Submitted:
17 April 2024
Posted:
17 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Obtaining Cellulosic and Active Fractions from RS
2.3. Film Preparation
2.3.1. TPS Monolayers
2.3.2. PLA Monolayers
2.3.3. Bilayer Preparation
2.4. Characterisation of the Films
2.4.1. Optical Properties
2.4.2. Tensile Properties
2.4.3. Barrier Properties
2.4.4. Bioactive Properties
2.5. Composting Properties of the Films
2.5.1. Compost Conditioning and Preparation of Synthetic Solid Residue
2.5.6. Disintegration Test
2.5.7. Biodegradation Test
2.6. Statistical Analysis
3. Results and Discussion
3.1. Changes in Physical Properties of the Films during Storage
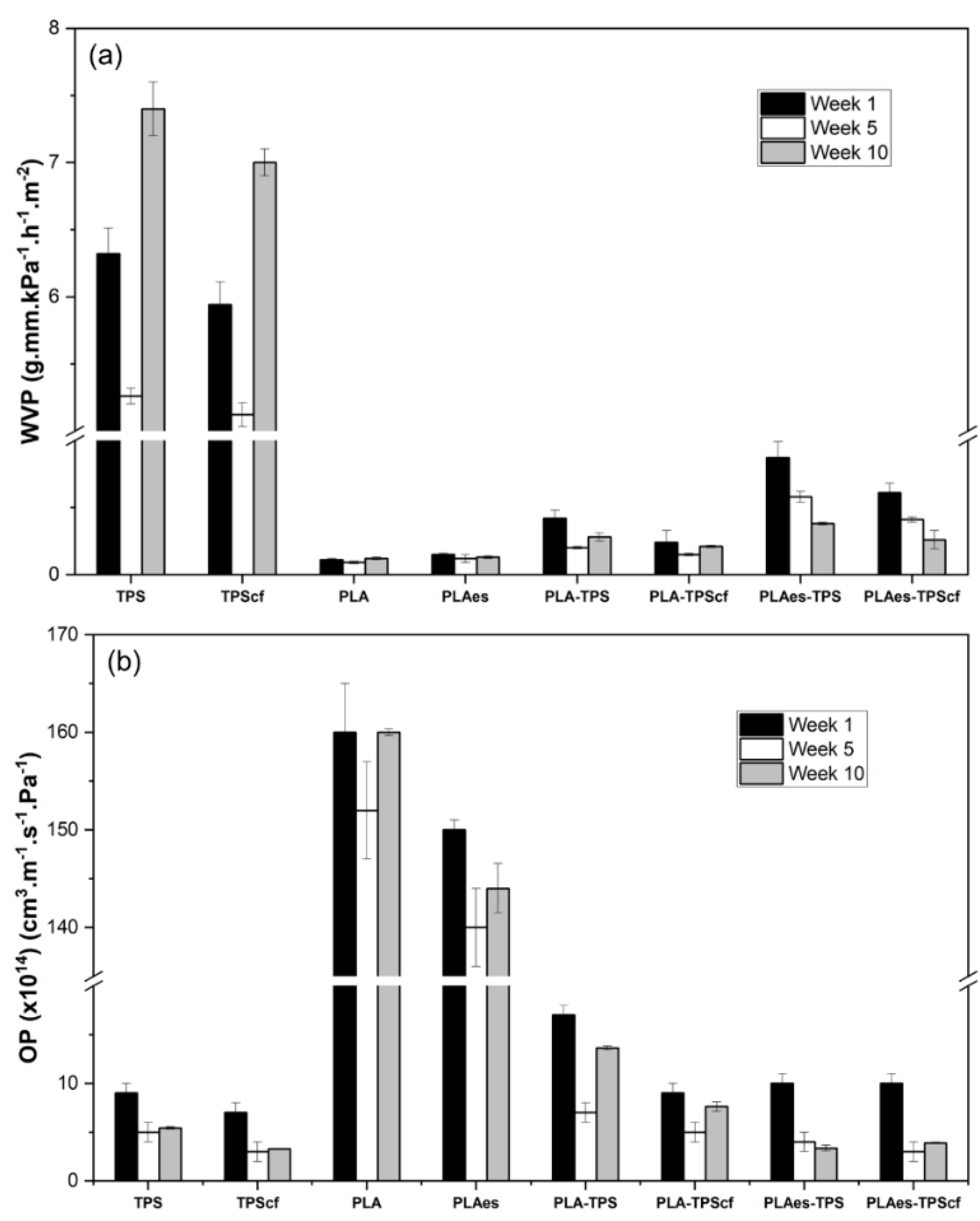
3.1.1. Barrier Properties
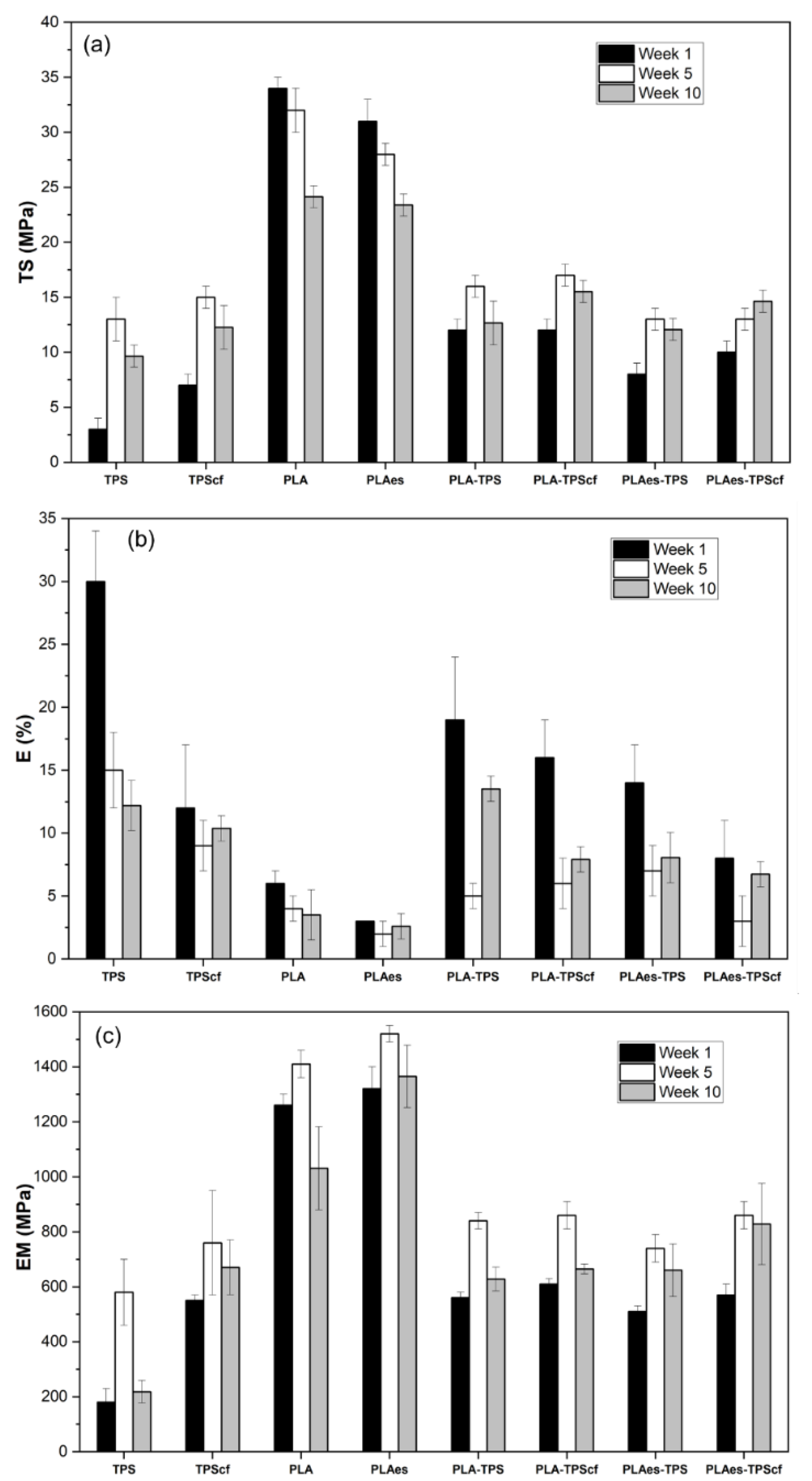
3.1.2. Tensile Properties
3.1.3. Optical Properties
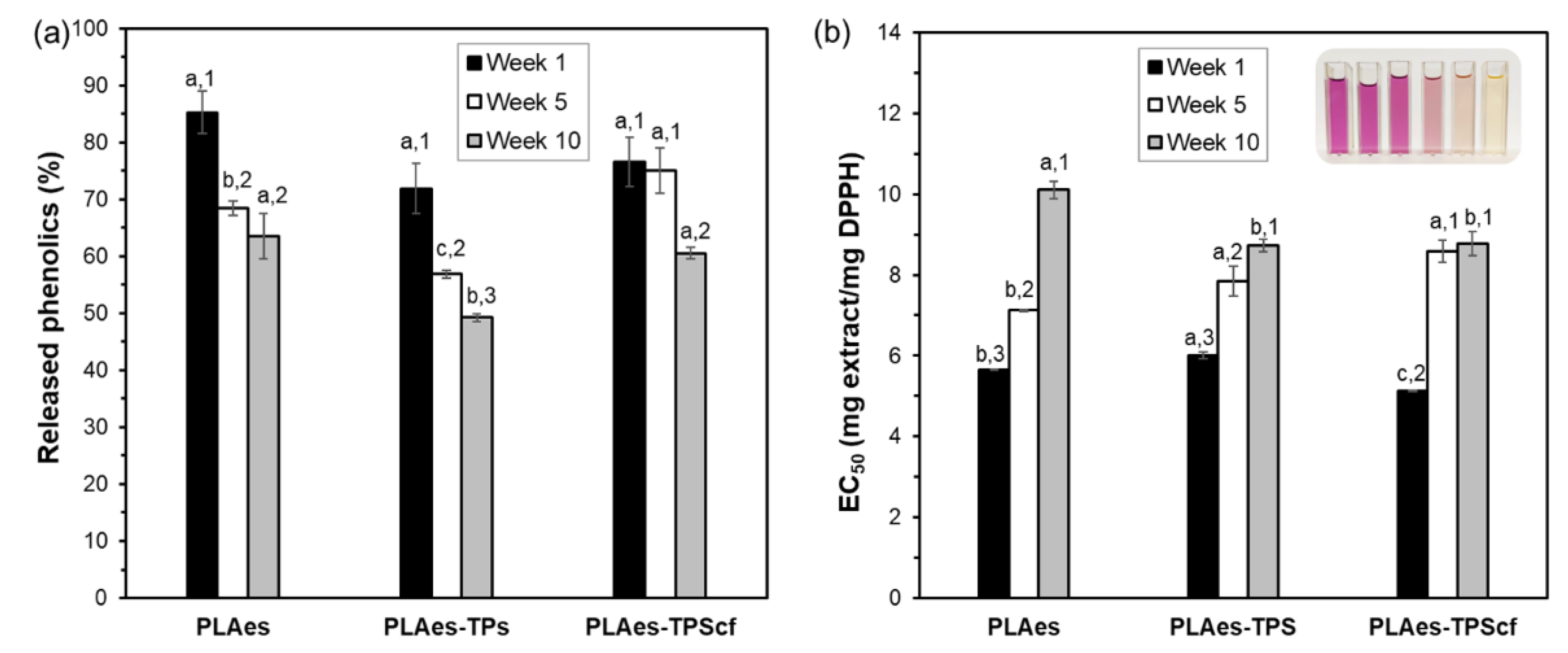
3.1.4. Bioactive Properties
3.2. Biodegradability of the Mono and Bilayer Films
3.2.1. Moisture Content, Thicknesses, and Elemental Carbon of the Films
3.2.2. Compost Characteristics
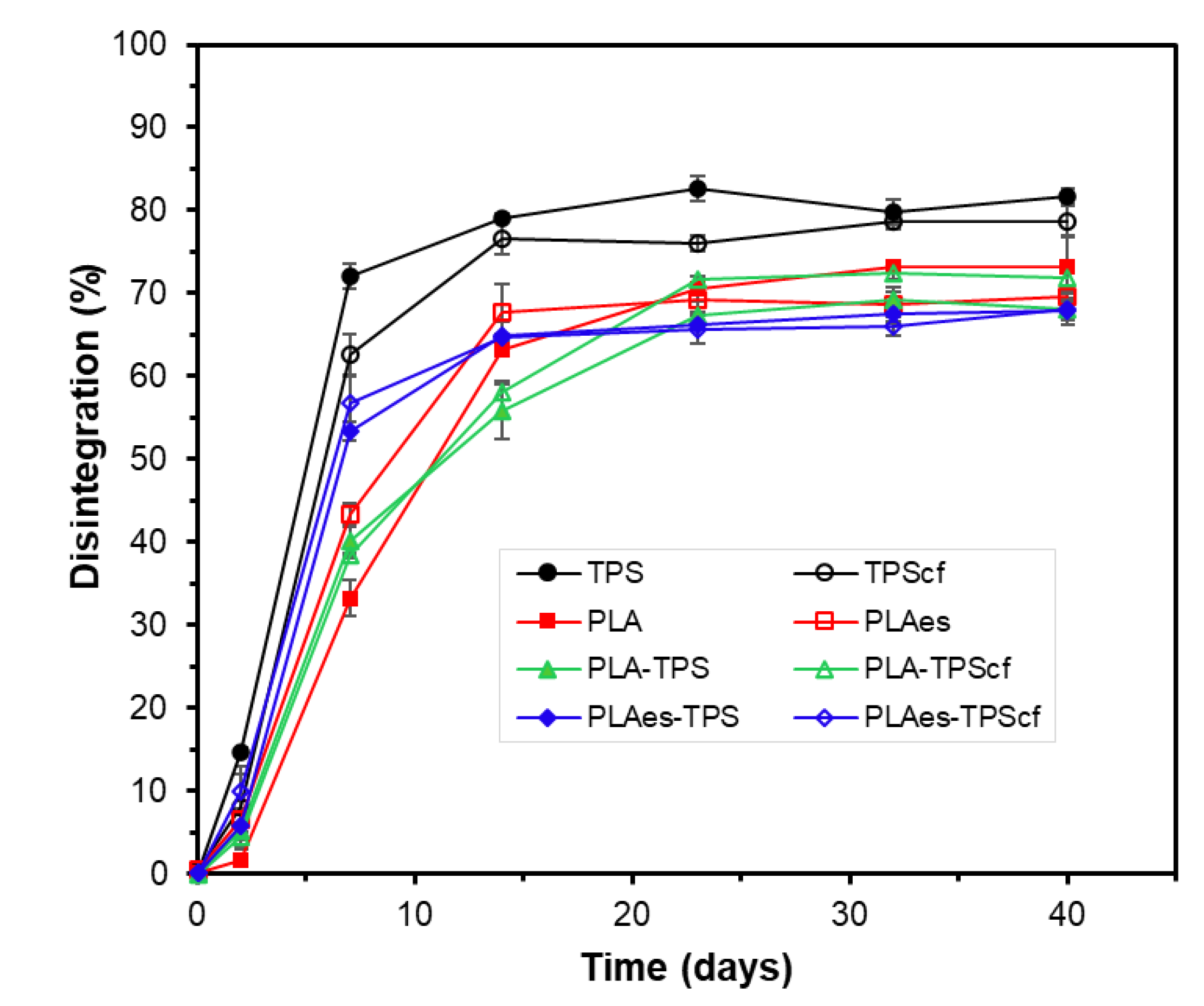
3.2.3. Disintegration Test
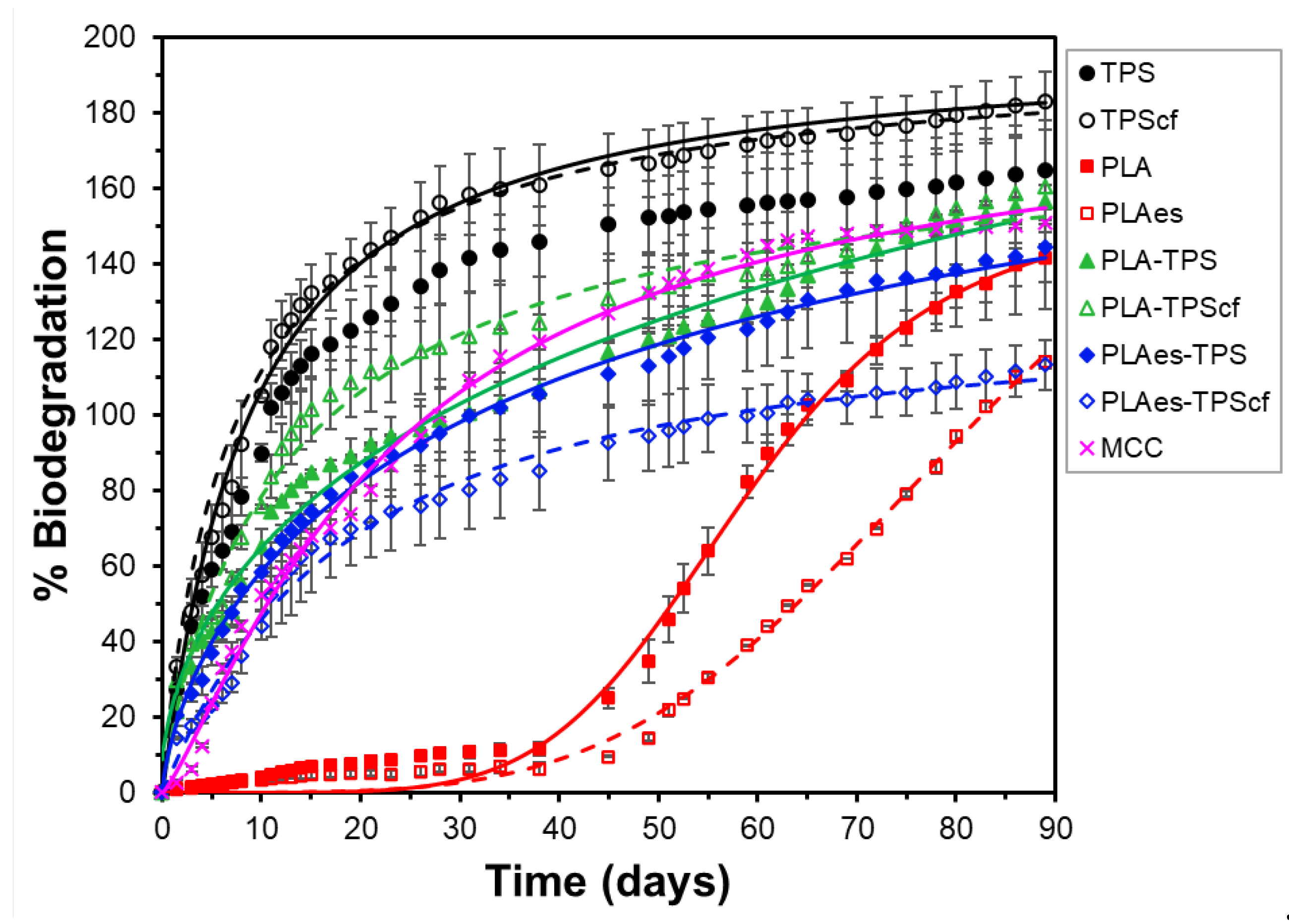
3.2.4. Biodegradation Test
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shlush, E.; Davidovich-Pinhas, M. Bioplastics for Food Packaging. Trends in Food Science & Technology 2022, 125, 66–80. [Google Scholar] [CrossRef]
- Thakur, S.; Chaudhary, J.; Sharma, B.; Verma, A.; Tamulevicius, S.; Thakur, V.K. Sustainability of Bioplastics: Opportunities and Challenges. Current Opinion in Green and Sustainable Chemistry 2018, 13, 68–75. [Google Scholar] [CrossRef]
