Submitted:
16 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
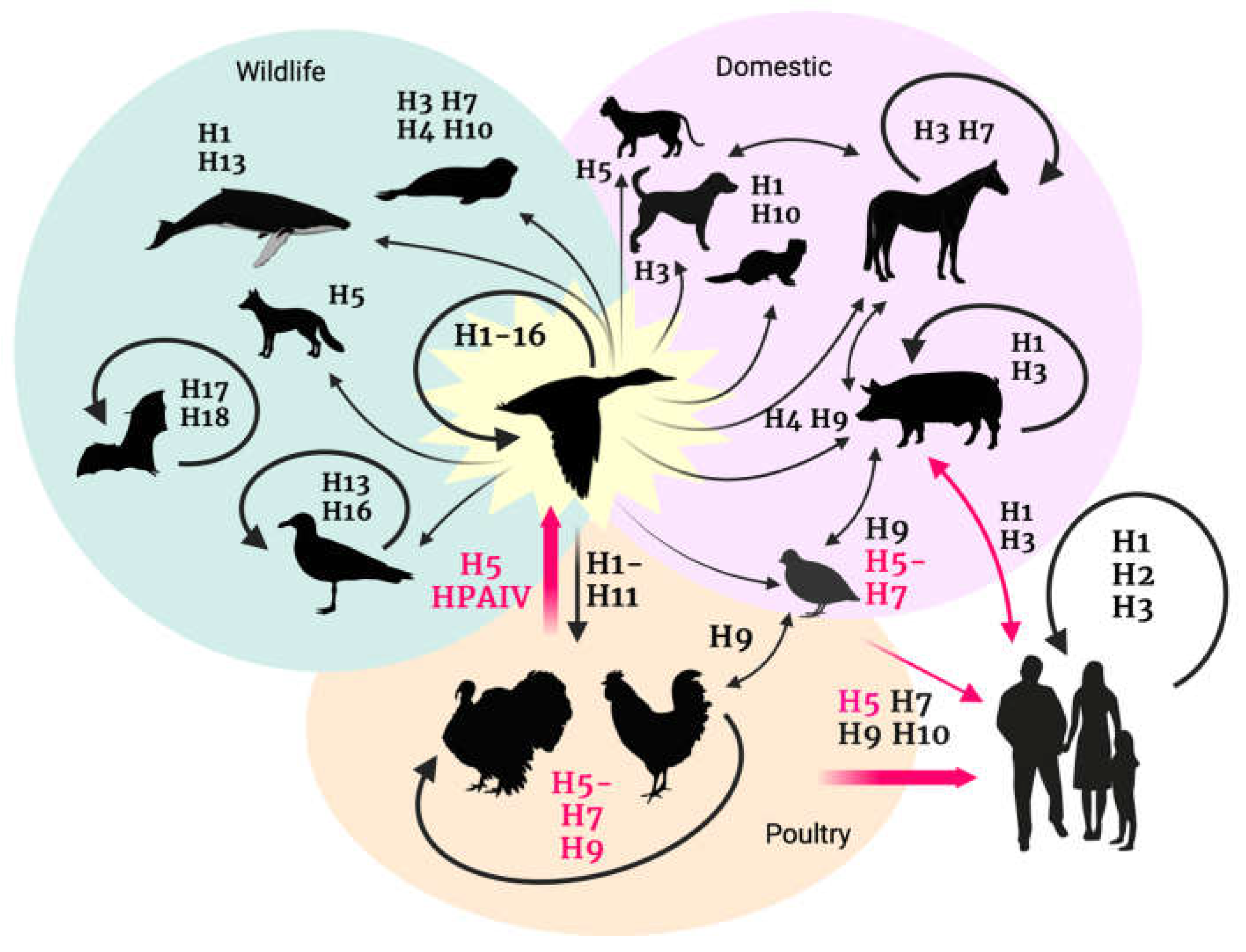
2. H5 Genetic Evolution and Epidemiology
3. Wild Aquatic Birds
3.1. Anseriformes Order
3.2. Charadriiformes Order
3.3. Other Aquatic Wild Birds
4. Game Fowls
5. Pigeons and Doves
6. Scavengers and Raptors
7. Domestic Poultry
7.1. Chickens
7.2. Turkeys
7.3. Domestic Ducks and Geese
8. Mammals
8.1. Farmed Animals and Pets
8.2. Wild Mammals
8.2.1. Marine Mammals
8.2.2. Terrestrial Mammals
9. Conclusions
Author Contributions
Acknowledgments
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Adriaenssens, E.M.; Alfenas-Zerbini, P.; Dempsey, D.M.; Dutilh, B.E.; Garcia, M.L.; Curtis Hendrickson, R.; et al. Recent changes to virus taxonomy ratified by the International Committee on Taxonomy of Viruses (2022). Arch Virol 2022, 167, 2429–2440. [Google Scholar] [CrossRef]
- Schoch, C.L.; Ciufo, S.; Domrachev, M.; Hotton, C.L.; Kannan, S.; Khovanskaya, R.; Leipe, D.; McVeigh, R.; O'Neill, K.; Robbertse, B.; et al. NCBI Taxonomy: a comprehensive update on curation, resources and tools. Database (Oxford) 2020, 2020. [Google Scholar] [CrossRef]
- Muramoto, Y.; Noda, T.; Kawakami, E.; Akkina, R.; Kawaoka, Y. Identification of novel influenza A virus proteins translated from PA mRNA. J Virol 2013, 87, 2455–2462. [Google Scholar] [CrossRef]
- Hutchinson, E.C.; Charles, P.D.; Hester, S.S.; Thomas, B.; Trudgian, D.; Martínez-Alonso, M.; Fodor, E. Conserved and host-specific features of influenza virion architecture. Nat Commun 2014, 5, 4816. [Google Scholar] [CrossRef]
- McCrone, J.T.; Woods, R.J.; Martin, E.T.; Malosh, R.E.; Monto, A.S.; Lauring, A.S. Stochastic processes constrain the within and between host evolution of influenza virus. Elife 2018, 7. [Google Scholar] [CrossRef]
- Lamb, R.; Krug, R. Orthomyxoviridae: The Viruses and Their Replication. In Fields Virology, Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, 2001; Volume 1. [Google Scholar]
- Fereidouni, S.; Starick, E.; Karamendin, K.; Genova, C.D.; Scott, S.D.; Khan, Y.; Harder, T.; Kydyrmanov, A. Genetic characterization of a new candidate hemagglutinin subtype of influenza A viruses. Emerg Microbes Infect 2023, 12, 2225645. [Google Scholar] [CrossRef]
- Kandeil, A.; Gomaa, M.R.; Shehata, M.M.; El Taweel, A.N.; Mahmoud, S.H.; Bagato, O.; Moatasim, Y.; Kutkat, O.; Kayed, A.S.; Dawson, P.; et al. Isolation and Characterization of a Distinct Influenza A Virus from Egyptian Bats. J Virol 2019, 93. [Google Scholar] [CrossRef]
- Tong, S.; Zhu, X.; Li, Y.; Shi, M.; Zhang, J.; Bourgeois, M.; Yang, H.; Chen, X.; Recuenco, S.; Gomez, J.; et al. New world bats harbor diverse influenza A viruses. PLoS Pathog 2013, 9, e1003657. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, Y.; Tefsen, B.; Shi, Y.; Gao, G.F. Bat-derived influenza-like viruses H17N10 and H18N11. Trends Microbiol 2014, 22, 183–191. [Google Scholar] [CrossRef]
- Alexander, D.J. An overview of the epidemiology of avian influenza. Vaccine 2007, 25, 5637–5644. [Google Scholar] [CrossRef]
- Alexander, D.J. A review of avian influenza in different bird species. Vet Microbiol 2000, 74, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Suarez, D.L. Highly pathogenic avian influenza. Rev Sci Tech 2000, 19, 463–482. [Google Scholar] [CrossRef] [PubMed]
- World Organization for Animal Health. International animal health code. Available online: www.oieint/eng/normes/MCode/A_00003htm (accessed on 18th of September 2023).
- World Organisation for Animal Health. Infection with high pathogenicity avian influenza viruses. Available online: https://www.woah.org/fileadmin/Home/eng/Health_standards/tahc/2023/chapitre_avian_influenza_viruses.pdf (accessed on 13th of October 2023).
- Xu, X.; Subbarao; Cox, N. J.; Guo, Y. Genetic characterization of the pathogenic influenza A/Goose/Guangdong/1/96 (H5N1) virus: similarity of its hemagglutinin gene to those of H5N1 viruses from the 1997 outbreaks in Hong Kong. Virology 1999, 261, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Xu, X.; Wan, X. Genetic characterization of an avian influenza A (H5N1) virus isolated from a sick goose in China. Zhonghua Shi Yan He Lin Chuang Bing Du Xue Za Zhi 1998, 12, 322–325. [Google Scholar] [PubMed]
- Marandino, A.; Tomás, G.; Panzera, Y.; Leizagoyen, C.; Pérez, R.; Bassetti, L.; Negro, R.; Rodríguez, S.; Pérez, R. Spreading of the High-Pathogenicity Avian Influenza (H5N1) Virus of Clade 2.3.4.4b into Uruguay. Viruses 2023, 15. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Bertran, K.; Kwon, J.H.; Swayne, D.E. Evolution, global spread, and pathogenicity of highly pathogenic avian influenza H5Nx clade 2.3.4.4. J Vet Sci 2017, 18, 269–280. [Google Scholar] [CrossRef] [PubMed]
- Kandeil, A.; Patton, C.; Jones, J.C.; Jeevan, T.; Harrington, W.N.; Trifkovic, S.; Seiler, J.P.; Fabrizio, T.; Woodard, K.; Turner, J.C.; et al. Rapid evolution of A(H5N1) influenza viruses after intercontinental spread to North America. Nat Commun 2023, 14, 3082. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.H.; Criado, M.F.; Swayne, D.E. Pathobiological Origins and Evolutionary History of Highly Pathogenic Avian Influenza Viruses. Cold Spring Harb Perspect Med 2021, 11. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Swayne, D.E. Pathobiology of Asian highly pathogenic avian influenza H5N1 virus infections in ducks. Avian Dis 2007, 51, 250–259. [Google Scholar] [CrossRef]
- (USDA), A.a.P.H.I.S. USDA, FDA and CDC Share Update on HPAI Detections in Dairy Cattle. (: Available online.
- Thompson, A.J.; Paulson, J.C. Adaptation of influenza viruses to human airway receptors. J Biol Chem 2021, 296, 100017. [Google Scholar] [CrossRef]
- Swayne, D.E. Understanding the complex pathobiology of high pathogenicity avian influenza viruses in birds. Avian Dis 2007, 51, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Long, J.S.; Mistry, B.; Haslam, S.M.; Barclay, W.S. Host and viral determinants of influenza A virus species specificity. Nature Reviews Microbiology 2019, 17, 67–81. [Google Scholar] [CrossRef] [PubMed]
- Steinhauer, D.A. Role of hemagglutinin cleavage for the pathogenicity of influenza virus. Virology 1999, 258, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Wiley, D.C.; Skehel, J.J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annual Review of Biochemistry 1987, 56, 365–394. [Google Scholar] [CrossRef] [PubMed]
- Wilson, I.A.; Skehel, J.J.; Wiley, D.C. Structure of the haemagglutinin membrane glycoprotein of influenza virus at 3 Å resolution. Nature 1981, 289, 366–373. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E. Avian influenza; John Wiley & Sons: 2009.
