- Kim, H. et al. The Preparation and Physiochemical Characterization of Tenebrio molitor Chitin Using Alcalase. Molecules vol. 28 (2023). [CrossRef]
- Islam, S., Bhuiyan, M. A. R. & Islam, M. N. Chitin and Chitosan: Structure, Properties and Applications in Biomedical Engineering. J Polym Environ 25, 854–866 (2017). [CrossRef]
- Emenike, E. C., Iwuozor, K. O., Saliu, O. D., Ramontja, J. & Adeniyi, A. G. Advances in the extraction, classification, modification, emerging and advanced applications of crystalline cellulose: A review. Carbohydrate Polymer Technologies and Applications vol. 6 (2023). [CrossRef]
- Shin, C.-S., Kim, D.-Y. & Shin, W.-S. Characterization of chitosan extracted from Mealworm Beetle (Tenebrio molitor, Zophobas morio) and Rhinoceros Beetle (Allomyrina dichotoma) and their antibacterial activities. Int J Biol Macromol 125, 72–77 (2019). [CrossRef]
- Coscueta, E. R., Reis, C. A. & Pintado, M. Phenylethyl Isothiocyanate Extracted from Watercress By-Products with Aqueous Micellar Systems: Development and Optimisation. Antioxidants vol. 9 Preprint at (2020). [CrossRef]
- Mesquita, R. B. R. & Rangel, A. O. S. S. Gas diffusion sequential injection system for the spectrophotometric determination of free chlorine with o-dianisidine. in Talanta vol. 68 268–273 (Elsevier, 2005). [CrossRef]
- Abidin, N. A. Z., Kormin, F., Abidin, N. A. Z., Anuar, N. A. F. M. & Bakar, M. F. A. The potential of insects as alternative sources of chitin: An overview on the chemical method of extraction from various sources. International Journal of Molecular Sciences vol. 21 1–25 (2020). [CrossRef]
- Kaya, M. et al. Comparison of physicochemical properties of chitins isolated from an insect (Melolontha melolontha) and a crustacean species (Oniscus asellus). Zoomorphology 133, 285–293 (2014). [CrossRef]
- Chae, K. S., Shin, C. S. & Shin, W. S. Characteristics of cricket (Gryllus bimaculatus) chitosan and chitosan-based nanoparticles. Food Sci Biotechnol 27, 631–639 (2018). [CrossRef]
- Yasmeen, S. et al. Chromium (VI) Ions Removal from Tannery Effluent using Chitosan-Microcrystalline Cellulose Composite as Adsorbent. Int Res J Pure Appl Chem 10, 1–14 (2016).
- Mahmoud, A. A. et al. FTIR Spectroscopy of Natural Bio - Polymers Blends. Journal o f Applied Sciences 4, 816–824 (2014).
- Varma, R. & Vasudevan, S. Extraction, Characterization, and Antimicrobial Activity of Chitosan from Horse Mussel Modiolus modiolus. ACS Omega 5, 20224–20230 (2020). [CrossRef]
- Yao, K., Li, J., Yao, F. & Yin, Y. Chitosan-Based Hydrogels. (Taylor & Francis Group, LLC, 2012).
- Kaya, M., Seyyar, O., Baran, T. & Turkes, T. Bat guano as new and attractive chitin and chitosan source. Front Zool 11, 59 (2014). [CrossRef]
- Amor, I. Ben, Hemmami, H., Laouini, S. E., Abdelaziz, A. G. & Barhoum, A. Influence of chitosan source and degree of deacetylation on antibacterial activity and adsorption of AZO dye from water. Biomass Convers Biorefin (2023). [CrossRef]
- Athavale, R. et al. Tuning the surface charge properties of chitosan nanoparticles. Mater Lett 308, 131114 (2022). [CrossRef]
- Warsito, M. F. & Agustiani, F. A review on factors affecting chitosan nanoparticles formation. in IOP Conference Series: Materials Science and Engineering vol. 1011 (IOP Publishing Ltd, 2021).
- Abedon, S. Chapter 1 - Phage Therapy Pharmacology: Calculating Phage Dosing. in (eds. Laskin, A. I., Sariaslani, S. & Gadd, G. M. B. T.-A. in A. M.) vol. 77 1–40 (Academic Press, 2011).
- Muñoz-Tebar, N., Pérez-Álvarez, J. A., Fernández-López, J. & Viuda-Martos, M. Chitosan Edible Films and Coatings with Added Bioactive Compounds: Antibacterial and Antioxidant Properties and Their Application to Food Products: A Review. Polymers vol. 15 (2023). [CrossRef]
- Lee, S., Sirich, T. L. & Meyer, T. W. Improving Solute Clearances by Hemodialysis. Blood Purification vol. 51 20–31 (2022). [CrossRef]
- Suri, R. S. KDOQI Hemodialysis Adequacy Clinical Practice Guideline Update 2015: What You Need to Know Presentation for National Renal Administrators’ Association. (2016).
- van Gelder, M. K., Abrahams, A. C., Joles, J. A., Kaysen, G. A. & Gerritsen, K. G. F. Albumin handling in different hemodialysis modalities. Nephrology Dialysis Transplantation 33, 906–913 (2018). [CrossRef]
- Said, N. et al. Iron oxide nanoparticles improved biocompatibility and removal of middle molecule uremic toxin of polysulfone hollow fiber membranes. J Appl Polym Sci 136, (2019). [CrossRef]
- Ji, H. et al. Advances in Enhancing Hemocompatibility of Hemodialysis Hollow-Fiber Membranes. Advanced Fiber Materials vol. 5 1198–1240 (2023). [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).