1. Introduction
Haemonchus contortus is a blood-feeding parasitic nematode living in the abomasum (stomach) of small ruminants that provokes gastritis, blood-loss in the abomasum, anorexia, anaemia, lethargy, weakness, weight loss, emaciation and, in severe cases, the death of young animals [
1,
2]. This and other genera/species of parasitic nematodes live in the gastrointestinal tract of small ruminants, where they cause severe deterioration of flock health and productivity. Gastrointestinal nematodiasis is controlled by the frequent and continuous administration of chemical anthelmintic drugs to the animals; however, the development of anthelmintic resistance in the parasites occasions an increasing inefficacy of these drugs that has raised the alarm regarding the use of these drugs by farmers around the world [
3,
4]. During recent decades, strategies other than the use of chemical anthelmintic drugs have been explored, including the use of natural antagonists of nematodes, such as a group of micro-fungi called nematode-trapping fungi [
5,
6]. Nematode-trapping fungi are regular microorganisms from the soil mycobiota living as saprophyte organisms and taking their nitrogen and carbon sources from decaying wood and litter; however, they can transform their saprophyte lifestyle into a predatory or parasitic one in the presence of nematodes such that, once trapped, they are used as a food source by fungi [
7]. The species
Arthrobotrys oligospora and
A. musiformis have been classified as Orbiliales fungi [
8]. These species develop three-dimensional adhesive nets from their mycelia where nematodes are trapped and destroyed for eventual use by fungi as their main source of nutrients [
9]. In addition to the mechanical capture exerted by trapping devices, nematode-trapping fungi possess other strategies to kill and penetrate nematodes using myco-chemical constituents, including enzymes and products derived from secondary metabolism [
10]. The objectives of the present study were to isolate nematode-trapping fungi and perform a taxonomical identification via morphological and molecular procedures as well to assess the nematocidal activity of their liquid culture filtrates, either individually or combined, and produced in two liquid media (sweet potato dextrose broth [SPDB] and Czapek-Dox Broth [CzDB]) against
Haemonchus contortus infective larvae and eventually to identify associated groups of myco-compounds.
2. Materials and Methods
2.1. Location
This study was performed at the Laboratory of Helminthology from the National Center of Disciplinary Research in Animal Health and Innocuity (CENID-SAI) in Jiutepec Municipality, Morelos State, Mexico. This centre belongs to INIFAP-Mexico (Agricultura, Mexican Government).
2.2. Nematodes
2.2.1. Obtaining the Free-Living Nematode Panagrellus redivivus for Use as Bait to Isolate Nematophagous Fungi
A population of the free-living nematode
P. redivivus was provided as a fish food by a local pet store in Jiutepec Municipality, Morelos, Mexico. For
en masse reproduction, nematodes were cultured in sterile plastic bowls containing sterile oat flakes (20 g), and 200 mL of sterile distilled water was added. Oat flakes and water were mixed and homogenized to finally obtain a humid mass that was used as a nutrient substrate for bacteria, and in turn, bacteria were used as the main source of food by the free-living nematodes. Bowls were covered with a cap of foil and gauze to prevent mosquitoes from entering. Cultures were incubated at room temperature (18–28 °C) for 7 days [
11]. After the incubation period, some culture material was collected using a metal spoon and dissolved in a glass of water, producing many
P. redivivus specimens swimming in an aqueous suspension. Nematodes were separated from the culture medium through a 74-µm sieve. This step was repeated several times until the nematodes were very clean. Nematodes were resuspended in sterile distilled water and sieved through a coffee filter. Nematodes were eventually recovered using the Baermann funnel technique [
12].
2.2.2. Procurement of Haemonchus Contortus Infective Larvae (L3)
A lamb artificially infected with
H. contortus was previously inoculated (
per os) with 350 infecting larvae per kilogram body weight. After a pre-patent period of 21 days, faecal samples taken directly from the rectum of this lamb tested positive for the presence of nematode eggs by the McMaster technique. Faeces of the infected lamb were collected directly from rectum of this animal. All rules regarding the treatment of animals and the prevention of unnecessary animal suffering were carefully followed according the Norma Oficial Mexicana (Official Mexican Standard) with official rule number NOM-052-ZOO-1995 (
http://www.senasica.gob.mx, accessed on 8 August 2023). Additionally, the Ley Federal de Sanidad Animal (Federal Law for Animal Health) DOF 07-06-2012 was strictly followed in accordance with the ethical standards outlined by INIFAP. Fresh faeces were ground in a plastic bowl and mixed with small pieces of polyurethane foam to obtain a porous mass that retained the oxygen necessary for the optimum development of nematode eggs [
13] (Iliev et al., 2018). Faecal cultures were incubated at room temperature (18–25 °C) for 7 days. After 5–7 days of elaboration of faecal cultures a rather large amount of
H. contortus infective larvae was collected using the Baermann Funnel technique for 24 h [
13,
14]. Infective larvae of the parasite were cleaned by using the differential centrifugation technique with 40% sucrose density gradients [
15]. After centrifugation for 5 min at 3500 rpm, a white ring in the interphase between water and sucrose corresponding to clean larvae was visualized. Larvae were removed with a Pasteur pipette and deposited into assay tubes, which were filled with water. In order to discard sucrose residue from the aqueous suspension of larvae, the assay tubes containing larvae in suspension were centrifuged at the same speed and spinning times, and larvae were sedimented to the base of the assay tubes. The supernatant was discarded and the tubes filled again with sterile water and centrifuged again. After three to four centrifugations, larvae in the sediment were free of sucrose residues.
2.3. Isolation of Nematophagous Fungi
Two 50-g samples of soil from a poultry farm in Cuernavaca Municipality, state of Morelos, Mexico were taken and transported in plastic bags to the Laboratory of Helminthology of CENID-SAI in Jiutepec, Morelos. A small soil sample (approximately 0.5 g) was sprinkled on sterile water agar plates. Plates were incubated at room temperature (18–25 °C) for three days. After this period, some drops of an aqueous suspension containing an undetermined number of specimens of the free-living nematode
P. redivivus were added to each plate in order to promote the growth of nematophagous fungi. After one week, the agar surface was observed under the microscope, and aerial structures typical of nematophagous fungi were seen, including trapping devices, conidiophores and trapped nematodes. These structures were transferred to fresh sterile water agar plates using a sterile metal needle. Plates were maintained at the same temperature, and aerial structures were again transferred to sterile water agar plates. This process was repeated until fungi were eventually obtained in pure culture [
16].
2.4. Procurement of Fungal Liquid Culture Filtrates
Fungi were cultivated in two different liquid culture media: SPDB and CzDB. Briefly, SPDB was prepared using 200 g of organic sweet potato (obtained from an organic farm at the Autonomous University of the State of Morelos (UAEM) in Cuernavaca, city). Sweet potato was peeled and chopped in small, square pieces of approximately 1 cm2. Pieces of sweet potato were cooked in 1 L of distilled water and boiled for 25 min. Subsequently, cooked medium was sieved using a piece of gauze to separate the solid material, and 20 g of dextrose were added to the liquid phase and the volume adjusted to 1 L with distilled water. The liquid was transferred to 250-ml flasks, depositing 50 mL of media in each flask (n=3). Flasks containing the medium were sterilized in an autoclave and allowed to cool. Fifty hundred microliters of penicillin (50 I.U./mL)/streptomycin (50 μg/mL) were added to each flask. This procedure was performed using a laminar flue cabinet. Later on, three plugs (1 cm2) obtained from the surface of a water agar plate containing a 21-day-old fungal culture were deposited in each flask. This procedure was performed for each of the two fungi. The same number of flasks with only liquid medium and without any fungal inoculum was used as a negative control. After the incubation period, liquid culture filtrates were obtained as follows: mycelia were separated by filtration using different kinds of filters and pore diameters, including a Whatman® #4 25 µm filter paper, and passed through three Millipore® filters (2 µm, 0.45 µm and 0.22 µm). Filtration was achieved using a Millipore filtration unit connected to a vacuum pump (Millipore High Performance Pump115 V/60 Hz, Darmstadt, Germany). Filtered media were concentrated using a rotatory evaporator (Buchi® R-300, Switzerland) to eliminate the greatest volume of water without altering the sample characteristics. Finally, evaporated material was lyophilised using a LABCONCO® FreeZone 4.5 plus, U.S.A lyophiliser.
2.5. Traditional Taxonomic Identification of Fungal Isolates by Morphometry
The morphological identification of fungi was performed by both macroscopic and microscopic observations. In the case of macroscopic characteristics of both fungi growing in water-agar and potato dextrose agar were described. Regarding the microscopic characteristics of fungi, aerial structures such as conidia, conidiophores, trapping devices and the presence or absence of chlamydospores, among other characteristics, were observed and analysed under the microscope (20x and 40x). Twenty-five conidia and conidiophores were randomly taken, and their dimensions, i.e. height and width, were measured and recorded. Taxonomic morphometric identification was achieved by comparing the characteristics of our isolates with those described in specialized taxonomic keys [
17,
18,
19]. Additionally, a set of microphotographs of the aerial structures of taxonomical importance were taken in a Leica DM6 B optical microscope.