- Chen, J.; Lee, K.H.; Steinhauer, D.A.; Stevens, D.J.; Skehel, J.J.; Wiley, D.C. Structure of the hemagglutinin precursor cleavage site, a determinant of influenza pathogenicity and the origin of the labile conformation. Cell 1998, 95, 409–417. [Google Scholar] [CrossRef] [PubMed]
- Gotoh, B.; Ogasawara, T.; Toyoda, T.; Inocencio, N.M.; Hamaguchi, M.; Nagai, Y. An endoprotease homologous to the blood clotting factor X as a determinant of viral tropism in chick embryo. Embo j 1990, 9, 4189–4195. [Google Scholar] [CrossRef] [PubMed]
- Klenk, H.D.; Garten, W. Host cell proteases controlling virus pathogenicity. Trends Microbiol 1994, 2, 39–43. [Google Scholar] [CrossRef]
- Stieneke-Gröber, A.; Vey, M.; Angliker, H.; Shaw, E.; Thomas, G.; Roberts, C.; Klenk, H.D.; Garten, W. Influenza virus hemagglutinin with multibasic cleavage site is activated by furin, a subtilisin-like endoprotease. Embo j 1992, 11, 2407–2414. [Google Scholar] [CrossRef] [PubMed]
- Swayne, D.E.; Pantin-Jackwood, M. Pathogenicity of avian influenza viruses in poultry. Dev Biol (Basel) 2006, 124, 61–67. [Google Scholar]
- Hulse, D.J.; Webster, R.G.; Russell, R.J.; Perez, D.R. Molecular determinants within the surface proteins involved in the pathogenicity of H5N1 influenza viruses in chickens. J Virol 2004, 78, 9954–9964. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Avian Influenza. <i>Manual of Diagnostic Tests and Vaccines for Terrestrial Animals </i>2012, pp. 37. World Organisation for Animal Health. Avian Influenza. Manual of Diagnostic Tests and Vaccines for Terrestrial Animals.
- Wilson, I.A.; Cox, N.J. Structural basis of immune recognition of influenza virus hemagglutinin. Annu Rev Immunol 1990, 8, 737–771. [Google Scholar] [CrossRef]
- Marcelin, G.; Sandbulte, M.R.; Webby, R.J. Contribution of antibody production against neuraminidase to the protection afforded by influenza vaccines. Rev Med Virol 2012, 22, 267–279. [Google Scholar] [CrossRef] [PubMed]
- Johansson, B.E.; Matthews, J.T.; Kilbourne, E.D. Supplementation of conventional influenza A vaccine with purified viral neuraminidase results in a balanced and broadened immune response. Vaccine 1998, 16, 1009–1015. [Google Scholar] [CrossRef]
- Suarez, D.L.; Schultz-Cherry, S. Immunology of avian influenza virus: a review. Developmental & Comparative Immunology 2000, 24, 269–283. [Google Scholar] [CrossRef]
- Cattoli, G.; Milani, A.; Temperton, N.; Zecchin, B.; Buratin, A.; Molesti, E.; Aly, M.M.; Arafa, A.; Capua, I. Antigenic drift in H5N1 avian influenza virus in poultry is driven by mutations in major antigenic sites of the hemagglutinin molecule analogous to those for human influenza virus. J Virol 2011, 85, 8718–8724. [Google Scholar] [CrossRef]
- Plotkin, J.B.; Dushoff, J. Codon bias and frequency-dependent selection on the hemagglutinin epitopes of influenza A virus. Proc Natl Acad Sci U S A 2003, 100, 7152–7157. [Google Scholar] [CrossRef] [PubMed]
- Bouvier, N.M.; Palese, P. The biology of influenza viruses. Vaccine 2008, 26, D49–D53. [Google Scholar] [CrossRef]
- Suttie, A.; Deng, Y.M.; Greenhill, A.R.; Dussart, P.; Horwood, P.F.; Karlsson, E.A. Inventory of molecular markers affecting biological characteristics of avian influenza A viruses. Virus Genes 2019, 55, 739–768. [Google Scholar] [CrossRef]
- Carnaccini, S.; Perez, D.R. H9 Influenza Viruses: An Emerging Challenge. Cold Spring Harb Perspect Med 2020, 10. [Google Scholar] [CrossRef]
- Pappas, C.; Aguilar, P.V.; Basler, C.F.; Solórzano, A.; Zeng, H.; Perrone, L.A.; Palese, P.; García-Sastre, A.; Katz, J.M.; Tumpey, T.M. Single gene reassortants identify a critical role for PB1, HA, and NA in the high virulence of the 1918 pandemic influenza virus. Proc Natl Acad Sci U S A 2008, 105, 3064–3069. [Google Scholar] [CrossRef]
- Webster, R.G.; Govorkova, E.A. Continuing challenges in influenza. Ann N Y Acad Sci 2014, 1323, 115–139. [Google Scholar] [CrossRef]
- Smith, G.J.; Donis, R.O. Nomenclature updates resulting from the evolution of avian influenza A(H5) virus clades 2.1.3.2a, 2.2.1, and 2.3.4 during 2013-2014. Influenza Other Respir Viruses 2015, 9, 271–276. [Google Scholar] [CrossRef]
- World Health Organization. Antigenic and genetic characteristics of zoonotic influenza A viruses and development of candidate vaccine viruses for pandemic preparedness.; WHO: Geneva, 2020. [Google Scholar]
- Kuiken, T.; Fouchier, R.A.M.; Koopmans, M.P.G. Being ready for the next influenza pandemic? Lancet Infect Dis 2023, 23, 398–399. [Google Scholar] [CrossRef]
- Zhao, K.; Gu, M.; Zhong, L.; Duan, Z.; Zhang, Y.; Zhu, Y.; Zhao, G.; Zhao, M.; Chen, Z.; Hu, S.; et al. Characterization of three H5N5 and one H5N8 highly pathogenic avian influenza viruses in China. Vet Microbiol 2013, 163, 351–357. [Google Scholar] [CrossRef]
- Lee, Y.J.; Kang, H.M.; Lee, E.K.; Song, B.M.; Jeong, J.; Kwon, Y.K.; Kim, H.R.; Lee, K.J.; Hong, M.S.; Jang, I.; et al. Novel reassortant influenza A(H5N8) viruses, South Korea, 2014. Emerg Infect Dis 2014, 20, 1087–1089. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; et al. Avian influenza overview September - December 2022. EFSA J 2023, 21, e07786. [Google Scholar] [CrossRef]
- Lee, D.H.; Torchetti, M.K.; Winker, K.; Ip, H.S.; Song, C.S.; Swayne, D.E. Intercontinental Spread of Asian-Origin H5N8 to North America through Beringia by Migratory Birds. J Virol 2015, 89, 6521–6524. [Google Scholar] [CrossRef]
- Lee, D.H.; Bahl, J.; Torchetti, M.K.; Killian, M.L.; Ip, H.S.; DeLiberto, T.J.; Swayne, D.E. Highly Pathogenic Avian Influenza Viruses and Generation of Novel Reassortants, United States, 2014-2015. Emerg Infect Dis 2016, 22, 1283–1285. [Google Scholar] [CrossRef]
- Lee, D.H.; Torchetti, M.K.; Hicks, J.; Killian, M.L.; Bahl, J.; Pantin-Jackwood, M.; Swayne, D.E. Transmission Dynamics of Highly Pathogenic Avian Influenza Virus A(H5Nx) Clade 2.3.4.4, North America, 2014-2015. Emerg Infect Dis 2018, 24, 1840–1848. [Google Scholar] [CrossRef]
- United States Department of Agriculture; Animal and Plant Health Inspection Service. July 2016 - June 2017 wild bird highly pathogenic avian influenza cases in the United States. Available online: https://www.aphis.usda.gov/animal_health/downloads/animal_diseases/ai/uspositivecases17.pdf (accessed on 13th of October 2023).
- Wu, H.; Peng, X.; Xu, L.; Jin, C.; Cheng, L.; Lu, X.; Xie, T.; Yao, H.; Wu, N. Novel reassortant influenza A(H5N8) viruses in domestic ducks, eastern China. Emerg Infect Dis 2014, 20, 1315–1318. [Google Scholar] [CrossRef]
- Lee, D.H.; Sharshov, K.; Swayne, D.E.; Kurskaya, O.; Sobolev, I.; Kabilov, M.; Alekseev, A.; Irza, V.; Shestopalov, A. Novel Reassortant Clade 2.3.4.4 Avian Influenza A(H5N8) Virus in Wild Aquatic Birds, Russia, 2016. Emerg Infect Dis 2017, 23, 359–360. [Google Scholar] [CrossRef]
- Li, M.; Liu, H.; Bi, Y.; Sun, J.; Wong, G.; Liu, D.; Li, L.; Liu, J.; Chen, Q.; Wang, H.; et al. Highly Pathogenic Avian Influenza A(H5N8) Virus in Wild Migratory Birds, Qinghai Lake, China. Emerg Infect Dis 2017, 23, 637–641. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian influenza; Brown, I. ; Kuiken, T.; Mulatti, P.; Smietanka, K.; Staubach, C.; Stroud, D.; Therkildsen, O.R.; et al. Avian influenza overview September – November 2017. EFSA J 2017, 15, e05141. [Google Scholar] [CrossRef]
- Lycett, S.J.; Pohlmann, A.; Staubach, C.; Caliendo, V.; Woolhouse, M.; Beer, M.; Kuiken, T. Genesis and spread of multiple reassortants during the 2016/2017 H5 avian influenza epidemic in Eurasia. Proc Natl Acad Sci U S A 2020, 117, 20814–20825. [Google Scholar] [CrossRef]
- European Food Safety Authority; Prevention, E. C.f.D.; Control; European Union Reference Laboratory for Avian influenza; Adlhoch, C.; Brouwer, A.; Kuiken, T.; Mulatti, P.; Smietanka, K.; Staubach, C.; et al. Avian influenza overview November 2017 - February 2018. EFSA J 2018, 16, e05240. [Google Scholar] [CrossRef]
- European Food Safety Authority; Prevention, E. C.f.D.; Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Brouwer, A.; Kuiken, T.; Miteva, A.; Mulatti, P.; Smietanka, K.; et al. Avian influenza overview August – November 2018. EFSA J 2018, 16, e05573. [Google Scholar] [CrossRef]
- European Food Safety Authority; Prevention, E. C.f.D.; Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Kuiken, T.; Niqueux, E.; Staubach, C.; Terregino, C.; et al. Avian influenza overview November 2019– February 2020. EFSA J 2020, 18, e06096. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; Staubach, C.; et al. Avian influenza overview September – December 2021. EFSA J 2021, 19, e07108. [Google Scholar] [CrossRef]
- European Food Safety Authority; Prevention, E. C.f.D.; Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; et al. Avian influenza overview December 2020 – February 2021. EFSA J 2021, 19, e06497. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; et al. Avian influenza overview December 2021 - March 2022. EFSA J 2022, 20, e07289. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; Staubach, C.; et al. Avian influenza overview June–September 2023. EFSA J 2023, 21, e08328. [Google Scholar] [CrossRef]
- Bevins, S.N.; Shriner, S.A.; Cumbee, J.C., Jr.; Dilione, K.E.; Douglass, K.E.; Ellis, J.W.; Killian, M.L.; Torchetti, M.K.; Lenoch, J.B. Intercontinental Movement of Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4 Virus to the United States, 2021. Emerg Infect Dis 2022, 28, 1006–1011. [Google Scholar] [CrossRef]
- Caliendo, V.; Lewis, N.S.; Pohlmann, A.; Baillie, S.R.; Banyard, A.C.; Beer, M.; Brown, I.H.; Fouchier, R.A.M.; Hansen, R.D.E.; Lameris, T.K.; et al. Transatlantic spread of highly pathogenic avian influenza H5N1 by wild birds from Europe to North America in 2021. Scientific Reports 2022, 12, 11729. [Google Scholar] [CrossRef]
- Günther, A.; Krone, O.; Svansson, V.; Pohlmann, A.; King, J.; Hallgrimsson, G.T.; Skarphéðinsson, K.H.; Sigurðardóttir, H.; Jónsson, S.R.; Beer, M.; et al. Iceland as Stepping Stone for Spread of Highly Pathogenic Avian Influenza Virus between Europe and North America. Emerg Infect Dis 2022, 28, 2383–2388. [Google Scholar] [CrossRef]
- Alkie, T.N.; Lopes, S.; Hisanaga, T.; Xu, W.; Suderman, M.; Koziuk, J.; Fisher, M.; Redford, T.; Lung, O.; Joseph, T.; et al. A threat from both sides: Multiple introductions of genetically distinct H5 HPAI viruses into Canada via both East Asia-Australasia/Pacific and Atlantic flyways. Virus Evolution 2022, 8. [Google Scholar] [CrossRef]
- Youk, S.; Torchetti, M.K.; Lantz, K.; Lenoch, J.B.; Killian, M.L.; Leyson, C.; Bevins, S.N.; Dilione, K.; Ip, H.S.; Stallknecht, D.E.; et al. H5N1 highly pathogenic avian influenza clade 2.3.4.4b in wild and domestic birds: Introductions into the United States and reassortments, December 2021–April 2022. Virology 2023, 587, 109860. [Google Scholar] [CrossRef]
- Ramey, A.M.; Scott, L.C.; Ahlstrom, C.A.; Buck, E.J.; Williams, A.R.; Kim Torchetti, M.; Stallknecht, D.E.; Poulson, R.L. Molecular detection and characterization of highly pathogenic H5N1 clade 2.3.4.4b avian influenza viruses among hunter-harvested wild birds provides evidence for three independent introductions into Alaska. Virology 2024, 589, 109938. [Google Scholar] [CrossRef]
- Youk, S.; Torchetti, M.K.; Lantz, K.; Lenoch, J.B.; Killian, M.L.; Leyson, C.; Bevins, S.N.; Dilione, K.E.; Ip, H.S.; Stallknecht, D.E.; et al. H5N1 highly pathogenic avian influenza clade 2.3.4.4b in wild and domestic birds: Introductions into the United States and reassortments, December 2021–April 2022. Virology 2023, 587, 109860. [Google Scholar] [CrossRef]
- Animal and Plant Health Inspection Service, U.S.D.o.A. 2022-2024 Confirmations of Highly Pathogenic Avian Influenza in Commercial and Backyard Flocks. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza/hpai-2022/2022-hpai-commercial-backyard-flocks (accessed on 9th August 2023).
