1. Introduction
Molluscs are extensively distributed around the world. Molluscs operate as secondary consumers, which means they devour primary consumers such as planktons before becoming food for higher consumers, hence preserving ecological equilibrium in the aquatic ecosystem. Bellamya bengalensis is a valuable bioresource for the freshwater environment (Ray et al. 2013). This edible mollusc is a filter feeder that lives on the diets of humans, fowl, and fish (Baby et al. 2010). Pyrethroids enter water bodies and are introduced to molluscs, which are not tolerable to them. They try to maintain their body activities using their toxic-resistant mechanisms, but an excess of pyrethroids increases stress by altering body metabolites, antioxidants, neurotransmitters, and so on. Pesticides are subsequently transferred to higher creatures by ingesting stressed molluscs, and this material may have a harmful influence on higher organisms. Molluscs serve as biofilters during eating. Pyrethroids are a type of synthetic insecticide linked to the natural pyrethrum, which is produced by chrysanthemum flowers (Chrysanthemum cinerariaefolium and Chrysanthemum coccineum). Pyrethrum, derived from the Chrysanthemum flower's granulated heads, has been used as an insecticide for centuries, possibly dating back to the first century AD (Hill et al., 1989). Permethrin, a light-stable compound for outdoor usage, was first marketed internationally in the late 1970s to combat insect pests on crops. In the decade afterward, the number of photo-stable insecticides has grown dramatically (Hill et al., 1989). Synthetic pyrethroid insecticides are often employed in agriculture, mosquito control, as well as the treatment of ectoparasitic diseases (Ansari et al., 2011). Every year, farmers use over one million metric tonnes of fertiliser and insecticides, compromising surface water as well as groundwater (Cruzeiro et al., 2016; Rodrigues et al., 2018). Pesticide concentrations in the environment are continually increasing. Before World War II, only 30 pesticides were recognised. Pesticide consumption peaked globally at over 1.8 billion metric tonnes per year between 1960 and 1980 (Renault 2011). The Ministry of Environmental Protection forecasts that Poland might have up to 60,000 metric tonnes of insecticide waste (Joanna et al., 2019). The most popular pesticide class is pyrethroids. Their excessive use raises the risk of water pollution. The farmers in Madurai, Tamil Nadu, India, frequently use this herbicide to improve their agricultural and aquaculture practices. China is the world's largest producer and user of pesticides, and synthetic pyrethroids have been identified by way of a widespread Environmental pollutant utilised in agriculture and animal production (Tang et al., 2018). These toxic substances are employed by way of insecticides popular soy-growing zones in Brazil (Hunt et al., 2016). These synthetic insecticides, specifically cypermethrin insecticides, are employed by way of an agricultural pesticide in paddy farming in the Philippines massively (Elfman et al., 2011). According to the report of Food and Agriculture Organisation of the United Nations (FAO), Asia and Europe have the most synthetic insecticide use. India, Pakistan, Ukraine, and Turkey each have greater consumption value than further nations (FAO, 2021). Although pyrethroids are utilised worldwide, thorough information on their use then detection is absent in various nations in addition areas, particularly popular nations with developing economies that are doubtful to have environmental restrictions and monitoring programmes. Quite a few synthetic pyrethroids currently have extensive aquatic toxicity data available in the scientific literature. Readers are encouraged to thoroughly research the literature in order to completely appreciate the effects of pyrethroids on aquatic molluscs. The goal of this study is to explore the issues associated with pyrethroid usage in molluscs. In this study, we will also look at the behavioural, physiological, and histological alterations that occur in molluscs following pyrethroid exposure. We shall attempt to determine the mechanism of action of pyrethroids and the function of antioxidants against these kinds of pyrethroids in molluscs.
2. Pyrethroid Profile
Since the 1980s, these insecticides have been widely used because of their high efficacy and limited toxicity in comparison to further compounds such for example organophosphorus and carbamic esters (Yoo et al., 2016). Pyrethroid pesticides are currently among the top three categories of pesticides (Xia et al., 2012). Pyrethroids are classified as agricultural (alpha-, medium-toxic) or urban (non-alpha-, low-toxic) based on their molecular structure. They are generally used for pest control in both agriculture and non-agriculture. Pyrethroids are often used in household goods, including shampoo and insect repellents (Gong et al., 2013). Pyrethroid insecticides are less environmentally harmful than typical pesticides, although they can still enter the food chain. They are very poisonous to aquatic creatures and may have long-term negative consequences for aquatic ecosystems (Zhao et al., 2014). Pyrethroids, due to their lipophilicity, are difficult to remove from an organism. Prolonged, low-dose exposure towards pyrethroids can encourage chronic disorders and harm the neurological, immunological, cardiovascular, and genetic systems, leading to teratogenicity, carcinogenicity, and mutagenicity (Ma 2009). Li et al. (2017) investigated the worldwide presence of pyrethroid pesticides in sediments and their harmful effects on aquatic invertebrates. Pyrethroids are utilised for monitoring public health to reduce pests, including roaches, mosquitoes, ticks, and flies, that can spread diseases (Weston et al., 2005). The current pyrethroid formulations are oil-based emulsifiable concentrations. The emulsifiable composition retains pyrethroids in liquid longer than technical chemicals. Piperonyl butoxide is widely used in pyrethroid preparations to increase the adverse impact of the active component. Piperonyl butoxide inhibits enzymes that detoxify pyrethroids (Werner I et al., 2008). Permethrin, cypermethrin, fenvalerate, and deltamethrin were previously thoroughly investigated, but there is less information on more contemporary products such as flucythrinate, bifenthrin, cyfluthrin, and lambda-cyhalothrin (
Table 1). Laboratory investigations with aquatic creatures in freshwater have revealed that certain invertebrates and fish are very vulnerable to this category of toxins (Hill et al., 1989). Pyrethroids are highly hazardous to aquatic creatures, including molluscs, amphibian tadpoles, and fish, as they are highly active insecticides with LC values over 1,000 micrograms per litre (Breistøl et al., 2015). Synthetic pyrethroids, used at the lowest rates, are most harmful to aquatic animals, with similar effects on species in field aquatic ecosystems after agricultural usage. Synthetic pyrethroids have high lipophilicity and poor water solubility, making them highly adsorbent to particulate matter. Pyrethroids are often a combination of stereoisomers, with varying toxicity levels (Liu et al., 2005). The synthetic pyrethroids are categorized into two distinct categories: type I and type II, resulting in distinct poisoning symptoms. Type II pyrethroids contain an alpha-cyano group, which can disrupt ion channels, including chloride and calcium channels, particularly sodium channels (Burr et al., 2004). They are more susceptible to biochemical and biological breakdowns due to modifications at many locations in the molecule. Such as a) the pentenone ring is usually replaced with a phenoxy-benzyl group; b) the isobutene group is replaced with a halogenated group composed of vinyl or a halogenated ring composed of phenyl; and c) a cyano group is replaced with the benzylic carbon. Both the first and second structural modifications boost the molecule's photostability while decreasing its vulnerability to oxidation. The third modification supports the ester bond's escape from hydrolysis. Photostable synthetic pyrethroids have water solubilities ranging from 1 to 10 pg/L and octanol/water partition coefficients of 10
4 to 10
7 (Coats et al., 1989). They are very harmful to several aquatic and marine species, especially fish (Coats et al., 1989). The activity of pyrethroids depends on their chemical configuration and steric conformation. Structural modifications can affect the activity of the pyrethroid by affecting detoxifying rates and the three-dimensional connection with receptors. High-activity compounds often have a (R) configuration at the cyclopentane ring's C-1 position. The toxicity of 16 cis isomers is greater than that of trans (Narahashi et al., 1984). Pyrethroids are typically composed of Cyclopropane carboxylic acid groups are linked to aromatic alcohols by a central ester (or ether) linkage. Changes in pyrethroid structure can enhance insecticidal efficacy and photostability, but may also affect non-target species (Soderlund et al., 2002). Pyrethroids are synergistically combined with organic chemicals like piperonyl butoxide, piperonyl sulfoxide, and sesamex to improve insecticide activity. These chemical compounds boost pesticide efficacy of pyrethroids. According to the Agency for Substances and Disease Registry (2003), commercial pyrethroids often include extremely hazardous inactive chemicals. Pyrethroid insecticides are more effective at targeting certain species compared to others. Organophosphates, organochlorines, and carbamates have an LD
50 ratio in rat and insect is less than 100, but pyrethroids have an LD50 ratio of more than 2000 (Katsuda et al., 1999). Pyrethroids can affect normal insect neuronal activity by altering the kinetics of voltage-sensitive sodium channels. This causes temporary influx in sodium ion permeability of the neuronal membrane, which is responsible for nerve activity (Bloomquist, 1993a). Casida discovered mechanisms for pyrethroid and metabolite degradation in the 1960s. The parent molecule undergoes biotransformation via esterase at its core ester link monooxygenases that are reliant on cytochrome P450 (Casida and Quistad, 1998). The effect of the initial hydrolytic or oxidative attack varies by pyrethroid component and isomer. Following ester cleavage, primary alcohol molecules, such as 3-phenoxybenzyl alcohol, are further oxidised to carboxylic acids via the aldehyde reaction. Cyano-substituted alcohols lose cyanide non-enzymatically to produce aldehyde (Soderlund et al., 2002). Certain ester fragmentation byproducts can become hydroxylated, and around of the hydroxylated ester intermediates may hydrolyze, resulting in hydroxylated ester fragmented byproducts. The early outcomes of hydrolytic assault undergo pairing with amino acids, sugars, or sulphates before excretion. (Soderlund et al., 1997). Pyrethroids have estrogenic and anti-progestogenic properties, as evidenced by epidemiological, clinical, and laboratory research. Thus, they are classed as endocrine-disrupting substances (Garey and Wolff, 1998). The primary structure of Class I pyrethroids is cyclopropane carboxyl esters. These consist of allethrin, bifenthrin, permethrin, resmethrin, and tetramethrin. Choreoathetosis and salivation are produced by class II pyrethroids, which include the cyano group. Pyrethroid effectiveness and selectivity for mollusc species depend on characteristics such as form and essential structural traits, particularly the chirality with cis/trans stereochemical composition throughout the cyclopropane circle, an ester, along with additional physical aspects (Khambay, 2002). Pyrethroids are toxic to aquatic animals, particularly molluscs and fish. Pyrethroids are excitotoxic to axons because they impede the voltage-gated sodium pathways in axonal membranes are closed, prohibiting neurons since repolarizing and depolarizing the membrane, thereby paralysing the organism. Pyrethroids can be improved by combining them by synergist piperonyl butoxide, an established inhibitor of microsomal P450 enzymes involved in pyrethroid metabolism (
Figure 2).
