1. Introduction
Tuberculosis is a respiratory tract disease caused by
Mycobacterium tuberculosis species complex that spread between people through aerosols containing as little as 1-3 bacilli [
1]. Although TB mostly affects the lungs, it may adversely impact the brain, kidneys, or spine. Despite being a treatable disease, tuberculosis still remains one of the leading causes of death globally, being the second most common single infectious agent-related cause of death (ranking above HIV/AIDS and only second to COVID-19 in 2022) [
2,
3,
4]. The COVID-19 pandemic has reversed years of global progress in tackling tuberculosis [
5] and, for the first time in over a decade, TB deaths had increased, and only now are starting to return to pre-pandemic levels, according to the World Health Organization’s 2023 Global TB report [
4].
Most cases of tuberculosis can be treated and the spread of infection can be prevented with prompt diagnosis and six months of first-line antibiotic treatment [
6]. However the increasing appearance of multidrug-resistant (MDR) and extensive drug-resistant (XDR) TB makes clear the need for new drugs that can be effective and used in shorter therapeutic regimens [
7]. Numerous novel therapeutic targets have been established in recent years with the effort to develop new effective anti-TB therapeutics, many of which targeting essential proteins implicated in the synthesis of the cell wall components [
8,
9]. One relevant example is DprE1, considered one of the promising targets for the development of new anti-TB drugs [
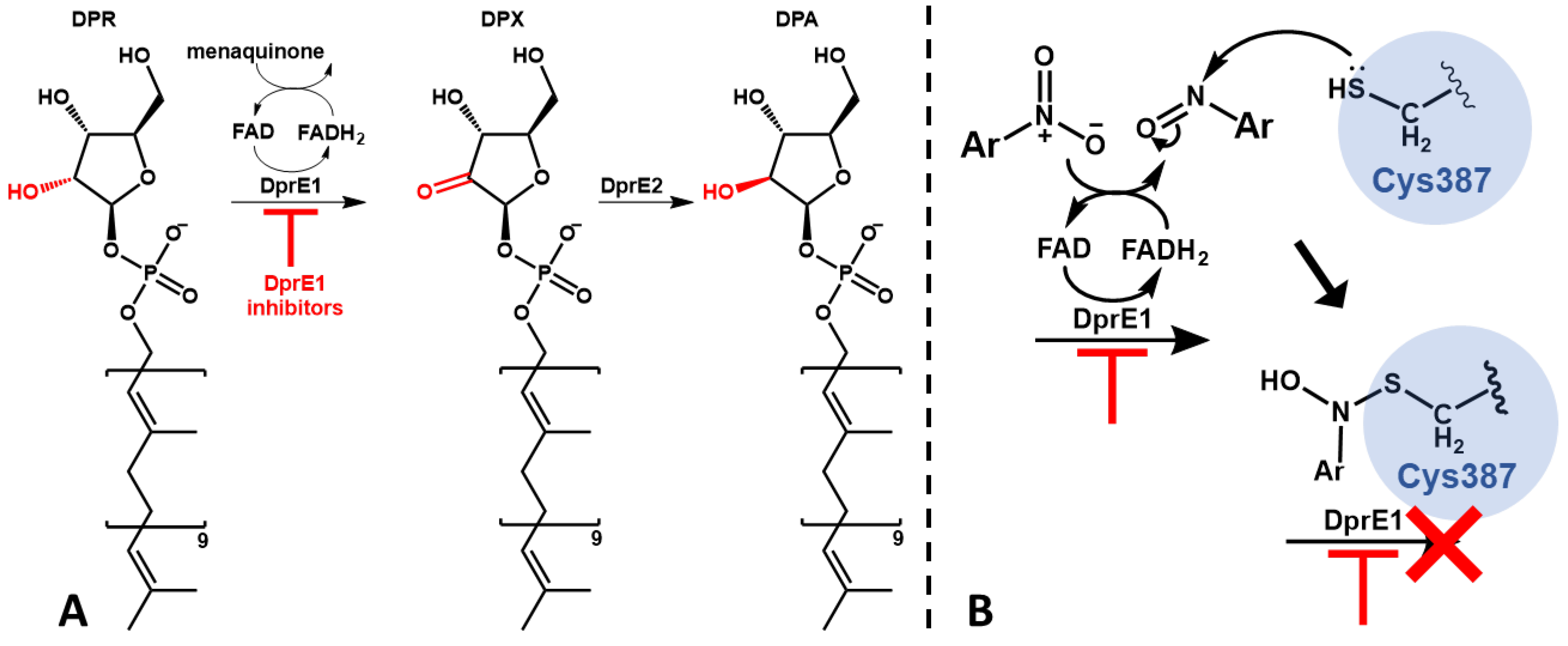
10]. DprE1 is a component of the DprE1-DprE2 complex, an heterodimeric protein that catalyzes the epimerization of decaprenylphosphoryl-D-ribose (DPR) to decaprenylphosphoryl-D-arabinose (DPA) (
Figure 1A) [
11]. DPA is a vital precursor in the production of both lipoarabinomannan and arabinogalactan, essential in cell wall biosynthesis [
12,
13]. One of the reasons that makes DprE1 so promising is its intracellular location, since by being located in the periplasmatic space of the mycobacteria the only barrier to the action of its inhibitors is the cell wall [
14].
Inhibitors of DprE1 are generally subdivided into covalent and non-covalent inhibitors. The mechanism of covalent inhibition is based on the formation of a non-reversible covalent bond between the inhibitor and the cysteine 387 residue (Cys387) of DprE1. For this to happen, covalent inhibitors make use of an aromatic nitro moiety that, upon interaction with the active site of DprE1, undergoes reduction of the nitro group to nitroso, which then forms a covalent adduct with Cys387 irreversibly hindering the protein’s function (
Figure 1B) [
15,
16]. This residue is profoundly conserved in mycobacteria, aside from in
Mycobacterium avium and
Mycobacterium aureum where cysteine is replaced by alanine and serine, respectively [
17,
18].
Figure 1.
Schematic representation of the DprE1-DprE2 complex function and its inhibition; (A) – Epimerization of DPR to DPA; (B) - Mechanism of activation and DprE1 inhibition of nitroaromatic suicide inhibitors.
Figure 1.
Schematic representation of the DprE1-DprE2 complex function and its inhibition; (A) – Epimerization of DPR to DPA; (B) - Mechanism of activation and DprE1 inhibition of nitroaromatic suicide inhibitors.
Our research group has studied benzoic acid and its derivatives for their antimycobacterial potential, specifically against Mtb H37Rv strain, attempting to develop alkyl esters as prodrugs capable of increasing the weak acids’ antitubercular activity [
19,
20]. We provided proof that mycobacteria may quickly hydrolyze a range of organic acid esters [
21,
22] and that esters demonstrate greater in vitro activity than that of the corresponding free organic acids [
23,
24], indicating that they are appropriate prodrugs for the substances, helping the molecules enter the cells and releasing the free acid.
In previous work we found that alkyl esters of 3,5-dinitrobenzoic acid showed very relevant antitubercular activities [
25]. The activity was much higher than anticipated by Zhang et al. [
19] based on the pka of the liberated free acid, indicating that the presence of the nitro groups might be important for the mechanism of action. Our inability to correlate the activity with ester hydrolysis rates led us to hypothesize that these compounds might act directly as active drugs rather than prodrugs. If the compounds could be acting as drugs, then a series of isosteres could be explored for activity.
Building on this, after exploring the potential of nitrobenzoates and nitrothiobenzoates [
26], we decided to explore the antimycobacterial potential of bioisosteric amide analogues. Only two 3,5-dinitrobenzamides with linear alkyl chains were reported in the literature as having antimycobacterial activity [
27] but they were somehow disregarded, as their activity was lower when compared with other more complex derivatives containing a terminal aromatic moiety (such as in the DNB1 or DNB2). The reason for that is probably because the authors unfortunately only explored the derivatives with C6 and C16
N-alkyl groups. Because we knew from our previous work with nitro containing esters that the alkyl chain could be optimized for activity by modifying the chain length of the compounds [
26], we decided to apply the same approach to the
N-alkyl benzamide analogues with the objective of improving their antimycobacterial efficacy.
