1. Introduction
To explore the factors that determine the structure and composition of biological communities has been one of the important themes of community ecology [
1,
2,
3]. This kind of approach is also vital for the conservation of biodiversity [
4]. In particular, conservation of living organisms is often practiced at the level of local ecosystems, and in this respect, exploring the determinants of community structure at the local habitat level is very important not only for understanding the formation of local communities, but also for promoting biodiversity conservation in the area [
5,
6].
In butterfly communities, many studies have been conducted and discussed to date on the determinants of the community structure and composition at the relatively broad geographic level [
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18,
19,
20,
21]. From the results of these studies, it is generally accepted that climate change and habitat alteration are two key determinants of butterfly community structure and composition at the relatively broad geographic level [
7,
9,
11,
12,
13,
14,
18,
20]. Meanwhile, at the local habitat level, various environmental factors have been reported to determine the structure and composition of butterfly communities. For example, physical environmental conditions (including climate) have been reported as determinants of the structure and composition of local butterfly communities [
22,
23,
24,
25]. Similarly, vegetation and landscape structure [
25,
26,
27], natural or anthropogenic disturbance and management [
22,
28,
29,
30,
31], and food resources [
23,
32] have also been reported as determinants of the structure and composition of local butterfly communities. Thus, unlike the case of relatively broad geographical levels, various cases have been reported for the determinants at the local habitat level, and it is assumed that the determination and the formation process of butterfly community structure will differ depending on the situation in each region. On the other hand, few studies have clarified the relationship between local butterfly community structure and the determinants, that is, how the determinants influence and function to determine the community structure and composition. Whilst, to clarify the mechanism and function of the determinants also lead to the conservation of local butterfly communities [
33].
In the present study, we chose a grassland and woodland area at the foot of Mount Fuji in central Japan as the study site. Previous studies [
34,
35] have shown that the area has a highly diverse butterfly fauna, including several Red Listed species. Moreover, it was an area that could categorize various environmental factors (habitat types, neighbouring plant community, management status, trampling pressure, and distance from the central part of the grassland) that are thought to affect the community structure of butterflies. Therefore, it was a very suitable area for exploring the environmental factors that determine the butterfly community structure. Under these conditions, we monitored butterfly communities in the area in 2009, and attempted to analyse the butterfly community structure and composition. Our goals of this study are (1) to clarify what environmental factors are most relevant to the determination of local butterfly community structure and composition in the area, (2) to explore how these environmental factors function in determining the local butterfly community structure and composition (i.e. the mechanism of community structure and composition determination), and (3) to clarify what kind of species groups the local butterfly community is composed of along the gradients of these environmental determinants.
2. Materials and Methods
2.1. Study Area
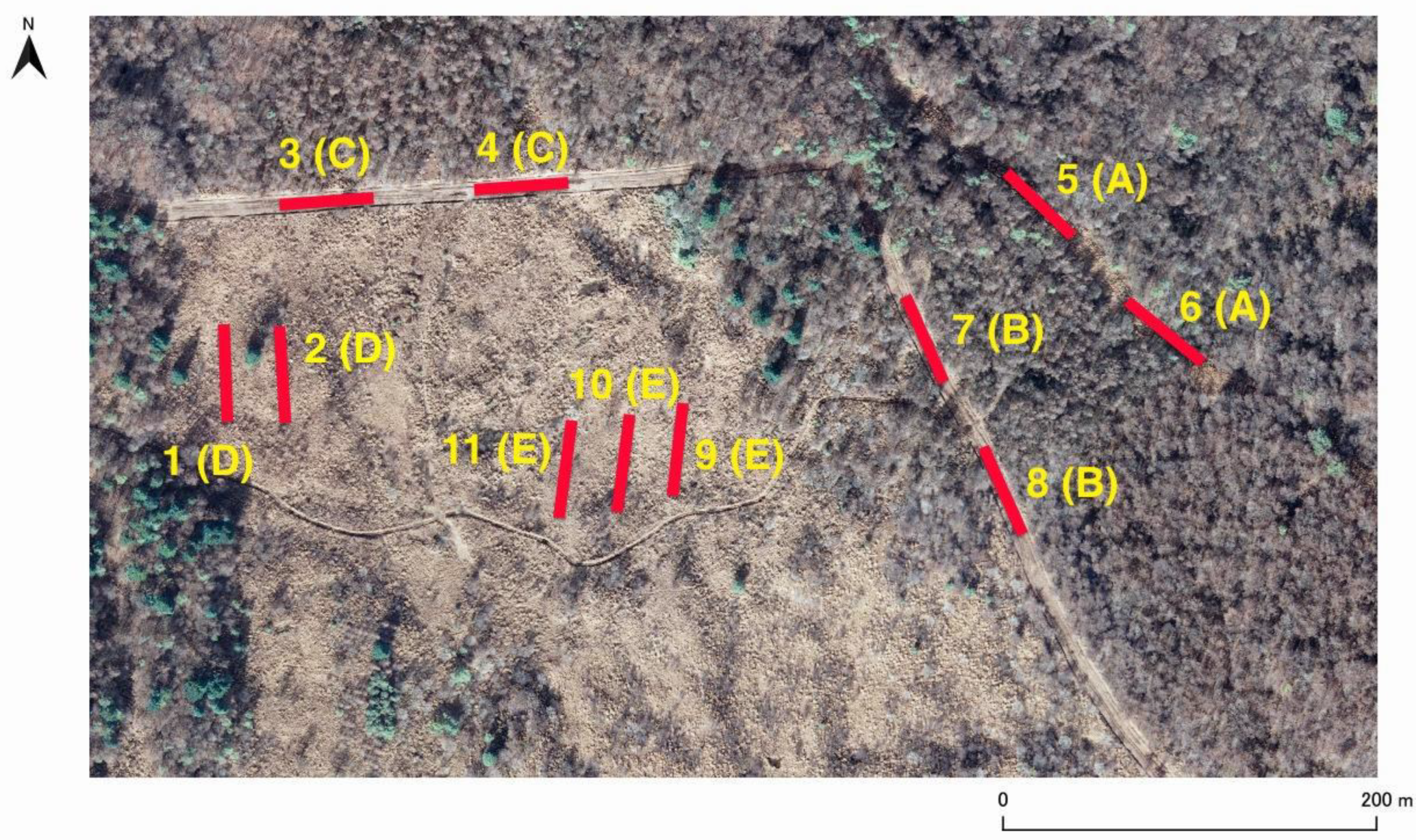
The study area was located in a grassland and woodland area (980 m a.s.l.) at the north-western foot of Mount Fuji, central Japan (35゜26’54”N, 138゜36’46”E). The terrain in this area is almost horizontal, but has irregular undulations with an elevation difference of about 8 m. The surface layer of this area is composed of scoria-like lava and volcanic ash resulting from past volcanic eruptions in the Mt. Fuji area. The study area consisted mainly of landscapes such as grasslands, forests, and firebreak belts at the edges (
Figure 1).
The grassland was used as a source of grass for fuel and forage until 60 years ago [
34]. After that, it was abandoned, but part of it became plantations. However, the afforestation was not successful, although the mowing management was carried out. This is probably due to severe weather conditions such as strong winds and extremely low temperatures in winter and severe soil conditions such as frozen soil [
36]. The management of the plantation (mowing) continued until 2005, but the plantation has been abandoned since then. The grassland at the time of this study (i.e., in 2009) was mostly dominated by poaceous grasses such as
Miscanthus sinensis,
Arundinella hirta and
Spodiopogon sibiricus. Various other herbaceous plant species were also present, including Red Listed plants such as
Tephroseris flammea,
Platanthera hologlottis, Swertia pseudochinensis and Vincetoxicum pycnostelma. Furthermore, in the grassland, several shrub trees such as
Rhamnus davurica,
Malus toringo, and
Euonymus sachalinensis were scattered due to the progress of secondary succession [
34].
The forest adjacent to the grassland consisted of a mixture of deciduous and coniferous trees and larch (
Larix kaempferi) plantations. The forest was separated from the grassland by a firebreak belt that was about 10m wide (2 km long) and was established in 1959 [
37]. Since 1961, all grasses and herbs in the firebreak belt have been mowed and removed annually in late autumn [
34].
In the study area, it was difficult to set many replicates in the same habitat type, because the area of each habitat type discriminated was small (see
Figure 1). While, it was necessary to take a 50 m transect for the survey of butterflies and to set up the transects apart from each other. As the result, we set up 2 or 3 transects in the same habitat type. The multiple transects of the same habitat type were located 20 m to 80 m away from other habitat types of transects. But all transects were located within the range of about 550 m × 250 m (
Figure 1), which is within the range of movement even for sedentary species [
38,
39].