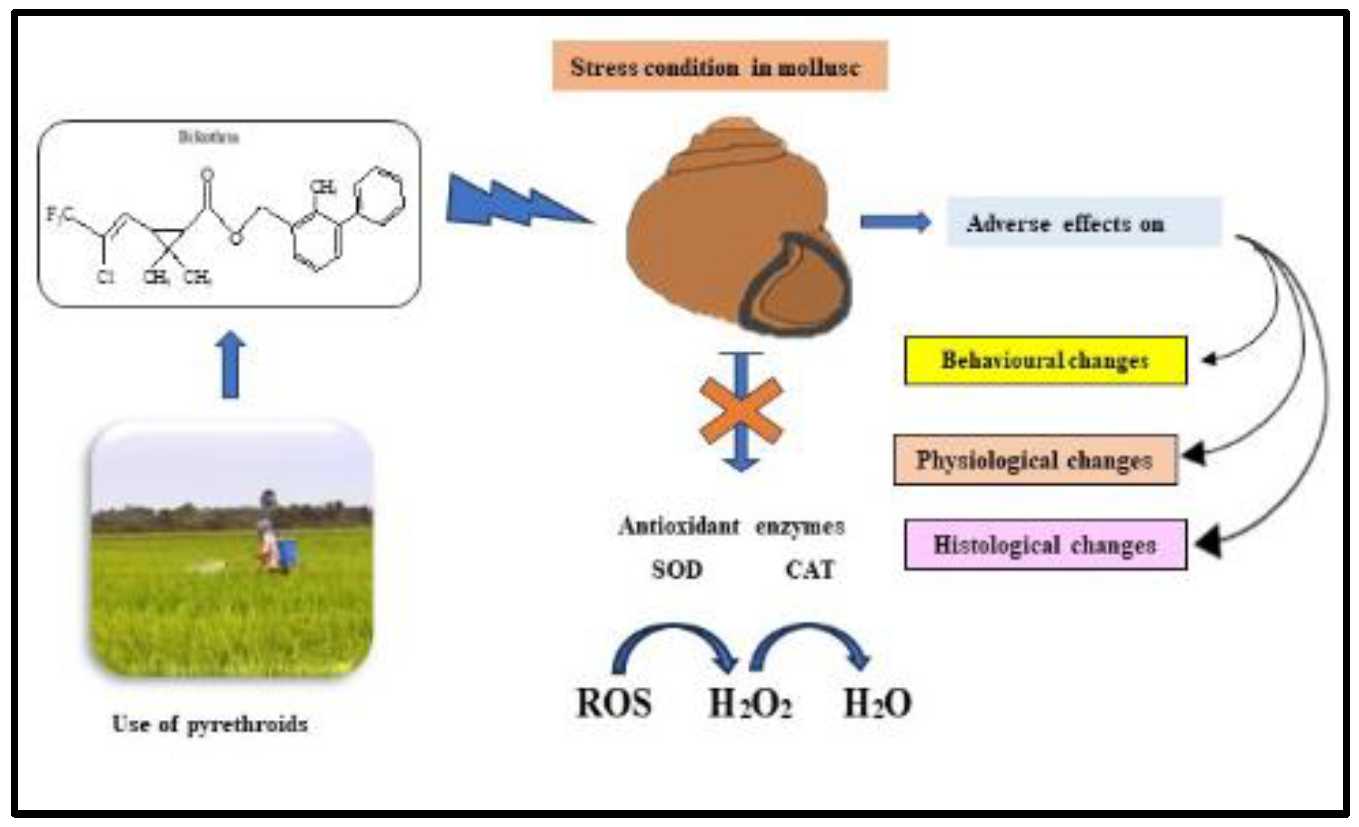
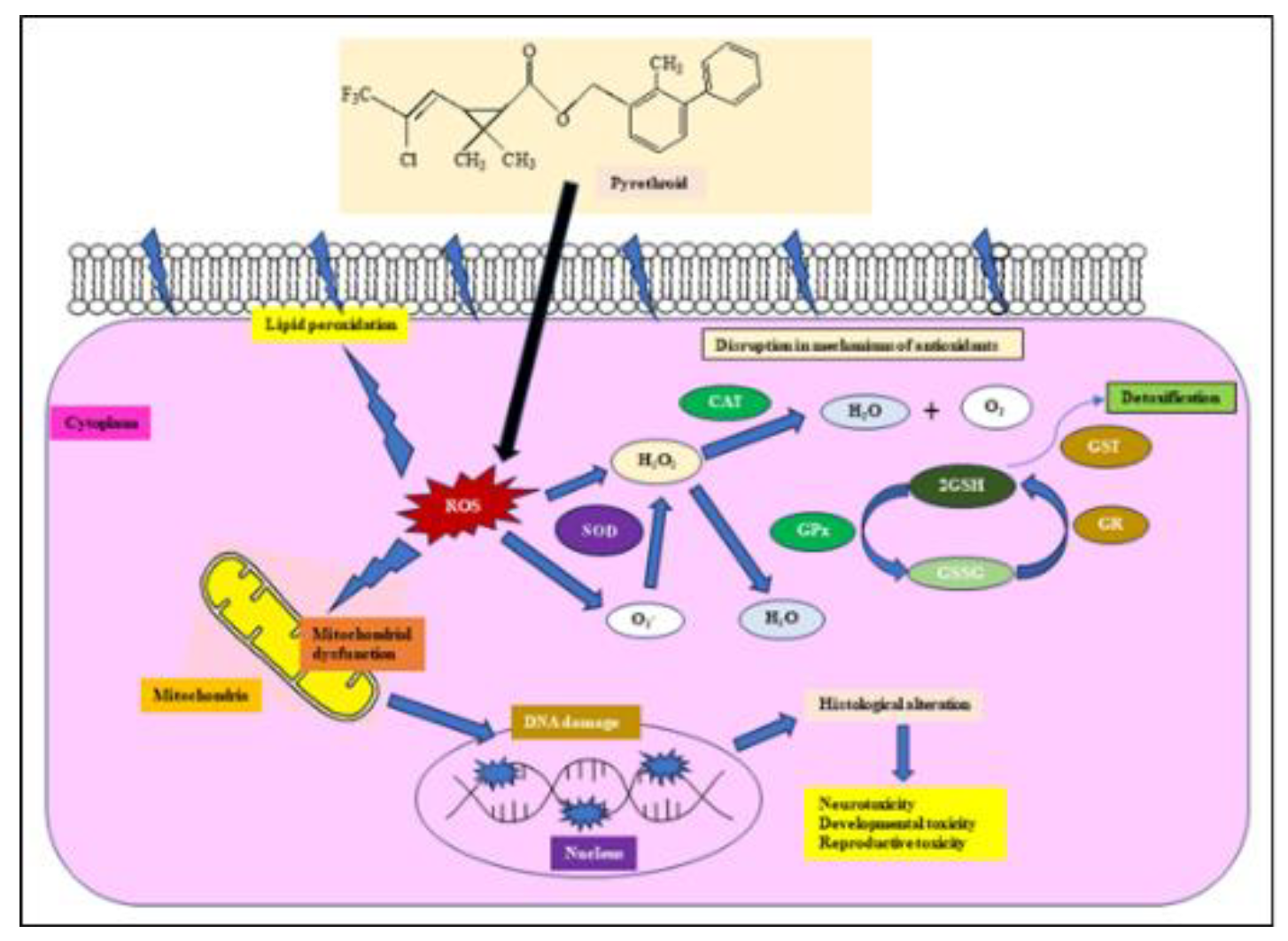
Figure 1.