2.6. Molecular Identification
Fungi were recovered from CzDB cultures and processed to obtain DNA using the Wizard® Genomic DNA Purification Kit (PROMEGA, USA). Genomic material was quantified using an IMPLEN (NanoPhotometer NP80) spectrophotometer. The endpoint PCR technique was performed following the procedures standardized at the Laboratory of Helminthology of CENID-SAI, INIFAP according to the methodology described by Tigano-Milani et al. (1995) [
20]. The Internal Transcribed Spacer (ITS)-1, ITS-2 and 5.8S complete regions and a partial sequence of small 18S and large 28S sub-unit regions were amplified. The selected primers were ITS5-forward (5′-TCC TCC GCT TAT TGA TAT GC-3′) and ITS4-reverse (5′-GGA AGT AAA AGT CGT AAC AAG G-3′) [
21].
The PCR technique is briefly described next. The reaction was carried out in a 20-µL total volume, containing 100 ng of gDNA, 10 µL GoTaq® Green Master Mix 2X (PROMEGA, USA), 1.5 µL of each primer at 20 µM and nuclease-free water to a total volume of 20 µL. The PCR method was carried out using a C1000 Touch® Thermal Cycler (BIORAD, Hercules, CA, USA). The PCR conditions were established as follows: initial denaturation at 94 °C for 3 min; an amplification stage, including 35 cycles of denaturation at 94 °C for 1 min, annealing at 42 °C for 90 s, extension at 72 °C for 90 s; and a final extension stage at 72 °C for 5 min.
The Wizard® SV Gel and PCR Clean-Up System (Promega, Madison, WI, USA) was used to purify the PCR products. Genomic material was sequenced at the Institute of Biotechnology of the National Autonomous University (IBT-UNAM), Cuernavaca city, Morelos, Mexico, using an Applied Biosystem Sequencer (7700; Thermo Fisher Scientific, Waltham, MA, USA). Similarity and coverage of sequences was achieved using the BLAST tool from NCBI. The sequences were compared with the closest sequences previously reported in the NCBI database. Molecular analysis was used to confirm the morphological identification [
22].
2.7. Assessing the Predatory Activity of Fungi against Haemonchus Contortus Infective Larvae
A square piece of water agar (1 cm2) from 10-day-old culture plates of A. oligospora was cut and deposited on the centre of a fresh sterile water agar plate (60 mm diameter). The same procedure was performed with A. musiformis (n=10). All plates were incubated at room temperature (18–25 °C) for 10 days. After this period, 10 other water agar plates without any fungus were prepared and used as a control group. Two hundred H. contortus infective larvae contained into 100 µL of PBS (7.2 pH) were individually added to each plate of the three experimental groups and incubated at the temperature mentioned above for 10 days. After incubation, the agar from every plate was individually transferred to a Baermann funnel apparatus where it remained for 24 h. The face of the agar on which nematodes and fungi were located was placed toward bottom of the assay tubes in order to allow non-trapped larvae to freely migrate and eventually remain sedimented at the bottom of the tubes. Non-trapped larvae from nematode/fungus interactions and whole larvae from control groups were recovered and quantified. The number of larvae in ten 5-µL aliquots from a 3-ml total volume recovered from experimental plates was quantified under a microscope (5×), and the mean numbers of larvae per group were estimated.
The percentage reduction attributed to the predatory effect exerted by fungi was estimated using the Abbott formula:
where:
RLCgroup = Recovered larvae from the control group
RLinteraction = Recovered larvae from the fungi/larvae interaction group
2.8. Assessing the Nematocidal Activity of Fungal Liquid Culture Filtrates against Haemonchus Contortus Infective Larvae
The interaction between Liquid Culture Filtrates (LCF) and
H. contortus infective larvae was carried out using 96-well microtiter plates (n=4). Six experimental groups were established using three different concentrations of LCF in each treatment (100, 50 and 25 mg/mL) as follows: 1)
A. oligospora LCF growth in SPDB; 2)
A. musiformis LCF growth in SPDB; 3) The combination of LCF from both fungi (using half of the volume used in individual LCF). Likewise, groups 4, 5 and 6 were similar to 1, 2 and 3, but LCF of fungi growing in CzDB were used instead of SPDB. Additionally, two groups containing only the SPDB and CzDB media were used to discard any possible lethal effect of these media on the nematodes. Likewise, a group with only PBS and one with 0.5% commercial ivermectin (Ivomec®) were used as negative and positive controls. LCF previously dried were dissolved in PBS (pH = 7.2). Fifty microliters of the corresponding filtrate and 50 µL of an aqueous larval suspension (in PBS) containing approximately 100
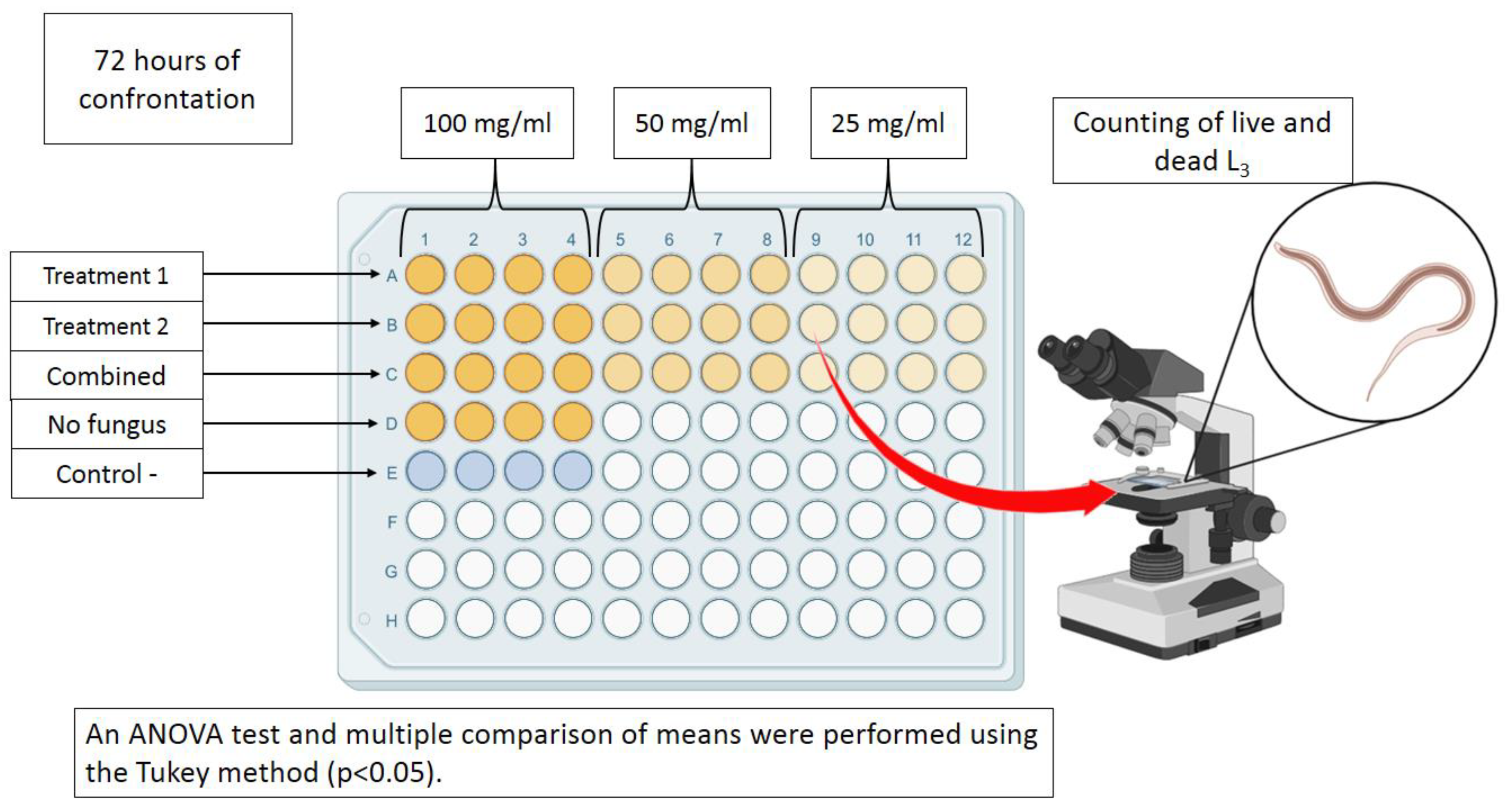
H. contortus infective larvae were deposited in every one of the four wells per treatment. All plates were incubated at room temperature (18–28 °C) for 72 h (
Figure 1).
After incubation, the volume of each well was observed under the microscope, and dead and live larvae were counted following the previously described procedure. Motionless larvae were determined to be alive or dead by applying a physical stimulus by touching its cuticle using a sharp metallic needle. Live larvae normally start to move actively after this stimulus. Larvae that remain motionless after this stimulus are considered as dead larvae [
23]. The whole experiment was repeated three times. Larval mortality obtained for each treatment and control group was calculated. The larval mortality attributed to the effect of LCF of each fungus and each concentration was estimated using the following formula:
where:
Meam L3 Treated group = Mean of larval mortality from treated group.
Mean L3 Control group = Mean of larval mortality from control group.
2.9. Microscopic Analysis of Haemonchus Contortus Infective Larvae Exposed to Fungal Liquid Culture Filtrates
After exposure of H. contortus larvae to LCF, a random selection of larvae from the different treatments was carried out in order to photograph possible morphological changes attributed to the effect of compounds present in the fungal filtrates. Larvae were photographed using a LEICA DM6 compound microscope using the program LAS V4.9 to document our findings.
