1. Introduction
Studies conducted since the 1920s have shown that isolated tissues can establish long-distance connections via fibrous hydrogels. In recent serial works, cell-cell mechanical communication has been shown using in-vitro experimental models [
1], of which mechanical signaling can occur on a large scale and be highly directional. Conversely, chemical signals don’t achieve such precise direction between individual cells [
2,
3]. Biomechanics are involved in numerous life processes. For example, long-range stress transmission across the cellular collectives guides endothelial gap junction formation [
4]. The fiber matrix is often involved in the perception and response to mechanical force. When cells use force to change the location and distribution of type I collagen (COL) and direction of their movement, the mechanical feedback led to the establishment of a linear tubular model of bistability [
5,
6]. Studies on collective cell migration and actomyosin, performed in clawed frog cells, have shown that contraction of rear cells likely drives the collective cell chemotaxis [
7,
8]. Force promotes growth of Integrin-mediated adhesion, and that of mature and newly-produced cytoskeletal proteins that enhance adhesion, of which process helps cells resist applied forces [
9]. Increasing evidence indicates that mechanical signaling plays important roles in cellular communication.
Mechanosensitive molecules mediate mechanical communications between cells over long distances. In response to mechanical signaling, Integrin can activate the GTPases RhoA and Rac, which affect cell migration, proliferation, and differentiation [
10,
11]. During mechanotransduction, Integrin mediates cell-substrate interactions and cellular resistance to mechanical loading, while dynamics and polarity determine cell velocity and directional persistence [
9,
12]. The large transmembrane mechanical-force sensor proteins Piezo1 and Piezo2 are mechanosensitive components [
13,
14]. Several rapid responses in mechanotransduction rely on mechano-gated and mechanosensitive ion channels [
15]. Many of these ion channels also mediate cell-cell adhesions and molecular structure of adhesion between cells and the extracellular matrix (ECM).
N-cadherin (N-cad), which maintains the integrity of adherens junctions, mediates the progenitor cell cycle, and can restrict the division and differentiation of neural progenitor cells [
16]. Piezo channels are functionally connected to the actin cytoskeleton via the cell junctional cadherin-β-Catenin complex [
17]. Various adhesion molecules bind cells to other cells, and mediate intercellular and cell-matrix communications. In cells with increased adhesion and cytoskeletal contractility, cell-matrix interactions become focalized and the migration mode is mesenchymal migration, which is used by fibroblasts, myoblasts, and many cancer cells [
18,
19,
20]. α-Catenin is required for acute cadherin-mediated mechanotransduction, while the vinculin-binding site of α-Catenin is required for force-dependent recruitment of actin [
21].
To study the mechanisms of distant mechanical cellular interactions, we developed a model based on the rapid assembly of airway smooth muscle (ASM) cells on Matrigel containing 0.5 mg/mL type I collagen (COL) [
22]. Our findings showed that cellular mechanical communication is induced by traction force, which is generated by cellular contractions and transmitted through the matrix hydrogel [
22]. This traction force is then sensed by Integrin and calcium channels (the critical mechanotransduction components localized at the endoplasmic reticulum and plasma membrane), and N-cadherin, resulting in stable traction-force regulated cell branching connections [
22,
23]. Recent studies have shown that partial localization of α-Catenin and Piezo1 in focal adhesions can regulate cell spreading and focal dynamics [
24,
25].
α-Catenin is thought to mainly act as a regulatory cell-adhesion protein that can bind to cadherin cytoplasmic domain, assemble a protein complex linked to the actin cytoskeleton, and mediate the stability of intercellular adhesions [
26]. A previous gene-targeting study showed that α-E-Catenin (referred as α-Catenin here) is required to form epithelial cell adhesions in mice [
27,
28]. This study explored the roles of α-Catenin (a mechanosensitive molecule involved in cadherin-mediated adhesion) and Piezo (a mechanosensitive ion channel) in long-distance mechanical communication between cells. We investigated whether α-Catenin affected the formation of stable cell-cell and cell-ECM adhesions. Additionally, we examined whether adhesion could be mediated by traction to induce cells to form stable connections to the matrix, and whether Piezo1 played a vital role in this mechanosensing process.
2. Materials and Methods
2.1. Cell Culture and Reagents
Primary airway smooth muscle (ASM) cells were purchased from Beina Biotech. Co. (Beijing), which were originated from 6-8-week old Sprague-Dawley female rats [
29]. All procedures involving animals were approved by the Ethics Committee of Changzhou University on Studies Ethics (Grant No. NSFC 11532003). ASM cells were cultured in low-glucose DMEM (Invitrogen) supplemented with 10% fetal bovine serum (FBS). Cells were cultured in a humidified incubator containing 5% CO
2 at 37 °C. The ASM cells used in experiments were generally within 10 times of passages during regular culture.
Matrigel was purchased from BD Biotechnology, and type I collagen was obtained from Advanced Biomatrix. Low-glucose DMEM, Opti-MEM, FBS, 0.25% trypsin, Lipofectamine 3000, Accutase, Ctnna1 siRNA was purchased from Thermo Fisher Scientific (USA). N-cadherin siRNA (N-cadherin siRNA, #M-091851-01-0005) was from Horizon Discovery. MISSION siRNA universal negative control (#1, SIC001-10nM), Human Plasma Fibronectin, DMSO were purchased from Sigma-Aldrich; GsMTx4 was purchased from MedChemExpress; GdCl3 was purchased from Aladdin. HiScript III 1st Strand cDNA Synthesis Kit (+gDNA wiper) was purchased from Vazyme.
The plasmids applied in this study have been described previously, including Integrin α5-EGFP [
30], mouse Piezo1-EGFP [
14] (referred as Piezo in this work), and Paxillin-dsRed [
31]. For inhibitor applications, cells were seeded at suitable densities in 6-well plates containing hydrogels, and allowed to incubate for 30 min, followed by the addition of 2 mL medium supplemented with the appropriate concentration of inhibitor. The concentrations of GsMTx4 and GdCl
3 were 3 μM [
2] and 100 μM [
32], respectively.