Our initial efforts focused on synthesizing a series of nitrobenzamides with
N-alkyl chains ranging from four to sixteen carbon atoms and evaluating their antitubercular activities. Encouraged by the results obtained on the activity of these compounds, we extended our study to include their performance in a macrophage infection model. Given the fears associated with nitroaromatic compounds in drug development [
28] the cytotoxicity of the compounds here reported was assessed using human macrophages. We also decided to study the stability of the compounds on buffer, plasma and in a mycobacterial homogenate. Buffer stability studies were used to evaluate the chemical stability of the compounds at pH 7.4 and plasma stability studies served to evaluate if the compounds exhibit stability that allows the determination of activity
in vivo. Stability in mycobacterial homogenate was examined to determine the effect of mycobacterial enzymes on the compounds. Since we had no access to purified DprE1 to directly test the inhibition of purified DprE1, we evaluated the effects of our compounds on various mycobacteria species known to have differing responses to DprE1 inhibitors. In parallel, computational docking studies were performed to analyze how our compounds fit within the DprE1 binding pocket, compared to the DNB1 and DNB2, known inhibitors of DprE1. These studies supported the potential of DprE1 as a possible target for our compounds, and lead to the discovery of new DNBs with antitubercular activities comparable to established benchmarks like DNB1.
3. Discussion
The benzamides presented here were easily obtained by reacting the acid chloride with the corresponding amide. Detailed synthesis procedures yields and results not presented in the manuscript are available in the Supplementary Material.
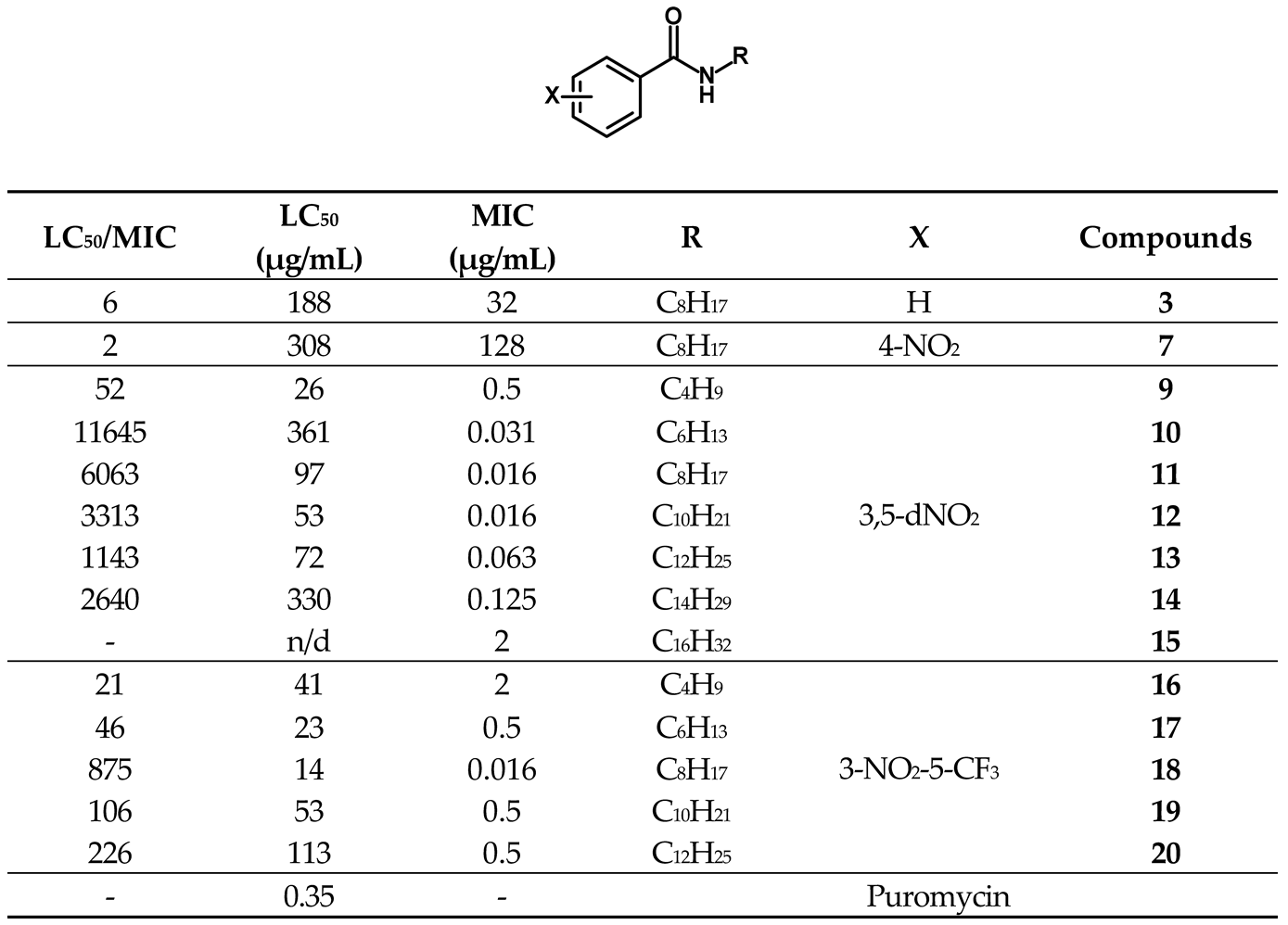
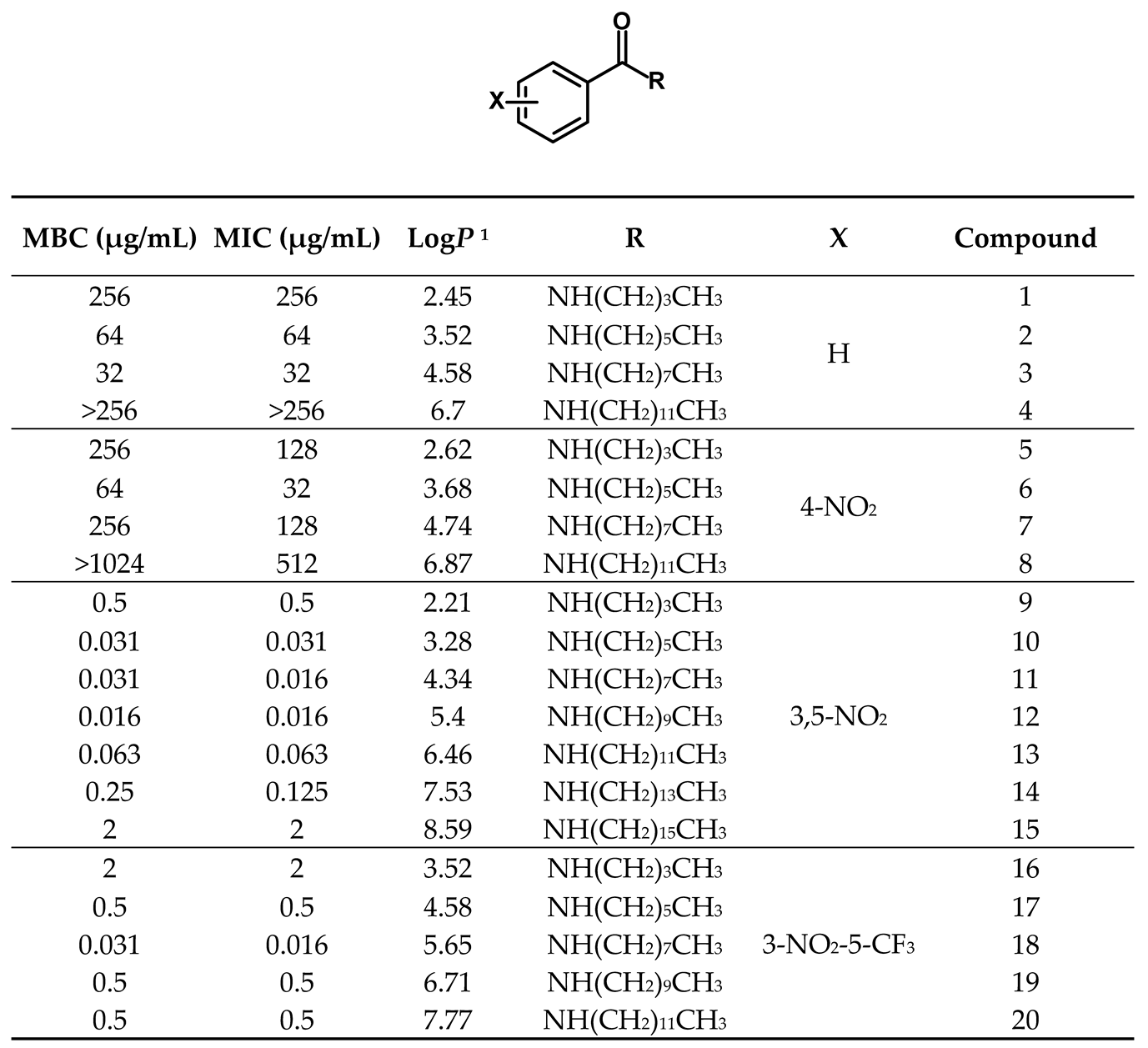
The large difference in activity observed between compounds 9-20 versus others suggests distinct mechanisms of action. The in vitro activity of these benzamides is primarily centered on the presence of a nitro group at the 3-position of the benzene ring. Substitution of this nitro group or its relocation to the 4-position significantly diminishes activity, echoing findings by Christophe et al. [
30].
