Submitted:
17 April 2024
Posted:
18 April 2024
You are already at the latest version
Abstract
Keywords:
1. Introduction
2. Results
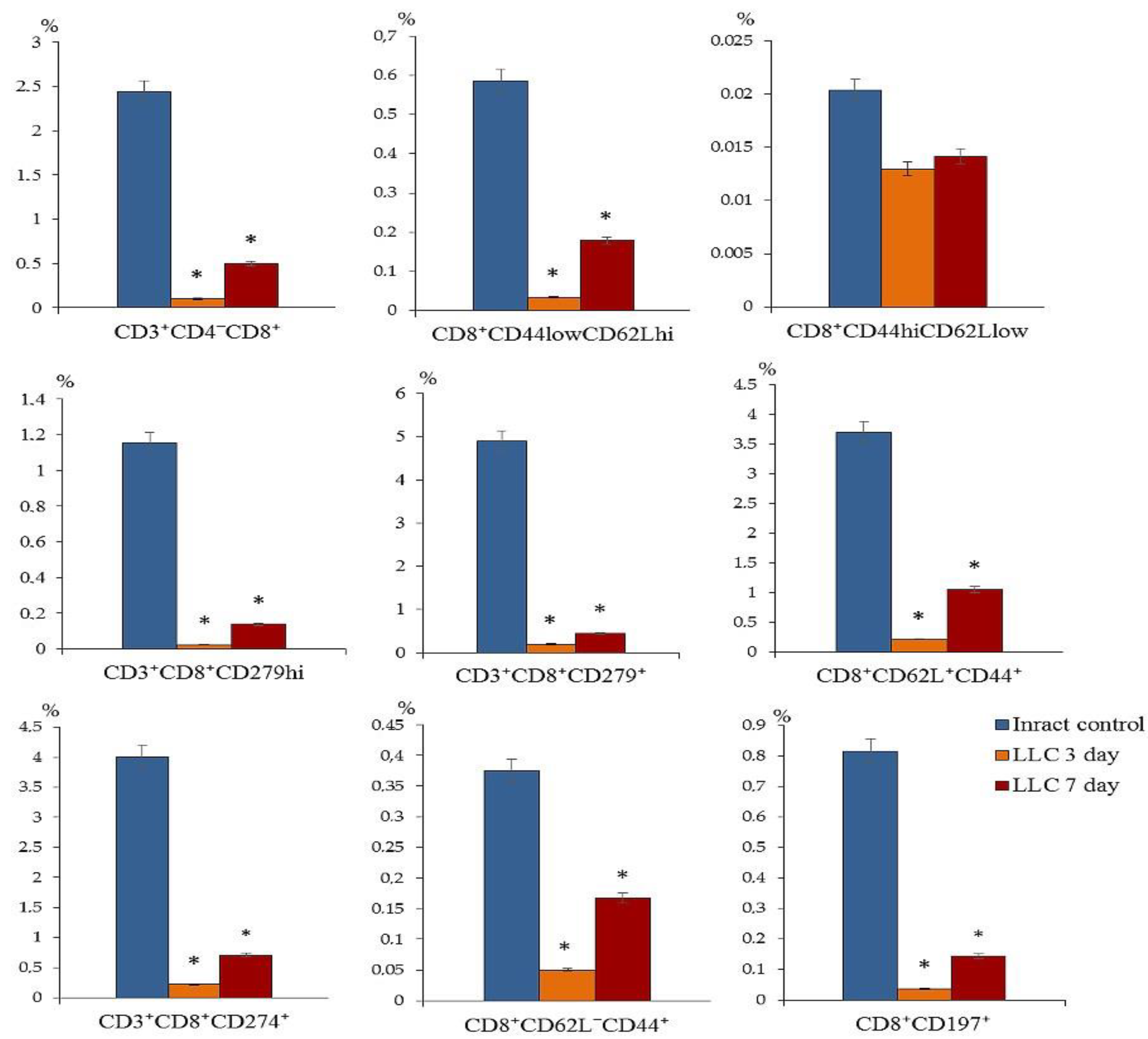
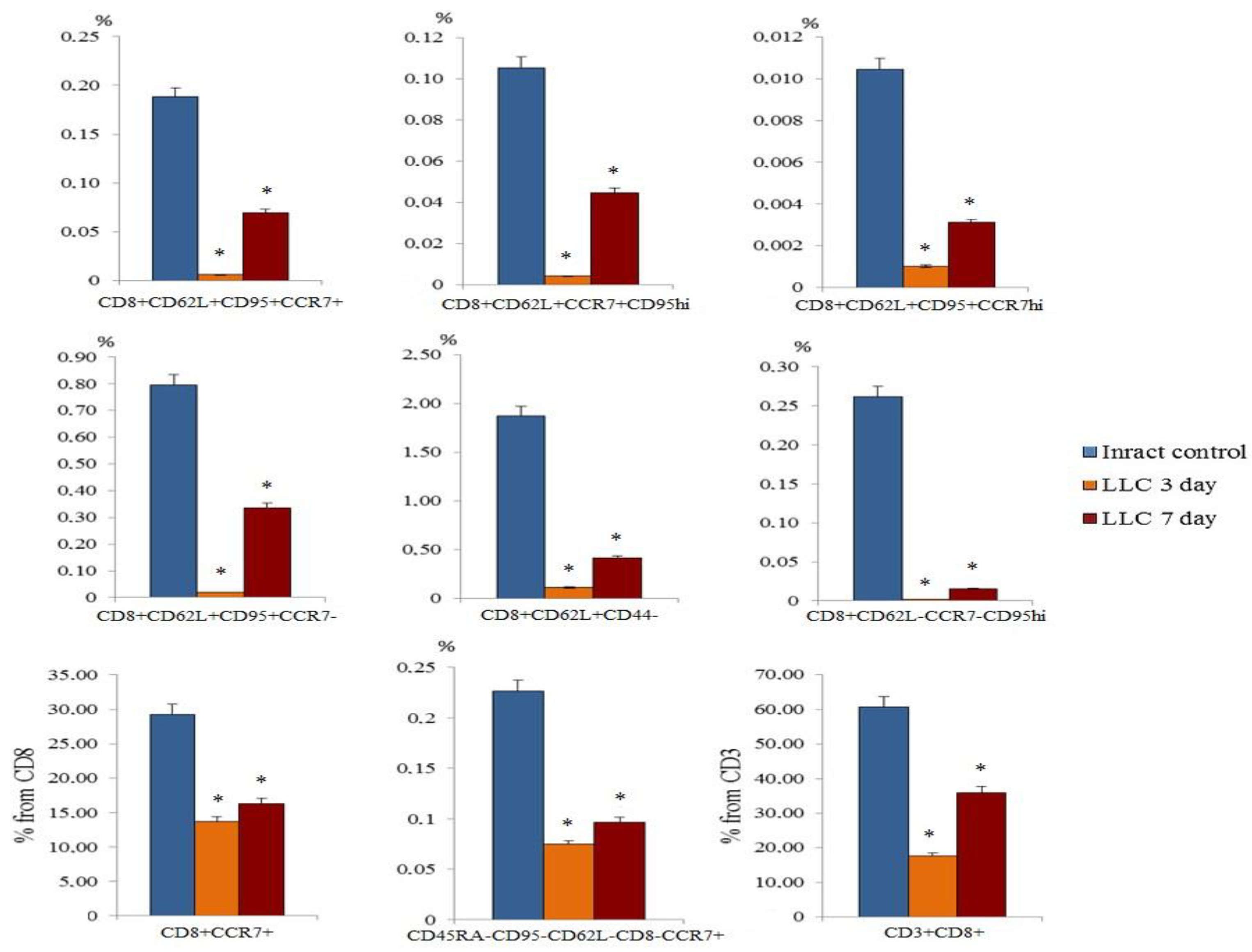
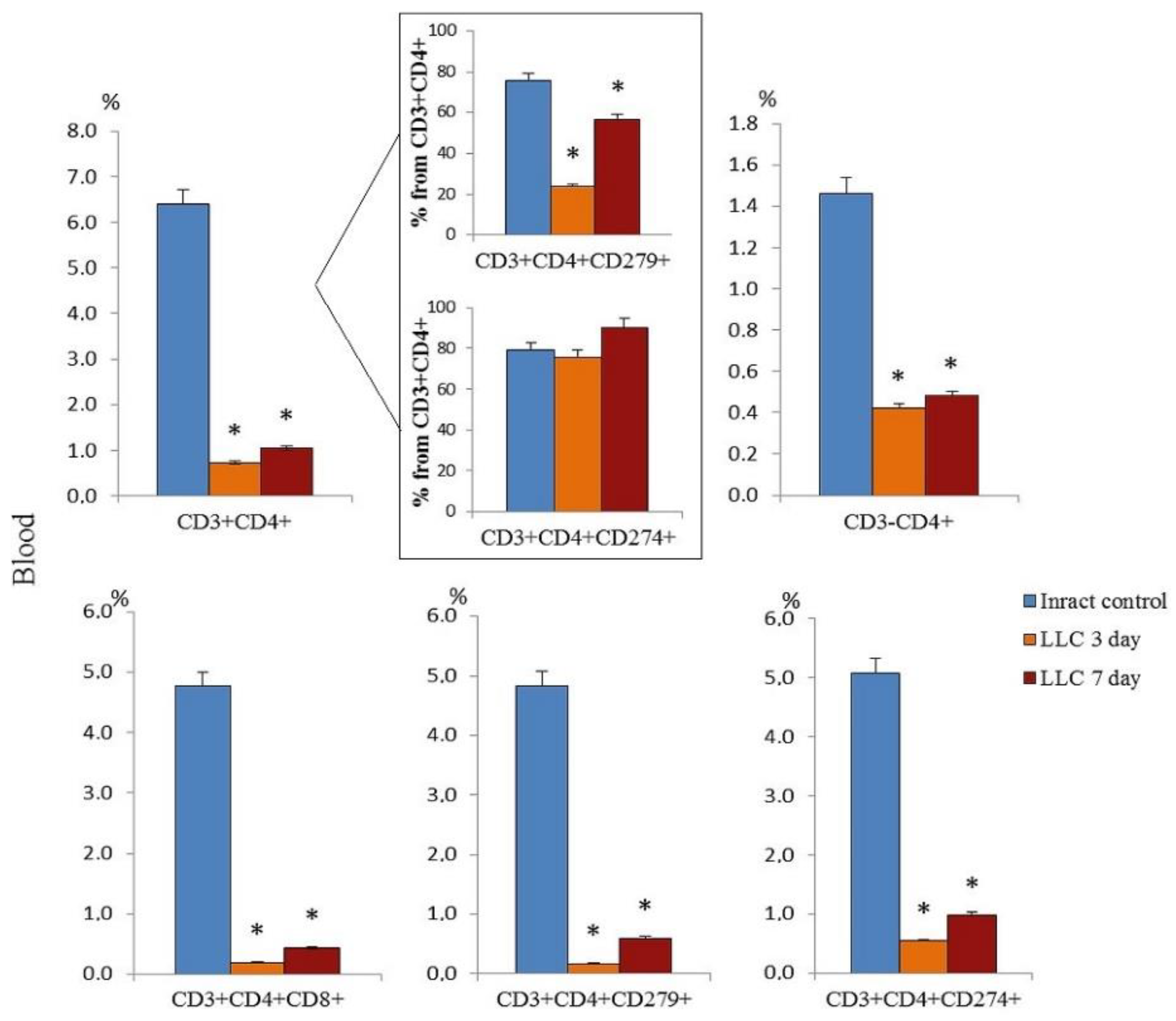
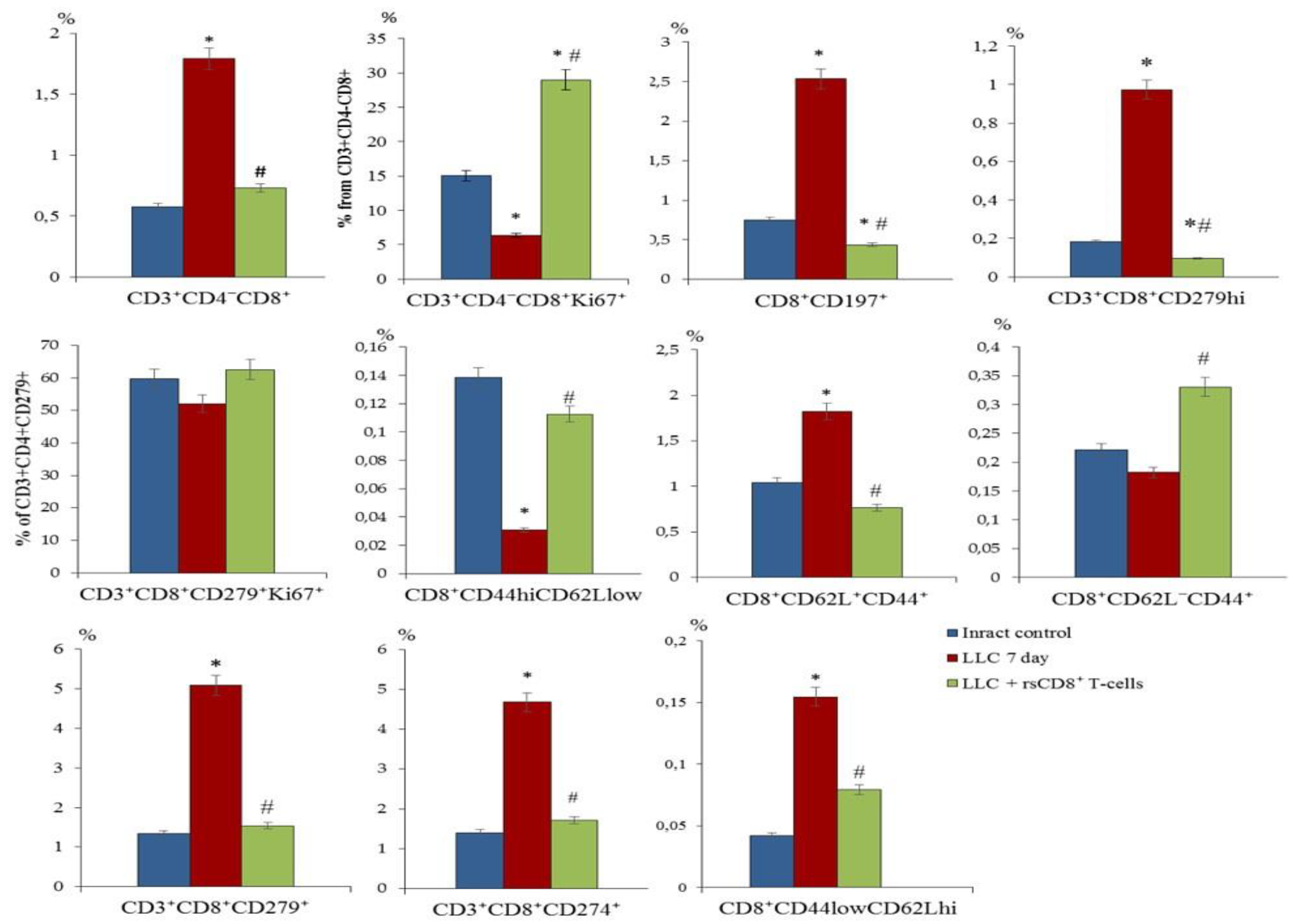
2.1. The Content of Different Populations of CD8+ T-Cells and CD4+ T-Cells in the Blood of Tumor-Bearing Mice after LLC Cell Implantation
2.2. The Amount of Circulating Tumor Cells in the Blood of Tumor-Bearing Mice after LLC Cell Implantation
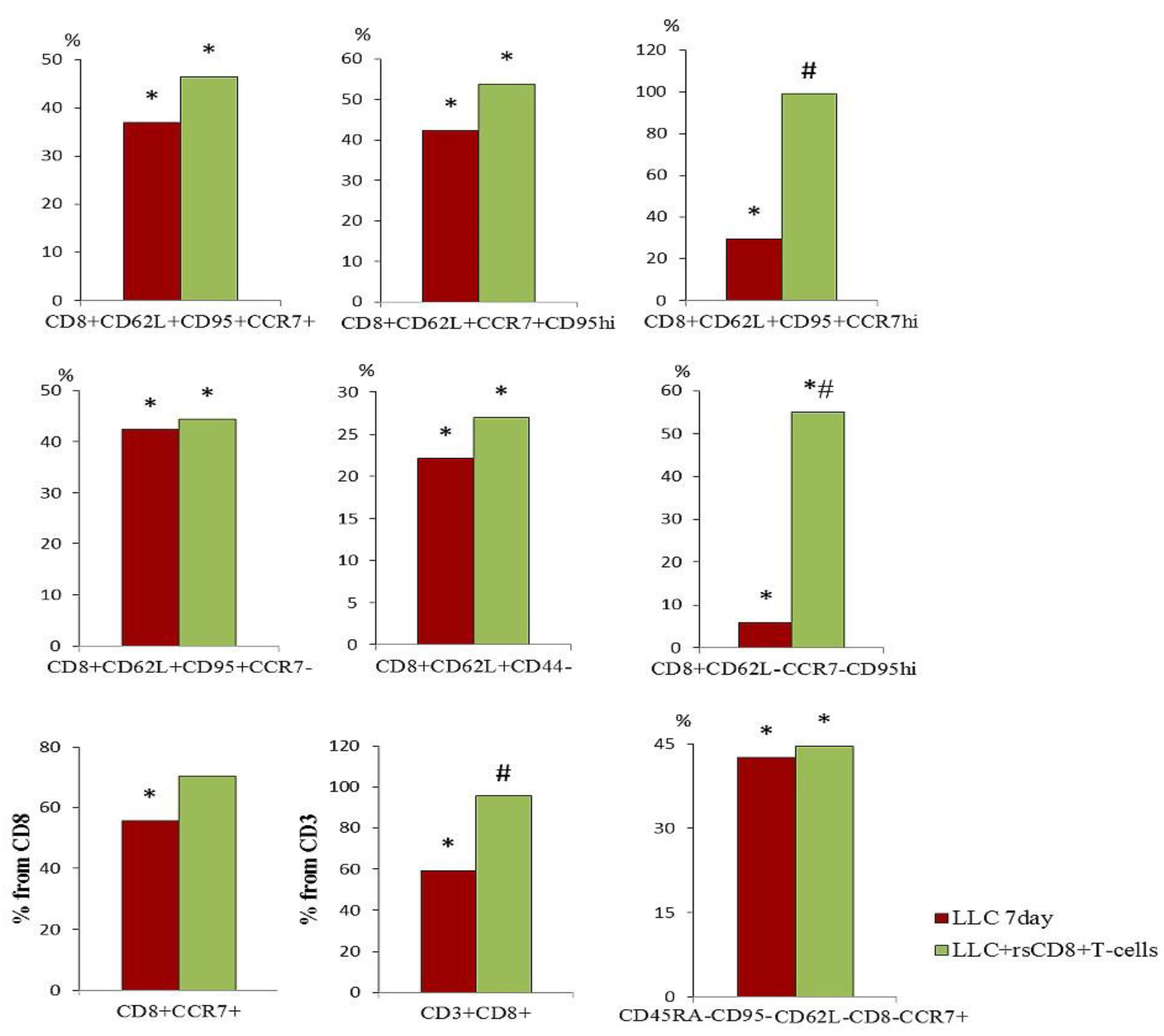
2.3. The Feature Assessment of rsCD8+T-Cells in Vitro and in Vivo
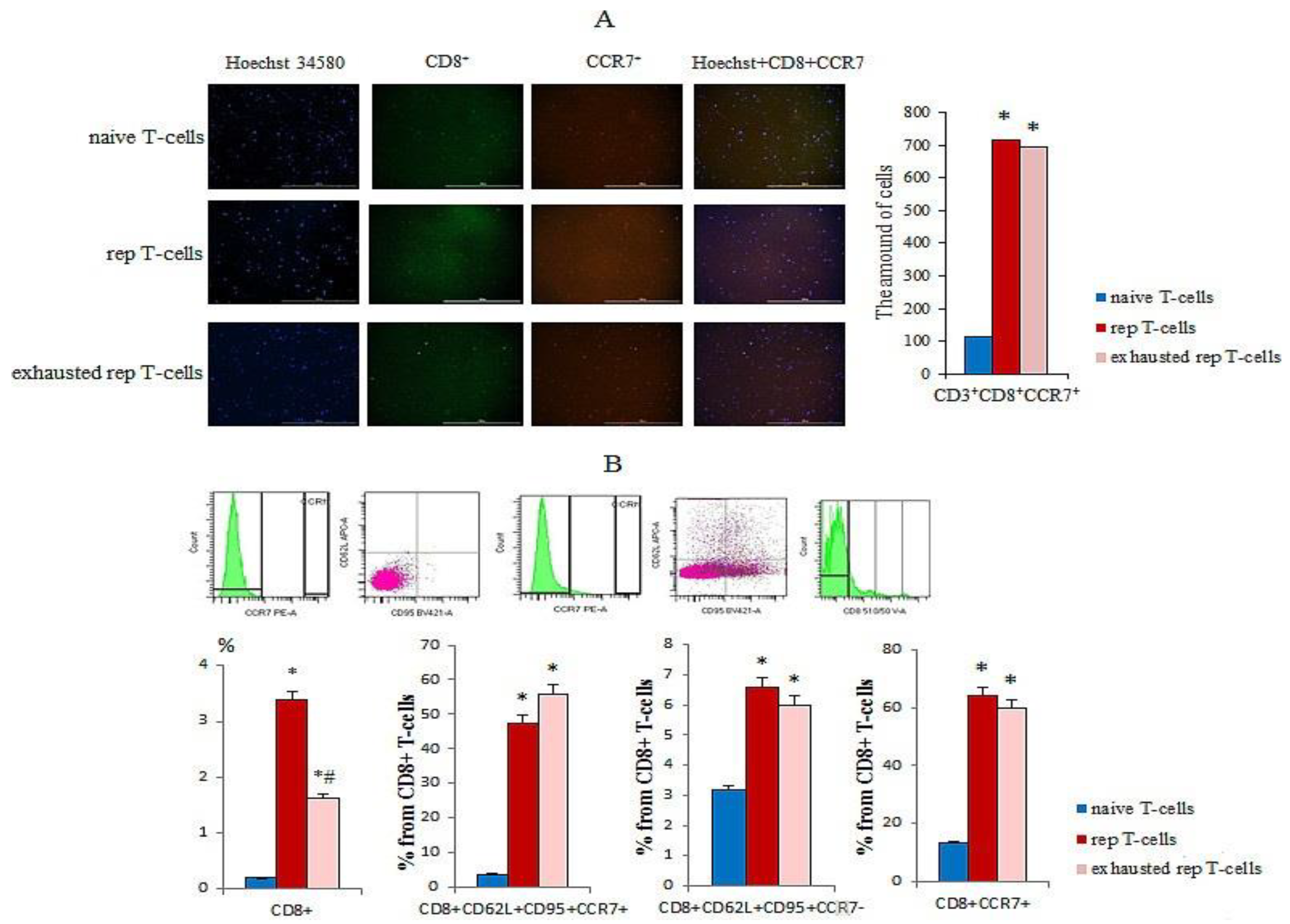
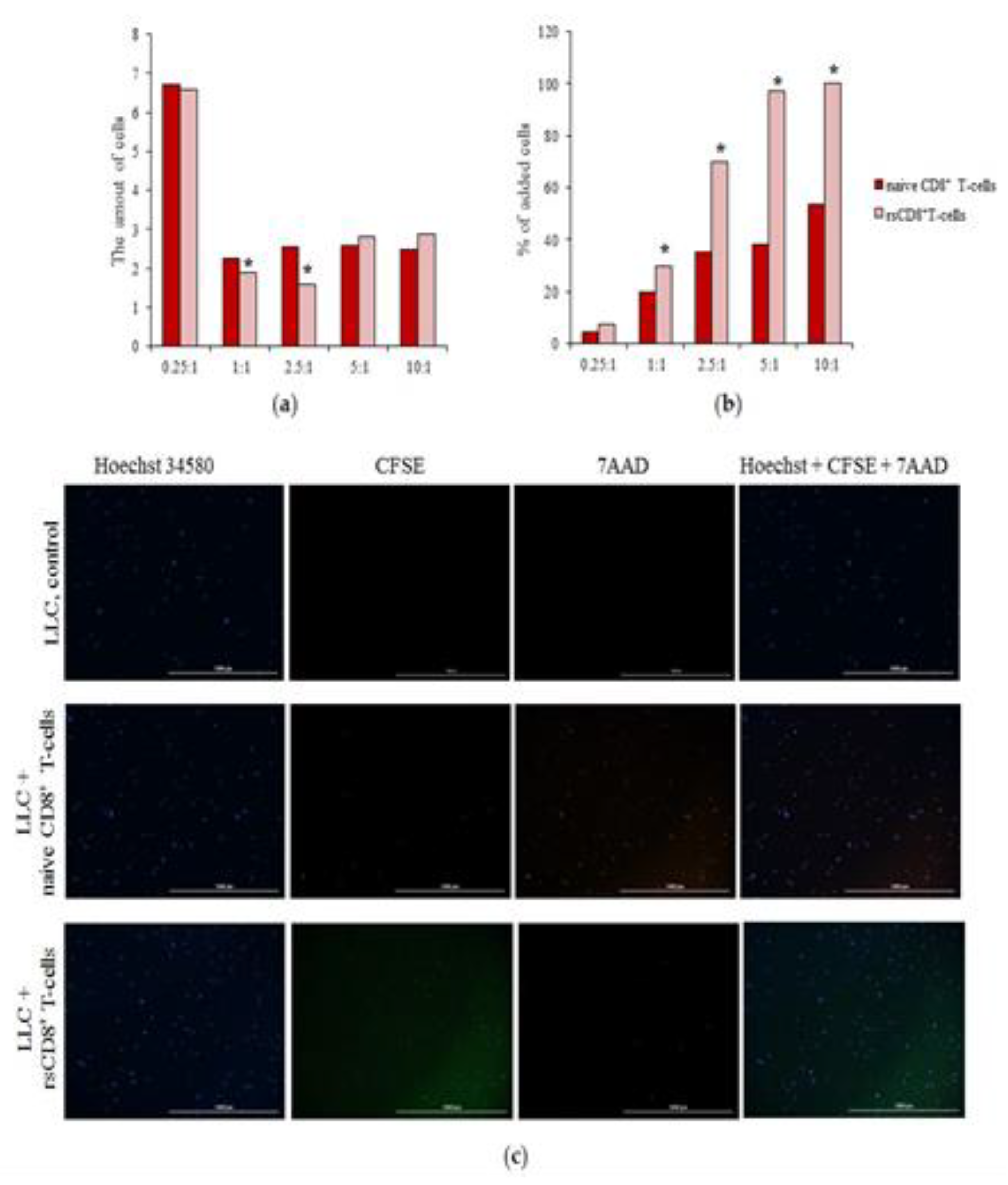
2.3.1. Study of Detection of the CCR7 Expression, Cytotoxicity, and Apoptosis of rsCD8+T-Cells in Vitro
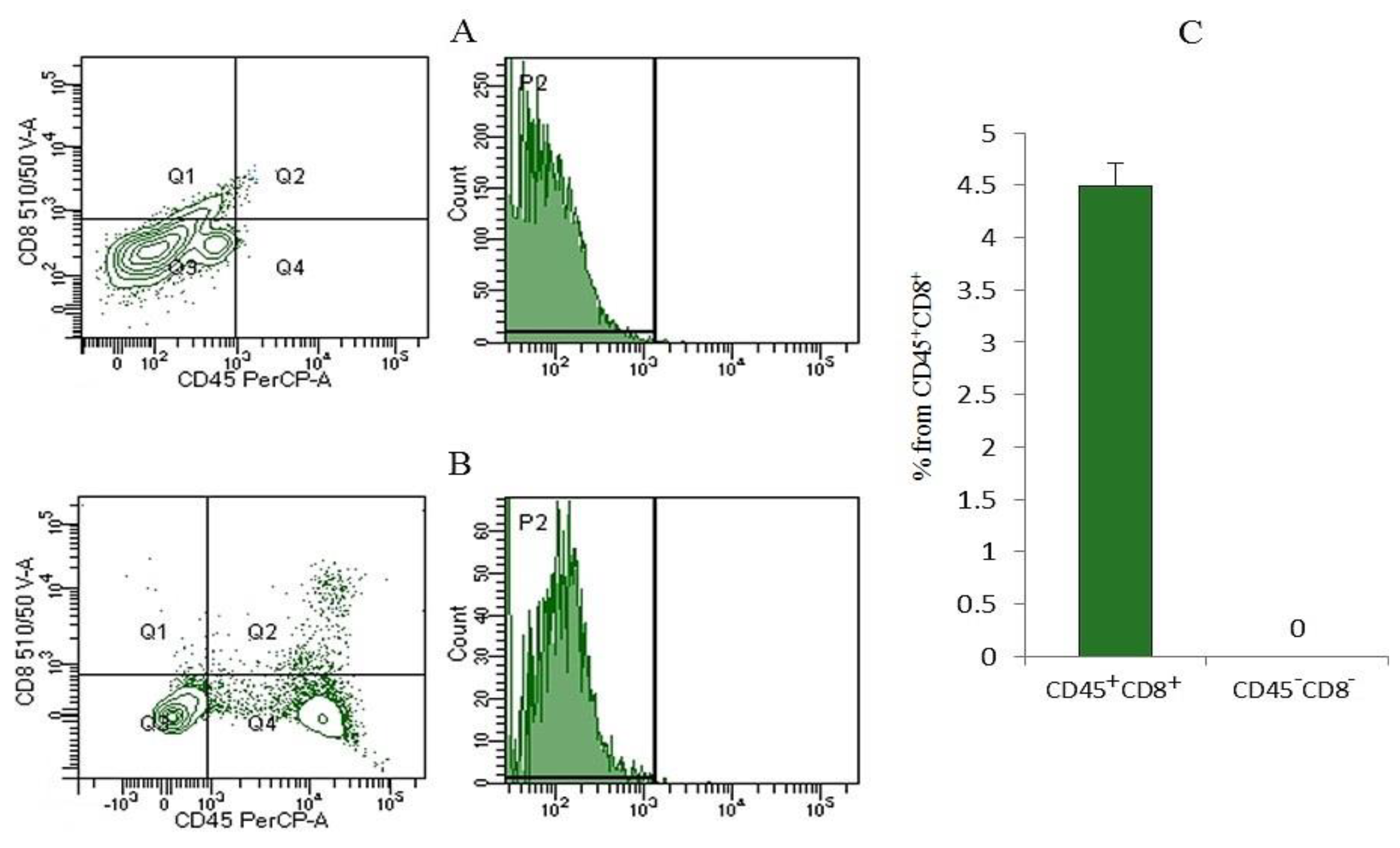
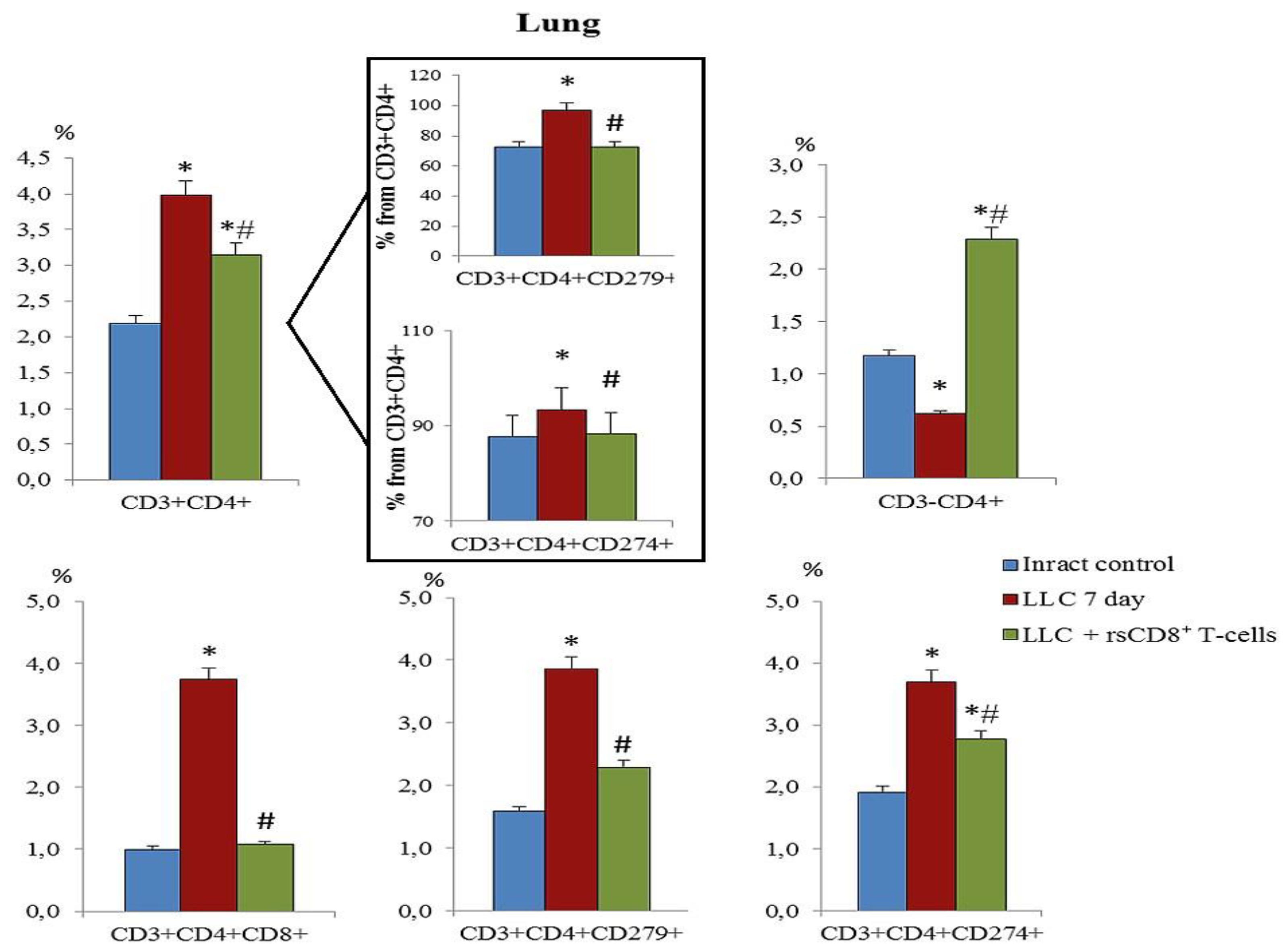
2.3.2. The rsCD8+ T-Cells Isolated from Mouse Spleen Migrated into the Lungs of Tumor-Bearing Mice
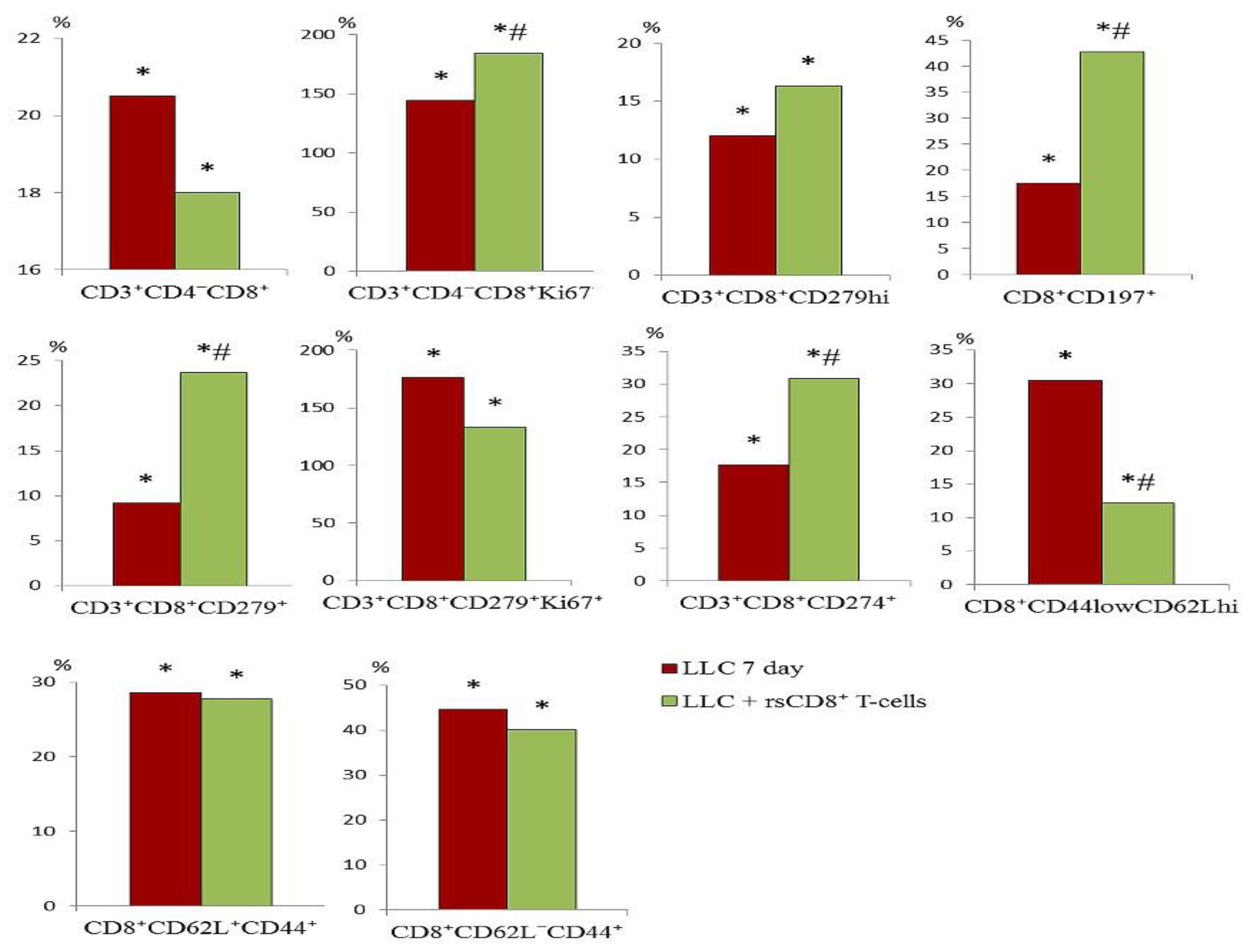