- Barnes, D.K.A.; Galgani, F.; Thompson, R.C.; Barlaz, M. Accumulation and Fragmentation of Plastic Debris in Global Environments. Phil. Trans. R. Soc. B 2009, 364, 1985–1998. [Google Scholar] [CrossRef]
- Sun, J.; Peng, Z.; Zhu, Z.-R.; Fu, W.; Dai, X.; Ni, B.-J. The Atmospheric Microplastics Deposition Contributes to Microplastic Pollution in Urban Waters. Water Research 2022, 225, 119116. [Google Scholar] [CrossRef]
- Ivar Do Sul, J.A.; Costa, M.F. The Present and Future of Microplastic Pollution in the Marine Environment. Environmental Pollution 2014, 185, 352–364. [Google Scholar] [CrossRef] [PubMed]
- Andrady, A.L.; Neal, M.A. Applications and Societal Benefits of Plastics. Phil. Trans. R. Soc. B 2009, 364, 1977–1984. [Google Scholar] [CrossRef]
- Peanparkdee, M.; Iwamoto, S. Bioactive Compounds from By-Products of Rice Cultivation and Rice Processing: Extraction and Application in the Food and Pharmaceutical Industries. Trends in Food Science & Technology 2019, 86, 109–117. [Google Scholar] [CrossRef]
- Singh, L.; Brar, B.S. A Review on Rice Straw Management Strategies. NEPT 2021, 20. [Google Scholar] [CrossRef]
- Sadh, P.K.; Chawla, P.; Bhandari, L.; Duhan, J.S. Bio-Enrichment of Functional Properties of Peanut Oil Cakes by Solid State Fermentation Using Aspergillus Oryzae. Food Measure 2018, 12, 622–633. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Application of Ultrasound Pre-Treatment for Enhancing Extraction of Bioactive Compounds from Rice Straw. Foods 2020, 9, 1657. [Google Scholar] [CrossRef]