The abstract is graphically represented.
Figure 1.
The abstract is graphically represented.
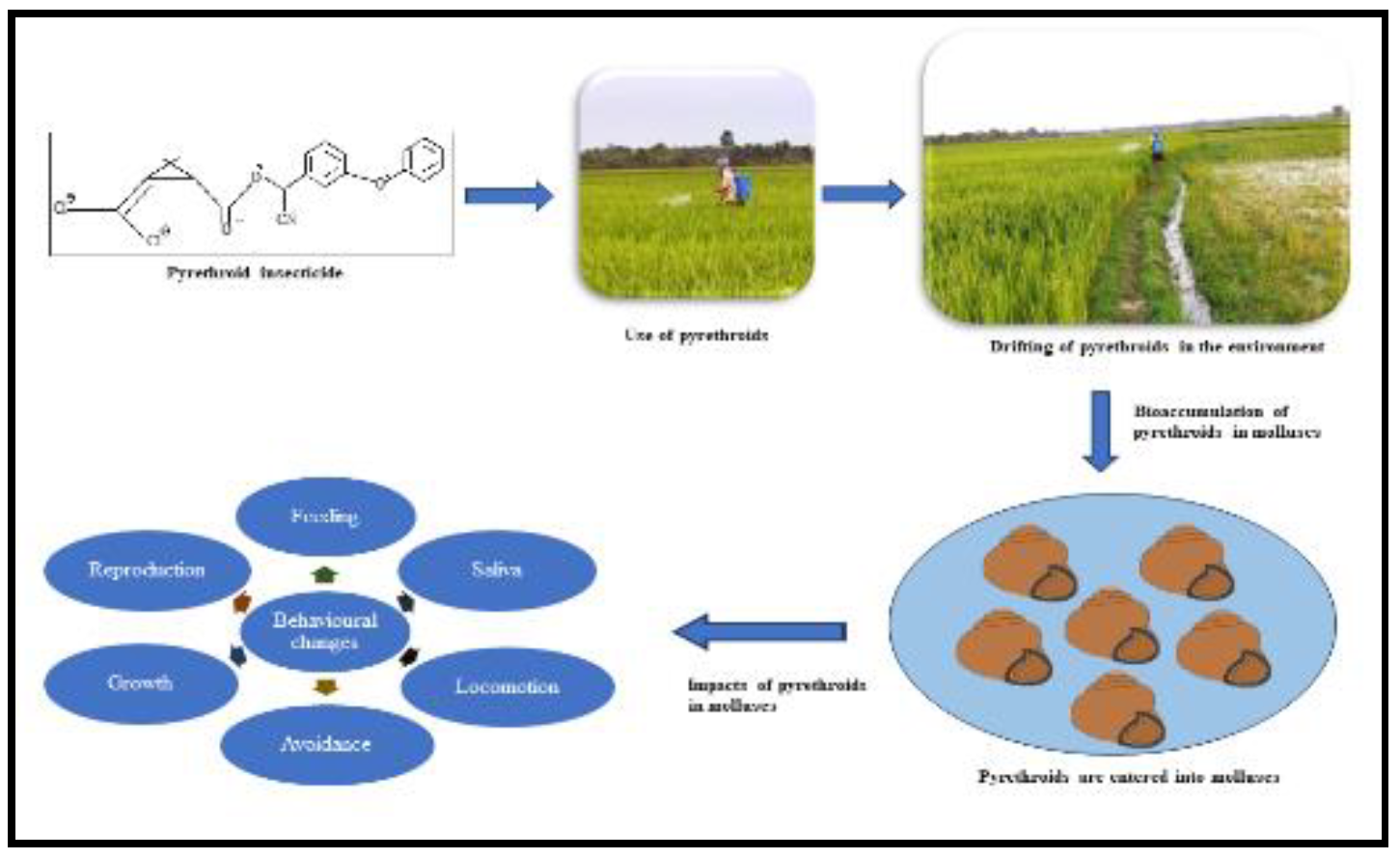
Figure 3.
Drifting of pyrethroids in aquatic environments shows negative impacts on nontargeted molluscs.
Figure 3.
Drifting of pyrethroids in aquatic environments shows negative impacts on nontargeted molluscs.
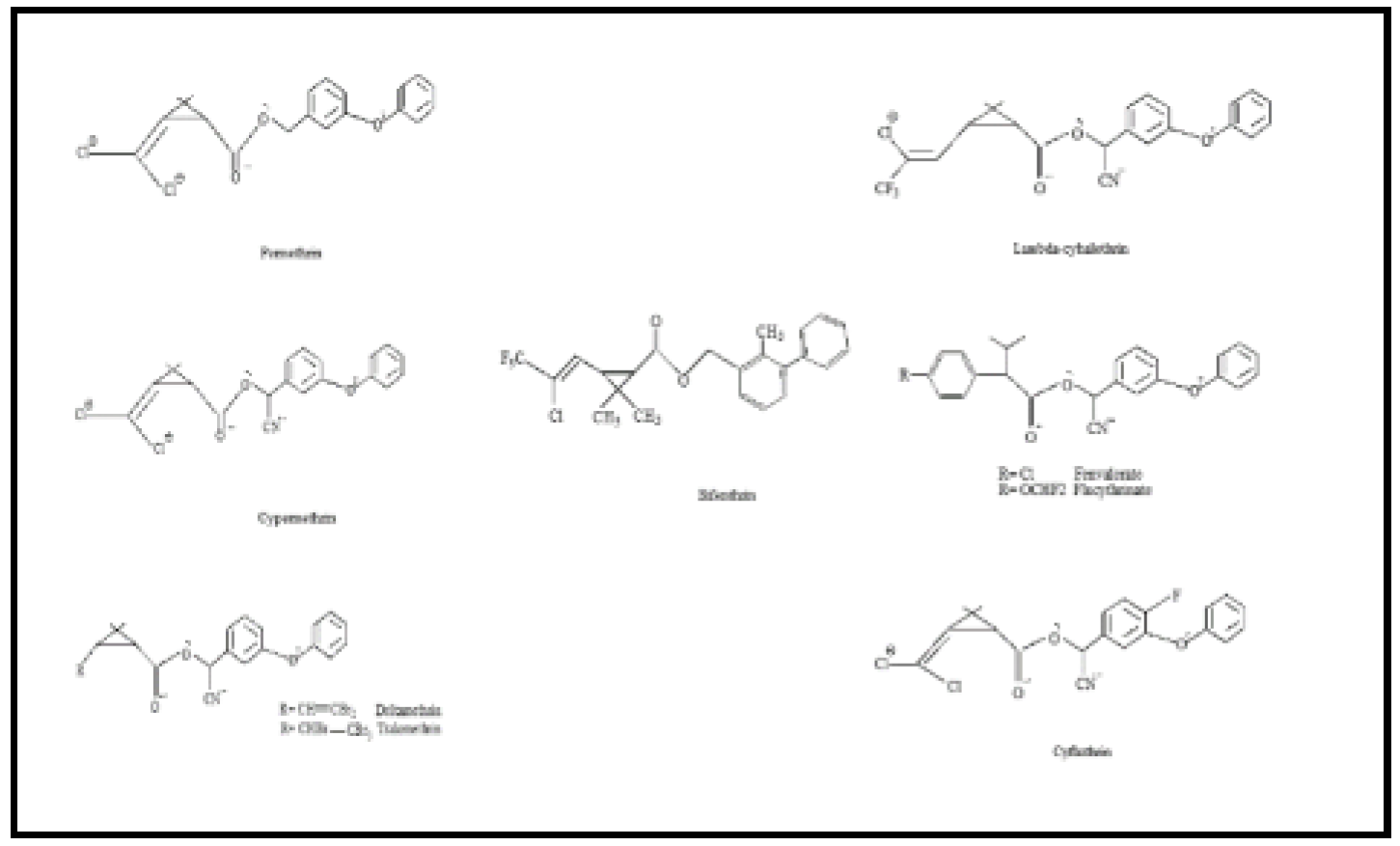
Permethrin
Permethrin is an ester that belongs to the cyclopropane carboxylate family. Cyclopropane-carboxylate ester is generated by esterifying alcohol groups in the form of phenoxybenzyl alcohol and replacing the cyclopropane ring using 2,2-dichloro vinyl as well as gem-dimethyl groups. Permethrin is functionally comparable to 3-(2,2-dichloro vinyl)-2 and 2-dimethyl cyclopropane carboxylic acids.
Cypermethrin
Cypermethrin is a carboxylic compound produced through the chemical combination of 3-(2,2-dichloro vinyl)-2,2-dimethyl cyclopropane carboxylic acid using the hydroxy (3-phenoxyphenyl) acetonitrile as an alcoholic hydroxy group. It is classed as a pyrethroid derivative insect repellant, agrochemical, along molluscicide. It contains a chlorinated hydrocarbon molecule, thus a propenonitrile, an aromatic ether, among others, as well as a cyclopropane carboxylate ester compound. It is functionally equivalent to 3-(2,2-dichloro vinyl)-2,2-dimethyl cyclopropane carboxylic acid.