2.4. Effect of rsCD8+ T-Cells on the Content of CD8+ T-Cells in the Blood and Lungs of Tumor-Bearing Mice
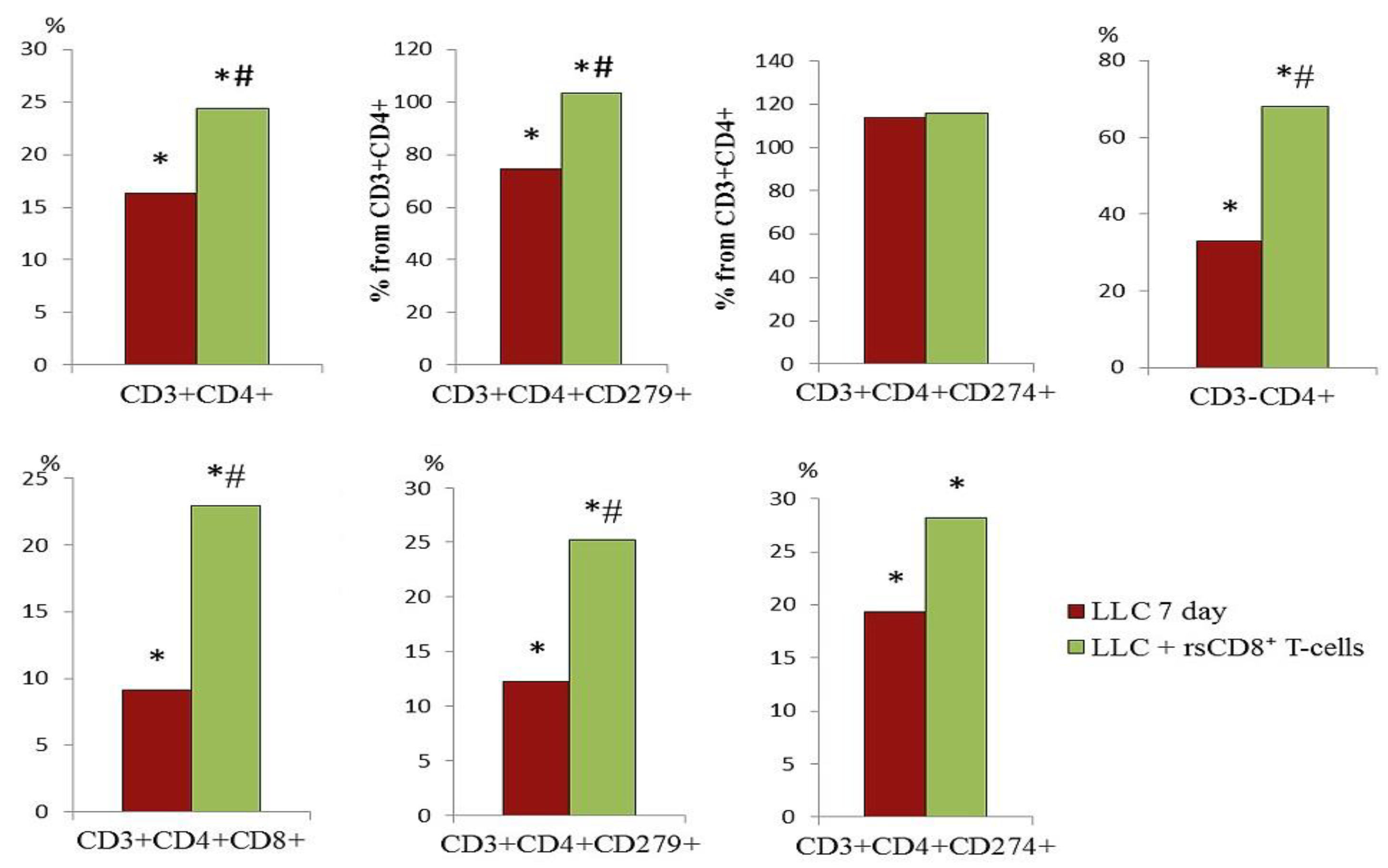
2.5. Effect of rsCD8+ T-Cells on the Content of CD4+ T-Cells in the Blood and Lungs of Tumor-Bearing Mice
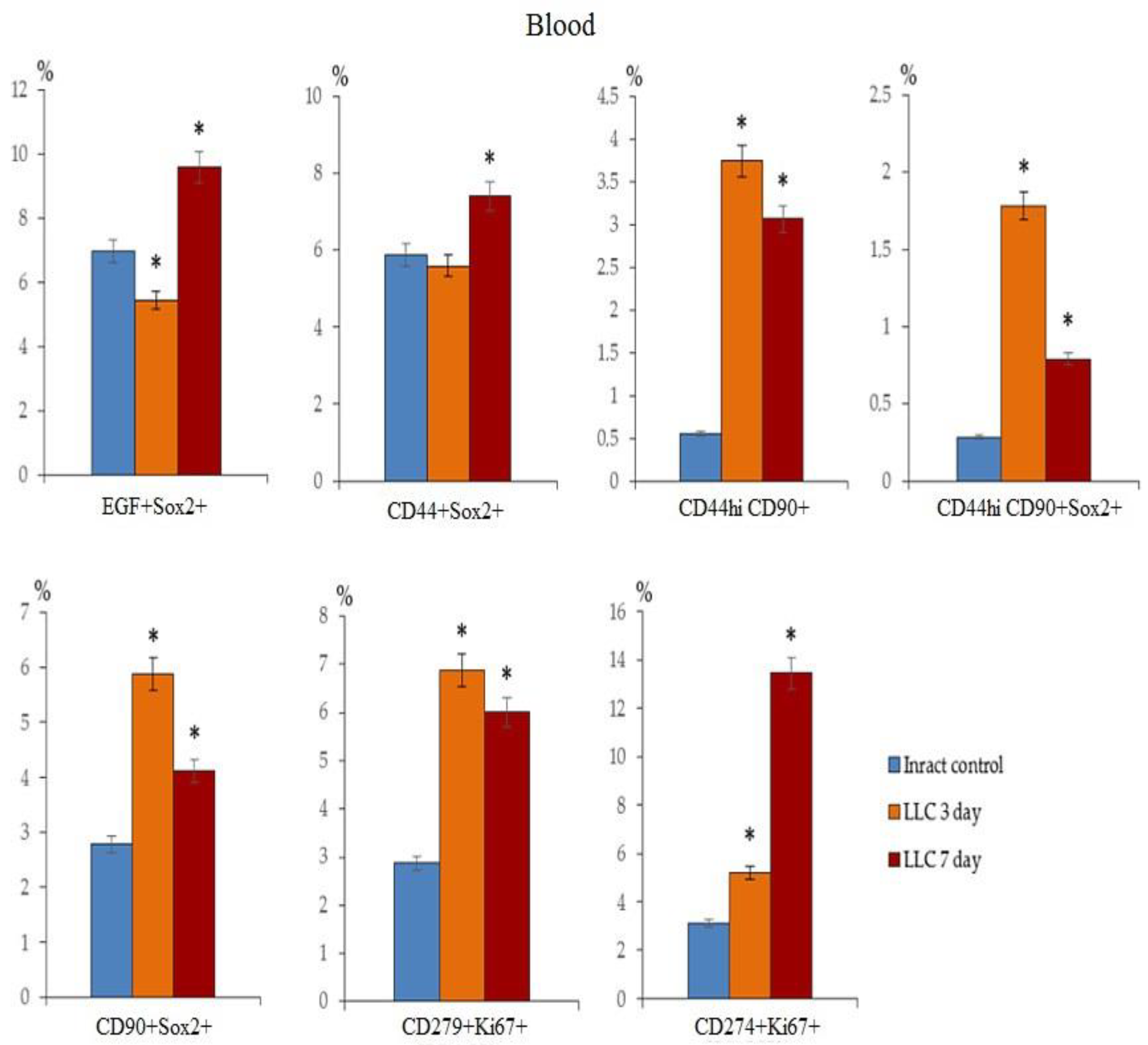
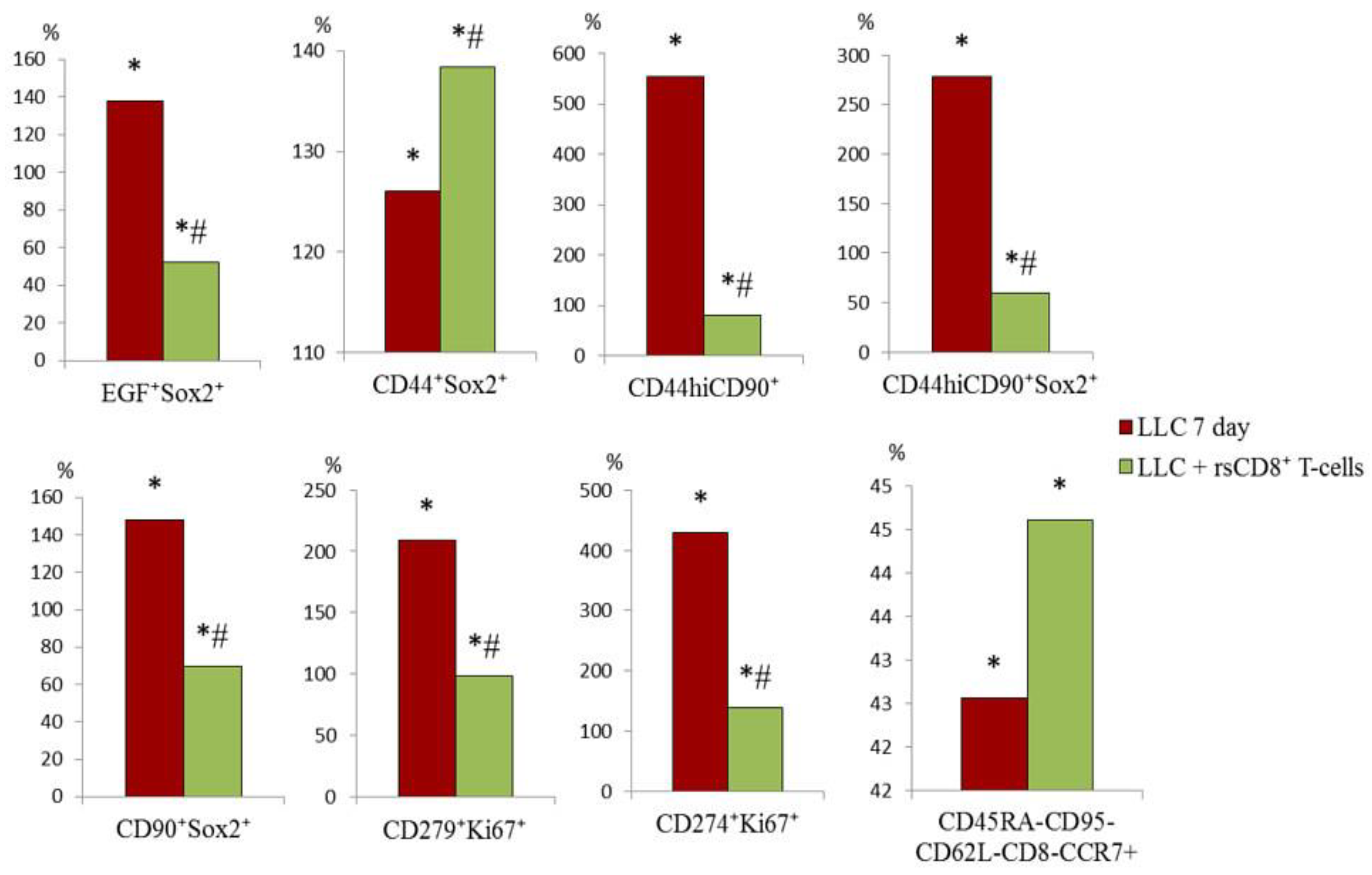
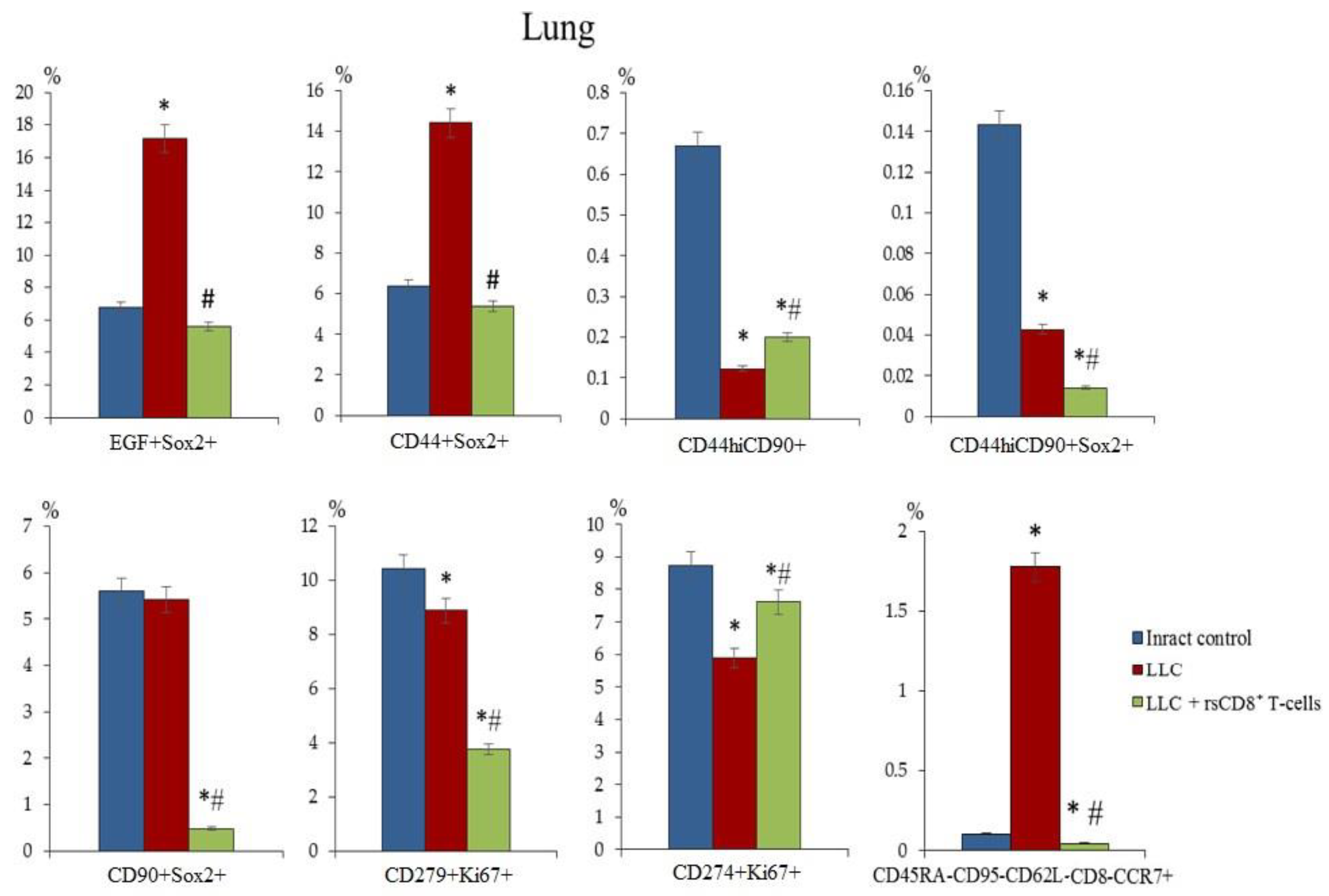
2.6. The rsCD8+ T-Cells Changes the Amount of Cancer Cells and Cancer Stem Cells in the Lungs and Blood of Tumor-Bearing Mice
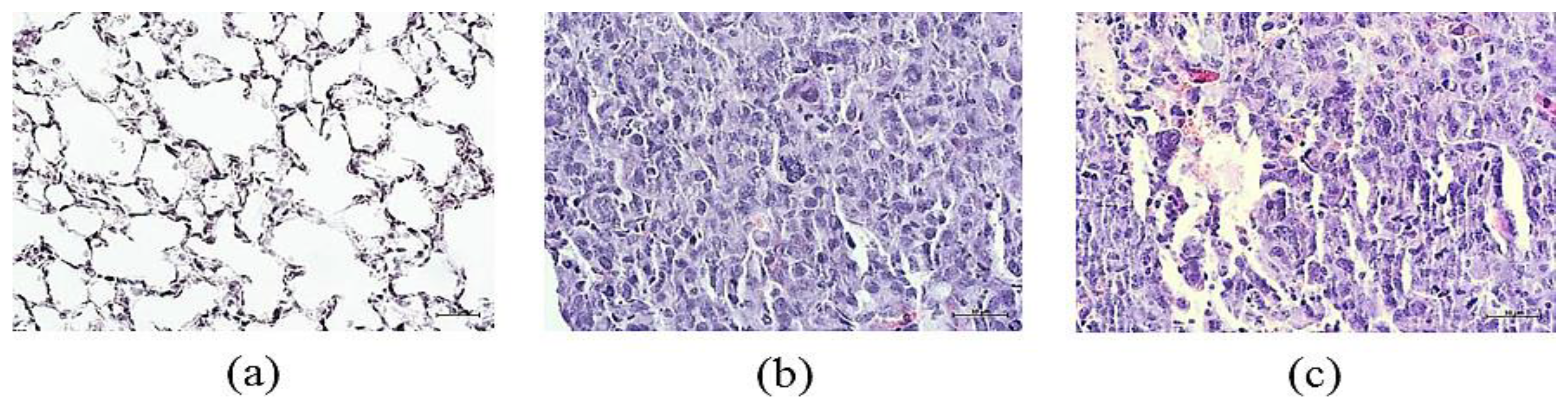
2.7. Lung Histology and Tumor Growth after rsCD8+ T-Cells Therapy
2.7.1. Lung Histology
2.7.2. Tumor Growth
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Lewis Lung Carcinoma Cell Line and Tumor Model
4.3. Isolation of Mononuclear Cells
4.4. Flow Cytometry
4.5. Magnetic Separation of CD8+ T-Cells
4.6. Reprogramming of Spleen CD8+ T-Cells
4.7. CD8+ T-Cells Injection
4.8. Detection of the CCR7 Expression, Cytotoxicity, and Apoptosis of rsCD8+T-Cells In Vitro
4.9. Histology of the Lungs
4.10. Assessment of Tumor Growth
4.11. Assessment of Tumor Volume