- Menzel, C.; González-Martínez, C.; Vilaplana, F.; Diretto, G.; Chiralt, A. Incorporation of Natural Antioxidants from Rice Straw into Renewable Starch Films. International Journal of Biological Macromolecules 2020, 146, 976–986. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Active Poly (Lactic Acid) Films with Rice Straw Aqueous Extracts for Meat Preservation Purposes. Food Bioprocess Technol 2023. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Arias, C.I.L.F.; Torres-Giner, S.; González-Martínez, C.; Chiralt, A. Valorization of Rice Straw into Cellulose Microfibers for the Reinforcement of Thermoplastic Corn Starch Films. Applied Sciences 2021, 11, 8433. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Influence of the Cellulose Purification Process on the Properties of Aerogels Obtained from Rice Straw. Carbohydrate Polymers 2023, 312, 120805. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Santana, L.G.; González-Martínez, C.; Chiralt, A. Combining Subcritical Water Extraction and Bleaching with Hydrogen Peroxide to Obtain Cellulose Fibres from Rice Straw. Carbohydrate Polymer Technologies and Applications 2024, 7, 100491. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Applying Ultrasound-Assisted Processing to Obtain Cellulose Fibres from Rice Straw to Be Used as Reinforcing Agents. Innovative Food Science & Emerging Technologies 2022, 76, 102932. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Using Rice Straw Fractions to Develop Reinforced, Active PLA-Starch Bilayers for Meat Preservation. Food Chemistry 2023, 405, 134990. [Google Scholar] [CrossRef] [PubMed]