The fact that most of the activity was maintained by substitution of the nitro group in the position 5 of the aromatic ring by a trifluoromethyl group is also in accordance with the literature [
31,
41]. In an interesting study, Li et al. developed nitrobenzamide compounds by simplifying the structure of PBTZ169 [
31]. They found that substituting the nitro group with a CF3 group slightly reduced the compounds' activity. However, the overall activity remained comparable to the original nitro derivatives, suggesting that a nitro group could be replaced with a CF3 group to address pharmacokinetic or toxicological concerns.
Lipophilicity is also important, but it only modulates the activity of each series with N-alkyl chain lengths of 6-10 carbons being optimal. Since our computational studies indicate that the lengths of the alkyl chain only interfere slightly with the position of the inhibitor in the binding pocket of DprE1, it seems that an optimum lipophilicity of the compounds is mainly needed to allow the compounds to access the periplasmatic space where DprE1 is located.
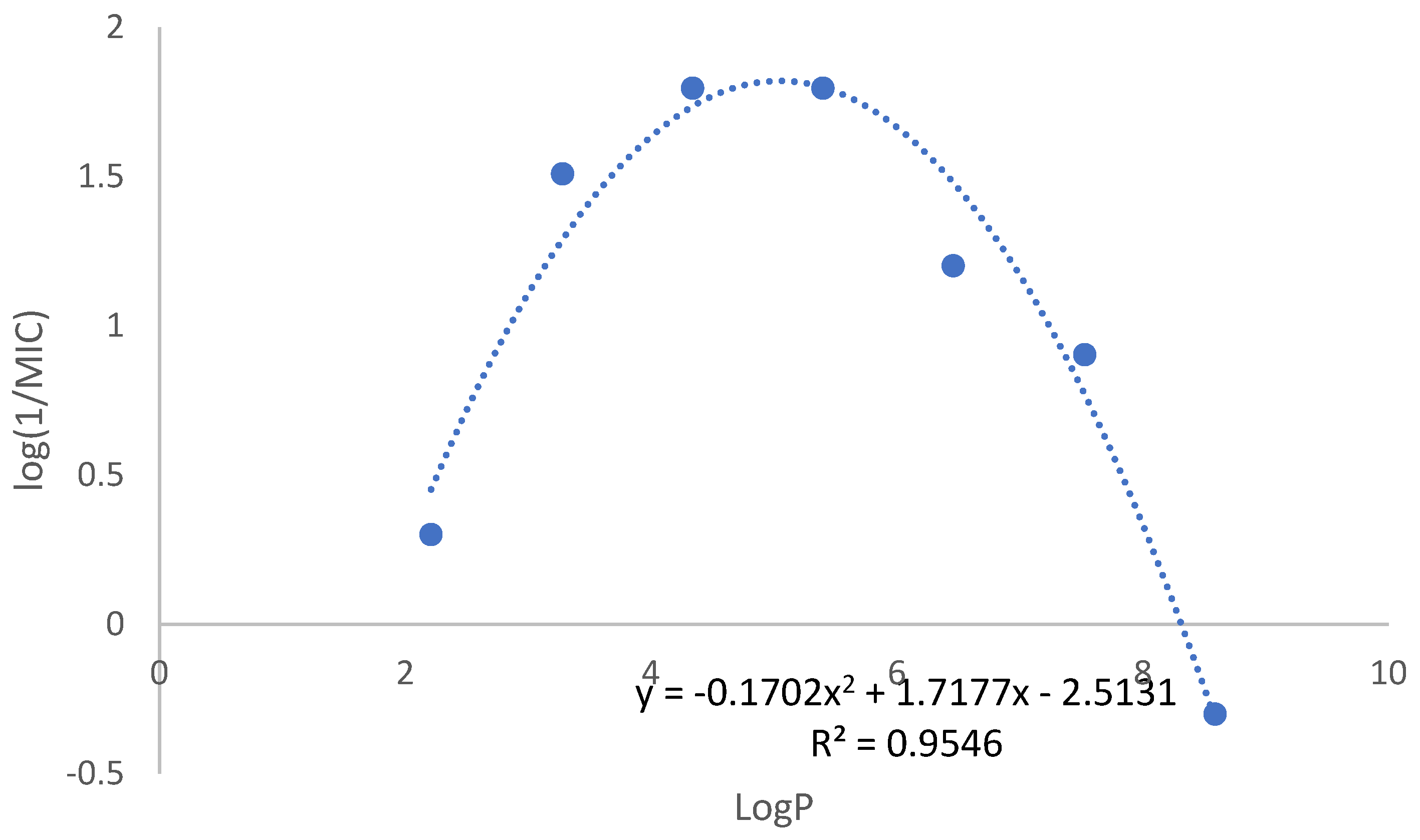
The relationship between logP and antimycobacterial activity in the 3,5-dinitro substituted derivatives indicates lipophilicity as a critical factor (
Figure 2). Considering mycobacterial cell wall lipidic rich composition, increasing lipophilicity may enhance membrane permeation however it also reduces water solubility, posing challenges in biological assays, especially for compounds with longer than ten carbon chain. Therefore, it is conceivable that longer alkyl chains have the capacity to exhibit comparable or even higher intrinsic DprE1 inhibition, but their solubility prevents its expression leading to the parabolic behavior observed in
Figure 2.