Fenvalerate
Fenvalerate is a carboxylic derivative derived from the proper condensation of 2-(4-chlorophenyl)-3-methylbutyric acid in cyano(3-phenoxyphenyl) methanol. This insecticide and molluscicide contain pyrethroid esters. The compound consists of an aromatic carboxylic ester and a monochlorobenzene derivative. It is functionally identical to 2-(4-chlorophenyl)-3-methylbutyric acid. Pyrethroids inhibit the sodium channel's closure, causing the sodium tail impulse with a delayed sodium inflow at the conclusion of hypolarization. Evidently, the pyrethroid particle maintains the activation gate accessible. Pyrethroids having an alpha-cyano group (such as fenvalerate) produce longer sodium tail flows than other pyrethroids.
Deltamethrin
Deltamethrin is a cyclopropane-carboxylate ester formed via proper condensation of 3-(2,2-dibromo vinyl)-2,2-dimethyl cyclopropane carboxylic acid with cyano (3-phenoxy phenyl) methanol. It is the active ingredient in the insecticide tralomethrin. This compound acts as a pyrethroid ester insecticide, pesticide, phosphoprotein phosphatase inhibitor, calcium channel agonist, and antifeedant. It contains an aromatic ether, an organobromine molecule, a nitrile, and a cyclopropane-carboxylate ester. It acts similarly to cis-3-(2,2-dibromo vinyl)-2,2-dimethyl cyclopropane carboxylic acid.
Flucythrinate
Flucythrinate is one type of insecticide that has a wide spectrum. The IUPAC name of flucythrinate is [cyano-(3-phenoxyphenyl) methyl]2-[4-(difluoromethoxy) phenyl]-3-methylbutanoateis. It is a new synthetic pyrethroid with potent insecticidal effects. This herbicide has a non-systemic action and affects the skin and gastrointestinal regions. It is efficient at controlling Lepidoptera, Homoptera, and Coleoptera in a variety of crops, fruits, vegetables, ornamentals, and flowers.
Bifenthrin
Bifenthrin is a carboxylic ester formed by the formal synthesis of cis-3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl cyclopropane carboxylic acid with [(2-methyl-1,1'-biphenyl)-3-yl] methanol. It serves as both a pyrethroid derivative and an acaricide. It contains organochlorine, organofluorides, and cyclopropane-carboxylate esters. It is functionally similar to cis-chrysanthemic acid.
Cyfluthrin
Cyfluthrin is a carboxylic ester formed by the chemical condensation of 3-(2,2-dichloroethenyl)-2,2-dimethyl cyclopropane carboxylic acid with (4-fluoro-3-phenoxyphenyl)(hydroxy)acetonitrile. The composition contains organochlorine, organofluorine, nitrile, aromatic ether, and cyclopropanecarboxylate ester. It serves as both a pyrethroid-derived insecticide and an agrochemical. It functions similarly to a 3-(2,2-dichloro vinyl)-2,2-dimethyl cyclopropane carboxylic acid. Its primary agricultural use has been to control chewing and sucking insects in cotton, turf, ornamentals, hops, cereal, corn, deciduous fruit, peanuts, potatoes, and vegetables. Cyfluthrin is also used to preserve public health and control structural pests.
Lambda-Cyhalothrin
Lambda-cyhalothrin is a 1:1 combination of two stereoisomers (S). -α-cyano-3-phenoxybenzyl-(Z)-(1R, 3R). -3-(2-chloro-3,3,3-trifluoroprop-1-enyl)-2,2-dimethyl cyclopropanecarboxylate, (R) -α-cyano-3-phenoxybenzyl-(Z)-(1S, 3S). -3-(2-chloro-3, 3-trifluoroprop-1-enyl) -2,2-dimethylcyclopropanecarboxylate. It was first discovered by Robson and Crosby in 1984. It was introduced into the market by ICI Chemicals (now Syngenta) in Central America and the Far East in 1985. Warrior, Scimitar, Karate, Demand, Icon, and Matador all use lambda-cyhalothrin as an active component. According to CDPR 2006, agricultural usage of lambda-cyhalothrin was around 30,000 lbs per year from 2000 to 2003 but climbed to over 40,000 lbs per year between 2004 and 2006 in California. Karate, one type of lambda-cyhalothrin, serves as an insecticide that acts both on the skin and in the gastrointestinal system, providing repellent characteristics. It is a new pyrethroid insecticide that is now known as Syngenta. These neurotoxic agents damage nerves by targeting the central nervous system. It is mostly absorbed via the gastrointestinal system by the absorption of dust and finely sprayed mist, and slightly through unaffected skin.
Introduction of Pyrethroids into the Water Bodies
Pesticides may flow into streams and rivers via rainfall and soil, eventually contaminating groundwater or aquatic ecosystems. Pyrethroids' hydrophobicity renders them susceptible to adsorption in soils, suspended particles, and sediments, making them important in aquatic environments. Chemicals delivered into aquatic environments via spray drift become absorbed by sediments, algae, plants, and other biologically active substrates. Pyrethroids in agricultural field sediments adhere to soil surfaces, and those that reach aquatic ecosystems via runoff are strongly bound to suspended material in the water. Pesticides in water can disrupt ecosystems and lead to higher death rates among native species (Edwards et al., 2013; Nowell et al., 2014).
3. Impacts of Pyrethroids on Molluscs
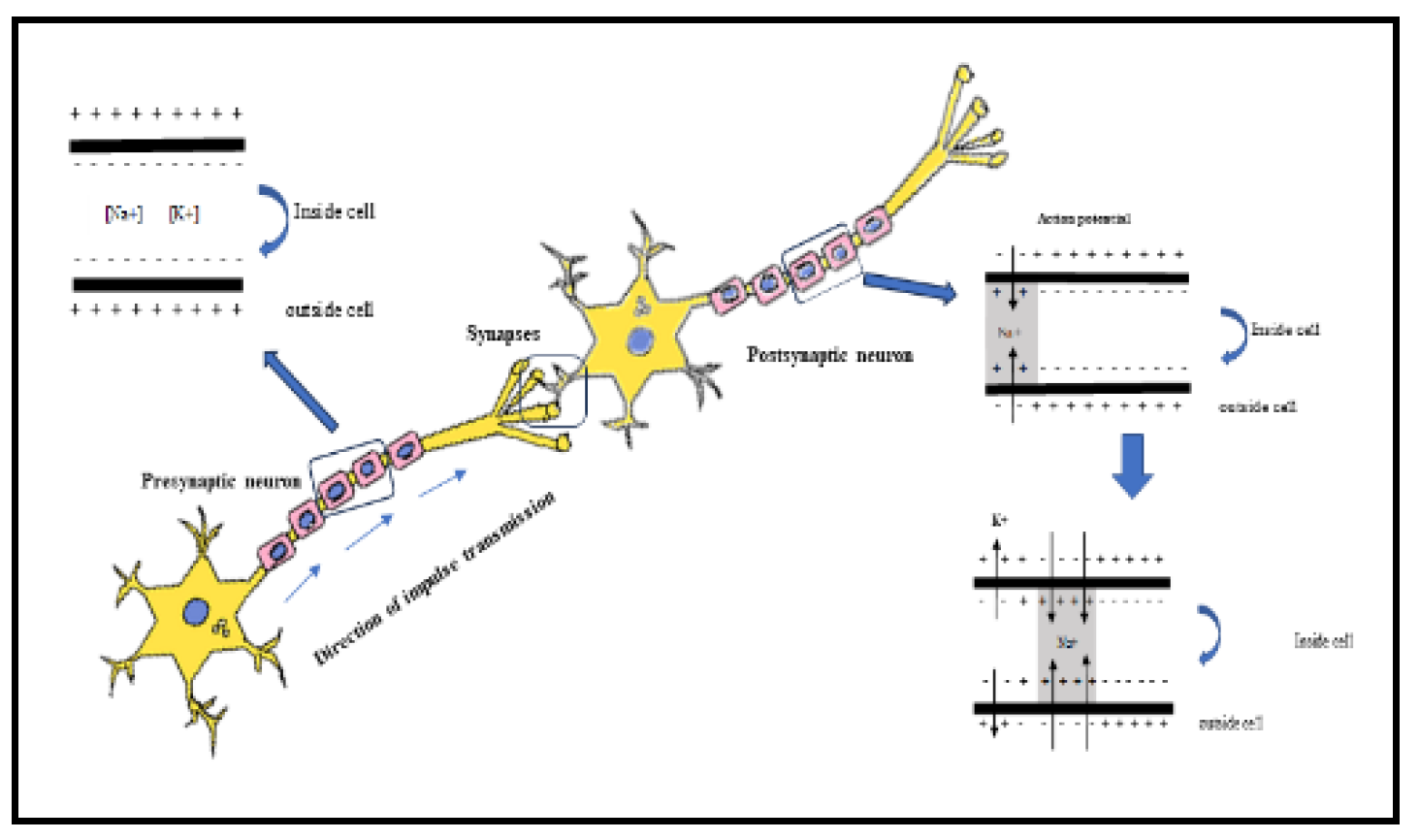
The reaction that occurs in the sodium channel is not the sole mode of action postulated for pyrethroids. Their impact on the central nervous system has prompted several researchers to propose activities such as antagonising gamma-aminobutyric acid (GABA)-mediated suppression, modulating nicotinic cholinergic transmission, increasing noradrenaline release, or acting on calcium ions. Because neurotransmitter-specific pharmacological treatments provide only little or no protection against poisoning, it is doubtful that one of these consequences is the predominant mode of action of pyrethroids, and most of the discharge of neurotransmitters is a result of increased salt entry. Pyrethroids are axonic poisons that target nerve fibres by binding to a protein that modulates the voltage-gated sodium channel. In general, this gate opens to stimulate the nerve and shuts to end the communication. Channels are passageways that allow ions to enter the axon and produce stimulation. When nerve cells possess accessible channels, they discharge repeatedly, leading to paralysis (Shafer and Meyer 2004). Pyrethroids bind to this gate and prevent it from closing. Which results in stimulation and convulsions of the nervous system in infected molluscs (
Figure 4). Contaminated molluscs lose control over their neurological system, resulting in uncoordinated movement. Pyrethroids are divided into Type I and Type II, depending on their poisoning symptoms. Type II pyrethroids differ from Type I by having an α-cyano group in their chemical structure. Unlike Type I pyrethroids, which primarily disrupt sodium channel activity in the central nervous system, Type II pyrethroids may interfere with chloride and calcium channels, both of which are essential for nerve function (Burr and Ray 2004). Pyrethroids are easily absorbed by cellular membranes and tissues due to their lipophilicity. Lambda-cyhalothrin enters the mollusc muscle, interrupting nerve transmission within minutes. This causes discontinuation of feeding, loss of muscle control, paralysis, and death.