2.2. Preparation of Hydrogel in the Polydimethylsiloxane (PDMS) Mold
The PDMS mold and cultivation patterns of the cell mesh structure were described in our previous study [
22]. A thin layer of PDMS (~500 μm in thickness) was generated as follows. First, the two liquid components included in the Sylgard 184 kit (Dow Corning) were mixed thoroughly at a mass ratio of 10:1. Then, this mixture was added to the tablet, air bubbles were removed by vacuum, and the sheet was cured in an oven at 70 °C for 3 h. The PDMS sheet was cut into pieces of appropriate size, and a hole with 0.6 cm in diameter was created in the middle using a mechanical puncher. Then, PDMS was sterilized overnight under a UV lamp in a cell culture hood, and attached onto the center of a glass-bottom dish (NEST). Prepared the liquid mixture with 100% Matrigel (~10 mg/mL) and pH-neutralized 1 mg/mL COL at the volume ratio of 1:1 on ice. Then, the ~50% Matrigel containing 0.5 mg/mL COL was added into the PDMS mold on ice. The assembly was placed into an incubator and allowed to solidify at 37 °C for 30 min. Then, 10-20 μL cell suspension was added on top of the hydrogel, and the mold was placed into the incubator for 30 min, followed by the addition of more culture medium.
2.3. Plasmid and siRNA Transfections
ASM cells were cultured to 40–50% confluence in 12-well plates (BIOFIL), and then transfected with 25 nM siRNA using Lipofectamine 3000 (#L3000-008, Thermo) per manufacturer’s protocol. After 8 h, culture medium was replaced with new DMEM supplemented with 10% FBS. The cells were then incubated for an additional 52 h and seeded onto a hydrogel.
For plasmid transfection, ASM cells were cultured to 50% of confluence in 12-well plates. Then, 1.5 µg biosensor DNA, 2.5 µL P3000, and 2.5 µL Lipofectamine 3000 were mixed to generate Lipid-DNA particles, which were added into the cell culture. After an 8-h incubation, culture medium was replaced with new DMEM supplemented with 10% FBS. The cells were incubated for an additional 30 h and then moved to the imaging processes.
For siRNA and DNA plasmid co-transfection, siRNA was transfected first, followed by the transfection of the DNA plasmid after 24 h. Then the medium was changed after 8 hours of incubation. Cell imaging was performed at approximately 30 h after transfection of the DNA plasmid.
2.4. Measurements of ctnna1 siRNA Transfection Efficiency Using qPCR
The down-regulation efficiency by ctnna1 siRNA was assessed by measuring the expression of mRNA using quantitative real-time PCR (qPCR). qPCR primer sequences for rat ctnna1 was as follows: GTGGGAGGCTCTCCCTAGAA (ctnna1 forward) and CCAGGGTTGTCACCTGTGTA (ctnna1 reverse). Control GAPDH primers were as follows: AGGTCGGTGTGAACGGATTTG (forward) and GGGGTCGTTGATGGCAACA (reverse) [
33]. Primers were synthesized by General Biosystems (Anhui, China). PowerUp SYBR Green Master Mix (#A25742, Applied Biosystems, CA) was used according to kit instructions.
2.5. Time-Lapse Microscopy and Fluorescence Imaging
The live-cell epi-microscopy system (Zeiss) was equipped with an X-Y-Z stage for multiple-position imaging, fine auto-focus function for long-duration time-lapse observation, and a constant temperature control chamber (37 °C, 5% CO2) to maintain cell viability over time. Imaging interval and duration were 30 min and 16–22 h, respectively. Observation and imaging were generally performed using an x10 objective. Fluorescence images were acquired using the Rhodamine (Paxillin) or FITC (Piezo or Integrin) channel. Peak emissions for Rhodamine and FITC were 683 and 519 nm, respectively.
2.6. Trajectory Analysis of Cell Movement
To track the trajectories of cell movement, the images acquired under the same field of view were imported into ImageJ in time sequences. Then, image size was converted to actual size using the “Set Scale” function. Using the “Manual tracking” function from the plugin list (Plugins –> Tracking –> Manual tracking –> Add track), time-sequence positions (x, y) and moving distances were generated automatically by continuously clicking the target cells through the first to last frame. The digital data file generated in ImageJ was collated and imported into MATLAB software to generate the final rate and velocity, trajectory and distance traveled (demonstrated in
Figure S1).
2.7. Number Counting and Fluorescence Quantification of Cellular Focal Adhesions
The nuclear areas showing high fluorescence outside focal adhesions were removed for the analysis of focal adhesion images by using ImageJ. The average fluorescence intensity in focal adhesions was calculated as follows. After selecting “Freehand selections”, the fluorescence-labeled protein region was selected, followed by “Measure” in the Analyze function on the plug-in list. These data were then imported into MATLAB software to ensure that all fluorescence-labeled proteins were counted by adjusting the “threshold” function. The total fluorescence area, average area, and number of focal adhesions were then obtained.
Fluorescence co-localization analysis of two proteins at focal adhesions was performed as follows. Two fluorescence images generated under the same field of view were imported into ImageJ, and the same position was selected to obtain fluorescence data from left to right. The exported data were then summarized using Origin2020. Finally, the fluorescence curves of the two proteins were obtained.
GraphPad and Origin2020 were used for statistical analysis and generation of graphs. The values on the graphs represent the mean ± S.D. (standard derivation) from their groups (in scattering dots). *, **, ***, and **** indicate p < 0.05, 0.01, 0.001, and 0.0001, respectively, for significant difference between each two groups as analyzed using Student’s t-test.