- Animal and Plant Health Inspection Service, U.S.D.o.A. 2022-2024 Detections of Highly Pathogenic Avian Influenza in Wild Birds. 2024.
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, E.; et al. Avian influenza overview December 2022 - March 2023. EFSA J 2023, 21, e07917. [Google Scholar] [CrossRef]
- Mariana, L.; Alejandra, G.-G.; Breno, M.-S.; Diana, J.; Patricia, B.; Carlos, C.-M.; Javier, J.; Walter, S.; Karl, P.; Lady, A.; et al. Highly pathogenic avian influenza A (H5N1) in marine mammals and seabirds in Peru. Nature Communications 2023, 14, 5489. [Google Scholar] [CrossRef]
- Castro-Sanguinetti, G.R.; González-Veliz, R.; Callupe-Leyva, A.; Apaza-Chiara, A.P.; Jara, J.; Silva, W.; Icochea, E.; More-Bayona, J.A. Highly pathogenic avian influenza virus H5N1 clade 2.3.4.4b from Peru forms a monophyletic group with Chilean isolates in South America. Scientific Reports 2024, 14, 3635. [Google Scholar] [CrossRef]
- Gamarra-Toledo, V.; Plaza, P.I.; Angulo, F.; Gutiérrez, R.; García-Tello, O.; Saravia-Guevara, P.; Mejía-Vargas, F.; Epiquién-Rivera, M.; Quiroz-Jiménez, G.; Martinez, P.; et al. Highly Pathogenic Avian Influenza (HPAI) strongly impacts wild birds in Peru. Biological Conservation 2023, 286, 110272. [Google Scholar] [CrossRef]
- Gamarra-Toledo, V.; Plaza, P.I.; Gutiérrez, R.; Inga-Diaz, G.; Saravia-Guevara, P.; Pereyra-Meza, O.; Coronado-Flores, E.; Calderón-Cerrón, A.; Quiroz-Jiménez, G.; Martinez, P.; et al. Mass Mortality of Marine Mammals Associated to Highly Pathogenic Influenza Virus (H5N1) in South America. bioRxiv, 2002. [Google Scholar] [CrossRef]
- Reischak, D.; Rivetti, A.V.; Otaka, J.N.P.; Domingues, C.S.; Freitas, T.d.L.; Cardoso, F.G.; Montesino, L.O.; da Silva, A.L.S.; Malta, F.; Amgarten, D.; et al. First report and genetic characterization of the highly pathogenic avian influenza A(H5N1) virus in Cabot's tern (Thalasseus acuflavidus), Brazil. Veterinary and Animal Science 2023, 22, 100319. [Google Scholar] [CrossRef]
- Rimondi, A.; Vanstreels, R.E.T.; Olivera, V.; Donini, A.; Lauriente, M.M.; Uhart, M. Highly Pathogenic Avian Influenza A(H5N1) Viruses from Multispecies Outbreak, Argentina, August 2023. Emerg Infect Dis 2024, 30. [Google Scholar] [CrossRef] [PubMed]
- Bennison, A.; Byrne, A.M.P.; Reid, S.M.; Lynton-Jenkins, J.G.; Mollett, B.; Sliva, D.D.; Peers-Dent, J.; Finlayson, K.; Hall, R.; Blockley, F.; et al. Detection and spread of high pathogenicity avian influenza virus H5N1 in the Antarctic Region. bioRxiv, 2011. [Google Scholar] [CrossRef]
- Authority, E.F.S.; Prevention, E.C.f.D.; Control; Influenza, E. U.R.L.f.A.; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Mirinavičiūtė, G.; Niqueux, É.; et al. Avian influenza overview September–December 2023. EFSA J 2023, 21, e8539. [Google Scholar] [CrossRef]
- Bi, Y.; Chen, Q.; Wang, Q.; Chen, J.; Jin, T.; Wong, G.; Quan, C.; Liu, J.; Wu, J.; Yin, R.; et al. Genesis, Evolution and Prevalence of H5N6 Avian Influenza Viruses in China. Cell Host Microbe 2016, 20, 810–821. [Google Scholar] [CrossRef]
- Kwon, J.H.; Noh, Y.K.; Lee, D.H.; Yuk, S.S.; Erdene-Ochir, T.O.; Noh, J.Y.; Hong, W.T.; Jeong, J.H.; Jeong, S.; Gwon, G.B.; et al. Experimental infection with highly pathogenic H5N8 avian influenza viruses in the Mandarin duck (Aix galericulata) and domestic pigeon (Columba livia domestica). Vet Microbiol 2017, 203, 95–102. [Google Scholar] [CrossRef]
- Takemae, N.; Tsunekuni, R.; Sharshov, K.; Tanikawa, T.; Uchida, Y.; Ito, H.; Soda, K.; Usui, T.; Sobolev, I.; Shestopalov, A.; et al. Five distinct reassortants of H5N6 highly pathogenic avian influenza A viruses affected Japan during the winter of 2016-2017. Virology 2017, 512, 8–20. [Google Scholar] [CrossRef]
- Elbe, S.; Buckland-Merrett, G. Data, disease and diplomacy: GISAID's innovative contribution to global health. Glob Chall 2017, 1, 33–46. [Google Scholar] [CrossRef]
- Koethe, S.; Ulrich, L.; Ulrich, R.; Amler, S.; Graaf, A.; Harder, T.C.; Grund, C.; Mettenleiter, T.C.; Conraths, F.J.; Beer, M.; et al. Modulation of lethal HPAIV H5N8 clade 2.3.4.4B infection in AIV pre-exposed mallards. Emerg Microbes Infect 2020, 9, 180–193. [Google Scholar] [CrossRef]
- Leyson, C.; Youk, S.S.; Smith, D.; Dimitrov, K.; Lee, D.H.; Larsen, L.E.; Swayne, D.E.; Pantin-Jackwood, M.J. Pathogenicity and genomic changes of a 2016 European H5N8 highly pathogenic avian influenza virus (clade 2.3.4.4) in experimentally infected mallards and chickens. Virology 2019, 537, 172–185. [Google Scholar] [CrossRef]
- Kim, Y.I.; Pascua, P.N.; Kwon, H.I.; Lim, G.J.; Kim, E.H.; Yoon, S.W.; Park, S.J.; Kim, S.M.; Choi, E.J.; Si, Y.J.; et al. Pathobiological features of a novel, highly pathogenic avian influenza A(H5N8) virus. Emerg Microbes Infect 2014, 3, e75. [Google Scholar] [CrossRef]
- Tarasiuk, K.; Kycko, A.; Świętoń, E.; Bocian, Ł.; Wyrostek, K.; Śmietanka, K. Homo- and Heterosubtypic Immunity to Low Pathogenic Avian Influenza Virus Mitigates the Clinical Outcome of Infection with Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4.b in Captive Mallards (Anas platyrhynchos). Pathogens 2023, 12, 217. [Google Scholar] [CrossRef]
- Gobbo, F.; Fornasiero, D.; De Marco, M.A.; Zecchin, B.; Mulatti, P.; Delogu, M.; Terregino, C. Active Surveillance for Highly Pathogenic Avian Influenza Viruses in Wintering Waterbirds in Northeast Italy, 2020-2021. Microorganisms 2021, 9. [Google Scholar] [CrossRef]
- Kleyheeg, E.; Slaterus, R.; Bodewes, R.; Rijks, J.M.; Spierenburg, M.A.H.; Beerens, N.; Kelder, L.; Poen, M.J.; Stegeman, J.A.; Fouchier, R.A.M.; et al. Deaths among Wild Birds during Highly Pathogenic Avian Influenza A(H5N8) Virus Outbreak, the Netherlands. Emerg Infect Dis 2017, 23, 2050–2054. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC); European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Mirinaviciute, G.; Niqueux, E.; et al. Avian influenza overview December 2022 - March 2023. EFSA J 2023, 21, e07917. [Google Scholar] [CrossRef]
- European Food Safety Authority; Aznar, I. ; Baldinelli, F.; Papanikolaou, A.; Stoicescu, A.; Van der Stede, Y. Annual Report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2020. EFSA J 2021, 19, e06953. [Google Scholar] [CrossRef]
- European Food Safety Authority; Aznar, I. ; Baldinelli, F.; Stoicescu, A.; Kohnle, L. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2021. EFSA J 2022, 20, e07554. [Google Scholar] [CrossRef]
- Pohlmann, A.; Stejskal, O.; King, J.; Bouwhuis, S.; Packmor, F.; Ballstaedt, E.; Hälterlein, B.; Hennig, V.; Stacker, L.; Graaf, A.; et al. Mass mortality among colony-breeding seabirds in the German Wadden Sea in 2022 due to distinct genotypes of HPAIV H5N1 clade 2.3.4.4b. J Gen Virol 2023, 104. [Google Scholar] [CrossRef]
- Bodewes, R.; Kuiken, T. Changing Role of Wild Birds in the Epidemiology of Avian Influenza A Viruses. Adv Virus Res 2018, 100, 279–307. [Google Scholar] [CrossRef]
- Pohlmann, A.; King, J.; Fusaro, A.; Zecchin, B.; Banyard, A.C.; Brown, I.H.; Byrne, A.M.P.; Beerens, N.; Liang, Y.; Heutink, R.; et al. Has Epizootic Become Enzootic? Evidence for a Fundamental Change in the Infection Dynamics of Highly Pathogenic Avian Influenza in Europe, 2021. mBio 2022, 13, e00609–00622. [Google Scholar] [CrossRef]
- CMS FAO CO-convened Scientific Task Force on Avian Influenza and Wild Birds. Scientific Task Force on Avian Influenza and Wild Birds Statement - July 2023. Available online: https://www.cms.int/sites/default/files/publication/avian_influenza_2023_aug.pdf (accessed on 14th of October 2023).
- IUCN. The IUCN Red List of Threatened Species. Version 2022-2. Available online: https://www.iucnredlist.org (accessed on 14th of October 2023).
- EAAFP. Updates of HPAI recorded in East Asian – Australasian Flyway. Available online: https://www.eaaflyway.net/updates-hpai-eaaf/ (accessed on 14th of October 2023).
- Alexandrou, O.; Malakou, M.; Catsadorakis, G. The impact of avian influenza 2022 on Dalmatian pelicans was the worst ever wildlife disaster in Greece. Oryx 2022, 56, 813–813. [Google Scholar] [CrossRef]
- Cunningham, E.J.A.; Gamble, A.; Hart, T.; Humphreys, E.M.; Philip, E.; Tyler, G.; Wood, M.J. The incursion of Highly Pathogenic Avian Influenza (HPAI) into North Atlantic seabird populations: an interim report from the 15th International Seabird Group conference. Seabird 2022, 34, 67–73. [Google Scholar] [CrossRef]
- U.S. Fish & Wildlife Service. California condors & HPAI update. Available online: https://www.fws.gov/program/california-condor-recovery/southwest-california-condor-flock-hpai-information-updates-2023 (accessed on 14th of October 2023).
- Animal and Plant Health Inspection Service; U.S. Department of Agriculture. USDA Takes Action to Help Protect Endangered California Condors From Highly Pathogenic Avian Influenza. Available online: https://www.aphis.usda.gov/aphis/newsroom/stakeholder-info/sa_by_date/sa-2023/ca-condor-hpai (accessed on 14th of October 2023).