Behavioural Changes
Environmental stress can have a significant impact on behaviour and survival (Byrne and O'Halloran, 2001). Behaviour offers a unique perspective that connects the relationship between an organism's physiology, ecology, and environment (Little and Brewer 2001). Ecotoxicology research indicates that observing behaviour can provide a sensitive way for monitoring sub-lethal toxicity. Behaviour helps organisms adapt to changing environments by responding to both internal and external stimuli. Behavioural changes are a holistic reaction. Alterations in reactions can lead to decreased wellness and survival, leading to negative repercussions for populations. Cypermethrin exposure causes mussels to reduce valve opening, display intermittent adductions, and respond to rapid and repetitive adductions, short and prolonged closures, and full and continuous closures for 400 and 800 lg/l Cypermethrin (Ait et al., 2011). Cypermethrin decreased the closure of valves in a time-dependent way. Exposure to Cypermethrin increased over time, leading to a faster onset of substantial effects. Cypermethrin concentrations increase valve closure times, comparable with how the pyrethroid deltamethrin decreases filtering function in
Anodonta cygnea (Kontreczky et al. 1997). According to a study on the effects of deltamethrin and malathion on
Helisoma duryi snails, these pesticides reduced the egg-laying capacity of the treated snails as compared to the control snails. The treated snails reduced their egg-laying capacity by up to 92.83% as compared to the control snails (Bakry et al., 2011). The study found that deltamethrin and malathion significantly kill
H. duryi snails, with LC50 values of 1.76 and 4.82 ppm, respectively (Bakry et al., 2011). Pyrethroid insecticides appear to be potentially harmful to non-target aquatic invertebrates as well as having a negative impact on their survival and reproductive success (Ray et al., 2013). Molluscs have strong calcareous shells or valves that provide major protection against infections and toxic substances. In the case of shell-less molluscs, the thick exterior cuticle covering known as the mantle or pollium is thought to serve a key physiological function in preventing toxin and disease entry. On the other hand, molluscs have soft body walls made up of cuticles, epidermis, and muscle, each of which is assumed to play an active role in innate immunological protection against environmental infections and poisons. The valves of mussels are kept closed to prevent unwanted agents from entering contaminated aquatic environments. In addition to providing outward protection, the mucus generated by the interior viscera adds another layer of protection against infections and toxic substances. The mucus functions as a protective barrier, prohibiting toxins from directly contacting epithelia. Mucus release is a key detoxification and evasion strategy in invertebrates. Toxic substances become caught within their mucus, resulting in their removal. The production of mucus is regarded as an exterior physiochemical barrier in mollusca. It is essential for aquatic organisms' immunological defence. Pollution of aquatic environments by these pyrethroids frequently causes breakdowns in the physicochemical barriers of the exterior body surface, allowing poisonous chemicals to enter the viscera of nontarget molluscs. Physiochemical barriers prevent the introduction of exogenous, harmful chemicals. Koprucu and Seker (2008) found that the synthetic pyrethroid deltamethrin was extremely harmful to
Unio elongatulus eucirrus at concentrations of 8.99, 8.09, 7.30, and 6.60 mg/L over 24, 48, 72, and 96 hours, correspondingly. Koprucu et al. (2010) found that the LC50 values of cypermethrin on Unio eucirrus were 96.50, 77.96, and 59.20 µg/l after 48 hours, 72 hours, and 96 hours, respectively. An investigation suggested that evaluated cypermethrin cytotoxicity within the snail,
Chilina parchappii, reported about 96-hour LC50 as 44.59 mg/L (San Juan et al., 2020). Once
Lymnaea acuminata was treated with sublethal doses of cypermethrin such as 4.0 mg/L, 8.0 mg/L, and 12.0 mg/L, individuals demonstrated improved oviposition, resulting in higher egg production (Tripathi and Singh, 2004). According to Joana et al. (2019), the toxic effects of these insecticides on mussels differ in terms of their effect on normal behaviour, such as shell opening, activity, and movement, in addition to the early beginning of mortality. This study presents scientific information regarding the effects of pesticides on indigenous freshwater bivalves in Poland. According to Renault (2011), pollution may harm whole trophic chains in aquatic habitats, making it a severe issue. This suggests that pyrethroids have become more harmful to aquatic invertebrates (
Figure 3).
Physiological Changes
According to a study on the effects of deltamethrin and malathion on Helisoma duryi snails by Bakry et al. (2011, the results revealed considerable decreases in the albumin level of the hemolymph. A decrease in albumin level may indicate injury to the hepatic parenchyma, which is where albumin originates (Rawi et al., 1995). Researchers observed that deltamethrin and malathion significantly increased glucose levels in snail hemolymph, which led to a considerable reduction in oxygen consumption during anaerobic respiration. Glycogen is the main source of serum glucose. Which is the most significant anaerobic energy source among anoxic-tolerant molluscs (Rawi et al., 1995). Pesticides can cause hypoxic or anoxic conditions, leading to enhanced glycogenolysis and elevated blood glucose levels. To replenish energy, the snail increases glycolysis, reducing glycogen content and increasing glucose levels in the hemolymph. They also reported that being exposed to deltamethrin and malathion in snail tissues decreased glycolytic enzyme activity, including lactate dehydrogenase, succinate dehydrogenase, ornithine aminotransferase, and arginase. According to Aboul-Zahab and El-Ansary (1992), succinate dehydrogenase had the lowest activity, perhaps because of inflated and damaged mitochondrial membranes and decreased Cristal levels, resulting in enzyme leakage. Snails exposed to pesticides have considerably decreased arginase and ornithine aminotransferase enzyme activity. Decreased enzyme activity may impact cells' capacity to eliminate endogenous toxic substances like ammonia, which is transformed into urea (Bakry et al., 2011). Scientists observed the dangerous consequences of pyrethroids, cypermethrin, and fenvalerate in freshwater molluscan invertebrates and classified them as immunotoxins due to their negative impact on host animal immunological parameters (Ray et al., 2013). ROS induces lipid peroxidation (LPO) and elevates calcium levels (Ca++), which leads to both genotoxicity and cell death in affected species (Ullah et al., 2018). Cypermethrin administration boosted antioxidant enzyme activity in the gills and digestive glands of U. gibbus, a freshwater mussel (Khazri et al., 2015). According to Idris et al. (2012, synthetic pyrethroid insectisides are harmful because they block the enzyme acetylcholinesterase, resulting in acetylcholine buildup in neuronal synaptic gaps. It also engages with sodium channels and depolarizes neurons.