3. Results
3.1. α-Catenin Regulates Cell Directed Migration and Reticular Structure Assembly in Distant Mechanical Communication
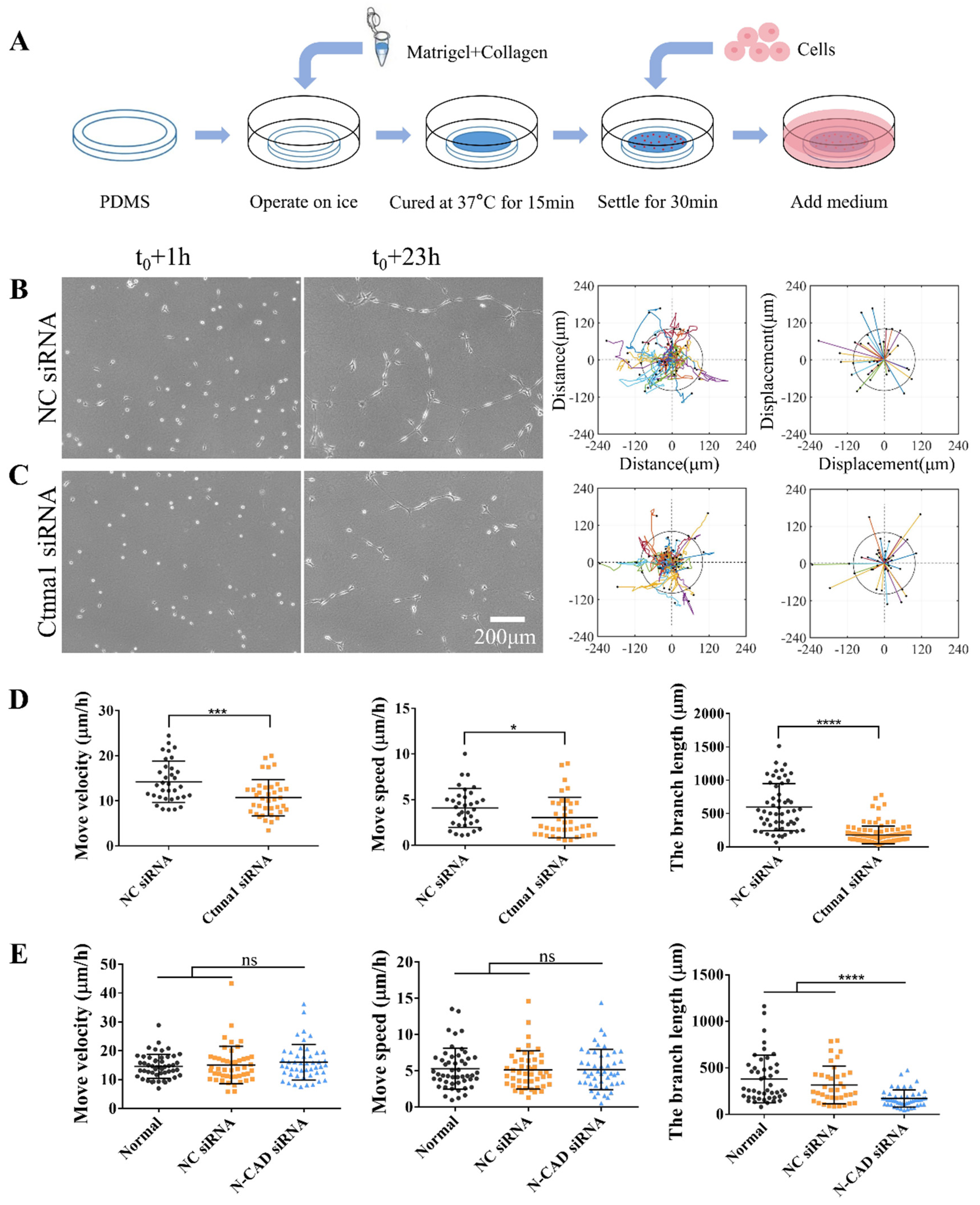
Previous studies have demonstrated the ability of cells to communicate over long distances through force. In this study, we expand upon our established model (
Figure 1A), in which ASM cell used traction forces through the hydrogel matrix to induce directed migration and rapid branch assembly [
22,
23]. We transfected ASM cells with α-Catenin siRNA (ctnna1 siRNA) to investigate mechanically induced long-distance cell communication and stable ligation. Derived from our previous studies, cell directional migration and branch connection were characterized as the efficiency index of traction force-induced mechanical communication. Time-lapse imaging was performed every 0.5 h throughout 22 h to characterize the directional migration of ASM cells on the hydrogel. Our results revealed that ASM cells transfected with α-Catenin siRNA exhibited reduced directed migration and low levels of branch assembly compared with those treated with control siRNA (
Figure 1B,C,
Supplementary Movie S1). Furthermore, the formed branch structures in α-Catenin siRNA-transfected ASM cells were unstable, resulting in more cell clusters and individual free cells (
Figure 1B,C).
QPCR analysis confirmed the downregulation of mRNA expression levels by ctnna1 siRNA in ASM cells (
Figure S1C). In our previous experiments, cell-cell junctional N-cadherin regulates cell branching assembly but not directed migration [
23] (repeated results shown in
Figure 1E, and
Supplementary Figure S2). Here, the junctional complex-associated α-Catenin influenced both cell migration rates and branching assembly, indicating a critical role in both cell-cell distant mechanosensation and stable adherens junctions. Together, these results indicate that α-Catenin, a traditionally known intercellular adhesion protein, plays a crucial role in the formation of stable branching structures between cells, and more study is required to elucidate its important impact on the rate of cell migration.
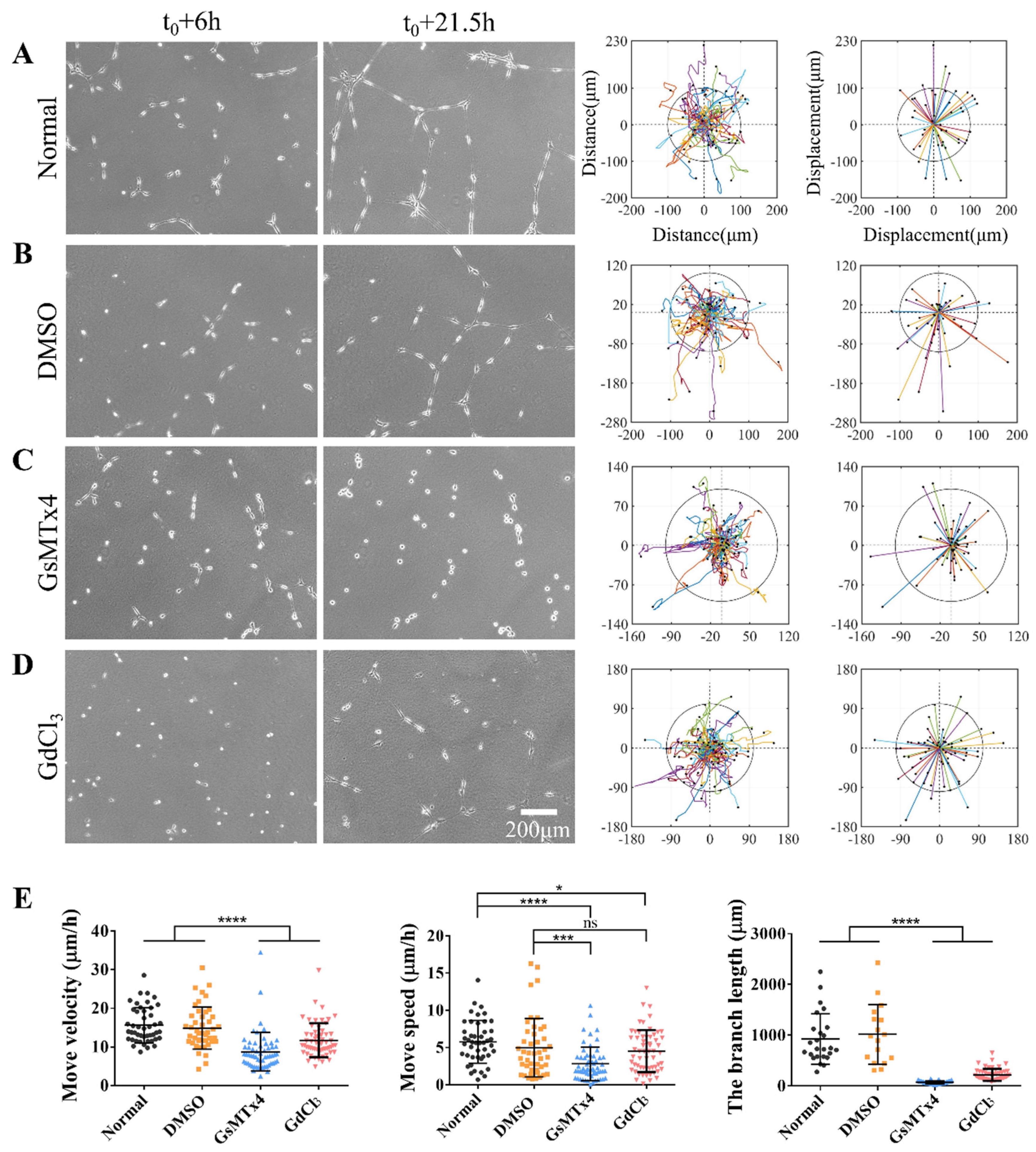
2.2. Piezo Regulates Directed Cell Migration and Branching Assembly
Piezo, an important mechanosensitive ion channel on cell membrane, is crucial in various mechanotransduction processes. When cells are subjected to mechanical stimuli, ion channels open to allow cations to enter through the plasma membrane, thereby inducing mechanotransduction [
34]. In this study, we examined the role of Piezo in cell mechanical communication on the directional migration of ASM cells and their assembly on the hydrogel. We used GsMTx4, a spider venom peptide that inhibits cation infiltration of the Piezo channel family, to treat ASM cells. Control cells were under normal culture or treated with DMSO (
Figure 2A,B). Our results indicate that the migration rate and velocity of ASM cells treated with GsMTx4 were significantly reduced compared to control cells, and resulted in inactive single cells unable to form a stable mesh structure (