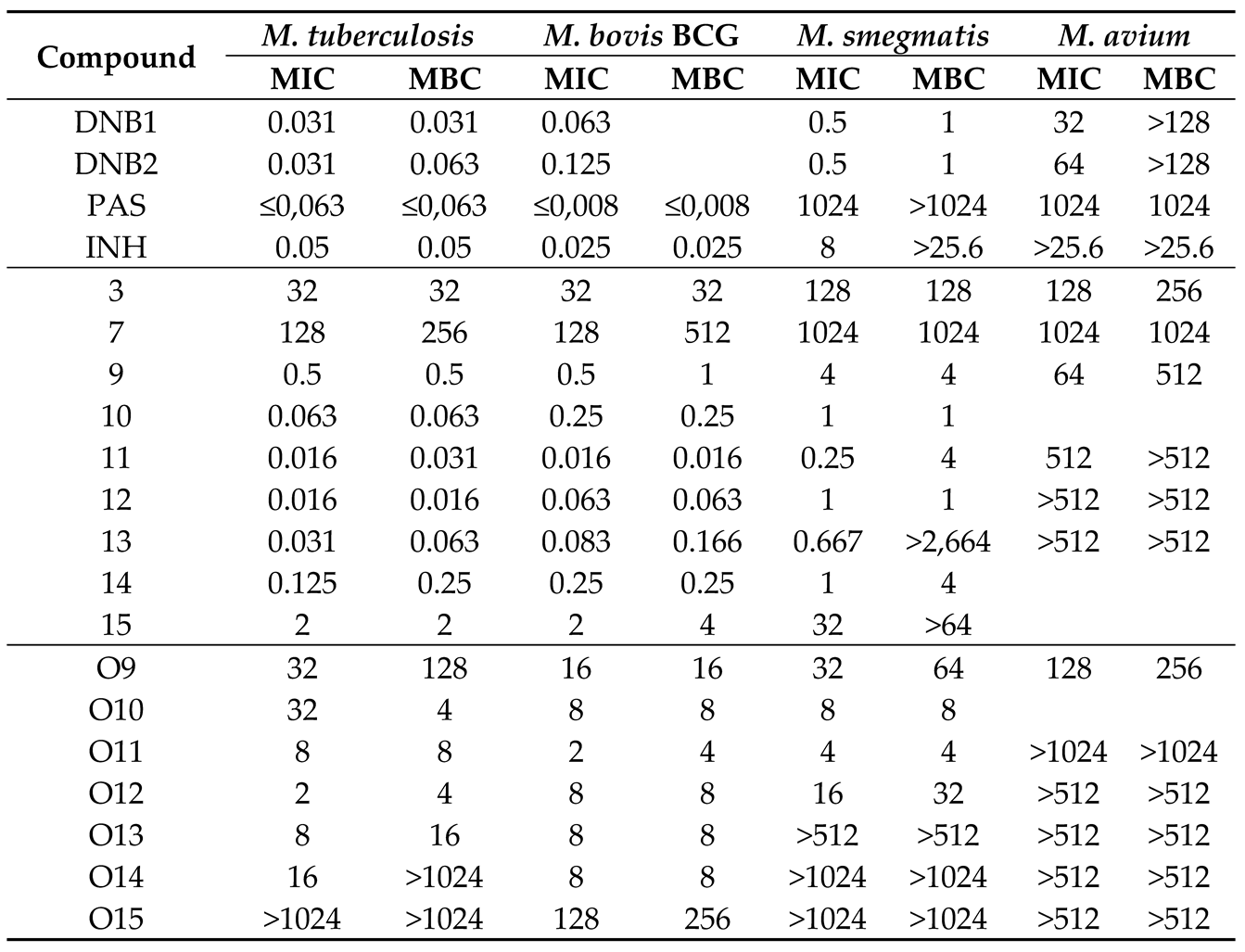
To support our hypothesis that our compounds inhibit DprE1, we evaluated their effectiveness against
M. tuberculosis,
M. bovis BCG, and
M. avium and compared these results with those of known DprE1 inhibitors, DNB1 and DNB2. Taking in consideration the limitation that
M. avium is naturally resistant to most antibiotics [
24], we can indeed observe that the amides 11 and 12 showed high activity in
M. bovis and
M. tuberculosis, that are naturally sensitive to DprE1 inhibitors, however, the activity is completely lost in
M. avium, which has a mutation in the DprE1 enzyme, having an alanine residue in the place of Cys387. This cysteine is fundamental for the mode of action of covalent DprE1 inhibitors as it is its thiol group that reacts with the nitroso group of the activated inhibitor (
Figure 1). Similar results were presented by the benzothiazinone BTZ043 which was tested by Caroline et al. [
17], where BTZ043 showed high activity in
M. tuberculosis, that, was lost in
M. avium. The NfnB nitroreductase is overexpressed in
M. smegmatis, which may cause the drug to become inactive due to the reduction of a nitro group to amine, which is unable to react with the cysteine residue. The fact that our more active 3,5-dinitrobenzamides presented activities over the four mycobacterial species that follow the described pattern of DprE1 sensitivity provided important clues that support the hypothesis of the disubstituted derivatives acting as DprE1 inhibitors. The remaining compounds tested did not present a similar spectrum of activity and presented intrinsically low activities against Mtb indicating that they either do not inhibit DprE1 or are unable to reach the periplasmic space where DprE1 is located [
12,
42].
One important aspect of any new antitubercular drug is its activity over dormant inactive cells [
43] We did not test our compounds in any model suitable to assess its activity over non-replicating
M. tuberculosis as the probable mechanism of action does not support a relevant activity over dormant cells, however this hypothesis should be kept open.
The cytotoxicity assessments indicated that despite their nitroaromatic nature, the compounds exhibit low toxicity relative to their antimycobacterial activity. This finding was particularly pronounced in compounds with MIC values below 0.5 µg/mL, where the effective concentrations were substantially lower than the cytotoxic levels, suggesting potential safety in therapeutic contexts. However, in the future, other toxicity studies (in silico, in vitro and in vivo) must be performed in parallel with the advancement of this family of compounds.
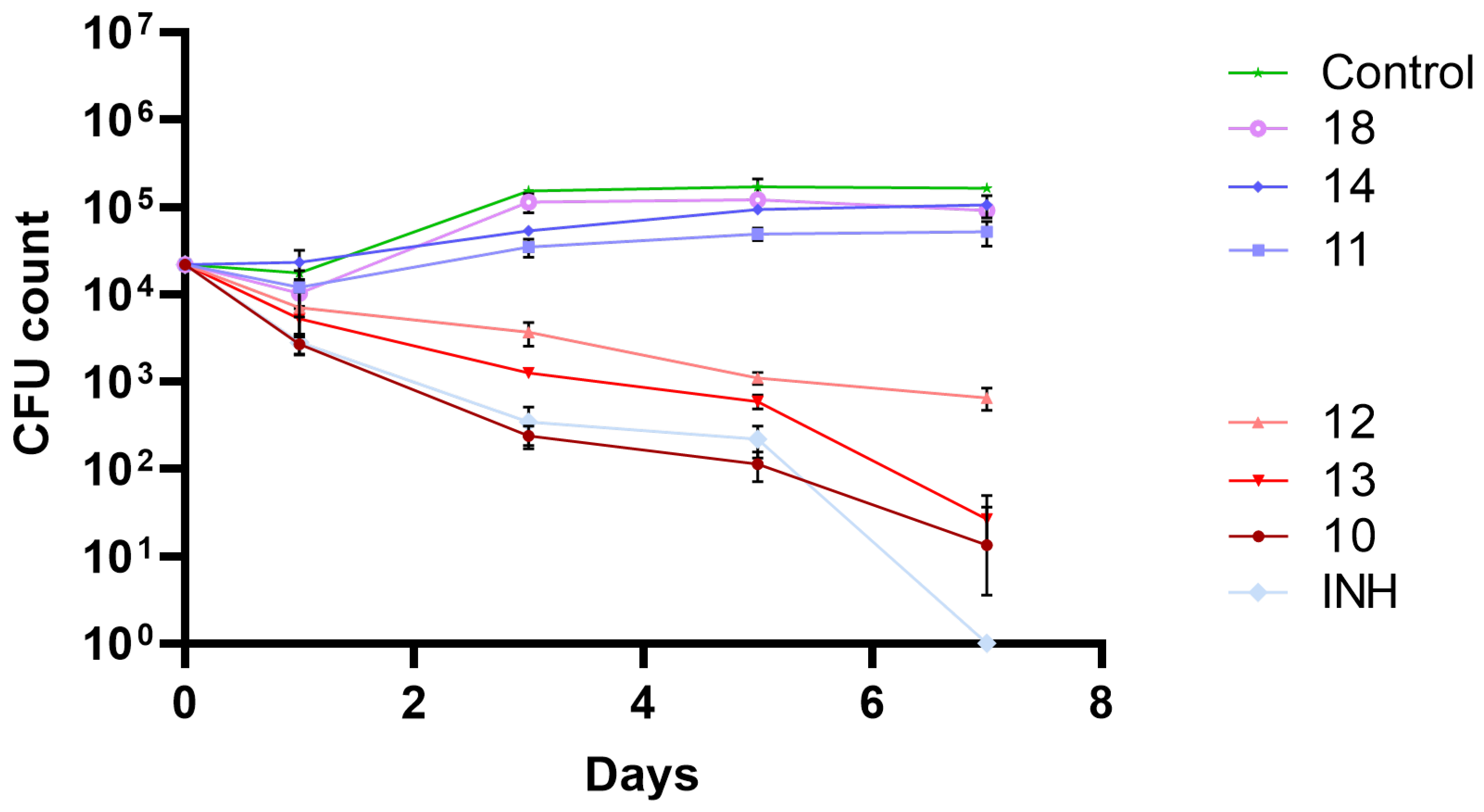
Since no significant impact on macrophage viability was observed at the MIC concentrations used, an infection model with Mtb infected macrophages was challenged with the most active compounds.