Histological Changes
Antioxidant defences fail to detoxify reactive oxygen species (ROS), leading to damage to biomolecules. Estimating the oxidative state of aquatic species involves monitoring protein and lipid oxidation levels and also assessing the activity of antioxidant enzymes (Regoli and Giuliani, 2014). According to Mahmoud et al. (2012), permethrin increased CAT activity in the digestive gland of the sea gastropod Hexaplex trunculus at varying doses but lowered it at higher concentrations. The authors suggest that this trend may be connected to the organism's energy level after pyrethroid exposure. The pyrethroid elicited comparable reactions in P. canaliculata, but there was no concentration- or time-dependent linear responses in lipid peroxidation. Cypermethrin had a short- and long-term influence on lipid oxidation in P. canaliculata gills, resulting in significantly elevated TBAR levels. However, in clams R. decussatus and V. decussate, the digestive gland was shown to be more vulnerable to phyretroid toxicity than the gills (Sellami et al., 2013; Sellami et al., 2015). The digestive gland, which detoxifies pesticides and produces large levels of ROS, may be responsible for these tissue-specific reactions (Sellami et al., 2015). The gill acts as a location for gas exchange and ion control, including catabolic waste elimination. subsequently also serves as a primary point of entry for dissolved toxicants from the surrounding water (Wei and Yang, 2015). This organ is sensitive to ROS damage, as seen in P. canaliculata. Cypermethrin and other xenobiotics can cause lipid peroxidation, which affects membrane fluidity and biomolecule integrity (Wei and Yang, 2015). Lipid peroxidation products, such as aldehydes and ketones, may accelerate the oxidation of proteins. Amino acid oxidation through glycation and glycoxidation processes can also produce these moieties (Dalle-Donne et al., 2003). According to Ahirrao and Phand (2015), cytomethrin exposure in Bellamya bengalensis causes a significant decrease in the concentration of ascorbic acid in reproductive phases. The molluscan defence mechanism relies on circulating hemocytes in the bloodstream. Hemocytes migrate throughout tissues in reaction to external substances, including infections. The major defensive mechanism involves phagocytosis, encapsulation, and the release of numerous cytotoxic chemicals (Carballal et al., 1997). Multicellular organisms often feature mobile phagocytic cells capable of recognising and eliminating externally harmful compounds. Phagocytosis is a recognised immune response and a diagnostic of water pollution (Oliver and Fisher, 1999). Fenvalerate administration dramatically reduced phagocytic indices in B. bengalensis, with a dose-dependent response. In molluscs, phenoloxidase is regarded as a vital defence enzyme. It plays a crucial role in the intracellular antibacterial defence system of oysters (Munoz et al., 2006). To produce active phenoloxidase, a prophenoloxidase activating system is activated by PAMPs such as lipopolysaccharide, peptidoglycan, laminarin, and β-1,3-glucan (Cerenius and Soderhall, 2004). Exposing B. bengalensis to fenvalerate affected its phenoloxidase activity. Fenvalerate therapy dramatically lowered hemocyte phagocytic indices in a dose-dependent manner. Male B. bengalensis showed a 56.6 ± 3.73 reduction in their phagocytic response as compared to the control sample when exposed to 3 ppm fenvalerate after 15 days of in vivo exposure (Mukherjee et al., 2023). The lysosome is an essential subcellular organelle that is involved in the destruction of foreign particles that have been absorbed. Following phagocytosis, the phagocytic vesicle containing ingested foreign particles fused with the lysosome to produce the phagolysosome. Since the lysosome plays a vital role in the production of numerous digesting enzymes, sustaining lysosome integrity in the membrane has received considerable scientific interest from an immunological perspective. Ray et al. (2013) investigated the effects of cypermethrin and fenvalerate on the lysosomal membrane integrity of hemocytes from Bellamya bengalensis and Lamellidens marginalis. The authors found pyrethroid-induced lysosomal membrane vulnerability in hemocytes, indicating significant damage and destabilisation of the lysosomal membrane.
Mechanism of Action of Pyrethroids and the Role of Antioxidants
Cypermethrin catabolism produces cyanohydrins, which are degraded into cyanide and aldehyde. These chemicals, along with other lipophilic conjugates, can cause ROS formation, resulting in DNA damage, lipid peroxidation, and protein carbonylation, altering normal cellular processes (Shashikumar and Rajini, 2010). Organisms have both enzymatic and non-enzymatic antioxidant defence mechanisms to neutralise ROS, including H2O2, superoxide (O
2-), and hydroxyl radical (OH
-), produced during aerobic metabolism. Enzymatic defences such as superoxide dismutase (SOD) and catalase (CAT) breakdown O
2- and H
2O
2 to create less harmful metabolites (Livingstone, 2001). Organisms can respond to increased ROS generation from xenobiotic exposure by increasing antioxidant enzyme activity in their cells. Enzyme activity is increased by transcriptional and post-translational control, promoting a defensive strategy (Regoli and Giuliani, 2014). SOD and CAT activity are widely used as indicators of pollution contamination in aquatic systems (Monserrat et al., 2007). Glutathione peroxidases and peroxiredoxins are part of the antioxidant mechanism and serve the same function as CAT. Scientists have increasingly focused on antioxidant enzymes and studied their expression in response to environmental stress (Tolomeo et al., 2016). Cypermethrin exposure increased SOD and CAT activity in the digestive gland of the freshwater mussel
Unio gibbus (Khazri et al., 2015). According to Regoli and Giuliani (2014), increased ROS levels may inhibit the alternating tendency of CAT activity. Superoxide radicals are thought to be the primary cause of CAT inhibition, resulting in high levels of H
2O
2 and reduced SOD activity (Wei and Yang, 2015). According to scientists, ROS can interfere with protein synthesis while impacting the functioning of enzymes. While SOD and CAT represent the first defence walls versus ROS, many researchers indicate that their responses differ significantly. Glutathion-S-Tranferase (GST), the biotransformation enzyme responsible for phase II metabolism in aquatic species, is extensively investigated. Its primary function is to detoxify electrophiles. This contains pesticide metabolites that combine with reduced glutathione to promote their excretion (Livingstone, 2001). Xenobiotics can trigger GST activity through transcription or stress-activated signalling pathways (Richardson et al., 2009). Some data indicate that GST has a role in cytomethrin conversion in aquatic organisms (Xu and Huang, 2017). The digestive gland is the primary location of phase I and II detoxification in molluscs. When enzymes in phase I fail to protect cells, GST increases its activity (Sellami et al., 2015). Pyrethroids can be the reason for serious damage to cells, which is caused by free oxidative radicals. These particles easily move into the membranes, which are made up of lipids. That induces permeability rates in lipid membranes in aquatic organisms. Even these excessive oxidative radicals can form reactive oxygen species (ROS) in aquatic organisms. These ROS can act as molecular cutters, which means they can damage DNA and proteins. Aquatic organisms, such as molluscs, have the ability to reduce the action of ROS. But when these particles produce more ROS in the body, the antioxidants fail or cannot control the normal cellular functions in aquatic organisms. These insecticides have several structural modifications and contain different moieties such as acid, alcohol, and esters. Several isomers are present in these insecticides that are stereospecific, which means they have different binding sites that are specific (Clark et al., 2012). In aquatic animals, exposure to pyrethroids can cause an increase in the overall quantity of cytochrome P-450 and related proteins, especially in the liver, within a few hours or days (Wilkinson et al., 1983). In order for freshwater species to detoxify pesticide compounds, oxidative degradation mechanisms must include cytochrome P-450 proteins (Alonso et al., 2023). They are the ones who bind and oxidise pesticide molecules directly. The inducer phenomenon is not limited to cytochrome P-450s; it also affects a wide range of other systems, including those linked to mixed-function oxidases, glutathione-S-transferases, and glucuronyl transferases (Loboda et al., 2016). Furthermore, the increased concentration of antioxidant enzymes in hepatopancreatic tissue, such as glutathione (GSH), superoxide dismutase (SOD), and catalase, which break down H
2O
2, is likely an early adaptive response to elevated oxidative stress in freshwater creatures intoxicated with pyrethroids (Sanchez-Hernandez et al., 2023). Redox (reduction/oxidation) cycle features, mitochondrial respiration, as well as reactive oxygen species (ROS), considered to be byproducts of detoxifying metabolism, are among the established mechanisms by which pesticides cause oxidative stress. In the redox cycle, the parent chemical is frequently enzymatically reduced first to form the pesticide radical by a reducer that depends on NADPH; it comprises cytochrome P450 reductase NADPH. The radical (O
2-) and parent chemical are created by giving up the unshared electron of the O
2 radical. The latter is susceptible to further cycles. Consequently, at each cycle turn, two potentially dangerous events have occurred: the creation of an oxyradical and the oxidation of a reductant (
Figure 5).
Conclusion
With rising population and food demand, pesticides and insecticides are increasingly used to protect plants and enhance productivity (FAO 2002). The widespread utilisation of these pyrethroids is projected to continue globally. According to research findings, pyrethroids pose a significant environmental threat to aquatic molluscs worldwide (Zhao et al., 2014). Researchers are mostly focused on larger water bodies, such as rivers, to evaluate the impacts of pyrethroids on aquatic life, whereas smaller ponds and streams are being overlooked. Pest management strategies should focus on preventing pyrethroid contamination of discharge and drift in waterbodies. The negative impacts of pyrethroids on nontargeted molluscs become a significant issue to be concerned about. Snail physiological changes cause reduced egg production and increased death rates in pyrethroid-affected snails (Bakry et al., 2011). Several behavioural alterations may indicate stress due to the hazardous environment (Ray et al., 2013). Behavioural alterations of molluscs reveal a distinct reaction to water contamination by various pyrethroids. More research is needed to use bioindicative signals from bivalves under contamination stress to better understand the interaction of pyrethroids in freshwater environments. To further assess the environmental impact of pyrethroids, it may be beneficial to analyse other biomarkers beyond oxidative stress. Previous findings provided by researchers clearly reveal that long-term exposure to pyrethroids can interfere with physiological functioning in molluscs (Rawi et al., 1995). More molecular research and understanding of molluscs are needed to efficiently implement the effects of pyrethroid insecticides. The impacts of various stressors on organisms, including prolonged toxic effects and interspecies interactions, are not widely recognised. More study is needed to determine the influence of pyrethroids on aquatic organisms. To evaluate the risks and biological threats, bioavailability-based stress evaluation and safety assessment are necessary measures. Establishing toxicity standards can aid in assessing the biological threats of pyrethroids to aquatic life.