In total, we set up 11 transects in five habitat types (A to E) (
Figure 1). Type A (two transects of Nos. 5 and 6) was a firebreak belt with mowing in the fall once a year, and surrounded on both sides by mixed forest of deciduous trees and conifers with a height of 10 m and more (treated as "forest - forest"). Type B (two transects of Nos. 7 and 8) was a firebreak belt with mowing in the fall once a year, and surrounded on one side by the mixed forest stated above and on the other side by shrubs 3-4 m high (treated as "forest - forest"). Type C (two transects of Nos. 3 and 4) was a firebreak belt with mowing in the fall once a year, and surrounded on one side by similar mixed forest to those stated above and on the other side by an abandoned grassland (treated as "grassland - forest"). Type D (two transects of Nos. 1 and 2) was an abandoned grassland that was mowed every year (1998 till 2005) up to 4 years before the present survey started (treated as "grassland - grassland"). Type E (three transects of Nos. 9, 10, and 11) was an abandoned grassland that has not been managed (mown) for several decades (treated as "grassland - grassland"). Type D and E transects were at least over 20 meters away from the edge of the nearest forest (
Figure 1).
2.2. Butterfly Survey
In the present study, we used the transect counts [
40,
41,
42], and recorded all adult butterflies observed within about 5 m on both sides and in front of each transect. The transects were visited under fine weather conditions between 10:00 and 14:00. Each transect was visited twice a month from May to September in 2009. Individuals that could not be identified immediately were captured by net, identified, and released. In the field, it is not possible to distinguish between
Pieris melete and
Pieris nesis. Therefore, these two congeneric species complex was treated as
Pieris spp. for the analysis.
2.3. Explanatory Variables
We selected five categorical environmental factors in each transect as explanatory variables that were thought to affect the butterfly community structure, such as habitat types (habitat), neighbouring plant community (nei.com), management status (manag), trampling pressure (tramp), and distance from the central part of the grassland (dist). The reasons for choosing each factor as an explanatory variable and their categorization are as follows.
1) Habitat type: since butterflies utilize species-specific dietary resources and breeding sites, different habitat types such as grasslands and forests may affect the butterfly community structure. The habitat type was divided into five categories (Rank 1: grassland where mowing continued until recently, Rank 2: grassland where mowing has not been performed for a long time, Rank 3: one side of the transect was grassland, and the other side was woodland, Rank 4: one side of the transect was shrubs, and the other side was woodland, and Rank 5: both sides of the transect were woodland). 2) Neighbouring plant community: butterflies usually depend on species-specific plants for food resources, so differences in plant communities near their habitat may affect the butterfly community structure. The neighbouring plant community was divided into three categories (Rank 1: both sides of the transect were grassland, Rank 2: one side of the transect was grassland, and the other side was woodland, and Rank 3: both sides of the transect were woodland). 3) Management status: since the presence or absence of management directly influences the condition of vegetation in habitats, which is the dietary resources for butterflies, so differences in management status may lead to differences in butterfly community structure. The management status in each transect was divided into three categories (Rank 1: mowing once a year, Rank 2: mowing stopped several years ago, and Rank 3: mowing stopped several decades ago). 4) Trampling pressure: since trampling pressure directly influences the condition of vegetation in the habitats, which is the dietary resources for butterflies, so differences in trampling pressure may lead to differences in butterfly community structure. The trampling pressure was divided into two ranks based on whether there was a path used by people along each transect (Rank 0: low trampling pressure, and Rank 1: high trampling pressure). 5) Distance from the central part of the grassland: as the distance from the centre of the grassland increases, the surrounding forest becomes closer, resulting in differences in vegetation structure and landscape structure. As a result, it is expected that the community structure of butterflies, which normally use different dietary plants and breeding sites, will be changed. The distance from the central part of the grassland was classified into 4 ranks (0, 1, 2, and 3) from the near side to the far side.
Table 1 shows the rank values of the five explanatory variables in each transect.
2.4. Data Analysis
To explore the environmental factors driving the butterfly community structure and composition, we used multivariate regression tree analysis (MRT [
43]) based on butterfly species abundance data in 11 transects (species-by-sites data set, see
Appendix A) as a response variable and the five categorical environmental variables (habitat types, neighbouring plant community, management status, trampling pressure, and distance from the central part of the grassland, see
Table 1) as explanatory variables. In the MRT settings, the one SE rule was used for pruning, and 10 groups cross validation was performed 100 times. MRT is characterized by the ability to handle multiple categorical variables as explanatory variables, and are reported to be more accurate than canonical correspondence analysis (CCA) and redundancy analysis (RDA), which are direct gradient analysis methods [
43]. When biological data are obtained from nearby transects as in the present study, there are often problems with spatial autocorrelation. However, MRT is a distribution-free and fully non-parametric data analysis tool, and can explore the relationships between multivariate response variables and explanatory variables by building a tree-like model without assuming a specified relationship or a distribution of the response variables [
44]. That is, MRT is a method that can also handle spatial autocorrelation cf. [
45]. MRT analysis was computed in the R software (version 2.12.0 [
46]) using the mvpart package [
47].
To examine the validity of the species groups discriminated by MRT analysis and the ordination and arrangement of all component species of the butterfly community, we performed principal component analysis (PCA) based on the results of the MRT analysis using the R software (version 2.12.0 [
46]) using the mvpart package [
47]. Eleven survey transects and all butterfly species observed were plotted jointly in a PCA biplot to evaluate habitat preferences of the species and identify characteristic species of the groups identified in MRT. In addition, we examined the contribution to the variance in the community of each component of the PCA.
In order to compare the utilization patterns of dietary resources of the characteristic species of the groups identified in the MRT and PCA analyses, we examined both the type of larval host plants and the species number of adult nectar source plants of them based on literature information. Fortunately, the information on larval host plants and adult nectar source plants of butterflies was well accumulated in Japan e.g. [
48,
49,
50,
51,
52,
53,
54,
55], so it was possible to analyse them. Larval host plants were divided into two types (herbaceous and woody plants) based on Unno and Aoyama [
48]. The species number of adult nectar source plants was determined based on those described in Fukuda
et al. [
49,
50,
51,
52]. The characteristic species of the butterfly groups were compared to the butterfly species on the Red List 2019 of Japan [
56] and butterfly species that corresponded to any of the Red List categories of Japan were determined. The Tukey test, which is a method of multiple comparisons of means to compare three or more groups simultaneously, was used using R to compare the utilization patterns of adult nectar plants among the characteristic species of the three groups (G1, G2 and G3) identified by MRT analysis.
3. Results
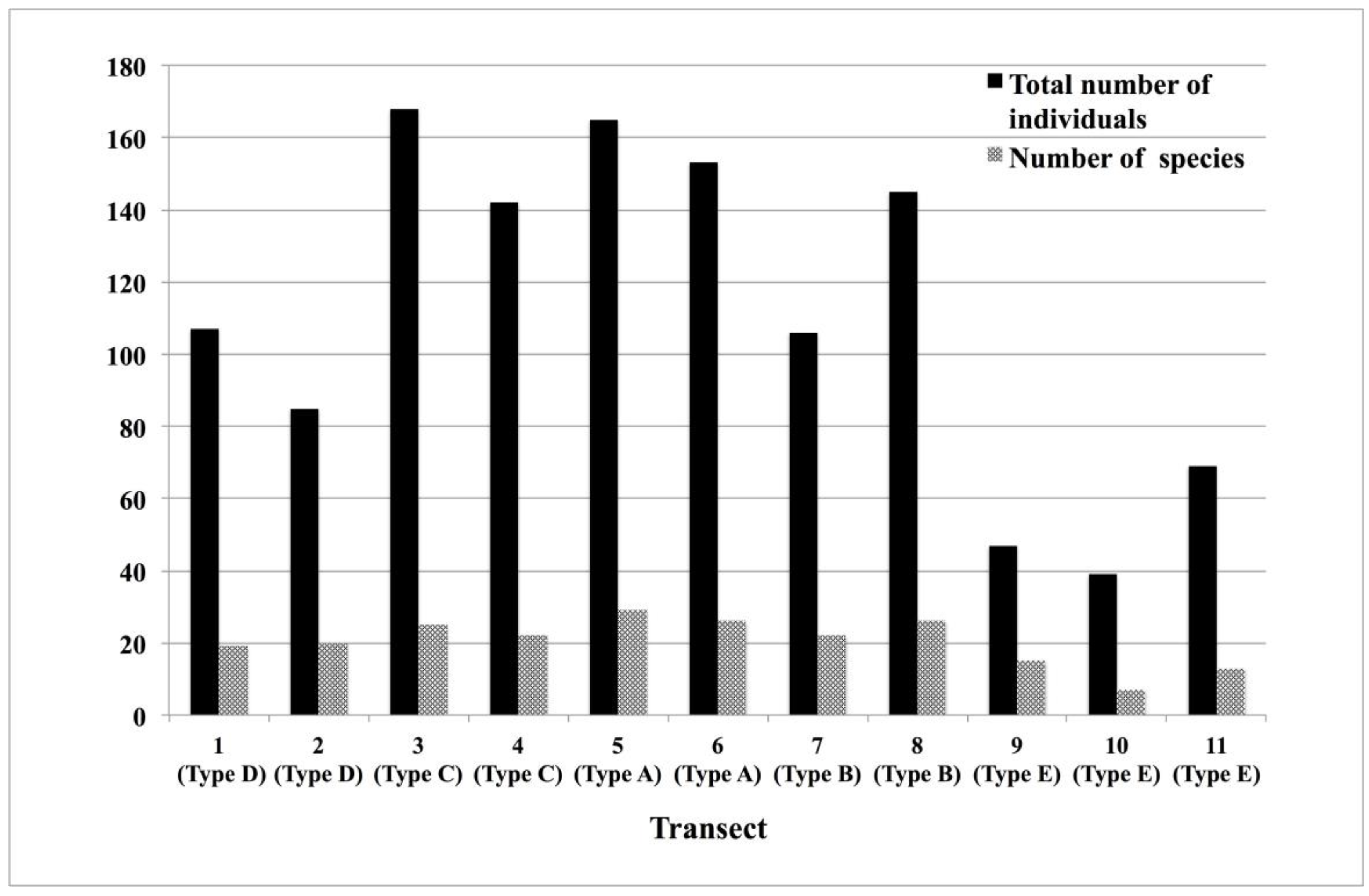
In the present study, we recorded a total of 1226 individuals of 48 butterfly species during the whole study period. The annual total number of individuals in each transect in all butterfly species recorded (corresponding to response variables) is shown in
Appendix A. Both the total numbers of species and individuals differed markedly between the studied transects (
Figure 2).