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stapelfeld, C.; Dammann, C.; Maser, E. Sex-specificity in lung cancer risk. Int. J. Cancer 2020, 146, 2376–2382. [Google Scholar] [CrossRef] [PubMed]
- Rossi, F.; Noren, H.; Jove, R.; Beljanski, V.; Grinnemo, K.H. Differences and similarities between cancer and somatic stem cells: therapeutic implications. Stem cell research & therapy 2020, 11, 489. [Google Scholar] [CrossRef] [PubMed]
- Poncette, L.; Bluhm, J.; Blankenstein, T. The role of CD4 T cells in rejection of solid tumors. Curr Opin Immunol. 2022, 74, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Raskov, H.; Orhan, A.; Christensen, J.P.; Gögenur, I. Cytotoxic CD8+ T-cells in cancer and cancer immunotherapy. Br. J. Cancer. 2021, 124, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- Skurikhin, E.G.; Pershina, O.; Ermakova, N.; Pakhomova, A.; Widera, D.; Zhukova, M.; Pan, E.; Sandrikina, L.; Kogai, L.; Kushlinskii, N.; et al. Reprogrammed CD8+ T-Lymphocytes Isolated from Bone Marrow Have Anticancer Potential in Lung Cancer. Biomedicines 2022, 10, 1450. [Google Scholar] [CrossRef] [PubMed]