Author Contributions
Conceptualization and Visualization, Writing - review & editing: Dr. Sangita Maiti Dutta, Writing - original draft and Writing - review & editing: Raja Saha.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Data Availability Statement
Every dataset that was examined for this study is openly accessible to the public.
Acknowledgments
We are grateful to the Higher Authorities of Biodiversity and Environmental Studies Research Centre, Midnapore City College, for giving us the chance to continue our research work in their institute.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Aboul-Zahab, A. O., & El-Ansary, A. (1992). Biological aspects of controlling snail hosts of bilharziasis using wild and cultivated plant extracts.
- Ahirrao, D. V., & Phand, D. L. (2015). Impact of cypermethrin on biochemical contents of a freshwater snail-Bellamya bengalensis. http://www.jebas.org.
- Ansari, R. A., Rahman, S., Kaur, M., Anjum, S., & Raisuddin, S. (2011). In vivo cytogenetic and oxidative stress-inducing effects of cypermethrin in freshwater fish, Channa punctata Bloch. Ecotoxicology and Environmental Safety, 74(1), 150-156. [CrossRef]
- Ait Ayad, M., Ait Fdil, M., & Mouabad, A. (2011). Effects of cypermethrin (pyrethroid insecticide) on the valve activity behavior, byssal thread formation, and survival in air of the marine mussel Mytilus galloprovincialis. Archives of environmental contamination and toxicology, 60, 462-470. [CrossRef]
- Baby, R. L., Hasan, I., Kabir, K. A., & Naser, M. N. (2010). Nutrient analysis of some commercially important molluscs of Bangladesh. Journal of Scientific Research, 2(2), 390-396. [CrossRef]
- Bakry, F. A., Hasheesh, W. S., & Hamdi, S. A. (2011). Biological, biochemical, and molecular parameters of Helisoma duryi snails exposed to the pesticides Malathion and Deltamethrin. Pesticide biochemistry and physiology, 101(2), 86-92. [CrossRef]
- Bloomquist, J. R. (1993). Neuroreceptor mechanisms in pyrethroid mode of action and resistance. Reviews in pesticide toxicology (USA), 2.
- Breistøl, A., Högstedt, G., & Lislevand, T. (2015). Pied Flycatchers Ficedula hypoleuca prefer ectoparasite-free nest sites when old nest material is present. Ornis Norvegica, 38, 9-13. [CrossRef]
- Burr, S. A., & Ray, D. E. (2004). Structure-activity and interaction effects of 14 different pyrethroids on voltage-gated chloride ion channels. Toxicological Sciences, 77(2), 341-346. [CrossRef]
- Byrne, P. A., & O'halloran, J. (2001). The role of bivalve molluscs as tools in estuarine sediment toxicity testing: a review. Hydrobiologia, 465, 209-217. [CrossRef]
- CARBALLAL, M. J., LÓPEZ, C., AZEVEDO, C., & VILLALBA, A. (1997). In vitrostudy of phagocytic ability ofMytilus galloprovincialisLmk. haemocytes. Fish & Shellfish Immunology, 7(6), 403-416. [CrossRef]
- Casida, J. E., & Quistad, G. B. (1998). Golden age of insecticide research: past, present, or future?. Annual review of entomology, 43(1), 1-16. [CrossRef]
- CDPR 2006. Pesticide Use Reporting: An Overview of California’s Unique Full Reporting System. Sacramento:California Department of Pesticide Regulation. Available: http://www.cdpr.ca.gov/docs/pur/purmain.htm [accessed 22 March 20007].
- Cerenius, L., & Söderhäll, K. (2004). The prophenoloxidase-activating system in invertebrates. Immunological reviews, 198, 116–126. [CrossRef]
- Chmist, J., Szoszkiewicz, K., & Drożdżyński, D. (2019). Behavioural Responses of Unio tumidus Freshwater Mussels to Pesticide Contamination. Archives of environmental contamination and toxicology, 77(3), 432–442. [CrossRef]
- Coats, J. R., Symonik, D. M., Bradbury, S. P., Dyer, S. D., Timson, L. K., & Atchison, G. J. (1989). Toxicology of synthetic pyrethroids in aquatic organisms: an overview. Environmental Toxicology and Chemistry: An International Journal, 8(8), 671-679. [CrossRef]
- Cruzeiro, C., Pardal, M. Â., Rodrigues-Oliveira, N., Castro, L. F. C., Rocha, E., & Rocha, M. J. (2016). Multi-matrix quantification and risk assessment of pesticides in the longest river of the Iberian peninsula. The Science of the total environment, 572, 263–272. [CrossRef]
- Dalle-Donne, I., Rossi, R., Giustarini, D., Milzani, A., & Colombo, R. (2003). Protein carbonyl groups as biomarkers of oxidative stress. Clinica chimica acta, 329(1-2), 23-38. [CrossRef]
- Data, F. P. Q. (2020). Available online: http://www. fao. org/faostat/en/# data. QC (accessed on 26 March 2021). http://www.fao.org/faostat/en/#search/pyrethroid.
- Edwards, C. (Ed.). (2013). Environmental pollution by pesticides (Vol. 3). Springer Science & Business Media.
- el-Ansary, A., el-Bardicy, S., Soliman, M. S., & Zayed, N. (2000). Sublethal concentration of Ambrosia maritima(Damsissa) affecting compatibility of Biomphalaria alexandrina snails to infection with Schistosoma mansoni through disturbing the glycolytic pathway. Journal of the Egyptian Society of Parasitology, 30(3), 809–819. PMID: 11198379.
- Elfman, L., Tooke, N. E., & Patring, J. D. (2011). Detection of pesticides used in rice cultivation in streams on the island of Leyte in the Philippines. Agricultural Water Management, 101(1), 81-87. [CrossRef]
- Garey, J., & Wolff, M. S. (1998). Estrogenic and antiprogestagenic activities of pyrethroid insecticides. Biochemical and biophysical research communications, 251(3), 855–859. [CrossRef]
- Gong, D. C. (2013). Pyrethroids pesticides residues and their behavior in a multimedium environment of Liangtan River Basin. School of Environment and Civil Engineering, Chongqing Unviersity, Chongqing, 93.
- Hill, I. R. (1989). Aquatic organisms and pyrethroids. Pesticide Science, 27(4), 429-457. [CrossRef]
- Hunt, L., Bonetto, C., Resh, V. H., Buss, D. F., Fanelli, S., Marrochi, N., & Lydy, M. J. (2016). Insecticide concentrations in stream sediments of soy production regions of South America. The Science of the total environment, 547, 114–124. [CrossRef]
- Idris, S. B., Ambali, S. F., & Ayo, J. O. (2012). Cytotoxicity of chlopyrifos and cypermethrin: The ameliorative effects of antioxidants. African Journal of Biotechnology, 11(99), 16461-16467.
- iusKatsuda, Y. (1999). Development of and future prospects for pyrethroid chemistry. Pesticide science, 55(8), 775-782.
- Khambay, B. P. (2002). Pyrethroid insecticides. Pesticide Outlook, 13(2), 49-54.
- Khazri, A., Sellami, B., Dellali, M., Corcellas, C., Eljarrat, E., Barceló, D., & Mahmoudi, E. (2015). Acute toxicity of cypermethrin on the freshwater mussel Unio gibbus. Ecotoxicology and environmental safety, 115, 62-66. [CrossRef]
- Kontreczky, C. S., Farkas, A., Nemcsók, J., & Salanki, J. (1997). Short-and Long-Term Effects of Deltamethrin on Filtering Activity of Freshwater Mussel (Anodonta cygneaL.). Ecotoxicology and environmental safety, 38(3), 195-199. [CrossRef]
- Köprücü, K., & Seker, E. (2008). Acute toxicity of deltamethrin for freshwater mussel, Unio elongatulus eucirrus bourguignat. Bulletin of environmental contamination and toxicology, 80(1), 1–4. [CrossRef]
- Köprücü, K., Yonar, S. M., & Şeker, E. (2010). Effects of cypermethrin on antioxidant status, oxidative stress biomarkers, behavior, and mortality in the freshwater mussel Unio elongatulus eucirrus. Fisheries science, 76, 1007-1013. [CrossRef]
- Li, H., Cheng, F., Wei, Y., Lydy, M. J., & You, J. (2017). Global occurrence of pyrethroid insecticides in sediment and the associated toxicological effects on benthic invertebrates: an overview. Journal of hazardous materials, 324, 258-271. [CrossRef]
- Little, E. E., & Brewer, S. K. (2001). Neurobehavioral toxicity in fish. Target organ toxicity in marine and freshwater teleosts, 141-176.