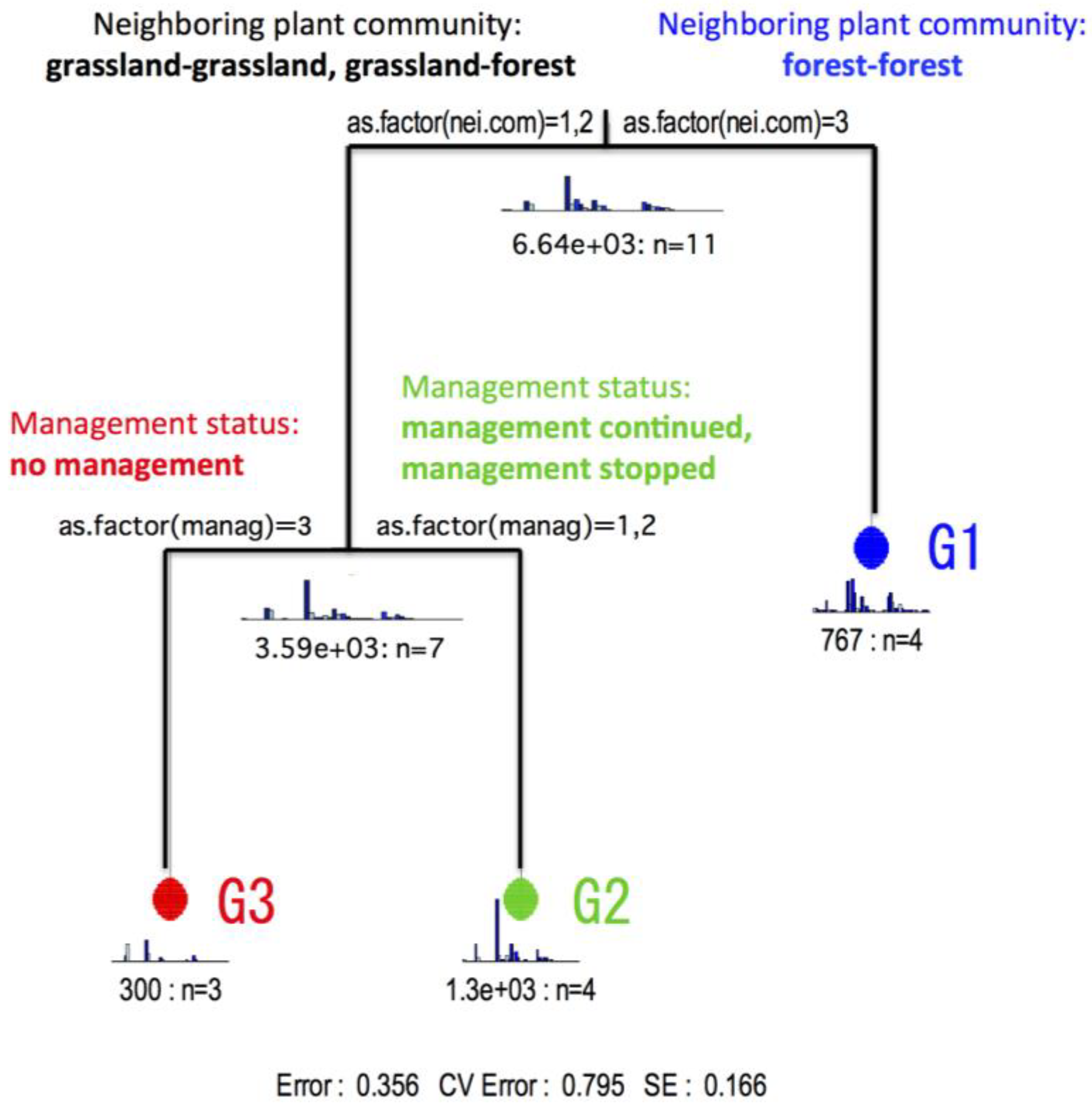
MRT analysis generated a 3-leaved MRT with the residual error (Error) = 0.356, the cross-validation error (CV Error) = 0.795 and the standard error (SE) = 0.166, and showed the total explained variation of 64.4% in the species composition (
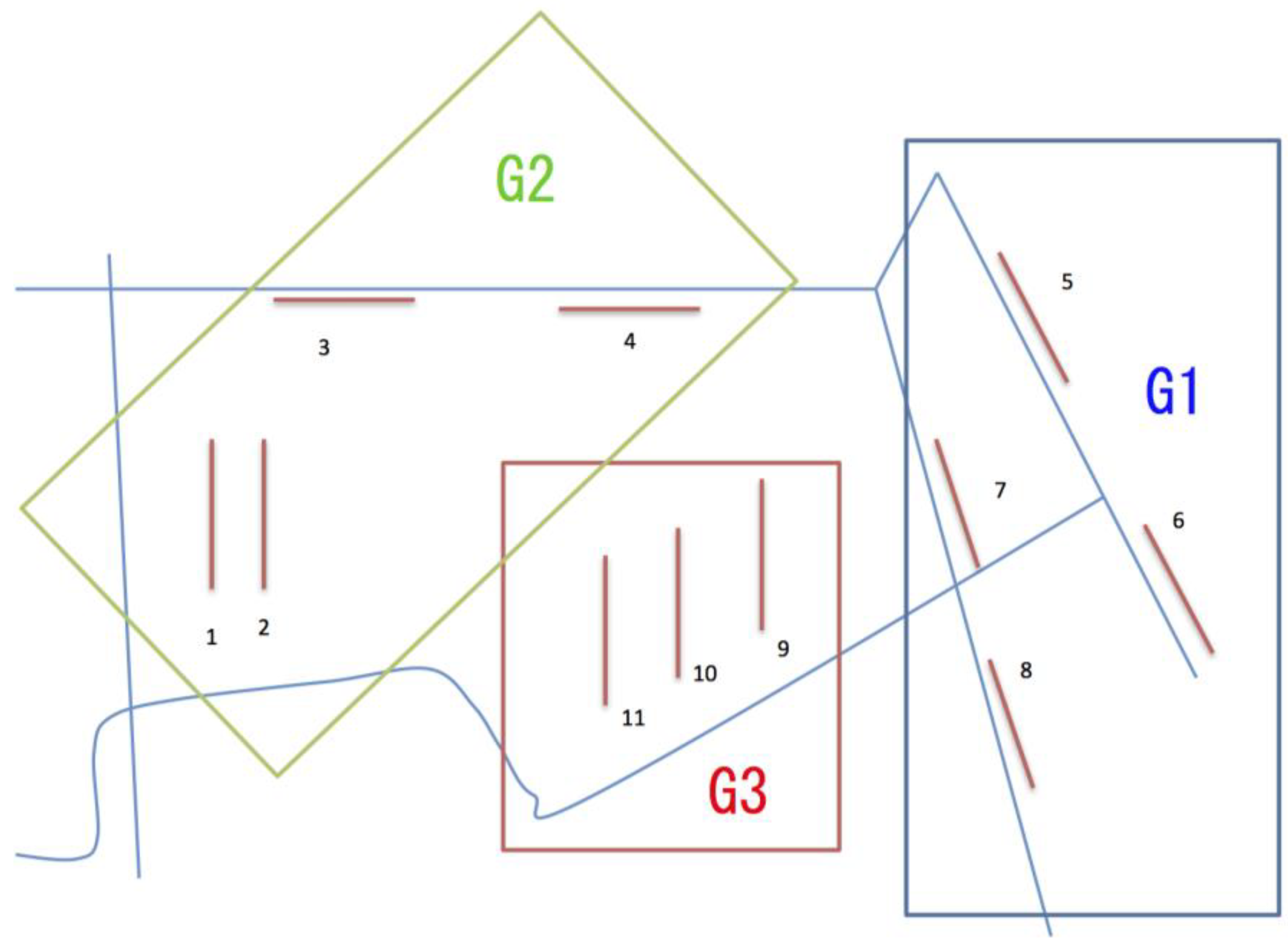
Figure 3). The explanatory variable discriminating between the two branches in the first node of the 3-leaved MRT was neighbouring plant community (nei.com). The group of the first leaf (G1) was characterized by the transect surrounded on both sides by forest (Rank 3), while the other group was characterized by the transect surrounded on one side by grassland and on the other side by forest (Rank 2), or the transect surrounded on both sides by grassland (Rank 1). The explanatory variable discriminating between the two branches in the second node of the MRT was management status (manag). The group of the second leaf (G2) was characterized by the transect with continuation of mowing once a year (Rank 1) or that where mowing was stopped a few years ago (Rank 2), while the group of the third leaf (G3) was characterized by the transect with mowing stopping quite a long time ago (Rank 3). Thus, the transects 5, 6, 7, 8 belonged to the group G1, the transects 1, 2, 3, 4 belonged to the group G2, and the transects 9, 10, 11 belonged to the group G3, and the positional relationship between the transects is shown in
Figure 4.