- Freitas, P.A.V.; González-Martínez, C.; Chiralt, A. Antioxidant Starch Composite Films Containing Rice Straw Extract and Cellulose Fibres. Food Chemistry 2023, 400, 134073. [Google Scholar] [CrossRef]
- Baghi, F.; Gharsallaoui, A.; Dumas, E.; Ghnimi, S. Advancements in Biodegradable Active Films for Food Packaging: Effects of Nano/Microcapsule Incorporation. Foods 2022, 11, 760. [Google Scholar] [CrossRef]
- Kaiser, K.; Schmid, M.; Schlummer, M. Recycling of Polymer-Based Multilayer Packaging: A Review. Recycling 2017, 3, 1. [Google Scholar] [CrossRef]
- Anukiruthika, T.; Sethupathy, P.; Wilson, A.; Kashampur, K.; Moses, J.A.; Anandharamakrishnan, C. Multilayer Packaging: Advances in Preparation Techniques and Emerging Food Applications. Comprehensive Reviews in Food Science and Food Safety 2020, 19, 1156–1186. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Chen, W.; Zhu, W.; McClements, D.J.; Liu, X.; Liu, F. A Review of Multilayer and Composite Films and Coatings for Active Biodegradable Packaging. npj Sci Food 2022, 6, 18. [Google Scholar] [CrossRef]
- Galdeano, M.C.; Mali, S.; Grossmann, M.V.E.; Yamashita, F.; García, M.A. Effects of Plasticizers on the Properties of Oat Starch Films. Materials Science and Engineering: C 2009, 29, 532–538. [Google Scholar] [CrossRef]
- Ghanbarzadeh, B.; Almasi, H.; Entezami, A.A. Improving the Barrier and Mechanical Properties of Corn Starch-Based Edible Films: Effect of Citric Acid and Carboxymethyl Cellulose. Industrial Crops and Products 2011, 33, 229–235. [Google Scholar] [CrossRef]
- Avérous, L.; Fringant, C.; Moro, L. Plasticized Starch–Cellulose Interactions in Polysaccharide Composites. Polymer 2001, 42, 6565–6572. [Google Scholar] [CrossRef]