- Skurikhin, E.G.; Pershina, O.; Ermakova, N.; Pakhomova, A.; Zhukova, M.; Pan, E.; Sandrikina, L.; Widera, D.; Kogai, L.; Kushlinskii, N.; Kubatiev, A.; Morozov, S.G.; Dygai, A. Cell Therapy with Human Reprogrammed CD8+ T-Cells Has Antimetastatic Effects on Lewis Lung Carcinoma in C57BL/6 Mice. Int. J. Mol. Sci. 2022, 23, 15780. [Google Scholar] [CrossRef] [PubMed]
- Salem, A.; Alotaibi, M.; Mroueh, R.; Basheer, H. A.; Afarinkia, K. CCR7 as a therapeutic target in Cancer. Biochimica et biophysica acta. Reviews on cancer 2021, 1875, 188499. [Google Scholar] [CrossRef]
- Xiong, Y.; Huang, F.; Li, X.; Chen, Z.; Feng, D.; Jiang, H.; Chen, W.; Zhang, X. CCL21/CCR7 interaction promotes cellular migration and invasion via modulation of the MEK/ERK1/2 signaling pathway and correlates with lymphatic metastatic spread and poor prognosis in urinary bladder cancer. International journal of oncology. 2017, 51, 75–90. [Google Scholar] [CrossRef]