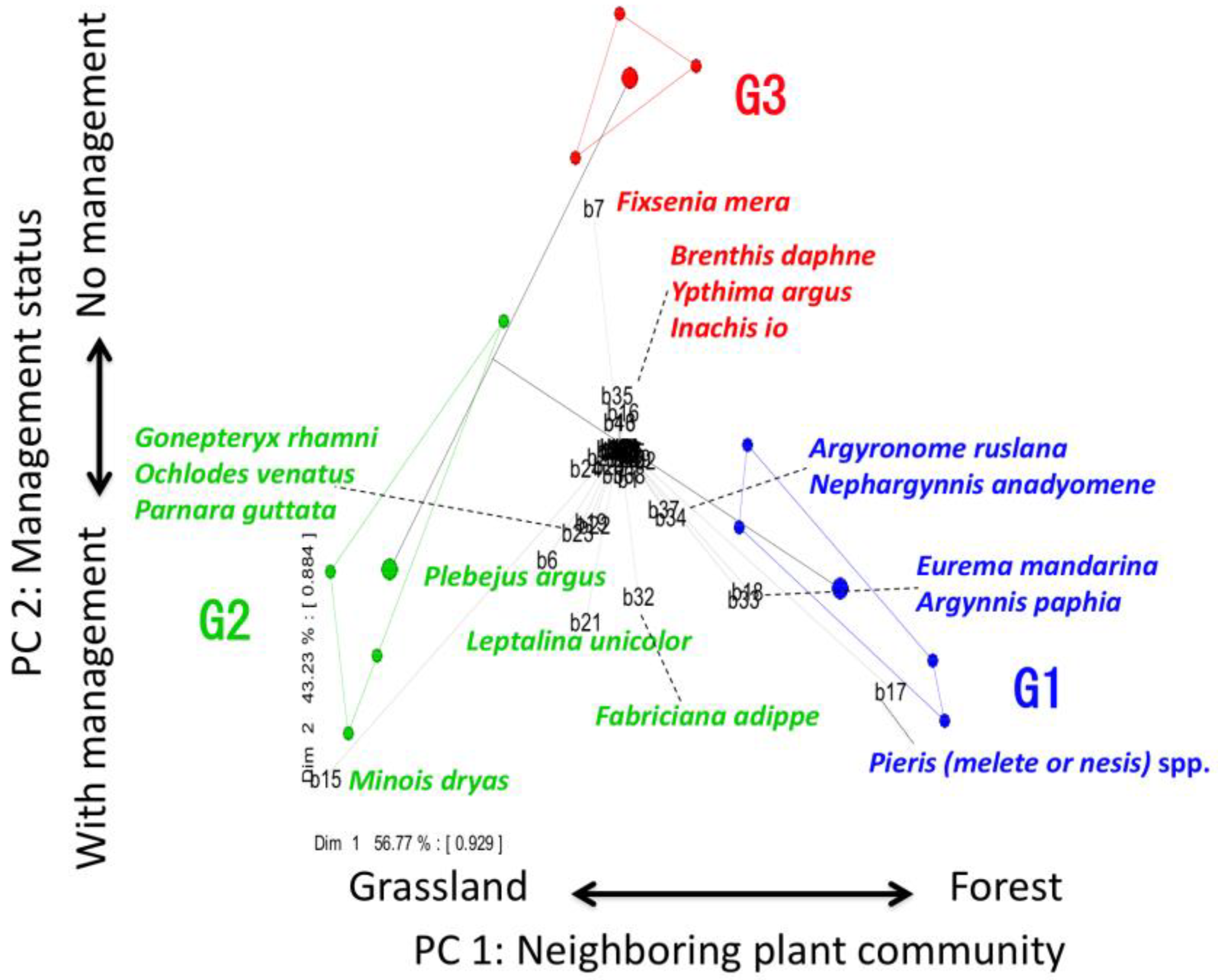
The groups (G1, G2, and G3) identified by MRT in
Figure 3 and 48 butterfly component species were plotted jointly in a PCA biplot (
Figure 5). The first axis of the PCA explained 42.6% of the total community variation (
Table 2), mainly separating the communities surrounded by forests from those surrounded by grasslands. The second axis of the PCA explained 36.1% of the variation (
Table 2), mainly separating the communities associated with management from the communities with no management. The first two axes of the PCA accounted largely for 78.8% of the variance in the community. The group G1 discriminated by MRT distributed on the right side of the first axis (forest-oriented), and the other groups (G2 and G3) distributed on the left side of the first axis (grassland-oriented). On the other hand, the group G3 distributed on the upper side of the second axis (no management-oriented), and the other groups (G1 and G2) distributed on the underside of the second axis (with management-oriented). Thus, the PCA also distinctly separated the 3 groups discriminated by MRT.
Pieris (melete or nesis) spp.,
Eurema mandarina,
Argynnis paphia,
Argyronome ruslana, and
Nephargynnis anadyomene were characteristic species of the group G1 and they were not Red Listed species (
Table 3).
Minois dryas,
Leptalina unicolor,
Fabriciana adippe, Plebejus argus,
Parnara guttata,
Ochlodes venatus, and
Gonepteryx rhamni were characteristic species of the group G2, three of which (
Leptalina unicolor,
Plebejus argus, and
Gonepteryx rhamni) were Red Listed species (
Table 3).
Fixsenia mera,
Brenthis daphne,
Ypthima argus, and
Inachis io were characteristic species of the group G3, one of which (
Brenthis daphne) was Red Listed species (
Table 3). According to
Figure 2 and
Figure 5, the number of butterfly component species was higher in the groups G1 and G2 than in the group G3.
The type of larval host plants and the number of herbaceous or woody species of adult nectar source plants in each of the characteristic species of the three butterfly groups (G1, G2, and G3) discriminated in the MRT analysis are shown in
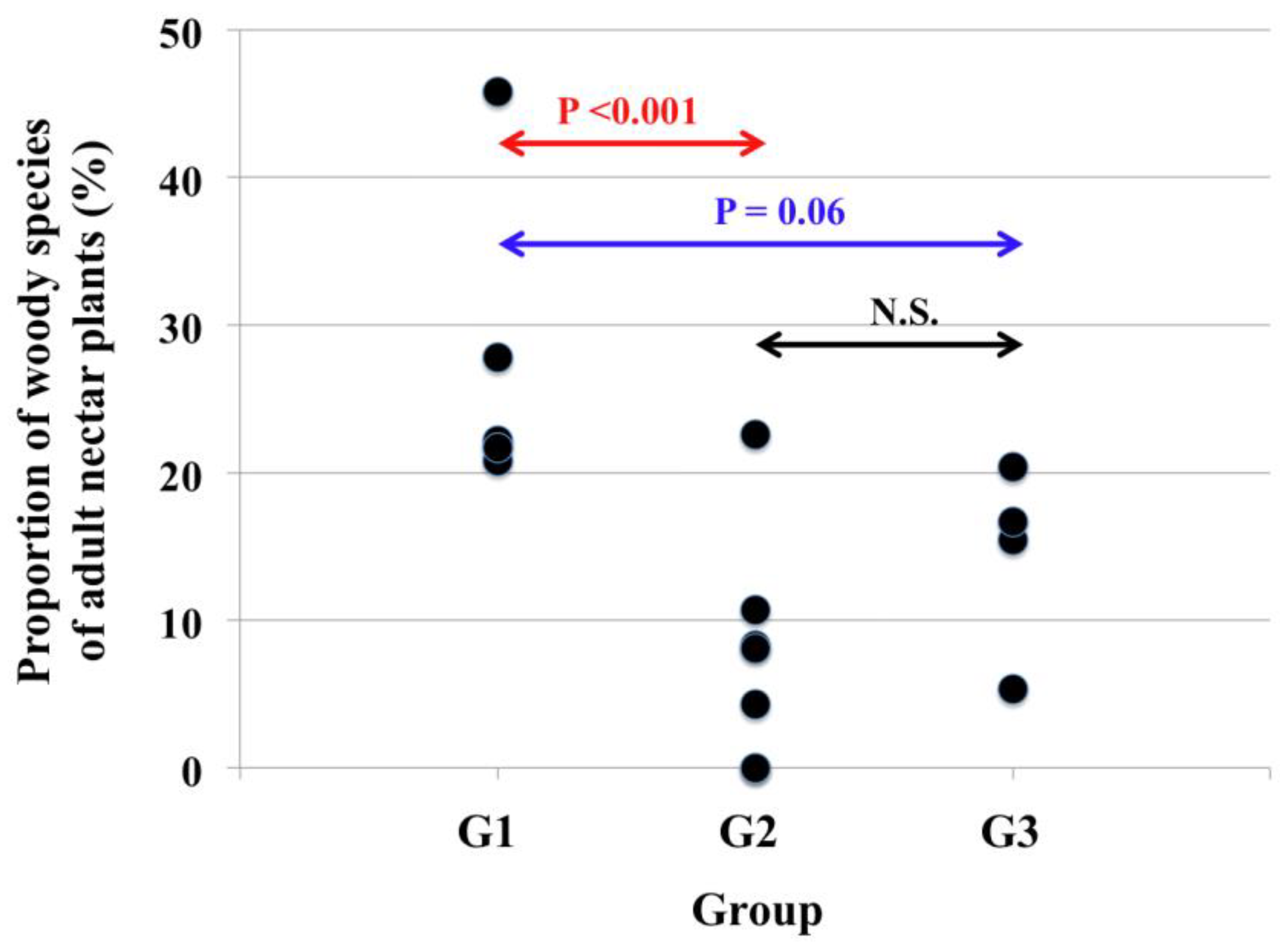
Table 3. In terms of larval host plants, the characteristic species of all groups are almost herb (grass) - feeders. While, in terms of adult nectar source plants, marked differences were detected among the characteristic species of the three groups. That is, the characteristic species of the group G1 use a higher percentage of woody nectar source plants than those of the other two groups G2 and G3 (
Figure 6 and the results of the Turkey test (
Table 4)).