Figure 3 allows to easily distinguish the compounds that lead to intracellular killing of mycobacteria (decreasing the CFU count number) to the ones that do not. For compounds 10, 12 and 13 it was observed pronounced intracellular mycobacterial death, although for compound 12 a diminished antitubercular activity was achieved. Interestingly, compound 11 did not show activity, despite being a compound with one of the lowest MIC values. Since compound 12 also showed a diminished effect when compared to compounds 10 and 13, it is possible that this loss of activity is due to the length of the alkyl group, or its associated physical properties, that, in this experimental setup, were detrimental to antitubercular activity, contrary to the in vitro experimental setup.
From
Table 2, is clear that amide isosteres of the 3,5-dinitrobenzoates have better activity over
M. tuberculosis than the esters. In previous work, ester analogues of the amides under study were associated with some susceptibility to hydrolysis [
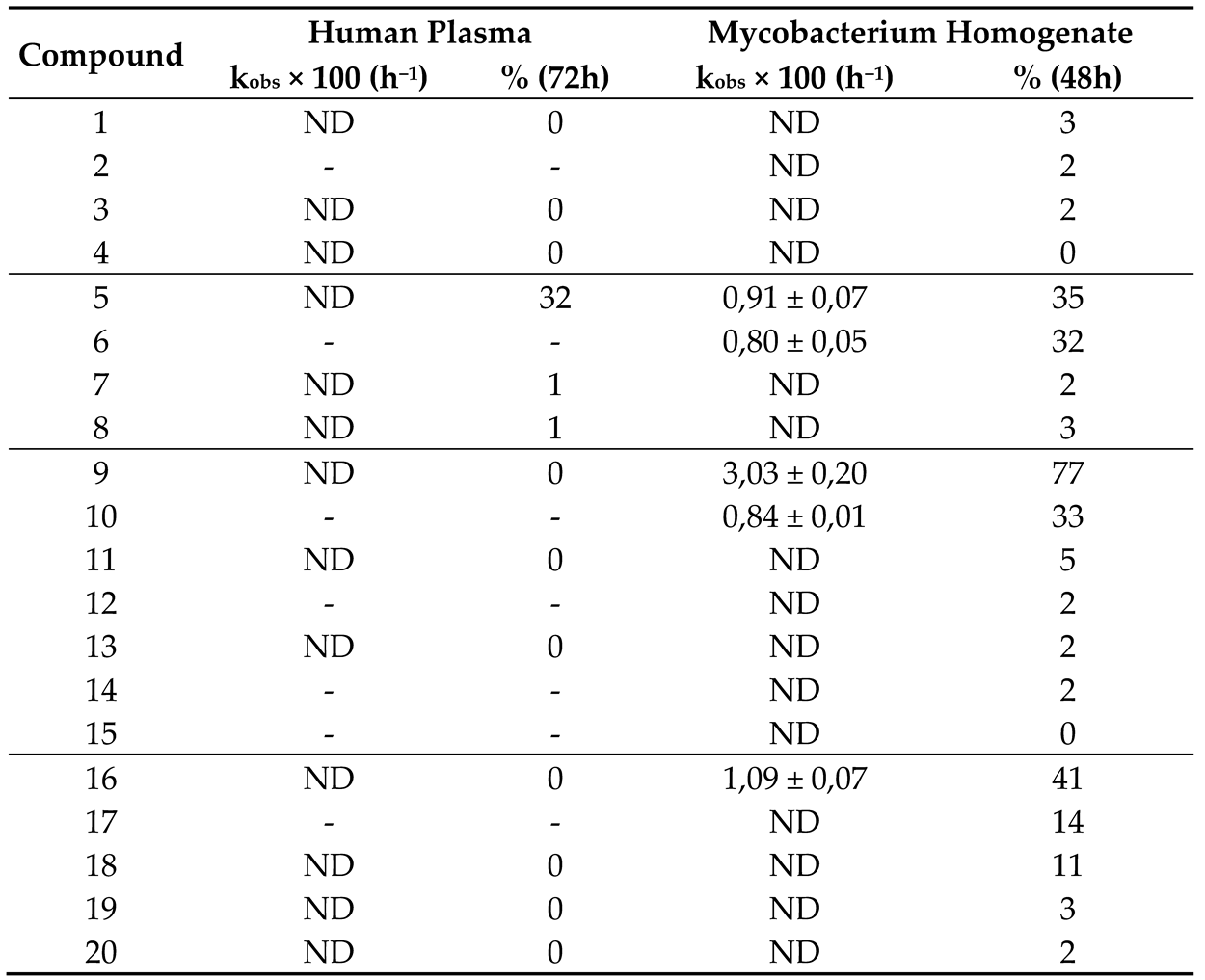
25]. However, here it was shown that the generality of amide derivatives was stable in all biological media assessed, and the most active compounds, both in vitro and in the macrophage model, were very stable.