2.10. Myco-Chemical Profile
A myco-quantitative reagent analysis was carried out using standard procedures with the proper reagents and methods. Alkaloids were determined using Dragendorff Mayer and Wagner´s reagents, and the Bornträger test was performed to identify the presence of coumarins. Likewise, the presence of flavonoids was investigated using Mg
2+ and HCl tests. On the other hand, the ferric chloride, gelatine and saline solution tests were carried out to identify tannins. Finally, the Lieberman–Burchard and Salkowski tests were used to identify the presence of Triterpenes [
24].
2.11. Statistical Analysis
Data obtained from the predatory activity assay of both fungal isolates were individually analysed by the Student’s t-test, where the larval reduction percentage was obtained by comparing the mean number of recovered larvae from the nematode/fungi interaction plates and from the control group without fungus. For the results of the nematocidal activity of LCF, a completely random model was used, and ANOVA was performed where the means of larval mortality in the different treatments was considered as the dependent variable. An orthogonal contrast by the Bonferroni method was performed to compare the effect of the combination of both LCF from fungi grown in the same media using the following coefficients: 0.5(AspT) + 0.5(AspG) – 1(combined, 50:50). A p value of 0.05 was used for all tests.
3. Results
3.1. Traditional Taxonomy (Morphometrics)
After the main structures of taxonomic interest were observed and analysed under the microscope (including conidia and conidiophore measurements) the morphological characteristics were compared with those described in taxonomic keys, and the authors eventually decided to classify these fungi as
A. oligospora and
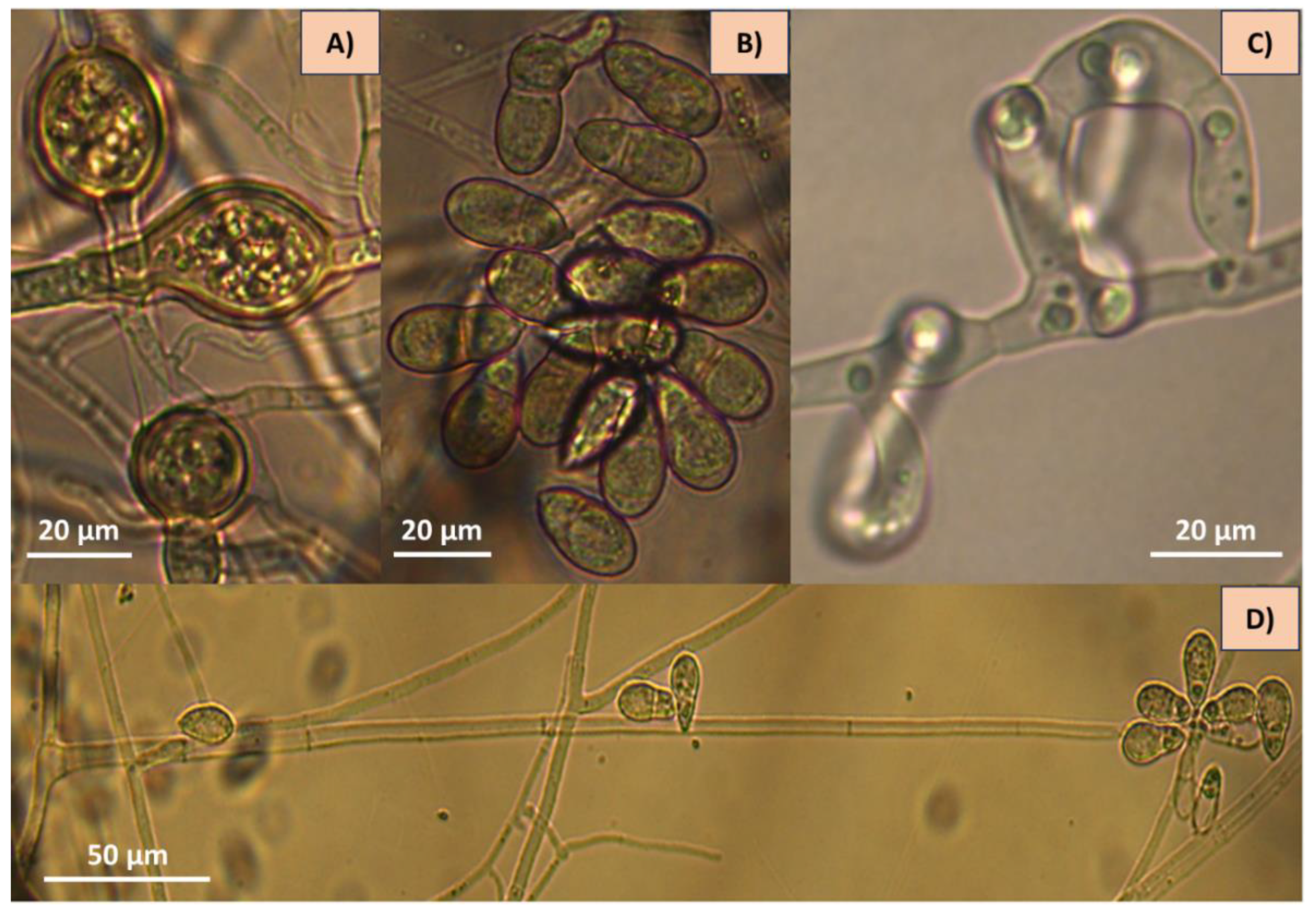
A. musiformis. The isolate AspT showed hyphae, conidia and hyaline conidiophores with repeated proliferation of conidia. Conidia showed a globose shape, lightly constricted in their septum. This fungus showed the presence of three-dimensional adhesive nets (
Figure 2).
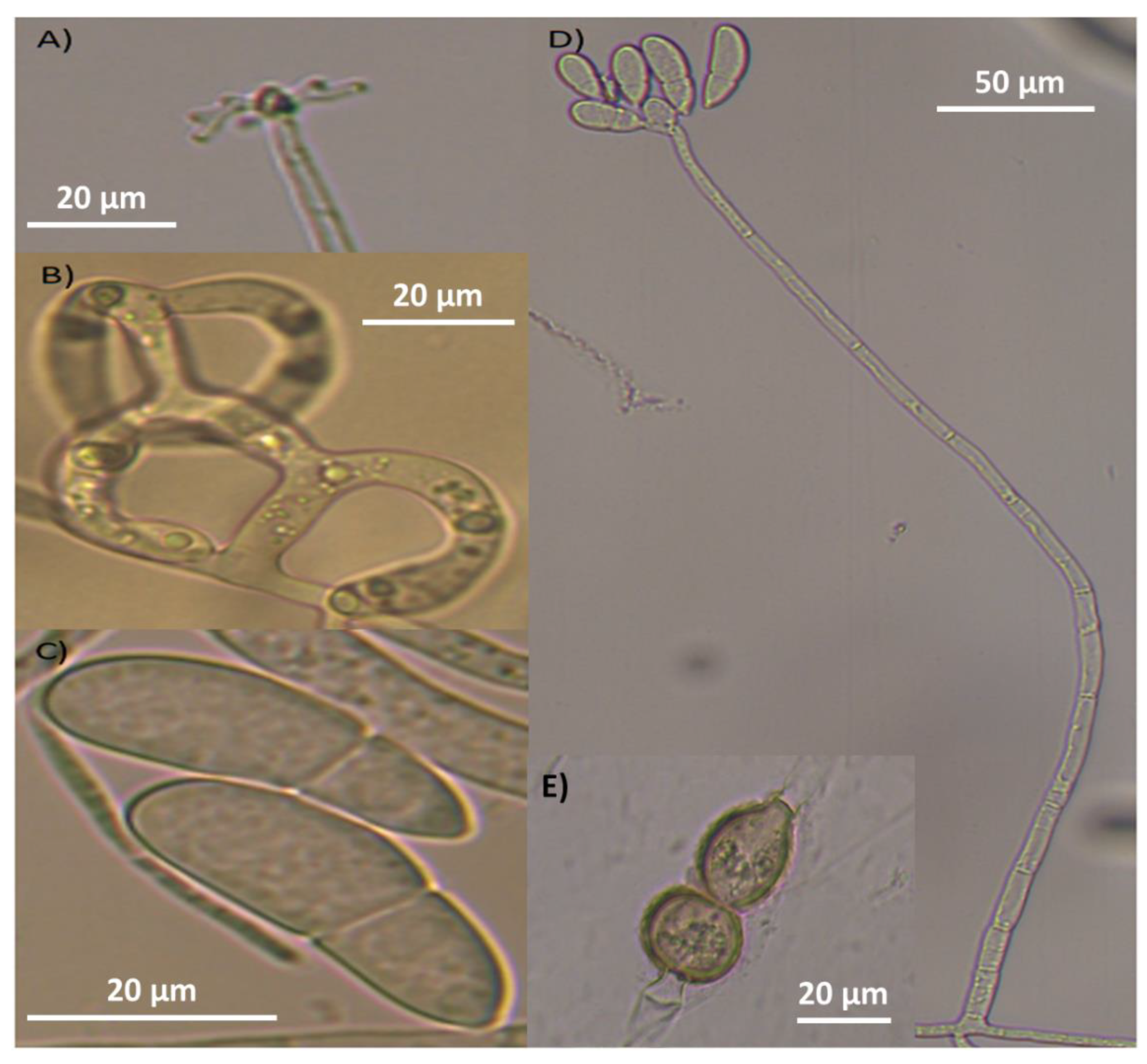
Likewise, the analysis of the isolate AspG showed hyaline hyphae, conidia and conidiophores. Nevertheless, conidiophores showed long and cylindrical denticles, produced by candelabra, where conidia were generated. Conidia were elongated and slightly curved, typical of
A. musiformis. This isolate showed the same trapping devices corresponding to the three-dimensional adhesive nets described above (
Figure 3).