4. Discussion
4.1. Determinants of Butterfly Community Structure and Composition at the Local Habitat Level
In the present study, we showed through the MRT analysis that the first important determinant of the butterfly community structure is neighbouring plant community. That is, it was found that the differences in the surrounding plant communities of the transects most influenced the determination of the structure and composition of the butterfly community. This result seems to be valid, because many butterflies depend on specific plants for their dietary resources during both larval and adult stages. In fact, there are already many studies that have shown that plant related factors are the determinants of butterfly community structure and composition at the local level [
15,
23,
25,
26,
32,
57,
58]. However, although it has been well investigated that the structure and composition of butterfly communities changes with different vegetation (the above studies), few attempts have been made to clarify its function and mechanism. In our study, due to the first determinant (neighbouring plant community), the butterfly community was divided into two groups, one of the transects surrounded by forests (G1) and the other of the transects surrounded mainly by grasslands (G2 and G3). Moreover, when the adult dietary habits of the characteristic species of those groups were analysed, it was found that the species of G2 and G3 mostly utilize herbaceous nectar plants, while those of G1 utilize more woody nectar plants (
Figure 6,
Table 3 and 4). Pocewicz
et al. [
59] argue for the importance of butterfly resource plant spatial distributions in determining butterfly population densities. Thus, a mechanism was suggested that the difference in the neighbouring plant communities causes the difference in the distribution of the nectar plant species of butterflies, and in turn, the difference in the species composition of the butterfly community (i.e. a species group that uses more woody nectar plants (G1) vs. a species group that uses herbaceous nectar plants (G2 or G3)). That is, from our results, it can be considered that the neighbouring plant communities functioned in determining the species composition of the butterfly community.
Next, we showed that the second important determinant of the butterfly community structure is management status. There are also many studies on how human management and the degree of natural or human-caused disturbance greatly influence the determination of butterfly community structure and composition at the local level [
9,
22,
24,
27,
29,
30,
31,
60,
61,
62,
63,
64]. In our study, due to the second determinant (management status), the butterfly community was divided into two groups, one of the transects with continuation of mowing once a year, or those where mowing was stopped a few years ago (G2) and the other of the transects with mowing stopping quite a long time ago (G3). It is known that active management promotes plant diversity and richness e.g. [
65]. In the previous studies at the present study site [
34,
35], it was found that the differences in the management status of each transect cause those in the amount of nectar plants in adult butterflies. That is, we know that the transects with management (mowing) had a larger amount of flowering plants (adult nectar resources) than those without management (mowing). Thus, it was suggested that the differences in the management status of the transects made the differences in the amount of adult nectar plants among the transects surrounded mainly by grasslands, and therefore, they made the differences in the structure of the butterfly community. Then, it is noteworthy that the number of butterfly species and their total population abundance were high in the group G2 and extremely low in the group G3 (
Figure 2 and
Figure 5). Generally, it is accepted that one of the local factors strongly affecting butterfly diversity and richness is the amount of flowering nectar producing plants e.g. [
32,
66,
67,
68]. From these, a mechanism was suggested that the difference in management status causes the difference in the abundance of the flowering nectar source plants of butterflies, and in turn, the difference in the species richness and abundance of the butterfly community (i.e. a species group with relatively high species richness and abundance (G2) vs. a species group with relatively low species richness and abundance (G3)). That is, from our results, it can be considered that the management status functioned in determining the species richness and abundance (diversity) of the butterfly community.
The results of PCA (
Figure 5) also strongly support the results and discussions of MRT analysis stated above. The first axis (PC1: neighbouring plant community) and the second axis (PC2: management status) of the PCA accounted for a cumulative 78.8% of the total community variation, providing evidence that these two factors were key determinants of the butterfly community. The group G2 (a group of herbaceous nectar feeders) was located on the left side of the first axis and the group G1 (a group of more woody nectar feeders) was located on the right side, and this axis reflected the environmental gradient from grassland to forest. Thus, it is clear that the first axis functioned in changing the species composition of the butterfly community (from grassland to woodland species). In the 2nd axis, the group that has been continuously managed or that has been managed until recently (G1 and G2) was located below the axis, and the group that has not been managed for a long time (G3) was located above the axis. Thus, the axis reflected the environmental gradient from with management to without management. Since the number of species belonging to each group of G1 and G2 was large, and the number of species belonging to group G3 is small, it is clear that the 2nd axis functioned in changing the species richness (diversity) the butterfly community.
Overall, it can be greatly emphasized that our study explored the determinants of butterfly community structure at the local habitat level and clarified their mechanism of action, and each determinant had a different role (function) in determining the local butterfly community structure. In addition, our results demonstrate that at the local habitat level, the environmental factors that strongly control the distribution and abundance of butterfly food resources, such as the present neighbouring plant community and management status, are the most contributing factors that determine the local butterfly community structure.
4.2. Characteristics of the Butterfly Groups Identified from the MRT and PCA
In the present study, it was found that the butterfly community was composed of three groups (G1, G2, and G3) with different characteristics. The transects (Nos. 5, 6, 7, and 8) surrounded by forests belonged to the group G1. The characteristic species of the group G1 were featured by larval dietary resources being mostly herbaceous plants, but adult ones being relatively more woody plants (
Table 3). The transects (Nos. 1, 2, 3, and 4) surrounded mainly by grassland belonged to the group G2. The characteristic species of the group G2 were featured by both larval and adult dietary resources being mostly herbaceous plants (
Table 3). The transects (Nos. 9, 10, and 11) surrounded by grassland belonged to the group G3. The characteristic species of the group G3 were featured by larval dietary resources being mostly herbaceous plants, but adult ones being slightly woody plants, as the values of
P in the Tukey test showed (
Table 4).
Based on the above, it was confirmed that the butterfly community in the study area was composed of the group (G2) associated with the grassland in the early successional stage maintained by management, the group (G3) associated with the grassland in the late successional stage with no management, and the group (G1) associated with mixed areas of grassland and forests. In addition, there may be a group associated with forest habitats only. However, in the present study, it was not possible to set up a survey transect associated only with forests, so the existence of the group is unclear.
Up to now, the species groupings in butterflies have been mainly made and discussed based on their life history characteristics and ecology [
12,
60,
61,
64,
69,
70,
71]. However, in comparison to this, there are not many studies on the species groupings based on their habitat structure and its successional stage. In this regard, our study revealed the existence of butterfly groups that establish on the gradient of secondary succession from grassland to forest habitats. In Japan, Inoue [
72] and Kobayashi
et al. [
73] reported in detail that the species composition and grouping of butterflies change clearly along the successive stages of deciduous forest development, supporting our results of this study. In the future, much research is needed to verify whether the species groupings of butterflies along these secondary successional stages have a generality. Furthermore, the analysis of which groups contain many endangered species and are linked to endangered characteristics (e.g., oligo-voltinism, narrow dietary breadth, narrow geographic distribution [
5,
6]) is an essential issue for the conservation of local biodiversity. In the present study, the largest number of Red Listed butterfly species was present among the characteristic species of the group G2 established in the early stage of secondary succession (
Table 3). This was in good agreement with the tendency of Japanese Red Listed butterfly species, that is, many of the Red Listed butterfly species are grassland species in rural areas of Japan [
56].