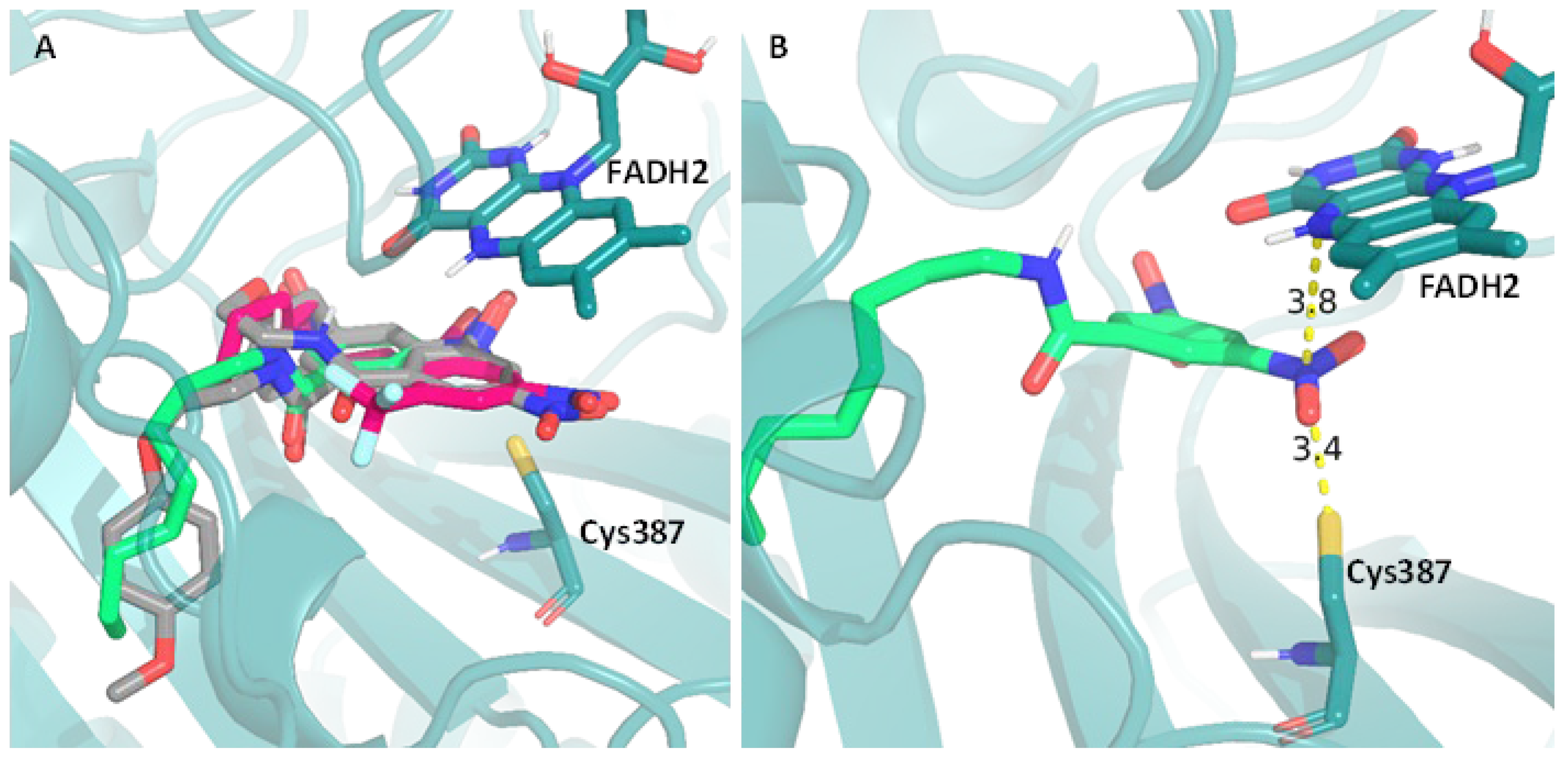
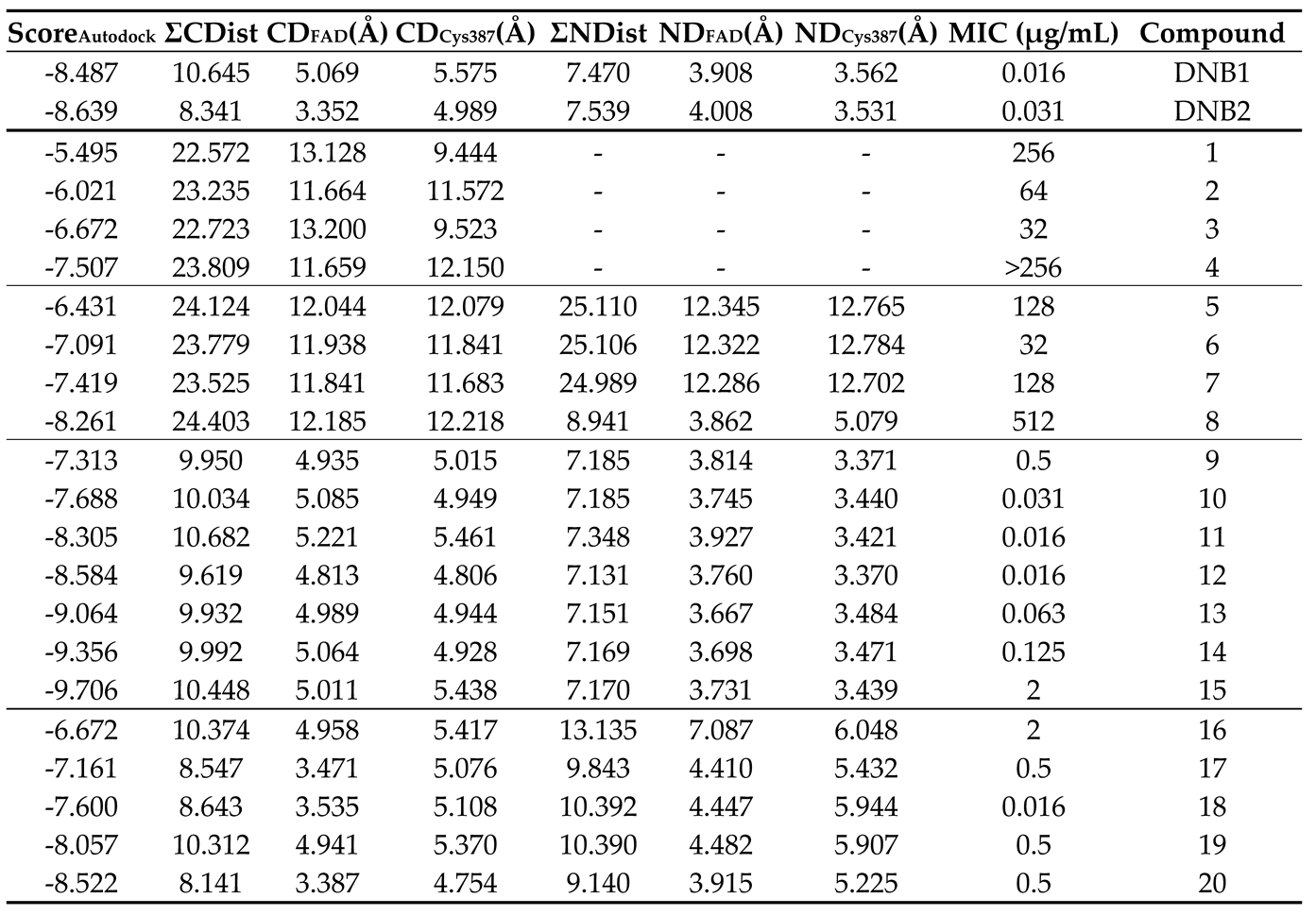
Finally, computational studies were performed aimed at assessing DprE1 as a possible target of action for the most active families of compounds, namely the 3,5-dinitro and 3-nitro-5-trifluoromethyl substituted derivatives. By comparing our compounds with the literature compounds DNB1 and DNB2, we can observe that, while the score values do not accurately allow to distinguish highly active (MIC ≤ 2 µg/mL) from less active (MIC > 2 µg/mL) compounds, the distances to the cysteine 387 residue and the FAD cofactor, both to the aromatic ring or the nitro group, allowed to make this distinction.
The similarity in distance measurements between our highly active compounds and established DprE1 inhibitors suggests a similar fit within the DprE1 binding pocket, reinforcing DprE1 as a suitable target for these compounds. Metrics such as ΣNDist and ΣCDist help distinguish between highly active and less active compound families, providing a valuable tool for future research and identifying potential new inhibitors.
It is important to note that these distance metrics do not vary significantly with different alkyl chain lengths because of the enzyme's broad binding pocket, which can accommodate large, branched chains like those in the natural substrate of DprE1. While this confirms the structural fit is crucial for inhibitory activity, it complicates the ability to rank compounds within families based on effectiveness.
Regarding the correlation between score values and MIC levels, no direct relationship was observed. Instead, score values increased linearly with alkyl chain length and logP values, indicating that differences in MIC are likely due to solubility and absorption challenges rather than binding affinity to DprE1.
4. Materials and Methods
Materials. Balanced salt solution, phosphate-buffered saline (PBS), Dulbecco’s modified Eagle’s medium (DMEM), and L-glutamine were purchased from Invitrogen. Sodium dodecyl sulphate (SDS), Triton X-100, benzoic acid, 4-nitrobenzoic acid, 3,5-dinitrobenzoic acid, 3-nitro-5-(trifluoromethyl)benzoic acid, n-butylamine, n-hexylamine, n-octylamine, n-decylamine, n-dodecylamine, n-tetradecylamine, n-hexadecylamine and trypan blue were purchased from Sigma-Aldrich Quimica SA. Middlebrook 7H10 agar was purchased from Difco (BD Difco, Franklin Lakes, NJ, USA). Microwell tissue culture plates were purchased from Nunc. All amides presented were synthesized according to the procedures described in this paper. Compounds were prepared in stock solutions of 40 mg/ml in dimethyl sulfoxide (DMSO – AppliChem Panreac). Isoniazid (Sigma-Aldrich) is a first line antibiotic against tuberculosis and was used as a positive control for M. tuberculosis killing.