- Stokstad, E. Deadly avian flu reaches Galápagos Islands. Science 2023. [Google Scholar] [CrossRef]
- Gobbo, F.; Zanardello, C.; Bottinelli, M.; Budai, J.; Bruno, F.; De Nardi, R.; Patregnani, T.; Catania, S.; Terregino, C. Silent Infection of Highly Pathogenic Avian Influenza Virus (H5N1) Clade 2.3.4.4b in a Commercial Chicken Broiler Flock in Italy. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Song, B.M.; Kang, H.M.; Lee, E.K.; Jung, J.; Kang, Y.; Lee, H.S.; Lee, Y.J. Pathogenicity of H5N8 virus in chickens from Korea in 2014. J Vet Sci 2015, 16, 237–240. [Google Scholar] [CrossRef]
- Takadate, Y.; Tsunekuni, R.; Kumagai, A.; Mine, J.; Kikutani, Y.; Sakuma, S.; Miyazawa, K.; Uchida, Y. Different Infectivity and Transmissibility of H5N8 and H5N1 High Pathogenicity Avian Influenza Viruses Isolated from Chickens in Japan in the 2021/2022 Season. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Kwon, J.H.; Bertran, K.; Lee, D.H.; Criado, M.F.; Killmaster, L.; Pantin-Jackwood, M.J.; Swayne, D.E. Diverse infectivity, transmissibility, and pathobiology of clade 2.3.4.4 H5Nx highly pathogenic avian influenza viruses in chickens. Emerg Microbes Infect 2023, 12, 2218945. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; et al. Avian influenza overview March - June 2022. EFSA J 2022, 20, e07415. [Google Scholar] [CrossRef]
- European Food Safety Authority, E.C.f.D.P.; Control, E.U.R.L.f.A.I.; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Melidou, A.; Mirinavičiūtė, G.; Niqueux, É.; Ståhl, K.; et al. Avian influenza overview April – June 2023. EFSA Journal 2023, 21, e08191. [Google Scholar]
- Vreman, S.; Kik, M.; Germeraad, E.; Heutink, R.; Harders, F.; Spierenburg, M.; Engelsma, M.; Rijks, J.; van den Brand, J.; Beerens, N. Zoonotic Mutation of Highly Pathogenic Avian Influenza H5N1 Virus Identified in the Brain of Multiple Wild Carnivore Species. Pathogens 2023, 12. [Google Scholar] [CrossRef]
- Appleby, M.C.; Mench, J.A.; Hughes, B.O. Poultry Behaviour and Welfare CABI Publishing: United Kingdom, 2004.
- Spackman, E.; Pantin-Jackwood, M.J.; Lee, S.A.; Prosser, D. The pathogenesis of a 2022 North American highly pathogenic clade 2.3.4.4b H5N1 avian influenza virus in mallards (Anas platyrhynchos). Avian Pathol 2023, 52, 219–228. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Fujii, K.; Sugie, Y.; Tsunekuni, R.; Nakayama, M.; Kobayashi, S. Comparative susceptibility of mallard (Anas platyrhynchos) to infection with high pathogenicity avian influenza virus strains (Gs/Gd lineage) isolated in Japan in 2004-2017. Vet Microbiol 2022, 272, 109496. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Leijten, L.; van de Bildt, M.W.G.; Poen, M.J.; Kok, A.; Bestebroer, T.; Richard, M.; Fouchier, R.A.M.; Kuiken, T. Long-Term Protective Effect of Serial Infections with H5N8 Highly Pathogenic Avian Influenza Virus in Wild Ducks. J Virol 2022, 96, e0123322. [Google Scholar] [CrossRef] [PubMed]
- Beerens, N.; Germeraad, E.A.; Venema, S.; Verheij, E.; Pritz-Verschuren, S.B.E.; Gonzales, J.L. Comparative pathogenicity and environmental transmission of recent highly pathogenic avian influenza H5 viruses. Emerg Microbes Infect 2021, 10, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Tanikawa, T.; Sakuma, S.; Yoshida, E.; Tsunekuni, R.; Nakayama, M.; Kobayashi, S. Comparative susceptibility of the common teal (Anas crecca) to infection with high pathogenic avian influenza virus strains isolated in Japan in 2004–2017. Vet Microbiol 2021, 263, 109266. [Google Scholar] [CrossRef] [PubMed]
- Tarasiuk, K.; Kycko, A.; Knitter, M.; Świętoń, E.; Wyrostek, K.; Domańska-Blicharz, K.; Bocian, Ł.; Meissner, W.; Śmietanka, K. Pathogenicity of highly pathogenic avian influenza H5N8 subtype for herring gulls (Larus argentatus): impact of homo- and heterosubtypic immunity on the outcome of infection. Veterinary Research 2022, 53, 108. [Google Scholar] [CrossRef]
- Kim, J.K.; Negovetich, N.J.; Forrest, H.L.; Webster, R.G. Ducks: the "Trojan horses" of H5N1 influenza. Influenza Other Respir Viruses 2009, 3, 121–128. [Google Scholar] [CrossRef]
- Campbell, L.K.; Magor, K.E. Pattern Recognition Receptor Signaling and Innate Responses to Influenza A Viruses in the Mallard Duck, Compared to Humans and Chickens. Front Cell Infect Microbiol 2020, 10, 209. [Google Scholar] [CrossRef]
- Webster, R.G.; Bean, W.J.; Gorman, O.T.; Chambers, T.M.; Kawaoka, Y. Evolution and ecology of influenza A viruses. Microbiol Rev 1992, 56, 152–179. [Google Scholar] [CrossRef] [PubMed]
- Evseev, D.; Magor, K.E. Innate Immune Responses to Avian Influenza Viruses in Ducks and Chickens. Vet Sci 2019, 6, 5. [Google Scholar] [CrossRef] [PubMed]
- Barber, M.R.W.; Aldridge, J.R.; Webster, R.G.; Magor, K.E. Association of RIG-I with innate immunity of ducks to influenza. Proc Natl Acad Sci U S A 2010, 107, 5913–5918. [Google Scholar] [CrossRef] [PubMed]
- Grund, C.; Hoffmann, D.; Ulrich, R.; Naguib, M.; Schinköthe, J.; Hoffmann, B.; Harder, T.; Saenger, S.; Zscheppang, K.; Tönnies, M.; et al. A novel European H5N8 influenza A virus has increased virulence in ducks but low zoonotic potential. Emerg Microbes Infect 2018, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.M.; Choi, J.G.; Kim, K.I.; Kim, B.S.; Batchuluun, D.; Erdene-Ochir, T.O.; Kim, M.C.; Kwon, J.H.; Park, C.K.; Lee, Y.J. Pathogenicity in domestic ducks and mice of highly pathogenic H5N1 clade 2.3.2.1 influenza viruses recently circulating in Eastern Asia. Vet Microbiol 2013, 167, 327–333. [Google Scholar] [CrossRef] [PubMed]
- Uchida, Y.; Mine, J.; Takemae, N.; Tanikawa, T.; Tsunekuni, R.; Saito, T. Comparative pathogenicity of H5N6 subtype highly pathogenic avian influenza viruses in chicken, Pekin duck and Muscovy duck. Transbound Emerg Dis 2019, 66, 1227–1251. [Google Scholar] [CrossRef] [PubMed]
- Leyson, C.M.; Youk, S.; Ferreira, H.L.; Suarez, D.L.; Pantin-Jackwood, M. Multiple Gene Segments Are Associated with Enhanced Virulence of Clade 2.3.4.4 H5N8 Highly Pathogenic Avian Influenza Virus in Mallards. J Virol 2021, 95, e0095521. [Google Scholar] [CrossRef] [PubMed]
- Slomka, M.J.; Puranik, A.; Mahmood, S.; Thomas, S.S.; Seekings, A.H.; Byrne, A.M.P.; Núñez, A.; Bianco, C.; Mollett, B.C.; Watson, S.; et al. Ducks Are Susceptible to Infection with a Range of Doses of H5N8 Highly Pathogenic Avian Influenza Virus (2016, Clade 2.3.4.4b) and Are Largely Resistant to Virus-Specific Mortality, but Efficiently Transmit Infection to Contact Turkeys. Avian Dis 2019, 63, 172–180. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.H.; Jin, S.Y.; Seo, S.H. Genetic and pathogenic analysis of a novel reassortant H5N6 influenza virus isolated from waterfowl in South Korea in 2016. Arch Virol 2017, 162, 3507–3510. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Shepherd, E.; DeJesus, E.; Smith, D.; Spackman, E.; Kapczynski, D.R.; Suarez, D.L.; Stallknecht, D.E.; Swayne, D.E. Pathogenicity and Transmission of H5 and H7 Highly Pathogenic Avian Influenza Viruses in Mallards. J Virol 2016, 90, 9967–9982. [Google Scholar] [CrossRef]
- Berhane, Y.; Kobasa, D.; Embury-Hyatt, C.; Pickering, B.; Babiuk, S.; Joseph, T.; Bowes, V.; Suderman, M.; Leung, A.; Cottam-Birt, C.; et al. Pathobiological Characterization of a Novel Reassortant Highly Pathogenic H5N1 Virus Isolated in British Columbia, Canada, 2015. Sci Rep 2016, 6, 23380. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Costa-Hurtado, M.; Bertran, K.; DeJesus, E.; Smith, D.; Swayne, D.E. Infectivity, transmission and pathogenicity of H5 highly pathogenic avian influenza clade 2.3.4.4 (H5N8 and H5N2) United States index viruses in Pekin ducks and Chinese geese. Vet Res 2017, 48, 33. [Google Scholar] [CrossRef]
- Hinshaw, V.S.; Webster, R.G.; Turner, B. The perpetuation of orthomyxoviruses and paramyxoviruses in Canadian waterfowl. Can J Microbiol 1980, 26, 622–629. [Google Scholar] [CrossRef] [PubMed]
- Webster, R.G.; Yakhno, M.; Hinshaw, V.S.; Bean, W.J.; Copal Murti, K. Intestinal influenza: Replication and characterization of influenza viruses in ducks. Virology 1978, 84, 268–278. [Google Scholar] [CrossRef] [PubMed]
- Caliendo, V.; Leijten, L.; Begeman, L.; Poen, M.J.; Fouchier, R.A.M.; Beerens, N.; Kuiken, T. Enterotropism of highly pathogenic avian influenza virus H5N8 from the 2016/2017 epidemic in some wild bird species. Vet Res 2020, 51, 117. [Google Scholar] [CrossRef]
- Caliendo, V.; Leijten, L.; van de Bildt, M.; Germeraad, E.; Fouchier, R.A.M.; Beerens, N.; Kuiken, T. Tropism of Highly Pathogenic Avian Influenza H5 Viruses from the 2020/2021 Epizootic in Wild Ducks and Geese. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Lean, F.Z.X.; Vitores, A.G.; Reid, S.M.; Banyard, A.C.; Brown, I.H.; Núñez, A.; Hansen, R.D.E. Gross pathology of high pathogenicity avian influenza virus H5N1 2021–2022 epizootic in naturally infected birds in the United Kingdom. One Health 2022, 14, 100392. [Google Scholar] [CrossRef]
- Lean, F.Z.X.; Núñez, A.; Banyard, A.C.; Reid, S.M.; Brown, I.H.; Hansen, R.D.E. Gross pathology associated with highly pathogenic avian influenza H5N8 and H5N1 in naturally infected birds in the UK (2020–2021). Vet Rec 2022, 190, e731. [Google Scholar] [CrossRef]
- European Food Safety Authority; Prevention, E. C.f.D.; Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C.; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, É.; et al. Avian influenza overview February – May 2021. EFSA J 2021, 19, e06951. [Google Scholar] [CrossRef]
- 148. Animal and Plant Health Agency. Annual report on surveillance for avian influenza in poultry and wild birds in Member States of the European Union in 2017, 2018.