The summarized information about measurements and observations of the main morphological characteristics of both isolates are shown in
Table 1.
3.2. Molecular Identification
Once nucleotide sequences from both fungi were analysed by alignment with the sequences previously reported in the NCBI database, query covers and similarity percentages were obtained (
Table 2 and
Table 3). The analysis shows a high similarity (98.52%–99.54%) between the strain AspT and sequences from the species
A. oligospora. On the other hand, sequences from AspG had high similarity with the species
A. eryuanensis (MT612105.1) and
A. musiformis (99.32% for both species).
3.3. Predatory Activity Assessment of the Two Nematophagous Fungal Isolates against Haemonchus Contortus Infective Larvae
The mean numbers of
H. contortus infective larvae recovered from agar plate groups with and without fungi are shown in
Table 4.
3.4. Microscopic Findings
Haemonchus contortus infective larvae trapped in three-dimensional adhesive nets of
A. oligospora and
A. musiformis are shown in
Figure 4A and
Figure 4B, respectively. In both images, hyphae are observed invading the nematode corps.
3.5. Assessment of the In Vitro Nematocidal Activity of Liquid Culture Filtrates of Arthrobotrys oligospora and Arthrobotrys musiformis Produced in SPDB and CzDoxB against Haemonchus contortus Infective Larvae
The results regarding the larval mortality of
H. contortus attributed to the effect of LCF of both nematophagous fungi either individually or in combination (50:50) grown in the two evaluated media are shown in
Table 5 and
Table 6, respectively.
On the other hand, the comparison between the larval mortality obtained in the different LCF concentrations with both fungi and both media (SPDB and CzDB) are shown in
Table 7 and
Table 8, respectively.
3.6. Microscopic Findings Regarding Haemonchus contortus Infective Larvae Exposed to Liquid Culture Filtrates of Two Nematophagous Fungi Grown in Sweet Potato Dextrose Broth and Czapek-Dox Broth
A set of microphotographs showing morphological changes identified in
H. contortus infective larvae after exposure to liquid culture filtrates of
A. musiformis and
A. oligospora are shown in
Figure 5. Images A and C show the aspects of
H. contortus infective larvae after 72 h exposure to LCF of
A. musiformis and
A. oligospora, respectively (grown in CzDB) assessed at 100 mg/mL. In these images a thickening of the larval bodies was observed in some areas; meanwhile, in other areas a diminishing of the body thickness was visualized. In both cases a clear loss of the intestinal cellular structural architecture was observed (c).
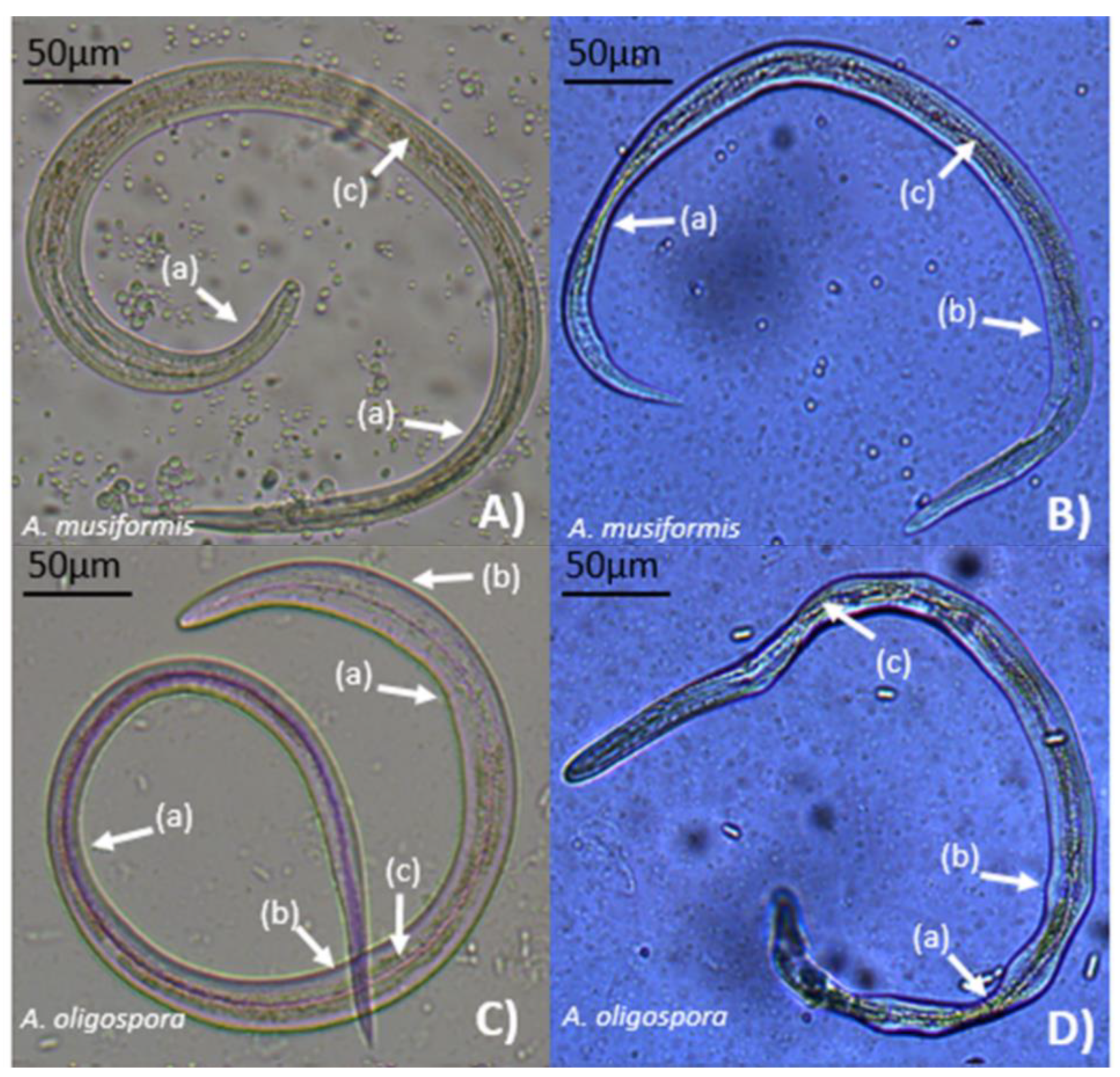
On the other hand, larvae exposed to LCF of both fungi grown in SPDB (B) and (D) showed similar damage, but more severe in SPDB, and a general deformation of larval bodies with a slimming in some areas of the nematode corps (a) and lose of turgor body in other areas (b) was observed.
Figure 5.
Microphotographs showing the aspect of Haemonchus contortus infective larvae after 72 h exposure to liquid culture filtrates obtained with two nematophagous fungi Arthrobotrys musiformis and A. oligospora grown in Czapek-Dox Broth (A) and (C) and Sweet Potato Dextrose Broth (B) and (D).
Figure 5.
Microphotographs showing the aspect of Haemonchus contortus infective larvae after 72 h exposure to liquid culture filtrates obtained with two nematophagous fungi Arthrobotrys musiformis and A. oligospora grown in Czapek-Dox Broth (A) and (C) and Sweet Potato Dextrose Broth (B) and (D).
3.7. Myco-Chemical Compound Profile
The results of the myco-quantitative reagent analysis obtained with LCF of both fungi in both culture media are shown in
Table 9.
4. Discussion
4.1. Traditional Taxonomy through Morphological Characteristics
At first sight, the growth of erect and long-stem apical conidiophores crowned by conidia clusters of globose form, as well as the presence of one septum almost at the middle of the conidia in the Asp-T strain suggested to us the presence of a nematophagous fungus belonging to the genus
Arthrobotrys [
18]. However, there are several species sharing these characteristics, and this fact can cause confusion in the taxonomic identification. Some of the species sharing these similar characteristics are
A. oligospora,
A. robusta,
A. superba,
A. conoides and
A. arthrobotryoides, among others. After observing the characteristics in more detail, including the measurements of some structures, i.e. conidia length and width, the number of conidia in the clusters, the presence or absence of branched conidiophores, the presence or absence of chlamydospores and particularly, the type of trapping devices, we were able to differentiate our isolate from other species. Because our isolate showed only unbranched conidiophores, and the measurements of conidia and the length of conidiophores were similar to those of species described in the taxonomical identification keys, we were able to discard several species that do not share these characteristics.