Figure 2C,E,
Movie S2). Similar results were obtained in ASM cells treated with GdCl
3, a calcium-sensitive agonist (
Figure 2D,E,
Movie S2). However, the effects of GdCl
3 were not as robust as those of GsMTx4, suggesting that GsMTx4 may have a more direct effect on the sensing of mechanical forces in ASM cells. Overall, our results indicate that Piezo as mechanosensitive component on cell membrane plays a crucial role in distant mechanical communication for directional migration and self-assembly of cells.
2.3. α-Catenin Influences Focal Localizations of Paxillin and Integrin α5
The above investigation of α-Catenin role in mechanical communication prompted us to consider the potential involvement in cell-matrix adhesion influencing cell migration rates. Hence, we transfected ASM cells with α-Catenin siRNA (ctnna1) for 24 h, followed by co-transfection with either fluorescent Paxillin-dsRed or Integrin α5-EGFP plasmid, of which both proteins are located at focal adhesions. Subsequently after plasmid transfection for 24–26 h, cells were seeded onto a 20-mm confocal dish coated with fibronectin (10 μg/mL), and microscope imaging was conducted after a 4-h rehydration period. The focal scaffold protein Paxillin is known to localize to discrete sites during cell attachment and interacts with various structures and signaling proteins, responding to Integrin-mediated cell adhesion [
35]. Integrin can induce the assembly of large complexes that bridge the ECM to the intracellular cytoskeleton [
36].
In our experiment, ASM cells expressing Integrin α5-EGFP didn’t display obvious focal adhesions seeding on the applied soft hydrogel containing 50% Matrigel and 0.5 mg/mL COL (
Figure S3). This might be due to the size of focal adhesions out of the resolution limit of our epi-fluorescence microscopy. Alternatively, to study the impact of α-Catenin on focal adhesions is still practical with ASM cells seeding on glass surfaces and showing visible focal adhesions. Our findings revealed that both normal cells and those transfected with control siRNA expressed clearly observable and intact fluorescent Paxillin in focal adhesions, but far less in cells transfected with ctnna1 siRNA (
Figure 3A). From statistical quantifications, the number of focal adhesions, fluorescence intensity of focal Paxillin, and total Paxillin-displayed adhesion area (per cell) were significantly reduced in cells bearing a knockdown of ctnna1 (
Figure 3B,C). ASM cells transfected with fluorescent Integrin α5 plasmids showed similar phenomena: siRNA knockdown of α-Catenin expression markedly reduced focal adhesion numbers, fluorescence intensity of focal Integrin, and total area of focal adhesions in the cells (
Figure 3D–F). Similar results were obtained when cells were seeded on Matrigel/COL solution-coated glass in mimicking the substrate of the hydrogel condition (
Figure S4A–D). These results suggest that the effects of α-Catenin on traction force-induced cell migration rate may be linked to focal localizations of Paxillin and Integrin α5 which play crucial roles in cell adhesions to drive migration.