- Brown, J.D.; Stallknecht, D.E.; Swayne, D.E. Experimental infection of swans and geese with highly pathogenic avian influenza virus (H5N1) of Asian lineage. Emerg Infect Dis 2008, 14, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Fereidouni, S.R.; Starick, E.; Beer, M.; Wilking, H.; Kalthoff, D.; Grund, C.; Häuslaigner, R.; Breithaupt, A.; Lange, E.; Harder, T.C. Highly Pathogenic Avian Influenza Virus Infection of Mallards with Homo- and Heterosubtypic Immunity Induced by Low Pathogenic Avian Influenza Viruses. PLOS ONE 2009, 4, e6706. [Google Scholar] [CrossRef]
- Costa, T.P.; Brown, J.D.; Howerth, E.W.; Stallknecht, D.E.; Swayne, D.E. Homo- and heterosubtypic low pathogenic avian influenza exposure on H5N1 highly pathogenic avian influenza virus infection in wood ducks (Aix sponsa). PLoS One 2011, 6, e15987. [Google Scholar] [CrossRef]
- Lv, X.; Li, X.; Sun, H.; Li, Y.; Peng, P.; Qin, S.; Wang, W.; Li, Y.; An, Q.; Fu, T.; et al. Highly Pathogenic Avian Influenza A(H5N8) Clade 2.3.4.4b Viruses in Satellite-Tracked Wild Ducks, Ningxia, China, 2020. Emerg Infect Dis 2022, 28, 1039. [Google Scholar] [CrossRef]
- Verhagen, J.H.; van der Jeugd, H.P.; Nolet, B.A.; Slaterus, R.; Kharitonov, S.P.; de Vries, P.P.; Vuong, O.; Majoor, F.; Kuiken, T.; Fouchier, R.A. Wild bird surveillance around outbreaks of highly pathogenic avian influenza A(H5N8) virus in the Netherlands, 2014, within the context of global flyways. Euro Surveill 2015, 20, 21069. [Google Scholar] [CrossRef] [PubMed]
- Poen, M.J.; Verhagen, J.H.; Manvell, R.J.; Brown, I.; Bestebroer, T.M.; van der Vliet, S.; Vuong, O.; Scheuer, R.D.; van der Jeugd, H.P.; Nolet, B.A.; et al. Lack of virological and serological evidence for continued circulation of highly pathogenic avian influenza H5N8 virus in wild birds in the Netherlands, 14 November 2014 to 31 January 2016. Euro Surveill 2016, 21. [Google Scholar] [CrossRef] [PubMed]
- Marchenko, V.Y.; Susloparov, I.M.; Kolosova, N.P.; Goncharova, N.I.; Shipovalov, A.V.; Durymanov, A.G.; Ilyicheva, T.N.; Budatsirenova, L.V.; Ivanova, V.K.; Ignatyev, G.A.; et al. Influenza A(H5N8) virus isolation in Russia, 2014. Arch Virol 2015, 160, 2857–2860. [Google Scholar] [CrossRef]
- van den Brand, J.M.A.; Verhagen, J.H.; Veldhuis Kroeze, E.J.B.; van de Bildt, M.W.G.; Bodewes, R.; Herfst, S.; Richard, M.; Lexmond, P.; Bestebroer, T.M.; Fouchier, R.A.M.; et al. Wild ducks excrete highly pathogenic avian influenza virus H5N8 (2014-2015) without clinical or pathological evidence of disease. Emerg Microbes Infect 2018, 7, 67. [Google Scholar] [CrossRef]
- Harder, T.; Maurer-Stroh, S.; Pohlmann, A.; Starick, E.; Höreth-Böntgen, D.; Albrecht, K.; Pannwitz, G.; Teifke, J.; Gunalan, V.; Lee, R.T.; et al. Influenza A(H5N8) Virus Similar to Strain in Korea Causing Highly Pathogenic Avian Influenza in Germany. Emerg Infect Dis 2015, 21, 860–863. [Google Scholar] [CrossRef]
- Germundsson, A.; Madslien, K.I.; Hjortaas, M.J.; Handeland, K.; Jonassen, C.M. Prevalence and subtypes of influenza A viruses in wild waterfowl in Norway 2006-2007. Acta Vet Scand 2010, 52, 28. [Google Scholar] [CrossRef]
- Hall, J.S.; Russell, R.E.; Franson, J.C.; Soos, C.; Dusek, R.J.; Allen, R.B.; Nashold, S.W.; TeSlaa, J.L.; Jónsson, J.E.; Ballard, J.R.; et al. Avian Influenza Ecology in North Atlantic Sea Ducks: Not All Ducks Are Created Equal. PLoS One 2015, 10, e0144524. [Google Scholar] [CrossRef]
- Alexander, D.J. Summary of avian influenza activity in Europe, Asia, Africa, and Australasia, 2002-2006. Avian Dis 2007, 51, 161–166. [Google Scholar] [CrossRef] [PubMed]
- Fouchier, R.A.; Olsen, B.; Bestebroer, T.M.; Herfst, S.; van der Kemp, L.; Rimmelzwaan, G.F.; Osterhaus, A.D. Influenza A virus surveillance in wild birds in Northern Europe in 1999 and 2000. Avian Dis 2003, 47, 857–860. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Walker, D.; Pryor, S.P.; Niles, L.; Chenghong, L.; Hinshaw, V.S.; Webster, R.G. Influenza A viruses of migrating wild aquatic birds in North America. Vector Borne Zoonotic Dis 2004, 4, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Krauss, S.; Obert, C.A.; Franks, J.; Walker, D.; Jones, K.; Seiler, P.; Niles, L.; Pryor, S.P.; Obenauer, J.C.; Naeve, C.W.; et al. Influenza in migratory birds and evidence of limited intercontinental virus exchange. PLoS Pathog 2007, 3, e167. [Google Scholar] [CrossRef] [PubMed]
- Wilson, H.M.; Hall, J.S.; Flint, P.L.; Franson, J.C.; Ely, C.R.; Schmutz, J.A.; Samuel, M.D. High seroprevalence of antibodies to avian influenza viruses among wild waterfowl in Alaska: implications for surveillance. PLoS One 2013, 8, e58308. [Google Scholar] [CrossRef] [PubMed]
- Pohlmann, A.; Starick, E.; Harder, T.; Grund, C.; Höper, D.; Globig, A.; Staubach, C.; Dietze, K.; Strebelow, G.; Ulrich, R.G.; et al. Outbreaks among Wild Birds and Domestic Poultry Caused by Reassorted Influenza A(H5N8) Clade 2.3.4.4 Viruses, Germany, 2016. Emerg Infect Dis 2017, 23, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Keawcharoen, J.; van Riel, D.; van Amerongen, G.; Bestebroer, T.; Beyer, W.E.; van Lavieren, R.; Osterhaus, A.D.; Fouchier, R.A.; Kuiken, T. Wild ducks as long-distance vectors of highly pathogenic avian influenza virus (H5N1). Emerg Infect Dis 2008, 14, 600–607. [Google Scholar] [CrossRef] [PubMed]
- Spackman, E.; Prosser, D.J.; Pantin-Jackwood, M.J.; Berlin, A.M.; Stephens, C.B. THE PATHOGENESIS OF CLADE 2.3.4.4 H5 HIGHLY PATHOGENIC AVIAN INFLUENZA VIRUSES IN RUDDY DUCK (OXYURA JAMAICENSIS) AND LESSER SCAUP (AYTHYA AFFINIS). J Wildl Dis 2017, 53, 832–842. [Google Scholar] [CrossRef] [PubMed]
- 168. CMS FAO CO-convened Scientific Task Force on Avian Influenza and Wild Birds. Scientific Task Force on Avian Influenza and Wild Birds statement. H5N1 Highly Pathogenic Avian Influenza in poultry and wild birds: Winter of 2021/2022 with focus on mass mortality of wild birds in UK and Israel.
- Division., F.a.A.O.o.t.U.N.A.H. 169. Division., F.a.A.O.o.t.U.N.A.H. Global Avian Influenza Viruses with Zoonotic Potential situation update.
- Waldenström, J.; van Toor, M.; Lewis, N.; Lopes, S.; Javakhishvili, Z.; Muzika, D.; Fouchier, R.A.M.; Brouwer, A. Active wild bird surveillance of avian influenza viruses, a report. EFSA Supporting Publications 2022, 19. [Google Scholar] [CrossRef]
- Floyd, T.; Banyard, A.C.; Lean, F.Z.X.; Byrne, A.M.P.; Fullick, E.; Whittard, E.; Mollett, B.C.; Bexton, S.; Swinson, V.; Macrelli, M.; et al. Encephalitis and Death in Wild Mammals at a Rehabilitation Center after Infection with Highly Pathogenic Avian Influenza A(H5N8) Virus, United Kingdom. Emerg Infect Dis 2021, 27, 2856–2863. [Google Scholar] [CrossRef]
- Globig, A.; Staubach, C.; Sauter-Louis, C.; Dietze, K.; Homeier-Bachmann, T.; Probst, C.; Gethmann, J.; Depner, K.R.; Grund, C.; Harder, T.C.; et al. Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4b in Germany in 2016/2017. Front Vet Sci 2017, 4, 240. [Google Scholar] [CrossRef] [PubMed]
- Peyrot, B.M.; Abolnik, C.; Anthony, T.; Roberts, L.C. Evolutionary dynamics of the clade 2.3.4.4B H5N8 high-pathogenicity avian influenza outbreaks in coastal seabirds and other species in southern Africa from 2017 to 2019. Transbound Emerg Dis 2022, 69, 3749–3760. [Google Scholar] [CrossRef] [PubMed]
- McPhail, G.M.; Collins, S.M.; Burt, T.V.; Careen, N.G.; Doiron, P.B.; Avery-Gomm, S.; Barychka, T.; English, M.D.; Giacinti, J.A.; Jones, M.E.B.; et al. Geographic, ecological, and temporal patterns of seabird mortality during the 2022 HPAI H5N1 outbreak on the island of Newfoundland. bioRxiv, 2001. [Google Scholar] [CrossRef]
- Rijks, J.M.; Leopold, M.F.; Kühn, S.; In 't Veld, R.; Schenk, F.; Brenninkmeijer, A.; Lilipaly, S.J.; Ballmann, M.Z.; Kelder, L.; de Jong, J.W.; et al. Mass Mortality Caused by Highly Pathogenic Influenza A(H5N1) Virus in Sandwich Terns, the Netherlands, 2022. Emerg Infect Dis 2022, 28, 2538–2542. [Google Scholar] [CrossRef] [PubMed]
- Abolnik, C.; Pieterse, R.; Peyrot, B.M.; Choma, P.; Phiri, T.P.; Ebersohn, K.; Heerden, C.J.V.; Vorster, A.A.; Zel, G.V.; Geertsma, P.J.; et al. The Incursion and Spread of Highly Pathogenic Avian Influenza H5N8 Clade 2.3.4.4 Within South Africa. Avian Dis 2019, 63, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Banyard, A.C.; Lean, F.Z.X.; Robinson, C.; Howie, F.; Tyler, G.; Nisbet, C.; Seekings, J.; Meyer, S.; Whittard, E.; Ashpitel, H.F.; et al. Detection of Highly Pathogenic Avian Influenza Virus H5N1 Clade 2.3.4.4b in Great Skuas: A Species of Conservation Concern in Great Britain. Viruses 2022, 14. [Google Scholar] [CrossRef] [PubMed]
- Camphuysen, C.; Gear, S.; Furness, B. Avian influenza leads to mass mortality of adult Great Skuas in Foula in summer 2022. 2022, 42, 312-323.