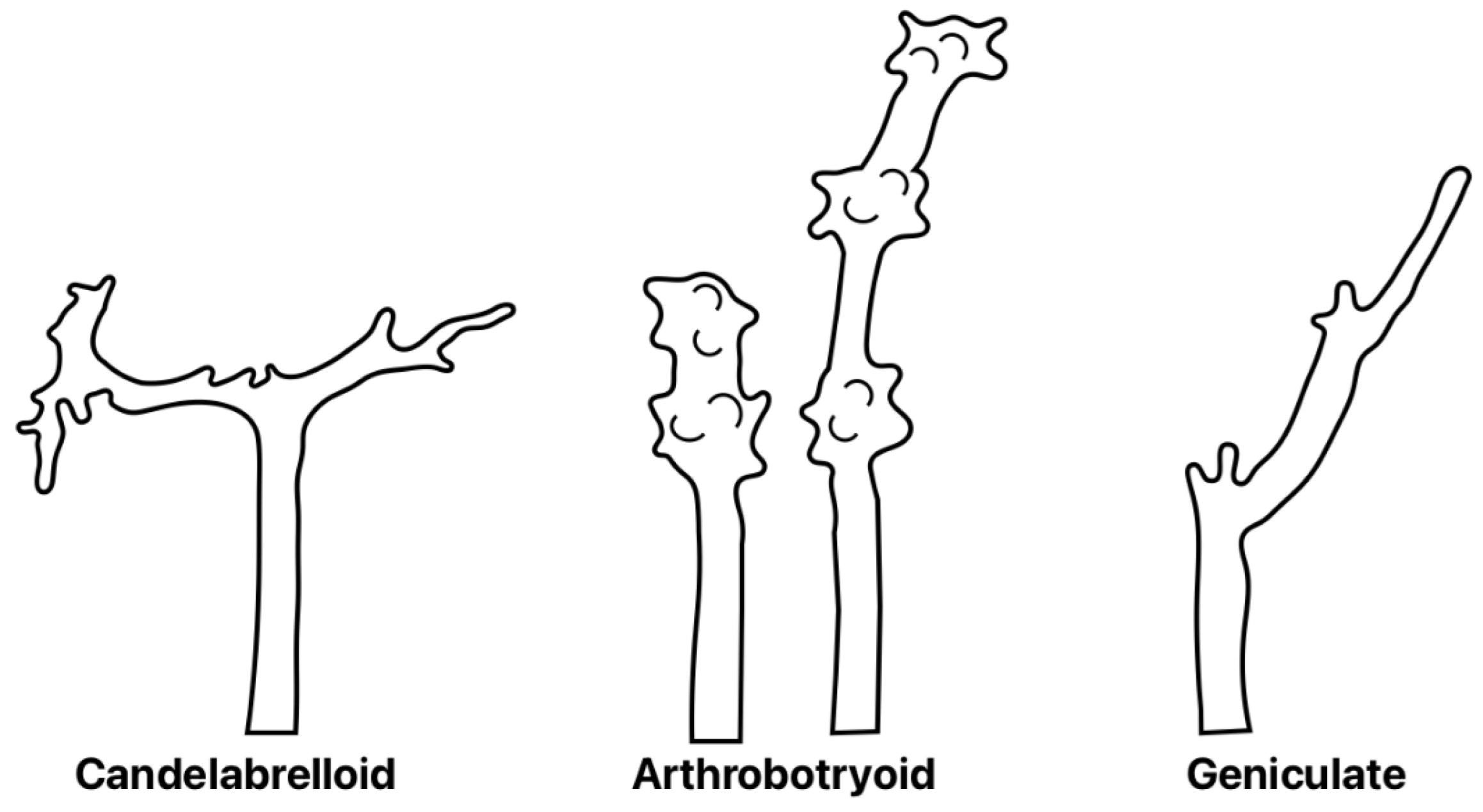
Additionally, our isolate showed an important taxonomical characteristic that is the type of conidiophore at the top, precisely where the conidiophore produces denticles where conidia are generated (conidiogenesis). Such denticles are typical of Arthrobotryoid conidiophores (
Figure 6A), and this characteristic differs from those of other species such as
A. musiformis and
A. javanica that, instead of these denticles, form a candelabrum where conidia are produced. The presence of conidiophores with denticle formation is typical of the species
A. oligospora (
Figure 6). It is important to mention that this isolate showed the presence of chlamydospores; as well as the formation of three-dimensional adhesive nets as trapping devices. These characteristics led us to classify our isolate as
A. oligospora on the basis of morphological analysis. Regarding the other fungal isolate recorded as Asp-G, this isolate showed a conidia cluster different to that of
A. oligospora. Instead, this fungus showed the presence of 4–8 obovoidal, elongated, slightly curved and slightly constricted conidia. There was a septum slightly situated below the middle of conidia. Elongated conidia were arranged laterally or slightly above the conidiophores. This isolate also showed the presence of the same trapping devices as
A. oligospora, three-dimensional adhesive nets. This fungus also showed the presence of chlamydospores but possessed candelabrelloid conidiophores typical of
A. musiformis. This structure resembles an elk horn where conidia are developed (
Figure 6B).
4.2. Molecular Taxonomy
After analysing the DNA sequences of the AspT strain and comparing this information with the sequences of isolates previously reported in the NCBI database, high coverage (96%–99%) and similarity (98.86%–99.54%) values with respect to fungi from the species
Orbilia oligospora and
A. oligospora were found. It seems that both species are synonyms according to recent nomenclature reported in the NCBI database [
25] (Taxonomy ID: 2813651, NCBI:txis2813651). On the other hand,
A. musiformis matched those sequences previously reported in the NCBI database with high levels of coverage (99.32) corresponding to
A. musiformis (MH855842.1., KP859624.1, OL454931.1) and
A. eryuanensis (MT612105.1). However, there are some remarkable morphological differences between
A. musiformis and
A. eryuanensis. For example, the length of erect conidiophores in
A. musiformis can reach up to 900 μM [
18]; meanwhile,
A. eryuanensis reach a length range of 110–308 µm, and this species also produces macro- and microconidia [
19].
4.3. Nematode-Predatory Activity of Fungal Isolates
The results regarding the predatory activity assessment show reduction percentages of
H. contortus infective larvae attributed to the predatory effect of both species of nematophagous fungi: moderate (around 45%) in
A. oligospora and higher than 70% in
A. musiformis. In the literature consulted, we found several reports where different strains of
A. musiformis obtained from different sources showed variable results in terms of their predatory activity against ruminant parasitic nematodes, i.e. Cao et al. (2018) [
26], using Chinese isolates of this species, reported a range of 89.02% to 94.80% reduction of infective larvae of the nematode
Trichostrongylus colubriformis [
26]. Likewise, an
A. musiformis strain isolated from a soil sample from Cuautla municipality, Morelos state, Mexico showed 71.54% larval mortality using
H. contortus (L3) as a target [
22]. Other isolates of
A. oligospora and
A. musiformis have shown variable results regarding predatory capability against infective larvae of ruminant parasitic nematodes and other nematode targets (
Table 10). In this regard, we can build a reflection, the biological behaviour of nematophagous fungi is a dynamic process and it depends on the adaptation to environmental circumstances of each microorganism from the soil microbiota; such variation in the ability to form traps and capture nematodes of different taxonomic groups can be attributed to the influence of diverse abiotic and biotic factors in fungal habitats [
27].
4.4. Nematocidal Activity of Fungal Liquid Culture Filtrates
When liquid culture filtrates of both fungi were used individually at the highest concentration (100 mg/mL) in the two assessed media, significant larval mortality was obtained. It is interesting that in both cases, when LCF were used, either individually or in combination, similar mortality values were found, ranging from 75.6% to 92.6%. No difference in the effect of individual or combined LCF was observed, since no statistical difference was observed in any of the cases. Although the concentration of LCF at which a lethal effect against
H. contortus was observed can be considered high, is clear that one or more mycocompounds derived from the secondary metabolism of both fungi are responsible for the nematocidal activity. This result is important, since it is the first step of compound purification using chromatographic procedures, i.e. gas chromatography–mass spectrometry (GCeMS), following a bio-guided assay to identify potential bioactive molecules as natural de-wormers against ruminant parasitic nematodes [
30,
32]. The technique of nuclear magnetic resonance could be an alternative method to identify the bioactive compound, although this procedure requires highly purified fractions [
33]. After a rigorous study on the safety of administration in animals, followed by its innocuity to human beings and the absence of a negative environmental impact, these compounds could have significant implications as natural anthelmintics to at least partially replace the use of chemically synthesized, commercially available drugs. Regarding the synergistic effect that we originally proposed as a hypothesis, we did not find this effect, since LCF of both fungi showed an important lethal effect against nematodes at the highest assessed concentration.
It is important to mention that at concentrations lower than 100 mg/mL, the results showed wide variability with very low ranges of lethality, in comparison with the highest concentration. The LCF of A. oligospora at 25 mg/mL in SPDB showed only 12.1% lethality; meanwhile, the highest lethality of A. musiformis (>51%) was observed at 50 mg/mL in CzDoxB. The large variability in the results at 25 and 50 mg/mL did not allow us to build a solid criterion about the bioactivity of these LCF.
4.5. Microscopic Analysis of Haemonchus contortus after Exposure to Fungal Liquid Culture Filtrates
After analysing the images captured by microscopy, we observed malformations in different sites of the larval bodies exposed to both LCFs. The larvae exposed to LCF from both fungi obtained from CzDoxB showed some morphological changes; however, the damage to those larvae exposed to LCF obtained from both fungi in SPBD showed more severe morphological alterations. These findings suggest that the culture media can influence the production of bioactive mycocompounds derived from the secondary metabolism of fungi. In future studies, we plan to use confocal laser scanning microscopy to perform a co-localization analysis to determine the site of action and the damage caused by metabolites responsible of the nematocidal activity [
34].