- Liu, W., Gan, J., Lee, S., & Werner, I. (2005). Isomer selectivity in aquatic toxicity and biodegradation of bifenthrin and permethrin. Environmental Toxicology and Chemistry: An International Journal, 24(8), 1861-1866. [CrossRef]
- Livingstone, D. R. (2001). Contaminant-stimulated reactive oxygen species production and oxidative damage in aquatic organisms. Marine pollution bulletin, 42(8), 656-666. [CrossRef]
- Ma, X. (2009). Research progress on analytical technique of pyrethroid pesticide residue. Journal of Anhui Agricultural Sciences, 28, 13775-13777.
- Mahmoud, N., Dellali, M., Aissa, P., & Mahmoudi, E. (2012). Acute toxicities of cadmium and permethrin on the pre-spawning and post-spawning phases of Hexaplex trunculus from Bizerta Lagoon, Tunisia. Environmental monitoring and assessment, 184, 5851-5861. [CrossRef]
- Monserrat, J. M., Martínez, P. E., Geracitano, L. A., Amado, L. L., Martins, C. M. G., Pinho, G. L. L., ... & Bianchini, A. (2007). Pollution biomarkers in estuarine animals: critical review and new perspectives. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 146(1-2), 221-234. [CrossRef]
- Mukherjee, S., & Mandal, C. (2023). Phagocytic response and phenoloxidase activity of the hemocytes of Bellamya bengalensis exposed to synthetic fenvalerate. Journal of Stress Physiology & Biochemistry, 19(3), 143-151.
- Muñoz, P., Meseguer, J., & Esteban, M. A. (2006). Phenoloxidase activity in three commercial bivalve species. Changes due to natural infestation with Perkinsus atlanticus. Fish & shellfish immunology, 20(1), 12–19. [CrossRef]
- Narahashi, T. (1984). Nerve membrane sodium channels as the target of pyrethroids. In Cellular and molecular neurotoxicology (pp. 85-108). Raven New York.
- Nowell, L. H., Norman, J. E., Moran, P. W., Martin, J. D., & Stone, W. W. (2014). Pesticide Toxicity Index--a tool for assessing potential toxicity of pesticide mixtures to freshwater aquatic organisms. The Science of the total environment, 476-477, 144–157. [CrossRef]
- Leah M Oliver William S Fisher (1999). Appraisal of prospective bivalve immunomarkers. Biomarkers : biochemical indicators of exposure, response, and susceptibility to chemicals, 4(6), 510–530. [CrossRef]
- Rawi, S. M., El-Gindy, H., Haggag, A. M., Abou El Hassan, A., & Abdel Kader, A. (1995). Few possible molluscicides from calendula Micrantha officinalis and Ammi majus plants. I. Physiological effect on B. alexandrina and B. truncatus. J. Egypt. Ger. Soc. Zool, 16, 69-75.
- Ray, M., Bhunia, A. S., Bhunia, N. S., & Ray, S. (2013). Density shift, morphological damage, lysosomal fragility and apoptosis of hemocytes of Indian molluscs exposed to pyrethroid pesticides. Fish & shellfish immunology, 35(2), 499–512. [CrossRef]
- Regoli, F., & Giuliani, M. E. (2014). Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Marine environmental research, 93, 106-117. [CrossRef]
- Renault, T. (2011). Effects of pesticides on marine bivalves: what do we know and what do we need to know. Pesticides in the Modern World–Risks and Benefits, 228-240. [CrossRef]
- Richardson, K. L., Gold-Bouchot, G., & Schlenk, D. (2009). The characterization of cytosolic glutathione transferase from four species of sea turtles: loggerhead (Caretta caretta), green (Chelonia mydas), olive ridley (Lepidochelys olivacea), and hawksbill (Eretmochelys imbricata). Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology, 150(2), 279-284. [CrossRef]
- Rodrigues, E. T., Alpendurada, M. F., Ramos, F., & Pardal, M. Â. (2018). Environmental and human health risk indicators for agricultural pesticides in estuaries. Ecotoxicology and environmental safety, 150, 224–231. [CrossRef]
- Fernández San Juan, M. R., Cortelezzi, A., Albornoz, C. B., Landro, S. M., Arrighetti, F., Najle, R., & Lavarías, S. M. L. (2020). Ecotoxicology and environmental safety toxicity of pyrethroid cypermethrin on the freshwater snail Chilina parchappii: Lethal and sublethal effects. Ecotoxicology and environmental safety, 196, 110565. [CrossRef]
- Sellami, B., Louati, H., Dellali, M., Aissa, P., Mahmoudi, E., Coelho, A. V., & Sheehan, D. (2014). Effects of permethrin exposure on antioxidant enzymes and protein status in Mediterranean clams Ruditapes decussatus. Environmental science and pollution research, 21, 4461-4472. [CrossRef]
- Sellami, B., Khazri, A., Mezni, A., Louati, H., Dellali, M., Aissa, P., ... & Sheehan, D. (2015). Effect of permethrin, anthracene and mixture exposure on shell components, enzymatic activities and proteins status in the Mediterranean clam Venerupis decussata. Aquatic toxicology, 158, 22-32. [CrossRef]
- Shafer, T. J., & Meyer, D. A. (2004). Effects of pyrethroids on voltage-sensitive calcium channels: a critical evaluation of strengths, weaknesses, data needs, and relationship to assessment of cumulative neurotoxicity. Toxicology and applied pharmacology, 196(2), 303-318. [CrossRef]
- Shashikumar, S., & Rajini, P. S. (2010). Cypermethrin-induced alterations in vital physiological parameters and oxidative balance in Caenorhabditis elegans. Pesticide Biochemistry and Physiology, 97(3), 235-242. [CrossRef]
- Soderlund, D. M., & Bloomquist, J. R. (1990). Molecular mechanisms of insecticide resistance. In Pesticide resistance in arthropods (pp. 58-96). Boston, MA: Springer US. [CrossRef]
- Soderlund, D. M., Clark, J. M., Sheets, L. P., Mullin, L. S., Piccirillo, V. J., Sargent, D., Stevens, J. T., & Weiner, M. L. (2002). Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology, 171(1), 3–59. [CrossRef]
- Tang, W., Wang, D., Wang, J., Wu, Z., Li, L., Huang, M., Xu, S., & Yan, D. (2018). Pyrethroid pesticide residues in the global environment: An overview. Chemosphere, 191, 990–1007. [CrossRef]
- Tolomeo, A. M., Carraro, A., Bakiu, R., Toppo, S., Place, S. P., Ferro, D., & Santovito, G. (2016). Peroxiredoxin 6 from the Antarctic emerald rockcod: molecular characterization of its response to warming. Journal of Comparative Physiology B, 186, 59-71. [CrossRef]
- Tripathi, P. K., & Singh, A. (2004). Toxic effects of cypermethrin and alphamethrin on reproduction and oxidative metabolism of the freshwater snail, Lymnaea acuminata. Ecotoxicology and environmental safety, 58(2), 227–235. [CrossRef]
- Ullah, S., Zuberi, A., Alagawany, M., Farag, M. R., Dadar, M., Karthik, K., Tiwari, R., Dhama, K., & Iqbal, H. M. N. (2018). Cypermethrin induced toxicities in fish and adverse health outcomes: Its prevention and control measure adaptation. Journal of environmental management, 206, 863–871. [CrossRef]
- Wei, K., & Yang, J. (2015). Oxidative damage induced by copper and beta-cypermethrin in gill of the freshwater crayfish Procambarus clarkii. Ecotoxicology and environmental safety, 113, 446-453. [CrossRef]
- Werner, I., & Moran, K. (2008). Effects of pyrethroid insecticides on aquatic organisms. Synthetic pyrethroids: Occurrence and behavior in aquatic environments, 991, 310-335.
- Weston, D. P., Holmes, R. W., You, J., & Lydy, M. J. (2005). Aquatic toxicity due to residential use of pyrethroid insecticides. Environmental Science & Technology, 39(24), 9778-9784. [CrossRef]
- Xiao, Y., Chen, S. H., Hu, W., & Hu, M. Y. (2012). New progress and prospect for the microbial degradation of pyrethroid pesticides. Chin. Agri. Sci. Bull, 28, 218-224.
- Xu, P., & Huang, L. (2017). Effects of α-cypermethrin enantiomers on the growth, biochemical parameters and bioaccumulation in Rana nigromaculata tadpoles of the anuran amphibians. Ecotoxicology and environmental safety, 139, 431-438. [CrossRef]
- Yoo, M., Lim, Y. H., Kim, T., Lee, D., & Hong, Y. C. (2016). Association between urinary 3-phenoxybenzoic acid and body mass index in Korean adults: 1st Korean National Environmental Health Survey. Annals of occupational and environmental medicine, 28, 1-8. [CrossRef]
- Zhao, L. N. (2014). Residue and risk assessment of 7 kinds of pyrethroids in water environment in the Pearl River Delta. Shanghai Ocean University.
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).