2.4. Partial Localization of Piezo at Focal Adhesions
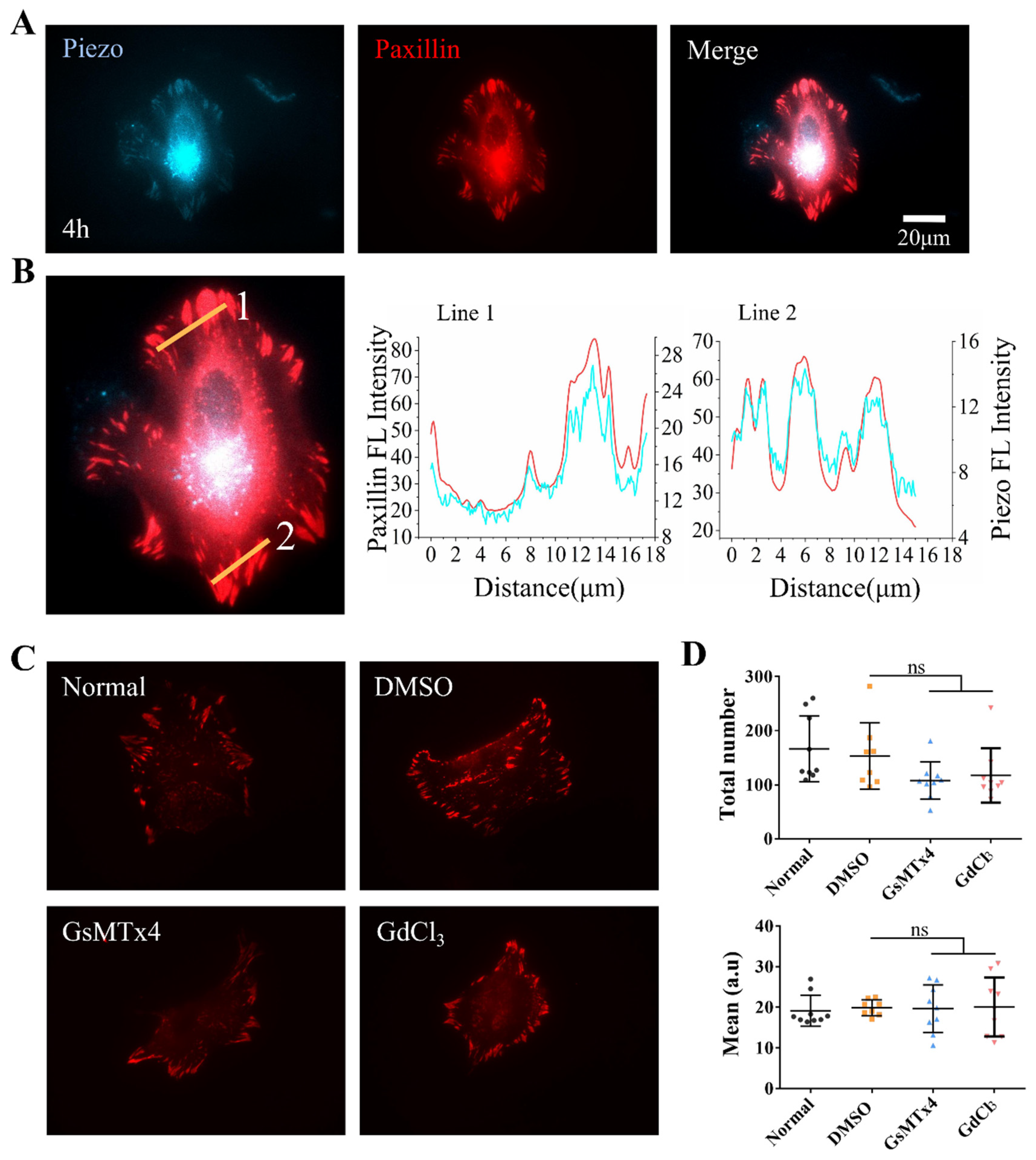
Our results indicate that the expression levels of α-Catenin could affect those of Paxillin and Integrin α5, resulting in an inability to form stable focal adhesions in ASM cells. Alteration of the cellular perception of mechanical forces via inhibition of Piezo also inhibited cellular motility and connectivity. Therefore, we further examined the relationship between Piezo and focal Paxillin in ASM cells.
Fluorescent Piezo and Paxillin were expressed into ASM cells by co-transfection, and cells were seeded on fibronectin-coated confocal dishes for imaging. The edges of normal ASM cells showed partial colocalization of mPiezo and Paxillin at focal adhesions after 4 h seeding (
Figure 4A,B). The colocalized fractions were still visible after 25 h, indicating Piezo having focal targeting (
Supplementary Figure S5A,B). Subsequently, we treated ASM cells expressing fluorescent Paxillin with the Piezo inhibitor GsMTx4 or GdCl
3. No significant differences in the total numbers of focal adhesions and fluorescence intensity of focal Paxillin were observed on the boundary regions of treated cells compared with the controls (
Figure 4C,D). Similar results were observed after 25 h (
Supplementary Figure S5C,D), indicating the assembly of cell focal adhesions less regulated by Piezo. Together, these data demonstrated localization of mechanosensitive Piezo at focal adhesions, which provides a possible linkage for cell sensing traction force in the hydrogel matrix via Piezo during cell mechanical communication.
To examine whether α-Catenin also regulates Piezo focal localization, ASM cells transfected with α-Catenin siRNA were further co-expressed with fluorescent Paxillin and Piezo. As shown in
Figure 5A,B, Piezo showed focal localizations along with Paxillin in the control groups, but the localization markedly decreased in α-Catenin siRNA-transfected group. It is noticed that only a fraction of cells (~50%) showed apparent Piezo focal locations under the normal condition, indicating not as stable as focal Paxillin or Integrin. Hence, α-Catenin regulated focal adhesion assembly, which also impacted on Piezo-focal targeting.
4. Discussion
Accumulating studies have provided evidence that cells are capable of communicating over long distance by transmitting mechanical signals through matrix substrates. The specific biological mechanisms underlying this phenomenon remain to be fully understood. In our present study, we investigated the mechanism of α-Catenin-mediated biological response during long-distance cellular communication. Our in-vitro model demonstrated that α-Catenin-mediated adhesions between cells, and between cells and matrix, can induce a rapid and accurate directional migration in cells, and promote the formation of a stable tissue mesh structure (summarized in
Figure 5C). Mechanical forces are essential for the formation of this mesh structure.
ASM cells transfected with control siRNA formed a mesh structure on hydrogels. However, ASM cells transfected with ctnna1 siRNA could not form a stable structure, and showed an inhibited migration rate compared with that of control group (
Figure 1B–D). These results indicate that α-Catenin promoted both directed migration speed and mesh structure formation during cell-to-cell distant mechanical communication.
In this study, we have once again underscored the significance of mechanical forces in long-distance intercellular communication. The Piezo, known as a mechanotransducer, plays a pivotal role in this process. In the Piezo ion channel, a specialized transduction structure converts mechanical forces into those used for cation transduction [
37]. Therefore, when inhibited the perception of force by inhibiting Piezo channel, we found that normal directional migration and self-assembly of cells on the hydrogel was markedly reduced (
Figure 2A–E). Particularly, Piezo showed localization at focal adhesions (
Figure 4A,B). These results provide mechanistic clue for Piezo in mechanosensing of the traction force transmitted through the hydrogel matrix. These findings provide evidence that mechanotransduction is imperative for the formation of intercellular communication and adhesion.