- Freitas, P.A.V.; Bas Gil, N.J.; González-Martínez, C.; Chiralt, A. Antioxidant Poly (Lactic Acid) Films with Rice Straw Extract for Food Packaging Applications. Food Packaging and Shelf Life 2022, 34, 101003. [Google Scholar] [CrossRef]
- Muller, J.; González-Martínez, C.; Chiralt, A. Poly(Lactic) Acid (PLA) and Starch Bilayer Films, Containing Cinnamaldehyde, Obtained by Compression Moulding. European Polymer Journal 2017, 95, 56–70. [Google Scholar] [CrossRef]
- Standard Test Method for Tensile Properties of Thin Plastic Sheeting (2).Pdf.
- ASTM. (2012). Standard Test Methods for Water Vapor Transmission of Materials. ASTM D882–12. American Society for Testing and Materials, 12. ASTM. (2012). Standard Test Methods for Water Vapor Transmission of Materials. ASTM D882–12. American Society for Testing and Materials, 12.
- ASTM. (2010). Standard Test Method for Oxygen Gas Transmission Rate Through Plastic Film and Sheeting Using s Coulometric Sensor. D3985-025. Annual Book of ASTM Standards, C, 1–7. [CrossRef]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a Free Radical Method to Evaluate Antioxidant Activity. LWT - Food Science and Technology 1995, 28, 25–30. [Google Scholar] [CrossRef]
- ISO 20200, Plastics - Determination of the Degree of Disintegration of Plastic Materials under Simulated Composting in a Laboratory-Scale Test, 2004.
- UNE-EN ISO 14885-1. Determinación de la biodegradabilidad aeróbica final de materiales plásticos en condiciones de compostaje controladas. Meétodo según el análisis de dióxido de carbono generado, 2013.