- European Food Safety Authority; European Centre for Disease Prevention and Control; European Union Reference Laboratory for Avian Influenza; Adlhoch, C. ; Fusaro, A.; Gonzales, J.L.; Kuiken, T.; Marangon, S.; Niqueux, E.; Staubach, C.; et al. Avian influenza overview December 2021 - March 2022. EFSA J 2022, 20, e07289. [Google Scholar] [CrossRef]
- Olsen, B.; Munster, V.J.; Wallensten, A.; Waldenström, J.; Osterhaus, A.D.; Fouchier, R.A. Global patterns of influenza a virus in wild birds. Science 2006, 312, 384–388. [Google Scholar] [CrossRef]
- Wille, M.; Lisovski, S.; Risely, A.; Ferenczi, M.; Roshier, D.; Wong, F.Y.K.; Breed, A.C.; Klaassen, M.; Hurt, A.C. Serologic Evidence of Exposure to Highly Pathogenic Avian Influenza H5 Viruses in Migratory Shorebirds, Australia. Emerg Infect Dis 2019, 25, 1903–1910. [Google Scholar] [CrossRef] [PubMed]
- Fusaro, A.; Zecchin, B.; Vrancken, B.; Abolnik, C.; Ademun, R.; Alassane, A.; Arafa, A.; Awuni, J.A.; Couacy-Hymann, E.; Coulibaly, M.B.; et al. Disentangling the role of Africa in the global spread of H5 highly pathogenic avian influenza. Nat Comm 2019, 10, 5310. [Google Scholar] [CrossRef]
- Molini, U.; Aikukutu, G.; Roux, J.P.; Kemper, J.; Ntahonshikira, C.; Marruchella, G.; Khaiseb, S.; Cattoli, G.; Dundon, W.G. Avian Influenza H5N8 Outbreak in African Penguins (Spheniscus demersus), Namibia, 2019. J Wildl Dis 2020, 56, 214–218. [Google Scholar] [CrossRef]
- Molini, U.; Yabe, J.; Meki, I.K.; Ouled Ahmed Ben Ali, H.; Settypalli, T.B.K.; Datta, S.; Coetzee, L.M.; Hamunyela, E.; Khaiseb, S.; Cattoli, G.; et al. Highly pathogenic avian influenza H5N1 virus outbreak among Cape cormorants (Phalacrocorax capensis) in Namibia, 2022. Emerg Microbes Infect 2023, 12, 2167610. [Google Scholar] [CrossRef] [PubMed]
- Beyit, A.D.; Meki, I.K.; Barry, Y.; Haki, M.L.; El Ghassem, A.; Hamma, S.M.; Abdelwahab, N.; Doumbia, B.; Ahmed Benane, H.; Daf, D.S.; et al. Avian influenza H5N1 in a great white pelican (Pelecanus onocrotalus), Mauritania 2022. Vet Res Commun 2023. [Google Scholar] [CrossRef] [PubMed]
- Bertran, K.; Dolz, R.; Majó, N. Pathobiology of avian influenza virus infection in minor gallinaceous species: a review. Avian Pathol 2014, 43, 9–25. [Google Scholar] [CrossRef] [PubMed]
- Grant, M.; Bröjer, C.; Zohari, S.; Nöremark, M.; Uhlhorn, H.; Jansson, D.S. Highly Pathogenic Avian Influenza (HPAI H5Nx, Clade 2.3.4.4.b) in Poultry and Wild Birds in Sweden: Synopsis of the 2020-2021 Season. Vet Sci 2022, 9. [Google Scholar] [CrossRef] [PubMed]
- Tammiranta, N.; Isomursu, M.; Fusaro, A.; Nylund, M.; Nokireki, T.; Giussani, E.; Zecchin, B.; Terregino, C.; Gadd, T. Highly pathogenic avian influenza A (H5N1) virus infections in wild carnivores connected to mass mortalities of pheasants in Finland. Infect Genet Evol 2023, 111, 105423. [Google Scholar] [CrossRef] [PubMed]
- Liang, Y.; Hjulsager, C.K.; Seekings, A.H.; Warren, C.J.; Lean, F.Z.X.; Núñez, A.; James, J.; Thomas, S.S.; Banyard, A.C.; Slomka, M.J.; et al. Pathogenesis and infection dynamics of high pathogenicity avian influenza virus (HPAIV) H5N6 (clade 2.3.4.4b) in pheasants and onward transmission to chickens. Virology 2022, 577, 138–148. [Google Scholar] [CrossRef] [PubMed]
- Brookes, S.M.; Mansfield, K.L.; Reid, S.M.; Coward, V.; Warren, C.; Seekings, J.; Brough, T.; Gray, D.; Núñez, A.; Brown, I.H. Incursion of H5N8 high pathogenicity avian influenza virus (HPAIV) into gamebirds in England. Epidemiol Infect 2022, 150, e51. [Google Scholar] [CrossRef] [PubMed]
- Stoimenov, G.M.; Goujgoulova, G.V.; Nikolov, B.; Hristov, K.; Teneva, A. Pathological Changes in Natural Infection of Pheasants with Highly Pathogenic Avian Influenza A (H5N8) in Bulgaria. J Vet Res 2019, 63, 497–502. [Google Scholar] [CrossRef] [PubMed]
- Abolnik, C. A current review of avian influenza in pigeons and doves (Columbidae). Vet Microbiol 2014, 170, 181–196. [Google Scholar] [CrossRef]
- Jeong, S.; Kwon, J.H.; Lee, S.H.; Kim, Y.J.; Jeong, J.H.; Park, J.E.; Jheong, W.H.; Lee, D.H.; Song, C.S. Subclinical Infection and Transmission of Clade 2.3.4.4 H5N6 Highly Pathogenic Avian Influenza Virus in Mandarin Duck (Aix galericulata) and Domestic Pigeon (Columbia livia domestica). Viruses 2021, 13. [Google Scholar] [CrossRef]
- Peters, M.; King, J.; Wohlsein, P.; Grund, C.; Harder, T. Genuine lethal infection of a wood pigeon (Columba palumbus) with high pathogenicity avian influenza H5N1, clade 2.3.4.4b, in Germany, 2022. Vet Microbiol 2022, 270, 109461. [Google Scholar] [CrossRef]
- Shriner, S.A.; Root, J.J. A Review of Avian Influenza A Virus Associations in Synanthropic Birds. Viruses 2020, 12. [Google Scholar] [CrossRef]
- Krone, O.; Globig, A.; Ulrich, R.; Harder, T.; Schinköthe, J.; Herrmann, C.; Gerst, S.; Conraths, F.J.; Beer, M. White-Tailed Sea Eagle (Haliaeetus albicilla) Die-Off Due to Infection with Highly Pathogenic Avian Influenza Virus, Subtype H5N8, in Germany. Viruses 2018, 10. [Google Scholar] [CrossRef]
- Günther, A.; Pohlmann, A.; Globig, A.; Ziegler, U.; Calvelage, S.; Keller, M.; Fischer, D.; Staubach, C.; Groschup, M.H.; Harder, T.; et al. Continuous surveillance of potentially zoonotic avian pathogens detects contemporaneous occurrence of highly pathogenic avian influenza viruses (HPAIV H5) and flaviviruses (USUV, WNV) in several wild and captive birds. Emerg Microbes Infect 2023, 12, 2231561. [Google Scholar] [CrossRef]
- Letsholo, S.L.; James, J.; Meyer, S.M.; Byrne, A.M.P.; Reid, S.M.; Settypalli, T.B.K.; Datta, S.; Oarabile, L.; Kemolatlhe, O.; Pebe, K.T.; et al. Emergence of High Pathogenicity Avian Influenza Virus H5N1 Clade 2.3.4.4b in Wild Birds and Poultry in Botswana. Viruses 2022, 14. [Google Scholar] [CrossRef]
- Caliendo, V.; Leijten, L.; van de Bildt, M.W.G.; Fouchier, R.A.M.; Rijks, J.M.; Kuiken, T. Pathology and virology of natural highly pathogenic avian influenza H5N8 infection in wild Common buzzards (Buteo buteo). Sci Rep 2022, 12, 920. [Google Scholar] [CrossRef]
- Swayne, D.E.; Suarez, D.L.; Sims, L.D. Influenza. In Diseases of Poultry; 2020; pp. 210-256.
- Penski, N.; Härtle, S.; Rubbenstroth, D.; Krohmann, C.; Ruggli, N.; Schusser, B.; Pfann, M.; Reuter, A.; Gohrbandt, S.; Hundt, J.; et al. Highly pathogenic avian influenza viruses do not inhibit interferon synthesis in infected chickens but can override the interferon-induced antiviral state. J Virol 2011, 85, 7730–7741. [Google Scholar] [CrossRef]
- Cao, Y.; Huang, Y.; Xu, K.; Liu, Y.; Li, X.; Xu, Y.; Zhong, W.; Hao, P. Differential responses of innate immunity triggered by different subtypes of influenza a viruses in human and avian hosts. BMC Med Genomics 2017, 10, 70. [Google Scholar] [CrossRef]
- Moulin, H.R.; Liniger, M.; Python, S.; Guzylack-Piriou, L.; Ocaña-Macchi, M.; Ruggli, N.; Summerfield, A. High interferon type I responses in the lung, plasma and spleen during highly pathogenic H5N1 infection of chicken. Vet Res 2011, 42, 6. [Google Scholar] [CrossRef] [PubMed]
- Park, M.-J.; Cha, R.M.; Kye, S.-J.; Lee, Y.-N.; Kim, N.-Y.; Baek, Y.-G.; Heo, G.-B.; Sagong, M.; Lee, K.-N.; Lee, Y.-J.; et al. Pathogenicity of H5N8 High Pathogenicity Avian Influenza Virus in Chickens and Ducks from South Korea in 2020–2021. Viruses 2021, 13, 1903. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-González, R.; Ramis, A.; Nofrarías, M.; Wali, N.; Valle, R.; Pérez, M.; Perlas, A.; Majó, N. Pathobiology of the highly pathogenic avian influenza viruses H7N1 and H5N8 in different chicken breeds and role of Mx 2032 G/A polymorphism in infection outcome. Vet Res 2020, 51, 113. [Google Scholar] [CrossRef]
- Yehia, N.; Erfan, A.M.; Adel, A.; El-Tayeb, A.; Hassan, W.M.M.; Samy, A.; Abd El-Hack, M.E.; El-Saadony, M.T.; El-Tarabily, K.A.; Ahmed, K.A. Pathogenicity of three genetically distinct and highly pathogenic Egyptian H5N8 avian influenza viruses in chickens. Poult Sci 2022, 101, 101662. [Google Scholar] [CrossRef]
- Pantin-Jackwood, M.J.; Spackman, E.; Leyson, C.; Youk, S.; Lee, S.A.; Moon, L.M.; Torchetti, M.K.; Killian, M.L.; Lenoch, J.B.; Kapczynski, D.R.; et al. Pathogenicity in Chickens and Turkeys of a 2021 United States H5N1 Highly Pathogenic Avian Influenza Clade 2.3.4.4b Wild Bird Virus Compared to Two Previous H5N8 Clade 2.3.4.4 Viruses. Viruses 2023, 15. [Google Scholar] [CrossRef]
- Prokopyeva, E.A.; Zinserling, V.A.; Bae, Y.C.; Kwon, Y.; Kurskaya, O.G.; Sobolev, I.A.; Kozhin, P.M.; Komissarov, A.; Fadeev, A.; Petrov, V.; et al. Pathology of A(H5N8) (Clade 2.3.4.4) Virus in Experimentally Infected Chickens and Mice. Interdiscip Perspect Infect Dis 2019, 2019, 4124865. [Google Scholar] [CrossRef]
- Matsuu, A.; Tanikawa, T.; Fujimoto, Y.; Yabuki, M.; Tsunekuni, R.; Sakuma, S.; Uchida, Y.; Saito, T. Different Sensitivity of Japanese Native-Bred Chickens to H5 Subtypes of Highly Pathogenic Avian Influenza Viruses. Avian Dis 2021, 65, 508–515. [Google Scholar] [CrossRef]
- Blohm, U.; Weigend, S.; Preisinger, R.; Beer, M.; Hoffmann, D. Immunological Competence of Different Domestic Chicken Breeds Against Avian Influenza Infection. Avian Dis 2016, 60, 262–268. [Google Scholar] [CrossRef]