5. Conclusions and Conservation Implications
In conclusion, our study demonstrated that neighbouring plant community and management status are very important environmental factors for determining the structure and composition of butterfly communities at the local habitat level. In particular, the neighbouring vegetation was related to the distribution of butterfly dietary resources and contributed much in determining the species composition of the community, while the management status was largely related to the amount of their dietary resources, affecting the species richness and diversity. Furthermore, it became clear that characteristic species groups were formed along the gradients of these two factors.
From the results of this study, it was considered essential to maintain the grassland habitats corresponding to the initial stage of secondary succession in order to conserve the Red Listed species and the species richness of the butterfly community, and for that purpose, it is very important to continue mowing management. Otherwise, as the group G3 of this study shows, abandonment of the management promotes secondary succession, reducing Red Listed species and reducing the butterfly species richness cf. [
57]. In general, it is well known that human activities and management are important for maintaining the high diversity of butterflies in semi-natural grassland e.g. [
63]. On the other hand, the maintenance of vegetation landscape diversity (existence of both grasslands and forests) was considered important for the conservation of butterfly community diversity (species composition). That is, homogenisation of vegetation landscapes will lead to a simplification of butterfly species composition cf. [
74,
75]. Overall, it can be concluded that continued mowing management and maintenance of vegetation landscape diversity are paramount to the conservation of the diversity (species composition and species richness) and the endangered species in the local butterfly communities in the region.
Author Contributions
Conceptualization, M.K.; methodology, M.K. and T.Y.; software, T.Y.; validation, M.K. and T.Y.; data analysis, T.Y.; investigation, M.K. and T.Y.; writing—original draft preparation, M.K.; writing—review and editing, M.K. and T.Y.; supervision, M.K.; project administration, M.K..; funding acquisition, M.K. and T.Y.; All authors have read and agreed to the published version of the manuscript.
Funding
The research was supported in part by both Grants-in-Aid for Scientific Research (B) (no. 17310138) and for Scientific Research (C) (no. 20510221) from the Japan Society for the Promotion of Science (JSPS) to M. Kitahara (Represent the applicant).
Data Availability Statement
The data presented in this study are available in the manuscript and
Appendix A.
Acknowledgments
We thank the members of the Mount Fuji Research Institute of Yamanashi Pref. for their suggestions, help, and cooperation for this study.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A List of Butterfly Species Observed in the Present Study, and the Annual Total Number of Individuals of Each Species in Each Transect, Corresponding to Response Variables
Table A1.
List of butterfly species observed in the present study, and the annual total number of individuals of each species in each transect, corresponding to response variables.
Table A1.
List of butterfly species observed in the present study, and the annual total number of individuals of each species in each transect, corresponding to response variables.
| |
Symbol in the PCA |
Transect (Habitat type) |
|
|
| Species |
1 (D) |
2 (D) |
3(C) |
4(C) |
5 (A) |
6 (A) |
7 (B) |
8 (B) |
9 (E) |
10 (E) |
11 (E) |
Total |
| Hesperiidae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Daimio tethys |
b26 |
0 |
1 |
0 |
1 |
1 |
1 |
0 |
1 |
0 |
0 |
0 |
5 |
| Choaspes benjaminii |
b30 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
1 |
| Leptalina unicolor |
b21 |
10 |
1 |
20 |
17 |
8 |
9 |
12 |
10 |
2 |
0 |
5 |
94 |
| Ochlodes venatus |
b22 |
2 |
5 |
13 |
3 |
2 |
4 |
5 |
5 |
0 |
0 |
3 |
42 |
| Ochlodes ochraceus |
b25 |
0 |
0 |
1 |
0 |
1 |
2 |
0 |
1 |
0 |
0 |
1 |
6 |
| Potanthus flavus |
b28 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
| Aeromachus inachus |
b24 |
5 |
0 |
5 |
1 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
13 |
| Thoressa varia |
b31 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Pelopidas mathias |
b29 |
0 |
1 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
| Pelopidas jansonis |
b27 |
2 |
0 |
1 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
5 |
| Parnara guttata |
b23 |
8 |
6 |
2 |
10 |
4 |
3 |
2 |
5 |
1 |
1 |
0 |
42 |
| Papilionidae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Parnassius glacialis |
b1 |
1 |
0 |
2 |
2 |
2 |
3 |
3 |
0 |
0 |
0 |
0 |
13 |
| Papilio xuthus |
b3 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
2 |
| Papilio protenor |
b4 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
1 |
| Papilio bianor |
b2 |
0 |
2 |
0 |
0 |
2 |
3 |
0 |
3 |
1 |
0 |
0 |
11 |
| Papilio maackii |
b5 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
| Pieridae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Eurema mandarina |
b18 |
0 |
1 |
2 |
5 |
18 |
16 |
7 |
7 |
0 |
0 |
1 |
57 |
| Gonepteryx rhamni |
b19 |
5 |
2 |
10 |
2 |
2 |
2 |
4 |
3 |
0 |
0 |
0 |
30 |
| Colias erate |
b20 |
0 |
0 |
4 |
3 |
0 |
0 |
1 |
3 |
0 |
0 |
1 |
12 |
|
Pieris (melete or nesis) spp. |
b17 |
0 |
1 |
4 |
0 |
32 |
30 |
12 |
15 |
2 |
0 |
0 |
96 |
| Lycaenidae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Artopoetes pryeri |
b9 |
0 |
0 |
0 |
0 |
0 |
3 |
0 |
0 |
0 |
0 |
0 |
3 |
| Rapala arata |
b11 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Fixsenia mera |
b7 |
0 |
7 |
3 |
3 |
0 |
0 |
0 |
4 |
9 |
6 |
23 |
55 |
| Lampides boeticus |
b12 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Pseudozizeeria maha |
b8 |
0 |
0 |
0 |
0 |
1 |
2 |
0 |
1 |
0 |
0 |
0 |
4 |
Table A1.
Cont.
| |
Symbol in the PCA |
Transect (Habitat type) |
|
|
| Species |
1 (D) |
2 (D) |
3(C) |
4(C) |
5 (A) |
6 (A) |
7 (B) |
8 (B) |
9 (E) |
10 (E) |
11 (E) |
Total |
| Celastrina argiolus |
b13 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
1 |
| Everes argiades |
b10 |
1 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
3 |
| Plebejus argus |
b6 |
2 |
2 |
27 |
17 |
6 |
7 |
11 |
3 |
3 |
4 |
4 |
86 |
| Curetis acuta |
b14 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Libytheinae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Libythea celtis |
b47 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
2 |
| Nymphalidae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Brenthis daphne |
b35 |
2 |
5 |
3 |
0 |
1 |
1 |
1 |
6 |
2 |
3 |
7 |
31 |
| Argyronome laodice |
b36 |
1 |
0 |
2 |
8 |
3 |
0 |
3 |
5 |
3 |
0 |
1 |
26 |
| Argyronome ruslana |
b34 |
4 |
2 |
2 |
0 |
11 |
7 |
3 |
3 |
2 |
0 |
0 |
34 |
| Argynnis paphia |
b33 |
2 |
0 |
1 |
7 |
13 |
14 |
9 |
13 |
1 |
0 |
0 |
60 |
| Nephargynnis anadyomene |
b37 |
2 |
1 |
0 |
2 |
6 |
7 |
2 |
4 |
0 |
0 |
0 |
24 |
| Damora sagana |
b44 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
1 |
| Fabriciana adippe |
b32 |
1 |
9 |
14 |
8 |
14 |
7 |
6 |
12 |
3 |
1 |
1 |
76 |
| Speyeria aglaja |
b41 |
0 |
0 |
0 |
0 |
1 |
0 |
1 |
0 |
0 |
0 |
0 |
2 |
| Argyreus hyperbius |
b42 |
0 |
0 |
1 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
2 |
| Limenitis camilla |
b39 |
0 |
0 |
0 |
0 |
1 |
1 |
1 |
2 |
0 |
0 |
0 |
5 |
| Limenitis glorifica |
b38 |
1 |
1 |
1 |
1 |
2 |
5 |
0 |
0 |
0 |
0 |
0 |
11 |
| Neptis sappho |
b43 |
0 |
0 |
0 |
0 |
2 |
0 |
0 |
0 |
0 |
0 |
0 |
2 |
| Neptis pryeri |
b45 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Polygonia c-aureum |
b40 |
0 |
0 |
0 |
1 |
2 |
0 |
1 |
1 |
0 |
0 |
0 |
5 |
| Inachis io |
b46 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
1 |
| Satyrinae |
|
|
|
|
|
|
|
|
|
|
|
|
|
| Ypthima argus |
b16 |
7 |
6 |
3 |
3 |
4 |
2 |
4 |
10 |
7 |
3 |
7 |
56 |
| Minois dryas |
b15 |
50 |
31 |
44 |
44 |
22 |
20 |
16 |
24 |
9 |
21 |
14 |
295 |
| Melanitis phedima |
b48 |
0 |
0 |
0 |
0 |
1 |
0 |
0 |
0 |
0 |
0 |
0 |
1 |
| Total |
|
107 |
85 |
168 |
142 |
165 |
153 |
106 |
145 |
47 |
39 |
69 |
1226 |
References
- MacArthur, R. H. Geographical ecology: patterns in the distribution of species; Harper & Row: New York, USA, 1972.