4.6. Mycochemical Compound Profile
The groups of secondary metabolites found after mycochemical compound analysis of LCF obtained in both liquid media (CzDoxB and SPDB) of the two nematophagous fungi (A. musiformis and A. ligospora) were alkaloids with a light reaction in the four treatments: coumarins with a light reaction in A. musiformis in SPDB and in A. oligospora in both media. Regarding the presence of triterpenes, this group of compounds was found with a light reaction in LCF of A. musiformis in SPDB and in A. oligospora in CzDoxB; however, in A. oligospora a positive reaction was found in SPDB and a strong reaction in CzDoxB. These findings show that A. oligospora, despite not showing high predatory activity of H. contortus infective larvae using physical mechanisms (three-dimensional adhesive nets), in certain respects, compensate for this lack of a strong predatory activity by producing a large number of secondary metabolites with nematocidal activity, which could contribute to immobilization and killing of nematodes.
5. Conclusions
In the present study, no difference was found in the nematocidal activity of LCF of A. oligospora and A. musiformis, used individually or in combination, against H. contortus infective larvae. Liquid culture filtrates of both fungi exerted an important nematocidal effect at the highest concentration (100 mg/mL) when used either individually or in a combined manner. The results of the present study contribute important information about the predatory activity of these species of nematophagous fungi and additionally about the nematocidal activity of LCF produced by these fungi that could have implications for future studies exploring the use of this LCF or the metabolites responsible for nematocidal activity as potential tools of control against sheep haemonchosis.
Author Contributions
Conceptualization, PMG and GPA; methodology, ACP; software, GABG; validation, AOJ and EGM; formal analysis, EJDN; investigation, ACP; resources, PMG; data curation, AOJ and GPA; writing—original draft preparation, PMG; writing—review and editing, PMG and GPA; supervision, PMG and GPA; project administration, PMG; funding acquisition, PMG. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The animal that acted as an egg donor of the parasite was maintained under indoor conditions, following the rules of care, respect and animal welfare and especial attention was put in avoiding any unnecessary animal suffering. These conditions were strictly performed according to the Good Management Practices policies established at INIFAP. The Norma Oficial Mexicana (Official Mexican Standard) with official rule number NOM-052-ZOO-1995 (
http://www.senasica.gob.mx, accessed on 6 February 2024) and the Ley Federal de Sanidad Animal (Federal Law for Animal Health) DOF 07-06-2012 were strictly applied (
https://www.gob.mx/cms/uploads/attachment/file/118761/LFSA.pdf, accessed on 6 February 2024) in accordance with the ethical standards outlined by INIFAP.
Acknowledgments
This study did not receive any specific budget. The reagents, consumables, and other materials as well as the equipment were supplied by the Helminthology Laboratory of CENID-SAI, INIFAP. This study formed part of the thesis work of Antonio Colinas Picazo to obtain the grade of DVM at the Universidad Mesoamericana, Cuernavaca, Morelos, Mexico, under the direction of Dr Pedro Mendoza-de Gives and tutored by MSc Gustavo Pérez-Anzúrez.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Besier, R.B.; Kahn, L.P.; Sargison, N.D.; Van Wyk, J.A. Diagnosis, treatment and management of Haemonchus contortus in small ruminants. Adv. Parasitol. 2016, 93, 181–238. [Google Scholar] [CrossRef] [PubMed]
- Flay, K.J.; Hill, F.I.; Muguiro, D.H. A Review: Haemonchus contortus infection in pasture-based sheep production systems, with a focus on the pathogenesis of anaemia and changes in haematological parameters. Animals 2022, 12, 1238. [Google Scholar] [CrossRef] [PubMed]
- Höglund, J.; Gustafsson, K.; Ljungström, B.L.; Skarin, M.; Varady, M.; Engström, F. Failure of ivermectin treatment in Haemonchus contortus infected-Swedish sheep flocks. Vet Parasitol Reg Stud Reports 2015, 1–2, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Martindah, E.; Sawitri, D.H.; Wardhana, A.H.; Ekawasti, F.; Dewi, D.A. Anthelmintic Resistance Status in Gastrointestinal Nematodes of Seven Different Breeds of Sheep in intensive management. IOP Conf. Ser. Earth Environ. 2023, 1174, 012030. [Google Scholar] [CrossRef]
- Jiang, X.; Xiang, M.; Liu, X. Nematode-trapping fungi. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Olmedo-Juárez, A.; Mendoza-de Gives, P. Control and prevention of nematodiasis in small ruminants: Background, challenges and outlook in Mexico. Rev. Mex. Cienc. Pecu. 2021, 12, 186–204. [Google Scholar] [CrossRef]
- Nordbring-Hertz, B. Morphogenesis in the nematode-trapping fungus Arthrobotrys oligospora—An extensive plasticity of infection structures. Mycologist 2004, 18, 125–133. [Google Scholar] [CrossRef]
- Index Fungorum. Available online: https://www.indexfungorum.org/names/namesrecord.asp?RecordID=149365 (accessed on 10 March 2024).
- Hyde, K.; Swe, A.; Zhang, K.Q. Nematode-Trapping Fungi. In Nematode-Trapping Fungi. Fungal Diversity Research Series; Zhang, K.Q., Hyde, K., Eds.; Springer: Dordrecht, 2014; Volume 23, pp. 1–12. [Google Scholar] [CrossRef]
- Rahman, M.U.; Chen, P.; Zhang, X.; Fan, B. Predacious Strategies of Nematophagous Fungi as Bio-Control Agents. Agronomy 2023, 13, 2685. [Google Scholar] [CrossRef]
- de Lara, R.; Castro, T.; Castro, J.; Castro, G. Cultivo del nematodo Panagrellus redivivus (Goodey, 1945) en un medio de avena enriquecida con Spirulina sp. Rev. Biol. Mar. Oceanogr. 2007, 42, 29–36. [Google Scholar] [CrossRef]
- Valcárcel-Sancho, F.; Rojo-Vázquez, F.A.; Olmeda-García, A.S.; Arribas-Novillo, B.; Márquez-Sopeña, L.; Fernando-Pat, N. Atlas de Parasitología Ovina. Editorial Servet. Zaragoza, España, 2009. 137p, ISBN 978-84-92569-05-2.
- Iliev, P.T.; Ivanov, A.; Prelezov, P. Effects of temperature and desiccation on survival rate of Haemonchus contortus infective larval stage. Trakia J. Sci. 2018, 16, 17. [Google Scholar] [CrossRef]
- Cedillo-Borda, M.; López-Arellano, M.E.; Reyes-Guerrero, D.E. In vitro assessment of ivermectin resistance and gene expression profiles of P-glycoprotein genes from Haemonchus contortus (L3). Bio-protocol 2020, Bio-101, e3851. [Google Scholar] [CrossRef]
- Reyes-Guerrero, D.E.; Cedillo-Borda, M.; Alonso-Morales, R.A.; Alonso-Díaz, M.A.; Olmedo-Juárez, A.; Mendoza-de-Gives, P.; López-Arellano, M.E. Comparative study of transcription profiles of the P-glycoprotein transporters of two Haemonchus contortus isolates: Susceptible and resistant to ivermectin. Mol. Biochem. Parasitol. 2020, 238, 111281. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Martínez, E.; Aguilar-Marcelino, L.; Hernandez-Romano, J.; Castañeda-Ramírez, G.S.; Mendoza-de Gives, P. Taxonomic and biological characterization and predatory activity of four nematophagous fungi isolates of Arthrobotrys species from Tapachula, Chiapas, Mexico. Arch. Agron. Soil Sci. 2023, 69, 327–343. [Google Scholar] [CrossRef]
- Dreschsler, C. Some hyphomycetes that prey on free-living terricolous nematodes. Mycologia 1937, 29, 447–552. [Google Scholar] [CrossRef]
- CAN Oorschot. Taxonomy of the Dactylaria complex V. A review of Arthrobotrys and allied genera. Stud. Mycol. 1985, 26, 61–96. [Google Scholar]
- Zhang, F.; Boonmee, S.; Bhat, J.D.; Xiao, W.; Yang, X.Y. New Arthrobotrys Nematode-Trapping Species (Orbiliaceae) from Terrestrial Soils and Freshwater Sediments in China. J. Fungi. 2022, 8, 671. [Google Scholar] [CrossRef] [PubMed]
- Tigano-Milani, M.S.; Honeycutt, R.J.; Lacey, L.A.; Assis, R.; McClelland, M.; Sobral, B.W. Genetic variability of Paecilomyces fumosoroseus isolates revealed by molecular markers. J. Invertebr. Pathol. 1995, 65, 274–282. [Google Scholar] [CrossRef] [PubMed]
- White, T.J.; Bruns, T.; Lee, S.J.W.T.; Taylor, J. Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. PCR protoc.: A Guide Methods Appl. 1990, 18, 315–322. [Google Scholar]