Paxillin, a multi-domain protein, localizes to cell adhesions through its LIM domain. Paxillin can bind to numerous proteins that affect the actin cytoskeletal tissue by directly interacting with β-Integrin at its tail or intermediate proteins [
38]. Integrins are mechanosensitive receptors on plasma membrane that facilitate cell-extracellular matrix adhesions [
39]. In this research, we investigated the influence of α-Catenin on the focal localizations of Paxillin and Integrin α5 in our in-vitro model. Previous research has shown that α-Catenin plays a role in cell adhesion [
40]. Our finding demonstrates that knockdown of α-Catenin expression in ASM cells inhibited focal localizations of Paxillin and Integrin α5, which inhibited directed cellular migration and formation of branching structures (
Figure 3A–F). These findings suggest that Paxillin and Integrin play essential roles within cells, and that α-Catenin expression levels can inhibit cell-cell and cell-stroma adhesions.
Our findings demonstrate that knocking down α-Catenin or inhibiting Piezo expression in ASM cells had a similar inhibitory effect. Therefore, we investigated whether Piezo mechanosensation was related to focal adhesions by co-expression with Paxillin. Co-transfection of Piezo and Paxillin plasmids into ASM cells showed that Piezo and Paxillin were partially colocalized (
Figure 4A,B), while reduced α-Catenin expression with siRNA inhibited Piezo-focal targeting (
Figure 5A,B). Taken together, the inhibition of Piezo resulted in attenuated directional migration of ASM cells in cell-to-cell mechanical communication (
Figure 2A–E). It is reasonable to speculate that cell mechanosensing of traction force in the hydrogel matrix is partially due to Piezo localized at cellular focal adhesions, although the role of non-focal Piezo wasn’t verified yet.
In summary, we show that α-Catenin was involved in cell adhesion and mechanical sensitivity. Besides connection of N-cadherin at adherens junctions to the actin cytoskeleton, α-Catenin was also functionally associated with focal Paxillin and Integrin α5, which affected the maturation of cell-matrix adhesions. We also verified the importance of Piezo in mechanical transduction and found a mechanistic clue between Piezo and cell mechanosensation in distant mechanical communication. Taking together with our recent study that cell traction force sensing induces distant mechanical communication [
22], this work further provides important insights to interpret the mechanism of molecular mechanotransduction (
Figure 5C).
Supplementary Materials
The following supporting information can be downloaded at the website of this paper posted on Preprints.org.
Author Contributions
M.O. and L.D. designed the research; Q.Z. performed the majority of experiments and data analysis; B.B. generated the program for focal adhesion measurements; Y.Z., J.L. and M.L. helped with experiments and resources; L.D. provided the setups of equipment; Q.Z., M.O., B.B. and L.D. prepared the paper.
Acknowledgments
The text in the paper received language editing service from LetPub. The work was assisted with help from Yan Pan, and Lei Liu (Changzhou University). This project was supported financially by National Natural Science Foundation of China (NSFC 12372312, 11872129), Program of “Jiangsu Specially-appointed Professor” (M.O.); National Natural Science Foundation of China (11902051 (B.B.), 12272063 (L.D.)).
Statement for No Conflict of Interest
The authors declare no conflicts of interest in this work.
References
- Alisafaei, F.; et al. Long-range mechanical signaling in biological systems. Soft Matter 2021, 17, 241–253. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; et al. Dynamically Reconstructed Collagen Fibers for Transmitting Mechanical Signals to Assist Macrophages Tracing Breast Cancer Cells. Advanced Functional Materials 2023, 33, 2211807. [Google Scholar] [CrossRef]
- Postma, M.; van Haastert, P.J. Mathematics of Experimentally Generated Chemoattractant Gradients. Methods Mol Biol 2016, 1407, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Hardin, C.C.; et al. Long-range stress transmission guides endothelial gap formation. Biochem Biophys Res Commun 2018, 495, 749–754. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; et al. Long-range force transmission in fibrous matrices enabled by tension-driven alignment of fibers. Biophys J 2014, 107, 2592–2603. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.L.; et al. Long-range mechanical force enables self-assembly of epithelial tubular patterns. Proc Natl Acad Sci USA 2012, 109, 5576–5582. [Google Scholar] [CrossRef]
- Sunyer, R.; et al. Collective cell durotaxis emerges from long-range intercellular force transmission. Science 2016, 353, 1157–1161. [Google Scholar] [CrossRef]
- Shellard, A.; et al. Supracellular contraction at the rear of neural crest cell groups drives collective chemotaxis. Science 2018, 362, 339–343. [Google Scholar] [CrossRef]
- Kolasangiani, R.; Bidone, T.C.; Schwartz, M.A. Integrin Conformational Dynamics and Mechanotransduction. Cells 2022, 11, 3584. [Google Scholar] [CrossRef]
- Chang, A.C.; et al. Single Molecule Force Measurements in Living Cells Reveal a Minimally Tensioned Integrin State. ACS Nano 2016, 10, 10745–10752. [Google Scholar] [CrossRef]
- Shyy, J.Y.; Chien, S. Role of integrins in endothelial mechanosensing of shear stress. Circ Res 2002, 91, 769–775. [Google Scholar] [CrossRef]
- Byron, A.; Morgan, M.R.; Humphries, M.J. Adhesion signalling complexes. Curr Biol 2010, 20, R1063–R1067. [Google Scholar] [CrossRef] [PubMed]
- Coste, B.; et al. Piezo proteins are pore-forming subunits of mechanically activated channels. Nature 2012, 483, 176–181. [Google Scholar] [CrossRef]
- Coste, B.; et al. Piezo1 and Piezo2 are essential components of distinct mechanically activated cation channels. Science 2010, 330, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.; et al. Mechanotransduction in gastrointestinal smooth muscle cells: Role of mechanosensitive ion channels. Am J Physiol Gastrointest Liver Physiol 2021, 320, G897–G906. [Google Scholar] [CrossRef] [PubMed]
- Chalasani, K.; Brewster, R.M. N-cadherin-mediated cell adhesion restricts cell proliferation in the dorsal neural tube. Mol Biol Cell 2011, 22, 1505–1515. [Google Scholar] [CrossRef]