- Pianka, E. R. Evolutionally Ecology, 4th ed.; Harper & Row: New York, USA,1988.
- Begon, M.; Harper, J. L.; Townsend, C. R. Ecology: individuals, populations and communities, 4th ed.; Blackwell Publishing, Ltd.: Oxford, UK, 2006.
- Ehrlich, P.R. Population biology of checkerspot butterflies and the preservation of global biodiversity. Oikos 1992, 63, 6-12. [CrossRef]
- Primack, R. B. Essentials of conservation biology, 5th ed.; Sinauer Associates, Inc.: Sunderland, Massachusetts, USA, 2010.
- Primack, R. B. A Primer of Conservation Biology, 5th ed.; Sinauer Associates, Inc.: Sunderland, Massachusetts, USA, 2012.
- Boggs, C. L.; Murphy, D. D. Community composition in mountain ecosystems: climatic determinants of montane butterfly distributions. Global Ecology and Biogeography Letters 1997, 6, 39-48. [CrossRef]
- Kerr, J. T.; Southwood, T. R. E.; Cihlar, J. Remotely sensed habitat diversity predicts butterfly species richness and community similarity in Canada. Proc. Natl. Acad. Sci. USA 2001, 98, 11365-11370. [CrossRef]
- Stefanescu, C.; Herrando, S.; Páramo, F. Butterfly species richness in the north-west Mediterranean Basin: the role of natural and human-induced factors. J. Biogeogr. 2004, 31, 905-915. [CrossRef]
- Davis, J. D.; Debinski, D. M.; Danielson, B. J. Local and landscape effects on the butterfly community in fragmented Midwest USA prairie habitats. Landscape Ecol. 2007, 22, 1341-1354. [CrossRef]
- Gonzalez-Megias, A.; Menendez, R.; Roy, D.; Brereton, T. O. M.; Thomas, C. D. Changes in the composition of British butterfly assemblages over two decades. Global Change Biol. 2008, 14, 1464-1474. [CrossRef]
- Forister, M. L.; McCall, A. C.; Sanders, N. J.; Fordyce, J. A.; Thorne, J. H.; O’Brien, J.; Waetjen, D. P.; Shapiro, A. M. Compounded effects of climate change and habitat alteration shift patterns of butterfly diversity. Proc. Natl. Acad. Sci. USA 2010, 107, 2088-2092. [CrossRef]
- Stefanescu, C.; Torre, I.; Jubany, J.; Páramo, F. Recent trends in butterfly populations from north-east Spain and Andorra in the light of habitat and climate change. J. Insect Conserv. 2011a, 15, 83-93. [CrossRef]
- Stefanescu, C.; Carnicer, J.; Penuelas, J. Determinants of species richness in generalist and specialist Mediterranean butterflies: the negative synergistic forces of climate and habitat change. Ecography 2011b, 34, 353-363. [CrossRef]
- Carneiro, E.; Mielke, O. H. H.; Casagrande, M. M.; Fiedler, K. Community structure of skipper butterflies (Lepidoptera, Hesperiidae) along elevational gradients in Brazilian Atlantic forest reflects vegetation type rather than altitude. PloS one 2014, 9 (10), e108207. [CrossRef]
- Chen, S.; Mao, L.; Zhang, J.; Zhou, K.; Gao, J. Environmental determinants of geographic butterfly richness pattern in eastern China. Biodiversity Conserv. 2014, 23, 1453-1467. [CrossRef]
- Fernández-Chacón, A.; Stefanescu, C.; Genovart, M.; Nichols, J. D.; Hines, J. E.; Páramo, F.; Turco, M.; Oro, D. Determinants of extinction-colonization dynamics in Mediterranean butterflies: the role of landscape, climate and local habitat features. J. Anim. Ecol. 2014, 83, 276-285. [CrossRef]
- Zografou, K.; Kati, V.; Grill, A.; Wilson, R. J.; Tzirkalli, E.; Pamperis, L. N.; Halley, J. M. Signals of climate change in butterfly communities in a Mediterranean protected area. PLoS One 2014, 9, e87245. [CrossRef]
- Acharya, B. K.; Vijayan, L. Butterfly diversity along the elevation gradient of eastern Himalaya, India. Ecol.Res. 2015, 30, 909-919. [CrossRef]
- Nieto-Sánchez, S.; Gutiérrez, D.; Wilson, R. J. Long-term change and spatial variation in butterfly communities over an elevational gradient: driven by climate, buffered by habitat. Divers. Distrib. 2015, 21, 950-961. [CrossRef]
- Vodă, R.; Dapporto, L.; Dincă, V.; Shreeve, T. G.; Khaldi, M.; Barech, G.; Rebbas, K.; Sammut, P.; Scalercio, S.; Hebert, P. D. N.; Vila, R. Historical and contemporary factors generate unique butterfly communities on islands. Sci. Rep. 2016, 6, 28828. [CrossRef]
- Cleary, D. F.; Genner, M. J.; Koh, L. P.; Boyle, T. J.; Setyawati, T.; de Jong, R.; Menken, S. B. Butterfly species and traits associated with selectively logged forest in Borneo. Basic Appl. Ecol. 2009, 10, 237-245. [CrossRef]
- Matteson, K. C.; Langellotto, G. A. Determinates of inner city butterfly and bee species richness. Urban Ecosystems 2010, 13, 333-347. [CrossRef]
- Kati, V.; Zografou, K.; Tzirkalli, E.; Chitos, T.; Willemse, L. Butterfly and grasshopper diversity patterns in humid Mediterranean grasslands: the roles of disturbance and environmental factors. J. Insect Conserv. 2012, 16, 807-818. [CrossRef]
- Checa, M. F.; Rodriguez, J.; Willmott, K. R.; Liger, B. Microclimate variability significantly affects the composition, abundance and phenology of butterfly communities in a highly threatened neotropical dry forest. Florida Entomologist 2014, 97, 1-14. [CrossRef]
- Collinge, S. K.; Prudic, K. L.; Oliver, J. C. Effects of local habitat characteristics and landscape context on grassland butterfly diversity. Conserv. Biol. 2003, 17, 178-187. [CrossRef]
- Lien, V.; Yuan, D. The differences of butterfly (Lepidoptera, Papilionoidea) communities in habitats with various degrees of disturbance and altitudes in tropical forests of Vietnam. Biodiversity Conserv. 2003, 12, 1099-1111. [CrossRef]
- Leps, J.; Spitzer, K. Ecological determinants of butterfly communities (Lepidoptera, Papilionoidea) in the Tam Dao mountains, Vietnam. Acta Entomologica Bohemoslovaca 1990, 87, 182-194.
- Spitzer, K.; Jaros, J.; Havelka, J.; Leps, J. Effect of small-scale disturbance on butterfly communities of an Indochinese montane rainforest. Biol. Conserv. 1997, 80, 9-15. [CrossRef]
- D’Aniello, B.; Stanislao, I.; Bonelli, S.; Balletto, E. Haying and grazing effects on the butterfly communities of two Mediterranean-area grasslands. Biodiversity Conserv. 2011, 20, 1731- 1741. [CrossRef]
- Kwon, T.S.; Kim, S.S.; Lee, C.M.; Jung, S.J. Changes of butterfly communities after forest fire. J. Asia-Pac. Entomol. 2013, 16, 361-367. [CrossRef]
- Clark, P. J.; Reed, J. M.; Chew, F. S. Effects of urbanization on butterfly species richness, guild structure, and rarity. Urban Ecosystems 2007, 10, 321-337. [CrossRef]
- New, T.R. Butterfly conservation, 2nd ed.; Oxford University Press: Melbourne, Australia, 1997.
- Kubo, M.; Kobayashi, T.; Kitahara, M.; Hayashi, A. Seasonal fluctuations in butterflies and nectar resources in a semi-natural grassland near Mt. Fuji, central Japan. Biodiversity Conserv. 2009, 18, 229-246. [CrossRef]
- Kubo, M.; Kobayashi, T.; Kitahara, M.; Hayashi, A. Effects of land management on species composition of butterflies and nectar resources in a semi-natural grassland near Mt. Fuji, central Japan. Vegetation Science 2011, 28, 49-62 (in Japanese with English summary).
- Kubo, M.; Matutani, J.; Hayashi, A. Factors of the failure of planting in Uenohara-area at the foot of Mt. Fuji. Bulletin of the Yamanashi Forestry Research Institute 2005, 24, 61–67 (in Japanese with English summary).