- Pérez-Anzúrez, G.; Olmedo-Juárez, A.; Von-Son de Fernex, E.; Alonso-Díaz, M.Á.; Delgado-Núñez, E.J.; López-Arellano, M.E.; González-Cortázar, M.; Zamilpa, A.; Ocampo-Gutierrez, A.Y.; Paz-Silva, A.; Mendoza-de Gives, P. Arthrobotrys musiformis (Orbiliales) Kills Haemonchus contortus Infective Larvae (Trichostronylidae) through Its Predatory Activity and Its Fungal Culture Filtrates. Pathogens 2022, 11, 1068. [Google Scholar] [CrossRef]
- Olmedo-Juárez, A.; Rojo-Rubio, R.; Zamilpa, A.; Mendoza-de Gives, P.; Arece-García, J.; López-Arellano, M.E.; von Son-de Fernex, E. In vitro larvicidal effect of a hydroalcoholic extract from Acacia cochliacantha leaf against ruminant parasitic nematodes. Vet. Res. Commun. 2017, 41, 227–232. [Google Scholar] [CrossRef]
- Delgado-Núñez, E.J.; Zamilpa, A.; González-Cortazar, M.; Olmedo-Juárez, A.; Cardoso-Taketa, A.; Sánchez-Mendoza, E.; Tapia-Maruri, D.; Salinas-Sánchez, D.O.; Mendoza-de Gives, P. Isorhamnetin: A nematocidal flavonoid from Prosopis laevigata leaves against Haemonchus contortus eggs and larvae. Biomolecules 2020, 10, 773. [Google Scholar] [CrossRef] [PubMed]
- Baral, H.O.; Weber, E.; Marson, G. Monograph of Orbiliomycetes (Ascomycota) based on vital taxonomy. Part II., 1st ed.; National Museum of Natural History: Luxembourg, Luxembourg, 2020; 1752p, ISBN 978-2-919877-24-9. [Google Scholar]
- Cao, X.; Xu, Q.; Wan, X.M.; Yang, X.C.; Jia, X.Y.; Xue, X.J.; Du, J.L.; Cai, K.Z. Isolation, identification, and in vitro predatory activity of nematophagous fungus Arthrobotrys musiformis and its interaction with nematodes using scanning electron microscopy. Biocontrol Sci. Techn. 2018, 28, 94–104. [Google Scholar] [CrossRef]
- Dhawan, S.C.; Narayana, R.; Babn, N.P. Influence of abiotic and biotic factors on growth of Paecilomyces lilacinus, Arthrobotrys oligospora and Pochonia chlamydosporia and parasitization of eggs/trapping of Meloidogyne incognita juveniles. Ann. Plant Prot. Sci. 2004, 12, 369–372. [Google Scholar]
- Alfaro-Gutiérrez, I.C.; Mendoza-de Gives, P.; Liébano Hernández, E.; López Arellano, M.E.; Valero Coss, R.O.; Hernández Velázquez, V.M. Nematophagous fungi (Orbiliales) capturing, destroying and feeding on the histotrophic larvae of Haemonchus contortus (Nematoda: Trichostrongylidae). Rev. Mex. Micol. 2011, 33, 29–35. [Google Scholar]
- Ojeda-Robertos, N.F.; Aguilar-Marcelino, L.; Olmedo-Juárez, A.; Luna-Palomera, C.; Peralta-Torres, J.A.; López-Arellano, M.E.; Mendoza-de Gives, P. In vitro predatory activity of nematophagous fungi isolated from water buffalo feces and from soil in the Mexican southeastern. Rev. Bras. Parasitol. Vet. 2019, 28, 314–319. [Google Scholar] [CrossRef] [PubMed]
- Jaramillo-Tlalapango, J.; Mendoza-de Gives, P.; Higuera-Piedrahita, R.I.; Ocampo-Gutiérrez, A.Y.; López-Arellano, M.E.; Pérez-Anzúrez, G.; Olmedo-Juárez, A.; Hernández-Romano, J.; Maza-López, J.; Delgado-Núñez, E.J.; González-Cortázar, M. Study of a Mexican isolate of Arthrobotrys musiformis (Orbiliales): Predatory behavior and nematocidal activity of liquid culture filtrates against Haemonchus contortus (Trichostrongylidae), protein profile and myco-constituent groups. Fungal Biol. 2023, 127, 1345–1361. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Yang, Z.; Jiang, Z.; Nizamani, M.M.; Zhang, H.; Liu, M.; Shan, W.; Wang, Y.; Li, K. Isolation, Identification, and Evaluation of the Predatory Activity of Chinese Arthrobotrys Species towards Economically Important Plant-Parasitic Nematodes. J. Fungi. 2023, 9, 1125. [Google Scholar] [CrossRef]
- Fiehn, O. Metabolomics by gas chromatography–mass spectrometry: Combined targeted and untargeted profiling. Curr. Protoc. Mol. Biol. 2016, 114, 30–34. [Google Scholar] [CrossRef]
- DiBello, M.; Healy, A.R.; Nikolayevskiy, H.; Xu, Z.; Herzon, S.B. Structure Elucidation of Secondary Metabolites: Current Frontiers and Lingering Pitfalls. Acc. Chem. Res. 2023, 56, 1656–1668. [Google Scholar] [CrossRef]
- Cortes-Morales, J.A.; Zamilpa, A.; Salinas-Sánchez, D.O.; González-Cortazar, M.; Tapia-Maruri, D.; Mendoza-de Gives, P.; Rivas-Gonzáles, J.M.; Olmedo-Juárez, A. In vitro ovicidal effect of p-coumaric acid from Acacia bilimekii aerial parts against Haemonchus contortus. Vet. Parasitol. 2023, 320, 109971. [Google Scholar] [CrossRef]
Figure 1.
Scheme representing the steps of the experimental process to evaluate the lethal effect of liquid culture filtrates obtained with two nematophagous fungi Arthrobotrys oligospora and A. musiformis growth in two culture media, Sweet Potato Dextrose Broth and Czapek-Dox Broth, against Haemonchus contortus infective larvae.
Figure 1.
Scheme representing the steps of the experimental process to evaluate the lethal effect of liquid culture filtrates obtained with two nematophagous fungi Arthrobotrys oligospora and A. musiformis growth in two culture media, Sweet Potato Dextrose Broth and Czapek-Dox Broth, against Haemonchus contortus infective larvae.
Figure 2.
Microphotographs of Arthrobotrys oligospora showing structures of taxonomic importance: A) chlamydospores, B) conidia, C) three-dimensional adhesive nets and D) conidiophore.km.
Figure 2.
Microphotographs of Arthrobotrys oligospora showing structures of taxonomic importance: A) chlamydospores, B) conidia, C) three-dimensional adhesive nets and D) conidiophore.km.
Figure 3.
Arthrobotrys musiformis structures of taxonomic importance: A) Candelabrum; B) Three-Dimensional adhesive net; C) Conidia; D) Conidiophore and E) Chlamydospores.
Figure 3.
Arthrobotrys musiformis structures of taxonomic importance: A) Candelabrum; B) Three-Dimensional adhesive net; C) Conidia; D) Conidiophore and E) Chlamydospores.
Figure 4.
Aspect of Haemonchus contortus infective larvae captured in three-dimensional adhesive nets of two nematophagous fungi, Arthrobotrys oligospora (A) and Arthrobotrys musiformis (B).
Figure 4.
Aspect of Haemonchus contortus infective larvae captured in three-dimensional adhesive nets of two nematophagous fungi, Arthrobotrys oligospora (A) and Arthrobotrys musiformis (B).
Figure 6.
Aspects of different types of apical extremes of conidiophores of different nematophagous fungi.
Figure 6.
Aspects of different types of apical extremes of conidiophores of different nematophagous fungi.
Table 1.
Main morphological characteristics observed in the two isolates of nematophagous fungi belonging to the genus Arthrobotrys and means of measurement of taxonomically important structures.
Table 1.
Main morphological characteristics observed in the two isolates of nematophagous fungi belonging to the genus Arthrobotrys and means of measurement of taxonomically important structures.
| Characteristic |
Strain 1 (AspT) |
Strain 2 (AspG) |
| Conidium shape |
Globose, slightly constricted in the septum (in occasions). |
Ellipsoidal to obovoidal, slightly curved |
| Septum |
One septum situated slightly below the half conidium |
One septum situated slightly below the half conidium |
| Number of conidia per conidiophore |
6 (4-8) |
7 (3-11) |
| Conidia length (µm) |
21.1 (19-23) |
32.7 (27-40) |
| Conidia width (µm) |
11.4 (10-12) |
13 (11-17) |
| Conidiophores length (µm) |
344 (193-429) |
291 (141-403) |
| Identified species |
Arthrobotrys oligospora |
Arthrobotrys musiformis |
Table 2.
Query covers and similarity percentages of the isolate (AspT) (Arthrobotrys oligospora) in relation to the first five isolates found in the NCBI-Blast alignment.
Table 2.
Query covers and similarity percentages of the isolate (AspT) (Arthrobotrys oligospora) in relation to the first five isolates found in the NCBI-Blast alignment.
| Isolate (Genus/Species) |
Query cover % |
Similarity % |
Gen Bank Accession number NCBI |
| Orbilia oligospora |
99 |
99.41 |
OQ781152.1 |
| O. oligospora |
98 |
98.86 |
MZ427471.1 |
| Arthrobotrys oligospora |
96 |
99.54 |
MF948413.1 |
| O. oligospora |
97 |
98.95 |
ON114061.1 |
| A. oligospora |
99 |
98.52 |
KC663625.1 |
Table 3.
Query covers and similarity percentages of the isolate (AspG) (Arthrobotrys musiformis) in relation to the first five isolates found in the NCBI-Blast alignment.
Table 3.