- Wang, J.; et al. Tethering Piezo channels to the actin cytoskeleton for mechanogating via the cadherin-β-catenin mechanotransduction complex. Cell Rep 2022, 38, 110342. [Google Scholar] [CrossRef] [PubMed]
- Gahmberg, C.G.; Grönholm, M. How integrin phosphorylations regulate cell adhesion and signaling. Trends Biochem Sci 2022, 47, 265–278. [Google Scholar] [CrossRef]
- Friedl, P.; et al. CD4+ T lymphocytes migrating in three-dimensional collagen lattices lack focal adhesions and utilize beta1 integrin-independent strategies for polarization, interaction with collagen fibers and locomotion. Eur J Immunol 1998, 28, 2331–2343. [Google Scholar] [CrossRef]
- Friedl, P.; Hegerfeldt, Y.; Tusch, M. Collective cell migration in morphogenesis and cancer. Int J Dev Biol 2004, 48, 441–449. [Google Scholar] [CrossRef]
- Barry, A.K.; et al. α-catenin cytomechanics--role in cadherin-dependent adhesion and mechanotransduction. J Cell Sci 2014, 127 Pt 8, 1779–1791. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; et al. Sensing Traction Force on the Matrix Induces Cell-Cell Distant Mechanical Communications for Self-Assembly. ACS Biomater Sci Eng 2020, 6, 5833–5848. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, M.; et al. Mechanical communication-associated cell directional migration and branching connections mediated by calcium channels, integrin β1, and N-cadherin. Front Cell Dev Biol 2022, 10, 942058. [Google Scholar] [CrossRef] [PubMed]
- Yao, M.; et al. Force- and cell state-dependent recruitment of Piezo1 drives focal adhesion dynamics and calcium entry. Sci Adv 2022, 8, eabo1461. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A.; et al. α-Catenin links integrin adhesions to F-actin to regulate ECM mechanosensing and rigidity dependence. J Cell Biol 2022, 221. [Google Scholar] [CrossRef] [PubMed]
- Kobielak, A.; Fuchs, E. Alpha-catenin: At the junction of intercellular adhesion and actin dynamics. Nat Rev Mol Cell Biol 2004, 5, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Vasioukhin, V.; et al. Hyperproliferation and defects in epithelial polarity upon conditional ablation of alpha-catenin in skin. Cell 2001, 104, 605–617. [Google Scholar] [CrossRef] [PubMed]
- Vasioukhin, V.; et al. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000, 100, 209–219. [Google Scholar] [CrossRef]
- Wang, Y.; et al. Evaluation of pharmacological relaxation effect of the natural product naringin on in vitro cultured airway smooth muscle cells and in vivo ovalbumin-induced asthma Balb/c mice. Biomed Rep 2016, 5, 715–722. [Google Scholar] [CrossRef]
- Ouyang, M.X.; et al. N-cadherin regulates spatially polarized signals through distinct p120ctn and β-catenin-dependent signalling pathways. Nature Communications 2013, 4. [Google Scholar] [CrossRef]
- Hu, Y.L.; et al. FAK and paxillin dynamics at focal adhesions in the protrusions of migrating cells. Scientific Reports 2014, 4. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; et al. Calcium Oxalate Induces Renal Injury through Calcium-Sensing Receptor. Oxid Med Cell Longev 2016, 2016, 5203801. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; et al. Gasoline exhaust damages spermatogenesis through downregulating α6-integrin and β1-integrin in the rat model. Andrologia 2018, 50, e13045. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; et al. Ion Permeation and Mechanotransduction Mechanisms of Mechanosensitive Piezo Channels. Neuron 2016, 89, 1248–1263. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E. Paxillin. Int J Biochem Cell Biol 1998, 30, 955–959. [Google Scholar] [CrossRef] [PubMed]
- Campbell, I.D.; Humphries, M.J. Integrin structure, activation, and interactions. Cold Spring Harb Perspect Biol 2011, 3. [Google Scholar] [CrossRef]
- Fang, X.Z.; et al. Structure, kinetic properties and biological function of mechanosensitive Piezo channels. Cell Biosci 2021, 11, 13. [Google Scholar] [CrossRef] [PubMed]
- Turner, C.E. Paxillin interactions. J Cell Sci 2000, 113 Pt 23, 4139–4140. [Google Scholar] [CrossRef]
- Koivisto, L.; et al. Integrin αvβ6: Structure, function and role in health and disease. Int J Biochem Cell Biol 2018, 99, 186–196. [Google Scholar] [CrossRef]
- Takeichi, M. Multiple functions of α-catenin beyond cell adhesion regulation. Curr Opin Cell Biol 2018, 54, 24–29. [Google Scholar] [CrossRef]
Figure 1.
α-Catenin regulates both branch formation and directed migration. ASM cells were transfected with α-Catenin (ctnna1) siRNA or control siRNA. After incubating for 52 h, the cells were seeded onto the hydrogel for time-lapse imaging.
(A) The experimental setup for cell culture on the hydrogel.
(B, C) Branch assembly of ASM cells transfected with control siRNA (NC) on the hydrogel, and migration analysis of trajectory plot (left) and displacement map (right) (B); transfected with ctnna1 siRNA (C);
(D) Statistical quantification of ASM cells movement rate and velocity (mean ± S.D., n = 34, 38, respectively) and branch length (n = 54, 118, respectively) under conditions (B-C).
(E) Statistical quantification of ASM cells transfected with control or N-CAD siRNA for the movement rate and velocity (mean ± S.D., n = 50, 47, and 48, respectively), and branch length (n = 45, 38, and 49, respectively). The cell images and migration trajectories are shown in
Figure S2.
Figure 1.
α-Catenin regulates both branch formation and directed migration. ASM cells were transfected with α-Catenin (ctnna1) siRNA or control siRNA. After incubating for 52 h, the cells were seeded onto the hydrogel for time-lapse imaging.
(A) The experimental setup for cell culture on the hydrogel.
(B, C) Branch assembly of ASM cells transfected with control siRNA (NC) on the hydrogel, and migration analysis of trajectory plot (left) and displacement map (right) (B); transfected with ctnna1 siRNA (C);
(D) Statistical quantification of ASM cells movement rate and velocity (mean ± S.D., n = 34, 38, respectively) and branch length (n = 54, 118, respectively) under conditions (B-C).
(E) Statistical quantification of ASM cells transfected with control or N-CAD siRNA for the movement rate and velocity (mean ± S.D., n = 50, 47, and 48, respectively), and branch length (n = 45, 38, and 49, respectively). The cell images and migration trajectories are shown in
Figure S2.