- Czystowska, M.; Gooding, W.; Szczepanski, M.J.; Lopez-Abaitero, A.; Ferris, R.L.; Johnson, J.T.; Whiteside, T.L. The immune signature of CD8(+)CCR7(+) T cells in the peripheral circulation associates with disease recurrence in patients with HNSCC. Clin Cancer Res. 2013, 15, 889–899. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics,2020. CA Cancer J Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sathaliyawala, T.; Kubota, M.; Yudanin, N.; Turner, D.; Camp, P.; Thome, J.J.; Bickham, K.L.; Lerner, H.; Goldstein, M.; Sykes, M.; et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013, 38, 187–197. [Google Scholar] [CrossRef] [PubMed]
- Gattinoni, L.; Speiser, D.E.; Lichterfeld, M.; Bonini, C. T memory stem cells in health and disease. Nat. Med. 2017, 23, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Jafarzadeh, N.; Boi, S.; Kundu, S.; Jiang, Z.; Fan, Y.; Lopez, J.; Nandre, R.; Zeng, P.; Alolaqi, F.; et al. MEK inhibition reprograms CD8+ T lymphocytes into memory stem cells with potent antitumor effects. Nat. Immunol. 2021, 22, 53–66. [Google Scholar] [CrossRef]
- Abken, H. Adoptive therapy with car redirected T cells: The challenges in targeting solid tumors. Immunotherapy 2015, 7, 535–544. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, H.; Mahmud, A.R.; Siddiquee, M.F.; Shahriar, A.; Biswas, P.; Shimul, M.E.K.; Ahmed, S. Z.; Ema, T.I.; Rahman, N.; Khan, M.A.; Mizan, M.F.R.; Emran, T. B. Role of T cells in cancer immunotherapy: Opportunities and challenges. Cancer pathogenesis and therapy 2022, 1, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Marcu, L.G.; Moghaddasi, L.; Bezak, E. Cannot Target What Cannot Be Seen: Molecular Imaging of Cancer Stem Cells. International journal of molecular sciences 2023, 24, 1524. [Google Scholar] [CrossRef] [PubMed]
- Poggiana, C.; Rossi, E.; Zamarchi, R. Possible role of circulating tumor cells in early detection of lung cancer. Journal of thoracic disease 2020, 12, 3821–3835. [Google Scholar] [CrossRef] [PubMed]
- Sathaliyawala, T.; Kubota, M.; Yudanin, N.; Turner, D.; Camp, P.; Thome, J.J.; Bickham, K.L.; Lerner, H.; Goldstein, M.; Sykes, M.; Kato, T.; Farber, D.L. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity 2013, 38, 187–197. [Google Scholar] [CrossRef]