Bacterial strains and cell lines. Bacteria broth culture medium Middlebrook 7H9 and solid culture medium Middlebrook 7H10 were purchased from Difco (BD Difco, Franklin Lakes, NJ, USA). All Mycobacterium spp. were cultivated in Middlebrook’s 7H9 medium supplemented with 10% OADC (oleic acid, albumin, dextrose, catalase) enrichment (BD Difco, Franklin Lakes, NJ, USA), 0.02% glycerol, and 0.05% tyloxapol (Merck, KGaA) incubated at 37 °C until exponential growth phase was achieved. M. tuberculosis H37Rv (ATCC 27294), M. avium (DSM44156) and M. bovis BCG (CIP 105050) were used for MIC evaluation. M. smegmatis (ATCC607, mc2 155) was used for homogenate preparation and for MIC determination.
Synthesis. 1. Acyl chloride synthesis: A solution of the chosen benzoic acid derivative in thionyl chloride (3 mL per mmol of acid) was refluxed for 5 h, leading to the formation of the desired acyl chloride. The excess thionyl chloride was removed by low pressure evaporation. The product was used without further purification. 2. General protocol to Amide synthesis: A solution of the appropriate acyl chloride (1 eq.) in dichloromethane was added dropwise to a solution of corresponding amine and triethylamine (1.5 eq.) in dichloromethane at 0 °C. When the reaction was complete (as assessed by TLC using hexane:ethyl acetate, 5:1 to 1:1, or ethyl acetate as eluent) the reaction mixture was filtered and the filtrate washed successively with 10 mL of distilled water and with 15 mL of saturated sodium bicarbonate solution. The dichloromethane solution was subsequently dried, and the solvent evaporated. The residue was purified by column chromatography (silica gel 60) using hexane: ethyl acetate, 5:1 to 1:1, or ethyl acetate as eluent. All compounds were characterized by 13C NMR, 1H NMR, IR and HRMS. The purity of the compounds was further tested by HPLC and TLC. Synthesis specifications, yields and structural data for compounds 11, 12 and 18 is described below. Data for the rest of the compounds is available in the Supplementary Material.
Synthesis of N-octyl-3,5-dinitrobenzamide (11). Following the described general procedure, 6 mmol (0,837 mL) of 3,5-dinitrobenzoyl chloride were dissolved in DCM (2,5 mL) and added to a solution in DCM (2,5 mL) of 9 mmol (1,480 mL) of n-octylamine and 6 mmol (0,833 mL) of triethylamine. N-octyl-3,5-dinitrobenzamide - yellow solid; Yield 43%; 1H NMR (300 MHz, Chloroform-d) δ 9.18 (t, J = 2.1 Hz, 1H), 8.95 (d, J = 2.1 Hz, 2H), 6.37 (s, 1H), 3.54 (td, J = 7.3, 5.7 Hz, 2H), 1.79 – 1.61 (m, 2H), 1.50 – 1.19 (m, 10H), 0.90 (t, J = 7.3 Hz, 3H). 13C RMN (300 MHz, Chloroform-d) δ 162.79 (C7), 120.95 (C4), 138.20 (C1), 127.17 (C2 and C6), 148.64 (C3 and C5), 40.89 (C9), 31.76 (C10), 29.44 29.23 29.16 26.98 (C11-C15), 14.05 (C16). Infra-red (IR) - (n, cm-1) - 1720,43 (C=O). HRMS (ESI+): m/z calculated for C15H21N3O5: 323.34446, found: 324.1562 (M+H+). Purity by HPLC 99,5%.
Synthesis of N-decyl-3,5-dinitrobenzamide (12). Following the described general procedure, 6 mmol (0,837 mL) of 3,5-dinitrobenzoyl chloride were dissolved in DCM (2,5 mL) and added to a solution in DCM (2,5 mL) of 9 mmol (1,776 mL) of n-decylamine and 6 mmol (0,833 mL) of triethylamine. N-decyl-3,5-dinitrobenzamide - yellow solid; Yield 41%; 1H NMR (300 MHz, Chloroform-d) δ 9.17 (t, J = 2.0 Hz, 1H), 8.96 (d, J = 2.1 Hz, 2H), 6.47 (s, 1H), 3.54 (td, J = 7.3, 5.7 Hz, 2H), 1.78 – 1.63 (m, 2H), 1.49 – 1.18 (m, 14H), 0.89 (t, J = 7.3 Hz, 3H). 13C RMN (300 MHz, Chloroform-d) δ 162.79 (C7), 120.94 (C4), 138.20 (C1), 127.18 (C2 and C6), 148.64 (C3 and C5), 40.90 (C9), 31.86 (C10), 29.52 29.44 29.28 26.98 22.65 (C11-C17), 14.08 (C18). Infra-red (IR) - (n, cm-1) - 1720,43 (C=O). HRMS (ESI+): m/z calculated for C17H25N3O5: 351.39762, found: 352.1869 (M+H+). Purity by HPLC 99,5%.
Synthesis of N-octyl-3-nitro-5-(trifluoromethyl)benzamide (18). Following the described general procedure, 6 mmol (0,967 mL) of 3-nitro-5-(trifluoromethyl)benzoyl chloride were dissolved in DCM (2,5 mL) and added to a solution in DCM (2,5 mL) of 9 mmol (1,480 mL) of n-octylamine and 6 mmol (0,833 mL) of triethylamine. N-octyl-3-nitro-5-(trifluoromethyl)benzamide - yellow solid; Yield 35%; 1H NMR (300 MHz, Chloroform-d) δ 8.76 (br. s, 1H), 8.60 (br. s, 1H), 8.41 (br. s, 1H), 6.40 (s, 1H), 3.50 (td, J = 7.3, 5.7 Hz, 2H), 1.75 – 1.55 (m, 2H), 1.48 – 1.16 (m, 10H), 0.88 (t, J = 7.3 Hz, 3H). 13C RMN (300 MHz, Chloroform-d) δ 163.77 (C7), 120.64 (C4), 137.63 (C1), 132.90 (q, JCF = 34.7 Hz, C5), 124.75 (C6), 129.97 (C2), 123.04 (CF3), 148.36 (C3), 40.77 (C9), 31.76 (C10), 29.44 29.23 29.16 26.97 22.60 (C11-С15), 14.03 (C16). Infra-red (IR) - (n, cm-1) - 1720,43 (C=O). HRMS (ESI+): m/z calculated for C16H21F3N2O3: 346.34482, found: 347.1496 (M+H+). Purity by HPLC 98,7%.
Compound characterization. 1H NMR and 13C-NMR spectra were recorded on a Bruker Ultra-Shield 300 MHz spectrometer, in the indicated solvent; chemical shifts are reported in parts per million (ppm), relative to tetramethylsilane (TMS). The spectra were referenced to the solvent peak and coupling constants (J) are quoted in hertz (Hz). The IR spectra were recorded on a 400 FTIR Nicolet Impact spectrometer between 4000 and 400 cm-1. High-resolution mass spectra (HRMS) were obtained on a Bruker Impact II quadrupole time-of-flight mass spectrometer (Bruker Daltoniks), and m/z values are reported in Daltons.
HPLC analysis: HPLC determinations were performed in a HPLC Hitachi LaChrom Ultra system comprising of two Hitachi L-2160U pumps, a Hitachi UV-L-2400U detector, a Hitachi L-2200U auto sampler, a Hitachi L-2300 column oven, a Merck-Hitachi D-2500 integrator and a Merck RP-8 column. The eluant was a mixture of acetonitrile (60% to 80%) and aqueous phosphate buffer with 5% of KH2PO4/H3PO4 0.025M (40% to 20%). The flow rate was 1 mL min-1 and the wavelength was set to 230 nm. All quantifications were evaluated using calibration curves from stock solutions. Purity by HPLC was determined using a Merck RP-8 column % 50% ACN in water for compounds 1, 2, 3, 5, 7, 9, 10, 11, 16, 17, 18 and 70% acetonitrile in water for the other compounds.