Figure 2.
Role of mechanosensitive Piezo channels during directed cell migration and self-assembly. Normal ASM cells were inoculated onto the hydrogel with or without an inhibitor in the culture media; DMSO were used for control cells. Cells were allowed to incubate for 6 h, and then time-lapse imaging was performed every 0.5 h for 15.5 h. (A-D) Cell images at 6 h and 21.5 h, trajectory analysis plots, and displacement maps are shown for normal state (A), control condition (DMSO) (B), treatment with GsMTx4 (3 μM) (C) or with GdCl3 (100 μM) (D). (E) Statistical quantification of ASM cells movement rate and velocity (n = 46, 45, 58, 58, respectively) and branch length (n = 25, 17, 49, 55, respectively) under conditions (A-D).
Figure 2.
Role of mechanosensitive Piezo channels during directed cell migration and self-assembly. Normal ASM cells were inoculated onto the hydrogel with or without an inhibitor in the culture media; DMSO were used for control cells. Cells were allowed to incubate for 6 h, and then time-lapse imaging was performed every 0.5 h for 15.5 h. (A-D) Cell images at 6 h and 21.5 h, trajectory analysis plots, and displacement maps are shown for normal state (A), control condition (DMSO) (B), treatment with GsMTx4 (3 μM) (C) or with GdCl3 (100 μM) (D). (E) Statistical quantification of ASM cells movement rate and velocity (n = 46, 45, 58, 58, respectively) and branch length (n = 25, 17, 49, 55, respectively) under conditions (A-D).
Figure 3.
The effect of α-Catenin on distribution of Paxillin or Integrin at focal adhesions in ASM cells. (A, D) Paxillin (A) or Integrin (D) fluorescence images in normal ASM cells and those transfected with control siRNA, ctnna1 siRNA. Fluorescence dots show Paxillin or Integrin α5 expression at focal adhesions. (B, E) Sample demonstrations for quantification of the numbers and average fluorescence of focal adhesions based on fluorescent Paxillin (B) and Integrin α5 (E). Details are described in Methods. (C, F) Statistical quantifications for the counted numbers of Paxillin-marked focal adhesions per cell (n = 17, 12, and 11, respectively) (C), or Integrin-labeled focal adhesions per cell (n = 14, 10, and 10, respectively) (F), averaged Paxillin or Integrin α5 fluorescence intensity in focal adhesions, and total area of focal adhesions per cell.
Figure 3.
The effect of α-Catenin on distribution of Paxillin or Integrin at focal adhesions in ASM cells. (A, D) Paxillin (A) or Integrin (D) fluorescence images in normal ASM cells and those transfected with control siRNA, ctnna1 siRNA. Fluorescence dots show Paxillin or Integrin α5 expression at focal adhesions. (B, E) Sample demonstrations for quantification of the numbers and average fluorescence of focal adhesions based on fluorescent Paxillin (B) and Integrin α5 (E). Details are described in Methods. (C, F) Statistical quantifications for the counted numbers of Paxillin-marked focal adhesions per cell (n = 17, 12, and 11, respectively) (C), or Integrin-labeled focal adhesions per cell (n = 14, 10, and 10, respectively) (F), averaged Paxillin or Integrin α5 fluorescence intensity in focal adhesions, and total area of focal adhesions per cell.
Figure 4.
Paxillin and Piezo colocalize at cellular focal adhesions. (A) Representative images show co-expressed fluorescent Paxillin and Piezo at focal adhesions in ASM cells spread on fibronectin-coated glass for 4 h. Fluorescence images show Piezo expression (left), and Paxillin expression (middle), and merged image shows both Piezo and Paxillin expression (right). (B) Measured fluorescence intensities of Piezo (blue) and Paxillin (red) across the selected lines at cellular focal adhesion regions. (C) Fluorescent Paxillin at focal adhesions in ASM cells with control groups, or treated with the inhibitor GsMTx4 or GdCl3. (D) Statistical quantifications of focal adhesion numbers and Paxillin fluorescence intensity (n=9, 8, 9, 9, respectively) at the conditions of (C).
Figure 4.
Paxillin and Piezo colocalize at cellular focal adhesions. (A) Representative images show co-expressed fluorescent Paxillin and Piezo at focal adhesions in ASM cells spread on fibronectin-coated glass for 4 h. Fluorescence images show Piezo expression (left), and Paxillin expression (middle), and merged image shows both Piezo and Paxillin expression (right). (B) Measured fluorescence intensities of Piezo (blue) and Paxillin (red) across the selected lines at cellular focal adhesion regions. (C) Fluorescent Paxillin at focal adhesions in ASM cells with control groups, or treated with the inhibitor GsMTx4 or GdCl3. (D) Statistical quantifications of focal adhesion numbers and Paxillin fluorescence intensity (n=9, 8, 9, 9, respectively) at the conditions of (C).
Figure 5.
The effect of α-Catenin on Piezo focal localization and a hypothetical model of molecular mechanosensation. (A, B) α-Catenin regulation of Piezo focal targeting. After transfection with control or α-Catenin siRNA, ASM cells were further transfected with fluorescent Piezo and Paxillin. The images of focal adhesions were taken (A), followed with Piezo-indicated focal number counting per cell (B). Only those cells showing visible focal adhesions were counted. (C) The illustration of hypothetical model for α-Catenin and Piezo in cell-to-cell mechanical communication on the hydrogel matrix. Cellular contraction generated traction force transmitted through the hydrogel; α-Catenin regulates both traction force-induced cell-cell adherens junctions and cell-matrix adhesions, while Piezo partially localized at focal adhesions could mechanosense the stretch in the matrix for cell distant communication.
Figure 5.
The effect of α-Catenin on Piezo focal localization and a hypothetical model of molecular mechanosensation. (A, B) α-Catenin regulation of Piezo focal targeting. After transfection with control or α-Catenin siRNA, ASM cells were further transfected with fluorescent Piezo and Paxillin. The images of focal adhesions were taken (A), followed with Piezo-indicated focal number counting per cell (B). Only those cells showing visible focal adhesions were counted. (C) The illustration of hypothetical model for α-Catenin and Piezo in cell-to-cell mechanical communication on the hydrogel matrix. Cellular contraction generated traction force transmitted through the hydrogel; α-Catenin regulates both traction force-induced cell-cell adherens junctions and cell-matrix adhesions, while Piezo partially localized at focal adhesions could mechanosense the stretch in the matrix for cell distant communication.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).