- Speiser, D.E.; Ho, P.C.; Verdeil, G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol. 2016, 16, 599–611. [Google Scholar] [CrossRef]
- Golubovskaya, V.; Wu, L. Different Subsets of T Cells, Memory, Effector Functions, and CAR-T Immunotherapy. Cancers 2016, 8, 36. [Google Scholar] [CrossRef] [PubMed]
- van der Leun, A.M.; Thommen, D.S.; Schumacher, T.N. CD8+ T cell states in human cancer: insights from single-cell analysis. Nature reviews. Cancer 2020, 20, 218–232. [Google Scholar] [CrossRef] [PubMed]
- Misslitz, A.; Pabst, O.; Hintzen, G.; Ohl, L.; Kremmer, E.; Petrie, H.T.; Förster, R. Thymic T cell development and progenitor localization depend on CCR7. The Journal of experimental medicine 2004, 200, 481–491. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Wu, Z.; Zuo, S.; Zhou, Y.; Chen, Y.; Wang, X. Establishment of orthotopic Lewis lung cancer model in mouse. Zhongguo Fei Ai Za Zhi 2010, 13, 42–47. [Google Scholar] [CrossRef] [PubMed]
- Justilien, V.; Fields, A.P. Utility and applications of orthotopic models of human non-small cell lung cancer (NSCLC) for the evaluation of novel and emerging cancer therapeutics. Curr. Protoc. Pharmacol. 2013, 62, 14.27.1–14.27.17. [Google Scholar] [CrossRef] [PubMed]
- Cardiff, R.D.; Miller, C.H.; Munn, R.J. Manual hematoxylin and eosin staining of mouse tissue sections. Cold Spring Harb. Protoc. 2014, 2014, 655–658. [Google Scholar] [CrossRef] [PubMed]
- Hather, G.; Liu, R.; Bandi, S.; Mettetal, J.; Manfredi, M.; Shyu, W.C.; Donelan, J.; Chakravarty, A. Growth rate analysis and efficient experimental design for tumor xenograft studies. Cancer Inform. 2014, 13, 65–72. [Google Scholar] [CrossRef]
- Tomayko, M.M.; Reynolds, C.P. Determination of subcutaneous tumor size in athymic (nude) mice. Cancer Chemother. Pharmacol. 1989, 24, 148–154. [Google Scholar] [CrossRef]
| Values/Parameters | Intact Control | LLC | rsCD8+ T-cells |
|---|---|---|---|
| Tumor volume, mm3 | 0 | 4.72 ± 2.85 # | 2.09±0.09 #,* |
| The average number of metastases | 0 | 2.60 ± 0.35 #,1 | 0.17±0.17#,* |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).