- Cano, A.I.; Cháfer, M.; Chiralt, A.; González-Martínez, C. Biodegradation Behavior of Starch-PVA Films as Affected by the Incorporation of Different Antimicrobials. Polymer Degradation and Stability 2016, 132, 11–20. [Google Scholar] [CrossRef]
- Hosseini, S.F.; Javidi, Z.; Rezaei, M. Efficient Gas Barrier Properties of Multi-Layer Films Based on Poly(Lactic Acid) and Fish Gelatin. International Journal of Biological Macromolecules 2016, 92, 1205–1214. [Google Scholar] [CrossRef]
- Siracusa, V. Food Packaging Permeability Behaviour: A Report. International Journal of Polymer Science 2012, 2012, 1–11. [Google Scholar] [CrossRef]
- Wang, S.; Li, C.; Copeland, L.; Niu, Q.; Wang, S. Starch Retrogradation: A Comprehensive Review. Comp Rev Food Sci Food Safe 2015, 14, 568–585. [Google Scholar] [CrossRef]
- Ke, T.; Sun, X. Melting Behavior and Crystallization Kinetics of Starch and Poly(Lactic Acid) Composites. J of Applied Polymer Sci 2003, 89, 1203–1210. [Google Scholar] [CrossRef]
- Acioli-Moura, R.; Sun, X.S. Thermal Degradation and Physical Aging of Poly(Lactic Acid) and Its Blends with Starch. Polymer Engineering & Sci 2008, 48, 829–836. [Google Scholar] [CrossRef]
- Vilarinho, F.; Stanzione, M.; Buonocore, G.G.; Barbosa-Pereira, L.; Sendón, R.; Vaz, M.F.; Sanches Silva, A. Green Tea Extract and Nanocellulose Embedded into Polylactic Acid Film: Properties and Efficiency on Retarding the Lipid Oxidation of a Model Fatty Food. Food Packaging and Shelf Life 2021, 27, 100609. [Google Scholar] [CrossRef]
- Collazo-Bigliardi, S.; Ortega-Toro, R.; Chiralt, A. Improving Properties of Thermoplastic Starch Films by Incorporating Active Extracts and Cellulose Fibres Isolated from Rice or Coffee Husk. Food Packaging and Shelf Life 2019, 22, 100383. [Google Scholar] [CrossRef]
- Fourati, Y.; Magnin, A.; Putaux, J.-L.; Boufi, S. One-Step Processing of Plasticized Starch/Cellulose Nanofibrils Nanocomposites via Twin-Screw Extrusion of Starch and Cellulose Fibers. Carbohydrate Polymers 2020, 229, 115554. [Google Scholar] [CrossRef] [PubMed]
- Andrade, J.; González-Martínez, C.; Chiralt, A. Antimicrobial PLA-PVA Multilayer Films Containing Phenolic Compounds. Food Chemistry 2022, 375, 131861. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Progress in Polymer Science 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Sapper, M.; Talens, P.; Chiralt, A. Improving Functional Properties of Cassava Starch-Based Films by Incorporating Xanthan, Gellan, or Pullulan Gums. International Journal of Polymer Science.
- Hernández-García, E.; Vargas, M.; Chiralt, A. Thermoprocessed Starch-Polyester Bilayer Films as Affected by the Addition of Gellan or Xanthan Gum. Food Hydrocolloids 2021, 113, 106509. [Google Scholar] [CrossRef]
- Benito-González, I.; López-Rubio, A.; Martínez-Sanz, M. High-Performance Starch Biocomposites with Celullose from Waste Biomass: Film Properties and Retrogradation Behaviour. Carbohydrate Polymers 2019, 216, 180–188. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Bi, X.; Huang, W.; Wu, J.; Hu, X.; Liao, X. Changes of Quality of High Hydrostatic Pressure Processed Cloudy and Clear Strawberry Juices during Storage. Innovative Food Science & Emerging Technologies 2012, 16, 181–190. [Google Scholar] [CrossRef]
- Tonon, R.V.; Brabet, C.; Hubinger, M.D. Anthocyanin Stability and Antioxidant Activity of Spray-Dried Açai (Euterpe Oleracea Mart.) Juice Produced with Different Carrier Agents. Food Research International 2010, 43, 907–914. [Google Scholar] [CrossRef]
- Tampau, A.; González-Martínez, C.; Chiralt, A. Biodegradability and Disintegration of Multilayer Starch Films with Electrospun PCL Fibres Encapsulating Carvacrol. Polymer Degradation and Stability 2020, 173, 109100. [Google Scholar] [CrossRef]
- Balaguer, M.P.; Villanova, J.; Cesar, G.; Gavara, R.; Hernandez-Munoz, P. Compostable Properties of Antimicrobial Bioplastics Based on Cinnamaldehyde Cross-Linked Gliadins. Chemical Engineering Journal 2015, 262, 447–455. [Google Scholar] [CrossRef]
- Silva, A.P.M.; Oliveira, A.V.; Pontes, S.M.A.; Pereira, A.L.S.; Souza Filho, M.D.S.M.; Rosa, M.F.; Azeredo, H.M.C. Mango Kernel Starch Films as Affected by Starch Nanocrystals and Cellulose Nanocrystals. Carbohydrate Polymers 2019, 211, 209–216. [Google Scholar] [CrossRef]
- Pavon, C.; Aldas, M.; López-Martínez, J.; Hernández-Fernández, J.; Arrieta, M.P. Films Based on Thermoplastic Starch Blended with Pine Resin Derivatives for Food Packaging. Foods 2021, 10, 1171. [Google Scholar] [CrossRef]
- Kale, G.; Auras, R.; Singh, S.P. Comparison of the Degradability of Poly(Lactide) Packages in Composting and Ambient Exposure Conditions. Packag Technol Sci 2007, 20, 49–70. [Google Scholar] [CrossRef]
- Chinaglia, S.; Tosin, M.; Degli-Innocenti, F. Biodegradation Rate of Biodegradable Plastics at Molecular Level. Polymer Degradation and Stability 2018, 147, 237–244. [Google Scholar] [CrossRef]
- Hernández-García, E.; Vargas, M.; Chiralt, A.; González-Martínez, C. Biodegradation of PLA-PHBV Blend Films as Affected by the Incorporation of Different Phenolic Acids. Foods 2022, 11, 243. [Google Scholar] [CrossRef]
- Arrieta, M.P.; Fortunati, E.; Dominici, F.; Rayón, E.; López, J.; Kenny, J.M. PLA-PHB/Cellulose Based Films: Mechanical, Barrier and Disintegration Properties. Polymer Degradation and Stability 2014, 107, 139–149. [Google Scholar] [CrossRef]
- Donlan, R.M. Biofilms: Microbial Life on Surfaces. Emerging Infectious Diseases 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Sheel, A.; Pant, D. Microbial Depolymerization. In; 2018; pp. 61–103.