Query covers and similarity percentages of the isolate (AspG) (Arthrobotrys musiformis) in relation to the first five isolates found in the NCBI-Blast alignment.
| Isolate (Genus/Species) |
Query cover % |
Similarity % |
Gen Bank accession number |
|
Arthrobotrys sp. |
96 |
99.49 |
ON383425.1 |
| A. eryuanensis |
96 |
99.32 |
MT612105.1 |
| A musiformis |
96 |
99.32 |
MH855842.1 |
| A musiformis |
96 |
99.32 |
KP859624.1 |
| A. musiformis |
96 |
99.32 |
OL454931.1 |
Table 4.
Results of the predatory activity of two nematophagous fungi Arthrobotrys oligospora (AspT) and A. musiformis (AspG) against Haemonchus contortus infective larvae on water agar plates.
Table 4.
Results of the predatory activity of two nematophagous fungi Arthrobotrys oligospora (AspT) and A. musiformis (AspG) against Haemonchus contortus infective larvae on water agar plates.
| Isolate (species) |
Recovered larvae Control group (Mean ± SE) |
Recovered larvae Treated group (Mean ± SE) |
Larval reduction % |
| Arthrobotrys oligospora |
192 ± 38.21 |
105 ± 26.42 |
45.14 a
|
| Arthrobotrys musiformis |
197 ± 60.53 |
57 ± 30.35 |
70.95 b
|
Table 5.
Mean of Haemonchus contortus dead and total recovered larvae and mean larval mortality percentages after 72 h exposure to Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained from Sweet Potato Dextrose Broth and the combination of both fungi.
Table 5.
Mean of Haemonchus contortus dead and total recovered larvae and mean larval mortality percentages after 72 h exposure to Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained from Sweet Potato Dextrose Broth and the combination of both fungi.
| Concentration (mg/mL) |
Fungal filtrate |
DL |
TL |
Mortality % |
SE |
Significance * |
| 0 |
A. musiformis |
6 |
119 |
5.03 |
0.87 |
1 |
| A. oligospora |
6 |
119 |
5.03 |
0.87 |
| Combination |
6 |
119 |
5.03 |
0.87 |
| 25 |
A. musiformis |
15 |
107 |
13.70 |
3.24 |
0.072 |
| A. oligospora |
12 |
96 |
12.14 |
2.74 |
| Combination |
21 |
101 |
20.54 |
3.95 |
| 50 |
A. musiformis |
25 |
104 |
24.50 |
5.08 |
0.456 |
| A. oligospora |
31 |
104 |
29.83 |
6.03 |
| Combination |
19 |
86 |
22.18 |
5.06 |
| 100 |
A. musiformis |
83 |
110 |
75.55 |
5.37 |
0.543 |
| A. oligospora |
82 |
104 |
78.29 |
2.65 |
| Combination |
79 |
99 |
79.84 |
3.04 |
Table 6.
Mean of Haemonchus contortus dead (DL) and total recovered larvae (TL) and mean larval mortality percentages after 72 h exposure to Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained from Czapek Dox Broth and the combination of both fungi.
Table 6.
Mean of Haemonchus contortus dead (DL) and total recovered larvae (TL) and mean larval mortality percentages after 72 h exposure to Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained from Czapek Dox Broth and the combination of both fungi.
| Concentration (mg/mL) |
Fungal filtrate |
DL |
TL |
Mortality % |
SE |
Significance * |
| 0 |
A. musiformis |
2 |
86 |
2.65 |
0.40 |
0.588 |
| A. oligospora |
2 |
80 |
2.12 |
0.38 |
| Combination |
2 |
80 |
2.12 |
0.38 |
| 25 |
A. musiformis |
22 |
81 |
27.08 |
3.42 |
0.011 |
| A. oligospora |
23 |
86 |
27.34 |
5.75 |
| Combination |
37 |
84 |
44.07 |
5.75 |
| 50 |
A. musiformis |
44 |
86 |
51.59 |
5.54 |
0.916 |
| A. oligospora |
38 |
89 |
43.03 |
8.84 |
| Combination |
36 |
75 |
48.29 |
7.96 |
| 100 |
A. musiformis |
83 |
91 |
91.35 |
3.05 |
0.308 |
| A. oligospora |
75 |
87 |
86.17 |
3.39 |
| Combination |
81 |
87 |
92.55 |
2.47 |
Table 7.
Results of Haemonchus contortus larval mortality percentages produced by Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained in Sweet Potato Dextrose Broth at different concentrations, either individually or combined.
Table 7.
Results of Haemonchus contortus larval mortality percentages produced by Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained in Sweet Potato Dextrose Broth at different concentrations, either individually or combined.
| Fungus |
Concentration (mg/mL) |
Mean ± SE |
Significance * |
| A. musiformis |
0 |
5.03 ± 0.87 |
a |
| 25 |
13.70 ± 3.24 |
ab |
| 50 |
24.50 ± 5.08 |
b |
| 100 |
75.55 ± 5.37 |
c |
| A. oligospora |
0 |
5.03 ± 0.87 |
a |
| 25 |
12.14 ± 2.74 |
a |
| 50 |
29.83 ± 6.03 |
b |
| 100 |
78.29 ± 2.65 |
c |
| Combination |
0 |
5.03 ± 0.87 |
a |
| 25 |
20.54 ± 3.95 |
b |
| 50 |
22.18 ± 5.06 |
b |
| 100 |
79.84 ± 3.04 |
c |
Table 8.
Results of Haemonchus contortus larval mortality percentages produced by Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained in Czapek-Dox Broth at different concentrations either individually or combined.
Table 8.
Results of Haemonchus contortus larval mortality percentages produced by Arthrobotrys musiformis and A. oligospora liquid culture filtrates obtained in Czapek-Dox Broth at different concentrations either individually or combined.
| Fungus |
Concentration (mg/mL) |
Mean ± SE |
Significance * |
| A. musiformis |
0 |
2.65 ± 0.40 |
a |
| 25 |
27.08 ± 3.42 |
b |
| 50 |
51.59 ± 5.54 |
c |
| 100 |
91.35 ± 3.05 |
d |
| A. oligospora |
0 |
2.12 ± 0.38 |
a |
| 25 |
27.34 ± 5.75 |
b |
| 50 |
43.03 ± 8.84 |
b |
| 100 |
86.17 ± 3.39 |
c |
| Combination |
0 |
2.12 ± 0.38 |
a |
| 25 |
44.07 ± 5.75 |
b |
| 50 |
48.29 ± 7.96 |
b |
| 100 |
92.55 ± 2.47 |
c |
Table 9.
Mycochemical profile obtained from liquid culture filtrates of two nematophagous fungi Arthrobotrys musiformis and A. oligospora grown in two culture media, Czapek-Dox Broth and Sweet Potato Dextrose Broth, by myco-quantitative reagent analysis.
Table 9.
Mycochemical profile obtained from liquid culture filtrates of two nematophagous fungi Arthrobotrys musiformis and A. oligospora grown in two culture media, Czapek-Dox Broth and Sweet Potato Dextrose Broth, by myco-quantitative reagent analysis.
| Metabolites and reagents |
Colorimetric Reaction |
Arthrobotrys musiformis |
Arthrobotrys oligospora |
| CzDoxB |
SPDB |
CCzDox |
SPDB |
| Alkaloids: Dragendorff |
Turbidity or precipitate (Red, to orange, white to cream or brown) |
+ |
+ |
+ |
+ |
| Mayer |
+ |
+ |
+ |
+ |
| Wagner |
+ |
+ |
+ |
+ |
| Coumarins: Bornträger |
Yellow florescence (UV) |
- |
+ |
+ |
+ |
| Flavonoids: Mg2+ and HCL |
Red, orange or violet |
- |
- |
- |
- |
| Tannins |
Hydrolizables (blue) |
- |
- |
- |
- |
| Iron chloride (FeCl3) |
Condensaded (green) |
- |
- |
- |
- |
| Confirmation |
|
|
|
|
|
| Gelatine solution |
White precipitate |
- |
- |
- |
- |
| Gelatine and saline solution |
White precipitate |
- |
- |
- |
- |
| Saline solution |
White precipitate |
- |
- |
- |
- |
| Triterpenes/Sterols: Liebermann-Buchard |
Blue, green-blue (Sterols) |
- |
- |
- |
- |
| Salkowski |
Red to purple (triterpene) |
- |
+ |
+ |
+ |
| Saponins: Water |
Foam formation |
- |
+ |
+++ |
++ |
Table 10.
Results of the in vitro predatory activity of Arthrobotrys musiformis and A. oligospora obtained from different sources and using different nematode targets.
Table 10.
Results of the in vitro predatory activity of Arthrobotrys musiformis and A. oligospora obtained from different sources and using different nematode targets.
| Isolate |
Source of Isolation |
Nematode target |
Predatory activity % |
Author (s) |
| A. oligospora |
Not available |
Meloidogyne incognita (**) |
79.6–87.5 |
[27] |
| A. musiformis |
Mossy soil, decaying plant material (a rotten trunk) and soil containing Brahea palm roots |
H. contortus (L3) |
>97 |
[28] |
| A. oligospora |
Faeces of water buffalo |
H. contortus (L3) |
>89 |
[29] |
| A. oligospora |
Soil and animal faeces |
Panagrellus redivivus (*) |
57.2 |
[16] |
| A. musiformis |
Soil sample |
H. contortus (L3) |
>74 |
[30] |
| A. oligospora |
Soil samples |
Aphelenchoides besseyi, Bursaphelenchus xylophilus and Ditylenchus destructor (**) |
54.6–97.3 |
[31] |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).