Mycobacterial homogenate preparation: A crude whole mycobacterial homogenate was prepared according to reference [
21]. A culture of exponentially growing
M. smegmatis ATCC607 variant mc
2 155 with an O.D. 600 nm of 0.8–1.0 was harvested by centrifugation at T = 4 °C for 10 min, washed and re-suspended in pH = 7.4 phosphate buffer saline PBS (25 mL for each 750 mL of the initial growing broth). The bacterial homogenate was prepared using an ultrasound probe with a sequence of five cycles of 2 min each. The homogenate was afterwards divided in 1 mL portions and kept at −80 °C until use. Total protein concentration was 1.4 mg mL
−1.
Stability studies. Conditions of incubations and preparation of samples: In all stability assays, the initial concentration of the compound under study was 5 × 10−4 M. All incubations were carried out at pH 7.4 and 37 °C under agitation using PBS as diluting agent. The benzamides were added from 2.5 × 10−2 M acetonitrile stock solutions. After incubation, aliquots of 50 μL were taken into vials processed as described below, injected into the HPLC and analysed for quantification of the corresponding acid and remaining benzamide. All quantifications were performed using calibration curves. Stability in buffer. 1600 µL pH 7.4 phosphate buffer saline PBS, 360 µL ACN and 40 µL a stock solution of the compound in ACN were mixed in a 2 mL vial. The solution was incubated at 37 °C and 50 µL aliquots were removed, mixed with 450 µL of ACN:H2O 1:1 and analysed by HPLC. Plasma stability. Pooled human plasma (640 µL), pH 7.4 phosphate buffered saline (160 µL) and 16 µL of a 2.5 × 10−2 M stock solution of the compound in ACN were mixed in a vial. The suspensions were incubated at 37 °C and 50 µL aliquots were removed, mixed with 450 µL of ACN:ZnSO4 1% 1:1 and centrifuged for 10 min at 15,000 rpm. The supernatant was then removed and analysed by HPLC. Homogenate stability. 948 µL pH 7.4 phosphate buffer, 32 µL of M. smegmatis homogenate and 20 µL of a 2.5 × 10−2 M stock solution of the compound in ACN were mixed in a vial. The solution was incubated at 37 °C and 50 µL aliquots were removed, mixed with 450 µL of ACN:H2O 1:1 and centrifuged for 10 min at 15,000 rpm. The supernatant was then removed and analysed by HPLC.
Determination of the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentrations (MBC): The MICs were determined by the broth microdilution method in 96-well plates. Briefly,
M. tuberculosis bacterial cultures in exponential growth phase were collected by centrifugation, washed with PBS and re-suspended in fresh culture medium. Clumps of bacteria were removed by ultrasonic treatment of the bacteria suspension in an ultrasonic water bath for 5 min followed by a low-speed centrifugation (500 x g) for 2 min. Single cell suspension was verified by microscopy. The microplates containing a bacterial suspension corresponding to approximately 10
5 colony-forming units per ml were incubated with the selected concentrations of the compounds. Every other day, the optical density of the wells was measured in a Tecan M200 spectrophotometer, following 30 s of orbital agitation. These values were used to produce the growth curves. At the 10th day of incubation, the MIC was determined, corresponding to the concentration with no visible turbidity. Optical density measurements were taken until the 15th day of incubation. The MBC values were determined using an established methodology [
44]. Briefly, following MIC determination, the bacterial samples were recovered from the MIC test microplates and plated in 7H10 + OADC solid medium. The MBC was determined following 3 weeks of incubation, corresponding to the concentration of compound that produced no colonies on the solid medium. Bacteria treated with DMSO solvent at the same proportions as present during the compound tests were used as a control. Isoniazid was used as a positive control for bacteria killing and assay validation following EUCAST guidelines (MIC = [0.03, 0.12] μg/mL). All MIC and MBC results presented comprise the mode value of a minimum of triplicate experiments.
Determination of macrophage viability after treatment with compounds: THP-1 Human monocytic cell line THP-1 (ATCC TIB202) was used to determine the effect of the compounds on cell viability. The cells were grown in RPMI 1640 (BD Difco, Franklin Lakes, NJ, USA) supplemented with 10 % fetal bovine serum (FBS; BD Difco, Franklin Lakes, NJ, USA), 10 mM HEPES (BD Difco, Franklin Lakes, NJ, USA), 1 mM sodium pyruvate, and maintained at 37 ºC with 5 % CO2. Differentiation of THP-1 monocytes into macrophages was induced for 48 h with 20 nM phorbol 12-myristate 13-acetate (PMA) following a 24 h resting period without PMA. Differentiated macrophages in 96-well plates, 5 × 104 cells per well, were treated with the compounds. After three days of treatment, cell viability was determined using PrestoBlue (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s indications. Briefly, the cells were washed with PBS and incubated with PrestoBlue 10 % (v/v) in cell culture medium. After 4 h of incubation the fluorescence of each well was measured in a Tecan M200 spectrophotometer (Em: 560 nm/Ex: 590 nm). Viability was calculated relatively to non-treated cells. Cells treated with DMSO solvent at the same proportions were used as a control. Puromycin was used as a positive control for cell death.
Macrophage Infection and intracellular bacteria killing following compounds treatment: Before infection, M. tuberculosis was cultivated for seven days at 37 °C and 5% CO2 until the exponential growth phase was reached. Bacterial suspensions were centrifuged and washed in phosphate-buffered saline (PBS) and resuspended in macrophage culture medium without antibiotics. Clumps of bacteria in the suspension were disrupted using an ultrasonic bath treatment for 5 min and removed using centrifugation at a low speed of 500× g for 1 min. The obtained single-cell suspension was verified through fluorescence microscopy and quantified by measuring the optical density at 600 nm. Then, the infection was performed with a multiplicity of infection (MOI) of 1 bacterium per macrophage for 3 h at 37 °C and 5% CO2. Following this incubation period, the cells were washed with PBS and the compounds (0.15 μg/mL) were added in fresh, complete medium. DMSO solvent was used as a negative control and isoniazid (0.15 μg/mL) was used as a positive control for bacteria killing. At the specified time-points, macrophages were disrupted using 0.05% Igepal and the resulting bacterial suspension was serial-diluted and plated in 7H10 OADC agar plates. Micro-colonies were counted following approximately 2 weeks of incubation at 37 °C.
Molecular docking: 3D models of the compounds under study were built and optimized (MMFF94forcefield) using the open-source cheminformatics toolkit RDK [
45]. Meeko library (
https://github.com/forlilab/Meeko) was used to obtain the corresponding PDBQT files, allowing all available torsions to rotate freely. The receptor structure was retrieved from the RCSB Protein Data Bank entry 4P8L (PDB: 4P8L), correspondent to the crystal structure of
M. tuberculosis DprE1 in complex with the non-covalent inhibitor Ty36c, and only the atoms pertaining to the chain A of the protein were considered. A PDBQT file of the receptor was obtained using AutoDockTools [
46], keeping only the polar hydrogen atoms. Molecular docking simulations were performed using AutoDock Vina 1.2.5 [
47,
48]. Two search spaces of 22 Å
3 and 30 Å
3 were used, centered on the position of the ligand from the crystallographic structure. For each compound, 5 independent docking runs were performed to increase sampling, using 3 scoring functions for pose selection: Vina, Vinardo and AutoDock. All poses were then sorted according to each of the scoring functions, selecting the lowest-energy conformations for each docking run and each compound. The coordinates of the 5 top poses for each compound were further used to calculate the distance from the N atom of their nitro groups and the center of their aromatic ring moiety to two atoms of the receptor: the S atom in the cysteine 387 residue and the N atom in the center portion of the isoalloxazine ring of the FAD cofactor that is in the binding pocket region (
Figure 4B).