- Shen, J.; Bartha, R. Priming Effect of Glucose Polymers in Soil-Based Biodegradation Tests. Soil Biology and Biochemistry 1997, 29, 1195–1198. [Google Scholar] [CrossRef]
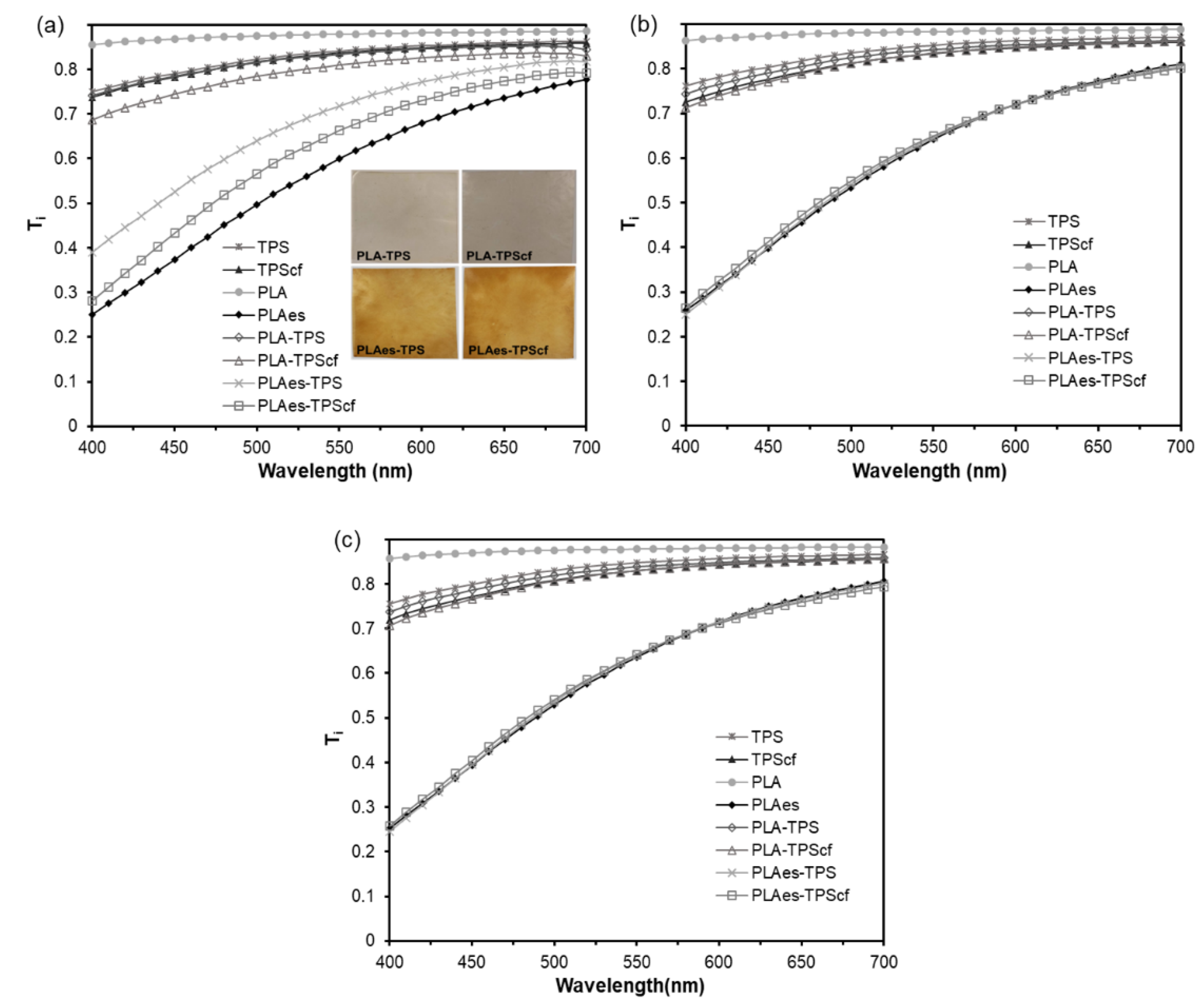

| Film | L* | Cab* | hab* | ||||||||
| S1 | S5 | S10 | S1 | S5 | S10 | S1 | S5 | S10 | |||
| PLA | 90.7 ± 0.2a,1 | 90.8 ± 0.1a,1 | 90.1 ± 0.3a,1 | 2.5 ± 0.13e,1 | 2.42 ± 0.12e,1 | 2.33 ± 0.17e,1 | 99.6 ± 0.8a,1 | 100.1 ± 0.21a,1 | 100.7 ± 0.5a,1 | ||
| PLAes | 67.6 ± 0.7f,1 | 68.3 ± 1.7f,1 | 66.2 ± 1.2f,1 | 34.57 ± 0.7a,1 | 32.85 ± 3.0a,1 | 33.72± 1.20a,1 | 77.1 ± 0.3f,1 | 79.0 ± 1.0f,1 | 78.5 ± 0.5f,1 | ||
| TPS | 88.5 ± 0.1b,1 | 89.0 ± 0.1b,1 | 87.8 ± 0.9b,1 | 7.57 ± 0.10d,1 | 7.3 ± 0.88d,1 | 7.12 ± 0.33d,1 | 92.5 ± 0.1b,1 | 94.7 ± 0.7b,1 | 95.4 ± 0.8b,1 | ||
| TPScf | 88.1 ± 0.2b,1 | 87.8 ± 0.3b,1 | 88.3 ± 0.7b,1 | 8.25 ± 0.35cd,1 | 8.7 ± 0.48cd,1 | 8.2 ± 0.28cd,1 | 92.6 ± 0.2b,1 | 92.4 ± 0.4b,1 | 92.1 ± 0.6b,1 | ||
| PLA-TPS | 87.3 ± 0.3b,1 | 88.4 ± 0.5b,1 | 87.9 ± 0.7b,1 | 7.58 ± 0.11d,1 | 7.3 ± 1.22d,1 | 7.1 ± 1.31d,1 | 92.2 ± 0.3b,1 | 95.1 ± 1.2b,1 | 93.1 ± 2.1b,1 | ||
| PLA-TPScf | 86.0 ± 0.5c,1 | 87.2 ± 0.3c,1 | 87.8 ± 0.4c,1 | 9.50 ± 0.31c,1 | 9.79 ± 0.36c,1 | 9.34 ± 0.22c,1 | 90.9 ± 0.4c,1 | 92.7 ± 0.5c,1 | 91.4 ± 0.5c,1 | ||
| PLAes-TPS | 76.9 ± 1.2d,1 | 74.5 ± 3.0d,1 | 75.4 ± 1.6d,1 | 27.92 ± 2.06b,1 | 25.83 ± 2.98b,1 | 25.14 ± 1.28b,1 | 83.4 ± 0.7d,1 | 80.4 ± 1.6d,1 | 80.7 ± 0.7d,1 | ||
| PLAes-TPScf | 72.2 ± 0.9e,1 | 72.0 ± 2.0e,1 | 73.1 ± 1.1e,1 | 34.41 ± 1.22a,1 | 34.32 ± 2.68a,1 | 34.24 ± 2.47a,1 | 81.0 ± 0.6e,1 | 81.0 ± 1.4e,1 | 80.5 ± 0.2e,1 | ||