- Ohwaki, A.; Hayami, S.; Kitahara, M.; Yasuda, T. The role of linear mown firebreaks in conserving butterfly diversity: Effects of adjacent vegetation and management. Entomol. Sci. 2018, 21, 112-123. [CrossRef]
- Hanski, I.; Kuussaari, M.; Nieminen, M. Metapopulation structure and migration in the butterfly Melitaea cinxia. Ecology 1994, 75, 747–762. [CrossRef]
- Lewis, O.; Thomas, C.; Hill, J.; Brookes, M.; Crane, T. P.; Graneau, Y.; Mallet, J.; Rose, O. Three ways of assessing metapopulation structure in the butterfly Plebejus argus. Ecol. Entomol. 1997, 22, 283-293. [CrossRef]
- Pollard, E.; Yates, T. J. Monitoring butterflies for ecology and conservation: the British butterfly monitoring scheme; Chapman & Hall: London, UK, 1993.
- Ishii, M. Transect counts of butterflies. In Decline and conservation of butterflies in Japan II; Yata, O., Ueda, K., Eds.; The Lepidopterological Society of Japan & the Nature Conservation Society of Japan: Osaka, Japan, 1993; pp. 91-101 (in Japanese with English summary).
- New, T.R. Butterfly conservation, 2nd ed.; Oxford University Press: Melbourne, Australia, 1997.
- De'ath, G. Multivariate regression trees: a new technique for modeling species–environment relationships. Ecology 2002, 83, 1105-1117. [CrossRef]
- Cao, T.; Wang, X.; Zhang, H. Energy bagging tree. Statistics and Its Interface 2016, 9, 171-181. [CrossRef]
- Shiyomi, M.; Chen, J. How do we analyze auto-correlated data? Japanese Journal of Grassland Science 2017, 63, 23-27 (in Japanese).
- R Development Core Team R: a language and environment for statistical computing (version 2.12.0); R Foundation for Statistical Computing: Vienna, Austria, 2012.
- De’ath, G. Mvpart: multivariate partitioning R package, version 1.2–6; 2006.
- Unno, K.; Aoyama, J. The butterflies of Japan; Shougakukan: Tokyo, Japan, 1981 (in Japanese).
- Fukuda, H.; Hama, E.; Kuzuya, T.; Takahashi, A.; Takahashi, M.; Tanaka, B.; Tanaka, H.; Wakabayashi, M.; Watanabe, Y. The life histories of butterflies in Japan, Vol. 1; Hoikusha:, Osaka, Japan, 1982 (in Japanese with English summary).
- Fukuda, H.; Hama, E.; Kuzuya, T.; Takahashi, A.; Takahashi, M.; Tanaka, B.; Tanaka, H.; Wakabayashi, M.; Watanabe, Y. The life histories of butterflies in Japan, Vol. 2; Hoikusha:, Osaka, Japan, 1983 (in Japanese with English summary).
- Fukuda, H.; Hama, E.; Kuzuya, T.; Takahashi, A.; Takahashi, M.; Tanaka, B.; Tanaka, H.; Wakabayashi, M.; Watanabe, Y. The life histories of butterflies in Japan, Vol. 3; Hoikusha:, Osaka, Japan, 1984a (in Japanese with English summary).
- Fukuda, H.; Hama, E.; Kuzuya, T.; Takahashi, A.; Takahashi, M.; Tanaka, B.; Tanaka, H.; Wakabayashi, M.; Watanabe, Y. The life histories of butterflies in Japan, Vol. 4; Hoikusha:, Osaka, Japan, 1984b (in Japanese with English summary).
- Endo, S.; Nihira, I. Larval food of Japanese butterflies; Group Tamamushi: Tokyo, Japan, 1990 (in Japanese).
- Nihira, I. Larval food plants of Japanese butterflies; Self-Published: Tokyo, Japan, 2004 (in Japanese).
- Saito, M. U.; Jinbo, U.; Yago, M.; Kurashima, O.; Ito, M. Larval host records of butterflies in Japan. Ecol. Res. 2016, 31, 491-491. [CrossRef]
- Ministry of the Environment of Japan Red List of Japanese insects in 2019 version; Available from https://ikilog.biodic.go.jp/Rdb/booklist. [Accessed 8 Oct. 2020].
- Stefanescu, C.; Penuelas, J.; Filella, I. Rapid changes in butterfly communities following the abandonment of grasslands: a case study. Insect Conserv. Diversity 2009, 2, 261-269. [CrossRef]
- Vu, L.V.; Bonebrake, T. C.; Vu, M.Q.; Nguyen, N. T. Butterfly diversity and habitat variation in a disturbed forest in northern Vietnam. Pan-Pac. Entomol. 2015, 91, 29-39. [CrossRef]
- Pocewicz, A.; Morgan, P.; Eigenbrode, S.D. Local and landscape effects on butterfly density in northern Idaho grasslands and forests. J. Insect Conserv. 2009, 13, 593-601. [CrossRef]
- Kitahara, M.; Fujii, K. Biodiversity and community structure of temperate butterfly species within a gradient of human disturbance: an analysis based on the concept of generalist vs. specialist strategies. Res. Popul. Ecol. 1994, 36, 187-199. [CrossRef]
- Kitahara, M.; Fujii, K. An island biogeographical approach to the analysis of butterfly community patterns in newly designed parks. Res. Popul. Ecol. 1997, 39, 23-35. [CrossRef]
- Kitahara, M.; Sei, K.; Fujii, K. Patterns in the structure of grassland butterfly communities along a gradient of human disturbance: further analysis based on the generalist/specialist concept. Popul. Ecol. 2000, 42, 135-144. [CrossRef]
- Schmitt, T.; Rákosy, L. Changes of traditional agrarian landscapes and their conservation implications: a case study of butterflies in Romania. Divers. Distrib. 2007, 13, 855-862. [CrossRef]
- Schmitt, T.; Ulrich, W.; Büschel, H.; Bretzel, J.; Gebler, J.; Mwadime, L.; Habel, J.C. The relevance of cloud forest fragments and their transition zones for butterfly conservation in Taita Hills, Kenya. Biodiv. Conserv. 2020, 29, 3191-3207. [CrossRef]
- Lanta, V.; Mudrák, O.; Liancourt, P.; Bartoš, M.; Chlumská, Z.; Dvorský, M.; Pusztaiová, Z.; Münzbergová, Z.; Sebek, P.; Čížek, L.; Doležal, J. Active management promotes plant diversity in lowland forests: a landscape-scale experiment with two types of clearings. For. Ecol. Manage. 2019, 448, 94-103. [CrossRef]
- Clausen, H.D.; Holbeck, H.B.; Reddersen, J. Factors influencing abundance of butterflies and burnet moths in the uncultivated habitats of an organic farm in Denmark. Biol. Conserv. 2001, 98, 167-178. [CrossRef]
- Pöyry, J.; Lindgren, S.; Salminen, J.; Kuussaari, M. Restoration of butterfly and moth communities in semi-natural grasslands by cattle grazing. Ecol. Appl. 2004, 14, 1656-1670. [CrossRef]
- Kuussaari, M.; Heliölä, J.; Luoto, M.; Pöyry, J. Determinants of local species richness of diurnal Lepidoptera in boreal agricultural landscapes. Agric. Ecosyst. Environ. 2007, 122, 366-376. [CrossRef]
- Shreeve, T.G.; Dennis, R.L.H.; Roy, D.B.; Moss, D. An ecological classification of British butterflies: ecological attributes and biotope occupancy. J. Insect Conserv. 2001, 5, 145-161. [CrossRef]
- Warren, M. S.; Hill, J. K.; Thomas, J. A.; Asher, J.; Fox, R.; Huntley, B.; Roy, D.B.; Telfer, M.G.; Jeffcoate, S.; Harding, P.; Jeffcoate, G.; Willis, S.G.; Greatorex-Davies, J.N.; Moss, D.; Thomas, C. D. Rapid responses of British butterflies to opposing forces of climate and habitat change. Nature 2001, 414, 65-69. [CrossRef]
- Wilson, R. J.; Gutierrez, D.; Gutierrez, J.; Monserrat, V. J. An elevational shift in butterfly species richness and composition accompanying recent climate change. Global Change Biol. 2007, 13, 1873-1887. [CrossRef]
- Inoue, T. Chronosequential change in a butterfly community after clear-cutting of deciduous forests in a cool temperate region of central Japan. Entomol. Sci. 2003, 6, 151-163. [CrossRef]
- Kobayashi, T.; Kitahara, M.; Ohkubo, T.; Aizawa, M. Relationships between the age of northern Kantou plain (central Japan) coppice woods used for production of Japanese forest mushroom logs and butterfly assemblage structure. Biodiv. Conserv. 2010, 19, 2147-2166. [CrossRef]
- Ekroos, J.; Heliölä, J.; Kuussaari, M. Homogenization of lepidopteran communities in intensively cultivated agricultural landscapes. J. Appl. Ecol. 2010, 47, 459-467. [CrossRef]
- Slancarova, J.; Benes, J.; Kristynek, M.; Kepka, P.; Konvicka, M. Does the surrounding landscape heterogeneity affect the butterflies of insular grassland reserves? a contrast between composition and configuration. J. Insect Conserv. 2014, 18, 1-12. [CrossRef]
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).