- Spickler, A.R.; Trampel, D.W.; Roth, J.A. The onset of virus shedding and clinical signs in chickens infected with high-pathogenicity and low-pathogenicity avian influenza viruses. Avian Pathol 2008, 37, 555–577. [Google Scholar] [CrossRef]
- Mosad, S.M.; El-Gohary, F.A.; Ali, H.S.; El-Sharkawy, H.; Elmahallawy, E.K. Pathological and Molecular Characterization of H5 Avian Influenza Virus in Poultry Flocks from Egypt over a Ten-Year Period (2009-2019). Animals (Basel) 2020, 10. [Google Scholar] [CrossRef]
- Bertran, K.; Swayne, D.E.; Pantin-Jackwood, M.J.; Kapczynski, D.R.; Spackman, E.; Suarez, D.L. Lack of chicken adaptation of newly emergent Eurasian H5N8 and reassortant H5N2 high pathogenicity avian influenza viruses in the U.S. is consistent with restricted poultry outbreaks in the Pacific flyway during 2014-2015. Virology 2016, 494, 190–197. [Google Scholar] [CrossRef]
- DeJesus, E.; Costa-Hurtado, M.; Smith, D.; Lee, D.H.; Spackman, E.; Kapczynski, D.R.; Torchetti, M.K.; Killian, M.L.; Suarez, D.L.; Swayne, D.E.; et al. Changes in adaptation of H5N2 highly pathogenic avian influenza H5 clade 2.3.4.4 viruses in chickens and mallards. Virology 2016, 499, 52–64. [Google Scholar] [CrossRef] [PubMed]
- Sironi, L.; Williams, J.L.; Moreno-Martin, A.M.; Ramelli, P.; Stella, A.; Jianlin, H.; Weigend, S.; Lombardi, G.; Cordioli, P.; Mariani, P. Susceptibility of different chicken lines to H7N1 highly pathogenic avian influenza virus and the role of Mx gene polymorphism coding amino acid position 631. Virology 2008, 380, 152–156. [Google Scholar] [CrossRef]
- Matsui, M.; Moriya, O.; Belladonna, M.L.; Kamiya, S.; Lemonnier, F.A.; Yoshimoto, T.; Akatsuka, T. Adjuvant activities of novel cytokines, interleukin-23 (IL-23) and IL-27, for induction of hepatitis C virus-specific cytotoxic T lymphocytes in HLA-A*0201 transgenic mice. J Virol 2004, 78, 9093–9104. [Google Scholar] [CrossRef]
- Park, S.C.; Song, B.M.; Lee, Y.N.; Lee, E.K.; Heo, G.B.; Kye, S.J.; Lee, K.H.; Bae, Y.C.; Lee, Y.J.; Kim, B. Pathogenicity of clade 2.3.4.4 H5N6 highly pathogenic avian influenza virus in three chicken breeds from South Korea in 2016/2017. J Vet Sci 2019, 20, e27. [Google Scholar] [CrossRef]
- Suba, S.; Nagarajan, S.; Saxena, V.K.; Kumar, M.; Vanamayya, P.R.; Rajukumar, K.; Gowthaman, V.; Jain, V.; Singh, D.P.; Dubey, S.C. Pathology of a H5N1, highly pathogenic avian influenza virus, in two Indian native chicken breeds and a synthetic broiler line. Indian J Exp Biol 2015, 53, 202–207. [Google Scholar]
- Tumpey, T.M.; Kapczynski, D.R.; Swayne, D.E. Comparative susceptibility of chickens and turkeys to avian influenza A H7N2 virus infection and protective efficacy of a commercial avian influenza H7N2 virus vaccine. Avian Dis 2004, 48, 167–176. [Google Scholar] [CrossRef]
- Aldous, E.W.; Seekings, J.M.; McNally, A.; Nili, H.; Fuller, C.M.; Irvine, R.M.; Alexander, D.J.; Brown, I.H. Infection dynamics of highly pathogenic avian influenza and virulent avian paramyxovirus type 1 viruses in chickens, turkeys and ducks. Avian Pathol 2010, 39, 265–273. [Google Scholar] [CrossRef]
- Malmberg, J.L.; Miller, M.; Jennings-Gaines, J.; Allen, S.E. Mortality in Wild Turkey (Meleagris gallopavo) Associated with Natural Infection with H5N1 Highly Pathogenic Avian Influenza Virus (HPAIV) Subclade 2.3.4.4. J Wildl Dis 2023. [Google Scholar] [CrossRef]
- Anis, A.; AboElkhair, M.; Ibrahim, M. Characterization of highly pathogenic avian influenza H5N8 virus from Egyptian domestic waterfowl in 2017. Avian Pathol 2018, 47, 400–409. [Google Scholar] [CrossRef]
- Sun, H.; Pu, J.; Hu, J.; Liu, L.; Xu, G.; Gao, G.F.; Liu, X.; Liu, J. Characterization of clade 2.3.4.4 highly pathogenic H5 avian influenza viruses in ducks and chickens. Vet Microbiol 2016, 182, 116–122. [Google Scholar] [CrossRef] [PubMed]
- Bányai, K.; Bistyák, A.T.; Thuma, Á.; Gyuris, É.; Ursu, K.; Marton, S.; Farkas, S.L.; Hortobágyi, E.; Bacsadi, Á.; Dán, Á. Neuroinvasive influenza virus A(H5N8) in fattening ducks, Hungary, 2015. Infect Genet Evol 2016, 43, 418–423. [Google Scholar] [CrossRef] [PubMed]
- Foret-Lucas, C.; Figueroa, T.; Coggon, A.; Houffschmitt, A.; Dupré, G.; Fusade-Boyer, M.; Guérin, J.-L.; Delverdier, M.; Bessière, P.; Volmer, R. In Vitro and In Vivo Characterization of H5N8 High-Pathogenicity Avian Influenza Virus Neurotropism in Ducks and Chickens. Microbiol Spectr 2023, 11, e04229–04222. [Google Scholar] [CrossRef] [PubMed]
- Beerens, N.; Heutink, R.; Bergervoet, S.A.; Harders, F.; Bossers, A.; Koch, G. Multiple Reassorted Viruses as Cause of Highly Pathogenic Avian Influenza A(H5N8) Virus Epidemic, the Netherlands, 2016. Emerg Infect Dis 2017, 23, 1974–1981. [Google Scholar] [CrossRef] [PubMed]
- Scheibner, D.; Breithaupt, A.; Luttermann, C.; Blaurock, C.; Mettenleiter, T.C.; Abdelwhab, E.M. Genetic Determinants for Virulence and Transmission of the Panzootic Avian Influenza Virus H5N8 Clade 2.3.4.4 in Pekin Ducks. J Virol 2022, 96, e00149–00122. [Google Scholar] [CrossRef] [PubMed]
- Pantin-Jackwood, M.; Swayne, D.E.; Smith, D.; Shepherd, E. Effect of species, breed and route of virus inoculation on the pathogenicity of H5N1 highly pathogenic influenza (HPAI) viruses in domestic ducks. Vet Res 2013, 44, 62. [Google Scholar] [CrossRef] [PubMed]
- Cagle, C.; To, T.L.; Nguyen, T.; Wasilenko, J.; Adams, S.C.; Cardona, C.J.; Spackman, E.; Suarez, D.L.; Pantin-Jackwood, M.J. Pekin and Muscovy ducks respond differently to vaccination with a H5N1 highly pathogenic avian influenza (HPAI) commercial inactivated vaccine. Vaccine 2011, 29, 6549–6557. [Google Scholar] [CrossRef] [PubMed]
- Cagle, C.; Wasilenko, J.; Adams, S.C.; Cardona, C.J.; To, T.L.; Nguyen, T.; Spackman, E.; Suarez, D.L.; Smith, D.; Shepherd, E.; et al. Differences in pathogenicity, response to vaccination, and innate immune responses in different types of ducks infected with a virulent H5N1 highly pathogenic avian influenza virus from Vietnam. Avian Dis 2012, 56, 479–487. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Cong, Y.; Sun, Y.; Han, J.; Gai, L.; Yang, T.; Liu, C.; Zhao, L.; Cong, Y. Isolation and Identification of Novel Highly Pathogenic Avian Influenza Virus (H5N8) Subclade 2.3.4.4b from Geese in Northeastern China. Appl Environ Microbiol 2023, 89, e0157222. [Google Scholar] [CrossRef] [PubMed]
- Animal and Plant Health Inspection Service; U.S. Department of Agriculture. 2022-2023 Detections of Highly Pathogenic Avian Influenza in Mammals. Available online: https://www.aphis.usda.gov/aphis/ourfocus/animalhealth/animal-disease-information/avian/avian-influenza/hpai-2022/2022-hpai-mammals (accessed on 7th August 2023).
- Agüero, M.; Monne, I.; Sánchez, A.; Zecchin, B.; Fusaro, A.; Ruano, M.J.; del Valle Arrojo, M.; Fernández-Antonio, R.; Souto, A.M.; Tordable, P.; et al. Highly pathogenic avian influenza A(H5N1) virus infection in farmed minks, Spain, October 2022. Euro Surveill 2023, 28, 2300001. [Google Scholar] [CrossRef]
- Lindh, E.; Lounela, H.; Ikonen, N.; Kantala, T.; Savolainen-Kopra, C.; Kauppinen, A.; Österlund, P.; Kareinen, L.; Katz, A.; Nokireki, T.; et al. Highly pathogenic avian influenza A(H5N1) virus infection on multiple fur farms in the South and Central Ostrobothnia regions of Finland, July 2023. Euro Surveill 2023, 28, 2300400. [Google Scholar] [CrossRef]
- Hervé, S.; Schmitz, A.; Briand, F.X.; Gorin, S.; Quéguiner, S.; Niqueux, É.; Paboeuf, F.; Scoizec, A.; Le Bouquin-Leneveu, S.; Eterradossi, N.; et al. Serological Evidence of Backyard Pig Exposure to Highly Pathogenic Avian Influenza H5N8 Virus during 2016-2017 Epizootic in France. Pathogens 2021, 10. [Google Scholar] [CrossRef]
- Rosone, F.; Bonfante, F.; Sala, M.G.; Maniero, S.; Cersini, A.; Ricci, I.; Garofalo, L.; Caciolo, D.; Denisi, A.; Napolitan, A.; et al. Seroconversion of a Swine Herd in a Free-Range Rural Multi-Species Farm against HPAI H5N1 2.3.4.4b Clade Virus. Microorganisms 2023, 11, 1162. [Google Scholar] [CrossRef] [PubMed]
- Domańska-Blicharz, K.; Świętoń, E.; Świątalska, A.; Monne, I.; Fusaro, A.; Tarasiuk, K.; Wyrostek, K.; Styś-Fijoł, N.; Giza, A.; Pietruk, M.; et al. Outbreak of highly pathogenic avian influenza A(H5N1) clade 2.3.4.4b virus in cats, Poland, June to July 2023. Euro Surveill 2023, 28. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention. Ask the Expert: Highly Pathogenic Avian Influenza A(H5N1) Viruses. Available online: https://www.cdc.gov/flu/avianflu/spotlights/2022-2023/avian-flu-highly-pathogenic.htm (accessed on 7th August 2023).
- Ma, W.; Kahn, R.E.; Richt, J.A. The pig as a mixing vessel for influenza viruses: Human and veterinary implications. J Mol Genet Med 2008, 3, 158–166. [Google Scholar] [CrossRef]
- Graaf, A.; Piesche, R.; Sehl-Ewert, J.; Grund, C.; Pohlmann, A.; Beer, M.; Harder, T. Low Susceptibility of Pigs against Experimental Infection with HPAI Virus H5N1 Clade 2.3.4.4b. Emerg Infect Dis 2023, 29, 1492–1495. [Google Scholar] [CrossRef]
- American Veterinary Medical Association (AVMA). Highly pathogenic avian influenza detected in TX, KS dairy cattle. 2024.
- Centers for Disease Control and Prevention. Highly Pathogenic Avian Influenza A (H5N1) Virus Infection Reported in a Person in the U.S. 2024.
- American Veterinary Medical Association (AVMA). Goat in Minnesota tests positive for HPAI. 2024.