| Sample | Moisture content (%) | Thickness (µm) | C (%) |
|---|---|---|---|
| TPS | 8.3 ± 0.2a | 0.171 ± 0.026bc | 40.3 ± 0.1 |
| TPScf | 8.0 ± 0.6a | 0.185 ± 0.025b | 40.2 ± 0.2 |
| PLA | 0.7 ± 0.1c | 0.146 ± 0.008c | 48.7 ± 0.1 |
| PLAes | 0.9 ± 0.1c | 0.144 ± 0.007c | 49.0 ± 0.5 |
| PLA-TPS | 6.9 ± 0.3b | 0.278 ± 0.033a | 45.0 ± 0.4 |
| PLA-TPScf | 6.7 ± 0.4b | 0.273 ± 0.012a | 43.8 ± 0.1 |
| PLAes-TPS | 6.7 ± 0.3b | 0.269 ± 0.013a | 44.7 ± 0.1 |
| PLAes-TPScf | 6.6 ± 0.1b | 0.262 ± 0.007a | 44.8 ± 0.2 |
| MCC | - | - | 42.2 ± 0.1 |
| Sample | VS (g.100 g-1 DS) | R (%) | Desintegration D73 (%) | |
|---|---|---|---|---|
| Pre-composting | Post-composting | |||
| SSR | 90.6 ± 1.8 | 88.3 ± 2.1 | 43.2 | - |
| TPS | - | 85.1 ± 1.2 | 48.1 | 84 ± 1 |
| TPScf | - | 83.1 ± 3.4 | 45.1 | 83 ± 1 |
| PLA | - | 82.4 ± 1.5 | 51.3 | 75 ± 1 |
| PLAes | - | 87.3 ± 2.7 | 49.2 | 80 ± 2 |
| PLA-TPS | - | 85.4 ± 1.5 | 48.2 | 75 ± 2 |
| PLA-TPScf | - | 83.4 ± 2.7 | 53.2 | 74 ± 3 |
| PLAes-TPS | - | 80.4 ± 2.4 | 47.6 | 70 ± 2 |
| PLAes-TPScf | - | 83.4 ± 1.2 | 49.2 | 71 ± 3 |
| Sample | n | k (days) | Bmax (%) | R2 |
|---|---|---|---|---|
| MCC | 1.2 | 23.6 | 185 | 0.995 |
| TPS | 1.0 | 20.1 | 198 | 0.994 |
| TPScf | 0.9 | 22.3 | 196 | 0.996 |
| PLA | 5.6 | 58.5 | 155 | 0.992 |
| PLAes | 4.2 | 84.0 | 125 | 0.992 |
| PLA-TPS | 0.4 | 35.2 | 163 | 0.998 |
| PLA-TPScf | 0.9 | 38.4 | 172 | 0.985 |
| PLAes-TPS | 0.7 | 45.8 | 147 | 0.996 |
| PLAes-TPScf | 1.0 | 39.4 | 129 | 0.990 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