- Briand, F.X.; Souchaud, F.; Pierre, I.; Beven, V.; Hirchaud, E.; Hérault, F.; Planel, R.; Rigaudeau, A.; Bernard-Stoecklin, S.; Van der Werf, S.; et al. Highly Pathogenic Avian Influenza A(H5N1) Clade 2.3.4.4b Virus in Domestic Cat, France, 2022. Emerg Infect Dis 2023, 29, 1696–1698. [Google Scholar] [CrossRef]
- Shin, D.L.; Siebert, U.; Lakemeyer, J.; Grilo, M.; Pawliczka, I.; Wu, N.H.; Valentin-Weigand, P.; Haas, L.; Herrler, G. Highly Pathogenic Avian Influenza A(H5N8) Virus in Gray Seals, Baltic Sea. Emerg Infect Dis 2019, 25, 2295–2298. [Google Scholar] [CrossRef]
- Postel, A.; King, J.; Kaiser, F.K.; Kennedy, J.; Lombardo, M.S.; Reineking, W.; de le Roi, M.; Harder, T.; Pohlmann, A.; Gerlach, T.; et al. Infections with highly pathogenic avian influenza A virus (HPAIV) H5N8 in harbor seals at the German North Sea coast, 2021. Emerg Microbes Infect 2022, 11, 725–729. [Google Scholar] [CrossRef]
- Puryear, W.; Sawatzki, K.; Hill, N.; Foss, A.; Stone, J.J.; Doughty, L.; Walk, D.; Gilbert, K.; Murray, M.; Cox, E.; et al. Highly Pathogenic Avian Influenza A(H5N1) Virus Outbreak in New England Seals, United States. Emerg Infect Dis 2023, 29, 786–791. [Google Scholar] [CrossRef] [PubMed]
- Gamarra-Toledo, V.; Plaza, P.I.; Gutiérrez, R.; Luyo, P.; Hernani, L.; Angulo, F.; Lambertucci, S.A. Avian flu threatens Neotropical birds. Science 2023, 379, 246. [Google Scholar] [CrossRef]
- Murawski, A.; Fabrizio, T.; Ossiboff, R.; Kackos, C.; Jeevan, T.; Jones, J.; Kandeil, A.; Walker, D.; Turner, J.; Patton, C.; et al. Highly pathogenic avian influenza A(H5N1) virus in a common bottlenose dolphin (Tursiops truncatus) in Florida. 2023. [CrossRef]
- Thorsson, E.; Zohari, S.; Roos, A.; Banihashem, F.; Bröjer, C.; Neimanis, A. Highly Pathogenic Avian Influenza A(H5N1) Virus in a Harbor Porpoise, Sweden. Emerg Infect Dis 2023, 29, 852–855. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Wei, C.J.; Kong, W.P.; Wu, L.; Xu, L.; Smith, D.F.; Nabel, G.J. Immunization by avian H5 influenza hemagglutinin mutants with altered receptor binding specificity. Science 2007, 317, 825–828. [Google Scholar] [CrossRef] [PubMed]
- Bordes, L.; Vreman, S.; Heutink, R.; Roose, M.; Venema, S.; Pritz-Verschuren, S.B.E.; Rijks, J.M.; Gonzales, J.L.; Germeraad, E.A.; Engelsma, M.; et al. Highly Pathogenic Avian Influenza H5N1 Virus Infections in Wild Red Foxes (Vulpes vulpes) Show Neurotropism and Adaptive Virus Mutations. Microbiol Spectr 2023, 11, e0286722. [Google Scholar] [CrossRef] [PubMed]
- Reperant, L.A.; van Amerongen, G.; van de Bildt, M.W.; Rimmelzwaan, G.F.; Dobson, A.P.; Osterhaus, A.D.; Kuiken, T. Highly pathogenic avian influenza virus (H5N1) infection in red foxes fed infected bird carcasses. Emerg Infect Dis 2008, 14, 1835–1841. [Google Scholar] [CrossRef] [PubMed]
- Hiono, T.; Kobayashi, D.; Kobayashi, A.; Suzuki, T.; Satake, Y.; Harada, R.; Matsuno, K.; Sashika, M.; Ban, H.; Kobayashi, M.; et al. Virological, pathological, and glycovirological investigations of an Ezo red fox and a tanuki naturally infected with H5N1 high pathogenicity avian influenza viruses in Hokkaido, Japan. Virology 2023, 578, 35–44. [Google Scholar] [CrossRef]
- Verhagen, J.H.; Fouchier, R.A.M.; Lewis, N. Highly Pathogenic Avian Influenza Viruses at the Wild-Domestic Bird Interface in Europe: Future Directions for Research and Surveillance. Viruses 2021, 13. [Google Scholar] [CrossRef]
| Species (age) |
Age | Virus | Route of infection (dose) | Morbidity* (%) |
Mortality (%) |
S or A | DT | Transmission | Ref. |
|---|---|---|---|---|---|---|---|---|---|
| Mallard | 2 wk | A/Tufted-duck/Denmark/11470/LWPL/2016 (H5N8) | IC (103EID50/mL) |
0/5 (0) | 0/5 (0) | n.a. | n.a. | No | [94] |
| IC (105EID50/mL) |
5/5 (100) | 4/5 (80) | S | 5.8 | Yes | ||||
| IC (107EID50/mL) |
5/5 (100) | 4/5(80) | S | 3.2 | Yes | ||||
| 2 wk | A/American Wigeon/South Carolina/22-000345-001/2022 (H5N1) | IC (103 EID50/mL) |
5/5 (100) | 1/5 (20)† | S | n.a. | Yes | [121] | |
| IC (105 EID50/mL) |
5/5 (100) | 1/5 (20)† | S | n.a. | Yes | ||||
| IC (107 EID50/mL) |
5/5 (100) | 4/5 (100)† | S | n.a. | Yes | ||||
| 6 wk | A/herring gull/Poland/MB082B/2016 (H5N8) | IO and IN (107EID50/mL) | 12/12 (100) | 7/12 (58) | S | 3-14 | n.a. | [96] | |
| 8 wk | A/mute swan/Shimane/ 3211A002/2017 (H5N8) |
IN (107EID50/mL) |
8/8 (100) | 0/8 (0) | A | n.a. | n.a. | [122] | |
| >9 mo | A/EurasianWigeon/NL/4/2016 (H5N8) | IC (107TCID50/mL) |
7/7 (100) | 0/7 (0) | A | n.a. | n.a. | [123] | |
| Tufted duck | >9 mo | IC (107TCID50/mL) |
7/7 (100) | 1/7 (14) | S | 4 | n.a. | ||
| Eurasian wigeon | 7 wk | A/duck/Neth/16014829-001005/2016 (H5N8) | IO and IT (106EID50/mL) |
9/10 (90) | 2/10 (20) | A | 7-8 | n.a. | [124] |
| A/duck/Neth/17017236-001005/2017 (H5N6) | IO and IT (106EID50/mL) |
10/10 (100) | 9/10 (90) | S | 3-6 | n.a. | |||
| Common teal | (n.d.) | A/mute swan/Shimane/ 3211A002/2017 (H5N8) |
IN (107EID50/mL) |
8/8 (100) | 0/8 (0) | A | n.a. | n.a. | [125] |
| Herring gull | 8 wk | A/herring gull/Poland/MB082B/2016 (H5N8) | IO and IN (107EID50/mL) |
12/12 (100) | 11/12 (90) | S | 1-6 | Yes | [126] |
| Species | Age | Virus | Route of infection (dose) | Morbidity* (%) |
Mortality (%) |
S or A | MDT | Transmission | Ref |
|---|---|---|---|---|---|---|---|---|---|
| Pekin duck | 1 wk | A/tufted duck/ Germany/AR8444-L01987/2016 (H5N8) |
IM1 (106TCID50/bird) |
10/10 (100) | 10/10 (100) | S | 2 | Yes | [132] |
| 5-6 wk | A/wigeon/Wales/052833/2016 (H5N8) |
IN and IO (107EID50/mL) |
8/8 (100) | 0/8 (0) | A | n.a. | Yes | [136] | |
| IO and IN (105EID50/mL) |
5/5 (100) | 1/5 (20) | S | n.a. | Yes | ||||
| IO and IN (103EID50/mL) |
5/5 (100) | 2/5 (40) | S | n.a. | Yes | ||||
| 7 wk | A/duck/Neth/16014829-001005/2016 (H5N8) |
IN and IT (107EID50/mL) |
10/10 (100) | 0/10 (0) | A | n.a. | n.a. | [124] | |
| A/duck/Neth/17017236-001005/2017 (H5N6) |
IN and IT (107EID50/mL) |
10/10 (100) | 6/10 (60) | S | ≥3 | n.a. | |||
| 14 mo | A/tufted duck/Germany/AR8444L01987/2016 (H5N8) | IN (106EID50/mL) |
10/10 (100) | 10/10 (100) | S | 1-8 | Yes | [93] | |
| adult | A/tufted duck/ Germany/AR8444-L01987/2016 (H5N8) |
IO and IN (106TCID50/bird) |
10/10 (100) | 2/10 (20) | A | 4-5 | Yes | [132] | |
| Domestic duck | 2 wk | A/mandarin duck/ Korea/H242/2020(H5N8) |
IN (107EID50/mL) |
5/5 (100) | 0/5 (0) | A | n.a. | Yes | [204] |
| A/duck/Korea/HD1/2017 (H5N6) |
IN (107EID50/mL) |
5/5 (100) | 0/5 (0) | A | n.a. | Yes | |||
| Muscovy duck | 1 wk | A/tufted duck/ Germany/AR8444-L01987/2016 (H5N8) |
IM1 (106TCID50/bird) |
10/10 (100) | 10/10 (100) | S | ≥2 | n.a. | [132] |
| Chicken – Ross 308 | 2 wk | A/Goose/Spain/ IA17CR02699/2017 (H5N8) |
IN (105ELD50/0.05mL) |
15/15 (100) | 1/15 (8) | S | 6 | n.a. | [205] |
| Chicken – White Leghorn | IN (105ELD50/0.05mL) |
13/13 (100) | 3/13 (25) | S | 3.7 | n.a. | |||
| 4 wk | A/common-coot/Egypt/CA285/2016 (H5N8) |
IN (107EID50/mL) |
10/10 (100) | 10/10 (100) | S | 8.1 | Yes | [206] | |
| A/duck/Egypt/F446/2017 (H5N8) | IN (107EID50/mL) |
10/10 (100) | 10/10 (100) | S | 2.5 | Yes | |||
| A/duck/Egypt/SS19/2017 (H5N8) |
IN (107EID50/mL) |
10/10 (100) | 10/10 (100) | S | 4.2 | Yes | |||
| A/American Wigeon/South Carolina/22-000345-001/2021 (H5N1) | IC (103.6EID50/mL) |
3/5 (60) | 3/5 (60) | S | 2.7 | Yes | [207] | ||
| IC (105.6EID50/mL) |
5/5 (100) | 5/5 (100) | S | 2 | Yes | ||||
| IC (107.6EID50/mL) |
5/5 (100) | 5/5 (100) | S | 1 | Yes | ||||
| A/Tufted duck/Denmark/11740-LWPL/2016 (H5N8) | IC (101.5EID50/mL) |
0/5 (0) | 0/5 (0) | A | n.a. | No | |||
| IC (102.8EID50/mL) |
1/5 (20) | 1/5 (20) | S | 3 | No | ||||
| IC (106.7EID50/mL) |
5/5 (100) | 5/5 (100) | S | 2 | Yes | ||||
| 5 wk | A/mandarin duck/ Korea/H242/2020 (H5N8) |
IN (107EID50/mL) |
5/5 (100) | 5/5 (100) | S | 4.3 | Yes | [204] | |
| A/duck/Korea/HD1/2017 (H5N6) |
IN (107EID50/mL) |
5/5 (100) | 5/5 (100) | S | 2.2 | Yes | |||
| 6 wk | A/tufted duck/ Germany/AR8444-L01987/2016 (H5N8) |
IV2 (n.d.) |
10/10 (100) | 10/10 (100) | S | 2 | Yes | [132] | |
| A/domestic duck/ Siberia/49feather/2016 (H5N8) |
IV2 (107EID50/mL) |
10/10 (100) | 10/10 (100) | S | 3 | n.a. | [208] | ||
| A/tufted-duck/ Denmark/11740/ 2016 (H5N8) |
IC (107EID50/mL) |
13/13 (100) | 13/13 (100) | S | 2.1 | No | [116] | ||
| A/turkey/Hungary/53433/2016 (H5N8) |
IC (107EID50/mL) |
13/13 (100) | 13/13 (100) | S | 2.1 | No | |||
| Turkey – Commercial BBW | 3 wk | A/American Wigeon/South Carolina/22-000345-001/2021 (H5N1) | IC (103.6EID50/mL) |
4/5 (80) | 5/5 (100) | S | 4.6 | Yes | [207] |
| IC (105.6EID50/mL) |
5/5 (100) | 5/5 (100) | S | 3.4 | Yes | ||||
| IC (107.6EID50/mL) |
5/5 (100) | 5/5 (100) | S | 2.6 | Yes | ||||
| A/Tufted duck/Denmark/11740-LWPL/2016 (H5N8) | IC (101.5EID50/mL) |
0/5 (0) | 0/5 (0) | A | n.a. | No | |||
| IC (102.8EID50/mL) |
0/5 (0) | 0/5 (0) | A | n.a. | No | ||||
| IC (106.7EID50/mL) |
5/5 (100) | 5/5 (100) | S | 3 | Yes | ||||
| 5-6 wk | A/wigeon/Wales/052833/2016 (H5N8) |
Contact to infected ducks | 12/12 (100) | 12/12 (100) | S | 2.96 to 8.54 | n.a. | [136] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





